Abstract
Introduction:
Intravascular fibrin clots and extravascular fibrin deposits are often implicated in the progression of liver fibrosis. However, evidence supporting a pathological role of fibrin in hepatic fibrosis is indirect and based largely on studies using anticoagulant drugs that inhibit activation of the coagulation protease thrombin, which has other downstream targets that promote fibrosis. Therefore, the goal of this study was to determine the precise role of fibrin deposits in experimental hepatic fibrosis.
Methods:
Liver fibrosis was induced in mice expressing mutant fibrinogen insensitive to thrombin-mediated proteolysis (i.e., locked in the monomeric form), termed FibAEK mice, and Factor XIII A2 subunit-deficient mice (FXIII−/−). Female wild-type mice, FXIII−/− mice, and homozygous FibAEK mice were challenged with carbon tetrachloride (CCl4), twice weekly for 4 or 6 weeks (1 ml/kg, ip).
Results:
Hepatic injury and fibrosis induced by CCl4 challenge were unaffected by FXIII deficiency or inhibition of thrombin-catalyzed fibrin polymer formation (in FibAEK mice). Surprisingly, hepatic deposition of cross-linked fibrin(ogen) was not reduced in CCl4-challenged FXIII−/− mice or FibAEK mice compared to wild-type mice. Rather, deposition of cross-linked hepatic fibrin(ogen) following CCl4 challenge was dramatically reduced in tissue transglutaminase-deficient mice (TGM2−/− mice). However, the reduction in cross-linked fibrin(ogen) in TGM2−/− mice did not affect CCl4-induced liver fibrosis.
Conclusions:
These results indicate that neither traditional fibrin clots, formed by the thrombin:FXIIIa pathway, nor atypical TGM2-cross-linked fibrin(ogen) contribute to experimental CCl4-induced liver fibrosis. Collectively, the results indicate that liver fibrosis occurs independently of intrahepatic fibrin(ogen) deposition.
Keywords: Coagulation, factor XIII, fibrin, fibrosis, thrombin
Introduction
Activation of the blood coagulation cascade is evident in experimental settings of chronic liver injury and fibrosis [1, 2]. This is evidenced by increases in plasma levels of biomarkers indicating generation of the coagulation protease thrombin and deposition of fibrin(ogen) within the liver [3–6]. Numerous studies over the last several decades support the hypothesis that activation of the coagulation cascade contributes to liver fibrosis. For example, administration of anticoagulant drugs or genetically reducing coagulation cascade activation reduces experimental liver fibrosis in rodents [6–10]. Mice with a pro-thrombotic state develop worse liver fibrosis than wild-type mice, and patients with hereditary hypercoagulable states show increased risk for development of liver fibrosis [9, 11–14]. Moreover, in a clinical study seeking to reduce the risk of portal vein thrombosis in patients with cirrhosis, anticoagulation was found to delay hepatic decompensation [15]. One ongoing clinical trial seeks to confirm and expand on this observation using rivaroxaban in patients with cirrhosis (NCT02643212). Thus, at present there is strong experimental and clinical evidence indicating a pathologic role for increased coagulation activity in liver fibrosis.
Several mechanisms are hypothesized to connect increased coagulation activity to the development of fibrosis, with most involving downstream substrates of the coagulation proteases Factor Xa (FXa) and thrombin. Activation of receptors for these proteases, namely protease activated receptor-1 (PAR-1) and PAR-2, has been shown to drive experimental fibrosis [5, 16, 17] and promote a pro-fibrogenic phenotype in cultured hepatic stellate cells [16, 18]. Another widely-accepted hypothesis is that thrombin exacerbates liver fibrosis by catalyzing the formation of intrahepatic fibrin clots, a component of microthrombi potentially driving parenchymal extinction [6, 19, 20]. Thrombin catalyzes conversion of soluble fibrinogen to fibrin monomers, which spontaneously polymerize. Thrombin also converts the inactive transglutaminase Factor XIII (FXIII) to its active form (FXIIIa), which then stabilizes and cross-links the fibrin polymers. Fibrin(ogen) deposits are present in the liver in many experimental settings of hepatic fibrosis [3, 4, 6, 21]. Formation of intrahepatic fibrin deposits by this process is proposed to promote fibrosis through multiple mechanisms, including the formation of a provisional matrix supporting subsequent deposition of collagen by activated hepatic stellate cells (i.e., liver myofibroblasts), and by blocking liver blood flow, causing liver damage and parenchymal extinction [22]. In fact, the formation of fibrin clots is frequently considered a primary mechanistic connection between hemostasis and liver disease [22–25]. Despite this, evidence supporting a role for fibrin in liver fibrosis has been indirect, and largely anchored in the protective effects afforded by anticoagulant drugs, which also inhibit the pro-fibrogenic PAR pathways [6, 26, 27]. Commonly used antibodies do not distinguish between fibrinogen and polymerized fibrin in tissues. Therefore, the term fibrin(ogen) has become convention to imply “either fibrinogen or fibrinogen-derived proteins,” including polymerized fibrin. Ultimately, addressing the specific role of thrombin-mediated fibrin clot formation in liver fibrosis has been challenging because of the multiple cellular targets of thrombin. Indeed, there are currently no published studies definitively documenting a pathologic role of thrombin-catalyzed fibrin polymer formation in liver fibrosis.
Here, using an established model of liver fibrosis induced by chronic carbon tetrachloride (CCl4) challenge, we document for the first time, the deposition of high molecular weight, cross-linked fibrin(ogen) tightly co-localized with collagen in the fibrotic liver. Moreover, using mice lacking the FXIII catalytic A2 subunit, and novel mice expressing a mutant form of fibrinogen that does not polymerize in response to thrombin (FibAEK mice), we test the longstanding, yet unverified hypothesis that thrombin-catalyzed fibrin clot formation contributes to the pathogenesis of liver fibrosis.
Materials and Methods
Mice:
Female mice expressing mutant fibrinogen that does not polymerize in response to thrombin (FibAEK mice) [28], mice lacking the catalytic FXIII-A2 subunit [29] (referred to as FXIII−/− mice), mice lacking tissue transglutaminase [30, 31] (TGM2−/− mice), and wild-type mice on an identical C57BL/6 background were used for these studies between 9–16 weeks of age. No mortality was observed over the duration of treatment for any genotype. Mice were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited facility at Michigan State University at approximately 22 ± 2 °C with alternating 12-hour light/dark cycles, and were provided ad libitum access to reverse-osmosis purified drinking water and rodent chow. All animal procedures were approved by Michigan State University (MSU) Institutional Animal Care and Use Committee.
Chronic CCl4 challenge and sample collection:
Mice were challenged with corn oil (vehicle) or 10% CCl4 in corn oil by intraperitoneal injection (10 ml/kg) twice weekly (i.e., Tuesday and Friday) for 4 or 6 weeks. Three days after the last injection of CCl4, under isoflurane anesthesia, the liver was excised and rinsed in phosphate-buffered saline. The left lateral lobe was fixed in 10% neutral-buffered formalin for approximately 96 hours and processed for routine histopathological analysis. Small sections of liver from different lobes were snap frozen in liquid nitrogen and saved for other analyses.
Histopathology and immunohistochemistry:
Hepatic fibrosis was quantified using multiple complementary approaches as described previously [32–36]. Staining of formalin-fixed paraffin-embedded liver sections to assess hepatic fibrosis was performed by the MSU Investigative Histopathology Laboratory, a division of Human Pathology. Briefly, paraffin-embedded livers were sectioned at 5 μm and stained with Gladstone-modified picrosirius red (PSR) to visualize hepatic fibrosis (i.e., collagen deposits) as described previously [37]. Expression of alpha-smooth muscle actin (αSMA) protein was detected by immunohistochemical labeling of paraffin-embedded liver tissues as described previously [37]. For quantification of the total area of PSR and αSMA staining, slides were first scanned using a Virtual Slide System VS110 (Olympus, Waltham, MA) with a 20x objective, and sample images were then digitally captured from the entire left lateral lobe (Visiopharm, Broomfield, CO) as described previously [32, 33, 37]. Total area of positive staining was calculated in an unbiased fashion from approximately 200 images per slide (captured with a 10x virtual objective) using a batch macro and color deconvolution tool in ImageJ Fiji [38].
Collagen type I and fibrin(ogen) were detected by immunofluorescent labeling of frozen liver sections. Briefly, frozen tissues were sectioned at 6 μm and fixed in 4% neutral-buffered formalin for 10 minutes. Following blocking in 10% normal goat serum, sections were incubated with rabbit anti-mouse collagen type I (Meridian Life Sciences, Memphis, TN, 1:500) or rabbit anti-human fibrinogen (Agilent Life Sciences, Santa Clara, CA, 1:2500) antibodies overnight at 4 °C. Staining was detected using Alexa Fluor 594-conjugated goat anti-rabbit IgG secondary antibody (Jackson Immunoresearch, West Grove, PA). Slides were scanned using a Virtual Slide System VS110, and the area of positive labeling was quantified in 4–5 randomly selected images (captured with a 4x virtual objective in OlyVIA software, Olympus) (approximately 40 mm2 of liver tissue) using a batch macro and threshold analysis tool in ImageJ.
Co-localization of collagen and fibrin(ogen) was detected in frozen liver tissues. Tissues were sectioned at 6 μm and fixed in 4% neutral-buffered formalin for 10 minutes, then blocked in 10% horse serum for 1 hour at room temperature. Sections were co-incubated with sheep anti-human fibrinogen (Bio-Rad, Hercules, CA, 1:1000) and rabbit anti-collagen type I (Meridian Life Sciences, 1:500) antibodies overnight at 4 °C. Staining was detected using highly cross-absorbed donkey anti-sheep IgG and goat anti-rabbit IgG secondary antibodies conjugated to Alexa Fluor 488 and 594, respectively (Jackson Immunoresearch). Fluorescently labeled slides were scanned using a Virtual Slide System VS110 with 20x objective.
RNA isolation, cDNA synthesis, and real-time PCR:
Total RNA was isolated from snap-frozen liver using TRI Reagent according to the manufacturer’s protocol (Molecular Research Center, Cincinnati, OH). 1 μg of total RNA was used to synthesize cDNA using a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Foster City, CA) and a C1000 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA). SYBR Green quantitative real-time PCR (qPCR) amplification was performed using a CFX Connect thermal cycler (Bio-Rad) with primers purchased from IDT (Coralville, IA) and PerfeCTa Sybr Green SuperMix (Quanta Biosciences, Beverly, MA). The expression of each gene was normalized to the geometric mean Ct of individual housekeeping genes, Hprt and Gapdh, and relative fold change was determined using the ΔΔCt method. Primer sequences used are previously described [33].
Detection of intrahepatic HMW fibrin(ogen) by automated capillary gel electrophoresis:
Intrahepatic levels of urea-insoluble (i.e., cross-linked [39, 40]), high molecular weight (HMW) fibrin(ogen) was determined as described previously [41] based on protocols adapted for determining fibrin content in venous thrombi [42]. Briefly, 70 mg of snap-frozen liver was homogenized in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, pH 7.5) supplemented with 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 1 mM PMSF and protease inhibitor (G Biosciences, St. Louis, MO). Samples were then rotated end-over-end for 1 hour at 4°C and spun at 7000xg for 15 minutes. Supernatant was saved for protein determination and the pellet was resuspended in solution containing 8 M urea, 40 mM DTT, 12.5 mM EDTA, rotated end-over-end at 60°C for 1 hour, and then incubated at 60°C overnight (~16 hours). The samples were then spun at 10,000 x g for 5 minutes and the supernatants were resolved on Wes 66–440 kDa 25-capillary gels using the Wes simple western approach (Protein Simple, San Jose, CA). Fibrin(ogen) was detected using polyclonal rabbit antibodies specific for fibrinogen α (Fibα) or γ (Fibγ) chains (1:100) (against human fibrinogen-α or fibrinogen-γ fusion proteins, Proteintech, Rosemont, IL, catalog numbers 20645–1-AP and 15841–1-AP) using the Wes Rabbit Master Kit and goat anti-rabbit HRP-conjugated secondary antibody (1:400, Jackson Immunoresearch) according to the manufacturer’s protocol (Protein Simple, San Jose, CA). Chemiluminescent signal was analyzed using Compass software (Protein Simple), and results are reported as area under the curve for fibrinogen peaks with molecular weight between 300–450 kDa.
Statistical Analysis:
Statistical significance was determined using the Student’s t-test or a two-way analysis of variance (ANOVA) with the Student-Newman-Keuls post hoc test, as appropriate. Differences were considered significant if p < 0.05.
Results
Deposition of cross-linked fibrin(ogen) in liver after chronic CCl4 challenge.
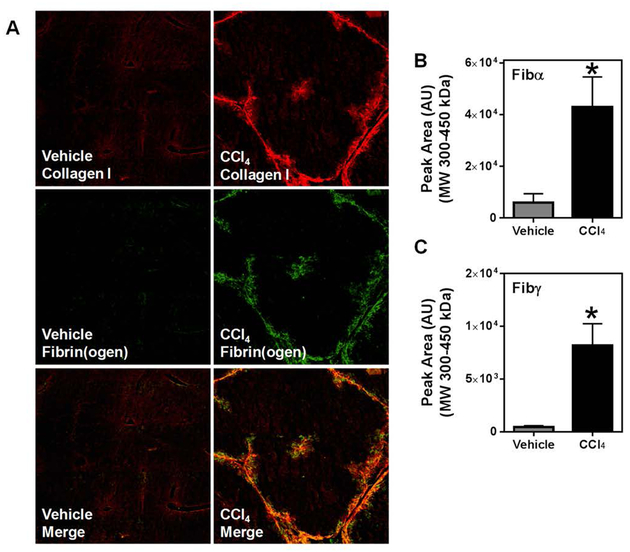
CCl4 challenge significantly increased deposition of collagen type I and fibrin(ogen) (Figure 1A) in livers of wild-type mice. Consistent with prior reports of fibrin(ogen) labeling in livers of CCl4-challenged animals [4, 6, 8], immunofluorescent detection revealed marked co-localization of fibrino(ogen) and collagen (Figure 1A, yellow). However, widely-applied strategies for immunolabeling of fibrin(ogen) in tissue sections do not distinguish between fibrinogen and cross-linked insoluble fibrin polymers. Therefore, we next determined the extent of fibrin(ogen) cross-linking in the insoluble fraction of livers of CCl4-challenged mice. First, we validated the specificity of commercial polyclonal antibodies specific for the Fibα and Fibγ chains (not shown). Next, we used capillary electrophoresis (Wes Simple Western) to determine levels of cross-linked Fibα and Fibγ chains deposited in the livers of CCl4-challenged mice (Figures 1B and 1C). Hepatic levels of cross-linked Fibα and Fibγ increased significantly in the livers of CCl4-challenged mice. The approximate molecular weights of the HMW cross-linked Fibα and Fibγ were similar, suggesting these species may be composed of Fibα and Fibγ cross-linked chains. Collectively, the results indicate that fibrin(ogen) deposits co-localize with collagen deposits, and that levels of cross-linked Fibα and Fibγ increase in livers of CCl4-challenged mice.
Fig. 1: Deposition of high molecular weight fibrin(ogen) co-localizes with collagen type I following chronic CCl4 exposure.
Wild-type mice were treated with CCl4 or vehicle for 4 weeks, and hepatic fibrin(ogen) and collagen deposition were assessed 3 days after the last injection. (A) Representative photomicrographs (4x virtual objective) of immunofluorescent labeling of collagen type I, fibrinogen, and image overlay in liver sections. HMW cross-linked Fibα (B) and Fibγ (C) detected in liver tissues. Data are presented as mean + SEM (n=6 mice per group). * p < 0.05 compared to vehicle-treated mice.
CCl4-induced liver fibrosis develops independently of FXIIIa transglutaminase activity.
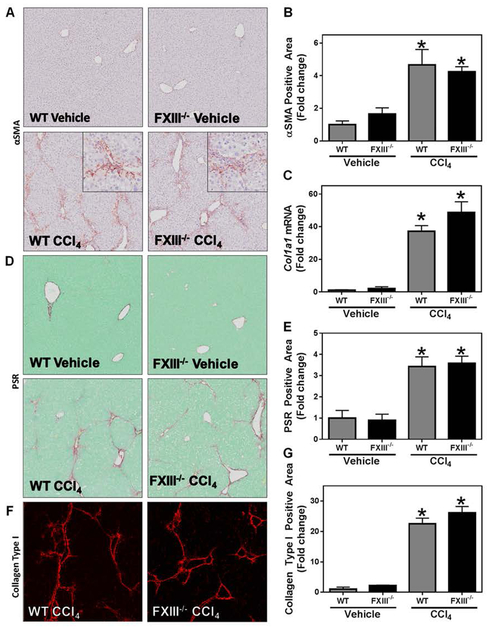
Intravascular fibrin clots are formed through a classical pathway initiated by thrombin-catalyzed fibrin polymer formation. Cross-linking of the fibrin polymer by the transglutaminase FXIIIa provides stability and contributes functionally to both hemostasis and thrombosis [43, 44]. FXIII is a heterodimeric protein comprised of two catalytic A subunits and two regulatory B subunits (A2B2). To address the role of FXIIIa in liver fibrosis, we induced liver fibrosis with CCl4 challenge in mice lacking the catalytic A2 subunit of FXIII (i.e, FXIII−/− mice) [29]. Chronic CCl4 challenge caused hepatic stellate cell activation, indicated by increased expression of αSMA (Figure 2A and B) and increased expression of collagen type I mRNA (Col1a1) (Figure 2C). A corresponding increase in collagen deposition was observed in the livers of CCl4-challenged wild-type mice, as measured by PSR staining and confirmed by immunofluorescent labeling for collagen type I protein (Figure 2D–G). Interestingly, αSMA levels and induction of Col1a1 after CCl4 challenge in FXIII−/− mice were similar to that of CCl4-challenged wild-type mice (Figure 2A–C). Moreover, this deficiency did not affect collagen deposition in CCl4-challenged mice (Figure 2D–G). The results indicate that CCl4-induced liver fibrosis develops independently of FXIIIa catalytic activity.
Fig. 2: Effect of FXIII catalytic A2 subunit deficiency on CCl4-induced experimental hepatic fibrosis.
Wild-type and FXIII−/− mice were treated with CCl4 or vehicle for 6 weeks and hepatic fibrosis was assessed 3 days after the last injection. (A) Representative photomicrographs (4x virtual objective, 20x insert) of hepatic αSMA labeling. (C) Hepatic Col1a1 expression. (D) Representative photomicrographs (4x virtual objective) of hepatic PSR staining. (F) Representative photomicrographs (4x virtual objective) of hepatic collagen type I labeling. (B, E, G) Quantification of positive staining area. Data are presented as mean + SEM (n=5–12 mice per group). * p < 0.05 compared to vehicle-treated mice.
CCl4-induced liver fibrosis occurs independently of thrombin-catalyzed fibrin polymer formation.
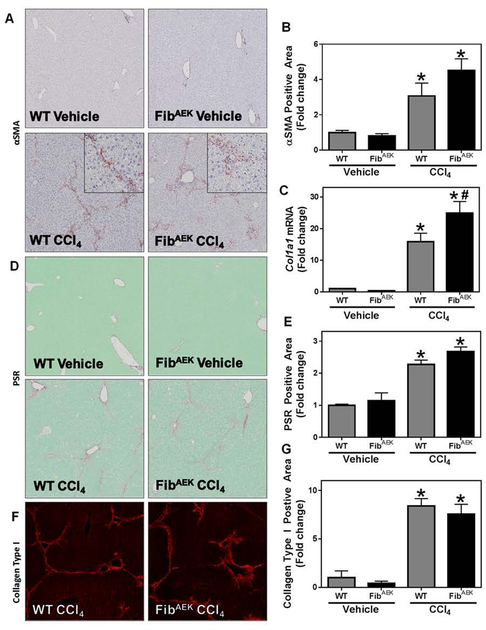
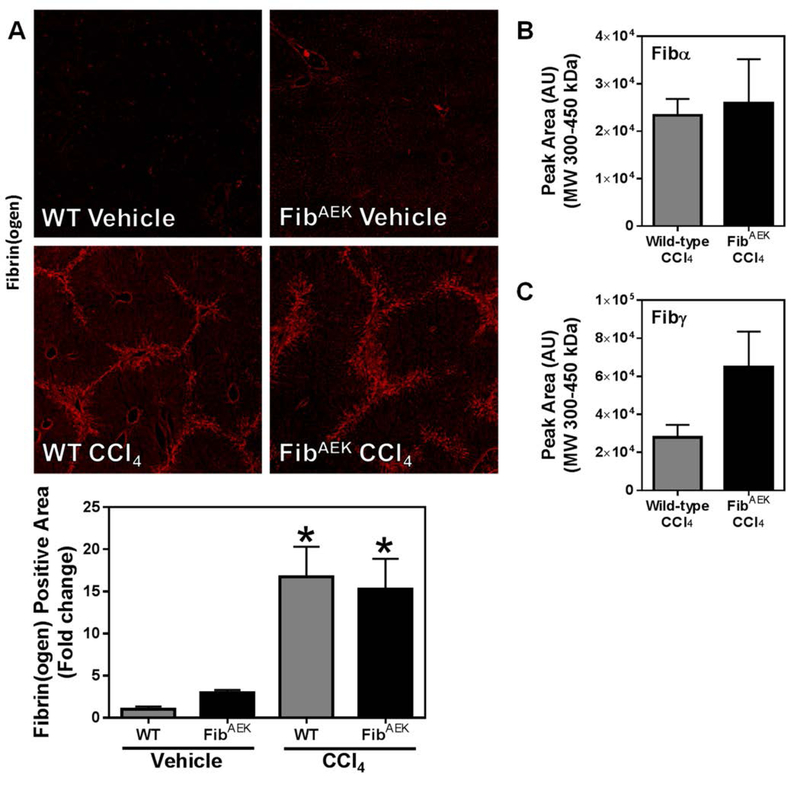
To isolate the precise role of thrombin-catalyzed fibrin polymer formation in hepatic fibrosis, we used FibAEK mice, which express a mutant fibrinogen incapable of forming fibrin polymer in response to thrombin [28]. Hepatic stellate cell activation, marked by increased expression of αSMA and Col1a1, was evident in CCl4-challenged wild-type mice (Figure 3A–C); this was reflected by a significant increase in hepatic collagen deposition, indicated by increased PSR staining and collagen type I deposition in the liver (Figure 3D–G). Interestingly, markers of stellate cell activation (e.g., αSMA expression), and collagen deposition were similarly increased in livers of CCl4-challenged FibAEK mice compared to wild-type mice; however, Col1a1 induction was significantly enhanced in livers of CCl4-challenged FibAEK mice. The results indicate that CCl4-induced liver fibrosis occurs independently of thrombin-catalyzed fibrin polymer formation.
Fig. 3: Effect of FibAEK expression on CCl4-induced experimental hepatic fibrosis.
Wild-type and FibAEK mice were treated with CCl4 or vehicle for 4 weeks and hepatic fibrosis was assessed 3 days after the last injection. (A) Representative photomicrographs (4x virtual objective, 20x insert) of hepatic αSMA labeling. (C) Hepatic Col1a1 mRNA expression. (D) Representative photomicrographs (4x virtual objective) of hepatic PSR staining. (F) Representative photomicrographs (4x virtual objective) of hepatic collagen type I labeling. (B, E, G) Quantification of positive staining area. Data are presented as mean + SEM (n=5–11 mice per group). * p < 0.05 compared to vehicle-treated mice, # p < 0.05 compared to wild-type mice of same treatment group.
Hepatic fibrin(ogen) deposition after chronic CCl4 challenge is mediated by a mechanism independent of thrombin-directed fibrin polymer formation or cross-linking by FXIIIa.
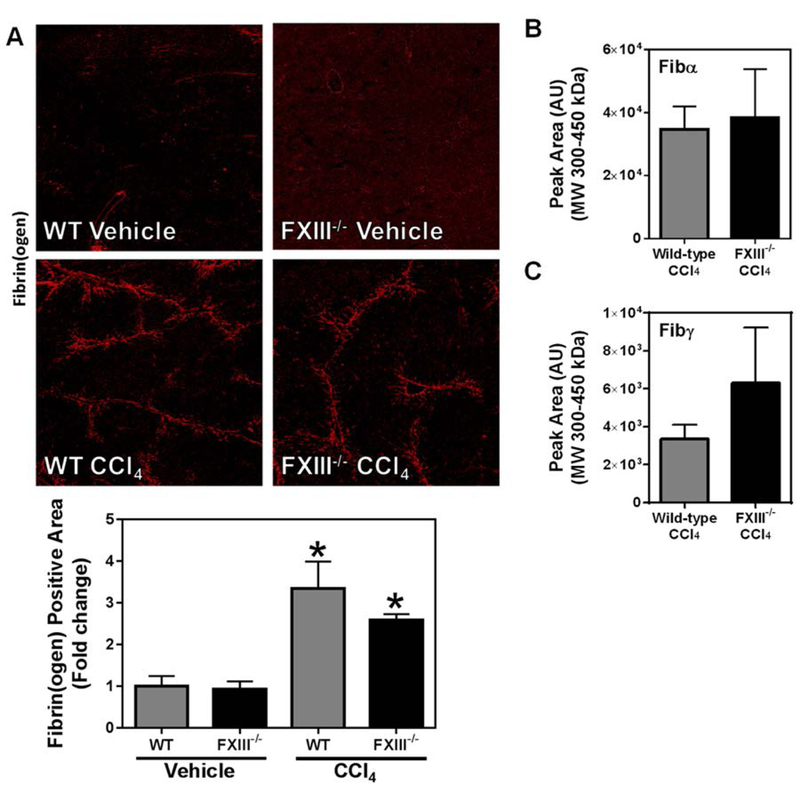
Thrombin-catalyzed fibrin polymer formation is a widely accepted prerequisite for FXIIIa-dependent cross-linking [45]. Moreover, under physiological conditions, FXIIIa is thought to be the primary transglutaminase to cross-link fibrin(ogen) [46]. Given the critical role that thrombin and FXIIIa play in traditional intravascular fibrin formation [47], we anticipated a reduction in hepatic fibrin(ogen) deposition in both FXIII−/− mice and FibAEK mice. However, to our surprise, hepatic fibrin(ogen) deposition driven by chronic CCl4 challenge was unaffected by FXIII catalytic A2 subunit deficiency or by the fibrinogenAEK mutation (Figures 4A and 5A, respectively). Even more surprising, neither FXIII catalytic A2 subunit deficiency, nor the absence of thrombin-catalyzed fibrin polymer formation (i.e., in FibAEK mice) significantly affected the levels of cross-linked Fibα and Fibγ (Figure 4B–C and Figure 5B–C, respectively) observed after chronic CCl4 treatment. Collectively, these results indicate that fibrin(ogen) deposits in the livers of CCl4-challenged mice are distinct species from those of traditional intravascular fibrin (i.e., are not mediated by thrombin-mediated polymerization or cross-linking by FXIIIa).
Fig. 4: Effect of FXIII catalytic A2 subunit deficiency on CCl4-induced hepatic fibrin(ogen) deposition.
Wild-type and FXIII−/− mice were treated with CCl4 or vehicle for 6 weeks, and hepatic fibrin(ogen) deposition was assessed 3 days after the last injection. (A) Representative photomicrographs (4x virtual objective) of immunofluorescent labeling of fibrin(ogen). Area of positive staining was measured as described in Materials and Methods. HMW cross-linked Fibα (B) and Fibγ (C) detected in liver tissues. Data are presented as mean + SEM (n=5–12 mice per group). * p < 0.05 compared to vehicle-treated mice.
Fig. 5: Effect of FibAEK expression on CCl4-induced hepatic fibrin(ogen) deposition.
Wild-type and FibAEK mice were treated with CCl4 or vehicle for 4 weeks, and hepatic fibrin(ogen) deposition was assessed 3 days after the last injection. (A) Representative photomicrographs (4x virtual objective) of immunofluorescent labeling of fibrin(ogen). Area of positive staining was measured as described in Materials and Methods. HMW cross-linked Fibα (B) and Fibγ (C) detected in liver tissues. Data are presented as mean + SEM (n=5–11 mice per group). * p < 0.05 compared to vehicle-treated mice.
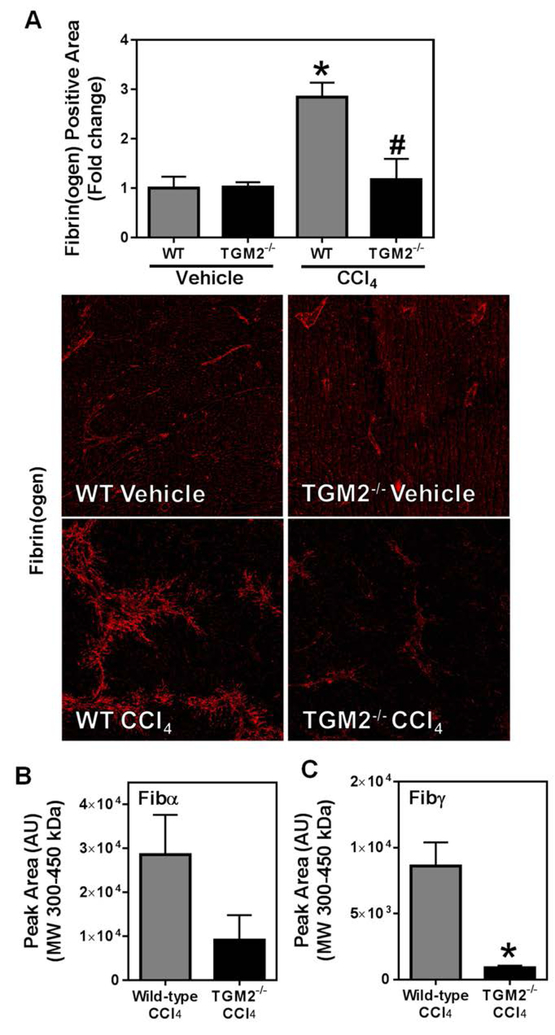
Tissue transglutaminase-2 (TGM2) cross-links fibrin(ogen) in experimental liver fibrosis.
To identify the FXIII-independent mechanism of fibrin(ogen) cross-linking, we considered TGM2, which is expressed at high levels in the liver and catalyzes hybrid FibAα-Fibγ chain cross-links [48]. However, no previous study has documented TGM2-cross-linked fibrin(ogen) in vivo. We found that TGM2 deficiency caused a near complete reduction in fibrin(ogen) staining in the livers of CCl4-treated mice compared to wild-type animals (Figure 6A). Of importance, plasma fibrinogen levels were similar in wild-type and TGM2−/− mice challenged with CCl4 (not shown). Compared to wild-type mice, HMW cross-linked Fibα tended to be reduced, and Fibγ levels were significantly reduced in CCl4-treated TGM2−/− mice (Figure 6B–C). These results indicate that TGM2 cross-links fibrinogen in experimental liver fibrosis in a manner consistent with in vivo assembly of cross-linked FibAα-Fibγ.
Fig. 6: Effect of TGM2 deficiency on CCl4-induced hepatic fibrinogen deposition.
Wild-type and TGM2−/− mice were treated with CCl4 or vehicle for 6 weeks, and hepatic fibrin(ogen) deposition was assessed 3 days after the last injection. (A) Representative photomicrographs (4x virtual objective) of labeling of fibrin(ogen) and quantitation of positive area. HMW cross-linked Fibα (B) and Fibγ (C) was detected in liver tissues. Data are presented as mean + SEM (n=5 mice per group). * p < 0.05 compared to vehicle-treated mice (A and D), or to wild-type CCl4-treated mice (C).
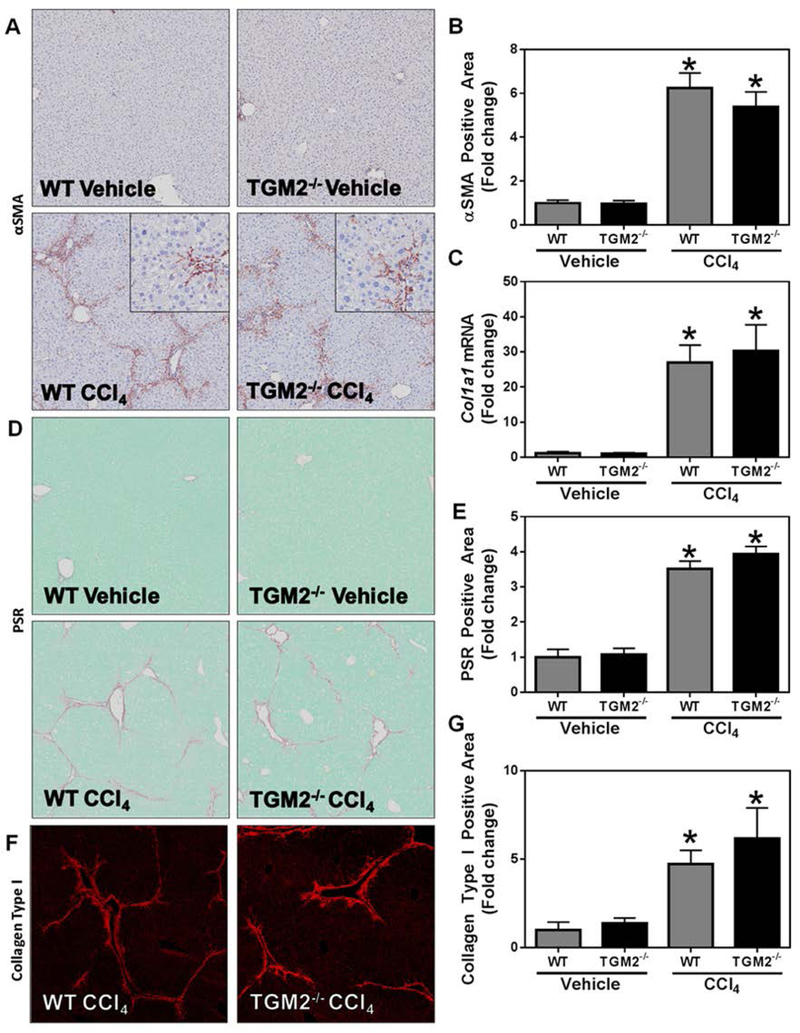
Notably, the absence of TGM2 and associated loss of cross-linked fibrin(ogen) did not impact the development of hepatocellular injury, as indicated by equivalent serum ALT activity (not shown). No obvious difference in inflammatory cell accumulation was evident in H&E-stained liver sections and inflammatory gene induction was comparable in wild-type and TGM2−/− mice challenged with CCl4 (not shown). Moreover, hepatic stellate cell activation, collagen gene expression, and hepatic collagen deposition were similar in wild-type and TGM2−/− mice challenged with CCl4, indicating no impact of TGM2 deficiency on hepatic fibrosis (Figure 7).
Fig. 7: Effect of TGM2 deficiency on CCl4-induced experimental hepatic fibrosis.
Wild-type and TGM2−/− mice were treated with CCl4 or vehicle for 6 weeks and hepatic fibrosis was assessed 3 days after the last injection. (A) Representative photomicrographs (4x virtual objective, 20x insert) of hepatic αSMA labeling. (C) Hepatic Col1a1 expression. (D) Representative photomicrographs (4x virtual objective) of hepatic PSR staining. (F) Representative photomicrographs (4x virtual objective) of hepatic collagen type I labeling. (B, E, G) Quantification of positive staining area. Data are presented as mean + SEM (n=5 mice per group). * p < 0.05 compared to vehicle-treated mice.
Discussion
Although it has been proposed that intrahepatic fibrin clots have a pathologic function in experimental hepatic fibrosis, evidence supporting this hypothesis is limited. To definitively identify the role of fibrin in liver fibrosis, we used experimental tools that precisely interfere with two distinct steps in traditional intravascular fibrin clot formation (i.e., thrombin-catalyzed fibrin polymerization and FXIIIa-mediated fibrin cross-linking) and discovered that traditional cross-linked fibrin polymers do not contribute to the development of experimental hepatic fibrosis. Moreover, in an equally surprising observation, our results suggest that hepatic fibrin(ogen) accumulation in experimental liver fibrosis is driven by a mechanism seemingly independent from blood coagulation. Collectively, these studies upend the widely held assumption that the thrombin-fibrinogen pathway contributes to experimental liver fibrosis.
The longstanding assumption that fibrin clots contribute to fibrosis has been anchored in studies using anticoagulant drugs. However, inhibition of upstream coagulation activity or thrombin itself blocks both proteolytic cleavage of fibrinogen as well as activation of other thrombin substrates, and therefore does not specifically implicate fibrin as an upstream mediator of liver fibrosis. Our study is the first to unambiguously test the role of thrombin-mediated fibrin polymer formation in experimental fibrosis by using mice expressing fibrinogenAEK, which cannot form fibrin polymer in response to thrombin. We found that selectively blocking the thrombin-fibrinogen pathway had no effect on liver fibrosis. This is critically important because other thrombin targets, including protease activated receptors (PARs), are still fully operative in the FibAEK mice. Strong evidence indicates that liver fibrosis is driven by thrombin-mediated (and FXa-mediated) activation of PARs, a family of tethered-ligand G-protein-coupled receptors [49, 50]. Thrombin is known to activate PAR-1 [51], and FXa can activate PAR-2 [52]. PAR-1 and PAR-2 are expressed by hepatic stellate cells, and activation of these receptors drives a myofibroblast-like phenotype in vitro [16, 53]. Importantly, experimental liver fibrosis is reduced in PAR-1-deficient and PAR-2-deficient mice [5, 16, 17, 54]. The discovery that fibrin is dispensable for development of experimental fibrosis supports the hypothesis that PAR-directed pro-fibrogenic pathways are an important mechanism connecting coagulation activity to liver fibrosis. Defining the profibrogenic effects of thrombin-PAR signaling may be particularly effective in mice lacking cross-linked fibrin(ogen) in liver (e.g., Fib−/− mice, TGM2−/− mice).
Traditional intravascular blood clotting includes thrombin-catalyzed fibrin polymer formation as a pre-requisite for cross-linking of fibrin(ogen) in physiological conditions [45], with FXIIIa serving as the predominant enzyme cross-linking fibrin(ogen) in vivo. It is assumed that fibrin(ogen) accumulates in the fibrotic liver as a traditional fibrin “clot,” formed as a consequence of these molecular steps. However, standard immunolabeling approaches used to detect fibrin(ogen) deposits do not distinguish these unique fibrin(ogen) cross-linking modifications. By adapting an approach used for quantification of cross-linked fibrin in venous thrombi [42], we identified an increase in HMW cross-linked alpha and gamma chains of fibrin(ogen) in the fibrotic liver. Notably, fibrin(ogen) cross-linking in the fibrotic liver was independent of thrombin-catalyzed fibrin polymerization. Moreover, we found that FXIII catalytic A2 subunit deficiency did not significantly reduce fibrin(ogen) cross-linking in the livers of CCl4-challenged mice. Collectively, these surprising results indicate that in experimental liver fibrosis, cross-linked fibrin(ogen) accumulates by a mechanism distinct from that of traditional intravascular fibrin. This result raises the question of whether fibrin(ogen) deposits in the injured liver, a universal feature of acute and chronic liver toxicity, always represent traditional fibrin.
The presence of cross-linked fibrinogen α and γ chains in livers of CCl4-challenged FibAEK mice suggested fibrin(ogen) polymer formation was not required for the mechanism of fibrin(ogen) cross-linking in the chronically-injured liver. Notably, soluble fibrinogen is a substrate of TGM2, which can cross-link fibrinogen in the absence of thrombin-mediated polymer formation [48]. Multiple hepatic cell types express TGM2, including hepatocytes and stellate cells [55]. Interestingly, TGM2 cross-linking of fibrin(ogen) also favors production of FibAα-Fibγ hybrid fibrin(ogen) [48, 56], consistent with the observed pattern of cross-linking in livers of CCl4-challenged mice. Of importance, we found that TGM2 cross-linking activity was critical to localize fibrin(ogen) to the injured liver, although the cellular source of TGM2 essential for this localization is not known. To our knowledge, this result offers the first evidence that fibrin(ogen) is a bona fide substrate of TGM2 transglutaminase activity in an in vivo setting, validating the documented in vitro biochemical activity of TGM2. Further supporting a direct, coagulation-independent role of TGM2, coagulation cascade activation was evident in both wild-type and TGM2−/− mice after acute CCl4 challenge (not shown). Both fibrinogen [48] and collagen [57] are substrates of TGM2, and prior studies indicate TGM2 can cross-link these two proteins together [58]. Indeed, it has been reported that in vitro activation of stellate cells (the primary collagen-producing cells in hepatic fibrosis) causes stellate cells to increase expression of TGM2 [55]. This phenomenon may potentially explain the co-localization of these proteins in the injured liver. This may also explain why interventions that reduce collagen deposition (including anticoagulants) also reduce hepatic fibrin(ogen) deposition, inverting the classical assumption that fibrin drives collagen deposition.
In the setting of CCl4-induced experimental hepatic fibrosis, intrahepatic fibrin(ogen) deposits do not appear to contribute to disease severity. Indeed, hepatic fibrin(ogen) deposition in CCl4-challenged TGM2−/− mice was dramatically reduced compared to wild-type animals with no effect on hepatic fibrosis, consistent with previous reports that TGM2 deficiency has no effect on development or resolution of hepatic fibrosis [59]. Notably, this confirms that the reduction in fibrin(ogen) deposits in livers of CCl4-challenged TGM2−/− mice was not indirectly affected by a reduction in disease severity. Overall, while fibrin(ogen) appears to not drive liver fibrosis in this experimental setting, the precise role of fibrin(ogen) in acute and chronic liver injury appears to relate to the stimulus for injury and experimental context [33, 41]. How precise changes in fibrin(ogen) structure affect these various functions in the injured liver should frame future studies.
The results presented here redefine the current understanding of the contribution of fibrin polymers to experimental hepatic fibrosis. Using two different, but complementary genetic approaches, we definitively demonstrate that experimental hepatic fibrosis occurs independently of thrombin-directed fibrin polymer formation and FXIIIa-mediated fibrin(ogen) cross-linking. Furthermore, our studies reveal a novel mechanism whereby deposition of fibrin(ogen) in the chronically-injured liver is driven by TGM2-directed fibrin(ogen) cross-linking. Collectively, these studies provide strong experimental evidence that the mechanism connecting coagulation activity to liver fibrosis occurs via a pathway independent of hepatic fibrin(ogen) deposition in the chronically damaged liver, suggesting a need to revisit dogma recurrently placing fibrin as central to experimental liver fibrosis.
Essentials.
Fibrin clots are often implicated in the progression of liver fibrosis
Liver fibrosis was induced in transgenic mice with defects in clot formation or stabilization
Liver fibrosis and fibrin(ogen) deposition do not require fibrin polymerization or Factor XIIIa
Fibrin(ogen) is an in vivo substrate of tissue transglutaminase in experimental liver fibrosis
Acknowledgements
The authors would like to thank Dr. Daniela Matei for generously providing TGM2−/− mice. Funding support from the National Institutes of Health (NIH R01 ES017537 and NIH T32 ES007255) is gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the NIH.
Footnotes
Disclosure of Conflict of Interest
The authors state that they have no conflict of interest.
References
- 1.Lisman T, Caldwell SH, Burroughs AK, Northup PG, Senzolo M, Stravitz RT, Tripodi A, Trotter JF, Valla DC, Porte RJ, Coagulation in Liver Disease Study G. Hemostasis and thrombosis in patients with liver disease: the ups and downs. J Hepatol. 2010; 53: 362–71. 10.1016/j.jhep.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 2.Tripodi A Hemostasis in Acute and Chronic Liver Disease. Semin Liver Dis. 2017; 37: 28–32. 10.1055/s-0036-1597770. [DOI] [PubMed] [Google Scholar]
- 3.Joshi N, Kopec AK, Towery K, Williams KJ, Luyendyk JP. The Antifibrinolytic Drug Tranexamic Acid Reduces Liver Injury and Fibrosis in a Mouse Model of Chronic Bile Duct Injury. The Journal of Pharmacology and Experimental Therapeutics. 2014; 349: 383–92. 10.1124/jpet.113.210880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neubauer K, Knittel T, Armbrust T, Ramadori G. Accumulation and cellular localization of fibrinogen/fibrin during short-term and long-term rat liver injury. Gastroenterology. 1995; 108: 1124–35. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan BP, Weinreb PH, Violette SM, Luyendyk JP. The coagulation system contributes to alphaVbeta6 integrin expression and liver fibrosis induced by cholestasis. Am J Pathol. 2010; 177: 2837–49. 10.2353/ajpath.2010.100425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vilaseca M, Garcia-Caldero H, Lafoz E, Garcia-Irigoyen O, Avila MA, Reverter JC, Bosch J, Hernandez-Gea V, Gracia-Sancho J, Garcia-Pagan JC. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology. 2017; 65: 2031–44. 10.1002/hep.29084. [DOI] [PubMed] [Google Scholar]
- 7.Abe W, Ikejima K, Lang T, Okumura K, Enomoto N, Kitamura T, Takei Y, Sato N. Low molecular weight heparin prevents hepatic fibrogenesis caused by carbon tetrachloride in the rat. J Hepatol. 2007; 46: 286–94. 10.1016/j.jhep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Cerini F, Vilaseca M, Lafoz E, Garcia-Irigoyen O, Garcia-Caldero H, Tripathi DM, Avila M, Reverter JC, Bosch J, Gracia-Sancho J, Garcia-Pagan JC. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2016; 64: 834–42. 10.1016/j.jhep.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Anstee QM, Goldin RD, Wright M, Martinelli A, Cox R, Thursz MR. Coagulation status modulates murine hepatic fibrogenesis: implications for the development of novel therapies. J Thromb Haemost. 2008; 6: 1336–43. 10.1111/j.1538-7836.2008.03015.x. [DOI] [PubMed] [Google Scholar]
- 10.Dhar A, Sadiq F, Anstee QM, Levene AP, Goldin RD, Thursz MR. Thrombin and factor Xa link the coagulation system with liver fibrosis. BMC Gastroenterol. 2018; 18: 60 10.1186/s12876-018-0789-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright M, Goldin R, Hellier S, Knapp S, Frodsham A, Hennig B, Hill A, Apple R, Cheng S, Thomas H, Thursz M. Factor V Leiden polymorphism and the rate of fibrosis development in chronic hepatitis C virus infection. Gut. 2003; 52: 1206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anstee QM, Dhar A, Thursz MR. The role of hypercoagulability in liver fibrogenesis. Clin Res Hepatol Gastroenterol. 2011; 35: 526–33. 10.1016/j.clinre.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Maharshak N, Halfon P, Deutsch V, Peretz H, Berliner S, Fishman S, Zelber-Sagi S, Rozovski U, Leshno M, Oren R. Increased fibrosis progression rates in hepatitis C patients carrying the prothrombin G20210A mutation. World J Gastroenterol. 2011; 17: 5007–13. 10.3748/wjg.v17.i45.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plompen EP, Darwish Murad S, Hansen BE, Loth DW, Schouten JN, Taimr P, Hofman A, Uitterlinden AG, Stricker BH, Janssen HL, Leebeek FW. Prothrombotic genetic risk factors are associated with an increased risk of liver fibrosis in the general population: The Rotterdam Study. J Hepatol. 2015; 63: 1459–65. 10.1016/j.jhep.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Villa E, Camma C, Marietta M, Luongo M, Critelli R, Colopi S, Tata C, Zecchini R, Gitto S, Petta S, Lei B, Bernabucci V, Vukotic R, De Maria N, Schepis F, Karampatou A, Caporali C, Simoni L, Del Buono M, Zambotto B, Turola E, Fornaciari G, Schianchi S, Ferrari A, Valla D. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012; 143: 1253–60 e1–4. 10.1053/j.gastro.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Fiorucci S, Antonelli E, Distrutti E, Severino B, Fiorentina R, Baldoni M, Caliendo G, Santagada V, Morelli A, Cirino G. PAR1 antagonism protects against experimental liver fibrosis. Role of proteinase receptors in stellate cell activation. Hepatology. 2004; 39: 365–75. 10.1002/hep.20054. [DOI] [PubMed] [Google Scholar]
- 17.Rullier A, Gillibert-Duplantier J, Costet P, Cubel G, Haurie V, Petibois C, Taras D, Dugot-Senant N, Deleris G, Bioulac-Sage P, Rosenbaum J. Protease-activated receptor 1 knockout reduces experimentally induced liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2008; 294: G226–35. 10.1152/ajpgi.00444.2007. [DOI] [PubMed] [Google Scholar]
- 18.Gaca MD, Zhou X, Benyon RC. Regulation of hepatic stellate cell proliferation and collagen synthesis by proteinase-activated receptors. J Hepatol. 2002; 36: 362–9. [DOI] [PubMed] [Google Scholar]
- 19.Simonetto DA, Yang HY, Yin M, de Assuncao TM, Kwon JH, Hilscher M, Pan S, Yang L, Bi Y, Beyder A, Cao S, Simari RD, Ehman R, Kamath PS, Shah VH. Chronic passive venous congestion drives hepatic fibrogenesis via sinusoidal thrombosis and mechanical forces. Hepatology. 2015; 61: 648–59. 10.1002/hep.27387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beier JI, Luyendyk JP, Guo L, von Montfort C, Staunton DE, Arteel GE. Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice. Hepatology. 2009; 49: 1545–53. 10.1002/hep.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Irigoyen O, Carotti S, Latasa MU, Uriarte I, Fernandez-Barrena MG, Elizalde M, Urtasun R, Vespasiani-Gentilucci U, Morini S, Banales JM, Parks WC, Rodriguez JA, Orbe J, Prieto J, Paramo JA, Berasain C, Avila MA. Matrix metalloproteinase-10 expression is induced during hepatic injury and plays a fundamental role in liver tissue repair. Liver Int. 2014; 34: e257–70. 10.1111/liv.12337. [DOI] [PubMed] [Google Scholar]
- 22.Anstee QM, Wright M, Goldin R, Thursz MR. Parenchymal extinction: coagulation and hepatic fibrogenesis. Clin Liver Dis. 2009; 13: 117–26. 10.1016/j.cld.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Beier JI, Arteel GE, McClain CJ. Advances in alcoholic liver disease. Curr Gastroenterol Rep. 2011; 13: 56–64. 10.1007/s11894-010-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beier JI, Arteel GE. Alcoholic liver disease and the potential role of plasminogen activator inhibitor-1 and fibrin metabolism. Exp Biol Med (Maywood). 2012; 237: 1–9. 10.1258/ebm.2011.011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poole LG, Arteel GE. Transitional Remodeling of the Hepatic Extracellular Matrix in Alcohol-Induced Liver Injury. Biomed Res Int. 2016; 2016: 3162670 10.1155/2016/3162670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsieh YC, Lee KC, Yang YY, Huang YH, Hsu WF, Lin HC. Dabigatran reduces liver fibrosis in thioacetamide-injured rats. Journal of Hepatology. 2017; 66: S657 10.1016/S0168-8278(17)31780-4. [DOI] [PubMed] [Google Scholar]
- 27.Duplantier JG, Dubuisson L, Senant N, Freyburger G, Laurendeau I, Herbert JM, Desmouliere A, Rosenbaum J. A role for thrombin in liver fibrosis. Gut. 2004; 53: 1682–7. 10.1136/gut.2003.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad JM, Gorkun OV, Raghu H, Thornton S, Mullins ES, Palumbo JS, Ko YP, Hook M, David T, Coughlin SR, Degen JL, Flick MJ. Mice expressing a mutant form of fibrinogen that cannot support fibrin formation exhibit compromised antimicrobial host defense. Blood. 2015; 126: 2047–58. 10.1182/blood-2015-04-639849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souri M, Koseki-Kuno S, Takeda N, Degen JL, Ichinose A. Administration of factor XIII B subunit increased plasma factor XIII A subunit levels in factor XIII B subunit knock-out mice. International Journal of Hematology. 2008; 87: 60–8. 10.1007/s12185-007-0005-z. [DOI] [PubMed] [Google Scholar]
- 30.Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001; 276: 20673–8. 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- 31.Iismaa SE, Aplin M, Holman S, Yiu TW, Jackson K, Burchfield JG, Mitchell CJ, O’Reilly L, Davenport A, Cantley J, Schmitz-Peiffer C, Biden TJ, Cooney GJ, Graham RM. Glucose homeostasis in mice is transglutaminase 2 independent. PLoS One. 2013; 8: e63346 10.1371/journal.pone.0063346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joshi N, Kopec AK, Ray JL, Cline-Fedewa H, Groeneveld DJ, Lisman T, Luyendyk JP. Von Willebrand factor deficiency reduces liver fibrosis in mice. Toxicol Appl Pharmacol. 2017; 328: 54–9. 10.1016/j.taap.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi N, Kopec AK, Ray JL, Cline-Fedewa H, Nawabi A, Schmitt T, Nault R, Zacharewski TR, Rockwell CE, Flick MJ, Luyendyk JP. Fibrin deposition following bile duct injury limits fibrosis through an alphaMbeta2-dependent mechanism. Blood. 2016; 127: 2751–62. 10.1182/blood-2015-09-670703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen-Lefebvre AT, Ajith A, Portik-Dobos V, Horuzsko DD, Arbab AS, Dzutsev A, Sadek R, Trinchieri G, Horuzsko A. The innate immune receptor TREM-1 promotes liver injury and fibrosis. The Journal of clinical investigation. 2018. 10.1172/JCI98156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puche JE, Lee YA, Jiao J, Aloman C, Fiel MI, Muñoz U, Kraus T, Lee T, Yee HF, Friedman SL. A Novel Murine Model to Deplete Hepatic Stellate Cells Uncovers Their Role In Amplifying Liver Damage. Hepatology (Baltimore, Md). 2013; 57: 339–50. 10.1002/hep.26053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu P-s, Nakamoto N, Ebinuma H, Usui S, Saeki K, Matsumoto A, Mikami Y, Sugiyama K, Tomita K, Kanai T, Saito H, Hibi T. C-C motif chemokine receptor 9 positive macrophages activate hepatic stellate cells and promote liver fibrosis in mice. Hepatology. 2013; 58: 337–50. doi: 10.1002/hep.26351. [DOI] [PubMed] [Google Scholar]
- 37.Joshi N, Kopec AK, O’Brien KM, Towery KL, Cline-Fedewa H, Williams KJ, Copple BL, Flick MJ, Luyendyk JP. Coagulation-driven platelet activation reduces cholestatic liver injury and fibrosis in mice. J Thromb Haemost. 2015; 13: 57–71. 10.1111/jth.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012; 9: 676–82. 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laki K, Lorand L. On the Solubility of Fibrin Clots. Science (New York, NY). 1948; 108: 280 10.1126/science.108.2802.280. [DOI] [PubMed] [Google Scholar]
- 40.Lorand L A study on the solubility of fibrin clots in urea. Hungarica acta physiologica. 1948; 1: 192–6. [PubMed] [Google Scholar]
- 41.Kopec AK, Joshi N, Cline-Fedewa H, Wojcicki AV, Ray JL, Sullivan BP, Froehlich JE, Johnson BF, Flick MJ, Luyendyk JP. Fibrin(ogen) drives repair after acetaminophen-induced liver injury via leukocyte αMβ2 integrin-dependent upregulation of Mmp12. Journal of Hepatology. 2017; 66: 787–97. 10.1016/j.jhep.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aleman MM, Byrnes JR, Wang JG, Tran R, Lam WA, Di Paola J, Mackman N, Degen JL, Flick MJ, Wolberg AS. Factor XIII activity mediates red blood cell retention in venous thrombi. The Journal of clinical investigation. 2014; 124: 3590–600. 10.1172/jci75386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hethershaw EL, Cilia La Corte AL, Duval C, Ali M, Grant PJ, Ariens RA, Philippou H. The effect of blood coagulation factor XIII on fibrin clot structure and fibrinolysis. J Thromb Haemost. 2014; 12: 197–205. 10.1111/jth.12455. [DOI] [PubMed] [Google Scholar]
- 44.Walton BL, Byrnes JR, Wolberg AS. Fibrinogen, red blood cells, and factor XIII in venous thrombosis. J Thromb Haemost. 2015; 13 Suppl 1: S208–15. 10.1111/jth.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mosesson MW. Fibrinogen structure and fibrin clot assembly. Semin Thromb Hemost. 1998; 24: 169–74. 10.1055/s-2007-995837. [DOI] [PubMed] [Google Scholar]
- 46.MM W. Fibrinogen and fibrin structure and functions. Journal of Thrombosis and Haemostasis. 2005; 3: 1894–904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 47.Byrnes JR, Wolberg AS. Newly-Recognized Roles of Factor XIII in Thrombosis. Semin Thromb Hemost. 2016; 42: 445–54. 10.1055/s-0036-1571343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murthy SN, Lorand L. Cross-linked A alpha.gamma chain hybrids serve as unique markers for fibrinogen polymerized by tissue transglutaminase. Proc Natl Acad Sci U S A. 1990; 87: 9679–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao P, Metcalf M, Bunnett NW. Biased Signaling of Protease-Activated Receptors. Frontiers in Endocrinology. 2014; 5 10.3389/fendo.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soh UJK, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. British Journal of Pharmacology. 2010; 160: 191–203. 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000; 407: 258 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 52.Krupiczojc MA, Scotton CJ, Chambers RC. Coagulation signalling following tissue injury: focus on the role of factor Xa. Int J Biochem Cell Biol. 2008; 40: 1228–37. 10.1016/j.biocel.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 53.Kallis YN, Scotton CJ, Mackinnon AC, Goldin RD, Wright NA, Iredale JP, Chambers RC, Forbes SJ. Proteinase activated receptor 1 mediated fibrosis in a mouse model of liver injury: a role for bone marrow derived macrophages. PLoS One. 2014; 9: e86241 10.1371/journal.pone.0086241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nault R, Fader KA, Kopec AK, Harkema JR, Zacharewski TR, Luyendyk JP. From the Cover: Coagulation-Driven Hepatic Fibrosis Requires Protease Activated Receptor-1 (PAR-1) in a Mouse Model of TCDD-Elicited Steatohepatitis. Toxicol Sci. 2016; 154: 381–91. 10.1093/toxsci/kfw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirza A, Liu SL, Frizell E, Zhu J, Maddukuri S, Martinez J, Davies P, Schwarting R, Norton P, Zern MA. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kappaB. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1997; 272: G281–G8. 10.1152/ajpgi.1997.272.2.G281. [DOI] [PubMed] [Google Scholar]
- 56.Shainoff JR, Urbanic DA, DiBello PM. Immunoelectrophoretic characterizations of the cross-linking of fibrinogen and fibrin by factor XIIIa and tissue transglutaminase. Identification of a rapid mode of hybrid alpha-/gamma-chain cross-linking that is promoted by the gamma-chain cross-linking. Journal of Biological Chemistry. 1991; 266: 6429–37. [PubMed] [Google Scholar]
- 57.Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodelling of extracellular matrices: the role of transglutaminases. Connect Tissue Res. 2000; 41: 1–27. [DOI] [PubMed] [Google Scholar]
- 58.Bowness JM, Tarr AH, Wiebe RI. Transglutaminase-catalysed cross-linking: a potential mechanism for the interaction of fibrinogen, low density lipoprotein and arterial type III procollagen. Thromb Res. 1989; 54: 357–67. [DOI] [PubMed] [Google Scholar]
- 59.Popov Y, Sverdlov DY, Sharma AK, Bhaskar KR, Li S, Freitag TL, Lee J, Dieterich W, Melino G, Schuppan D. Tissue Transglutaminase Does Not Affect Fibrotic Matrix Stability or Regression of Liver Fibrosis in Mice. Gastroenterology. 2011; 140: 1642–52. 10.1053/j.gastro.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]