Abstract
Overall Goal: This study was designed to evaluate the impact of pentosan polysulfate (PPS) treatment on mice with mucopolysaccharidosis (MPS) type IIIA (Sanfilippo A syndrome; OMIM 252900).
Protocol: Three groups of MPS IIIA mice were evaluated: 1-week-old mice treated with subcutaneous (subQ) PPS at 25 mg/kg once weekly for 31 weeks (group 1); 5-month-old mice treated with subQ PPS once weekly at 50 mg/kg for 12 weeks (group 2); and 5-week-old mice treated by continual intracerebroventricular (ICV) PPS infusion for 11 weeks (60 μg/kg/day). Treated MPS IIIA mice and controls were assessed by measuring plasma cytokine levels, histologic analyses of systemic organs, and analyses of various neuroinflammatory, neurodegenerative, and lysosomal disease markers in their brains. Neurobehavioral testing also was carried out.
Results: As seen in other MPS animal models, subQ PPS treatment reduced plasma cytokine levels and macrophage infiltration in systemic tissues. ICV administration did not elicit these systemic effects. SubQ PPS administration also significantly impacted brain neuropathology, inflammation, and behavior. The effect of early subQ treatment was more significant than dose. Surprisingly, ICV PPS treatment had intermediate effects on most of these brain markers, perhaps due to the limited dose and/or duration of treatment. Consistent with these neuropathological findings, we also observed significant improvements in the hyperactivity/anxiety and learning behaviors of the MPS IIIA mice treated with early subQ PPS.
Electronic supplementary material
The online version of this article (10.1007/8904_2018_96) contains supplementary material, which is available to authorized users.
Keywords: Central nervous system, Mucopolysaccharidosis type IIIA, Neuroinflammation, Pentosan polysulfate
Introduction
Mucopolysaccharidosis type III (MPS III), or Sanfilippo syndrome, refers to one of four inherited neurodegenerative lysosomal storage disorders (LSDs) caused by the deficiency of enzymes involved in heparan sulfate (HS) degradation, including sulfamidase (MPS IIIA; EC 3.10.1.1; Kresse 1973), α-N-acetylglucosaminidase (MPS IIIB; EC 3.2.1.50; von Figura 1977), heparan-α-glucosaminide N-acetyltransferase (MPS IIIC; EC 2.3.1.78; Klein et al. 1978), and N-acetylglucosamine 6-sulfatase (MPS IIID; EC 3.1.6.14; Kresse et al. 1980). Among the Sanfilippo diseases, MPS IIIA is the most common subtype and is considered the more severe form, characterized by progressive mental deterioration, distinct behavioral disturbances, dementia, and a markedly shortened lifespan.
There is currently no effective treatment for the MPS III diseases and the clinical management remains mainly supportive. Hematopoietic stem cell transplantation (HSCT) has been performed in patients with MPS IIIA and IIIB, but the neurological benefits have been questioned (Boelens et al. 2010). Direct delivery of recombinant human heparan-N-sulfatase to the central nervous system (CNS) by intrathecal injection in MPS IIIA patients also has been evaluated, but such an approach is considered challenging due to the enzyme’s relatively short half-life, the limited distribution of enzyme throughout the CNS after injection, and the potential toxic effects associated with the procedure (Jones et al. 2016). Several gene therapy clinical trials also are under evaluation (Gaffke et al. 2017; Tardieu et al. 2017).
Our previous studies have demonstrated that systemic administration of the FDA/EMA approved drug, pentosan polysulfate (PPS), reduced inflammation and glycosaminoglycan (GAG) storage in MPS VI rats and dogs, and resulted in significant pathological and clinical improvements (Schuchman et al. 2013; Frohbergh et al. 2014; Simonaro et al. 2016). Based on these findings, a proof-of-concept phase 1/2 clinical trial also has been undertaken in MPS I patients, and the safety and potential efficacy of this approach has recently been published (Hennermann et al. 2016). We have hypothesized that PPS should be effective in other MPS types as well since it inhibits the NFkbeta pathway that is activated in many of these diseases, and a recent clinical study in Japanese MPS II patients suggests that this hypothesis may be correct (Orii et al. 2016). Of particular relevance to the use of PPS in the neurologic MPS III diseases is the fact that our studies in MPS I dogs showed that subcutaneous (subQ) treatment reduced inflammatory markers not only in systemic organs and plasma but also in the cerebrospinal fluid (CSF) (Simonaro et al. 2016). Recent findings suggest that the blood brain barrier (BBB) may be structurally and functionally impaired in MPS IIIA and MPS IIIB patients (Garbuzova-Davis et al. 2011, 2013), which may facilitate the development of such systemic therapies for these disorders.
The present study was therefore designed to validate the impact of PPS on a mouse model of MPS IIIA. Although the pathophysiological mechanisms underlying the CNS manifestation of this disease are not fully understood, previous studies have revealed that neuroinflammation and neurodegeneration are significant neuropathological phenotypes of the diseased brain (Wilkinson et al. 2012; Archer et al. 2014). Two approaches for PPS administration were evaluated: once weekly subQ injection and continual intracerebroventricular (ICV) infusion. The effects on neuroinflammation, neurodegeneration, and behavior were examined.
Materials and Methods
Experimental Animals
MPS IIIA (Bhaumik et al. 1999) and age-matched, wild-type mice were bred, housed, and maintained in the animal facility of the Icahn School of Medicine at Mount Sinai. All animal protocols were approved by the Mount Sinai Institutional Animal Care and Use Committee (protocol #08-0108), and were performed in accordance with NIH guidelines. All mice were genotyped using established protocols (Gliddon and Hopwood 2004). Euthanasia was performed using carbon dioxide inhalation.
PPS Administration
PPS (Bene pharmaChem, Germany) administration was performed in MPS IIIA mice through ICV infusion or subQ injections. For ICV, 5-week-old MPS IIIA mice were anesthetized in an induction chamber with 4% isoflurane, and were maintained anesthetized during stereotaxic surgery with 1.5% isoflurane. The scalp was incised and holes were drilled at appropriate locations in the skull with a dental drill, using the following coordinates: AP – 0.5 mm from Bregma, 1.1 mm from midline. The cannula of a brain infusion kit (Alzet, Cupertino, CA) was aseptically implanted into the right lateral ventricle through the hole, and the cannula was secured to the skull with cyanoacrylate adhesive. The infusion system was connected to a mini osmotic pump (Model 2006, Alzet), which was placed subcutaneously between the scapulae. The pump was assembled and prepared according to the manufacturer’s instructions, and the fully assembled pump was soaked and primed in bacteriostatic sodium chloride at 37°C for 60 h. The mice were infused with 60 μg/kg of PPS/day for up to 11 weeks. This PPS dose was based on previous ICV studies in patients with Creutzfeldt-Jakob disease (CDJ) (Terada et al. 2010). Local (bacitracin ointment) and systemic antibiotic (ampicillin 100 mg/kg, intramuscular every 12 h for the first 48 h post-op) treatments were administered to prevent post-operative infections. Buprenorphine (0.1–0.5 mg/kg subQ) was administered twice daily for the first 72 h, and then on an as-needed basis if the animals appeared to be in pain. A total of 11 MPS IIIA mice received stereotaxic surgery (8 received PPS infusions and 3 received sham saline infusions). Three of the ICV PPS-infused animals died during weeks 3–7 post-implantation from infections or other procedure related complications. The remaining three sham and five PPS-infused animals were sacrificed at week 16 and analyzed.
For subQ PPS treatment, the MPS IIIA mice were divided into two groups (n = 10 per group) that were treated for up to 31 weeks: group 1 animals received 25 mg/kg (human equivalent dose 1 mg/kg) once weekly starting at 1 week of age, and group 2 animals received 50 mg/kg (human equivalent dose 2 mg/kg) once weekly starting at 5 months of age. All control mice (wild-type and sham treated MPS IIIA) were age-matched. PPS was prepared and administered as described previously (Schuchman et al. 2013). No adverse events from PPS treatment were noted in the animals.
Plasma Immunoassays
Blood samples were collected into heparin tubes from the control and subQ treated animals at various intervals (16, 24 and 32 weeks post-treatment). For animals receiving ICV PPS infusion, the blood samples were collected at the time of euthanasia (16 weeks of age; after 11 weeks of infusion). After centrifugation, plasma stored at −20°C. The plasma inflammatory cytokines interleukin-1α (IL-1α), macrophage inflammatory protein-1α (MIP-1α), monocyte chemoattractant protein-1 (MCP-1), and granulocyte colony stimulating factor (G-CSF) were measured using mouse enzyme-linked immunosorbent assay (ELISAs) kits according to the manufacturer’s protocols. Mouse ELISA kits for IL-1α (catalog #MLA00), MIP-1α (catalog #MMA00), MCP-1 (catalog #MJE00), and G-CSF (catalog #MCS00) were purchased from R & D Systems (Minneapolis, MN). All immunoassays were performed in triplicate.
Histology and Immunohistochemistry
Following subQ PPS treatment, the treated and sham treated MPS IIIA mice, and wild-type, age-matched littermate mice were perfused with 0.9% saline. Organs including liver, spleen, and kidney were removed, fixed in 4% paraformaldehyde and paraffin embedded, and tissue sections were cut with a cryostat (Leica Biosystems, Buffalo Grove, IL). The sections were then stained with hematoxylin and eosin for histological examination.
To quantify the storage vacuoles in the livers, spleens, and kidneys, at least three slides/tissue were examined from each mouse. Three laboratory technicians were blinded to the study groups, and assessed the number of vacuoles with the following scoring system: 0 = between 0 and 10 vacuoles; 1 = between 10 and 20 vacuoles; 2 = between 21 and 30 vacuoles; 3 = between 31 and 40 vacuoles; 4 = between 41 and 50 vacuoles; 5 = greater than 50 vacuoles. Slides were scored at the same magnification and for the same set area of each stained section. Scores were presented as means +/− standard deviation and Student’s t test was used to analyze data between untreated and treated groups. The results were considered significant at p < 0.001 (Supplementary Fig. 1). Statistics were performed using Sigma Stat 3.1 (Systat Software).
Brains also were removed and placed in the same fresh fixative for at least 12 h, and cut using a vibratome (Leica Biosystems, Buffalo Grove, IL). The sections were washed with phosphate buffered saline (PBS), and preincubated with 3% H2O2 for 15 min to remove endogenous peroxidase activity. To minimize nonspecific immunostaining, the sections were incubated for 60 min with a blocking solution of 2% bovine serum albumin (BSA) in PBS. The sections were then reacted overnight at 4°C with primary antibody against glial fibrillary acidic protein (GFAP; 1:1,000), lysosomal integral membrane protein-2 (Limp-2; 1:250), heparan sulfate (HS; 1:100), ganglioside (GM3; 1:125–1:750), isolectin B4 (IL-B4) (1:10) or P-taus262 (1:10–1:20), respectively, in blocking solution plus 0.3% Triton X-100. Following several washes in PBS for 30 min, the sections were reacted with a biotin-conjugated secondary antibody against the primary IgG or IgM for 60 min at room temperature. The immunoreaction was completed by the avidin-biotin-peroxidase method using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA) suitable for the secondary antibody, and the color reaction was visualized using the diaminobenzidine (DAB) substrate system, enhanced with 2% ammonium nickel(II).
The rabbit polyclonal antibody against GFAP was purchased from Dako/Agilent (Santa Clara, CA), the monoclonal anti-Limp-2 antibody was purchased from Santa Cruz Biotechnology (Dallas, TX), the mouse monoclonal antibody against HS and the mouse monoclonal antibody against GM3 were purchased from Amsbio LLC (Cambridge, MA), and the rabbit polyclonal antibody against phosphorylated tau (P-taus262) was purchased from Abcam (Cambridge, MA). For detection of IL-B4, peroxidase-conjugated lectin from Bandeiraea (Griffonia) simplicifolia was purchased from Sigma (St. Louis, MO, USA). The biotinylated secondary antibodies: goat anti-rabbit IgG, rabbit anti-goat IgG, goat anti-mouse IgM, and the Vectastain ABC kits were purchased from Vector Laboratories. The DAB chromogen and substrate were purchased from Thermo Scientific (Waltham, MA).
To obtain semi-quantitative data regarding the effects of PPS treatment, for each of the above staining at least three slides were read per mouse. Two independent laboratory technicians read the slides and were blinded to the treatment group. A scoring system that assessed both the intensity and the number of positive signals per slide was established, with 0 being the least and 5 being the most. Slides were scored at the same magnification and for the same set area of each stained section. Tissues (liver, spleen, and kidney) also were collected for total GAG assays and homogenized according to the BLYSCAN GAG kit #NC0287381 (Fisher Scientific, Waltham, MA).
Neurobehavioral Evaluations
To evaluate the acquisition of skilled behavior (motor skill learning) in mice, a modified rotarod test that emphasizes the learning aspect of the test and minimizes other factors was used (Shiotsuki et al. 2010). The rotarod apparatus was equipped with automatic timers and falling sensors (IITC/Life Science, Woodland Hills, CA), and the mice were individually placed on a 3.75″ diameter drum. The surface of the drum was covered with hard chloroethylene, which does not permit gripping on the surface. Prior to the training sessions, all of the mice were habituated to stay on the stationary drum for 5 min. Habituation was repeated daily for 3 min just before the session. Instead of the typical rotation acceleration, the rotation in this modified test was set at a relatively slow but steady speed (14 rpm, 3.9 m/min on the surface, with a starting speed of 5 rpm, and 15 s ramp to the top speed), to make the task feasible for the animals to learn. The animal was placed back on the drum immediately after falling, up to five times in one session. A fall was overlooked when the animal remained on the drum for 120 s. To evaluate long-term memory of the learned motor skill, the test was repeated daily for four consecutive days. The latency to falling was recorded automatically by photocells and the total latencies on the rod on each day was analyzed.
To assess anxiety-like and/or repetitive-like behaviors of the animals, a marble-burying test was performed (Boivin et al. 2017; Thomas et al. 2009). Standard clean cages (26 cm × 48 cm × 20 cm) were used to habituate and test the animals. Unscented wood chip bedding was added to each cage to a depth of 5 cm and the bedding surface was leveled by inserting another cage of the same size onto the surface of the bedding. The impressed parallel lines on the bedding surface were used for marble placement, and 20 standard glass toy marbles (assorted styles and colors, 15 mm diameter) were gently placed on the surface of the bedding in 5 rows of 4 marbles/row. Following each test, the marbles were washed in mild laboratory detergent, rinsed extensively in distilled-deionized water, and dried at least 1 day prior to each use. During the habituation phase of the test, the mice were introduced to cages without any marbles and allowed to explore for 90 min and then removed. During the testing phase each mouse was placed in the cage with 20 marbles placed on the bedding surface, and allowed to explore for 30 min. At the end of the test, mice were removed from the cage and the number of marbles buried in bedding up to 2/3 of their depth was counted. All of the mice in their home cages were transported to the testing room 60 min before the start of testing.
Statistics
A Student’s t-test was used to compare values between two groups, and ANOVA with Tukey post hoc analysis was used to compare values between three groups. All statistical analyses were performed using the Sigma Stat software (Systat Software, Inc., Point Richmond, CA).
Results
Effects of PPS Treatment on Systemic Pathology and Inflammation in MPS IIIA Mice
Histological examinations revealed moderate to severe, multifocal infiltration of large foamy macrophages containing cytoplasmic vacuoles in the livers, spleens, and kidneys of sham treated MPS IIIA mice at 32 weeks of age (Fig. 1). A significant reduction of these macrophages and vacuoles was observed following weekly subQ injections of PPS in both groups of treated mice, with a more pronounced effect in group 1 animals (Supplementary Fig. 1). Subcutaneous treatment also attenuated systemic inflammation in both groups, as indicated by ELISA immunoassays of the plasma inflammatory markers IL-1α, MCP-1, MIP-1α, and G-CSF (Fig. 2). Sham treated MPS IIIA mice exhibited age-progressive (16, 24 and 32 weeks) increases in each of these cytokines, and group 1 animals exhibited significantly reduced levels at each age. Group 2 animals (who were started on treatment at 5 months of age) were only assessed at 32 weeks of age, and PPS significantly reduced the levels as well. In comparison, MPS IIIA animals receiving PPS via ICV infusion at the dose of 60 μg/kg/day for 11 weeks did not exhibit reduced cytokine levels in their plasma (Fig. 2), nor did they exhibit a reduction in macrophage infiltration into the systemic tissues (data not shown). It is notable that the levels of these cytokines were higher in the ICV treated mice at 16 weeks of age compared to sham treated 16-week control MPS IIIA mice, suggesting that an inflammatory reaction was occurring from the insertion of the infusion pumps and/or the local, continuous administration of PPS.
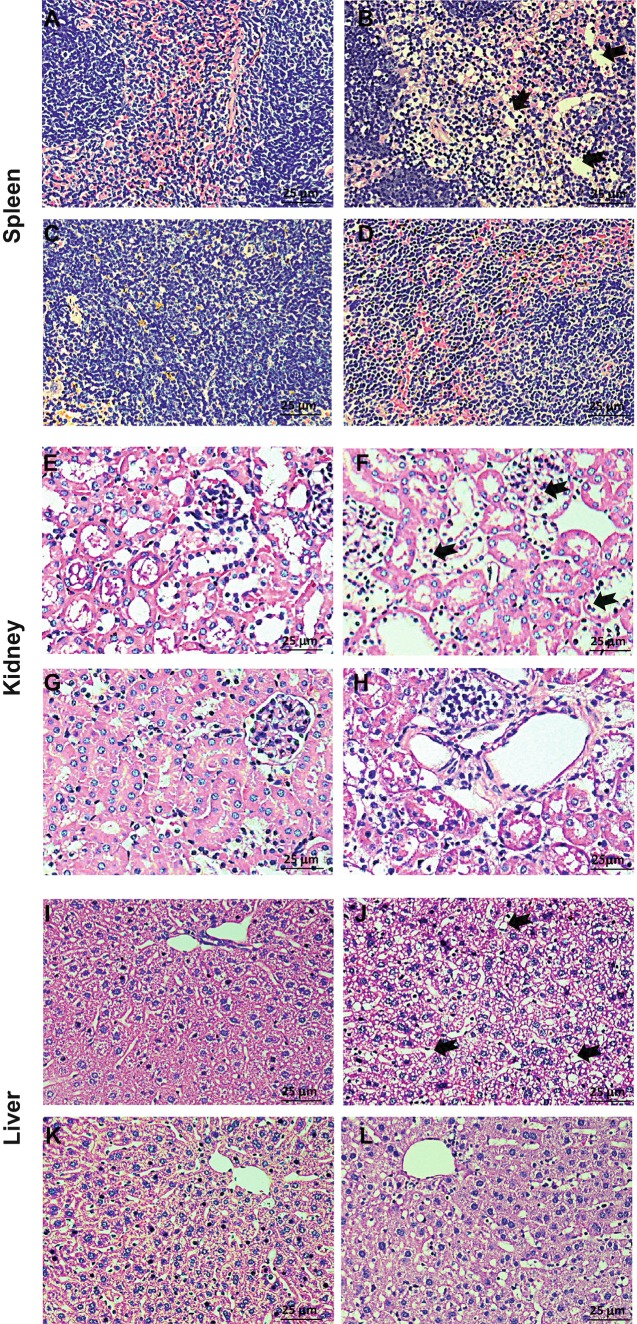
Fig. 1.
Systemic effects of subQ PPS administration in MPS IIIA mice as assessed by histopathology. Panels (a, e, and i) are representative images from wild-type mice; panels (b, f, and j) are representative images from sham treated MPS IIIA mice; panels (c, g, and k) are representative images from MPS IIIA mice treated at 1 week of age for 31 weeks, once weekly with 25 mg/kg PPS (group 1), and panels (d, h, and l) are representative images from MPS IIIA mice treated at 5 months of age for 12 weeks, once weekly with 50 mg/kg PPS (group 2). A reduction of macrophages and vacuoles was evident in MPS IIIA mice treated with subQ PPS. Black arrows point to representative storage vacuoles
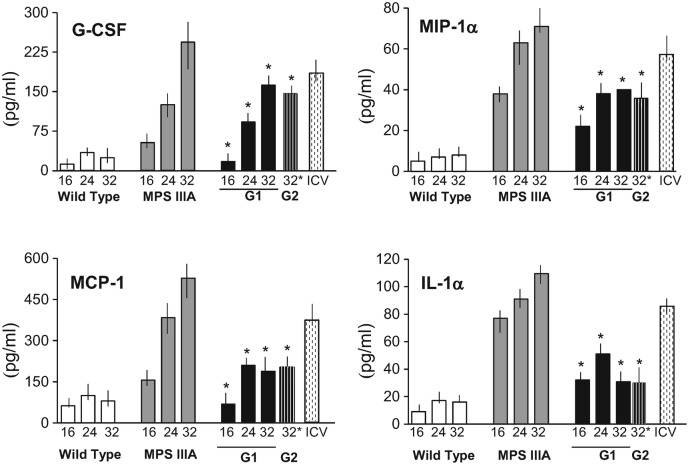
Fig. 2.
Plasma cytokine levels in MPS IIIA mice treated with PPS. IL-1α, MCP-1, MIP-1α, and G-CSF were measured in the plasma of wild-type, sham treated MPS IIIA, and PPS treated MPS IIIA mice at various intervals (16, 24 and 32 weeks) by ELISA. G1, group 1; G2, group 2 (n = 10/group). ICV mice (n = 5) were only assessed at the end of the treatment period (16 weeks). Cytokine levels were higher in the ICV treated when compared to the untreated age-matched animals probably due to the fact that the PPS was not released systemically. *p < 0.05 compared to sham treated MPS IIIA mice of the same age
We also measured total GAGs in the livers, spleens, and kidneys of the subQ treated PPS animals, although this analysis is complicated by the fact that PPS cross-reacts with most of the established GAG assays, and the fact that that animals were euthanized within 48 h after the last PPS injection, a time when the levels of PPS remain high in the tissues. As shown in Supplementary Fig. 2, we did observe reductions in liver or spleen GAGs in the treated mice, although we hypothesize that this is due to the remaining PPS and the fact that the drug is known to accumulate in these tissues after injection (Greenslade et al. 1983). In contrast, in the spleen where less residual PPS will be present, significant GAG reductions were observed.
Effects of PPS Treatment on Neuropathological Markers in MPS IIIA Mice
SubQ Administration
Immunohistochemical assessments of various brain regions were carried out for both groups of subQ treated animals. Overall, the impact of PPS on the CNS was much more evident in the group 1 animals who started treatment at 1 week of age and were treated for 31 weeks. Although double the dose was used in the group 2 animals (50 vs. 25 mg/kg), little or no evidence of CNS effects was found. This could be due to the fact that treatment was started at 5 months of age, and/or the fact that the duration of treatment was only 12 weeks.
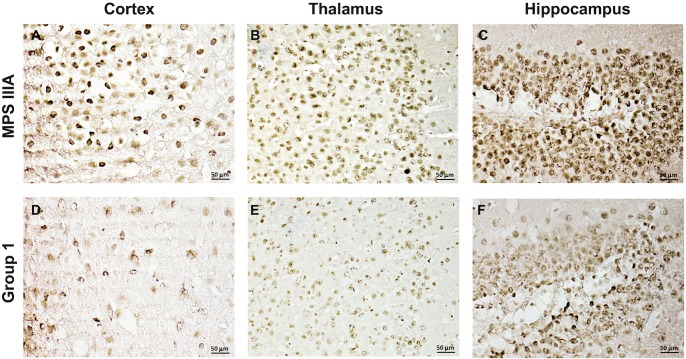
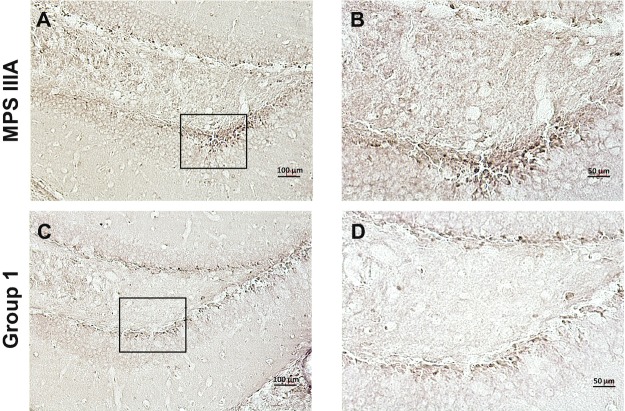
For example, sham treated MPS IIIA animals exhibited significant astrocyte activation, as evidenced by strongly positive GFAP staining in different brain regions, including the thalamus, cortex, and hippocampus, and this staining was greatly reduced in the group 1 treated animals (Fig. 3). Intense staining for Limp-2, an important regulator of lysosomal/endosomal transport that is frequently elevated in tissues from lysosomal storage disease animal models, including MPS IIIA mice and dogs (Beard et al. 2017; Reczek et al. 2007; King et al. 2015, 2017), also was found in the hippocampus of sham treated MPS IIIA mice, and this staining was attenuated in the group 1 PPS treated animals as well (Fig. 4a–d). Neuronal staining for HS, the accumulating GAG substrate in MPS IIIA, also was found in different brain regions of the sham treated mice, including the cortex, thalamus, and hippocampus, and this was reduced in the group 1 treated mice as well (Fig. 5).
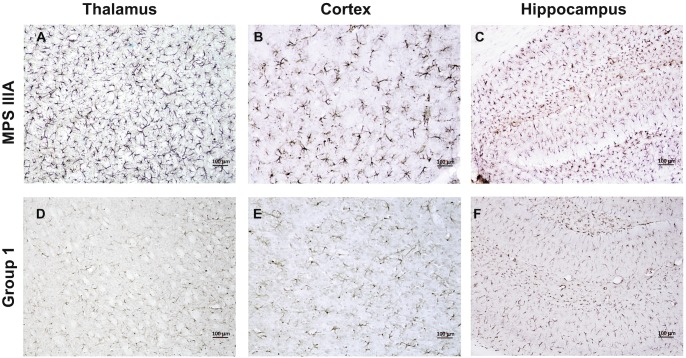
Fig. 3.
GFAP immunostaining in the thalamus, cortex, and hippocampus of MPS IIIA mice treated by subQ PPS. Panels (a–c) show representative images from sham treated MPS IIIA mice at 32 weeks of age. Panels (d–f) show representative images from subQ treated (group 1) PPS treated mice at the same age
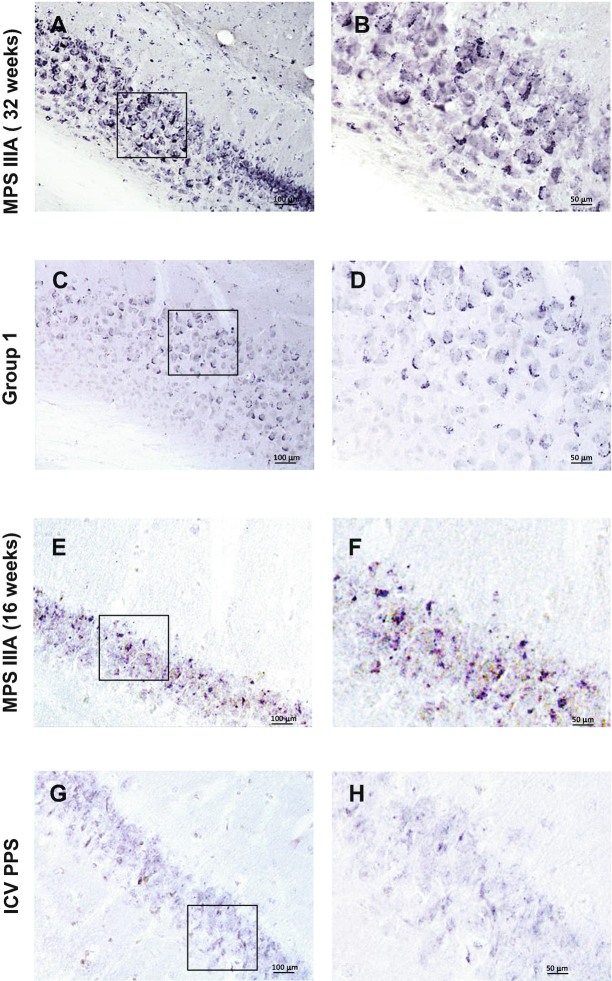
Fig. 4.
Limp-2 immunostaining in the hippocampus of MPS IIIA mice treated with PPS. Panels (a, b) show representative images from 32-week-old, sham treated MPS IIIA mice, and panels (c, d) show representative images from group 1 treated mice of the same age. Panels (e, f) show representative images from 16-week-old sham treated MPS IIIA mice, and panels (g, h) show images from mice of the same age treated by ICV infusion. The boxes in panels (a, c, e, and g) show the areas of magnification
Fig. 5.
HS immunostaining in the thalamus, cortex, and hippocampus of MPS IIIA mice treated by subQ PPS. Panels (a–c) show representative images from sham treated 32-week-old MPS IIIA mice, and panels (d–f) show representative images from group 1 treated animals
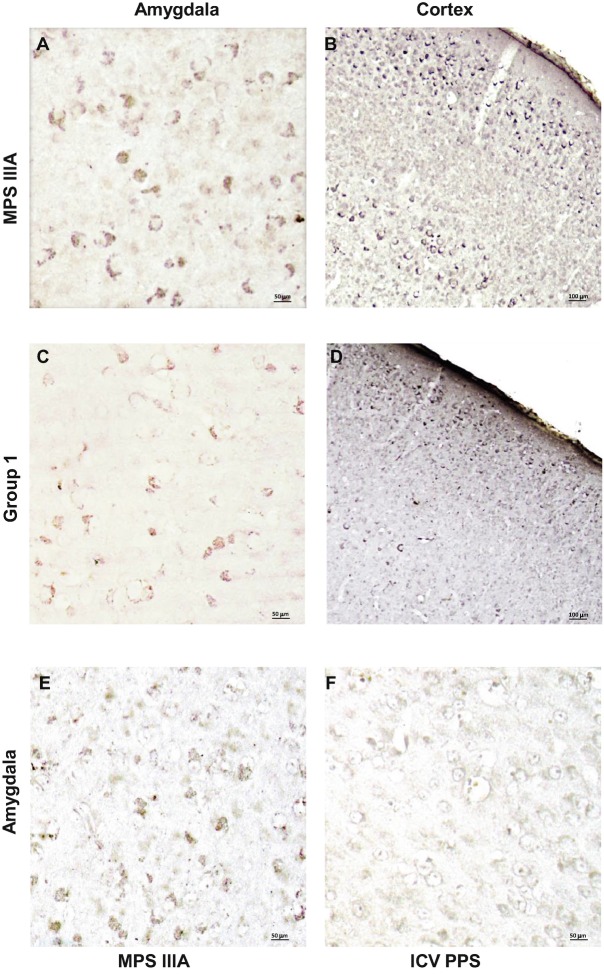
The ganglioside GM3 is a secondary substrate that accumulates in the CNS of many neurologic lysosomal storage disease mouse models, including MPS III models (Ryazantsev et al. 2007; Dawson et al. 2012), and staining for this glycolipid was evident in the amygdala and cortex of the sham treated MPS IIIA mice (Fig. 6a). Compared to the sham treated animals, PPS treated group 1 animals exhibited reduced GM3 staining in both of these regions (Fig. 6a). Finally, to evaluate neurodegeneration in the MPS IIIA mice, a neurodegenerative marker, phosphorylated-tau protein, P-taus262, also was examined. Figure 7 shows that accumulation of P-taus262 was found in the hippocampus of sham treated MPS IIIA mice, especially in the dentate gyrus. In contrast, in PPS treated group 1 mice P-taus262 staining was markedly reduced.
Fig. 6.
GM3 immunostaining in the amygdala and cortex of PPS treated MPS IIIA mice. Panels (a, b) show representative images from 32-week-old, sham injected MPS IIIA mice. Panels (c, d) show representative images from group 1 mice treated with PPS. Panels (e, f) show representative images from the amygdala of 16-week-old sham and ICV treated MPS IIIA mice, respectively
Fig. 7.
P-taus262 immunostaining of MPS IIIA mice treated by subQ PPS. Panels (a, b) show representative images from sham treated, 32-week-old MPS IIIA mice, and panels (c, d) show representative images from 32-week-old group 1 mice treated by PPS
ICV Infusion
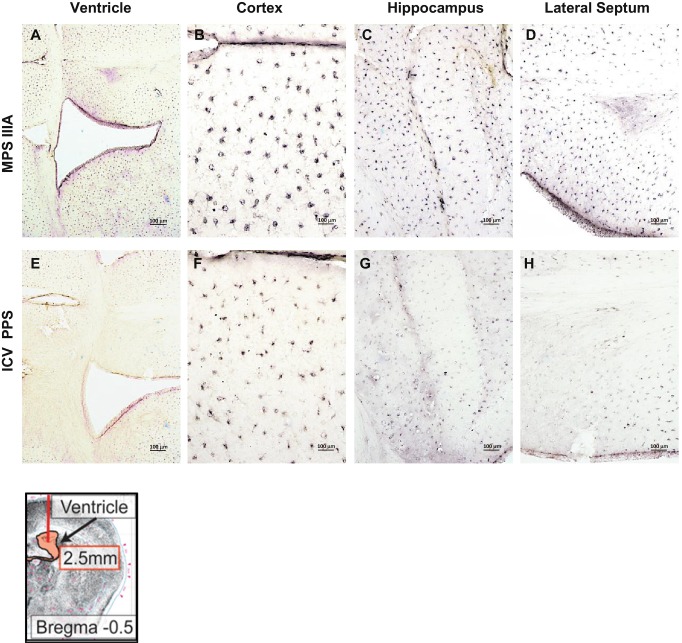
Following 11 weeks of continuous PPS ICV infusion (60 μg/kg/day), the impact on several of these neuropathological markers also was assessed. For example, Fig. 4e–h shows the reduction of Limp-2 staining, and Fig. 6 shows the reduction of GM3 staining. Although the neuroinflammatory marker GFAP was not assessed in these animals, we did observe a marked reduction in IL-B4 staining, a marker of microglial activation, in the region immediately surrounding the site of infusion, as well as in the cortex, hippocampus, and lateral septum (Fig. 8). We did not observe differences in HS or P-taus262 staining in the ICV treated group.
Fig. 8.
IL-B4 staining in MPS IIIA mice treated with PPS by ICV infusion. Panels (a–d) show representative images from 16-week-old, sham treated MPS IIIA mice (ventricle, cortex, hippocampus and lateral septum, respectively). Panels (e–h) show representative images from MPS IIIA mice of the same age that received ICV PPS infusions. A schematic showing the site of injection (arrow) is provided in the lower left
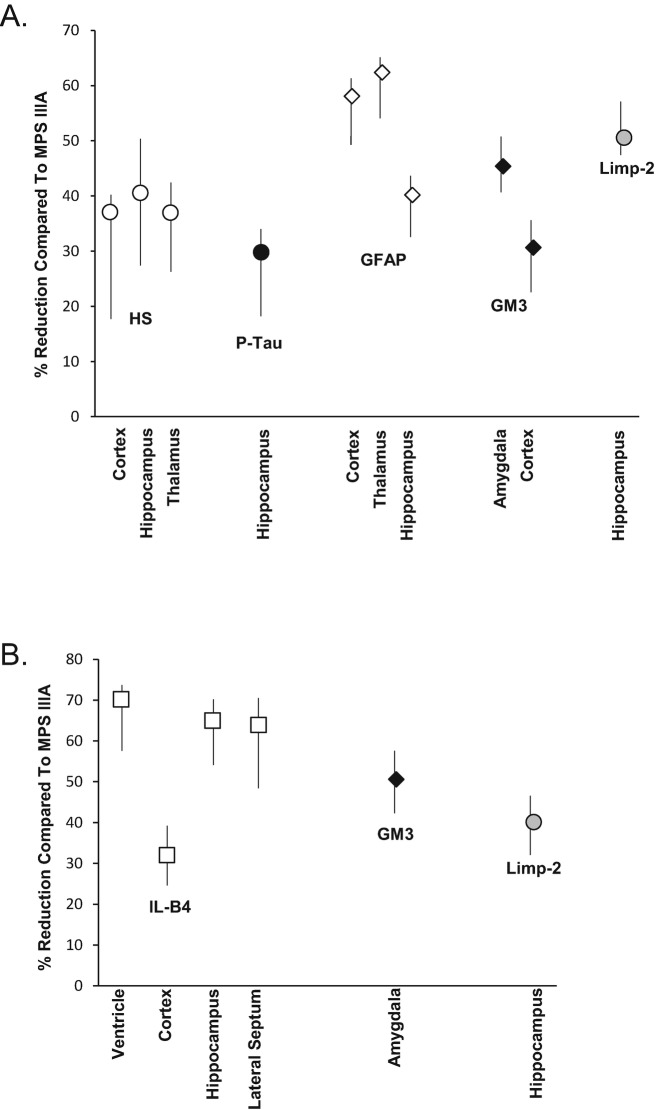
Figure 9 summarizes semi-quantitative data obtained from the analysis of neuropathologic markers in various brain regions areas of subQ and ICV PPS treated MPS IIIA mice. Group 1 mice showed a reduction from 28 to 61% in HS, p-Tau, GFAP, GM3, and Limp-2 when compared to sham treated, age-matched MPS IIIA mice (Fig. 9a). A significant reduction of neuroinflammation (GFAP staining) also was found, between 39–61% in the cortex, thalamus, and hippocampus, and a 36–41% reduction in HS also was seen in these same regions.
Fig. 9.
Semi-quantitative analysis of neuropathologic markers in PPS treated MPS IIIA mice. Semi-quantitative analysis of the staining intensities was determined in PPS and sham treated MPS IIIA mice as described in the Materials and Methods. Two blinded readers examined and scored at least three slides per mouse per group. The percent reduction in staining is shown in the PPS vs. the age-matched MPS IIIA control mice for subQ (group 1, panel a) and ICV (panel b) treated animals. The mean values are graphed from the six determinations (two readers and three slides each), and the vertical lines indicate the ranges
ICV treated animals exhibited a significant reduction of IL-B4, a marker for microglia, also indicating an anti-inflammatory effect of PPS (Fig. 9b). The ventricle, hippocampus, and lateral septum showed the most significant effects (64–70% reduction) when compared to the cortex (32%), which is the region furthest from insertion site of the PPS pump. Overall, both subQ and ICV PPS treatment led to a reduction in neuropathologic markers.
Effects of PPS Treatment on Behavioral Testing in the MPS IIIA Mice
Behavioral testing was performed on the sham vs. group 1 treated MPS IIIA mice. We did not assess the group 2 or ICV treated animals due to the later onset of the treatments and/or shorter duration. Marble burying performance was used to assess anxiety-like hyperactivity and obsessive-compulsive behavior (Fig. 10a). Sham treated MPS IIIA animals buried more marbles in the 30 min test period compared to wild-type littermates (wild-type, 3.3 ± 2.5; MPS IIIA, 9.0 ± 2.6; n = 10 group), and in the group 1 treated animals there was a significant reduction towards normal (4.1 ± 2.8; n = 10/group). We also assessed motor skill learning using a modified rotarod test. As shown in Fig. 10b, during the testing phase (days 3 and 4) untreated MPS IIIA mice exhibited poor performance (longer latency) compared to wild-type mice, indicating poor learning skills. In contrast, the group 1 treated PPS animals exhibited a learning behavior that was intermediate and significantly improved by day 4.
Fig. 10.
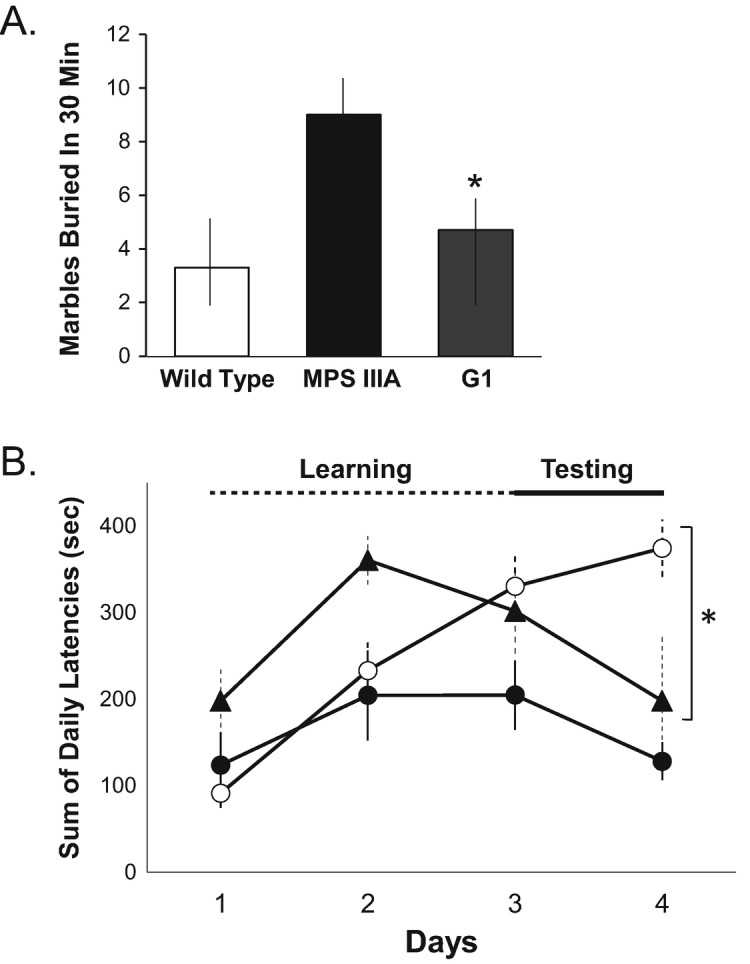
Behavioral testing in MPS IIIA mice treated by subQ PPS. (a) A marble burying test was used to assess hyperactivity and obsessive-compulsive behavior, and was performed as described in the Materials and Methods. The mean values are graphed for age-matched (32-week-old) wild-type, sham treated MPS IIIA (MPS IIIA), and group 1 MPS IIIA mice treated with subQ PPS (Q1). n = 10 per group. The vertical lines indicate the ranges from three independent test determinations. *p < 0.05 in the G1 PPS treated vs. sham treated MPS IIIA groups. (b) A modified rotarod test was used to assess skilled learning behavior, and was performed as described in the Materials and Methods. The test was repeated one session per day for 4 consecutive days. The first 2 days were considered the learning period, and the final 2 days the testing period. The test was repeated at least three times per group. The mean values are graphed and the vertical lines indicate the ranges. *p < 0.05 comparing group 1 treated (black triangles) to sham treated (white circles) MPS IIIA mice on day 4. Black circles indicated wild-type mice. All mice were 32 weeks of age at the time of testing. n = 10 per group
Discussion
Previous studies from our lab and others have shown that PPS has potent anti-inflammatory properties in various disease models and patients (e.g., Schuchman et al. 2013; Frohbergh et al. 2014; Simonaro et al. 2016; Hardi et al. 2016; Sanden et al. 2017; Hennermann et al. 2016). Consistent with these reports, once weekly subQ PPS administration to MPS IIIA mice attenuated systemic inflammation, as shown by the reduction of the elevated plasma inflammatory markers IL-1α, MCP-1, MIP-1α, and G-CSF (Fig. 2). TNF-α was not significantly elevated in this particular mouse model, and was therefore not evaluated. Overall, there was a significant and similar impact on the plasma cytokines in both groups of subQ treated mice. In addition, reduced macrophage infiltration was observed in the livers, kidneys, and spleens of both groups, although the effects were more pronounced in group 1 mice (Fig. 1).
These findings are in agreement with the potent anti-inflammatory effects of PPS we have observed in the other MPS models (Frohbergh et al. 2014; Simonaro et al. 2016). Further, they suggest that the age at which treatment is initiated is more important than the dose, since group 1 animals were treated with half the dose of group 2 (25 vs. 50 mg/kg), but treatment was started at 1 week of age (as compared to 5 months). This suggests that for optimal clinical effects in patients, treatment should be initiated as early as possible to prevent irreversible damage. It is also important to note that group 1 animals were treated for a longer period of time (31 weeks), as compared to 12 weeks for group 2 animals.
As expected, ICV infusion for up to 11 weeks (60 μg/kg/day) did not reduce plasma cytokine levels or systemic tissue macrophage infiltration, which confirms that the ICV administration procedure was specific for the CNS. Of note, the cytokine levels in the ICV mice were moderately elevated compared to sham treated controls, indicating that an inflammatory reaction might have occurred in these mice from the insertion of the infusion pumps and/or the continuous, local administration of the drug.
Importantly, subQ PPS administration also had an impact on the brains of the MPS IIIA mice, suggesting that the administered PPS may cross the BBB. It is important to note that impairment of the BBB has been previously described in this mouse model (Garbuzova-Davis et al. 2011), as well as in patients with MPS IIIA and IIID (Garbuzova-Davis et al. 2013). We have also previously detected reduction in CSF cytokine levels in MPS I dogs treated by subQ PPS (Simonaro et al. 2016), which further suggests penetration of the drug into the CNS following systemic administration. In the current study, at a dose of 25 mg/kg, once weekly for 31 weeks (starting at 1 week of age; group 1), subQ PPS treatment reduced astrocyte activation, as evidenced by GFAP staining (Fig. 3), and attenuated overexpression of Limp-2 (Fig. 4), HS (Fig. 5), and GM3 (Fig. 6). Although it is well known that PPS is a potent anti-inflammatory drug that impacts peripheral organs (Wu et al. 2011; Herrero et al. 2015; Simonaro et al. 2016), this is the first clear evidence that systemic administration of the drug also has an impact on the brain.
Subcutaneous PPS administration also resulted in a reduction of the neurodegenerative marker, P-taus262, in the MPS IIIA mice (Fig. 3). The presence of hyperphosphorylated tau protein and tau aggregates in patients with Niemann–Pick disease type C (Love et al. 1995), and in mouse models of MPS IIIA (Bhaumik et al. 1999), IIIB (Ohmi et al. 2009), and IIIC (Martins et al. 2015), has been previously shown, and it has even been suggested that the MPS III diseases should be considered “tauopathies,” a diverse group of diseases associated with dementia, including Alzheimer’s disease. The current finding that PPS attenuated the abnormal accumulation of P-taus262 in MPS IIIA mice therefore suggests that PPS might have neuroprotective effects in other neurodegenerative diseases, in line with an earlier report that PPS exerted neuroprotective actions in a rodent model of ischemia (Sakurai-Yamashita et al. 2006).
PPS also acts competitively with endogenous HS to bind prion protein on the cell surface, and several studies have indicated its efficacy in animal models and patients with CJD (Larramendy-Gozalo et al. 2013). For example, intracerebral administration of PPS increased the survival of mice in a dose-dependent manner after prion infection (Farquhar et al. 1999; Bone et al. 2008; Honda et al. 2012), and continuous intraventricular infusion of PPS in seven CJD patients was well tolerated over a large dose range (11–110 μg/kg/day). Survival in all seven exceeded the mean survival of untreated patients.
In addition to subQ administration, PPS was directly delivered to the CNS of 5-week-old MPS IIIA mice via continual ICV infusion (60 μg/kg/day) for up to 11 weeks, and overall the findings were intermediate between those observed in the group 1 and 2 subQ treated animals. Clear reductions of Limp-2 and GM3 staining were observed, but there were no changes in HS or P-taus262. Although we did not perform GFAP staining on these mice, we did observe a marked reduced of IL-B4, another marker of neuroinflammation that indicates microglial activation. While it is somewhat surprising that we did not observe more significant neurological effects via this direct CNS delivery method, it is also important to note that we used a relatively low PPS dose and that the animals could only be maintained on treatment for a maximum of 11 weeks due to clogging of the infusion pumps and/or the risk of infection.
Finally, we assessed the behavioral phenotype of the treated MPS IIIA mice using two different tests. We focused these analyses on the group 1 treated animals since we observed the most significant neuropathological effects in these mice. Behavioral testing was performed at the end of the treatment period (32 weeks of age). When assessed with the marble burying test, the sham treated MPS IIIA animals showed anxiety-like hyperactivity and obsessive-compulsive behavior compared to age-matched healthy littermates, as indicated by more marbles buried during the 30 min test period (Fig. 9a). A significant difference in the number of buried marbles between the PPS treated and control MPS IIIA mice also was observed, suggesting that early subQ PPS treatment might attenuate the neurobehavioral deficits in this disease. We confirmed these findings using a modified rotarod test (Shiotsuki et al. 2010). After a brief learning period on the apparatus, sham treated PPS mice exhibited poor performance (longer latency) during the testing period, indicating a reduced learning skill. Group 1 PPS treated animals exhibited significantly reduced latency, consistent with improved learning behavior. Overall, these behavioral improvements were consistent with the neuropathological improvements we observed in this group of PPS treated mice.
In conclusion, the current study shows for the first time that subQ PPS treatment reduced neuroinflammatory and neurodegenerative markers in the brains of MPS IIIA mice and that neurobehavioral deficits were attenuated as well. The impact of starting the treatment early (1 week of age vs. 5 months) appeared to be greater than doubling the dose in older animals (25 vs. 50 mg/kg). ICV PPS infusion had an intermediate effect, although treatment was only evaluated at a low dose and for a relatively short period of time. There are several limitations to this study that should be considered, including the relatively small number of animals evaluated (particularly with regard to the ICV protocol), and the variability in some of the behavioral testing. In addition, assessment of the neuropathological markers by immunocytochemistry, while informative, is difficult to quantify. We attempted to do this by having two independent readers blindly assess the slides (at least three images per mouse per group), and score the staining intensity and frequency. Overall, the data supports the conclusion that subQ PPS administration could be beneficial in MPS IIIA and perhaps other neurological LSDs, particularly if the treatment is started early in life, and should be evaluated further. Furthermore, the findings on improvement of systemic inflammation and macrophage infiltration in this model support previous findings from MPS I and VI animal studies, and two proof-of-concept clinical studies in MPS I and II patients (Frohbergh et al. 2014; Simonaro et al. 2016; Hennermann et al. 2016; Orii et al. 2016).
Electronic Supplementary Material
Semi-quantitative assessment of storage vacuoles in the livers, spleens, and kidneys of MPS IIIA mice treated by subQ PPS. Scoring was performed by three independent laboratory technicians who were blinded to the treatment groups. The scoring system is described in the Materials and Methods. At least three slides were scored per tissue per mouse. *p = <0.001 compared treated to age-matched untreated mice (PPTX 34 kb)
Total GAG analysis of liver, spleen, and kidney extracts of MPS IIIA mice treated by subQ PPS. Total GAGs were determined by the Blyscan method using tissue extracts prepared from each mouse. n = 10 per group (PPTX 36 kb)
Acknowledgements
The research was funded by the Stop Sanfilippo Foundation.
Abbreviations
- BBB
Blood brain barrier
- BSA
Bovine serum albumin
- CNS
Central nervous system
- CSF
Cerebral spinal fluid
- DAB
Diaminobenzidine
- ELISA
Enzyme-linked immunosorbent assays
- GAG
Glycosaminoglycan
- GCS-F
Granulocyte colony stimulating factor
- GFAP
Glial fibrillary acidic protein
- GM3
Monosialodihexosylganglioside
- HS
Heparan sulfate
- HSCT
Hematopoietic stem cell transplantation
- ICV
Intracerebroventricular
- IL1α
Interleukin-1 alpha
- IL-B4
Isolectin B4
- kg
Kilogram
- Limp-2
Lysosomal integral membrane protein-2
- LSD
Lysosomal storage disorder
- MCP-1
Monocyte chemoattractant protein-1
- mg
Milligram
- MIP-1α
Macrophage inflammatory protein-1 alpha
- MPS
Mucopolysaccharidosis
- PBS
Phosphate buffered saline
- PPS
Pentosan polysulfate
- subQ
Subcutaneous
Synopsis of Article
Subcutaneous pentosan polysulfate treatment reduces neuroinflammation and neurodegeneration in the brain of MPS IIIA mice.
Conflict of Interest
Calogera M. Simonaro: inventor on patent related to the use of pentosan polysulfate for the treatment of lysosomal storage disorders; licensed to Plexcera Therapeutics.
Calogera M. Simonaro has received a research grant from the Stop Sanfilippo Foundation (Spain).
Edward H. Schuchman: inventor on patent related to the use of pentosan polysulfate for the treatment of lysosomal storage disorders; licensed to Plexcera Therapeutics.
Edward H. Schuchman: co-founder and owns equity in Plexcera Therapeutics.
Ningning Guo, Victor A. DeAngelis and Changzhi Zhu declare that they have no conflict of interest.
Animal Rights
All institutional and national guidelines for the care and use of laboratory animals were followed. All animal protocols were approved by the Mount Sinai Institutional Animal Care and Use Committee (protocol #08-0108), and were performed in accordance with NIH guidelines.
Contributions of Individual Authors
Calogera M. Simonaro: concept and design, analysis and interpretation of data, drafting of article, revising of article for important intellectual content. Corresponding author.
Edward H. Schuchman: concept and design, analysis and interpretation of data, drafting of article, revising of article for important intellectual content.
Ningning Guo: conducting experiments, analysis and interpretation of data, and drafting of article.
Victor A. DeAngelis: conducting experiments and analysis of data.
Changzi Zhu: conducting experiments and analysis of data.
References
- Archer LD, Langford-Smith KJ, Bigger BW, Fildes JE. Mucopolysaccharide diseases: a complex interplay between neuroinflammation, microglial activation and adaptive immunity. J Inherit Metab Dis. 2014;37(1):1–12. doi: 10.1007/s10545-013-9613-3. [DOI] [PubMed] [Google Scholar]
- Beard H, Hassiotis S, Gai WP, Parkinson-Lawrence E, Hopwood JJ, Hemsley KM. Axonal dystrophy in the brain of mice with Sanfilippo syndrome. Exp Neurol. 2017;295:243–255. doi: 10.1016/j.expneurol.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Bhaumik M, Muller VJ, Rozaklis T. A mouse model for mucopolysaccharidosis type III A (Sanfilippo syndrome) Glycobiology. 1999;9(12):1389–1396. doi: 10.1093/glycob/9.12.1389. [DOI] [PubMed] [Google Scholar]
- Boelens JJ, Prasad VK, Tolar J, Wynn RF, Peters C. Current international perspectives on hematopoietic stem cell transplantation for inherited metabolic disorders. Pediatr Clin N Am. 2010;1:123–145. doi: 10.1016/j.pcl.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Boivin JR, Piekarski DJ, Wahlberg JK, Wilbrecht L. Age, sex, and gonadal hormones differently influence anxiety- and depression-related behavior during puberty in mice. Psychoneuroendocrinology. 2017;85:78–87. doi: 10.1016/j.psyneuen.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone L, Belton L, Walker AS, Darbyshire J. Intraventricular pentosan polysulphate in human prion diseases: an observational study in the UK. Eur J Neurol. 2008;15(5):458–464. doi: 10.1111/j.1468-1331.2008.02108.x. [DOI] [PubMed] [Google Scholar]
- Dawson G, Fuller M, Helmsley KM, Hopwood JJ. Abnormal gangliosides are localized in lipid rafts in Sanfilippo (MPS3a) mouse brain. Neurochem Res. 2012;37(6):1372–1380. doi: 10.1007/s11064-012-0761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar C, Dickinson A, Bruce M. Prophylactic potential of pentosan polysulphate in transmissible spongiform encephalopathies. Lancet. 1999;353(9147):117. doi: 10.1016/S0140-6736(98)05395-1. [DOI] [PubMed] [Google Scholar]
- Frohbergh M, Ge Y, Meng F, et al. Dose responsive effects of subcutaneous pentosan polysulfate injection in mucopolysaccharidosis type VI rats and comparison to oral treatment. PLoS One. 2014;9(6):e100882. doi: 10.1371/journal.pone.0100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffke Lidia, Pierzynowska Karolina, Piotrowska Ewa, Węgrzyn Grzegorz. How close are we to therapies for Sanfilippo disease? Metabolic Brain Disease. 2017;33(1):1–10. doi: 10.1007/s11011-017-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Louis MK, Haller EM, Derasari HM, Rawls AE, Sanberg PR. Blood-brain barrier impairment in an animal model of MPS IIIB. PLoS One. 2011;6(3):e16601. doi: 10.1371/journal.pone.0016601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Mirtyl S, Sallot SA, Hernandez-Ontiveros DG, Haller E, Sanberg PR. Blood-brain barrier impairment in MPS III patients. BMC Neurol. 2013;13:174. doi: 10.1186/1471-2377-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliddon BL, Hopwood JJ. Enzyme-replacement therapy from birth delays the development of behavior and learning problems in mucopolysaccharidosis type IIIA mice. Pediatr Res. 2004;56(1):65–72. doi: 10.1203/01.PDR.0000129661.40499.12. [DOI] [PubMed] [Google Scholar]
- Greenslade D, Ward J, Hopkins R (1983) (3H)-sodium pentosan polysulfate (SP 54): a study of the elimination of radioactivity following subcutaneous administration to the rat. Report No. 3484-218/5, Hazleton Laboratories Europe
- Hardi P, Nagy T, Fazekas G, et al. Sodium pentosan polysulfate reduced renal ischemia-reperfusion-induced oxidative stress and inflammatory responses in an experimental animal model. J Vasc Res. 2016;53(3–4):230–242. doi: 10.1159/000452246. [DOI] [PubMed] [Google Scholar]
- Hennermann JB, Gökce S, Solyom A, Mengel E, Schuchman EH, Simonaro CM. Treatment with pentosan polysulphate in patients with MPS I: results from an open label, randomized, monocentric phase II study. J Inherit Metab Dis. 2016;39(6):831–837. doi: 10.1016/j.bbacli.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Herrero LJ, Foo SS, Sheng KC, Chen W, Forwood MR, Bucala R, et al. Pentosan polysulfate: a novel glycosaminoglycan-like molecule for effective treatment of alphavirus-induced cartilage destruction and inflammatory disease. J Virol. 2015;89(15):8063–8076. doi: 10.1128/JVI.00224-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, Sasaki K, Minaki H, Masui K, Suzuki SO, et al. Proteaseresistant PrP and PrP oligomers in the brain in human prion diseases after intraventricular pentosan polysulfate infusion. Neuropathology. 2012;32(2):124–132. doi: 10.1111/j.1440-1789.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- Jones SA, Breen C, Heap F, et al. A phase 1/2 study of intrathecal heparan-N-sulfatase in patients with mucopolysaccharidosis IIIA. Mol Genet Metab. 2016;118(3):198–205. doi: 10.1016/j.ymgme.2016.05.006. [DOI] [PubMed] [Google Scholar]
- King B, Marshall N, Beard H, et al. Evaluation of enzyme dose and dose-frequency in ameliorating substrate accumulation in MPS IIIA huntaway dog brain. J Inherit Metab Dis. 2015;38(2):341–350. doi: 10.1007/s10545-014-9790-8. [DOI] [PubMed] [Google Scholar]
- King B, Marshall NR, Hassiotis S, et al. Slow, continuous enzyme replacement via spinal CSF in dogs with the paediatric-onset neurodegenerative disease, MPS IIIA. J Inherit Metab Dis. 2017;40(3):443–453. doi: 10.1007/s10545-016-9994-1. [DOI] [PubMed] [Google Scholar]
- Klein U, Kresse H, von Figura K. Sanfilippo syndrome type C: deficiency of acetyl-CoA: alpha-glucosaminide N-acetyltransferase in skin fibroblasts. Proc Natl Acad Sci U S A. 1978;75(10):5185–5189. doi: 10.1073/pnas.75.10.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresse H. Mucopolysaccharidosis 3A (Sanfilippo A disease): deficiency of a heparin sulfamidase in skin fibroblasts and leucocytes. Biochem Biophys Res Commun. 1973;54(3):1111–1118. doi: 10.1016/0006-291X(73)90807-3. [DOI] [PubMed] [Google Scholar]
- Kresse H, Paschke E, von Figura K, Gilberg W, Fuchs W. Sanfilippo disease type D: deficiency of N-acetylglucosamine-6-sulfate sulfatase required for heparan sulfate degradation. Proc Natl Acad Sci U S A. 1980;77(11):6822–6826. doi: 10.1073/pnas.77.11.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larramendy-Gozalo C, Barret A, Daudigeous E, et al. Comparison of CR36, a new heparin mimetic, and pentosan polysulfate in the treatment of therapies for human prion diseases. Am J Neurodegener Dis. 2013;2(3):176–186. doi: 10.1099/vir.0.82286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S, Bridges LR, Case CP. Neurofibrillary tangles in Niemann-Pick disease type C. Brain. 1995;118(Pt 1):119–129. doi: 10.1093/brain/118.1.119. [DOI] [PubMed] [Google Scholar]
- Martins C, Hůlková H, Dridi L, et al. Neuroinflammation, mitochondrial defects and neurodegeneration in mucopolysaccharidosis III type C mouse model. Brain. 2015;138(Pt 2):336–355. doi: 10.1093/brain/awu355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmi K, Kudo LC, Ryazantsev S, Zhao HZ, Karsten SL, Neufeld EF. Sanfilippo syndrome type B, a lysosomal storage disease, is also a tauopathy. Proc Natl Acad Sci U S A. 2009;106(20):8332–8337. doi: 10.1073/pnas.0903223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orii K, Tomatsu S, Suzuki Y, et al. Safety study of sodium pentosan polysulfate for adult patients with mucopolysaccharidosis type II. Mol Genet Metab. 2016;117(2):S88. doi: 10.1016/j.ymgme.2015.12.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczek D, Schwake M, Schröder J, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131(4):770–783. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Ryazantsev S, Yu WH, Zhao HZ, Neufeld EF, Ohmi K. Lysosomal accumulation of SCMAS (subunit c of mitochondrial ATP synthase) in neurons of the mouse model of mucopolysaccharidosis III B. Mol Genet Metab. 2007;90(4):393–401. doi: 10.1016/j.ymgme.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai-Yamashita Y, Kinugawa H, Niwa M. Neuroprotective effect of pentosan polysulphate on ischemia-related neuronal death of the hippocampus. Neurosci Lett. 2006;409(1):30–34. doi: 10.1016/j.neulet.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Sanden C, Mori M, Jogdand P, et al. Broad Th2 neutralization and anti-inflammatory action of pentosan polysulfate sodium in experimental allergic rhinitis. Immun Inflamm Dis. 2017;5(3):300–309. doi: 10.1002/iid3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchman EH, Ge Y, Lai A, et al. Pentosan polysulfate: a novel therapy for the mucopolysaccharidoses. PLoS One. 2013;8:e54459. doi: 10.1371/journal.pone.0054459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotsuki H, Yoshimi K, Shimo Y, et al. A rotarod test for evaluation of motor skill learning. J Neurosci Methods. 2010;189(2):180–185. doi: 10.1016/j.jneumeth.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Simonaro CM, Tomatsu S, Sikora T, et al. Pentosan polysulfate: oral versus subcutaneous injection in mucopolysaccharidosis type I dogs. PLoS One. 2016;11(4):e0153136. doi: 10.1371/journal.pone.0153136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M, Zérah M, Gougeon ML, et al. Intracerebral gene therapy in children with mucopolysaccharidosis type IIIB syndrome: an uncontrolled phase 1/2 clinical trial. Lancet Neurol. 2017;16(9):712–720. doi: 10.1016/S1474-4422(17)30169-2. [DOI] [PubMed] [Google Scholar]
- Terada T, Tsuboi Y, Obi T, et al. Less protease-resistant PrP in a patient with sporadic CJD treated with intraventricular pentosan polysulphate. Acta Neurol Scand. 2010;121(2):127–130. doi: 10.1111/j.1600-0404.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204(2):361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Figura K. Human alpha-n-acetylglucosaminidase. 2. Activity towards natural substrates and multiple recognition forms. Eur J Biochem. 1977;80(2):535–542. doi: 10.1111/j.1432-1033.1977.tb11909.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson FL, Holley RJ, Langford-Smith KJ, et al. Neuropathology in mouse models of mucopolysaccharidosis type I, IIIA and IIIB. PLoS One. 2012;7(4):e35787. doi: 10.1371/journal.pone.0035787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Guan TJ, Zheng S, et al. Inhibition of inflammation by pentosan polysulfate impedes the development and progression of severe diabetic nephropathy in aging C57B6 mice. Lab Investig. 2011;91(10):1459–1471. doi: 10.1038/labinvest.2011.93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Semi-quantitative assessment of storage vacuoles in the livers, spleens, and kidneys of MPS IIIA mice treated by subQ PPS. Scoring was performed by three independent laboratory technicians who were blinded to the treatment groups. The scoring system is described in the Materials and Methods. At least three slides were scored per tissue per mouse. *p = <0.001 compared treated to age-matched untreated mice (PPTX 34 kb)
Total GAG analysis of liver, spleen, and kidney extracts of MPS IIIA mice treated by subQ PPS. Total GAGs were determined by the Blyscan method using tissue extracts prepared from each mouse. n = 10 per group (PPTX 36 kb)