Abstract
Background
Pterostilbene has a proven chemopreventive effect for colon carcinogenesis but suffers low bioavailability limitations and therefore unable to reach the colonic tissue.
Objective and methodology
To overcome the issue of low bioavailability, pterostilbene was formulated into an oral colon targeted beads by ionic gelation method using pectin and zinc acetate. Optimization was carried out by 23 factorial design whereby the effect of pectin concentration (X1), zinc acetate concentration (X2) and pterostilbene:pectin ratio (X3) were studied on entrapment efficiency (Y1) and in vitro drug release till 24 h (Y2). The optimized beads were characterized for shape and size, swelling and surface morphology. The optimized beads were uniformly coated with Eudragit S-100 using fluidized bed coater. Optimized coated beads were characterized for in vitro drug release till 24 h and surface morphology. Pharmacokinetic and organ distribution study were performed in rats to ascertain the release of pterostilbene in colon.
Results
The optimized formulation comprised of 2% w/v of pectin concentration (X1), 2% w/v of zinc acetate concentration (X2) and 1:4 of pterostilbene:pectin ratio (X3), which showed a satisfactory entrapment efficiency (64.80%) and in vitro release (37.88%) till 24 h. The zinc pectinate beads exhibited sphericity, uniform size distribution, adequate swelling and rough surface. The optimized coated beads achieved 15% weight gain, displayed smooth surface and optimum drug release. Pterostilbene from optimized coated beads appeared in the plasma at 14 h and reached the Cmax at 22 h (Tmax), whereas plain pterostilbene exhibited Tmax of 3 h.
Discussion and conclusion
Thus, larger distribution of pterostilbene was obtained in the colonic tissue compared to stomach and small intestinal tissues. Thus, delayed Tmax and larger distribution of pterostilbene in colonic tissue confirmed the targeting of beads to colon.
Keywords: Colon targeted system, Eudragit S-100, 23 factorial design, Organ distribution, Pterostilbene, Zinc pectinate beads
1. Introduction
Colorectal cancer (CRC) is the third leading cancer diagnosed all over the world and constitutes 10% of all cancers majorly affecting the elderly aged more than 60 years (Lin et al., 2015, Patel, 2014, Ricchi et al., 2003). The progression of colorectal cancer is a long process involving a series of pre-malignant lesions (Bond, 1993, Das et al., 2007, Jacobson and Neugut, 1994, Karin Gwyn, 2002, Kelloff et al., 2004, Levine and Ahnen, 2012, Nasrallah et al., 2014, Pasi and Robert, 2000, Stewart et al., 2006). Embarking the treatment at pre-malignant stages is the best approach to deal with malignancy, since cancer treatments decrease the quality of life of patients attributed to the side effects and cost of treatment (Harun and Ghazali, 2012, Kroenke et al., 2010, Shukla and Pal, 2004). Chemoprevention not only retards, reverses or halts the process of cancer progression but also aid in enhancing the defensive mechanisms of the body to combat pre-malignant lesions (Das et al., 2007, Sporn, 1976, Umar et al., 2001). Chemoprevention can be achieved by adopting various strategies including physical activity, diet (folate, omega 3, vitamins, dietary fibers, calcium, etc.), fruits and vegetables (blueberries, broccoli, etc.) and drugs (NSAIDs) (Das et al., 2007, Sporn, 1976, Umar et al., 2001).
Nutraceuticals are class of compounds that aid in chemoprevention with less side effects (Ansari et al., 2013, Pan et al., 2011, Rajasekaran et al., 2008). Pterostilbene, a stilbenoid phytoalexin and a natural dimethoxy analogue of resveratrol has shown its therapeutic potential in wide range of diseases including neurological, cardiovascular, metabolic, cancer and hematologic disorders. Despite its role in chemoprevention, the beneficial effect of pterostilbene on colonic tissue is constrained due to its low oral bioavailability largely attributed to its low solubility and high metabolism (Chiou et al., 2011, Chiou et al., 2010, Estrela et al., 2013, McCormack and McFadden, 2013, McCormack and McFadden, 2012, Paul et al., 2010, Paul et al., 2009). When given as an oral suspension, the bioavailability was relatively low (F = 15.9% ± 7.8). However, in solution form pterostilbene showed bioavailability up to 59.2 ± 19.6% (Yeo et al., 2013). Sulphate and glucuronide conjugation are two major metabolic pathways for pterostilbene metabolism. When administered orally, pterostilbene is majorly metabolized by liver and enterocytes into pterostilbene-4′-sulfate. Another important metabolite includes pterostilbene-4′-glucuronide, which was only found in the liver. Regarding distribution, the amount of pterostilbene was remarkably higher in liver followed by kidney, lungs, brain, heart, muscles, testes and blood (Azzolini et al., 2014, Dellinger et al., 2013). Hence, it can be established that low solubility, high metabolism and higher distribution in different tissues remains the main stumbling block that prevents pterostilbene from reaching the colon. The present study is aimed at addressing these concerns by formulating an oral multiunit colon targeted drug delivery system of pterostilbene. The performance of this delivery system was assessed using its in vitro and in vivo release profile.
Colon targeted drug delivery system (CoDDS) has remarkably progressed in recent years. It has shown encouraging results in treating local complications such as Chron’s disease, Ulcerative colitis, Colorectal cancer (Amidon et al., 2015, Patel, 2014). The objective of CoDDS is to deliver the drugs safely to the colon by protecting it from release, absorption and enzymatic degradation in stomach and small intestine (Philip and Philip, 2010). CoDDS involves different approaches including (i) Conventional approach like pH dependent system, microbially triggered system (prodrug and polysaccharide) and time dependent approach and (ii) Novel approach which include pressure controlled system, osmotically controlled system and integration of pH sensitive and microbial triggered system (e.g. CODES™) (Krishnaiah and Khan, 2012, Philip and Philip, 2010). Multiunit drug delivery system (MDDS) refers to the usage of pellets, beads, granules, microsphere, spheroids, mini tabs, microparticles and nanoparticles. Multiunit system offers several advantages over single unit system that include easy passage through GIT, uniformly distribution throughout the GIT causing uniform absorption and enhanced bioavailability, prevention of dose dumping thus causes reduction in systemic toxicity and local irritation (Ali Asgar and Chandran, 2006, Roy and Shahiwala, 2009).
The current research deals with formulation, optimization and characterization of pterostilbene loaded matrix type biodegradable beads using pectin as biodegradable polymer and zinc acetate as crosslinking agent (microbially triggered system). The optimized beads were coated with Eudragit S-100 as a pH dependent polymer (pH dependent system). Further, oral pharmacokinetic and organ distribution studies were performed in rats to confirm the colonic release of pterostilbene from optimized coated beads.
2. Materials and methods
2.1. Materials
Pterostilbene was purchased from Nanjing Zelang Medical Technology Co. Ltd, Nanjing, China. Efavirenz was as a gift sample from Cipla, Mumbai, India. Pectin, amidated with low methoxylated grade (CF020) was a generous gift from Herbstreith and Fox, Werder, Germany. Eudragit S-100 was a gift sample from Evonik India Pvt Ltd. BL Pectinase was purchased from Biolaxi Corporation, Mumbai, India. Zinc acetate, isopropyl alcohol and triethyl citrate was obtained from S.D Fine Chemicals Ltd, Mumbai. All other materials used in the study were of analytical grade and were used as received.
2.2. Formulation of pterostilbene loaded biodegradable zinc pectinate beads
Zinc pectinate beads containing pterostilbene were prepared using ionotropic gelation technique (El-Gibaly, 2002, Gadalla et al., 2016, Kawadkar et al., 2010) with some modifications. The aqueous solution of pectin (2% w/v and 3% w/v) containing drug (50–100 mg) was prepared. The homogenous polymer drug dispersion (10 ml) was filled in a glass burette with a hypodermic needle of 18 gauge fixed at its tip. Polymer drug dispersion was released dropwise (1 ml/min) in 200 ml zinc acetate (1% w/v and 2% w/v) stirred at 500 rpm. The dropping height was maintained at 0.5 cm from the needle tip to the surface of crosslinking solution. The beads were left for curing in the crosslinking solution for 2 h with constant stirring at 500 rpm. After 2 h the beads were filtered, washed with distilled water, separated and dried at room temperature. For complete moisture removal, beads were dried in the hot air oven at 60 °C until no changes in weight was observed. Beads were collected and stored in a closed container at RT for further analysis.
2.3. Optimization of pterostilbene loaded biodegradable zinc pectinate beads
Formulation was optimized by 23 full factorial design using Design Expert Version 7.0.0 software (Stat-Ease, 2005). Three independent variables were varied at higher levels (+1) and lower levels (−1). The independent variables were pectin concentration (X1), zinc acetate concentration (X2) and pterostilbene to pectin ratio (X3) as shown in Table 1. The levels of independent variables were decided from preliminary trials. While, entrapment efficiency (Y1) and in vitro release in 24 h (Y2) were chosen as dependent variables.
Table 1.
Independent variables and their levels.
| Independent variables | Lower levels (−1) | Higher levels (+1) |
|---|---|---|
| a. Pectin concentration (% w/v) (X1) |
2 | 3 |
| b. Zinc acetate concentration (% w/v) (X2) |
1 | 2 |
| c. Pterostilbene:pectin ratio (X3) |
1:4 | 1:3 |
2.4. Characterization of pterostilbene loaded biodegradable zinc pectinate beads
2.4.1. Size and shape
Randomly selected 50 beads were measured for its length and breadth using optical microscopy and the mean particle size was calculated. Shape was calculated based on the elongation ratio (ER) as (Das et al., 2010):
Beads with ER = 1 is considered as perfectly spherical while ER > 1 indicates deviation from sphericity.
2.4.2. Entrapment efficiency
Crushed beads (5 mg) was added in 10 ml of methanol and sonicated for 10 min to ensure complete drug extraction. After centrifugation, 0.5 ml supernatant was removed and diluted to 10 ml with methanol-water (1:1). The content of pterostilbene was analysed using UV–visible spectrophotometry (Jasco V-530, India) at a λ max of 317 nm. Entrapment efficiency in percentage was calculated by the given formula (Das et al., 2011, Zhang et al., 2014):
2.4.3. Swelling
Swelling studies was performed in a water bath shaker at 37 ± 0.2 °C. Pre-weighed beads were placed in a phosphate buffer (pH 7.4) and allowed to swell. Beads were removed at various time intervals, blotted with filter paper to remove excess water and their change in weights were recorded until an equilibrium weight was achieved. The degree of swelling was calculated by:
where Wi is the initial weight of beads before swelling, Wf is the final weight of beads at equilibrium swelling.
2.4.4. In vitro drug release study
The drug release study was carried out using USP apparatus type II (TDT-08L, Electrolab, India) at 100 rpm. Drug release was measured in 900 ml enzyme free phosphate buffer (pH 7.4) maintained at 37 ± 0.2 °C mimicking the colonic pH. Beads equivalent to 10 mg of drug were added into the media. Aliquots (5 ml) were withdrawn and replaced with the same amount of fresh buffer at predetermined time interval until 24 h. The samples were analyzed using UV–visible spectrophotometry (Jasco V-530, India) at 317 nm (El-Gibaly, 2002).
2.5. Coating of pterostilbene loaded biodegradable zinc pectinate beads
The optimized batch of zinc pectinate beads were coated using Eudragit S-100, a colon targeted pH dependent polymer using mini fluidized bed coater (V.J instruments, India) (Kadam and Gattani, 2010) with some modifications. Eight percent (w/w) polymer solution was prepared in isopropyl alcohol (IPA) and acetone (7:3). The solution was plasticized with triethyl citrate (2.5% w/w of dry polymer) and talc (15% w/w of dry polymer) was added as anti-tacking agent. The desired level of coating was achieved with 15% of weight gain. Since organic coating was used, to prevent loses due to unwanted solvent evaporation 30% overages were prepared. The samples were removed after desired level of weight gain was achieved and stored in a suitable container for further analysis.
The amount of solvent used for coating is calculated as:
To achieve 15% (34.5 g) weight gain on 30 g of beads, 4.5 g of solid content was applied. Hence, if 9.4 g of solid content equals to 8 g of polymer, then 4.5 g of solid content equals to 3.82 g of polymer. Similarly, ratios of other ingredients were calculated (Table 2).
Table 2.
Preparation of coating solution.
| Ingredients | Quantity given | Quantity taken | Overages (30% of quantity taken) |
|---|---|---|---|
| Eudragit S-100 | 8 g | 3.82 g | 1.14 g |
| Triethyl citrate | 0.2 g | 0.09 g | 0.02 g |
| Talc | 1.2 g | 0.57 g | 0.17 g |
| IPA | 87.41 g | 41.84 g | 12.55 g |
| Acetone | 37.46 g | 17.93 g | 5.37 g |
2.6. In vitro drug release study of pterostilbene loaded coated zinc pectinate beads
The drug release study was carried out as described in Section 2.4.4 with the exception of the use of release media. Drug release was measured for 2 h in 0.1N HCl (pH 1.2) to simulate stomach environment. To mimic small intestinal condition, the drug release study was continued for the next 2 h in phosphate buffer (pH 6.8). Phosphate buffer of pH 7.4 was used with and without enzyme for another 20 h mimicking the colonic environment (Atyabi et al., 2005, Mura et al., 2003).
2.7. Morphological examination of pterostilbene loaded uncoated and coated zinc pectinate beads
Surface morphology of pterostilbene loaded uncoated (F4) and coated zinc pectinate beads containing pterostilbene was determined by Scanning Electron microscope (SEM). Sample was kept on an aluminum sample holder onto which double-sided carbon tape (Ted Pella Inc., California, US) was placed. The assembly was positioned in the SEM chamber, which was maintained at a constant pressure of 80 Pa. and operated in a low vacuum mode. Photographs of samples were taken to inspect their surface appearance and shape.
2.8. In vivo study
2.8.1. Bioanalytical method development of pterostilbene using HPLC
Bioanalytical method was developed and validated for rat plasma, stomach tissue, small intestinal tissue and colonic tissue. Briefly, 20 μL working standard of pterostilbene (drug) and efavirenz (IS) was spiked in 160 μL of blank plasma or tissues homogenate (stomach, small intestine, colon). To this mixture, 1 ml of cold acetonitrile was added, vortexed for 2 min and centrifuged. The supernatant was collected and evaporated to dryness. After evaporation, dry deposits was reconstituted using 200 μL of mobile phase, vortexed and injected (100 μL) into the HPLC system (Jasco PU-980, India). Sample analysis was done by reverse phase HPLC method using Inertsil ODS-3 column (4.6 mm i.d × 250 mm, 5 μm) at RT using Borwin software version 1.50. Mobile phase of acetonitrile and water (70:30) was used isocratically with a flow rate of 0.8 ml/min. The drug and internal standard was detected at 7.9 and 10.2 min, respectively, at a wavelength of 240 nm. Calibration curve (0.1–10 mg/ml) for both plasma and tissues homogenate was found to be linear (R2 = 0.999) with a recovery of >90%. The percentage RSD for repeatability and intermediate precision was 1.16% and 4.91%, respectively. The samples were stable for three-freeze thaw cycles.
2.8.2. Pharmacokinetic analysis
Male Wistar rats, weighing 250–300 g, were obtained from Bharat Serum and Vaccines Limited, Thane, India. All animals used in the experiments received care in compliance with the guidelines of The Committee for the Purpose of Control and Supervision of Experiments on Animals. The experimental protocol was approved by the Institutional Ethical Committee, Bombay College of Pharmacy, Mumbai, India (approval no. CPCSEA – BCP/2015 – 01/08).
The rats were randomly divided into two groups (n = 8) receiving plain pterostilbene suspended in 0.5% sodium CMC and optimized coated beads, respectively. Both the groups were further divided into two subgroups for blood withdrawals at alternate time points. The rats were fasted 12 h prior to dosing having free access to water. The dose of pterostilbene in both the groups was kept to 40 mg/kg p.o. Formulation was administered orally along with water with the help of infant feeding tube (No. 8) due to large particle size (1 mm). Blood (0.5 ml) was withdrawn from both the groups at 5 min, 15 min, 30 min, 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 25, 30 and 48 h through retro orbital plexus under anaesthesia. The blood was collected in the micro centrifuge tubes containing EDTA. Plasma samples (supernatant) were collected by centrifuging the blood samples at 5000 rpm for 10 min and stored at −20 °C until HPLC analysis.
The pharmacokinetic parameters such as Cmax, Tmax were obtained from plasma concentration versus time curve whereas AUC0–48h was calculated by linear trapezoidal rule and compared between plain drug and optimized coated beads.
2.8.3. Organ distribution study
Sixteen rats were used in this study to assess the distribution of pterostilbene in stomach, small intestine and colon after intragastric administration of plain pterostilbene and optimized coated beads. The rats were randomly divided into four groups (n = 4), each group was given a single dose of 40 mg/kg plain pterostilbene and optimized coated beads as described in Section 2.7.2. At 24 and 48 h after drug administration, animals were sacrificed and the entire GI tract was excised, mesenteric and fatty tissues were separated. The GI tract was segmented into stomach, small intestine, and colon. Organs were weighed, minced and homogenized, homogenate was centrifuged at 6000 rpm for 15 min at 4 °C, supernatants were collected and the drug content in the supernatant was determined by HPLC.
2.9. Statistical analysis
All the experimental data are presented as mean ± standard deviation (Mean ± SD). ANOVA (Design Expert version 7.0.0) was used to examine the significance in differences among results. The unpaired Student’s t-test was applied to the in vivo study. Differences at p < 0.05 were considered statistically significant.
3. Results and discussion
Colon targeted beads were formulated by coating Eudragit S-100 over zinc pectinate beads. On entering the colon the coating dissolves exposing pectin to the enzymes secreted by microbiota, thus, degrading pectin and releases the drug in the colon. Thus, the double dependent approach provide the successful delivery of drug to the colon with negligible release in the upper GIT. (Ahmad et al., 2012, Rahman et al., 2008).
3.1. Formulation of pterostilbene loaded biodegradable zinc pectinate beads
With regards to the selection of excipient, among various polysaccharides (Shah et al., 2011), pectin, a non-starch linear heteropolysaccharide obtained from the cell walls of plant holds a promising carrier for targeting drugs to colon. Pectin is a long chain of D-galacturonic acid residues with rhamnose forming a part of the polymer backbone while arabinose and galactose form a side chains (Kosaraju, 2005, Morris et al., 2010). Pectin is classified based on the esterification and amidation of carboxyl group into high methoxy (HM) or low methoxy (LM) pectin and amidated or non-amidated pectin, respectively (Morris et al., 2010, Sriamornsak, 2003). De-esterification of HM pectin using ammonia give rise to amidated LM pectin which is usually used in the CoDDS due to its advantage of forming more efficient gel at low calcium concentration than LM pectin alone (Chourasia and Jain, 2004, Shah et al., 2011, Sriamornsak, 1998). Apart from the physicochemical properties, pectin also holds pharmacological benefits that priorities its use in the treatment and prevention of colorectal cancer (Wong et al., 2011).
Report (Sinha and Kumria, 2001) affirms that pectin is practically soluble in aqueous medium; therefore, oral delivery of drug to the colon becomes a challenging task. Hence, the attention moved in developing strategies, which would make pectin least soluble in aqueous medium but can easily be degraded in colon. Das et al., 2010, Sriamornsak and Nunthanid, 1998 have depicted that the use of crosslinking agents like calcium and zinc aid in strengthening the pectin matrix making the polymer less soluble in aqueous medium. In this process, intermolecular cross-links develop between the negatively charged carboxyl groups of pectin and the positively charged divalent metal ions (Morris et al., 2010, Sriamornsak, 2003). It has been reported that zinc forms a stronger crosslink with pectin than calcium at the same concentration (El-Gibaly, 2002), thus, zinc pectinate was considered to be a favorable carrier for colonic delivery than calcium pectinate. The zinc pectinate system was further coated with a pH dependent polymer (e.g. Eudragit S-100) that would dissolve at colonic pH.
3.2. Optimization of pterostilbene loaded biodegradable zinc pectinate beads
Pectin concentration (X1), zinc acetate concentration (X2) and pterostilbene to pectin ratio (X3) were optimized for entrapment efficiency (Y1) and in vitro drug release (Y2) (Table 3).
Table 3.
Optimization of biodegradable zinc pectinate beads using 23 full factorial design.
| Formulation code | Factors |
Responses |
|||
|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | |
| Pectin concentration % (w/v) | Zinc acetate concentration % (w/v) | Pterostilbene:pectin ratio | EE (%) Mean ± SD (n = 3) |
In vitro drug release till 24 h (%) Mean ± SD (n = 3) |
|
| F1 | 2.00 | 1.00 | 1:4 | 71.37 ± 2.27 | 96.48 ± 4.86 |
| F2 | 3.00 | 1.00 | 1:4 | 47.31 ± 1.06 | 100.00 ± 1.21 |
| F3 | 3.00 | 2.00 | 1:4 | 56.42 ± 1.11 | 22.46 ± 3.86 |
| F4 | 2.00 | 2.00 | 1:4 | 64.80 ± 0.85 | 37.88 ± 2.78 |
| F5 | 2.00 | 2.00 | 1:3 | 71.58 ± 3.13 | 64.26 ± 1.05 |
| F6 | 2.50 | 1.50 | 1:3.5 | 77.13 ± 0.12 | 97.12 ± 0.89 |
| F7 | 2.50 | 1.50 | 1:3.5 | 80.01 ± 0.54 | 81.29 ± 0.55 |
| F8 | 3.00 | 2.00 | 1:3 | 68.70 ± 1.75 | 9.86 ± 1.75 |
| F9 | 2.50 | 1.50 | 1:3.5 | 75.97 ± 0.70 | 93.65 ± 2.66 |
| F10 | 2.00 | 1.00 | 1:3 | 45.47 ± 1.11 | 100.00 ± 4.58 |
| F11 | 3.00 | 1.00 | 1:3 | 70.63 ± 2.48 | 93.50 ± 3.87 |
| F12 | 2.50 | 1.50 | 1:3.5 | 72.74 ± 0.81 | 94.51 ± 2.99 |
The effect of individual independent variables was studied on each dependent variables. The results of individual dependent variables from 12 batches were subjected to multiple regression analysis with an appropriate model selection which gave a first order polynomial equation for each dependent variable, which is as follows:
| (1) |
where, Y – Dependent variables, b – Regression coefficients for first order polynomial, X – Independent variables.
In the equation, X1, X2 and X3 represents the effect on responses when each factors are changed individually from low to high level. The interaction terms (X1X2, X2X3, X1X3 and X1X2X3) demonstrate the effect on responses when all the factors are changed simultaneously. Models were evaluated by analysis of variance (ANOVA) which suggests a significant model and model terms when p < 0.05. Surface plot and Pareto chart were also plotted for both responses.
The selected model (3FI) for each dependent variables was found to be significant with a p value of 0.0293 and 0.0097 for Y1 and Y2, respectively. The regression coefficient for the selected model was 0.9682. The entrapment efficiency was significantly affected by the interaction of factors AC and ABC having a p value of 0.0076 and 0.0143 respectively, such that the interaction of AC (26.56%) affects more than ABC (16.96%). This can also be represented by Pareto chart (Fig. 1) where the contribution of each factors are shown sequentially. The model term significantly affecting (p < 0.05) the entrapment efficiency crosses the t value limit and the factors that are very close or beyond the bonferroni’s limits have highest impact on entrapment efficiency. The first order polynomial equation for entrapment efficiency is as follows:
| (2) |
Fig. 1.
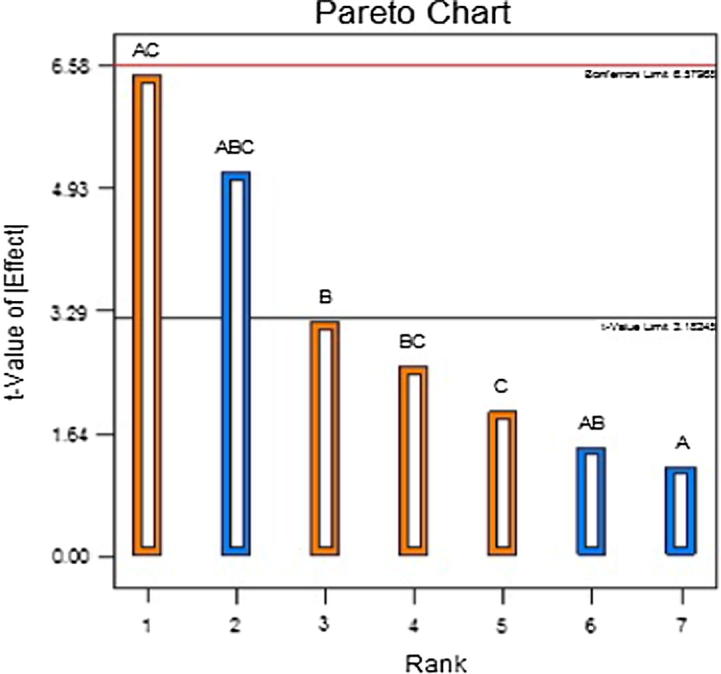
Pareto chart for entrapment efficiency.
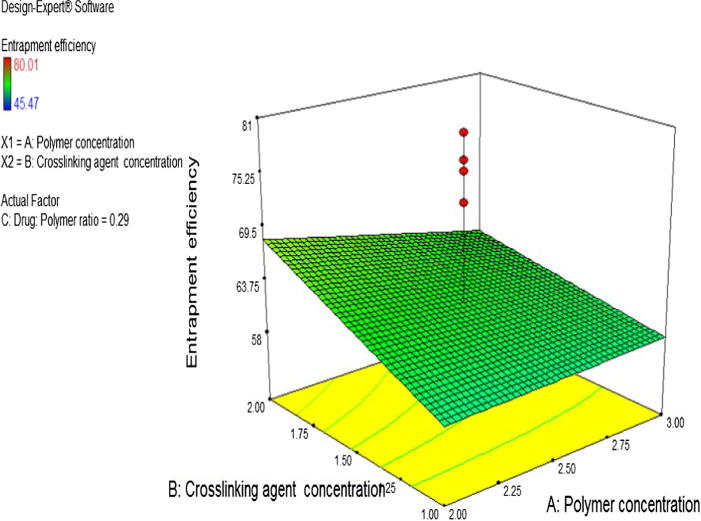
Surface plot for response Y1 were also developed as shown in Fig. 2.
Fig. 2.
Surface plot for entrapment efficiency.
For in vitro drug release, factor A (p < 0.0356), B (p < 0.0010) and interaction of AB (p < 0.0441) has significant effect such that factor B i.e. concentration of zinc acetate affects the highest having a contribution (Fig. 3) of 69.16% followed by factor A (5.61%) and interaction of AB (4.73%). The regression coefficient of the model was 0.9850. The first order polynomial equation for in vitro drug release is as follows:
| (3) |
Fig. 3.
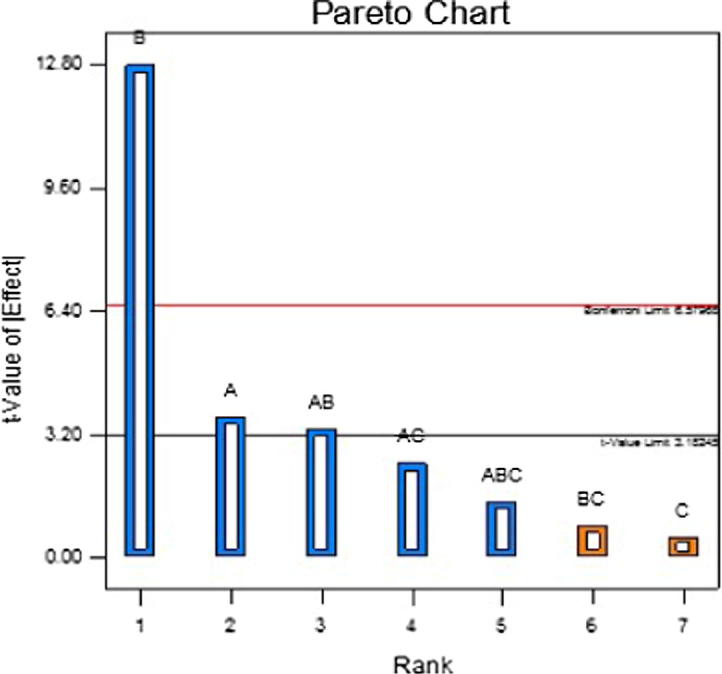
Pareto chart for in vitro drug release.
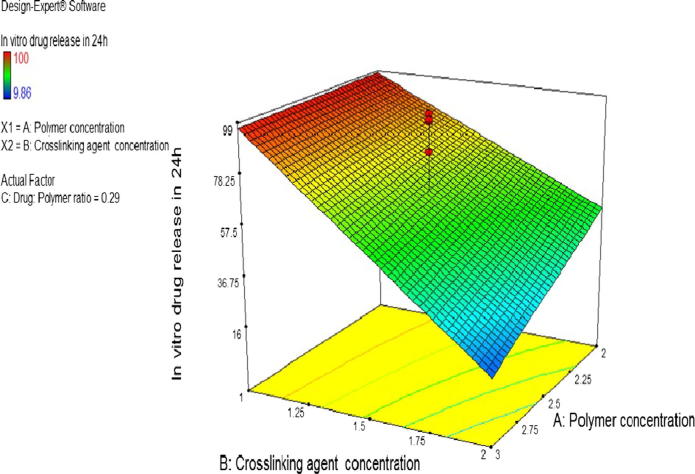
Surface plot of response Y2 is shown in Fig. 4.
Fig. 4.
Surface plot of in vitro drug release.
3.2.1. Effect of pectin concentration
The effect of pectin concentration was analyzed on EE (Y1) and in vitro drug release profile (Y2). It was observed that pectin does not have significant effect on the EE (p > 0.05). This is further shown in the Pareto chart (Fig. 1) where factor A is below the t value limit which indicates that the factor is not significant. This is in agreement with the study carried by Das et al. (2010) where the entrapment efficiency was said to be independent of pectin concentration such that higher or lower pectin concentrations causes neither increase nor decrease in the drug entrapment. With regards to the effect of pectin concentration on in vitro release, changes in pectin concentration causes a significant change in pterostilbene release profile (p < 0.05). From the first order polynomial equation (Eq. (3) of in vitro drug release the effectivity coefficient of X1 has a negative sign, thereby, indicating an inversely proportional effect on the corresponding response. It can be noted from Table 3 and Fig. 4 that drug release from biodegradable beads made with 3% w/v pectin concentration (F8) was lower than biodegradable beads prepared with 2% w/v pectin concentration (F5). This is mainly attributed to the formation of denser matrix which increases the drug retention behavior of the beads. Additionally, increase in pectin concentration causes increase in diffusional path length that the drug molecules have to transverse. This is in concordance with the previous reports by Das et al., 2010, Kawadkar et al., 2010.
3.2.2. Effect of zinc acetate concentration
The effect of zinc acetate concentration was analysed on the EE and in vitro drug release profile. It was observed that the p value for factor B is 0.0516 which is marginally away from 0.05, therefore, zinc acetate concentration can be considered to have a significant effect on the EE with a total contribution of 6.33%. This is shown in the Pareto chart (Fig. 1) where factor B is about to touch the t value limit that can be considered as an indication of significant factor. From the first order polynomial equation of EE the effectivity coefficient of X2 has a positive sign, thereby, indicating a direct proportional effect on the corresponding response. It is seen from Table 3 and Fig. 2 that the EE of biodegradable beads made with 2% w/v zinc acetate concentration (F5) was higher than that of biodegradable beads prepared with 1% w/v zinc acetate concentration (F10). This is in concordance with the reported study (Kawadkar et al., 2010) where the entrapment efficiency increases with increase in the crosslinking agent concentration. This may be explained by the formation of higher degree of crosslink between polymeric mesh networks that would aid in the formation of tightly bound matrix strongly trapping the drug and preventing its discharge from zinc pectinate biodegradable beads (Kawadkar et al., 2010).
With regards to the effect of zinc acetate concentration on in vitro release, changes in the zinc acetate concentration causes a significant change in pterostilbene release profile (p < 0.05). This can be clearly seen from the Pareto chart (Fig. 3) where factor B crosses the Bonferroni limit which indicates that factor B has highest impact on drug release profile compared to others. From the first order polynomial equation of in vitro drug release the effectivity coefficient of X2 has a negative sign, thereby, indicating a decreasing effect on the corresponding response. It is seen from Table 3 that release from biodegradable beads made with 2% w/v zinc acetate concentration (F5) was lower than biodegradable beads prepared using 1% w/v zinc acetate concentration (F10). This is in agreement with almost all the previous established reports (El-Gibaly, 2002, Gadalla et al., 2016, Kawadkar et al., 2010, Maestrelli et al., 2008) that attributed higher gel strength to be the reason for retarded drug release at higher zinc acetate concentrations. The increased amount of zinc ions leads to a greater degree of crosslinks between pectin in terms of number and strength as well an aggregation of pectin molecules leads to higher gel strength (El-Gibaly, 2002, Wakerly et al., 1997). Similar results were reported for indomethacin loaded calcium pectinate gel beads (Sriamornsak and Nunthanid, 1998).
3.2.3. Effect of pterostilbene:pectin ratio
The impact of pterostilbene:pectin was evaluated in the ratio of 1:3 and 1:4. It was observed that pectin does not show any significant effect on the EE (p > 0.05) as well as on drug release (p > 0.05). This is further shown in the Pareto chart (Figs. 1 and 3) where factor C is below the t value limit which is an indication of insignificant factor.
3.3. Characterization of pterostilbene loaded biodegradable zinc pectinate beads
The complete characteristics of uncoated zinc pectinate beads containing pterostilbene is given in Tables 3 and 4.
Table 4.
Characterization of zinc pectinate beads (Mean ± SD, n = 3).
| Formulation code | Shape (ER) | Size (mm) | Swelling (%) |
|---|---|---|---|
| F1 | 1.1 ± 0.02 | 0.96 ± 1.23 | 102.66 ± 1.52 |
| F2 | 0.9 ± 0.03 | 1.08 ± 2.35 | 97.95 ± 1.23 |
| F3 | 1 ± 0.02 | 1.04 ± 4.32 | 81.51 ± 1.40 |
| F4 | 1 ± 0.03 | 1.04 ± 2.89 | 144.00 ± 0.75 |
| F5 | 1 ± 0.04 | 0.99 ± 4.77 | 124.91 ± 2.30 |
| F6 | 1 ± 0.07 | 1.07 ± 1.58 | 140.19 ± 3.62 |
| F7 | 1 ± 0.05 | 1.06 ± 2.57 | 137.62 ± 2.09 |
| F8 | 1 ± 0.01 | 1.18 ± 0.96 | 138.94 ± 1.08 |
| F9 | 1 ± 0.03 | 1.04 ± 1.02 | 138.48 ± 2.51 |
| F10 | 1.1 ± 0.04 | 1.00 ± 3.87 | 88.41 ± 2.36 |
| F11 | 1 ± 0.09 | 1.11 ± 4.56 | 118.60 ± 0.96 |
| F12 | 1 ± 0.08 | 1.05 ± 0.99 | 14.21 ± 1.55 |
3.3.1. Shape and size
Shape of beads were measured in terms of ER. All the formulation batches exhibited good sphericity as indicated by their acceptable elongation ratio (Table 4). However, formulations with lowest pectin and zinc acetate concentration of 2% w/v and 1% w/v, respectively (F1 and F10), possessed higher elongation ratio values, presumably due to less denser matrix and lower degree of crosslinking that causes depression of their weak surface during drying (Maestrelli et al., 2008).
The size of beads from different batches (F1–F12) exhibited diameter ranging between 0.96 ± 1.23 and 1.18 ± 0.96 mm. The smaller size of 0.96 ± 1.23 mm was mainly due to low pectin and drug content (F1) compared to formulation (F8) where both pectin and drug concentration was high leading to large beads of size 1.18 ± 0.96 mm. Low pectin and drug content produced a less viscous solution and yielded smaller droplets, which in turn resulted in smaller beads (Jose et al., 2011). This is in agreement with other reports (Jose et al., 2011, Sriamornsak and Nunthanid, 1998) that propose similar observations.
3.3.2. Swelling
Swelling increases on increasing the polymer concentration and decreases on increasing the drug loading. On increasing the pectin concentration swelling increases from 124.91% ± 2.30 (F5) to 138.94% ± 1.08 (F8). This behavior is due to the presence of high amount of polymer in the matrix that imbibes more water and show high swelling compared to the matrix that contain low polymer concentration. Moreover, it was well observed in batch F1 and F10 wherein the drug loading was varied by keeping pectin and zinc acetate concentration constant. Lesser swelling of 88.41% ± 2.36 was noted in F10 due to increase drug loading compared to F1 (102.66% ± 1.52) wherein the drug loading was found to be lower. These observations and explanations are in accordance with the study carried by Das et al., 2010, Kawadkar et al., 2010.
3.4. Coating of pterostilbene loaded biodegradable zinc pectinate beads
Coating was achieved using Eudragit S-100, an anionic pH sensitive copolymers of methacrylic acid and methyl methacrylate that dissolves above pH 7. Coating was done on the optimized formula (F4) using mini fluidized bed coater. To coat the optimized zinc pectinate beads upto 15% of its weight gain, 8% of polymer was selected. While performing coating in fluidized bed coater, major problem that can arise is gun choking. To prevent this, a total dispersion of 7% with a nozzle diameter of 0.5 mm and an optimum spray rate of polymeric dispersion was used. The ability of Eudragit S-100 to form a film is appreciable compared to other polymers. Therefore, incorporating high amount of plasticizer can lead to film formation on gun which disallows the spraying of polymeric dispersion. In order to prevent this, the amount of plasticizer used was 2.5% w/w of dry polymer which was optimum to get a uniform coat without cracks. Sticking of beads usually occur during the process of organic coating. To overcome this problem, talc (15% w/w of dry polymer) is used as an anti-sticking agent. Use of talc more than an optimum level leads to dust formation after drying. Another important parameter is product temperature which beyond 32 °C and below 24 °C leads to spray drying of polymeric dispersion and sticking of beads, respectively. As a result, product temperature was kept in the range of 28–32 °C. The process offered a smooth and uniform coating by keeping the fluidization air and atomization pressure in the range of 0.40–0.60 bar. After complete layering of the polymeric dispersion, the beads were dried in the equipment for 10 min.
3.5. In vitro drug release study of pterostilbene loaded coated zinc pectinate beads
The in vitro release profile of coated beads is presented in Table 5. The release of pterostilbene was recorded in 0.1N HCl for 2 h, in phosphate buffer (pH 6.8) for another 2 h and finally in phosphate buffer (pH 7.4) with and without enzyme (BL Pectinase) till 24 h. The utility of enzyme allows to mimic the colonic environment since in the colon pectinolytic bacteria resides which cleaves the pectin bond leading to drug release in the colon. From the release profile it was observed that a coat (15% weight gain) of Eudragit S-100 was able to protect pterostilbene release in pH 1.2 and 6.8 thereby releasing in pH 7.4 wherein coating of Eudragit S-100 dissolves and the drug releases from the biodegradable beads. Unexpectedly, release studies carried out in the presence of enzyme did not showcase the expected improvement in pterostilbene release. In similarity with some reports (Liu et al., 2003, Sriamornsak, 1998, Vandamme et al., 2002), pectinolytic enzymes seemed not to be very effective in promoting the degradation of pectin beads. Moreover, some authors (Chambin et al., 2006, Semde et al., 2000) as well, did not find any significant effect of pectinolytic enzyme on pellets and beads made from pectin. Bourgeois et al., 2006 observed that only a small amount of divalent ions actually take part in pectinate matrix formation, while most of them remain in the formulation under free form. Therefore, it could be hypothesized that the free divalent ions present in beads, due to their ability to reduce enzymatic activities, could induce a decline in pectinolytic enzyme activity (Maestrelli et al., 2008). The other reason that may be ascribed is the optimum activity of pectinase enzyme is at pH 6, thus, providing a medium of pH 7.4 may hamper the enzymatic activity which could not show any significant effect on the release profile of zinc pectinate beads.
Table 5.
In vitro drug release profile of coated beads (Mean ± SD, n = 3).
| Time (h) | Coated beads (without enzyme) (%) | Coated beads (with enzyme) (%) |
|---|---|---|
| 0.08 | 1.17 ± 3.52 | 1.15 ± 4.21 |
| 0.5 | 1.75 ± 2.36 | 1.64 ± 6.70 |
| 1 | 1.40 ± 1.25 | 2.09 ± 8.95 |
| 2 | 1.49 ± 2.58 | 2.40 ± 6.21 |
| 4 | 10.10 ± 3.22 | 11.90 ± 7.85 |
| 6 | 24.44 ± 4.11 | 28.36 ± 15.24 |
| 8 | 55.11 ± 9.87 | 67.25 ± 9.54 |
| 10 | 91.97 ± 12.57 | 98.95 ± 7.65 |
| 12 | 94.80 ± 10.54 | 99.99 ± 2.11 |
| 24 | 99.09 ± 1.58 | 100.00 ± 0.87 |
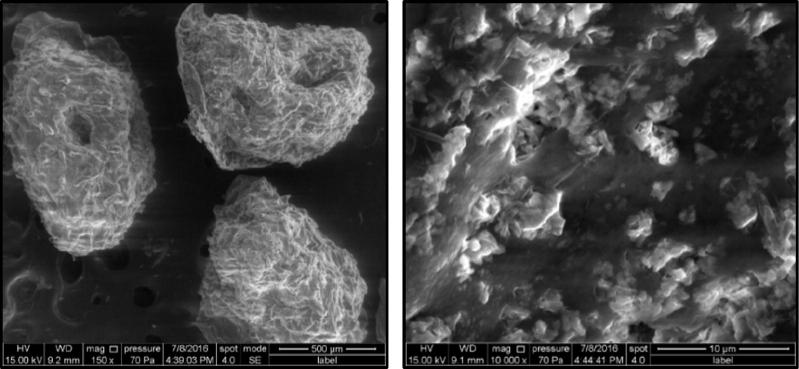
3.6. Morphological examination of pterostilbene loaded uncoated and coated zinc pectinate beads
Surface morphology of pterostilbene loaded uncoated (F4) and coated beads was observed by SEM and the images are shown in Fig. 5a and Fig. 5b. The SEM images of uncoated beads revealed that the beads show dense fibrous, crosslinked network structure. The surface of uncoated beads show rough and rugged surface which indicates that the drug crystals are embedded in the bead matrix. Slight depression and absence of ideal spherical morphology can probably be attributed to the drying process that causes certain invaginations in the beads, as is commonly reported (Kawadkar et al., 2010). On the other hand, surface morphology of coated beads showed a smooth surface that can be easily differentiated from the rough surface of uncoated beads. Coating of Eudragit S-100 on zinc pectinate beads was even and uniform. On higher magnification, the haziness of the coat can easily be identified.
Fig. 5a.
SEM images of uncoated beads (F4).
Fig. 5b.
SEM images of coated beads (coated F4).
3.7. In vivo study
3.7.1. Pharmacokinetic study
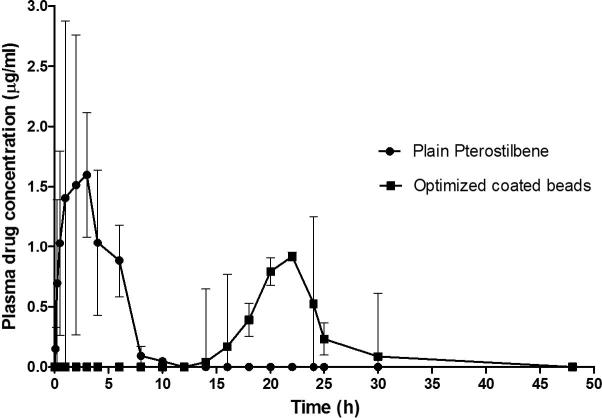
In vivo pharmacokinetic studies of plain pterostilbene and optimized coated beads were performed in rats. The pharmacokinetic parameters evaluated are listed in Table 6. Plasma drug concentrations following administration of the plain drug and optimized coated beads were plotted against time (Fig. 6). The study showed longer Tmax value for optimized coated beads than plain pterostilbene. However, Cmax and AUC0–48h values were higher in case of plain drug than optimized coated beads. In group 1, pterostilbene was detectable in plasma at 5 min (0.15 ± 0.17 μg/ml). Drug concentration was rapidly increased and reached to maximum concentration (1.59 ± 0.51 μg/ml) at 3 h, and then quickly decreased. Drug was not detectable in plasma at 12 h. In case of group 2, the drug was not detectable in plasma upto 12 h following administration of optimized coated beads. Thereafter, maximum concentration (0.91 ± 1.44 μg/ml) of pterostilbene was detected in plasma at 22 h, since enzymatic degradation of polysaccharides by colonic microflora is a slow process that requires several hours for completion (Rahman et al., 2008, Yang et al., 2002). Drug concentration in plasma gradually decreased with time such that the drug was not detectable in plasma at 48 h.
Table 6.
Pharmacokinetic parameters of plain pterostilbene and optimized coated beads (Mean ± SD, n = 4).
| Pharmacokinetic parameters | Group 1 (Plain pterostilbene) |
Group 2 (Optimized coated beads) |
|---|---|---|
| Tmax (h) | 3 | 22 |
| Cmax (µg/ml) | 1.59 ± 0.51 | 0.91 ± 1.44 |
| AUC0–48h (µg h ml−1) | 6.45 ± 1.16 | 4.05 ± 3.27 |
Fig. 6.
Plasma concentrations time profile of pterostilbene after a single oral dose of plain pterostilbene (Group 1) and optimized coated beads (Group 2) (p > 0.05).
Measurement of drug concentration in blood was performed to determine drug release in the GI tract following administration of the formulation. Many researchers used pharmacokinetic study to predict in vivo colon specific drug release (Bourgeois et al., 2008, Das et al., 2011, Krishnaiah et al., 2003, Munjeri et al., 1998, Musabayane et al., 2000, Wu et al., 2008, Zhao et al., 2008). It is well established that drug in any formulation needs about 5–6 h for its arrival to the colon (Das et al., 2011, Liu et al., 2003). Hence, plain pterostilbene cannot be considered as colon specific since the drug starts appearing in 5 min and the concentration was maximum at 3 h. On the other hand, no drug was detected in plasma till 12 h after administration of optimized coated beads. Absorption was delayed in case of optimized coated beads, indicated by its longer Tmax, indicating that the optimized coated beads prevented drug release in the upper part of the GI tract and releases it in the colon. The higher Cmax and AUC0–48 with plain pterostilbene is due to the absorption of maximum amount of drug from the upper part of GI tract, which has a large surface area available for absorption. The lower value of Cmax and AUC0–48 in case of optimized coated beads would account for the low systemic bioavailability. Thus, minimal amount of drug is available systemically for interaction with non-target site such that most of the drug resides in the colonic tissue for longer duration for its desired local action at colonic sites. This signifies that Eudragit S-100 coated zinc pectinate beads loaded with pterostilbene showed in vivo colon specific release.
3.7.2. Organ distribution study
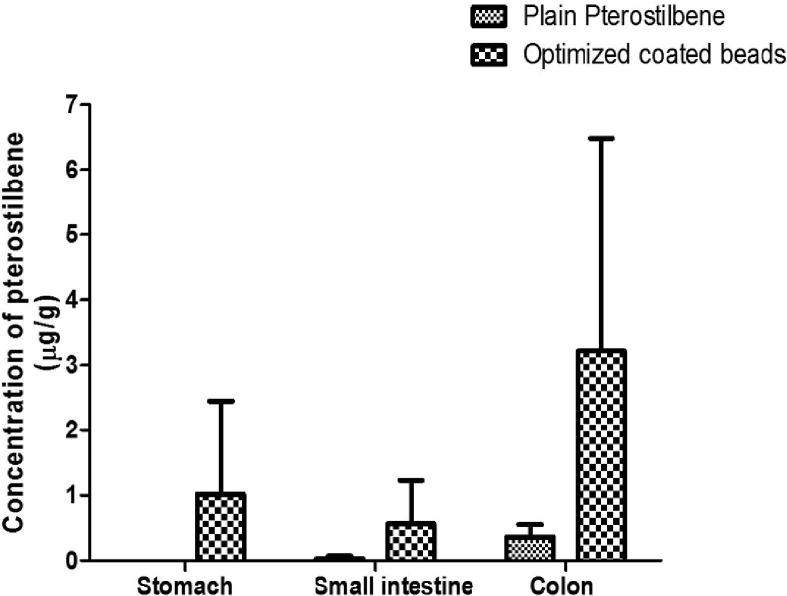
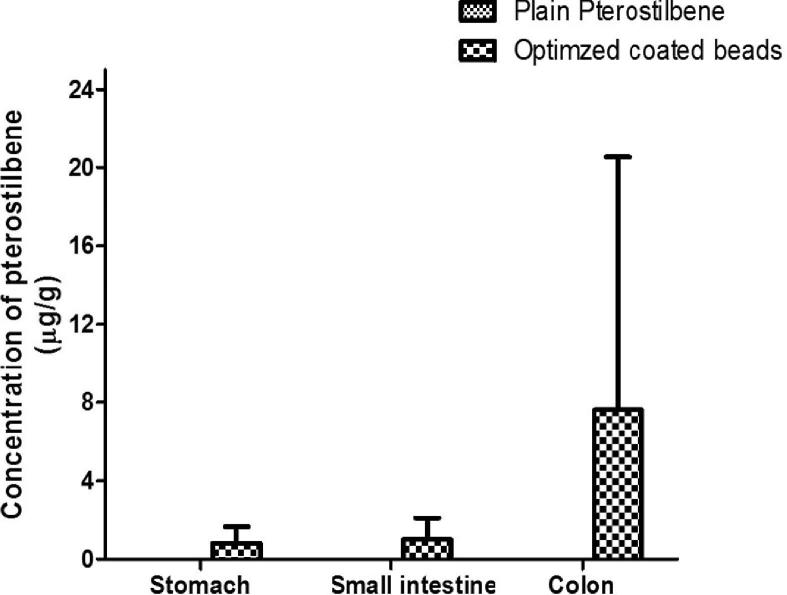
Organ distribution study was performed to further confirm the colon targeting potential of optimized coated beads by measuring the drug concentration in the stomach, small intestinal and colonic tissues. Rats were sacrificed at 24 h and 48 h after oral administration of plain drug and optimized coated beads. The amount of drug distributed in unit mass of stomach, small intestine and colonic tissue were measured at 24 h and 48 h. Pterostilbene was not detected in the stomach at 24 h when rats were administered with plain pterostilbene while the amount in small intestine and colonic tissues were 0.03 ± 0.04 μg/g and 0.36 ± 0.19 μg/g, respectively. The amount of pterostilbene in stomach, small intestine and colonic tissue from the optimized coated beads at 24 h include 1.02 ± 0.43 μg/g, 0.57 ± 0.66 μg/g and 3.22 ± 3.26 μg/g, respectively (Fig. 7a). Pterostilbene was undetectable throughout the GIT at 48 h when given as a plain drug whereas, on administration of optimized coated beads, highest level (7.61 ± 12.91 μg/g) was measured in colonic tissues at 48 h compared to stomach (0.83 ± 0.84 μg/g) and small intestinal tissue (1.01 ± 1.09 μg/g) (Fig. 7b). Thus, highest amount of pterostilbene was achieved in the colonic tissue with optimized coated beads at 24 and 48 h with minimum detection in stomach and small intestine compared to plain pterostilbene. Thus, the high amount of pterostilbene in the colonic tissue indicates that the optimized coated beads were intact during transit through the stomach and small intestine which causes minimal drug release in the upper part of the GIT compared to colon. It was evident that the amount of pterostilbene in colonic tissue was increased by 2 folds from 24 h to 48 h thus providing a greater scope for chemoprevention at the local site due to high tissue retention of pterostilbene.
Fig. 7a.
Distribution of pterostilbene in different tissues at 24 h post administration. Stomach (p < 0.05), Small Intestine (p > 0.05), Colon (p > 0.05).
Fig. 7b.
Distribution of pterostilbene in different tissues at 48 h post administration. Stomach (p > 0.05), Small Intestine (p > 0.05), Colon (p > 0.05).
4. Conclusion
Colorectal cancer is the third widespread cancer mostly affecting old age people. Chemoprevention with nutraceutical at pre-cancerous stage not only reduces the chances of cancer progression but also reduces the side effects associated with chemotherapy. Pterostilbene, an upcoming nutraceutical has an established chemopreventive effect but possesses a bioavailability problem limiting its reach to the colonic tissue. To overcome this issue, a multiunit colon targeted delivery system of pterostilbene was developed using a double dependent approach. Firstly, zinc pectinate beads loaded with pterostilbene were formulated using ionotropic gelation technique which was supposed to get degraded by the colonic bacteria. The formulation was optimized using 23 full factorial design. The optimized formulation was coated using mini fluidized bed coater with Eudragit S-100 a polymer that dissolves above pH 7. The coating on zinc pectinate beads was assessed by in vitro drug release. Pharmacokinetic and organ distribution study of optimized coated beads in rats confirmed the release of pterostilbene in colon. Therefore, this kind of formulation targeting the chemopreventive drug pterostilbene to the colon can be effective due to local action to delay or prevent development of colon cancer.
Acknowledgements
We acknowledge AICTE for providing a postgraduate contingency to carry out the research. We kindly acknowledge Herbstriet and Fox (Werder, Germany) for providing me a gift sample of pectin and Evonik for providing a gift sample of Eudragit S-100 (Evonik, India). In addition, major contribution of V.J instruments (Karanja, Maharashtra) for helping in providing fluidised bed coater with technical support and expert advice is worth mentioning. We would also like to acknowledge National Center for Nanosciences and Nanotechnology, University of Mumbai for SEM facilities. We warmly thank Dr. (Mrs.) Mala D Menon and Dr. (Mrs.) Ujwala Shinde for permitting me to use their facilities. Last but not the least, I am obliged to all my friends and seniors who gave me round the clock support for anything required that keep me moving on the path of research.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad M.Z., Akhter S., Anwar M., Ahmad F.J. Assam bora rice starch based biocompatible mucoadhesive microsphere for targeted delivery of 5-fluorouracil in colorectal cancer. Mol. Pharm. 2012;9:2986–2994. doi: 10.1021/mp300289y. [DOI] [PubMed] [Google Scholar]
- Ali Asgar L.F., Chandran S. Multiparticulate formulation approach to colon specific drug delivery: current perspectives. J. Pharm. Pharm. Sci. 2006;9:327–338. [PubMed] [Google Scholar]
- Amidon S., Brown J.E., Dave V.S. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech. 2015;16:731–741. doi: 10.1208/s12249-015-0350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari S., Chauhan B., Kalam N., Kumar G. Current concepts and prospects of herbal nutraceutical: a review. J. Adv. Pharm. Technol. Res. 2013;4:4–8. doi: 10.4103/2231-4040.107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atyabi F., Majzoob S., Iman M., Salehi M., Dorkoosh F. In vitro evaluation and modification of pectinate gel beads containing trimethyl chitosan, as a multi-particulate system for delivery of water-soluble macromolecules to colon. Carbohydr. Polym. 2005;61:39–51. [Google Scholar]
- Azzolini M., La Spina M., Mattarei A., Paradisi C., Zoratti M., Biasutto L. Pharmacokinetics and tissue distribution of pterostilbene in the rat. Mol. Nutr. Food Res. 2014;58:2122–2132. doi: 10.1002/mnfr.201400244. [DOI] [PubMed] [Google Scholar]
- Bond J.H. Polyp guideline: diagnosis, treatment, and surveillance for patients with nonfamilial colorectal polyps. The Practice Parameters Committee of the American College of Gastroenterology. Ann. Intern. Med. 1993;119:836–843. doi: 10.7326/0003-4819-119-8-199310150-00010. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Gernet M., Pradeau D., Andremont A., Fattal E. Evaluation of critical formulation parameters influencing the bioactivity of beta-lactamases entrapped in pectin beads. Int. J. Pharm. 2006;324:2–9. doi: 10.1016/j.ijpharm.2006.05.059. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Tsapis N., Honnas H., Andremont A., Shakweh M., Besnard M., Fattal E. Colonic delivery of β-lactamases does not affect amoxicillin pharmacokinetics in rats. J. Pharm. Sci. 2008;97:1853–1863. doi: 10.1002/jps.21115. [DOI] [PubMed] [Google Scholar]
- Chambin O., Dupuis G., Champion D., Voilley A., Pourcelot Y. Colon-specific drug delivery: influence of solution reticulation properties upon pectin beads performance. Int. J. Pharm. 2006;321:86–93. doi: 10.1016/j.ijpharm.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Chiou Y.S., Tsai M.L., Nagabhushanam K., Wang Y.J., Wu C.H., Ho C.T., Pan M.H. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J. Agric. Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103. [DOI] [PubMed] [Google Scholar]
- Chiou Y.S., Tsai M.L., Wang Y.J., Cheng A.C., Lai W.M., Badmaev V., Ho C.T., Pan M.H. Pterostilbene inhibits colorectal aberrant crypt foci (ACF) and colon carcinogenesis via suppression of multiple signal transduction pathways in azoxymethane-treated mice. J. Agric. Food Chem. 2010;58:8833–8841. doi: 10.1021/jf101571z. [DOI] [PubMed] [Google Scholar]
- Chourasia M.K., Jain S.K. Polysaccharides for colon targeted drug delivery. Drug Deliv. J. Deliv. Target. Ther. Agents. 2004;11:129–148. doi: 10.1080/10717540490280778. [DOI] [PubMed] [Google Scholar]
- Das D., Arber N., Jankowski J.A. Chemoprevention of colorectal cancer. Digestion. 2007;76:51–67. doi: 10.1159/000108394. [DOI] [PubMed] [Google Scholar]
- Das S., Chaudhury A., Ng K.Y. Preparation and evaluation of zinc-pectin-chitosan composite particles for drug delivery to the colon: role of chitosan in modifying in vitro and in vivo drug release. Int. J. Pharm. 2011;406:11–20. doi: 10.1016/j.ijpharm.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Das S., Ng K.-Y., Ho P.C. Formulation and optimization of zinc-pectinate beads for the controlled delivery of resveratrol. AAPS PharmSciTech. 2010;11:729–742. doi: 10.1208/s12249-010-9435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger R.W., Garcia A.M.G., Meyskens F.L., Jr. Differences in the glucuronidation of resveratrol and pterostilbene; altered enzyme specificity and potential gender differences. Drug Metab. Pharmacokinet. 2013;29:112–119. doi: 10.2133/dmpk.dmpk-13-rg-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gibaly I. Oral delayed-release system based on Zn-pectinate gel (ZPG) microparticles as an alternative carrier to calcium pectinate beads for colonic drug delivery. Int. J. Pharm. 2002;232:199–211. doi: 10.1016/s0378-5173(01)00903-6. [DOI] [PubMed] [Google Scholar]
- Estrela J.M., Ortega A., Mena S., Rodriguez M.L., Asensi M. Pterostilbene: biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013;50:65–78. doi: 10.3109/10408363.2013.805182. [DOI] [PubMed] [Google Scholar]
- Gadalla H.H., Soliman G.M., Mohammed F.A., El-sayed A.M. Development and in vitro/in vivo evaluation of Zn-pectinate microparticles reinforced with chitosan for the colonic delivery of progesterone. Drug Deliv. 2016;23:2541–2554. doi: 10.3109/10717544.2015.1028602. [DOI] [PubMed] [Google Scholar]
- Harun Z., Ghazali A.R. Potential chemoprevention activity of pterostilbene by enhancing the detoxifying enzymes in the HT-29 cell line. Asian Pac. J. Cancer Prev. 2012;13:6403–6407. doi: 10.7314/apjcp.2012.13.12.6403. [DOI] [PubMed] [Google Scholar]
- Jacobson J.S., Neugut A.I. Interpreting preeursor studies: what polyp trials tell us about large-bowel cancer. J. Natl Cancer Inst. 1994;86:1648–1649. doi: 10.1093/jnci/86.21.1648. [DOI] [PubMed] [Google Scholar]
- Jose S., Prema M.T., Chacko A.J., Thomas A.C., Souto E.B. Colon specific chitosan microspheres for chronotherapy of chronic stable angina. Colloids Surf. B Biointerf. 2011;83:277–283. doi: 10.1016/j.colsurfb.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Kadam V.D., Gattani S.G. Development of colon targeted multiparticulate pulsatile drug delivery system for treating nocturnal asthma. Drug Deliv. 2010;17:343–351. doi: 10.3109/10717541003762821. [DOI] [PubMed] [Google Scholar]
- Karin Gwyn F.A.S. Chemoprevention of colorectal cancer. Am. J. Gastroenterol. 2002;97:13–21. doi: 10.1111/j.1572-0241.2002.05435.x. [DOI] [PubMed] [Google Scholar]
- Kawadkar J., Chauhan M.K., Ram A. Evaluation of potential of Zn-pectinate gel (ZPG) microparticles containing mesalazine for colonic drug delivery. DARU. 2010;18:211–220. [PMC free article] [PubMed] [Google Scholar]
- Kelloff G.J., Schilsky R.L., Alberts D.S., Day R.W., Guyton K.Z., Pearce H.L., Peck J.C., Phillips R., Sigman C.C. Colorectal adenomas: a prototype for the use of surrogate end points in the development of cancer prevention drugs colorectal adenomas: a prototype for the use of surrogate end points in the development of cancer prevention drugs. Clin. Cancer Res. 2004;10:3908–3918. doi: 10.1158/1078-0432.CCR-03-0789. [DOI] [PubMed] [Google Scholar]
- Kosaraju S.L. Colon targeted delivery systems: review of polysaccharides for encapsulation and delivery. Crit. Rev. Food Sci. Nutr. 2005;45:251–258. doi: 10.1080/10408690490478091. [DOI] [PubMed] [Google Scholar]
- Krishnaiah Y.S.R., Khan M.A. Strategies of targeting oral drug delivery systems to the colon and their potential use for the treatment of colorectal cancer. Pharm. Dev. Technol. 2012;17:521–540. doi: 10.3109/10837450.2012.696268. [DOI] [PubMed] [Google Scholar]
- Krishnaiah Y.S.R., Satyanarayana V., Kumar B.D., Karthikeyan R.S., Bhaskar P. In vivo pharmacokinetics in human volunteers: Oral administered guar gum-based colon-targeted 5-fluorouracil tablets. Eur. J. Pharm. Sci. 2003;19:355–362. doi: 10.1016/s0928-0987(03)00139-8. [DOI] [PubMed] [Google Scholar]
- Kroenke Kurt, Theobald Dale, Wu Jingwei, Loza Julie K., Carpenter Janet S., Tu W. The association of depression and pain with health-related quality of life, disability, and health care use in cancer patients. J. Pain Symptom Manage. 2010;40:327–341. doi: 10.1016/j.jpainsymman.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J.S., Ahnen D.J. Adenomatous polyps of the colon. NEJM N. Engl. J. Med. 2012;6:2551–2557. doi: 10.1056/NEJMcp063038. [DOI] [PubMed] [Google Scholar]
- Lin C., Ng H., Pan W., Chen H., Zhang G., Bian Z., Lu A., Yang Z. Exploring different strategies for efficient delivery of colorectal cancer therapy. Int. J. Mol. Sci. 2015;16:26936–26952. doi: 10.3390/ijms161125995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Fishman M.L., Kost J., Hicks K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials. 2003;24:3333–3343. doi: 10.1016/s0142-9612(03)00213-8. [DOI] [PubMed] [Google Scholar]
- Maestrelli F., Cirri M., Corti G., Mennini N., Mura P. Development of enteric-coated calcium pectinate microspheres intended for colonic drug delivery. Eur. J. Pharm. Biopharm. 2008;69:508–518. doi: 10.1016/j.ejpb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- McCormack D., McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxidative Med. Cell. Longev. 2013;2013:1–15. doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack D., McFadden D. Pterostilbene and cancer: current review. J. Surg. Res. 2012;173:e53–e61. doi: 10.1016/j.jss.2011.09.054. [DOI] [PubMed] [Google Scholar]
- Morris G., Kök S., Harding S., Adams G. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol. Genet. Eng. Rev. 2010;27:257–284. doi: 10.1080/02648725.2010.10648153. [DOI] [PubMed] [Google Scholar]
- Munjeri O., Hodza P., Osim E.E., Musabayane C.T. An investigation into the suitability of amidated pectin hydrogel beads as a delivery matrix for chloroquine. J. Pharm. Sci. 1998;87:905–908. doi: 10.1021/js9801283. [DOI] [PubMed] [Google Scholar]
- Mura P., Maestrelli F., Cirri M., González Rodríguez M.L., Rabasco Alvarez A.M. Development of enteric-coated pectin-based matrix tablets for colonic delivery of theophylline. J. Drug Target. 2003;11:365–371. doi: 10.1080/10611860310001639130. [DOI] [PubMed] [Google Scholar]
- Musabayane C.T., Munjeri O., Bwititi P., Osim E.E. Orally administered, insulin-loaded amidated pectin hydrogel beads sustain plasma concentrations of insulin in streptozotocin-diabetic rats. J. Endocrinol. 2000;164:1–6. doi: 10.1677/joe.0.1640001. [DOI] [PubMed] [Google Scholar]
- Nasrallah A., Saykali B., Al Dimassi S., Khoury N., Hanna S., El-Sibai M. Effect of StarD13 on colorectal cancer proliferation, motility and invasion. Oncol. Rep. 2014;31:505–515. doi: 10.3892/or.2013.2861. [DOI] [PubMed] [Google Scholar]
- Pan M.H., Lai C.S., Wu J.C., Ho C.T. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol. Nutr. Food Res. 2011;55:32–45. doi: 10.1002/mnfr.201000412. [DOI] [PubMed] [Google Scholar]
- Pasi J.A., Robert M.J. Chemoprevention of colon cancer. N. Engl. J. Med. 2000;342:1960–1968. doi: 10.1056/NEJM200006293422606. [DOI] [PubMed] [Google Scholar]
- Patel M.M. Getting into the colon: approaches to target colorectal cancer. Expert Opin. Drug Deliv. 2014;11:1343–1350. doi: 10.1517/17425247.2014.927440. [DOI] [PubMed] [Google Scholar]
- Paul S., de Castro A.J., Lee H.J., Smolarek A.K., So J.Y., Simi B., Wang C.X., Zhou R., Rimando A.M., Suh N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the beta-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis. 2010;31:1272–1278. doi: 10.1093/carcin/bgq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Rimando A.M., Lee H.J., Ji Y., Reddy B.S., Suh N. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev. Res. 2009;2:650–657. doi: 10.1158/1940-6207.CAPR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip A.K., Philip B. Colon targeted drug delivery systems: a review on primary and novel approaches. Oman Med J. 2010;25:79–87. doi: 10.5001/omj.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman Z., Kohli K., Zhang S.-Q., Khar R.K., Ali M., Charoo N.A., Tauseef M., Shamsher A.A.A., Mohammed N.N., Repka M.A. In-vivo evaluation in rats of colon-specific microspheres containing 5-fluorouracil. J. Pharm. Pharmacol. 2008;60:615–623. doi: 10.1211/jpp.60.5.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran A., Sivagnanam G., Xavier R. Nutraceuticals as therapeutic agents: a review. Res. J. Pharm. Technol. 2008;1:328–340. [Google Scholar]
- Ricchi P., Zarrilli R., Di Palma A., Acquaviva A.M. Nonsteroidal anti-inflammatory drugs in colorectal cancer: from prevention to therapy. Br. J. Cancer. 2003;88:803–807. doi: 10.1038/sj.bjc.6600829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Shahiwala A. Multiparticulate formulation approach to pulsatile drug delivery: current perspectives. J. Control. Release. 2009;134:74–80. doi: 10.1016/j.jconrel.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Semde R., Amighi K., Devleeschouwer M.J., Moriss A. Effect of pectinolytic enzymes on the theophylline release from pellets coated with water insoluble polymers containing pectin HM or calcium pectinate. Int. J. Pharm. 2000;197:169–179. doi: 10.1016/s0378-5173(99)00465-2. [DOI] [PubMed] [Google Scholar]
- Shah N., Shah T., Amin A. Polysaccharides: a targeting strategy for colonic drug delivery. Expert Opin. Drug Deliv. 2011;8:779–796. doi: 10.1517/17425247.2011.574121. [DOI] [PubMed] [Google Scholar]
- Shukla Y., Pal S.K. Dietary cancer chemoprevention: an overview. Int. J. Hum. Genet. 2004;4:265–276. [Google Scholar]
- Sinha V.R., Kumria R. Polysaccharides in colon-specific drug delivery. Int. J. Pham. 2001;224:19–38. doi: 10.1016/s0378-5173(01)00720-7. [DOI] [PubMed] [Google Scholar]
- Sporn M.B. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976;36:2699–2702. [PubMed] [Google Scholar]
- Sriamornsak P. Chemistry of pectin and its pharmaceutical uses: a review. Silpakorn Univ. Int. J. 2003;3:206–228. [Google Scholar]
- Sriamornsak P. Investigation of pectin as a carrier for oral delivery of proteins using calcium pectinate gel beads. Int. J. Pharm. 1998;169:213–220. [Google Scholar]
- Sriamornsak P., Nunthanid J. Calcium pectinate gel beads for controlled release drug delivery: I. Preparation and in vitro release studies 1. Int. J. Pham. 1998;160:207–212. [Google Scholar]
- Stewart S.L., Wike J.M., Kato I., Lewis D.R., Michaud F. A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer. 2006;107:1128–1141. doi: 10.1002/cncr.22010. [DOI] [PubMed] [Google Scholar]
- Umar A., Viner J.L., Hawk E.T. The future of colon cancer prevention. Ann. N. Y. Acad. Sci. 2001;952:88–108. doi: 10.1111/j.1749-6632.2001.tb02730.x. [DOI] [PubMed] [Google Scholar]
- Vandamme T.F., Lenourry A., Charrueau C., Chaumeil J.C. The use of polysaccharides to target drugs to the colon. Carbohydr. Polym. 2002;48:219–231. [Google Scholar]
- Wakerly Z., Fell J., Attwood D., Parkins D. Studies on amidated pectins as potential carriers in colonic drug delivery. J. Pharm. Pharmacol. 1997;49:622–625. doi: 10.1111/j.2042-7158.1997.tb06856.x. [DOI] [PubMed] [Google Scholar]
- Wong T.W., Colombo G., Sonvico F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech. 2011;12:201–214. doi: 10.1208/s12249-010-9564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Deng D., Lu Y., Wu W. Biphasic release of indomethacin from HPMC/pectin/calcium matrix tablet: II. Influencing variables, stability and pharmacokinetics in dogs. Eur. J. Pharm. Biopharm. 2008;69:294–302. doi: 10.1016/j.ejpb.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Yang L., Chu J.S., Fix J.A. Colon-specific drug delivery: new approaches and in vitro/in vivo evaluation. Int. J. Pharm. 2002;235:1–15. doi: 10.1016/s0378-5173(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Yeo S.C.M., Ho P.C., Lin H.S. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: the impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol. Nutr. Food Res. 2013;57:1015–1025. doi: 10.1002/mnfr.201200651. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shi X.X., Yu Y.F., Zhao S.C., Song H.W., Chen A.B., Shang Z.H. Preparation and characterization of vanillin cross-linked chitosan microspheres of pterostilbene. Int. J. Polym. Anal. Charact. 2014;19:83–93. [Google Scholar]
- Zhao X.-L., Li K.-X., Zhao X.-F., Pang D.-H., Chen D.-W. Study on colon-specific 5-Fu pH-enzyme Di-dependent chitosan microspheres. Chem. Pharm. Bull. (Tokyo) 2008;56:963–968. doi: 10.1248/cpb.56.963. [DOI] [PubMed] [Google Scholar]