Abstract
Rodent and clinical studies have documented that myeloid cell infiltration of tumors is associated with poor outcomes, neutrophilia and lymphocytopenia. This contrasts with increased lymphocyte infiltration of tumors, which is correlated with improved outcomes. Lifestyle parameters, such as obesity and diets with high levels of saturated fat and/or omega (ω)-6 polyunsaturated fatty acids (PUFAs), can influence these inflammatory parameters, including an increase in extramedullary myelopoiesis (EMM). While tumor secretion of growth factors (GFs) and chemokines regulate tumor-immune-cell crosstalk, lifestyle choices also contribute to inflammation, abnormal pathology and leukocyte infiltration of tumors. A relationship between obesity and high-fat diets (notably saturated fats in Western diets) and inflammation, tumor incidence, metastasis and poor outcomes is generally accepted. However, the mechanisms of dietary promotion of an inflammatory microenvironment and targeted drugs to inhibit the clinical sequelae are poorly understood. Thus, modifications of obesity and dietary fat may provide preventative or therapeutic approaches to control tumor-associated inflammation and disease progression. Currently, the majority of basic and clinical research does not differentiate between obesity and fatty acid consumption as mediators of inflammatory and neoplastic processes. In this review, we discuss the relationships between dietary PUFAs, inflammation and neoplasia and experimental strategies to improve our understanding of these relationships. We conclude that dietary composition, notably the ratio of ω−3 vs ω−6 PUFA regulates tumor growth and the frequency and sites of metastasis that together, impact overall survival (OS) in mice.
Keywords: Inflammation, Immune escape, Tumor induction, Tumor progression, TAM, Infiltration, MDSC, PUFA, High fat diet
Introduction
Diet composition affects the onset and progression of chronic degenerative diseases, including cancer, that are controlled in part by inflammatory processes.1, 2 Growing evidence indicates that diet and its composition critically influence human health and immunity via secretion of adipokines, and their regulation of metabolic pathways. Dietary ω−3 PUFA has been a focus due to its anti-inflammatory, immunomodulatory and potential anticancer activity.1, 3, 4 In this review, we discuss the systemic expansion, as well as, local/regional infiltration of immune and myeloid cells, which can support or inhibit tumor initiation and progression in a phenotypic dependent manner. As an example, tumor-associated macrophages (TAMs) have direct tumoricidal activity and can induce antitumor T-cell responses; but can also suppress cytotoxic T-lymphocyte (CTL) numbers and functions; thereby, facilitating tumor growth and progression (Figure 1). Tumor infiltration by myeloid cells is regulated, in part, by tumor-secreted GFs and chemokines, and as discussed herein, PUFAs, all of which control the migration, expansion and tissue infiltration of myeloid progenitors5, 6. Growing epidemiological, experimental, and clinical evidence suggests that ω−3 PUFAs have a role in the control of neoplastic cell growth and relapse and, by improving the efficacy of radiation and chemotherapy, reduce therapy-associated secondary complications.7, 8 These bioactivities may be related to the immunomodulatory and anti-inflammatory activities of ω−3 PUFA, as this can influence inflammatory processes, notably those induced by ω−6 PUFA bioactivity.1, 3, 4
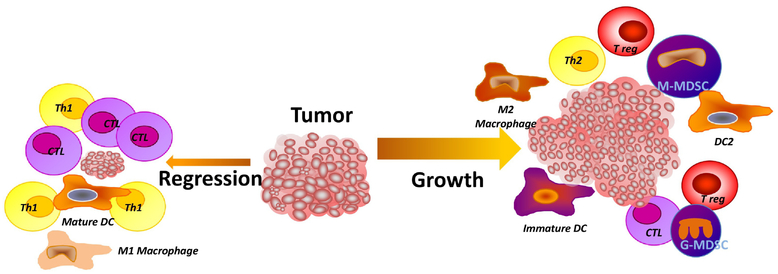
Fig. 1.
Leukocytes that infiltrate a tumor can regulate their growth rate, progression and may facilitate metastasis. Tumor regression is associated with tumor infiltration by dendritic cells (DCs), cytotoxic T cells (CTL) and type 1 T-helper cells (Th-1). Contrasting with this, tumor growth is facilitated by immune mediated immunosuppression and neoangiogenesis in association with infiltration by myeloid-derived suppressor cells, (MDSCs), immature DCs, pDCs, M2 macrophages, as well as T regulatory (T-reg) cells and a low frequency of CD4+ and CD8+ effector T cells. Further, the expansion of and infiltration by myeloid cell populations, including immunosuppressive sub-populations, is regulated in part, by colony stimulating factors (CSFs), and chemokines secreted by tumor cells, dietary ω−6 poly-unsaturated fatty acids (PUFAs) and saturated fatty acids (SFAs).
Although, myeloid cell infiltration of tumors is predominantly pro-tumorigenic9, myeloid cells can also inhibit tumor growth including activated macrophage tumor cytotoxicity as measured in vitro10. Despite the lack of a correlation between immunogenicity, metastatic propensity and the frequency of TAMs11–13, TAM infiltration is associated with poor outcomes14 and rapid disease progression15, 16. Myeloid-derived suppressor cells (MDSCs) are increased in the circulation of tumor bearing (TB) hosts and within tumors, and are associated with an immunosuppressive tumor microenvironment17–21. This immunosuppressive activity occurs via multiple mechanisms, including reactive oxygen species (ROS), nitric oxide (NO) synthetase (NOS-2) and arginase, as well as the secretion of immunosuppressive cytokines22. Preclinical and clinical studies have shown that macrophages and MDSCs can stimulate tumor growth11, 23, while immune augmenting M1 macrophages and/or dendritic cells (DC) −1 cells contribute to antitumor T-cell responses, although, often insufficient for tumor rejection24. A low frequency of M1 macrophages, or an increase in infiltrating M2 macrophages, DC2s, and MDSCs are associated with a poor prognosis and an increase in tumor relapse following primary tumor resection25, 26. In contrast to tumor infiltration by myeloid cells, numerous clinical studies have demonstrated a correlation between tumor-infiltrating lymphocytes (TILs) and disease free survival (DFS) and OS in cancer patients27, 28. However, the relationship is dependent on the infiltrating lymphocyte phenotype, density and location. For example, infiltration by CD4+ T-regulatory (T-reg) cells is associated with poor outcomes, while infiltration by CD8+ cytotoxic effector cells is associated with positive outcomes29. In this review, we focus on the potential role of ω−3 PUFAs as a therapeutic adjuvant agent, highlighting their immunomodulatory effects and potential for beneficial effects, as well as the pro-tumorigenic activity of the inflammatory ω−6 PUFAs.
Tumor Infiltrating Inflammatory Cells and Patient Outcomes.
Human health and disease are controlled by both genetic and environmental factors. Numerous studies have examined the relationship between dietary habits and the types and amounts of essential fatty acids, particularly PUFA and resultant tumor development30. The ω−3 PUFAs have anti-cancer effects based on in vitro and in vivo studies31–33. Thus, the addition of eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA) to tumor cell cultures, but not normal cells, is cytotoxic34. This bioactivity is predominantly associated with long-chain (LC) ω−3 PUFA as compared to the shorter chain plant ω−3, α-linolenic acid (ALA)35. Perhaps of greater import is the finding that co-culture or pre-exposure of tumor cells with LC ω−3 PUFA enhances the cytotoxicity of antimitotic and other chemotherapeutic drugs against tumor cells36. Several additional anticancer mechanisms have been proposed including an alteration in the growth of tumor cells, interference with the cell cycle, increasing cell death via necrosis or apoptosis37, 38, inhibition of angiogenesis and metastasis39 and down regulation of inflammation and inflammatory cell infiltration of tumors40, 41. Based on these and other studies LC ω−3 PUFAs are being examined in a therapeutic context in combination with traditional adjuvant therapy in patients with cancer42. Similar to inflammatory cells, tumor specific CTLs can also infiltrate tumors, and their frequency is predictive of improved outcomes43. However, a subset of CD4+ infiltrating lymphocytes can suppress antitumor T-cell functions and are identified as suppressive T-cells (i.e., T-regulatory cells (T-regs)). In contrast, infiltrating CD8+ CTLs have anti-tumor activity but generally occur at low frequencies and with low avidities44 such that, they have minimal ability to control tumor growth45. Nonetheless, a high frequency of tumor infiltrating T-cells (primarily CD8+ T-cells) is associated with improved outcomes46–53.
A better understanding of the regulatory events and pharmacophores, such as the PUFAs that can regulate tumor infiltration with antitumor T-cells, is needed to predict outcomes and develop novel therapeutic modalities54. In addition to immunoregulatory activity by infiltrating immune cells, some tumor and myeloid cells, including macrophages, polymorphonuclear neutrophils (PMN) and MDSCs, express immunosuppressive checkpoint mediators, such as PD-L155, resulting in cellular interactions that inhibit T-cell proliferation and functions. An effective antitumor immune response can occur, following a coordinated response, by innate and adaptive immune cells. Critical components include DC and macrophage presentation of tumor associated antigens (TAAs) and neoantigens, upregulation of costimulatory ligands, and increased cytokine and chemokine secretion, resulting in the induction of CTL responses, migration of activated T-cells to the tumor microenvironment, and T-cell tumoricidal activity. Tumor-specific T-cell responses also contribute to the recruitment of innate effector cells to the tumor microenvironment, including macrophages, DCs, gamma/delta (γ/δ) T-cells, natural killer (NK) cells and natural killer T-cells (NKT) cells, which are capable of tumor cell cytotoxicity, independent of T-cell receptor (TCR)-mediated T-cell recognition. Nonetheless, the immune system has developed potent mechanisms to maintain homeostasis and limit potentially dangerous complications due to an exuberant immune response. These negative regulatory feedback loops, are usurped by tumors, allowing them to evade immune surveillance, inhibit CTL responses, resulting in obstacles to the initiation and propagation of successful antitumor adaptive immune responses. The cellular mediators, mechanisms of action and molecular mediators of both pro- and anti-tumorigenic infiltrating cells are summarized in Table 1 and 2.
Table 1.
Tumor Inhibitory Cellular Mediators
| Cellular Mediators | Functions | Molecular Mediators |
|---|---|---|
| Cluster of differentiation 4 positive (CD4+) T helper 1 cell (Th1) | Immune recognition of tumor associate antigens (TAAs) bound to major histocompatibility complex (MHC) class II molecules; contribution to dendritic cell (DC) and macrophage activation; natural killer (NK) and natural killer T cell (NKT) cell activation; cytotoxic T-lymphocyte (CTL) generation; induction of B-cell differentiation. | IL-2, interferon gamma (IFN-γ). interleukin 12 (IL-12), tumor necrosis factor-beta (TNF-β), |
| CD8+ CTL | Immune recognition of TAAs bound to MHC class I molecules; | perforin, granzyme B, Fas ligand (FasL); IFN-γ, IL-2, |
| M1 (classically activated macrophages) | TAA presentation; expression of co-stimulatory molecules; Fc gamma receptor (FcR)-mediated antibody -dependent cellular cytotoxicity (ADCC). | IL-1β and TNF-α; release of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) |
| CD4+ Th17 | Stimulation and expansion of Th1 and CTLs | IFN-γ, IL-2, TNF, chemokine ligand 20 (CCL20), chemokine ligand (CXCL)9, CXCL10 |
| Dendritic cell 1 (DC1) (CD11c+, conventional DCs) mature DCs | Immunogenic cross-presentation of TAAs; expression of costimulatory molecules such as CD40, CD80, and CD86 | IFNs, TNF-α, IL-1, IL-4, IL-6, IL-10, IL-12, and IL-23. |
| NK cells | Recognition of cells with down-regulated or absent MHC expression; cytotoxicity through perforin granzyme and other mechanisms | perforin, granzyme B, FasL; IFN-γ, IL-2, TNF-β |
| Gamma/delta T-cells (γ/δ T-cells) | Immune recognition of tumor-derived phosphoantigens or stress ligands; contact-dependent cytokine production, tumor and viral cytotoxicity | IFN-γ, TNF, IL-17, FasL, perforin, granzyme |
| NKT cells | Immune recognition through both NK membrane receptors and an invariant CD1d restricted T-cell receptor (TCR); | IFN-γ, IL-4, perforin, granzyme B and FasL |
| B-cells | Immune recognition of soluble and membrane TAAs; expression of co-stimulatory molecules; ADCC and Ag presentation | secretion of TAA-specific antibodies; IL-12, TNF-β |
Table 2.
Pro-tumorigenic cellular mediators
| Cellular Mediators | Functions | Molecular Mediators |
|---|---|---|
| Regulatory T-cell/cluster of differentiation 4 positive, T helper 2 cell (T-reg/CD4+ Th2) | Mediate immune homeostasis via suppression of inflammation and cytotoxic T-lymphocyte type T1 T-helper cells (CTL Th1) responses, and maintenance of peripheral tolerance | Expression of lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin mucin 3 (TIM-3), glucocorticoid-induced tumor necrosis factor receptor (TNFR)-related protein (GITR), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed death-1 (PD-1); local consumption of interleukin 2 (IL-2), secretion of IL-10, IL-4, IL-35, IL-5, IL-6, transforming growth factor beta (TGF-β), granzyme B, and perforin |
| Cluster of differentiation 8 positive (CD8+) | Inhibition of CTL and CD4+ Th1 responses | IL-4, IL-5, IL-10 |
| T helper 17 cells (Th17) | Promoting angiogenesis via VEGF production, neutrophil recruitment/infiltration | IL-8, IL-6, vascular endothelial cell growth factor (VEGF), prostaglandin E2 (PGE2), TGF-β, chemokine ligand 1 (CXCL1), CXCL5, CXCL8, and IL-1 |
| Myeloid-derived suppressor cells (MDSC), granulocyte-like (G-MDSC), monocyte-like (M-MDSC) and inhibitor (iMDSC), | Depletion of arginine; disruption of interleukin-2 (IL-2) receptor signaling; inhibit lymphocyte trafficking; increase T-reg activation by CD40-CD40L, inhibit T-cell function and antigen presentation, stimulate neovascularization, downregulation of CD3ζ | Reactive oxygen species (ROS), nitrate oxide synthetase (NOS), arginase 1 (Arg1), IL-10, TGF-β |
| Regulatory B cells (B-regs) | Inhibition of T-cell function | Secretion of IL-10 and TGF-β. |
| M2, tumor associated macrophages (TAM) alternatively activated macrophages (Macs) | Promote angiogenesis, activate Th2 and CD8 cells, support tumor progression | Secretion of arginase, cyclo-oxygenase 2 (COX2), IL-10, chemokine ligand 22 (CCL22), programmed death-ligand 1 (PDL1) |
| Dendritic cell 2 (DC2) CD123+, plasmacytoid (pDC) | Low levels of co-stimulatory molecule expression. | Secretion of IL-10, IDO, and TGF-β |
| Granulocytes | Increased secretion of toxic peptides | Secretion of ROS, atopic mediators, and IL-4 |
Innate and Adaptive Antitumor Cellular Mechanisms
Dietary supplementation with PUFAs, especially LC ω−3 PUFAs, has pro-resolving effects on both innate and adaptive immunity via multiple mechanisms. This includes effects on numerous cell phenotypes that coordinate the host response against tumors. Thus, resolvins, metabolites from LC ω−3 PUFAs have endogenous pro-resolution activity that protects against aberrant / uncontrolled innate inflammatory responses56. The present paradigm suggests that the activation of DCs, an innate immune cell, initiates the development of adaptive immune responses against tumors. Classically activated macrophages (M1s) are part of the tumor microenvironment, with a functional role limiting tumor progression. In early tumors, macrophages have an inflammatory, tumoricidal phenotype. Important features of M1 macrophages include the expression of iNOS, ROS and the secretion of the NK and type 1 T-cell stimulating cytokine IL-12. Further, M1 macrophages phagocytose and kill bacteria, viruses and tumor cells, and secrete proinflammatory cytokines57. M1s also promote indirect cytotoxicity by activating adaptive immune responses58.
Granulocytes, specifically neutrophils, may also have a role in tumor regression, including cytotoxicity via Fas/Fas Ligand interactions, as well as, ROS. Eosinophils can also infiltrate tumors, with potential antitumor activity via their secretion of cytotoxic factors including major basic protein, cationic protein and peroxidase; however, it is unclear whether granulocytes exert direct anti-tumor activities59.
NK cells are also highly cytotoxic, innate immune effectors with cytotoxicity, via perforin and granzyme-dependent mechanisms. NK cells have an array of different activating and inhibitory receptors facilitating recognition of stress ligands on tumor cells, which can regulate the levels of major histocompatibility complex (MHC) expression60, 61. It is noted that the anti-tumor activity of NK cells is largely limited to single tumor cells or micro-metastases.
Classical (αβ+) CD8 T-cells recognize peptides presented by class I MHC on the membranes of Ag-presenting cells (APCs)62, 63 and tumor cell, intracellular antigens (Ags), following phagocytosis, are subjected to proteolysis with antigenic (Agic) epitopes bound within the peptide-binding groove of the MHC molecule, and the peptide-MHC complexes transported to and inserted into the plasma membrane of APCs for T-cell recognition. In addition, CD4+ T-cells recognize Ags in the context of MHC class II molecules, primarily expressed by APCs. T helper cell differentiation occurs via the secretion of cytokines that ‘help’ activate B cells, NK cells, and CD8+ CTLs. A wide variety of T helper cell subsets with differing functional roles have been identified based on their function (Th1, Th2, Th17, etc.).
Following activation by APCs, and with CD4+ T-cell help, CTLs develop a direct cell mediated cytotoxicity. Upon differentiation and activation, these T-cells undergo programmed cell death and/or exhaustion, preventing over-activation of immunity, thereby limiting autoimmune responses. These lymphocyte responses can also be regulated by LC ω−3 PUFAs, including promotion of CD4+ Th1 cell differentiation64 and the modulation of T-reg cells65.
Numerous randomized clinical trials have reported anti-inflammatory activity by marine LC ω−3 PUFAs and improved clinical parameters in rheumatoid arthritis patients66, 67. These effects are mostly attributed to EPA, (C20:5) and DHA, (C22:6) via two mechanisms. First, they interfere with the enzymatic conversion of arachidonic acid (AA, C20:4) an ω−6 PUFA to pro–inflammatory prostaglandins (PGs) and leukotrienes (LTs). Second, EPA is a direct precursor in the biosynthetic pathway of anti-inflammatory PGs (series-3) and LTs (series-5). Dietary ω−3 LC-PUFAs replace AA in the phospholipid bilayer of cell and then alters the membrane composition and fluidity, as well as cell signaling, gene transcription and metabolism of proresolving mediators40, 41, 68, 69. A recent meta-analysis of nine clinical trials, with 475 colorectal cancer (CRC) patients, evaluated the effects of ω−3 PUFA on cytokines and/or acute phase proteins levels70. In a stratified analyses, a reduction in IL-6 levels was observed in surgical patients that received 0.2 g/kg of fish oil parenterally during the postoperative period and the albumin levels were increased in the surgical patients that received >2.5 g/d of EPA and DHA orally during the preoperative period. In patients undergoing chemotherapy, supplementation of 0.6 g/d of EPA and DHA for 9 week significantly reduced C-reactive protein (CRP) levels, and the CRP:albumin ratio. The authors concluded that LC ω−3 PUFA supplementation has benefits on reducing some inflammatory mediators, but that these benefits were specific to distinct supplementation protocols based on duration, dose and route of administration.
Dietary PUFA Regulation of Myeloid Cell Function
The regulatory activity of PUFAs on immunity appears to affect myeloid cells. It has been demonstrated that a diet rich in ω−6 PUFAs enhanced the accumulation of MDSCs, which are negative immune regulators71. This was observed with both cultured murine bone marrow cells and in vivo, in mice fed diets enriched in ω−6 PUFAs. In these studies, mice were fed a linseed oil based diet containing 45% of the shorter ω−3 PUFA, ALA, or a sunflower oil diet containing 45% LA, an ω−6 PUFA. The results suggested that the bioactivity of PUFAs occurred through janus kinase-signal transducer and activator of transcription (JAK-STAT3) signalling, such that a JAK inhibitor almost completely inhibited the bioactivity of PUFAs on MDSCs. Thus, it was concluded that the immune modulatory activity of PUFAs may be mediated, in part, by diet.
High fat diets are associated with non-alcoholic fatty liver disease (NAFLD), and with Kupffer cells, which are hepatic resident macrophages, representing 20–25% of the non-parenchymal cells in the liver. NAFLD, which is characterized by chronic systemic low grade inflammation, including a critical contribution by Kupffer cells72. The increased production of proinflammatory cytokines and eicosanoids, by ω−6 PUFA metabolism, can enhance Kupffer cell secretion of inflammatory cytokines, resulting in NFκβ activation with further worsening of inflammation and fibrosis73. Recently, several studies have suggested that lipid accumulation in adipose tissues of obese hosts, promoted infiltrating macrophages with an M1 polarization shift; while M2 phenotype macrophages were found in lean adipose tissue74, 75. Thus, high-fat diets can decrease the frequency of Kupffer cells with an M1-predominant phenotype and result in increased secretion of pro-inflammatory cytokines. Further, ω−3 PUFAs polarize Kupffer cells/macrophages to a predominantly M1 phenotype while ω−6 PUFAs polarize Kupffer cells/macrophages to an M2 phenotype, in association with the activation of NFkβ signalling and peroxisome proliferator-activated receptors (PPAR)-ƴ respectively76, 77. The up-regulation of PPAR-ƴ induces macrophage polarization from an M1-predominant phenotype to an M2 phenotype78. Because dietary fish oil, LC ω−3 PUFA, results in decreased PGE-2 production, LC ω−3 PUFAs are anti-inflammatory, and enhance secretion of Th1-type cytokines, and decrease MHC II expression, NK cell activity, and lymphocyte proliferation. Consistent with this hypothesis, the culture of human neutrophils with the LC ω−3 PUFAs, EPA or DHA inhibits superoxide production and phagocytosis79. Similarly, the incubation of murine peritoneal macrophages with EPA or DHA has been reported to inhibit MHC II expression80. In one study, human monocytes were cultured with EPA or DHA, resulting in a decrease in the proportion of human leukocyte antigens-DR or DP (HLA-DR or –DP) positive monocytes following addition of IFN-γ81 depressing Ag presentation82. Similarly, adding fish oil to rodent diets has been shown to decrease superoxide and hydrogen peroxide secretion by macrophages83. Experiments comparing diets with safflower oil versus fish oil have been found to decrease peak plasma levels of TNF-α, IL-1β, and IL-6 following lipopolysaccharide (LPS) injection84. Indeed, parenteral nutrition that includes fish oil can decrease serum TNF-α, IL-6, and IL-8 levels in burned rats, compared with animals given ω−6 PUFA–rich parenteral nutrition79. However, these studies utilized super-pharmacologic doses, contrasting with most rodent studies using dietary fish oil in which EPA plus DHA comprise up to 30% of the lipid fatty acids and up to 12% of energy. Conclusions from studies, such as these, have been refined by using relatively low levels of EPA or DHA (4.4% of total FAs or 1.7% of dietary energy), documenting that these levels are sufficient to result in anti-inflammatory activities85.
T-cell Immunosuppression and PUFA
LC ω−6 PUFAs are proinflammatory86 as they can be metabolized to AA and subsequently by COX-/LOX- to inflammatory lipid mediators including (PGs) and leukotrienes (LTs)87. These AA metabolites are well known for their tumor-promoting effects and the COX downstream molecule PGE-2 can enhance tumor growth by stimulating the development of tolerogenic DCs and Tregs. 5-LOX metabolites involve 4 series leukotrienes (LTs) and have a role in stimulating tumor growth and progression88. In contrast, the ω−3 fatty acids, EPA and DHA, modulate COX/LOX activities by forming less potent metabolites such as three series PGEs and five series LTs. Further, the lipoxygenase products from AA metabolism stimulate the expansion and differentiation of myeloid progenitor cells89 such as MDSCs. Tolerogenic DCs contribute to T-cell regulatory functions by suppressing their activation via peripheral tolerance. In steady state conditions, tissue-resident immature DCs internalize/process and present Ags from tumors. These DCs, identified as DC2s, are poorly immunogenic, and do not secrete proinflammatory cytokines, and express low levels of costimulatory molecules. Further, DC2s secrete immunosuppressive cytokines, including IL-10 and TGF-β that are key mediators in the induction of T-reg cell differentiation. Indoleamine 2,3-dioxygenase (IDO) secretion by these DCs also contributes to immune tolerance90. Alternatively activated macrophages (M2s) are differentiated from monocytes by IL-4 stimulation. M2s facilitate tumor angiogenesis, support tumor progression, invasion and metastasis, and contribute to immunosuppression by secreting IL-10, facilitating the development of IL-4-secreting Th2 cells, and provide a positive feedback for the development of additional M2 macrophages. CCL22, produced by M2 macrophages, also recruit T-regs to suppress CTL functions. Further, PD-L1, expressed by M2 macrophages, contributes to the apoptosis of activated T-cells91.
Similar to M2 macrophages, MDSCs; a heterogeneous population of immature myeloid cells with potent immunosuppressive activity also infiltrates tumors. MDSCs can be either of monocytic, granulocytic or immature origin20, 92. In the blood of cancer patients, MDSCs lack lineage (LIN) markers for lymphocytes (CD19 and CD3) and NK cells (CD56) and thus express an LIN−HLADR−CD11b+ phenotype93, 94 that can be further segregated based on expression of CD14 (monocytic), CD15 (granulocytic) or CD33+CD14-CD15− (immature) expression20, 95. A positive correlation between the frequency of MDSCs and tumor stage has been reported for numerous tumor pathologies92. MDSCs inhibit T-cell activation via arginase, iNOS, ROS or reactive nitrogen species (RNS) as well as secretion of immunosuppressive cytokines96. Further, MDSCs deplete nutrients necessary for lymphocyte function, disrupt IL-2 receptor signaling, interfere with lymphocyte trafficking, promote activation of T-regs by CD40-CD40L ligation, suppress CD3-zeta (ζ) expression and secrete IL-10 or TGF-β97, 98.
T-regs are divided into 2 major populations, one that develops in the thymus99 and one that is induced in the PB100 by TGF-β. In homeostatic conditions, T-regs limit the induction and expression of autoimmunity, inhibit bystander tissue destruction and maintain tolerance to self-antigens101. In cancer patients, the T-reg frequency in the PB is increased compared to normal individuals102. In addition, there are high numbers of tumor infiltrating T-regs103. T-regs are phenotypically identified as CD4+ T-cells that co-express forkhead box P3 (Foxp3) and CD25102. Further, T-regs can express one or more checkpoint inhibitory molecules; including, but not limited to, lymphocyte activating gene-3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM3), glucocorticoid-induced tumor necrosis factor receptor (GITR), cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4), and programmed death-1 (PD-1), which can directly suppress immune cells102, 104. T-regs can also indirectly suppress effector T-cells by depleting local IL-2, which is needed for the survival of actively dividing effector T-cells105. Indirect suppression is also associated with the secretion of immunosuppressive cytokines, including IL-10 and TGF-β106. In contrast to immunosuppressive myeloid cells, recent research has focused on direct PUFA regulation of T-regs.
Diets Rich in ω−6 PUFAs Increase the Risk of Inflammatory Diseases
Numerous studies have suggested that dietary PUFAs regulate inflammatory responses. Western diets include fatty acids (FAs) from animal sources, which are mainly saturated fatty acids (SFAs) and FAs from plants that are predominantly ω−6 PUFAs. In contrast, some FAs derived from plant-based oils, and fatty fish consist mainly of ω−3 PUFA. Rodent and clinical studies have shown that hosts given diets rich in ω−6 PUFAs have an increased risk of inflammatory diseases, including asthma, rheumatoid arthritis and inflammatory bowel disease107. In contrast, diets with high levels of LC ω−3 PUFAs are anti-inflammatory, with a decreased risk of inflammatory diseases107. Further, PUFAs can be oxidized to either pro-inflammatory or pro-resolving lipid mediators (Figure 2), both of which have potent immune modulatory capacities108. Pro-inflammatory mediators, notably PGs and LTs, are secreted in response to “foreign” substances and cleared by pro-resolving lipid mediators, restoring tissue homeostasis109. Diets with high levels of the ω−3 PUFAs, shorter chain ALA, and more critically, LC EPA and DHA are associated with regulation of the incidence and severity of inflammation70. The beneficial effects of dietary FAs include the anti-inflammatory metabolites LTs, thromboxanes (TX), resolvins and a decrease in the levels of inflammatory cytokines. The ω−3 PUFAs differ from the ω−6 PUFAs based on the position of their double bonds in the acyl chain, such as linoleic acid (LA) as compared to arachidonic acid (AA) found with ω−6 PUFA containing diets. (Figure 2) However, the inflammatory and immune augmenting aspects of PUFAs are not clearly separated based on the number and placement of double bonds, counting from the methyl end of the FA (i.e., ω−3 vs ω−6). The addition of the dietary shorter ω−3 PUFA, ALA, an essential FA, and the main precursor of LC ω−3 PUFAs, enhances secretion of superoxides from macrophages and neutrophils110, resulting in cellular adhesion to endothelial cells111 and pro-inflammatory effects in vitro. Further, ALA can slow the proliferation of rodent and human lymphocytes following mitogen stimulation112–114, supporting immunosuppressive activity by ALA. Consistent with these in vitro observations, studies in which rodents were given a high-fat diet, rich in ALA, resulted in a decreased mitogen-stimulation of lymphocyte proliferation and NK cell activity115. In vitro studies using the ω−6 PUFA; AA, have demonstrated increased inflammation associated changes, including enhanced superoxide release110, neutrophil attachment to endothelial cells111, and IL-1β secretion by macrophages116. Mice fed diets with high ω−6 PUFA levels, in a dose dependent manner, resulted in increased levels of LTE-4 and PGE-2 following zymosan stimulation in vivo117. Thus, diets high in AA result in increased angiotensinogen, IL-6 and monocyte chemoattractant protein (MCP)-1 and increased expression of the proinflammatory transcription factor; nuclear factor κβ (NFκβ)118. In other studies using rats fed diets with a high ALA composition for 8 weeks, a decreased superoxide production was observed by peritoneal macrophages in response to phorbol esters119, and an increase in TNF secretion by resident macrophages, although no effect on TNF production was observed with inflammatory macrophages120. Thus, the effects of the shorter ω−3 PUFA, ALA on lymphocyte functions appears to be dependent on ALA levels and total PUFA diet content and composition121. These observations with ALA and ω−6 PUFA contrast with the bioactivity of the LC ω−3 PUFA with 20 or more carbon atoms such as EPA, and DHA, which are anti-inflammatory and immune augmenting122. Diets incorporating LC ω−3 PUFAs are anti-inflammatory, in part due to a decrease in metabolism of ω−6 PUFA into inflammatory eicosanoids, cytokines, and stimulation of ROS and NOS mediators123. Clinically, EPA and DHA dietary supplementation can decrease intestinal damage and improve gut histology in patients with inflammatory bowel disease124, as well as decreased arthritic lesions including joint pain, number of tender and swollen joints, and duration of morning stiffness125.
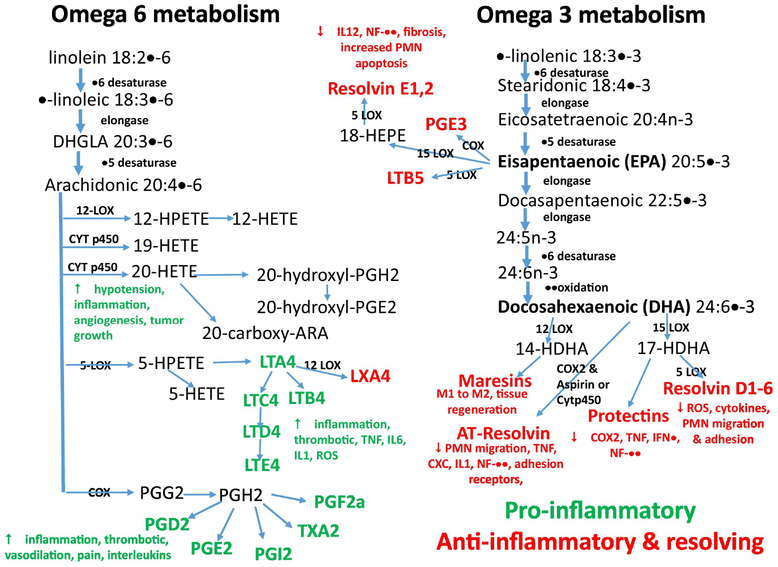
Fig. 2.
This figure is an outline of eicosanoid mediator synthesis pathways from arachidonic acid (AA) and resolvin related mediators from α-linolenic acid (ALA) and their inflammatory and anti-inflammatory functions. COX, cyclooxygenase; CYT p450 cytochrome, p450; chemokine subtype, CXC; HETE, hydroxyeicosatetraenoic acid; HDHA, hydroxyldocosahexaenoic acid; HPETE, hydroperoxyeicosatetraenoic acid; HPDHA, hydroperoxydocosahexaenoic acid; HPEPE, hydroperoxyeicosapentaenoic acid; IL, interleukin, IFN, interferon; LOX, lipoxygenase; LT, leukotriene; LX, lipoxin; PG, prostaglandin; PMN, polymorphonuclear leukocytes; ROS, reactive oxygen synthetase; TNF, tumor necrosis factor, TX, thromboxane.
Dynamic Anti-inflammatory Activities of ω−3 PUFA
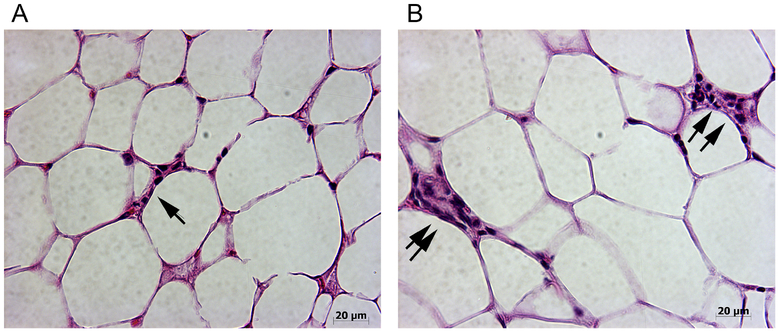
One of the challenges of dietary studies, with PUFA regulation of inflammation, is that obesity is associated with a chronic low-grade inflammation and increased levels of free fatty acids, pro-inflammatory cytokines, hormones and circulating macrophages126, 127. Adipose tissue can secrete metabolites that either promote or resolve an inflammatory response128. Additionally, excess energy is normally stored in adipose tissue129, resulting in enlarged fat cells (hypertrophy) and increased numbers, primarily through hyperplasia of pre-adipocytes, to store the excess triglycerides (TG). This hypertrophy and hyperplasia of adipose cells increases oxygen consumption, resulting in hypoxia129, activation of cellular stress pathways and autonomous inflammation due to pro-inflammatory cytokine secretion129. This also results in myeloid infiltration of adipose tissue, (including mammary glands), surrounding both dead and dying adipocytes, where they form crown-like structures (CLS) and a phenotypic shift of the adipose tissue macrophages, releasing pro-inflammatory cytokines that induce ROS and activate inflammatory signalling pathways in neighbouring adipocytes130. An example of the impact of differing dietary PUFA composition on CLS formation in mammary fat pad (MFP) is shown in Figure 3. MFPs from groups of mice receiving isocaloric and isolipidic diets containing either ω−3 PUFA (Figure 3A), or ω−6 PUFA (Figure 3B) diets by pair feeding for 20 weeks, were analyzed for CLS in 10 high power fields per sample (N=10/group). The results from our studies showed that there was a significant increase in CLS in the mammary fat pad (MFP) of mice receiving ω−6 diets (Figure 3B) compared to the mice consuming ω−3 diets (Figure 3A). These observations are consistent with the recent report on hepatocyte secretion of dipeptidyl peptidase 4 and adipose inflammation including CLCs.131
Fig. 3:
Dietary PUFA regulation of mammary adipose tissue inflammation
Crown-like-structures (CLS) were analysed in H & E stained sections of mammary fat pad from mice that received the differing PUFA composition diets for 20 weeks5. MFP from mice fed ω−3 diet (Fig. 3A) and ω−6 diet (Fig. 3B). Mice given the ω−3 diet had fewer and smaller CLS relative to mice given an ω−6 diet. Single arrow indicates small CLS and double arrows indicated large CLS. Note the difference in size of the adipocytes in the MFPs of mice on the different diets, i.e. the adipocytes are significantly larger in the mice given ω−6 diets. Images were taken at 400x magnification.
Obesity contributes to the tumor microenvironment by increasing inflammation, and the presence of free fatty acids (FFAs)132. High levels of proinflammatory adipokines contribute to the content of inflammatory cell content within the tumor microenvironment133, 134 through autocrine and paracrine activation of signalling pathways including NF-κβ135, STAT3 and extracellular regulated kinase (ERK)1/2, all of which stimulate tumor cell proliferation, which can inhibit apoptosis136. In contrast, adiponectin, secreted by white adipose tissue, has anti-proliferative effects for breast cancer cells, but is down-regulated in obese patients137. Thus, low levels of adiponectin increase the risk of breast cancer in obese women138.
A number of clinical trials have assessed the therapeutic activity of diets supplemented with fish oil in inflammatory diseases, including Crohn’s disease, psoriasis, ulcerative colitis, rheumatoid arthritis, multiple sclerosis, and lupus139. Many placebo-controlled, double-blinded trials with dietary fish oil, undertaken in patients with chronic inflammatory diseases, have documented significant benefits. The evidence for clinical activity by fish oil is greatest in rheumatoid arthritis, where LC ω−3 PUFA consumption results in a concentration-dependent decrease in inflammatory enzymes, including ones that degrade cartilage, COX-2, but not COX-1 expression and TNF-α and IL-1β expression in cultured articular chondrocytes140. The mechanisms of action with LC ω−3 PUFAs in patients with arthritis have been postulated to be a competition between the canonical ω−6 substrate AA resulting in eicosanoids with lower inflammatory activity141. Further, LC ω−3 PUFAs can be metabolized into anti-inflammatory, bioactive lipid mediators including resolvins, protectins and maresins, which can resolve inflammation with significantly more activity than their lipid precursors142. The associated paradigm shift, based on these observations, suggests that the resolving phase of inflammation is not passive, but involves actively downregulated endogenous anti-inflammatory mediators143. This contrasts with ω−6 PUFA metabolites, including PGD-2, LTD-4, LTC-4, and LTE-4, which mediate asthmatic bronchoconstriction. Although AA is a precursor to LTs and has a role in allergic inflammation, PGE-2 can also regulate macrophage and lymphocyte functions. Thus, dietary consumption of the ω−6 PUFA LA, as the precursor of AA, is causally linked to allergic diseases and supports a potential treatment strategy using LC ω−3 PUFAs144.
Dietary ω−3 Regulation of Murine Tumor Growth
Clinically, a number of differing associations have been reported between PUFA consumption / composition and inflammation; due in part to confounding factors including genetic susceptibility, tissue microenvironments, stress, obesity, age, caloric intake and dietary duration. Murine models have suggested a number of mechanisms that associate dietary PUFA with tumor initiation and progression, secondary to systemic and tissue inflammation. These studies include a number of pathologic conditions such as infections, autoimmune and inflammatory conditions, neoplasia and obesity with neutrophilia, splenomegaly and multifocal, hepatic extramedullary myelopoiesis (i.e., the formation of myeloid tissue outside of the bone marrow)5, 145. These inflammatory conditions, in association with tumor initiation, are regulated by multiple risk factors, including hormones, obesity, diet, and age. However, following tumor initiation and growth, inflammation is controlled by tumor secretion of GFs, as well as, existing risk factors. Thus, the tumor inflammatory microenvironment is associated with cross talk between host immunity and tumor-secreted GFs. As an example, the cellular microenvironment of mammary glands incorporates, primarily, adipocytes, hormonal responsive epithelial cells, and stromal cells, as well as, infiltrating immune cells, resulting in mammary glands that can act as both an endocrine and as an immune organ146. This stimulates a progressive increase in tumor infiltrating inflammatory cells, including MDSCs, M2 macrophages, DC2s, and granulocytes, during tumor initiation and progression from normal tissue to dysplastic cells147.
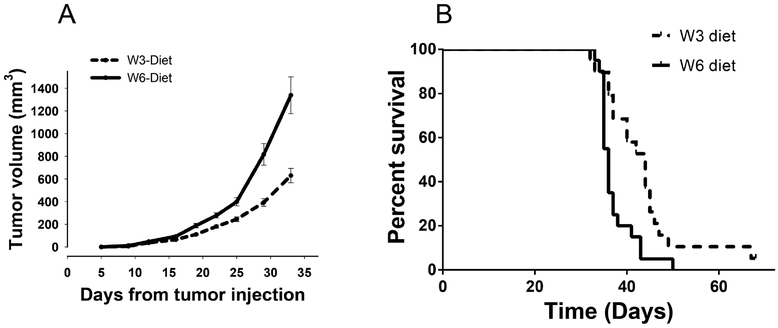
The role of dietary PUFA during tumor progression and metastasis has been examined in syngeneic, and xenograft mammary cancer models. In a xenograft model using MDA-MB-435 tumor cells, athymic nude mice were injected with tumor cells following establishment of the mice on diets of either LA, EPA or DHA. These studies revealed significantly delayed tumor growth and metastasis in the mice fed an EPA or DHA diet, including a reduction in AA levels in the tumor membrane phospholipids148. The results from one of our studies are shown in (Figure 4). In this study, two groups of mice received diets differing in PUFA composition using pair fed, isocaloric and isolipidic liquid diets (unpublished results). Ten weeks following initiation of the diets, the mice received orthotopic injections of 4T1 mammary tumor cells. The results show that mice consuming a LC ω−3 PUFA diet had significantly slower growing tumors (Figure 4A) and prolonged survival (Figure 4B) compared to the mice receiving an ω−6 PUFA diet. Interestingly, when subgroups of mice were autopsied 35 days post orthotopic injection, the mice consuming the ω−6 based diets were observed to have a significantly greater number and frequency of pulmonary, hepatic, renal, cardiac and bone marrow metastases. Inhibition of inflammatory cells, as discussed elsewhere in this review, is associated with slower growth of primary tumors and potentially a reduction in the frequency of metastases. This suggests that dietary PUFA composition is not only critical to tumor initiation, but also modulates tumor growth and the extent of metastasis and metastatic sites. Further, in murine studies, when EPA and DHA are provided as neoadjuvant therapy, the number of pulmonary metastases are significantly decreased compared to mice on an LA diet149. Similar immune-augmenting and therapeutic activities were reported in studies with R3230RC and MCF-7 breast adenocarcinoma tumor models150, 151, including a reduced number of MDSCs152. In a tumor survival study, mice were switched from an 8% corn oil (1% ALA) diet to an 8% canola oil (10% ALA) diet, when the mice had developed an average primary tumor volume of 60 mm3. In this study, tumor growth was significantly lower in mice fed the ω−3, canola oil diet as compared to the ω−6, corn oil cohort.153 Based on these and other preclinical studies, it appears that dietary intervention may be used with therapeutic intent.
Fig. 4: Dietary PUFA regulation of mammary tumor growth and survival:
Groups of mice fed ω−6 and ω−3 diets for 10 weeks5, were injected orthotopically with 4T1 cells. Tumor volume was recorded twice a week and plotted with average tumor volume per dietary group (Fig. 4A) (n=20). Survival days were compared between the dietary groups (Fig. 4B) (n=20) (p<0.05).
Murine studies with interventions using LC ω−3 PUFA and autochthonous, chemically induced mammary tumors support these observations. In an autochthonous 7, 12-dimethylbenz (α) anthracene (DMBA) induced mammary tumor model, mice on a fish oil diet had a significantly reduced tumor incidence, growth and metastasis154, 155. The LC ω−3 diet affected tumor induction and growth that correlated with reduced AA serum levels, suppressed tumor cell proliferation, protection against DNA single strand breaks, and an increase in apoptosis marker expression155–157. Similarly, in a tumor model with N-methyl-N-nitrosourea (MNU)-induced rat mammary tumors, diets with varying fat composition were compared, including an SFA diet, a monounsaturated fat (MUFA) diet, an ω−6 PUFA alone diet or diets with different ratios of ω−6 : ω−3 PUFA diets. It was found that a diet incorporating a 1:1 ratio of ω−6 : ω−3 PUFA was most effective in preventing mammary tumor development as compared to the other dietary groups. Studies into causal relationships revealed that this diet group had decreased transcription of cyclooxygenase-2 (COX-2), and 5-lipoxygenase (5-LOX) in mammary tissues and PPAR-γ levels158. Together, these and other studies directly support a role for LC ω−3 PUFA in controlling the inflammatory tumor microenvironment by the upregulation of PPAR-γ157, 158. When dietary LC ω−3 PUFA content was increased to an ω−6 : ω−3 ratio of 1:14.6, as compared to 1:0.7, a 60% decrease in tumor growth was observed159. Similar studies, using a therapy model with orthotopic 4T1 mammary tumors, in which a 5% fish oil diet was initiated when the hosts had developed primary tumors that were 8–10 mm3 in diameter, resulted in significantly reduced growth and metastasis, which correlated with decreased tumor cell proliferation160.
The ability of LC ω−3 PUFAs to downregulate inflammatory mediators and increase proteins, associated with apoptosis, supports the importance of exogenous regulation of the tumor microenvironment. However, the regulatory mechanisms are unclear. In-vivo studies, focused on cellular phenotypes, have examined the effect of dietary LC ω−3 PUFA on inflammatory cells in animal models with both LPS and tumor induced inflammation. However, the majority of murine models use diets that are not isocaloric and are rarely pair-fed, raising questions regarding mechanisms based on obesity verses dietary composition. Since obesity itself is inflammatory, clarifying the effects of obesity associated inflammation, as opposed to diet regulation, is crucial to determining the actual effects of dietary components in tumor initiation and progression. Thus, the use of an animal model using an isocaloric, isolipidic liquid diet that allows pair feeding and controlled dietary caloric intake is required to assess dietary impacts on weight and adipose changes, as well as dissociate effects between obesity and dietary composition, such as PUFA composition.
Dietary LC ω−3 PUFA and Improved Cancer Patient Outcomes
The local tumor microenvironment includes tumor cells, extracellular matrix; endothelial cells, stromal cells, fibroblasts, adipocytes and critically infiltrating inflammatory and adaptive immune cells. These microenvironmental elements have a role in regulating tumorigenesis, tumor growth, invasion and metastasis. The infiltrating immune cells, particularly CTLs, serve as regulatory factors in the tumor microenvironment161, 162. In cancer patients, it has been documented that the infiltration of immune cells provided an independent positive prognostic factor using immunohistochemistry (IHC) and hematoxylineosin (HE) staining.163 Studies, into the type of infiltrating immune cells (e.g., CD3+, CD8+, and FOXP3+ T lymphocytes) and the density or location of infiltrating T-cells, contribute to a prognostic correlation with positive outcomes for patients with CRC50, 164–170. This correlation is also observed in a variety of other tumors, including ovarian cancer and breast cancer171–174. These studies have been extended clinically to include a meta-analysis assessing the impact of tumor-infiltrating inflammatory cells on outcomes, including a meta study incorporating 30 studies involving 2,988 patients.175 These studies examined the associations between CRC survival and generalized tumor inflammatory infiltrates (N=12) and T lymphocyte subsets (N=18). Pooled analyses revealed that a significant, generalized tumor inflammatory infiltrate was associated with improved cancer-specific survival (CS), OS and DFS. Stratification by cellular location and T lymphocyte subset indicated that in the tumor microenvironment, CD3+, CD8+ and FoxP3+ cellular infiltrates were not significant prognostic markers for OS or CS. In contrast, a high frequency of infiltrating CD8+, but not CD3+ or FoxP3+ T-cell cells were predictive of an increased OS. Furthermore, a high frequency of tumor infiltrating CD3+ cells at the invasive tumor margin was also associated with improved OS and DFS.175
Consistent with the effect of LC ω−3FA on tumor infiltrating immune cells, is an inverse relationship between dietary LC ω−3FA consumption and the risk of developing CRC, as reported within case-control studies by Murff et al.176 and Habermann et al.177. However, the benefits were limited such that, in one study176 an increased ω−3 PUFA intake was associated with a reduced risk of colorectal adenomas in women, whereas in another trial178 an inverse association was observed between low DHA intake and an increase in the risk of CRC in patients with specific genetic variants that resulted in higher levels of proinflammatory mediators. More recently, a relationship between LC ω−3FA intake and survival in the CALGB 89803 randomised trial of adjuvant chemotherapy for completely resected stage III CRC (n=1,264) was investigated retrospectively178. Patients in the highest quartile of LC ω−3FA dietary intake had an increased disease-free survival (DFS) compared with the lowest quartile. Notably, this relationship appeared to be greater for patients with high CRC COX-2 expression178. Numerous clinical studies have examined adjuvant supplemental therapy with LC ω−3FA179. In one positive example breast cancer patients with high dietary DHA had a significantly longer time to disease progression and survival as compared to patients with lower incorporation of supplemented DHA180.
IHC analyses of infiltrating immune cells, particularly CD3+ T lymphocytes in the primary tumor, provide a biomarker predicting good clinical outcomes in most cancer pathologies181–183. Furthermore, basic histological quantification of T lymphocyte density, cytotoxicity and memory, by CD3+, CD8+, and CD45RO+ markers respectively, demonstrate that an increase in T lymphocyte infiltration was associated with significant improvements in a patient’s DFS and OS165, 182, 184. In CRC, identifying the location of infiltrating CTLs, assessed as CD3+CD8+ T-cells in two areas within the center (CT) and invading margin (IM) of the primary tumor, provides an accurate prediction of clinical outcomes165. The quantification of the density, phenotype, and location (CT or IM) of infiltrating CTL results in what has been termed an Immunoscore185–187. Indeed, the analysis of CD3+ cell infiltration surpasses the gold standard of diagnosis by tumor-stage, lymph node, and metastatic invasion. The assessment, of a tumor Immunoscore, subsets patients into five categories based on the location in the tumor (CT and IM) of CD3+ and CD8+ T-cells.188, 189.
In addition to leukocytic infiltration of tumors, circulating hematological inflammatory markers, including the neutrophil–lymphocyte ratio (NLR), can predict the survival of cancer patients190. This has been extensively studied, documenting the prognostic value of the NLR in multiple, but not all, tumor pathologies and disease stages. Over 60 studies (>37,000 patients) have been examined to assess the prognostic value of the NLR191. In parallel with these studies, there are reports of a relationship between plasma proinflammatory cytokine levels and elevated NLR (>5)192, 193 providing insight into underlying mechanisms. Further, there is potentially a relationship between the frequency of circulating myeloid cells and an elevated NLR and an increase in peritumoral macrophages192. Together, these observations suggest that the NLR reflects, in part, innate immunity and myeloid cell infiltration of tumors, providing an easily measurable biomarker that is predictive of OS and progression free survival (PFS).
TILs occur primarily at the tumor interface with the surrounding stroma194. Thus, while infiltrating leukocytes may have prognostic significance, subsets of infiltrating cells, may be a more accurate predictor. As discussed above, infiltrating CD8+ T-cells, are a critical component of tumor-specific adaptive immunity. CTLs are a cellular mediator that can be prognostic of positive outcomes. Further, immunosuppressive myeloid cells, including MDSCs, DC2s and M2 macrophages, emphasize the criticality of assessing the infiltrating myeloid cell-to-CD8+ lymphocyte ratio in cancer tissues. Several studies have focused on this parameter, concluding that infiltrating CD66+ myeloid cells provide an independent prognostic factor for poor DFS and OS195. This observation has been extended with the finding that the infiltrating NLR (iNLR) determined as a CD66+ : CD8+ cell ratio, documents a relationship with OS and tumor stage196.
In association with immunoregulatory properties, a patient’s lifestyle, preceding and following diagnosis and therapeutic interventions, may help control cancer initiation, progression38, and responses to therapeutic interventions197. Specifically, patients who consume a high-fat diet; one with high levels of saturated fat, or one with high levels of ω−6 PUFAs, frequently exhibit neutrophilia, that can facilitate tumor initiation and progression, resulting in poor outcomes198, 199. Conversely, diets that contain a high ω−3 PUFA content have been associated with lower inflammation, lower EMM and better clinical outcomes5. We posit, herein, that dietary LC ω−3 PUFA may increase the infiltration of tumor specific CTLs, decrease myeloid cell infiltration and improve intraturmoral survival of T-cells, contributing to improved patient outcomes.
Epidemiological studies into the incidence and progression of breast cancer in American women of Japanese descent, have been compared to that of women in Japan. The results from one study indicated a significantly higher breast cancer incidence in American women compared to Japanese women200. This conclusion was supported by the observation that children from Japanese immigrants to America, but not the immigrants themselves, had breast cancer rates similar to the general American population201. In the 1990s, dietary components were found to be implicated in these different incidences202. These relatively weak correlative epidemiologic studies were considered plausible, as preclinical experiments demonstrated that LC ω−3 PUFAs could reduce pro-inflammatory cytokines, inflammation and cancer development203. Similarly, high fat diets have been shown to increase the risk of breast cancer and aggressive prostate cancers204. Case–controlled studies have documented an inverse relationship between the dietary ω−6 and LC ω−3 PUFAs ratio and the incidence of breast cancer, supporting the importance of the relative ratio of ω−6 and LC ω−3 LC-PUFAs in the diet205. In an epidemiological study of 56,007 French women over 8 years, the risk of breast cancer was reported to be unrelated to dietary PUFA consumption overall. Rather, a significant risk was associated with the ratio of dietary ω−6 vs. LC ω−3 PUFAs, which was inversely related to LC ω−3 PUFA levels in women with the highest intake of ω−6 PUFAs, indicating interactions due to PUFA consumption206. The decreased risk of developing breast cancer with LC ω−3 PUFA consumption was confirmed in a case controlled study207, where a population based study showed all-cause mortality was reduced 16–34% in women consuming high levels of LC ω−3 PUFAs208. Indeed, in the last 20 years, data has accumulated suggesting that high ω−6 PUFA consumption is pro-inflammatory, likely involving COX-2 secretion and NFκβ activation, resulting in an increased incidence of cancer and all-cause mortality. In contrast, a high consumption of LC ω−3 PUFA is protective against neoplasia, resulting in the downregulation of NFκβ, a decreased incidence of cancer and neoplasia associated all-cause mortality209. Indeed, in a meta-analysis of 11 independent prospective studies, it was suggested that a decrease in the dietary ω−6 : LC ω−3 PUFA ratio significantly lowered the risk of breast cancer210. Even though some studies have shown no association between a heightened diet of ω−6 : LC ω−3 fatty acid ratios and breast cancer development, the risk of developing breast cancer was directly associated with increasing ω−6 : LC ω−3 PUFA ratios211.
Recent studies have investigated the underlying mechanisms in these observations, and their relationship to innate and acquired immune cells in the tumor microenvironment. The regulatory activity of LC ω−3 PUFA on macrophage functions, has been documented with the use of antagonists to GPR120, which is expressed by some myeloid cell populations and acts as a PUFA receptor212. This is supportive of a role for LC ω−3 PUFA mediation of anti-inflammatory effects via this receptor. However, the nuclear receptor PPAR-γ also acts as a receptor for PUFAs and the regulatory mechanisms of LC ω−3 and ω−6 PUFA on obesity213, postmenopausal breast cancer214 and microenvironmental inflammation64, suggesting a need for additional studies. Changes in the lipid content of cell membranes associated with LC ω−3 and ω−6 PUFA consumption may regulate oncogenic signalling via the regulating of lipid raft profiles and a reduction in cytokine production215. Further, PUFAs contribute to the regulation of BM and extramedullary hematopoiesis at sites such as the spleen216, 217 and may also induce the expansion of MDSCs71.
Summary
Dietary consumption of PUFAs may not only affect inflammation and the incidence and progression of neoplasia, but also may provide an interventional strategy with positive clinical outcomes for cancer patients and patients with other pathologies via the regulation of inflammation. In general, increased dietary ω−6 PUFA consumption is associated with a heightened risk of breast cancer due to direct effects on the mammary gland and promotion of a pro-inflammatory tumor microenvironment. In contrast, dietary LC ω−3 PUFAs have protective effects and suppress ω−6 PUFA associated inflammation. The nutritional recommendation has been that individuals should decrease dietary ω−6 PUFA intake and increase LC ω−3 PUFA consumption with an intake of at least 500 mg/day of dietary LC ω−3 PUFA218, easily achievable with 2 weekly servings of oily fish, supporting prevention of cancer and cardiac disease and a dietary ratio of ω−6 : ω−3 PUFA of approximately 8:1 or lower219. PPAR-γ and GPR120 agonists also have potential use as neoplastic chemopreventive drugs; although their use, initially, is perhaps better targeted towards either high-risk individuals or as a therapeutic intervention. Regardless, there remains a compelling need to document that both pharmacophores and dietary regulation of PUFAs have clinically significant anti-cancer activities. Future trials should address this question as well as the impact on tumor infiltrating cells subtypes. We stress that translational/preclinical studies should utilize isocaloric and isolipidic, pair fed diets to control the regulation of immunity and inflammation by obesity versus dietary FAs. Further, great care must be taken to differentiate dietary control of tumor growth as opposed to metastasis, as these biologic parameters are interrelated. In our experience, fatty diets impact not only primary tumor growth, but also the extent and critically, sites of metastasis, all of which are typically unstudied but clinically and highly relevant since they are often the ultimate cause of the patient’s demise.
Highlights:
This review discusses the relationships between dietary PUFAs, lipid mediators, inflammation and neoplasia and experimental strategies to improve our understanding of these relationships.
Discusses the need for isocaloric, isolipidic and pair-fed models to separate mechanisms based on obesity verses dietary composition
Discusses our understanding of dietary PUFAs regulation of inflammation and neoplastic progression as an interventional strategy for cancer patients.
Discusses recent findings on PUFA regulation of not only tumor initiation, but also tumor growth and the extent and sites of metastasis.
Acknowledgements:
James E. Talmadge, Timothy R. McGuire and John Graham Sharp are members of the Fred and Pamela Buffet Cancer Center supported by 30CA036727. John Graham Sharp and Timothy R. McGuire receive support via the Children’s Hospital/UNMC Pediatric Cancer Research Group, from the State of Nebraska. Agricultural Research Division and Office of Research and Economic Development, University of Nebraska-Lincoln, National Institutes of Health, RO1-DK07076
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Saraswoti Khadge, University of Nebraska Medical Center, 986495 Nebraska Medical Center, Omaha, Nebraska 68198-6495, Business: 402-559-8256, Fax: 402-559-4990, saraswotikhadge@gmail.com
John Graham Sharp, University of Nebraska Medical Center, 986395 Nebraska Medical Center Omaha, Nebraska 68198-6395, Business: 402-559-4390, Fax: 402-559-3400, jsharp@unmc.edu.
Timothy R. McGuire, University of Nebraska Medical Center, 986045 Nebraska Medical Center Omaha, Nebraska 68198-6045, Business: 402-559-8224, trmcguir@unmc.edu.
Geoffrey M. Thiele, University of Nebraska Medical Center, 986350 Nebraska Medical Center Omaha, Nebraska 68198-6350, Business: 402-559-7010, Fax: 402-559-0604, gthiele@unmc.edu.
Paul Black, University of Nebraska-Lincoln, 1901 Vine Street N200 Beadle Lincoln, Nebraska 68588-0664, Business: 402-472-2932, Fax: 402-472-7842, pblack2@unl.edu.
Concetta DiRusso, University of Nebraska-Lincoln, 1400 R St 300 Canfield Lincoln, Nebraska 68588-0664, Business: 402-472-6504, Fax: 402-472-7842, cdirusso2@unl.edu.
Leah Cook, University of Nebraska Medical Center, 986495 Nebraska Medical Center Omaha, Nebraska 68198-6495, Business: 402-559-1234, Fax: 402-559-5900, leah.cook@unmc.edu.
Lynell W. Klassen, University of Nebraska Medical Center, 986495 Nebraska Medical Center, Omaha, Nebraska 68198-6495, Business: 402-559-7520, Fax: 402-559-0604, lklassen@unmc.edu.
James E. Talmadge, University of Nebraska Medical Center, 986495 Nebraska Medical Center Omaha, Nebraska 68198-6495, Business: 402-559-5639, Fax: 402-559-4990, jtalmadg@unmc.edu.
References
- [1].Krusinska B, Hawrysz I, Wadolowska L, Slowinska MA, Biernacki M, Czerwinska A, Golota JJ: Associations of Mediterranean Diet and a Posteriori Derived Dietary Patterns with Breast and Lung Cancer Risk: A Case-Control Study. Nutrients 2018, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Simopoulos AP: Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother 2006, 60:502–7. [DOI] [PubMed] [Google Scholar]
- [3].Black HS, Rhodes LE: Potential Benefits of Omega-3 Fatty Acids in Non-Melanoma Skin Cancer. J Clin Med 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Miccadei S, Masella R, Mileo AM, Gessani S: omega3 Polyunsaturated Fatty Acids as Immunomodulators in Colorectal Cancer: New Potential Role in Adjuvant Therapies. Frontiers in immunology 2016, 7:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Khadge S, Sharp JG, Thiele GM, McGuire TR, Klassen LW, Duryee MJ, Britton HC, Dafferner AJ, Beck J, Black PN, DiRusso CC, Talmadge J: Dietary omega-3 and omega-6 polyunsaturated fatty acids modulate hepatic pathology. The Journal of nutritional biochemistry 2018, 52:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kang JX, Wan JB, He C: Concise review: Regulation of stem cell proliferation and differentiation by essential fatty acids and their metabolites. Stem cells (Dayton, Ohio) 2014, 32:1092–8. [DOI] [PubMed] [Google Scholar]
- [7].Eltweri AM, Thomas AL, Metcalfe M, Calder PC, Dennison AR, Bowrey DJ: Potential applications of fish oils rich in omega-3 polyunsaturated fatty acids in the management of gastrointestinal cancer. Clin Nutr 2017, 36:65–78. [DOI] [PubMed] [Google Scholar]
- [8].Nabavi SF, Bilotto S, Russo GL, Orhan IE, Habtemariam S, Daglia M, Devi KP, Loizzo MR, Tundis R, Nabavi SM: Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev 2015, 34:359–80. [DOI] [PubMed] [Google Scholar]
- [9].Eccles SA, Alexander P: Macrophage content of tumours in relation to metastatic spread and host immune reaction. Nature 1974, 250:667–9. [DOI] [PubMed] [Google Scholar]
- [10].Varesio L, Blasi E, Thurman GB, Talmadge JE, Wiltrout RH, Herberman RB: Potent activation of mouse macrophages by recombinant interferon-gamma. Cancer Res 1984, 44:4465–9. [PubMed] [Google Scholar]
- [11].Key M, Talmadge JE, Fidler IJ: Lack of correlation between the progressive growth of spontaneous metastases and their content of infiltrating macrophages. J ReticuloendothelSoc 1982, 32:387–96. [PubMed] [Google Scholar]
- [12].Evans R, Lawler EM: Macrophage content and immunogenicity of C57BL/6J and BALB/cByJ methylcholanthrene-induced sarcomas. Int J Cancer 1980, 26:831–5. [DOI] [PubMed] [Google Scholar]
- [13].Steele RJ, Eremin O, Brown M, Hawkins RA: A high macrophage content in human breast cancer is not associated with favourable prognostic factors. BrJ Surg 1984, 71:456–8. [DOI] [PubMed] [Google Scholar]
- [14].Sun XF, Zhang H: Clinicopathological significance of stromal variables: angiogenesis, lymphangiogenesis, inflammatory infiltration, MMP and PINCH in colorectal carcinomas. MolCancer 2006, 5:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pollard JW: Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004, 4:71–8. [DOI] [PubMed] [Google Scholar]
- [16].Mantovani A, Allavena P, Sica A, Balkwill F: Cancer-related inflammation. Nature 2008, 454:436–44. [DOI] [PubMed] [Google Scholar]
- [17].Gabrilovich DI, Ostrand-Rosenberg S, Bronte V: Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012, 12:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chioda M, Peranzoni E, Desantis G, Papalini F, Falisi E, Samantha S, Mandruzzato S, Bronte V: Myeloid cell diversification and complexity: an old concept with new turns in oncology. Cancer metastasis reviews 2011, 30:27–43. [DOI] [PubMed] [Google Scholar]
- [19].Talmadge JE: Pathways Mediating the Expansion and Immunosuppressive Activity of Myeloid-Derived Suppressor Cells and Their Relevance to Cancer Therapy. Clin Cancer Res 2007, 13:5243–8. [DOI] [PubMed] [Google Scholar]
- [20].Talmadge JE, Gabrilovich DI: History of myeloid-derived suppressor cells. Nat Rev Cancer 2013, 13:739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D: Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 2011, 121:4015–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gabrilovich DI, Nagaraj S: Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009, 9:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Alexander P, Eccles SA, Gauci CL: The significance of macrophages in human and experimental tumors. AnnNYAcad Sci 1976, 276:124–33. [DOI] [PubMed] [Google Scholar]
- [24].Sinha P: Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Research 2005, 65:11743–51. [DOI] [PubMed] [Google Scholar]
- [25].Okubo M, Kioi M, Nakashima H, Sugiura K, Mitsudo K, Aoki I, Taniguchi H, Tohnai I: M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci Rep 2016, 6:27548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lin D, Wang X, Choi SYC, Ci X, Dong X, Wang Y: Immune phenotypes of prostate cancer cells: Evidence of epithelial immune cell-like transition? Asian J Urol 2016, 3:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Okabe M, Toh U, Iwakuma N, Saku S, Akashi M, Kimitsuki Y, Seki N, Kawahara A, Ogo E, Itoh K, Akagi Y: Predictive factors of the tumor immunological microenvironment for long-term follow-up in early stage breast cancer. Cancer Sci 2017, 108:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS: Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 2014, 32:2959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Talmadge JE, Donkor M, Scholar E: Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev 2007, 26:373–400. [DOI] [PubMed] [Google Scholar]
- [30].MacLennan MB, Clarke SE, Perez K, Wood GA, Muller WJ, Kang JX, Ma DW: Mammary tumor development is directly inhibited by lifelong n-3 polyunsaturated fatty acids. J Nutr Biochem 2013, 24:388–95. [DOI] [PubMed] [Google Scholar]
- [31].Marks F, Muller-Decker K, Furstenberger G: A causal relationship between unscheduled eicosanoid signaling and tumor development: cancer chemoprevention by inhibitors of arachidonic acid metabolism. Toxicology 2000, 153:11–26. [DOI] [PubMed] [Google Scholar]
- [32].Sawada N, Inoue M, Iwasaki M, Sasazuki S, Shimazu T, Yamaji T, Takachi R, Tanaka Y, Mizokami M, Tsugane S, Japan Public Health Center-Based Prospective Study G: Consumption of n-3 fatty acids and fish reduces risk of hepatocellular carcinoma. Gastroenterology 2012, 142:1468–75. [DOI] [PubMed] [Google Scholar]
- [33].Sasazuki S, Inoue M, Iwasaki M, Sawada N, Shimazu T, Yamaji T, Takachi R, Tsugane S, Japan Public Health Center-Based Prospective Study G: Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int J Cancer 2011, 129:1718–29. [DOI] [PubMed] [Google Scholar]
- [34].Begin ME, Ells G, Das UN, Horrobin DF: Differential killing of human carcinoma cells supplemented with n-3 and n-6 polyunsaturated fatty acids. Journal of the National Cancer Institute 1986, 77:1053–62. [PubMed] [Google Scholar]
- [35].LeMay-Nedjelski L, Mason-Ennis JK, Taibi A, Comelli EM, Thompson LU: Omega-3 Polyunsaturated Fatty Acids Time-Dependently Reduce Cell Viability and Oncogenic MicroRNA-21 Expression in Estrogen Receptor-Positive Breast Cancer Cells (MCF-7). International journal of molecular sciences 2018, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Menendez JA, Lupu R, Colomer R: Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur J Cancer Prev 2005, 14:263–70. [DOI] [PubMed] [Google Scholar]
- [37].Wang S, Wu J, Suburu J, Gu Z, Cai J, Axanova LS, Cramer SD, Thomas MJ, Perry DL, Edwards IJ, Mucci LA, Sinnott JA, Loda MF, Sui G, Berquin IM, Chen YQ: Effect of dietary polyunsaturated fatty acids on castration-resistant Pten-null prostate cancer. Carcinogenesis 2012, 33:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zanoaga O, Jurj A, Raduly L, Cojocneanu-Petric R, Fuentes-Mattei E, Wu O, Braicu C, Gherman CD, Berindan-Neagoe I: Implications of dietary omega-3 and omega-6 polyunsaturated fatty acids in breast cancer. Exp Ther Med 2018, 15:1167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Baracos VE, Mazurak VC, Ma DW: n-3 Polyunsaturated fatty acids throughout the cancer trajectory: influence on disease incidence, progression, response to therapy and cancer-associated cachexia. Nutr Res Rev 2004, 17:177–92. [DOI] [PubMed] [Google Scholar]
- [40].Mori TA, Beilin LJ: Omega-3 fatty acids and inflammation. Curr Atheroscler Rep 2004, 6:461–7. [DOI] [PubMed] [Google Scholar]
- [41].Calder PC: Dietary modification of inflammation with lipids. Proc Nutr Soc 2002, 61:345–58. [DOI] [PubMed] [Google Scholar]
- [42].Vaughan VC, Hassing MR, Lewandowski PA: Marine polyunsaturated fatty acids and cancer therapy. Br J Cancer 2013, 108:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Church SE, Galon J: Regulation of CTL infiltration within the tumor microenvironment. Adv Exp Med Biol 2017, 1036:33–49. [DOI] [PubMed] [Google Scholar]
- [44].Karanikas V, Zamanakou M, Soukou F, Kerenidi T, Gourgoulianis KI, Germenis AE: Naturally occurring tumor-specific CD8+ T-cell precursors in individuals with and without cancer. Immunol Cell Biol 2010, 88:575–85. [DOI] [PubMed] [Google Scholar]
- [45].Gervois N, Guilloux Y, Diez E, Jotereau F: Suboptimal activation of melanoma infiltrating lymphocytes (TIL) due to low avidity of TCR/MHC-tumor peptide interactions. Journal of Experimental Medicine 1996, 183:2403–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, Kudoh S, Ochiai A: Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008, 113:1387–95. [DOI] [PubMed] [Google Scholar]
- [47].Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G: Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. NEnglJ Med 2003, 348:203–13. [DOI] [PubMed] [Google Scholar]
- [48].Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W: Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine 2004, 10:942–9. [DOI] [PubMed] [Google Scholar]
- [49].Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van dV, Fleuren GJ, Kuppen PJ: Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest 2004, 84:493–501. [DOI] [PubMed] [Google Scholar]
- [50].Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H: CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998, 58:3491–4. [PubMed] [Google Scholar]
- [51].Piersma SJ, Jordanova ES, van Poelgeest MIE, Kwappenberg KMC, van der Hulst JM, Drijfhout JW, Melief CJM, Kenter GG, Fleuren GJ, Offringa R, van der Burg SH: High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Research 2007, 67:354–61. [DOI] [PubMed] [Google Scholar]
- [52].Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H: Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 2001, 61:5132–6. [PubMed] [Google Scholar]
- [53].Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH: Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 2009, 29:1093–102. [DOI] [PubMed] [Google Scholar]
- [54].Mlecnik B, Van den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, Pairet G, Jouret-Mourin A, Gigot JF, Hubert C, Danse E, Dragean C, Carrasco J, Humblet Y, Valge-Archer V, Berger A, Pages F, Machiels JP, Galon J: Comprehensive intrametastatic immune quantification and major impact of immunoscore on survival. J Natl Cancer Inst 2018, 110. [DOI] [PubMed] [Google Scholar]
- [55].Gorgun G, Samur MK, Cowens KB, Paula S, Bianchi G, Anderson JE, White RE, Singh A, Ohguchi H, Suzuki R, Kikuchi S, Harada T, Hideshima T, Tai YT, Laubach JP, Raje N, Magrangeas F, Minvielle S, Avet-Loiseau H, Munshi NC, Dorfman DM, Richardson PG, Anderson KC: Lenalidomide enhances immune checkpoint blockade-induced immune response in multiple myeloma. Clin Cancer Res 2015, 21:4607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, Taguchi R, Masuda K, Sasaki K, Urabe D, Inoue M, Arai H: Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J Biol Chem 2012, 287:10525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Heusinkveld M, van der Burg SH: Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med 2011, 9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Biswas SK, Mantovani A: Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010, 11:889–96. [DOI] [PubMed] [Google Scholar]
- [59].Piccard H, Muschel RJ, Opdenakker G: On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 2012, 82:296–309. [DOI] [PubMed] [Google Scholar]
- [60].Talmadge JE, Meyers KM, Prieur DJ, Starkey JR: Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J Natl Cancer Inst 1980, 65:929–35. [PubMed] [Google Scholar]
- [61].Donadon M, Hudspeth K, Cimino M, Di Tommaso L, Preti M, Tentorio P, Roncalli M, Mavilio D, Torzilli G: Increased infiltration of natural killer and T cells in colorectal liver metastases improves patient overall survival. J Gastrointest Surg 2017, 21:1226–36. [DOI] [PubMed] [Google Scholar]
- [62].Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F: Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Frontiers in immunology 2018, 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kurts C, Cannarile M, Klebba I, Brocker T: Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J Immunol 2001, 166:1439–42. [DOI] [PubMed] [Google Scholar]
- [64].Calder PC: Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015, 1851:469–84. [DOI] [PubMed] [Google Scholar]
- [65].Song M, Nishihara R, Cao Y, Chun E, Qian ZR, Mima K, Inamura K, Masugi Y, Nowak JA, Nosho K, Wu K, Wang M, Giovannucci E, Garrett WS, Fuchs CS, Ogino S, Chan AT: Marine omega-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer Characterized by Tumor-Infiltrating T Cells. JAMA Oncol 2016, 2:1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Calder PC: n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 2006, 83:1505S–19S. [DOI] [PubMed] [Google Scholar]
- [67].Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA: Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent Fatty Acids 2016, 107:24–9. [DOI] [PubMed] [Google Scholar]
- [68].Barden AE, Mas E, Mori TA: n-3 Fatty acid supplementation and proresolving mediators of inflammation. Curr Opin Lipidol 2016, 27:26–32. [DOI] [PubMed] [Google Scholar]
- [69].Volker D, Fitzgerald P, Major G, Garg M: Efficacy of fish oil concentrate in the treatment of rheumatoid arthritis. J Rheumatol 2000, 27:2343–6. [PubMed] [Google Scholar]
- [70].Mocellin MC, Camargo CQ, Nunes EA, Fiates GM, Trindade EB: A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin Nutr 2016, 35:359–69. [DOI] [PubMed] [Google Scholar]
- [71].Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, Yin H, Zhou J: Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol 2013, 43:2943–55. [DOI] [PubMed] [Google Scholar]
- [72].Baffy G: Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 2009, 51:212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Byrne CD: Fatty liver: role of inflammation and fatty acid nutrition. Prostaglandins Leukot Essent Fatty Acids 2010, 82:265–71. [DOI] [PubMed] [Google Scholar]
- [74].Lumeng CN, Bodzin JL, Saltiel AR: Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007, 117:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Morris DL, Singer K, Lumeng CN: Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care 2011, 14:341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z: EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int 2005, 67:867–74. [DOI] [PubMed] [Google Scholar]
- [77].Tai CC, Ding ST: N-3 polyunsaturated fatty acids regulate lipid metabolism through several inflammation mediators: mechanisms and implications for obesity prevention. J Nutr Biochem 2010, 21:357–63. [DOI] [PubMed] [Google Scholar]
- [78].Luo W, Xu Q, Wang Q, Wu H, Hua J: Effect of modulation of PPAR-gamma activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease. Sci Rep 2017, 7:44612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hayashi N, Tashiro T, Yamamori H, Takagi K, Morishima Y, Otsubo Y, Sugiura T, Furukawa K, Nitta H, Nakajima N, Suzuki N, Ito I: Effect of intravenous omega-6 and omega-3 fat emulsions on nitrogen retention and protein kinetics in burned rats. Nutrition 1999, 15:135–9. [DOI] [PubMed] [Google Scholar]
- [80].Khair-el-Din TA, Sicher SC, Vazquez MA, Wright WJ, Lu CY: Docosahexaenoic acid, a major constituent of fetal serum and fish oil diets, inhibits IFN gamma-induced Ia-expression by murine macrophages in vitro. J Immunol 1995, 154:1296–306. [PubMed] [Google Scholar]
- [81].Hughes DA, Southon S, Pinder AC: (n-3) Polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes in vitro. J Nutr 1996, 126:603–10. [DOI] [PubMed] [Google Scholar]
- [82].Hughes DA, Pinder AC: N-3 polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes and inhibit antigen-presentation in vitro. Clin Exp Immunol 1997, 110:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hubbard NE, Somers SD, Erickson KL: Effect of dietary fish oil on development and selected functions of murine inflammatory macrophages. J Leukoc Biol 1991, 49:592–8. [DOI] [PubMed] [Google Scholar]
- [84].Sadeghi S, Wallace FA, Calder PC: Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology 1999, 96:404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Peterson LD, Thies F, Sanderson P, Newsholme EA, Calder PC: Low levels of eicosapentaenoic and docosahexaenoic acids mimic the effects of fish oil upon rat lymphocytes. Life Sci 1998, 62:2209–17. [DOI] [PubMed] [Google Scholar]
- [86].Ghosh S, Novak EM, Innis SM: Cardiac proinflammatory pathways are altered with different dietary n-6 linoleic to n-3 alpha-linolenic acid ratios in normal, fat-fed pigs. Am J Physiol Heart Circ Physiol 2007, 293:H2919–27. [DOI] [PubMed] [Google Scholar]
- [87].Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C: Health implications of high dietary omega-6 polyunsaturated Fatty acids. Journal of nutrition and metabolism 2012, 2012:539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Janakiram Naveena B., M A, Lang Mark L., Rao Chinthalapally V.: Immune modulation by agents used in the prevention and treatment of colon and pancreatic cancers. Cancer Immunology: Cancer Immunotherapy for Organ-Specific Tumors 2015:249–75. [Google Scholar]
- [89].Greene ER, Huang S, Serhan CN, Panigrahy D: Regulation of inflammation in cancer by eicosanoids. Prostaglandins & other lipid mediators 2011, 96:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Fitzgerald-Bocarsly P, Dai J, Singh S: Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev 2008, 19:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Szebeni GJ, Vizler C, Kitajka K, Puskas LG: Inflammation and cancer: extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediators Inflamm 2017, 2017:9294018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ: Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009, 58:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ostrand-Rosenberg S, Fenselau C: Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol 2018, 200:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI: HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010, 207:2439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI: Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016, 7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Anani W, Shurin MR: Targeting Myeloid-Derived Suppressor Cells in Cancer. Adv Exp Med Biol 2017, 1036:105–28. [DOI] [PubMed] [Google Scholar]
- [97].Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK: Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol 2012, 22:275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Appleby LJ, Nausch N, Heard F, Erskine L, Bourke CD, Midzi N, Mduluza T, Allen JE, Mutapi F: Down Regulation of the TCR Complex CD3zeta-Chain on CD3+ T Cells: A Potential Mechanism for Helminth-Mediated Immune Modulation. Frontiers in immunology 2015, 6:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Levine AG, Hemmers S, Baptista AP, Schizas M, Faire MB, Moltedo B, Konopacki C, Schmidt-Supprian M, Germain RN, Treuting PM, Rudensky AY: Suppression of lethal autoimmunity by regulatory T cells with a single TCR specificity. J Exp Med 2017, 214:609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B: Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 2006, 203:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA: CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006, 203:1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Tarhini AA, Butterfield LH, Shuai Y, Gooding WE, Kalinski P, Kirkwood JM: Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA4 antibody and interferon-alpha or TLR-9 agonist and GM-CSF with peptide vaccination. J Immunother 2012, 35:702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chao JL, Savage PA: Unlocking the complexities of tumor-associated regulatory T-cells. J Immunol 2018, 200:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Spellman A, Tang SC: Immunotherapy for breast cancer: past, present, and future. Cancer Metastasis Rev 2016, 35:525–46. [DOI] [PubMed] [Google Scholar]
- [105].Seledtsov VI, Goncharov AG, Seledtsova GV: Clinically feasible approaches to potentiating cancer cell-based immunotherapies. Hum Vaccin Immunother 2015, 11:851–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR Jr., Muller W, Rudensky AY: Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008, 28:546–58. [DOI] [PubMed] [Google Scholar]
- [107].Wall R, Ross RP, Fitzgerald GF, Stanton C: Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev 2010, 68:280–9. [DOI] [PubMed] [Google Scholar]
- [108].Serhan CN: Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 2007, 25:101–37. [DOI] [PubMed] [Google Scholar]
- [109].Serhan CN, Chiang N, Van Dyke TE: Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008, 8:349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Badwey JA, Curnutte JT, Robinson JM, Berde CB, Karnovsky MJ, Karnovsky ML: Effects of free fatty acids on release of superoxide and on change of shape by human neutrophils. Reversibility by albumin. J Biol Chem 1984, 259:7870–7. [PubMed] [Google Scholar]
- [111].Bates EJ, Ferrante A, Smithers L, Poulos A, Robinson BS: Effect of fatty acid structure on neutrophil adhesion, degranulation and damage to endothelial cells. Atherosclerosis 1995, 116:247–59. [DOI] [PubMed] [Google Scholar]
- [112].Soyland E, Nenseter MS, Braathen L, Drevon CA: Very long chain n-3 and n-6 polyunsaturated fatty acids inhibit proliferation of human T-lymphocytes in vitro. Eur J Clin Invest 1993, 23:112–21. [DOI] [PubMed] [Google Scholar]
- [113].Santoli D, Phillips PD, Colt TL, Zurier RB: Suppression of interleukin 2-dependent human T cell growth in vitro by prostaglandin E (PGE) and their precursor fatty acids. Evidence for a PGE-independent mechanism of inhibition by the fatty acids. J Clin Invest 1990, 85:424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kelly JP, Parker CW: Effects of arachidonic acid and other unsaturated fatty acids on mitogenesis in human lymphocytes. J Immunol 1979, 122:1556–62. [PubMed] [Google Scholar]
- [115].Calder PC: Dietary fatty acids and the immune system. Nutr Rev 1998, 56:S70–83. [DOI] [PubMed] [Google Scholar]
- [116].Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC, et al. : The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med 1989, 320:265–71. [DOI] [PubMed] [Google Scholar]
- [117].German JB, Lokesh B, Kinsella JE: The effect of dietary fish oils on eicosanoid biosynthesis in peritoneal macrophages is influenced by both dietary N-6 polyunsaturated fats and total dietary fat. Prostaglandins Leukot Essent Fatty Acids 1988, 34:37–45. [DOI] [PubMed] [Google Scholar]
- [118].Siriwardhana N, Kalupahana NS, Fletcher S, Xin W, Claycombe KJ, Quignard-Boulange A, Zhao L, Saxton AM, Moustaid-Moussa N: n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-kappaB-dependent mechanisms. J Nutr Biochem 2012, 23:1661–7. [DOI] [PubMed] [Google Scholar]
- [119].Babu US, Bunning VK, Wiesenfeld P, Raybourne RB, O’Donnell M: Effect of dietary flaxseed on fatty acid composition, superoxide, nitric oxide generation and antilisterial activity of peritoneal macrophages from female Sprague-Dawley rats. Life Sci 1997, 60:545–54. [DOI] [PubMed] [Google Scholar]
- [120].Turek JJ, Schoenlein IA, Bottoms GD: The effect of dietary n-3 and n-6 fatty acids on tumor necrosis factor-alpha production and leucine aminopeptidase levels in rat peritoneal macrophages. Prostaglandins Leukot Essent Fatty Acids 1991, 43:141–9. [DOI] [PubMed] [Google Scholar]
- [121].Jeffery NM, Newsholme EA, Calder PC: Level of polyunsaturated fatty acids and the n-6 to n-3 polyunsaturated fatty acid ratio in the rat diet alter serum lipid levels and lymphocyte functions. Prostaglandins Leukot Essent Fatty Acids 1997, 57:149–60. [DOI] [PubMed] [Google Scholar]
- [122].Turchini GM, Nichols PD, Barrow C, Sinclair AJ: Jumping on the omega-3 bandwagon: distinguishing the role of long-chain and short-chain omega-3 fatty acids. Crit Rev Food Sci Nutr 2012, 52:795–803. [DOI] [PubMed] [Google Scholar]
- [123].Simopoulos AP: Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 2002, 21:495–505. [DOI] [PubMed] [Google Scholar]
- [124].Wild GE, Drozdowski L, Tartaglia C, Clandinin MT, Thomson AB: Nutritional modulation of the inflammatory response in inflammatory bowel disease--from the molecular to the integrative to the clinical. World J Gastroenterol 2007, 13:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].James M, Proudman S, Cleland L: Fish oil and rheumatoid arthritis: past, present and future. Proc Nutr Soc 2010, 69:316–23. [DOI] [PubMed] [Google Scholar]
- [126].Ahima RS: Digging deeper into obesity. J Clin Invest 2011, 121:2076–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Vlassov AV, Magdaleno S, Setterquist R, Conrad R: Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012, 1820:940–8. [DOI] [PubMed] [Google Scholar]
- [128].Galic S, Oakhill JS, Steinberg GR: Adipose tissue as an endocrine organ. Mol Cell Endocrinol 2010, 316:129–39. [DOI] [PubMed] [Google Scholar]
- [129].de Luca C, Olefsky JM: Inflammation and insulin resistance. FEBS Lett 2008, 582:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Correa LH, Correa R, Farinasso CM, de Sant’Ana Dourado LP, Magalhaes KG: Adipocytes and Macrophages Interplay in the Orchestration of Tumor Microenvironment: New Implications in Cancer Progression. Frontiers in immunology 2017, 8:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ghorpade DS, Ozcan L, Zheng Z, Nicoloro SM, Shen Y, Chen E, Bluher M, Czech MP, Tabas I: Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature 2018, 555:673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Roberts DL, Dive C, Renehan AG: Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med 2010, 61:301–16. [DOI] [PubMed] [Google Scholar]
- [133].Trayhurn P, Wood IS: Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 2004, 92:347–55. [DOI] [PubMed] [Google Scholar]
- [134].Vona-Davis L, Rose DP: The obesity-inflammation-eicosanoid axis in breast cancer. J Mammary Gland Biol Neoplasia 2013, 18:291–307. [DOI] [PubMed] [Google Scholar]
- [135].Ben-Neriah Y, Karin M: Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol 2011, 12:715–23. [DOI] [PubMed] [Google Scholar]
- [136].Honma S, Shimodaira K, Shimizu Y, Tsuchiya N, Saito H, Yanaihara T, Okai T: The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocr J 2002, 49:371–7. [DOI] [PubMed] [Google Scholar]
- [137].Ryan AS, Berman DM, Nicklas BJ, Sinha M, Gingerich RL, Meneilly GS, Egan JM, Elahi D: Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care 2003, 26:2383–8. [DOI] [PubMed] [Google Scholar]
- [138].Vona-Davis L, Rose DP: Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer 2007, 14:189–206. [DOI] [PubMed] [Google Scholar]
- [139].Calder PC: Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 2008, 52:885–97. [DOI] [PubMed] [Google Scholar]
- [140].Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B: n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem 2000, 275:721–4. [DOI] [PubMed] [Google Scholar]
- [141].Flower RJ, Perretti M: Controlling inflammation: a fat chance? J Exp Med 2005, 201:671–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Norling LV, Serhan CN: Profiling in resolving inflammatory exudates identifies novel anti-inflammatory and pro-resolving mediators and signals for termination. J Intern Med 2010, 268:15–24. [DOI] [PubMed] [Google Scholar]
- [143].Gilroy DW, Lawrence T, Perretti M, Rossi AG: Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov 2004, 3:401–16. [DOI] [PubMed] [Google Scholar]
- [144].Rueter K, Haynes A, Prescott SL: Developing primary intervention strategies to prevent allergic disease. Curr Allergy Asthma Rep 2015, 15:40. [DOI] [PubMed] [Google Scholar]
- [145].Jackson JD, Yan Y, Brunda MJ, Kelsey LS, Talmadge JE: Interleukin-12 enhances peripheral hematopoiesis in vivo. Blood 1995, 85:2371–6. [PubMed] [Google Scholar]
- [146].Ouchi N, Parker JL, Lugus JJ, Walsh K: Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011, 11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hussein MR, Hassan HI: Analysis of the mononuclear inflammatory cell infiltrate in the normal breast, benign proliferative breast disease, in situ and infiltrating ductal breast carcinomas: preliminary observations. J Clin Pathol 2006, 59:972–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Rose DP, Connolly JM, Rayburn J, Coleman M: Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J Natl Cancer Inst 1995, 87:587–92. [DOI] [PubMed] [Google Scholar]
- [149].Rose DP, Connolly JM, Coleman M: Effect of omega-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin Cancer Res 1996, 2:1751–6. [PubMed] [Google Scholar]
- [150].Mandal CC, Ghosh-Choudhury T, Yoneda T, Choudhury GG, Ghosh-Choudhury N: Fish oil prevents breast cancer cell metastasis to bone. Biochem Biophys Res Commun 2010, 402:602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Gonzalez MJ, Schemmel RA, Gray JI, Dugan L Jr., Sheffield LG, Welsch CW: Effect of dietary fat on growth of MCF-7 and MDA-MB231 human breast carcinomas in athymic nude mice: relationship between carcinoma growth and lipid peroxidation product levels. Carcinogenesis 1991, 12:1231–5. [DOI] [PubMed] [Google Scholar]
- [152].Talmadge J: Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clinical Cancer Research 2007, 13:5243–8. [DOI] [PubMed] [Google Scholar]
- [153].Hardman WE: Dietary canola oil suppressed growth of implanted MDA-MB 231 human breast tumors in nude mice. Nutr Cancer 2007, 57:177–83. [DOI] [PubMed] [Google Scholar]
- [154].Manna S, Janarthan M, Ghosh B, Rana B, Rana A, Chatterjee M: Fish oil regulates cell proliferation, protect DNA damages and decrease HER-2/neu and c-Myc protein expression in rat mammary carcinogenesis. Clin Nutr 2010, 29:531–7. [DOI] [PubMed] [Google Scholar]
- [155].Noguchi M, Minami M, Yagasaki R, Kinoshita K, Earashi M, Kitagawa H, Taniya T, Miyazaki I: Chemoprevention of DMBA-induced mammary carcinogenesis in rats by low-dose EPA and DHA. Br J Cancer 1997, 75:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Manna S, Chakraborty T, Ghosh B, Chatterjee M, Panda A, Srivastava S, Rana A, Chatterjee M: Dietary fish oil associated with increased apoptosis and modulated expression of Bax and Bcl-2 during 7,12-dimethylbenz(alpha)anthracene-induced mammary carcinogenesis in rats. Prostaglandins Leukot Essent Fatty Acids 2008, 79:5–14. [DOI] [PubMed] [Google Scholar]
- [157].Simopoulos AP: The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002, 56:365–79. [DOI] [PubMed] [Google Scholar]
- [158].Wei N, Wang B, Zhang QY, Mi MT, Zhu JD, Yu XP, Yuan JL, Chen K, Wang J, Chang H: Effects of different dietary fatty acids on the fatty acid compositions and the expression of lipid metabolic-related genes in mammary tumor tissues of rats. Nutr Cancer 2008, 60:810–25. [DOI] [PubMed] [Google Scholar]
- [159].Jiang W, Zhu Z, McGinley JN, El Bayoumy K, Manni A, Thompson HJ: Identification of a molecular signature underlying inhibition of mammary carcinoma growth by dietary N-3 fatty acids. Cancer Res 2012, 72:3795–806. [DOI] [PubMed] [Google Scholar]
- [160].Xue M, Wang Q, Zhao J, Dong L, Ge Y, Hou L, Liu Y, Zheng Z: Docosahexaenoic acid inhibited the Wnt/beta-catenin pathway and suppressed breast cancer cells in vitro and in vivo. J Nutr Biochem 2014, 25:104–10. [DOI] [PubMed] [Google Scholar]
- [161].Liotta LA, Kohn EC: The microenvironment of the tumour-host interface. Nature 2001, 411:375–9. [DOI] [PubMed] [Google Scholar]
- [162].Li H, Fan X, Houghton J: Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 2007, 101:805–15. [DOI] [PubMed] [Google Scholar]
- [163].Jass JR: Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol 1986, 39:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, Satomi S: Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. BrJ Cancer 2004, 91:1711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F: Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313:1960–4. [DOI] [PubMed] [Google Scholar]
- [166].Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B: Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 2009, 27:186–92. [DOI] [PubMed] [Google Scholar]
- [167].Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ: Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology 2009, 137:1270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, Tsang KY, Licchetta A, Mannucci S, Loiacono L, Tassone P, Francini G, Tagliaferri P: Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother 2010, 33:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, Vermorken JB: Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol 2010, 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Chew A, Salama P, Robbshaw A, Klopcic B, Zeps N, Platell C, Lawrance IC: SPARC, FOXP3, CD8 and CD45 correlation with disease recurrence and long-term disease-free survival in colorectal cancer. PLoS One 2011, 6:e22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K: Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005, 102:18538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Tomsova M, Melichar B, Sedlakova I, Steiner I: Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol Oncol 2008, 108:415–20. [DOI] [PubMed] [Google Scholar]
- [173].Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR: Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011, 29:1949–55. [DOI] [PubMed] [Google Scholar]
- [174].Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO: CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res 2012, 14:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, Peng H, Cui L, Li C: Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer 2014, 110:1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Murff HJ, Shrubsole MJ, Cai Q, Smalley WE, Dai Q, Milne GL, Ness RM, Zheng W: Dietary intake of PUFAs and colorectal polyp risk. The American journal of clinical nutrition 2012, 95:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Habermann N, Ulrich CM, Lundgreen A, Makar KW, Poole EM, Caan B, Kulmacz R, Whitton J, Galbraith R, Potter JD, Slattery ML: PTGS1, PTGS2, ALOX5, ALOX12, ALOX15, and FLAP SNPs: interaction with fatty acids in colon cancer and rectal cancer. Genes & nutrition 2013, 8:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Van Blarigan EL, Fuchs CS, Niedzwiecki D, Ye X, Zhang S, Song M, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson A, Atienza D, Messino M, Kindler H, Venook A, Ogino S, Giovannucci EL, Meyerhardt JA: Marine omega-3 Polyunsaturated Fatty Acid and Fish Intake after Colon Cancer Diagnosis and Survival: CALGB 89803 (Alliance). Cancer Epidemiol Biomarkers Prev 2018, 27:438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Mazurak VC: n-3 polyunsaturated fatty acid supplementation during cancer chemotherapy. Journal of Nutrition \& Intermediary Metabolism 2016, 5:107–16. [Google Scholar]
- [180].Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O: Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. British journal of cancer 2009, 101:1978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Angell H, Galon J: From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol 2013, 25:261–7. [DOI] [PubMed] [Google Scholar]
- [182].Fridman WH, Pages F, Sautes-Fridman C, Galon J: The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012, 12:298–306. [DOI] [PubMed] [Google Scholar]
- [183].Galon J, Angell HK, Bedognetti D, Marincola FM: The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013, 39:11–26. [DOI] [PubMed] [Google Scholar]
- [184].Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J: Effector memory T cells, early metastasis, and survival in colorectal cancer. NEnglJ Med 2005, 353:2654–66. [DOI] [PubMed] [Google Scholar]
- [185].Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, Botti G, Tatangelo F, Delrio P, Maio M, Laghi L, Grizzi F, Asslaber M, D’Arrigo C, Vidal-Vanaclocha F, Zavadova E, Chouchane L, Ohashi PS, Hafezi-Bakhtiari S, Wouters BG, Roehrl M, Nguyen L, Kawakami Y, Hazama S, Okuno K, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Wang Y, Kopetz S, Sinicrope FA, Scripcariu V, Ascierto PA, Marincola FM, Fox BA, Pages F: Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014, 232:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, Masucci GV, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O’Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D’Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA: Cancer classification using the Immunoscore: a worldwide task force. J Transl Med 2012, 10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Galon J, Pages F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA: The immune score as a new possible approach for the classification of cancer. J Transl Med 2012, 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, Galon J: Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011, 29:610–8. [DOI] [PubMed] [Google Scholar]
- [189].Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J: In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009, 27:5944–51. [DOI] [PubMed] [Google Scholar]
- [190].Han F, Shang X, Wan F, Liu Z, Tian W, Wang D, Liu Y, Wang Y, Zhang B, Ju Y: Clinical value of the preoperative neutrophil-to-lymphocyte ratio and red blood cell distribution width in patients with colorectal carcinoma. Oncol Lett 2018, 15:3339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ: The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013, 88:218–30. [DOI] [PubMed] [Google Scholar]
- [192].Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T, Soejima Y, Maehara Y: Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 2013, 58:58–64. [DOI] [PubMed] [Google Scholar]
- [193].Kantola T, Klintrup K, Vayrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig KH, Napankangas J, Makela J, Karttunen TJ, Tuomisto A, Makinen MJ: Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer 2012, 107:1729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G: Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 2011, 71:5601–5. [DOI] [PubMed] [Google Scholar]
- [195].Wang J, Jia Y, Wang N, Zhang X, Tan B, Zhang G, Cheng Y: The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med 2014, 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coelle C, Mouroux J, Hofman P: Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer 2012, 118:1726–37. [DOI] [PubMed] [Google Scholar]
- [197].Chagas TR, Borges DS, de Oliveira PF, Mocellin MC, Barbosa AM, Camargo CQ, Del Moral JAG, Poli A, Calder PC, Trindade E, Nunes EA: Oral fish oil positively influences nutritional-inflammatory risk in patients with haematological malignancies during chemotherapy with an impact on long-term survival: a randomised clinical trial. J Hum Nutr Diet 2017, 30:681–92. [DOI] [PubMed] [Google Scholar]
- [198].do Carmo LS, Rogero MM, Paredes-Gamero EJ, Nogueira-Pedro A, Xavier JG, Cortez M, Borges MC, Fujii TM, Borelli P, Fock RA: A high-fat diet increases interleukin-3 and granulocyte colony-stimulating factor production by bone marrow cells and triggers bone marrow hyperplasia and neutrophilia in Wistar rats. Exp Biol Med (Maywood) 2013, 238:375–84. [DOI] [PubMed] [Google Scholar]
- [199].Rosales C: Neutrophil: A cell with many roles in inflammation or several cell types? Front Physiol 2018, 9:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Berg JW: Can nutrition explain the pattern of international epidemiology of hormone-dependent cancers? Cancer Res 1975, 35:3345–50. [PubMed] [Google Scholar]
- [201].Tominaga S: Cancer incidence in Japanese in Japan, Hawaii, and western United States. Natl Cancer Inst Monogr 1985, 69:83–92. [PubMed] [Google Scholar]
- [202].Goodstine SL, Zheng T, Holford TR, Ward BA, Carter D, Owens PH, Mayne ST: Dietary (n-3)/(n-6) fatty acid ratio: possible relationship to premenopausal but not postmenopausal breast cancer risk in U.S. women. J Nutr 2003, 133:1409–14. [DOI] [PubMed] [Google Scholar]
- [203].Calder PC: N-3 polyunsaturated fatty acids and immune cell function. Adv Enzyme Regul 1997, 37:197–237. [DOI] [PubMed] [Google Scholar]
- [204].Rose DP: Dietary fatty acids and cancer. Am J Clin Nutr 1997, 66:998S–1003S. [DOI] [PubMed] [Google Scholar]
- [205].Simonsen N, van’t Veer P, Strain JJ, Martin-Moreno JM, Huttunen JK, Navajas JF, Martin BC, Thamm M, Kardinaal AF, Kok FJ, Kohlmeier L: Adipose tissue omega-3 and omega-6 fatty acid content and breast cancer in the EURAMIC study. European Community Multicenter Study on Antioxidants, Myocardial Infarction, and Breast Cancer. Am J Epidemiol 1998, 147:342–52. [DOI] [PubMed] [Google Scholar]
- [206].Thiebaut AC, Chajes V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, Berrino F, Riboli E, Benichou J, Clavel-Chapelon F: Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer 2009, 124:924–31. [DOI] [PubMed] [Google Scholar]
- [207].Kim J, Lim SY, Shin A, Sung MK, Ro J, Kang HS, Lee KS, Kim SW, Lee ES: Fatty fish and fish omega-3 fatty acid intakes decrease the breast cancer risk: a case-control study. BMC Cancer 2009, 9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Khankari NK, Bradshaw PT, Steck SE, He K, Olshan AF, Shen J, Ahn J, Chen Y, Ahsan H, Terry MB, Teitelbaum SL, Neugut AI, Santella RM, Gammon MD: Dietary intake of fish, polyunsaturated fatty acids, and survival after breast cancer: A population-based follow-up study on Long Island, New York. Cancer 2015, 121:2244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Bagga D, Anders KH, Wang HJ, Glaspy JA: Long-chain n-3-to-n-6 polyunsaturated fatty acid ratios in breast adipose tissue from women with and without breast cancer. Nutr Cancer 2002, 42:180–5. [DOI] [PubMed] [Google Scholar]
- [210].Yang B, Ren XL, Fu YQ, Gao JL, Li D: Ratio of n-3/n-6 PUFAs and risk of breast cancer: a meta-analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer 2014, 14:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Chajes V, Torres-Mejia G, Biessy C, Ortega-Olvera C, Angeles-Llerenas A, Ferrari P, Lazcano-Ponce E, Romieu I: omega-3 and omega-6 Polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiol Biomarkers Prev 2012, 21:319–26. [DOI] [PubMed] [Google Scholar]
- [212].Im DS: Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur J Pharmacol 2016, 785:36–43. [DOI] [PubMed] [Google Scholar]
- [213].Bjursell M, Xu X, Admyre T, Bottcher G, Lundin S, Nilsson R, Stone VM, Morgan NG, Lam YY, Storlien LH, Linden D, Smith DM, Bohlooly YM, Oscarsson J: The beneficial effects of n-3 polyunsaturated fatty acids on diet induced obesity and impaired glucose control do not require Gpr120. PLoS One 2014, 9:e114942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Chung H, Lee YS, Mayoral R, Oh DY, Siu JT, Webster NJ, Sears DD, Olefsky JM, Ellies LG: Omega-3 fatty acids reduce obesity-induced tumor progression independent of GPR120 in a mouse model of postmenopausal breast cancer. Oncogene 2015, 34:3504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Turk HF, Chapkin RS: Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 2013, 88:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Xia S, Li XP, Cheng L, Han MT, Zhang MM, Shao QX, Xu HX, Qi L: Fish Oil-Rich Diet Promotes Hematopoiesis and Alters Hematopoietic Niche. Endocrinology 2015, 156:2821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Schumann T, Adhikary T, Wortmann A, Finkernagel F, Lieber S, Schnitzer E, Legrand N, Schober Y, Nockher WA, Toth PM, Diederich WE, Nist A, Stiewe T, Wagner U, Reinartz S, Muller-Brusselbach S, Muller R: Deregulation of PPARbeta/delta target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget 2015, 6:13416–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD: Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121:586–613. [DOI] [PubMed] [Google Scholar]
- [219].Committee DGA: 2010 Dietary Guidelines Advisory Committee Report. 2010.