Abstract
Hippocampal sharp-wave ripples are brief high-frequency (120 – 250 Hz) oscillatory events that support mnemonic processes during sleep and awake behavior. Although ripples occurring during sleep are believed to facilitate memory consolidation, waking ripples may also be involved in planning and memory retrieval. Recent work from our group determined that normal aging results in a significant reduction in the peak oscillatory frequency and rate-of-occurrence of ripples during sleep (Wiegand et al., 2016) that may contribute to age-associated memory decline. It is unknown, however, how aging alters waking ripples. We investigated whether characteristics of waking ripples undergo age-dependent changes. Sharp-wave ripple events were recorded from the CA1 region of the hippocampus in old (n = 5) and young (n = 6) F344 male rats as they performed a place-dependent eyeblink conditioning task. Several novel observations emerged from this analysis. First, although aged rats expressed more waking ripples than young rats during track running and reward consumption, this effect was eliminated, and, in the case of track-running, reversed when time spent in each location was accounted for. Thus, aged rats emit more ripples, but young rats express a higher ripple rate. This likely results from reduced locomotor activity in aged animals. Furthermore, although ripple rates increased as young rats approached rewards, rates did not increase in aged rats, and rates in aged and young animals were not affected by eyeblink conditioning. Finally, although the oscillatory frequency of ripples was lower in aged animals during rest, frequencies in aged rats increased during behavior to levels indistinguishable from young rats. Given the involvement of waking ripples in memory retrieval, a possible consequence of slower movement speeds of aged animals is to provide more opportunity to replay task-relevant information and compensate for age-related declines in ripple rate during task performance.
Keywords: oscillation, working memory, reactivation
Introduction
Hippocampal sharp-wave ripples (Buzsaki, 1986; Csicsvari et al., 1999) are high-frequency (120 – 250 Hz) oscillatory events believed to support mnemonic processes during sleep (Wilson and McNaughton, 1994), awake immobility (Kudrimoti et al., 1999), and awake behavior (Foster and Wilson, 2006). The activities of populations of hippocampal neurons become coordinated during ripples, and this activity can reactivate neural responses associated with spatial exploration (O’Neill et al., 2010; Carr et al., 2011; Jadhav et al., 2012; Roumis and Frank, 2015; Wikenheiser and Redish, 2015). While ripples are implicated in memory processes, their specific function depends on the animal’s behavioral state. For example, reactivation during waking ripples is implicated in spatial learning and memory retrieval (Jadhav et al., 2012; Nokia et al., 2012; Wu et al., 2017), prospective and retrospective working memory (Singer et al., 2013), and updating navigational strategies (Dupret et al., 2010; Pfeiffer and Foster, 2013). Replay during rest-associated ripples, on the other hand, may facilitate the gradual consolidation of memories within broad hippocampal-subcortical-cortical networks (Wilson and McNaughton, 1994; Pennartz et al., 2004; Girardeau et al., 2009, 2014; Ego-Stengel and Wilson, 2010; Nokia et al., 2012).
Normal aging is associated with a decline in the capacity to consolidate memories during sleep (e.g., Pace-Schott and Spencer, 2015), and to retrieve and utilize spatial (Lester et al., 2017) and object-guided (Burke et al., 2010, 2011) memories during waking behavior. The neural basis for these effects are not known. Investigations of rest-associated ripples in aged animals suggests that features of ripples such as their frequency and capacity to organize neural activity contributes to age-associated memory decline. For example, CA1 single-unit activity is delayed relative to ripple onset in aged animals (Kanak et al., 2013), and CA1 principal neurons in aged animals have increased burst firing (Smith et al., 2000), although this study did not directly analyze ripple events. Furthermore, Gerrard et al. (2001) demonstrated that aged and young rats express intact memory trace reactivation during rest-associated ripples, but that the temporal organization of reactivated events is reduced in aged animals (Gerrard et al., 2008). Similarly, Wiegand et al. (2016) observed reduced reactivation in aged rats, and this reduction was largely due to a reduced rate of occurrence of ripples in aged animals. Wiegand et al. (2016) also demonstrated that the oscillatory frequency of ripples was reduced by approximately 15 Hz in aged animals. It is important to note that in this study, age-associated changes in the rate of occurrence, oscillatory frequency of ripples, and the strength of reactivation did not correlate with measures of learning. This suggests that the relationship between ripples and age-associated memory decline requires further investigation.
The effects of normal aging on features of waking ripples have not been investigated. This is an important question to address given their potential role in working memory (Gupta et al., 2010; Jadhav et al., 2012), decision making (Diba and Buzsaki, 2007; Jadhav et al., 2012; Singer et al., 2013), memory consolidation for goal locations (Foster and Wilson, 2006; O’Neill et al., 2006; Carr et al., 2011), and reward-driven learning (Foster and Wilson, 2006; Ambrose et al., 2016). Here we investigated whether the rate of occurrence and oscillatory frequency of waking ripples are affected by aging, and whether ripples were impacted by salient aversive events (eye shocks). Analyses were performed on waking ripples acquired from the CA1 region of the hippocampus using data described in Schimanski et al. (2013). The behavior consisted of a spatial eyeblink conditioning task that required rats to shuttle clockwise and counter-clockwise on a semi-circular track for food reward. One location in each running direction was associated with a randomly delivered electric shock to the eyelid. Given the involvement of ripples in variable reinforcement learning (Ambrose et al., 2016), we predicted that ripple rates would increase after animals received an eye shock. We also predicted that aging would reduce the rate of ripple events and the oscillatory frequency of ripples, as has been previously reported for rest-associated ripples (Wiegand et al., 2016).
Materials and Methods
Subjects and behavioral pre-training
Data were analyzed from 6 young adult (9 – 12 months) and 5 old (25 – 28 months) male Fischer-344 rats that were obtained from the National Institute on Aging colony at Charles River. Rats were kept on a 12:12 h reversed light cycle and housed individually. All experiments were performed as described in Schimanski et al. (2013), following the guidelines of the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals using protocols approved by the University of Arizona Institutional Animal Care and Use Committee. The animals used in the present study are the same as those used in Schimanski et al. (2013).
Prior to behavioral or electrophysiological experiments, the Morris swim task was administered over four consecutive days to assess motor ability, vision, and spatial learning (Morris, 1984), as described in full by Barnes et al. (1996). Rats then underwent food restriction to 85% of free-feeding body weight and were pre-trained on a shuttle task on a linear maze for food reinforcement until reaching 80 laps within 45 min. Once physiological experiments began, animals were exposed to the spatial trace eyeblink task (described below). As reported in Schimanski et al. (2013), aged animals exhibited significant deficits in the spatial version of the water maze task relative to young animals (Fig. 1B), but performed no differently than young rats on the spatial eyeblink task.
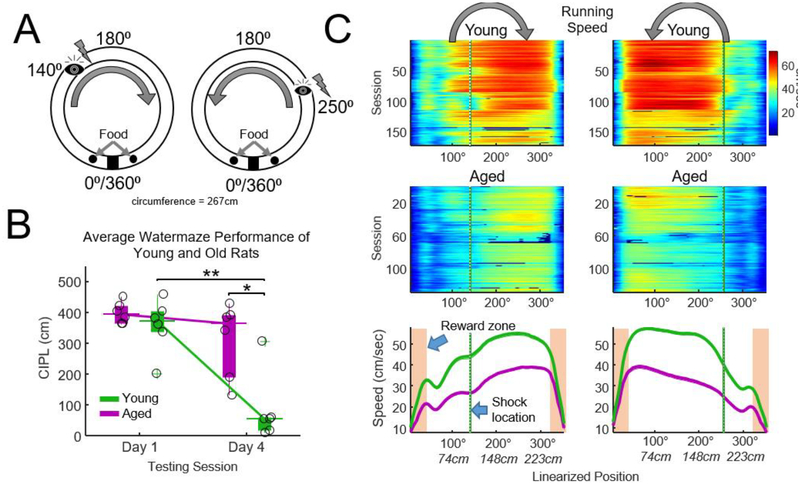
Figure 1.
Task design and behavior. A) Schematic of track and procedures for the spatial eyeblink task. Rats alternated between running clockwise and counterclockwise on a circular track (85 cm diameter, 267 cm circumference) for food reward on either side of a barrier. After five laps in either direction, electrical stimulation to the eyelid was delivered on a 50% pseudorandom schedule at the positions indicated by the ‘eye’. Rats typically ran 37 laps in each direction during a given recording session. EMG signals from the right eyelid were analyzed to determine if blinking occurred. A 30-minute rest period preceded and followed each track-running session, and two track-running sessions occurred on each day. B) Spatial watermaze performance measured prior to implantation. The corrected integrated path length (CIPL) scores indicated that age rats were impaired at finding the escape platform relative to young rats on the final day (day 4) of testing (t-test (age), * p = 0.01, d = 1.95, paired t-test (day) ** p = 0.004, Bonferroni-Holm correction for 2 tests). Thick vertical bars indicate the interquartile range, thin lines indicate the median, and circles indicate the CIPL score for each animal. C) Running speed for clockwise (left) and counterclockwise (right) traversals for each recording session. Color indicates mean speed (cm/sec). Aged animals were considerably slower than young rats. Shaded boxes indicate the reward/food zones. Green vertical lines indicate the shock zones. Thick lines indicate mean running speed across sessions (n = 146 sessions young, n = 119 sessions aged).
Surgical and electrophysiological recording procedures
All rats received eyelid wire implantations for monitoring of spatial eyeblink conditioning behavior as well as a chronic microdrive or “hyperdrive” holding 12 independently-adjustable tetrode recording probes (McNaughton et al., 1983; Wilson and McNaughton, 1993). Prior to surgery, rats began antibiotic treatments (either 10-day ampicillin cycle or 5-day sulfamethoxazole and trimethoprim oral suspension cycle). On the day of surgery, rats were anesthetized using 1.0 – 2.0% isoflurane in oxygen (flow rate 1.5 L/min) and placed into a stereotaxic apparatus. The hyperdrive was centered over a right hemispheric craniotomy made at 2.0 mm lateral and 3.8 mm posterior to Bregma. Tetrodes were constructed of four twisted polyimide-coated nichrome wires (13 μm diameter) and were driven to an initial depth of 1 mm at surgery. Over the next 14 days, tetrodes were lowered to optimally record extracellular spikes from CA1 pyramidal cells (~2 mm from brain surface). Two additional tetrodes whose four wires had been shorted together acted as references and were placed in or near the corpus callosum and hippocampal fissure. A ground screw and all wires were secured to the skull using dental acrylic.
Neural signals were amplified through a unity-gain headstage and programmable amplifiers (Neuralynx Inc.). Electrophysiological signals were recorded by the Cheetah Data Acquisition System (Neuralynx Inc.). A 1 ms window was recorded surrounding each candidate action potential. Single-unit activity was digitized at 32 kHz, amplified 500 – 5000 times, and bandpass filtered between 0.6 and 6 kHz. Local-field activity was recorded from a subset of tetrodes, bandpass filtered from 0.5 to 600 Hz, and sampled at 1893 Hz. Video tracking data were obtained with an overhead CCD camera. Movement during rest was calculated by finding the first derivative of the tracking data acquired from the CCD camera and subsequently squaring and smoothing the result.
Pre-training and tetrode adjustment continued for ~14 days after surgery until behavior improved and clear CA1 action potentials were observed on the majority of tetrodes. At least 30 recording sessions were acquired from 10 rats (6 young and 4 old). Data was also acquired from a fifth aged rat. This rat, however, became ill after 15 days of recording and so only these sessions were acquired and analyzed from this animal.
Behavioral procedures
On day 1 of the spatial-eyeblink conditioning experiment, rats were taken to a dedicated room for calibration of the level of electrical current necessary to induce an eyeblink. The eyelid stimulus was a 100 ms, 100 Hz train of bipolar square pulses 5 ms long, delivered through the wires implanted in the right eyelid using a Master-8 from A.M.P.I. and stimulus isolator A365 from World Precision Instruments. Every day before the experiment, current was modulated in order to induce a complete eye blink (typical range, 0.1 – 0.6 mA). After calibration of the eyeblink current, rats were brought to a dimly lit recording room containing a circular track (85 cm diameter), a towel-lined clay flowerpot, and numerous visual cues (see Schimanski et al., 2013). Rats were placed in the pot to rest quietly alone for a minimum of 30 min before and after the behavioral task. Task: Rats ran 10 laps for food reward without eye shocks, and on lap 11, rats received a blink-inducing electrical stimulation at two locations (at 140 and 250 degrees, see Fig. 1A) ‒ one in the clockwise and one in the counter-clockwise direction. Stimulation was delivered with a probability of 50%. Rats ran on the track for a maximum of 74 laps or until ceasing locomotion. The number of laps was controlled between young and aged rat pairs by allowing the young rats to run the same number of laps as their yoked aged pair. The two within-day recording sessions were separated by an average of 157.5 +/− 4.0 min. This protocol was repeated for 31 days/rat (with the exception of 1 old rat)
Statistical analyses
Student’s t-tests and ANOVA were used except when specified otherwise. Tukey-Kramer (ANOVA) and Bonferroni-Holm corrections were performed to adjust for familywise error. Alpha was set at 0.05 (two-tailed). Statistical analyses were performed using Matlab (The MathWorks, Inc., Natick, Massachusetts) and R (R Core Team, 2013).
Identification of putative ripple events
In order to reduce the impact of high-frequency artifacts on the local-field potential signal from action potentials (Ray and Maunsell, 2011), local-field activity was first “de-spiked” by removing a 4 ms window of data around spikes measured on the same tetrode and replacing the absent data with a spline (Zanos et al., 2011). Signals were then bandpass filtered (115 to 250 Hz, 8th order IIR Butterworth filter). We chose 115 Hz as a lower band given our previous finding that ripple oscillation frequencies are ~15 Hz lower in aged relative to young rats with a lower bound approaching 120 Hz (Wiegand et al., 2016). A candidate high-frequency event was identified when the envelope of the absolute value of the filtered signal exceeded 3 sds above the mean, lasted for at least 30 ms, and had a peak frequency in the PSD from 115 to 240 Hz of ≥ 120 Hz. Candidate ripples with a peak frequency < 120 Hz were eliminated in order to reduce contamination from high-gamma events. The 3 sd threshold was lower than the 5 sd threshold used in our previous investigation (Wiegand et al., 2016) as waking ripples have been reported to be 30 – 60% lower in amplitude relative to rest-associated ripples (O’Neill et al., 2006). The time of ripple onset was determined by moving backward in time from the point of threshold detection until power fell to 2.5 sds above the mean. The same procedure was applied forward in time to determine event offset. Peak oscillation frequency was determined by constructing a spectrogram using a complex Morelet wavelet convolution (cwtft() in Matlab) for the ripple and then identifying the frequency with the highest power.
Low-gamma power during ripple events was also determined by first filtering the local-field signal for each ripple event (±100 ms was added on start and end of each ripple to reduce edge effects) with an 8th order Butterworth band-pass filter (20–50 Hz). The envelope of this signal was computed and averaged to determine power in μV.
Results
Aged rats exhibited reduced watermaze performance and running speed.
Watermaze performance was assessed prior to surgery. Young but not aged animals improved performance on the spatial water maze, as expressed as reduced path length, from the first to the second testing session (Fig. 1B, paired t-test, p young = 0.004, p old > 0.05). Performance between young and aged animals differed significantly on the second testing session (t-test, p = 0.01, d = 1.95). These data were also presented in Schimanski et al. (2013).
Motor performance on the circular track was assessed by measuring running speed during task performance. Data for all experimental sessions are summarized in the color plots in Fig. 1C for rats running in clockwise and counter clockwise directions and when animals approached the reward zones (tan shaded region, bottom plot). Mean running speed, as calculated when animals were outside of the reward zones, was reduced in aged rats when compared to young rats (two-sample t-test, p = 0.0003, d = 3.5, mean young (n = 6) = 40.9 cm/sec, mean aged (n = 5) = 24.76 cm/sec).
Aged rats produced more ripples in the reward zones, but at the same rate as young rats.
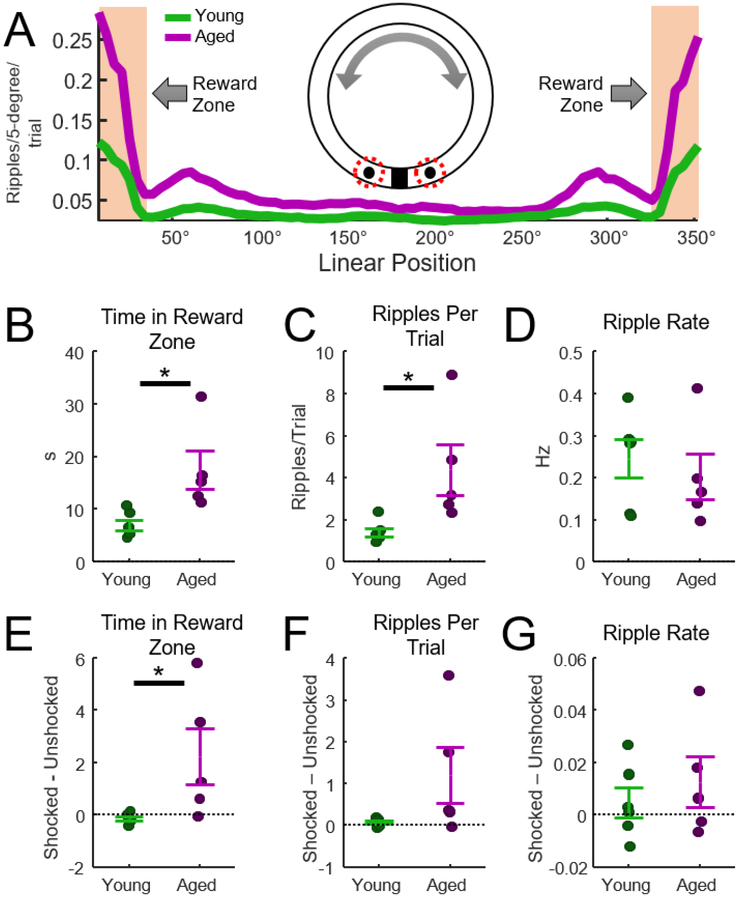
Consistent with previous work, the majority of ripples occurred when rats were near reward locations. This is illustrated in Fig. 2A which indicates the mean number of ripples observed on each trial, organized by the location of the rat on the track. The shaded region indicates the reward zone. Analysis of behavior indicated that aged rats spent significantly more time in the reward zones relative to young rats (Fig. 2B, t-test, t = −3.0, p = 0.02, d =−1.7). Analysis of ripples in the reward zone indicated that aged rats also produced more ripples-per-trial relative to young animals (Fig. 2C, t = −2.7, p = 0.03, d =−1.5). Because aged rats spent more time in reward zones, it was conceivable that the rate of ripple occurrence was similar in aged and young rats despite these differences in the number of observed ripples. This hypothesis was tested by normalizing ripple counts by the time spent in the reward zones (ripple rate in Hz). Consistent with this prediction, no difference in ripple rate was observed between aged and young rats (Fig. 2D, t = 0.61, p = 0.36). Thus, relative to young rats, aged animals spent more time in reward zones, produced more ripples, but produced these ripples at the same rate.
Figure 2.
Analysis of ripples at reward zones. A) Average number of ripples per trial at each location on the track (clockwise and counterclockwise trials were combined). The number of ripples/trial was largest when animals were in the reward zones (shaded region). Clockwise and counterclockwise trials were combined in this analysis, making this plot symmetrical. Values were not normalized by time spent at the reward zone (see Figure 6B). B) Aged rats spent significantly more time in the reward zones (t-test, t = −3.0, p = 0.02, d = −1.7, n = 6 young, n = 5 aged rats). C) There were significantly more ripples per trial for the aged rats (t = −2.7, p = 0.03, d = −1.5). D) The rate of ripple events in the reward zone was not different between aged and young rats (p = 0.36). E) Comparison of total time spent in the feeder zone after rats did or did not receive an eye shock. Aged rats spent more time in the feeder zone relative to young rats (t = −2.4, p = 0.04, d = −1.4). F) The number of ripples per trial did not differ between trials with and without eye shocks (p = 0.1). G) The rate of ripple events did not differ between trials with and without eye shocks (p = 0.5).
Effect of the eye shocks on waking ripples in the reward zone.
Given the salience of eye shocks and involvement of ripples in memory processing, we hypothesized that the number and rate of ripple events would increase on trials following eye shocks. To test this, we divided trials into shocked and un-shocked trials (eye shocks were delivered on pseudo randomly selected trials, see Methods), and assessed behavior and ripple activity. A within-session difference measure was computed for each session, and these within-session values were averaged for each animal. To illustrate, the difference measure for the effect of eye-shock events on time spent in the reward zone was measured as the time in reward zone on eye shock trials minus the time spent on un-shocked trials. These within-session measures were averaged for each rat. This analysis revealed that aged rats spend more time in the reward zone following a shock when compared to young rats (Fig. 2E, t-test, t = −2.4, p = 0.04, d = −1.4). No effect of shock was observed in the number of ripples per trial (t = −1.8, p = 0.1), or in the rate of ripple events (t = −0.7, p = 0.5). Taken together, these results indicate that eye shocks affected the time spent in the reward zone for the aged but not for young animals; however, shocks did not alter the quantity or the rate of ripple events in either aged or young rats.
Age-related differences in ripple occurrence during task behavior.
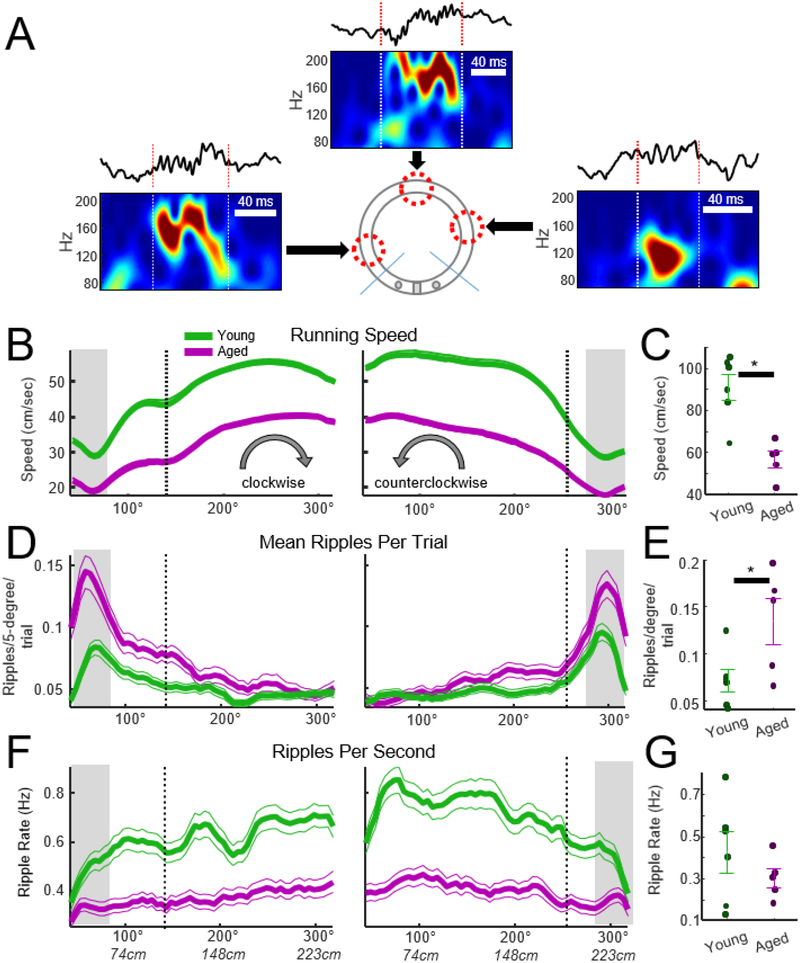
Although the quantity of ripple events was high at reward locations, ripples also occur during active task performance, and these ripples may be involved in retrospective and prospective memory processing. The following analyses investigated whether aging or eye shock events impact ripple occurrence during active task performance. Active behavior was defined as the region of the track that was 30 degrees (22 cm) beyond the reward zones. Specifically, all regions beyond the tan shaded regions in Fig. 2A were considered for the following analysis of task behavior. Examples of three ripples identified during task performance are presented in Fig. 3A. As indicated in Fig. 3B, running speed dipped at the start of each trail and increased as rats approached the reward zone. Inspection of head position from the tracking record revealed that this dip in movement speed at the start of the trial corresponded to the moment when rats turned around 180° from the reward site and faced the destination reward zone. Analysis of running speed during this ‘dip’ in movement speed, indicated as the gray shaded regions in Fig. 3, indicated that aged rats moved more slowly than young rats at the start of each trial (Fig. 3C, t = 5.7, p = 0.005, d = 2.2, n = 5 aged, n = 6 young). Furthermore, analysis of the number of ripples per trial indicated that more ripples occurred in aged rats at the start of each trial (Fig. 3E, t = - 2.4, p = 0.039, d = −1.4). This effect was likely due to aged rats running more slowly at the start of each trail as no age-related difference was observed when the number of ripples per unit time (ripple rate) at the start zone was calculated (Fig. 3G, t = 1.0, p = 0.50). Although the present analysis focused on the start of each trial, data in Fig. 3F suggests that ripple rates may differ between groups when rats moved beyond the start zone. These questions are addressed in subsequent analyses.
Figure 3.
Waking ripples during the spatial eyeblink behavior. A) Examples of waking ripples from an aged rat observed during conditioning. Arrows point to the location on the track where the ripple occurred. The waveform and wavelet spectrogram are presented for each ripple. B) Running speed for aged and young rats outside of the reward zone. Running speed in both aged and young rats ‘dipped’ at the start of each trial and increased until animals approached the reward zone (n = 146 sessions young, n = 119 sessions aged). The x axis indicates the linearized position of the animal in degrees. Vertical dashed lines indicate the location where animals received an eyelid shock on 50% of trials. Shocked trails were eliminated from the averages so that ripple counts could be assessed without contamination from electrical artifact. C) Running speed at the start of each trial, with the start indicated by the gray horizontal bars in B, was slower for aged rats (t-test, t = 5.7, p = 0.005, d = 2.2, n = 5 aged, n = 6 young rats, error bars = SEM). D) Average number of ripples per trial during track running. E) To assess whether the incidence of ripple events differed between aged and young animals, the average number of ripples per trial was computed at the start of the clockwise and counterclockwise journeys. The average number of ripples per trial was larger in aged animals (t-test, t = −2.4, p = 0.039, d = −1.4, n = 5 aged, n = 6 young). F) To determine if the increased number of ripples in aged rats was due to their slower running speed, the ripples per trial were normalized by the total time spent at each spatial location, resulting in a measure of ripples per second. G) The mean ripple rate was not different between aged and young animals at the start of each trial (t = 1.1, p = 0.50), suggesting that the increase in the incidence of ripples at the start of each journey observed in D was due to animals moving more slowly.
Ripple rates are lower in reward zones relative to track running.
Although more ripples occurred when rats were within the reward zone (e.g., Fig. 2A), young and especially aged rats spent considerable time in this region (Fig. 2B). Consequently, it was conceivable that the rate of ripple occurrence differed between reward consumption and track running. Track running was defined as periods when rats were outside of the reward zone (unshaded region in Fig. 2A). Paired t-tests indicated that both aged and young rats expressed higher ripple rates during track running (Fig. 4A, p young = 0.03, p aged = 0.03, Bonferroni-Holm correction). This indicates that although more ripples occurred in the reward zones, the rate of ripple occurrence was greater during active task performance in aged and young rats.
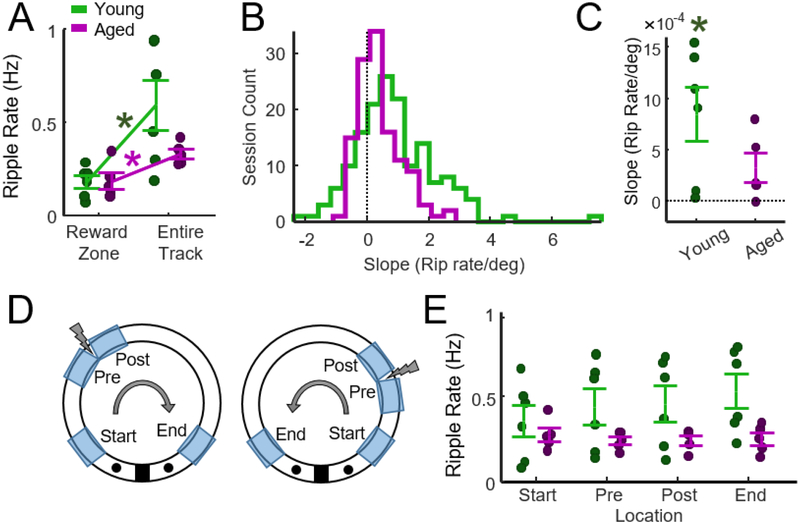
Figure 4.
Changes in ripple rate during track running and reward consumption. A) Although more ripples occurred when rats were in the Reward Zone, ripple rates were lower in the reward zone relative to track running. Paired t-tests indicated that both aged and young rats expressed higher ripple rates during track running (p young = 0.03, p aged= 0.03, Bonferroni-Holm correction). B) The slope of the regression line that fit the relationship between location (degrees) and ripple rate (Fig. 3F) was used to determine whether ripple rates increased as animals approached the reward zone. A positive slope indicates that ripple rate increased as animals approached the reward zone. The histograms present slopes for all analyzed sessions (n = 146 young, n = 119 aged). C) Slopes for young and aged rats did not differ (two-sample t-test, t = 1.6, p = 0.14, n = 6 young, n = 5 aged). Slopes for young (one-sample t-test, p = 0.048), but not aged (p = 0.089) rats were greater than zero (p values Bonferroni corrected for 2 tests). D) Schematic of zones on the track used to identify changes in ripple rate as a function of location. Each zone was 30° in extent. Clockwise and counter clockwise journeys were combined. E) Average ripple rate at each location on the track. Two-way ANOVA (age x location) revealed a main effect of age (F = 11.3, p = 0.002) but not location (F = 0.3, p = 0.83) and no interaction (F = 0.52, p = 0.67).
Ripple rates increase as young rats run towards reward zones.
Ripples are associated with reward-driven learning and ripples can cluster around reward zones. To determine if ripple rates increased as rats ran towards reward zones, a regression line was fit to the relationship between the location (degrees) and ripple rate measured on each session and the slope of this line was determined. The regression fit was performed such that a positive slope indicated that ripple rate increased as animals ran towards the reward zone regardless of whether rats ran in the clockwise or counter-clockwise direction. Slopes were first computed for each session and the distribution of these slopes for aged and young rats is presented in Fig. 4B (n = 146 sessions young, n = 119 sessions aged). These values were then averaged for each animal for statistical analysis. Slopes for young and aged rats did not differ (two-sample t-test, t = 1.6, p = 0.14, n = 6 young, n = 5 aged). However, the mean slope for young (one-sample t-test, p = 0.048), but not aged (p = 0.089) rats was greater than zero (p values Bonferroni corrected for 2 tests).
The rate of occurrence of ripples is higher in young rats during track running.
Data summarized in Fig. 3F suggests that the rate of ripple events was higher in young rats during track-running. It was also hypothesized that the location of the shock zone may also influence the rate of ripple events. Specifically, we predicted that ripple rates would increase as rats approached or left the shock zone. To explore these hypotheses, track running was divided into four zones on the track: the start zone, the zone preceding the shock, the zone following the shock, and the zone preceding the reward (Fig. 4D). Mean ripple rates for each animal are presented in Fig. 4E. Two-way ANOVA (age x location) revealed a main effect of age (F = 11.3, p = 0.002) but not location (F = 0.3, p = 0.83) and no interaction (F = 0.52, p = 0.67). This indicated that ripple rate was greater in young rats during track running, and that the location of the shock did not impact ripple rate.
Age-associated differences in ripple oscillatory frequency during sleep are not present in waking ripples.
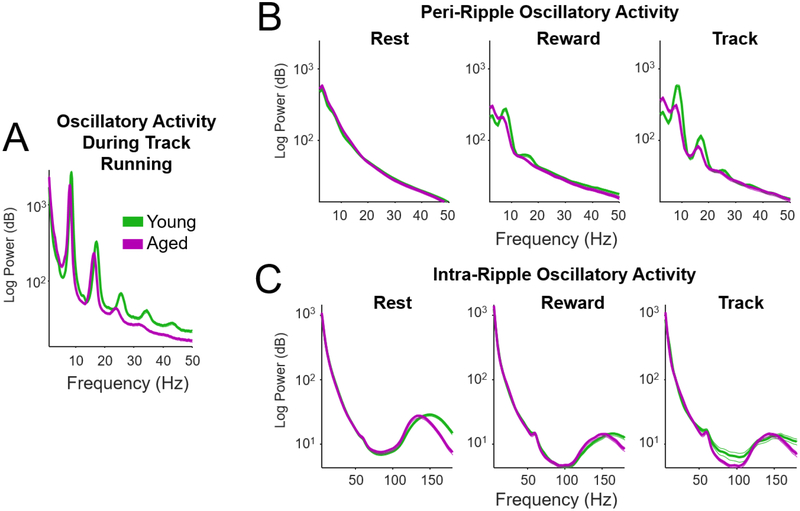
Our previous investigation of sleep-associated ripples indicated that the peak oscillatory frequency of ripples in aged rats is significantly lower than in young rats (Wiegand et al., 2016). We investigated whether such age-associated changes in frequency are also found in waking ripples. Prior to performing this analysis, oscillatory power during waking and rest states and during ripples were analyzed. Fig. 5 presents power spectral density estimates that summarize oscillatory responses during maze running (Fig. 5A), during peri-ripple periods (Fig. 5B), and during ripples (Fig. 5C). As expected, track running was associated with strong theta-band oscillations and theta-band harmonics (Fig. 5A). Oscillatory activity surrounding, but not including, ripple events was also analyzed by performing the power spectral estimate on the signal acquired during the 500 ms interval preceding ripple onset and 500 ms interval following ripple offset (Fig. 5B). No theta was visible in the spectral response during Rest, although some theta power was evident during Reward and Track epochs. Finally, oscillatory activity during ripple events was analyzed (Fig. 5C). The power spectral densities indicated a clear peak in the ripple band (130–150 Hz) for all epochs. The absence of clear high-gamma (80–120 Hz) peak in the power spectral densities indicated that detected ripples were not significantly contaminated by behavior-associated gamma oscillations.
Figure 5.
Oscillatory responses in aged and young rats. A) Power spectral density (PSD) of hippocampal oscillatory power during periods of track running (mean across all sessions and animals). Note strong theta power and theta harmonics during track running. The mean (thick lines) and SEM (thin lines) were averaged across experimental sessions (n young = 186, n aged = 139). B) Power spectral density plots for the peri-ripple periods (the 500 ms interval preceding ripple onset and 500 ms interval following ripple offset) for rest, reward consumption, and track running. Theta was detected during track running and reward consumption, although theta power was considerably lower during peri-ripple periods than the during track running (see A). C) Mean PSDs during ripple events. The interval 150 ms before ripple onset and after ripple offset were included in the PSD estimate to limit filter distortion due to edge effects. The mean peak frequency was above the maximum high-gamma frequency that is typically reported (high-gamma typically reported to be between 80 and 120 Hz).
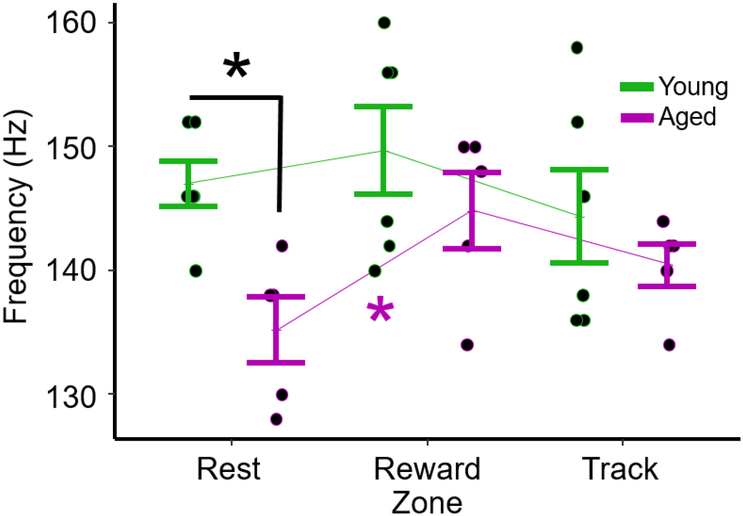
The next objective was to determine if the peak oscillatory frequency of ripples in aged rats was significantly lower than in young rats. Peak oscillatory frequency was quantified as the frequency of peak power in the wavelet spectrogram (see Methods). Fig. 6 plots the mean of this measure of oscillatory frequency for each rat during rest and during performance of the eyeblink conditioning task. Two-way ANOVA (age x behavioral state) revealed a significant effect of age (F = 8.08, p = 0.008) but not behavioral state (F = 2.25, p = 0.12). Post hoc comparisons revealed an effect of age during rest (t-test, t = 3.7, p = 0.02, Bonferroni correction for 3 comparisons), but not during reward consumption (p = 0.65) or track running (p = 0.65). To determine if the lower oscillatory frequencies observed during rest in aged rats increased during behavior, paired t-tests were performed that compared Rest to Reward and Rest to Track. Frequencies increased from Rest to Reward in aged rats (t = −3.87, p = 0.04, Bonferroni corrected for 2 comparisons), but not from Rest to Track (t = −2.15, p = 0.20).
Figure 6.
Oscillatory frequency of ripples during rest and behavior. Mean (±SEM) oscillatory frequency of ripples for aged (n = 5) and young (n = 6) rats during sleep, reward consumption, and track running. Two-way ANOVA (age x behavioral state) revealed a significant effect of age (F = 8.08, p = 0.008) but not behavioral state F = 2.25, p = 0.12). Post hoc comparisons revealed an effect of age during sleep (t test, t = 3.7, p = 0.02, Bonferroni correction), but not at the reward zone (p = 0.65) or track-running (p = 0.65). To determine if the lower oscillatory frequencies observed during rest in aged rats increased during behavior, paired t-tests were performed that compared Rest to Reward and Rest to Track. Frequencies increased from Rest to Reward in aged rats (t = −3.87, p = 0.04, Bonferroni correction for 2 comparisons), but not from Rest to Track (t = −2.15, p = 0.20).
We also investigated whether the power of low-gamma oscillations during ripple events was reduced in aged animals. This hypothesis was motivated by observations that increased low-gamma power during waking ripples is associated with enhanced memory trace reactivation (Carr et al, 2012). Using this approach and averaging by animal, we did not observe an effect of age (two-way ANOVA, Fage = 0.58, p = 0.45, Fphase = 0.61, p = 0.55, Fint =0.27, p = 0.76).
Ripple duration and ripple doublets.
The strength of memory-trace reactivation is positively associated with the duration of ripple events and the presence of ripple bursting (e.g., “ripple doublets”)(Davidson et al, 2009; Sirota et al, 2003). Consequently, we investigated whether ripple duration or the occurrence of ripple bursts are altered in aged rats. Analysis of ripple duration during rest, track-running, and during reward consumption revealed no effect of age or behavioral phase on ripple duration (Fage = 1.41, p = 0.25; Fphase = 3.34, p = 0.051). We quantified the extent of bursting of ripple events using the established Local Variance measure (Shinomoto et al, 2009) which evaluates the extent to which sequences of inter-event intervals exceed values expected by a Poisson random process (values > 1 suggest bursting and < 1 suggests a more regular pattern). Local Variance was measured for aged and young rats and for the sleep, reward consumption, and track running phases of the task. A two-way ANOVA revealed no effect of age (F = 0.58, p = 0.45) nor interaction between age and behavioral phase (F = 0.69, p = 0.51). Thus, we did not find support for the hypothesis that age affects the clustering of ripples in time. A significant effect of behavioral phase was observed (F = 5.64, p = 0.009).
Discussion
Waking ripples are implicated in spatial learning, memory consolidation, and memory retrieval (Dupret et al., 2010; Jadhav et al., 2012; Nokia et al., 2012; Pfeiffer and Foster, 2013; Wu et al., 2017). The present study is the first to investigate how features of waking ripples are impacted by normal aging. We observed that aged rats expressed more waking ripples during reward consumption and task performance, and that this increase was likely a result of aged animals moving more slowly and spending more time at reward zones. Indeed, when normalized for time, ripple rates (ripples per unit time) during active task performance were actually higher in young rats (Fig. 4E). Furthermore, although previous work has shown that aged and young rats learned the eyeblink conditioning task at similar rates (Schimanski et al., 2013), we found that aged rats spent more time in the reward zones after receiving an eye shock. Although this suggests that aged animals are more impacted by aversive eye shocks than young rats, ripple rates were not affected by eye shocks in either aged or young animals (Figs. 2, 4). What follows is a detailed discussion of these observations.
Behavior.
Behavioral testing using a spatial water maze revealed that the aged animals used in this study exhibited impaired spatial learning or memory recall. In contrast, and as shown in Schimanski et al. (2013), aged and young rats learned the eyeblink task at similar rates. In the present study, we observed that aged rats ran at ~40% of the speed of young rats, and that aged rats spent considerably more time in the reward zones. More surprising was the finding that aged rats spent more time in reward zones following eye shocks (Fig. 2E). This suggests that aged rats are more impacted by the aversive eye shocks than young animals. Indeed, there is evidence from rodent work that repeated exposure to stressful events enhances the emotional stress responses in aged but not in young rats (Sapolsky et al., 1983, 1986; Shoji and Mizoguchi, 2010). Conceivably, the additional time spent in the reward zone following a shock could be adaptive as it would provide more opportunities for ripple events and thus support memory consolidation. However, no significant increase in ripple counts following eye shocks was observed (Fig. 2F). Future experiments could be designed to examine this question by manipulating ripple counts by systematically modulating running speeds in young animals (e.g., treadmill).
Behavior-dependent effects on waking ripple quantity and rate.
Aged rats expressed more waking ripples than young rats during reward consumption and task performance. This may not be surprising given the reduced movement speed and activity of aged rats, and the known inverse relationship between ripple count and running speed (Jadhav et al., 2015). Indeed, when normalized for time spent at each location, the rate of ripple events was larger in young rats during task performance (Fig. 4E), but not during reward consumption. Thus, aged rats generated more waking ripples near reward locations, but young rats expressed higher ripple rates during task performance. The age-related decline in ripple rate during waking behavior is consistent with previous reports of reduced rest-associated ripple rates in aged animals (Wiegand et al., 2016).
The fact that ripple counts are higher in aged rats while ripple rates are higher in young animals begs the question of whether the quantity or the rate of ripple events are more important for mnemonic processing. This is an important unresolved question. If ripple quantity is critical, then it is conceivable that slower movement of the aged rats and the corresponding increase in ripple counts could help aged animals compensate for age-related memory decline and reduced ripple rates. Indeed, this may account for the absence of an age-associated reduction in spatial eyeblink learning observed in the eyeblink task used in the present study (Schimanski et al., 2013). Future studies that explore the relationship between the quantity and the rate of ripple occurrence on task performance will help resolve this issue. Furthermore, examination of the content of waking ripples through reconstruction (e.g., Foster and Wilson, 2006) could determine the extent to which ripples reactivate salient aversive and rewarding events, and how reactivation is affected by ripple rate and aging. Indeed, the aversive conditioning paradigm used in the present study could strongly impact reactivation as a recent study reported that reactivation during waking ripples replays trajectories through locations in which rats received foot shocks (Wu et al., 2017).
Potential mechanisms for reduced ripple rates in aged animals.
Ripple generation is thought to result from the depolarization of relatively few CA1 pyramidal neurons following CA3 discharge (Stark et al., 2014). Thus, waking- and rest- associated reductions in ripple rate may reflect an age-related reduction in functional synaptic innervation of CA1 from CA3 (Barnes et al., 1992). Reduced innervation could impair the capacity of CA3 to trigger CA1 ripple events (Wiegand et al., 2016).
Oscillatory frequency of ripples increases from rest to behavior in aged rats.
Previous work from our laboratory reported that the oscillatory frequency of rest-associated ripples in aged rats is ~14 Hz lower than in young animals (aged = 132 Hz, young = 146 Hz; Wiegand et al., 2016). In the present study we compared oscillatory frequencies of ripples during rest and behavior. Although the rest-associated differences in oscillatory frequency were observed, these differences disappeared during behavior (Fig. 6). In fact, we observed that ripple frequencies in aged rats increased during behavior to levels that were indistinguishable from young rats. This suggests that physiological features of behavior, such as the altered neuromodulatory context or neuronal excitability, may restore age-associated reductions in ripple oscillation frequency. Such adaptations may be important for learning in aged animals given that ripple oscillations may coordinate the timing of action potentials so that that they occur within the narrow temporal window required for synaptic plasticity (< 20 ms; Bi ansd Poo, 1998; Jensen and Lisman, 2005).
Conclusions and Future directions
To conclude, aged and young animals expressed significant differences in the number and the rate of waking ripples. Surprisingly, ripple rates did not change with the delivery of eye shocks, suggesting a limited involvement of waking ripples in aversive associative learning. We also observed that although the oscillatory frequency of ripples is lower in aged rats during rest, ripple frequencies during behavior were indistinguishable between age groups. This suggests that age-associated physiological changes that slow ripple oscillations during rest may be circumvented by physiological changes evoked by behavior. A limitation of the present study was the fact that no age difference was observed on the eyeblink task (Schimanski et al., 2013), despite significant differences being observed in the Morris water maze. Consequently, future studies of waking ripples that use tasks that place more cognitive demands on aged rats, such as set-shifting and multi-item spatial working memory tasks (e.g., Bizon et al., 2012) may reveal significant age-associated differences in ripple properties.
Acknowledgements
McKnight Brain Research Foundation NIH Grant R37 AG012609 NIH Grant R01 AG003376
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- Ambrose RE, Pfeiffer BE, Foster DJ. 2016. Reverse Replay of Hippocampal Place Cells Is Uniquely Modulated by Changing Reward. Neuron [Internet] 91:1124–1136. Available from: http://dx.doi.org/10.1016Zj.neuron.2016.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Foster TC, McNaughton BL. 1992. Region-specific age effects on AMPA sensitivity: electrophysiological evidence for loss of synaptic contacts in hippocampal field CA1. Hippocampus [Internet] 2:457–68. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1284976 [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, McNaughton BL. 1996. Functional integrity of NMDA-dependent LTP induction mechanisms across the lifespan of F-344 rats. Learn Mem 3:124–137. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. 1998. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18:10464–10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Foster TC, Alexander GE, Glisky EL. 2012. Characterizing cognitive aging of working memory and executive function in animal models. Front Aging Neurosci 4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. 2011. Age-Associated Deficits in Pattern Separation Functions of the Perirhinal Cortex: A Cross species Consensus. Behav Neurosci 125:836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Wallace JL, Nematollahi S, Uprety AR, Barnes CA. 2010. Pattern separation deficits may contribute to age-associated recognition impairments. Behav Neurosci [Internet] 124:559–73. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3071152&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G 1986. Hippocampal sharp waves: their origin and significance. Brain Res [Internet] 398:242–252. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3026567 [DOI] [PubMed] [Google Scholar]
- Carr MF, Jadhav SP, Frank LM. 2011. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci [Internet] 14:147–53. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3215304&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari tJozsef, Hirase H, Czurko A, Mamiya A, Buzsaki G. 1999. tFast Network Oscillations in the Hippocampal CA1 Region of the Behaving Rat. J Neurosci 19:RC20–RC20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diba K, Buzsaki G. 2007. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci [Internet] 10:1241–2. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2039924&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J. 2010. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci [Internet] 13:995–1002. Available from: http://www.nature.com/doifinder/10.1038/nn.2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego-Stengel V, Wilson MA. 2010. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus [Internet] 20:1–10. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2801761&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. 2006. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature [Internet] 440:680–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16474382 [DOI] [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes CA. 2008. Sequence reactivation in the hippocampus is impaired in aged rats. J Neurosci [Internet] 28:7883–90. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2703197&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard JL, Kudrimoti H, McNaughton BL, Barnes CA. 2001. Reactivation of hippocampal ensemble activity patterns in the aging rat. Behav Neurosci 115:1180–1192. [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. 2009. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci [Internet] 12:1222–1223. Available from: 10.1038/nn.2384 [DOI] [PubMed] [Google Scholar]
- Girardeau G, Cei A, Zugaro M. 2014. Learning-induced plasticity regulates hippocampal sharp wave-ripple drive. J Neurosci [Internet] 34:5176–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24719097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AS, van der Meer M a a, Touretzky DS, Redish a D. 2010. Hippocampal replay is not a simple function of experience. Neuron [Internet] 65:695–705. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20223204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. 2012. Awake hippocampal sharp-wave ripples support spatial memory. Science [Internet] 336:1454–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22555434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Rothschild G, Roumis DK, Frank LM. 2015. Coordinated Excitation and Inhibition of Prefrontal Ensembles during Awake Hippocampal Sharp-Wave Ripple Events. Neuron:113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. 2005. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci 28:67–72. [DOI] [PubMed] [Google Scholar]
- Kanak DJ, Rose GM, Zaveri HP, Patrylo PR. 2013. Altered network timing in the CA3-CA1 circuit of hippocampal slices from aged mice. PLoS One [Internet] 8:e61364 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3620228&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL. 1999. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci 19:4090–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester AW, Moffat SD, Wiener JM, Barnes CA, Wolbers T. 2017. The Aging Navigational System. Neuron 95:1019–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, O’Keefe J, Barnes CA. 1983. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods 8:391–7. [DOI] [PubMed] [Google Scholar]
- Morris R 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60. [DOI] [PubMed] [Google Scholar]
- Nokia MS, Mikkonen JE, Penttonen M, Wikgren J. 2012. Disrupting neural activity related to awake-state sharp wave-ripple complexes prevents hippocampal learning. Front Behav Neurosci [Internet] 6:84 Available from: http://www.frontiersin.org/Journal/10.3389/fnbeh.2012.00084/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. 2010. Play it again: reactivation of waking experience and memory. Trends Neurosci 33:220–229. [DOI] [PubMed] [Google Scholar]
- O’Neill J, Senior T, Csicsvari J. 2006. Place-selective firing of CA1 pyramidal cells during sharp wave/ripple network patterns in exploratory behavior. Neuron [Internet] 49:143–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16387646 [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RMC. 2015. Sleep-dependent memory consolidation in healthy aging and mild cognitive impairment. Curr Top Behav Neurosci [Internet] 25:307–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24652608 [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL. 2004. The ventral striatum in off-line processing: ensemble reactivation during sleep and modulation by hippocampal ripples. J Neurosci [Internet] 24:6446–6456. Available from: http://www.jneurosci.org/content/24/29/6446.short [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer BE, Foster DJ. 2013. Hippocampal place-cell sequences depict future paths to remembered goals. Nature [Internet] 497:74–81. Available from: http://www.nature.com/doifinder/10.1038/nature12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Maunsell JHR. 2011. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol [Internet] 9 Available from: http://dx.plos.org/10.1371/journal.pbio.1000610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumis DK, Frank LM. 2015. Hippocampal sharp-wave ripples in waking and sleeping states. Curr Opin Neurobiol [Internet] 35:6–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26011627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. 1983. The adrenocortical stress-response in the aged male rat: impairment of recovery from stress. Exp Gerontol [Internet] 18:55–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6683660 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. 1986. The Neuroendocrinology of Stress and Aging: The Glucorticoid Cascade Hypothesis. Endocr Rev 7:284–301. [DOI] [PubMed] [Google Scholar]
- Schimanski LA, Lipa P, Barnes CA. 2013. Tracking the course of hippocampal representations during learning: when is the map required? J Neurosci [Internet] 33:3094–106. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3700421&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomoto S, Kim H, Shimokawa T, Matsuno N, Funahashi S, Shima K, Fujita I, Tamura H, Doi T, Kawano K, Inaba N, Fukushima K, Kurkin S, Kurata K, Taira M, Tsutsui K-I, Komatsu H, Ogawa T, Koida K, Tanji J, Toyama K. 2009. Relating Neuronal Firing Patterns to Functional Differentiation of Cerebral Cortex. PLoS Comput Biol [Internet] 5:e1000433 Available from: http://dx.plos.org/10.1371/journal.pcbi.1000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji H, Mizoguchi K. 2010. Acute and repeated stress differentially regulates behavioral, endocrine, neural parameters relevant to emotional and stress response in young and aged rats. Behav Brain Res [Internet] 211:169–177. Available from: http://dx.doi.org/10.1016Zj.bbr.2010.03.025 [DOI] [PubMed] [Google Scholar]
- Singer ACC, Carr MFF, Karlsson MPP, Frank LMM. 2013. Hippocampal SWR Activity Predicts Correct Decisions during the Initial Learning of an Alternation Task. Neuron [Internet] 77:1163–1173. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23522050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Gerrard JL, Barnes CA, McNaughton BL. 2000. Effect of age on burst firing characteristics of rat hippocampal pyramidal cells. Neuroreport [Internet] 11:3865–3871. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11117505 [DOI] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsaki G. 2014. Pyramidal Cell-Interneuron Interactions Underlie Hippocampal Ripple Oscillations. Neuron [Internet] 83:467–480. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0896627314005455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand J-PL, Gray DT, Schimanski LA, Lipa P, Barnes CA, Cowen SL. 2016. Age Is Associated with Reduced Sharp-Wave Ripple Frequency and Altered Patterns of Neuronal Variability. J Neurosci [Internet] 36:5650–60. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.3069-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Redish AD. 2015. Decoding the cognitive map: ensemble hippocampal sequences and decision making. Curr Opin Neurobiol 32:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. 1993. Dynamics of the hippocampal ensemble code for space. Science [Internet] 261:1055–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8351520 [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science [Internet] 265:676–679. Available from: http://www.sciencemag.org/content/265/5172/676.short [DOI] [PubMed] [Google Scholar]
- Wu C-T, Haggerty D, Kemere C, Ji D. 2017. Hippocampal awake replay in fear memory retrieval. Nat Neurosci [Internet] 20:571–580. Available from: http://www.nature.com/doifinder/10.1038/nn.4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos TP, Mineault PJ, Pack CC. 2011. Removal of spurious correlations between spikes and local field potentials. J Neurophysiol 105:474–486. [DOI] [PubMed] [Google Scholar]