Abstract
Objective
In obese patients undergoing caloric restriction, there are several potential mechanisms involved in the improvement of metabolic outcomes. The present study further explores whether caloric restriction can modulate endoplasmic reticulum (ER) stress and mitochondrial function, as both are known to be mechanisms underlying inflammation and insulin resistance (IR) during obesity.
Methods
A total of 64 obese patients with BMI ≥35 kg/m2 underwent a dietary program consisting of 6 weeks of a very-low-calorie diet followed by 18 weeks of low-calorie diet. We evaluated changes in the metabolic and inflammatory markers -TNFα, hsCRP, complement component 3 (C3c), and retinol binding protein 4 (RBP4)-, in the ER stress markers and modulators -eIF2α-P, sXBP1, ATF6, JNK-P, CHOP, GRP78, and SIRT1-, and in mitochondrial function parameters -mitochondrial reactive oxygen species (mROS), glutathione peroxidase 1 (GPX1), cytosolic Ca2+, and mitochondrial membrane potential.
Results
The dietary intervention produced an 8.85% weight loss associated with enhanced insulin sensitivity, a less marked atherogenic lipid profile, and a decrease in systemic inflammation (TNFα, hsCRP) and adipokine levels (RBP4 and C3c). Chronic ER stress was significantly reduced (ATF6-CHOP, JNK-P) and expression levels of SIRT1 and GRP78 – a Ca2+-dependent chaperone – were increased and accompanied by the restoration of Ca2+ depots. Furthermore, mROS production and mitochondrial membrane potential improvement were associated with the up-regulation of the antioxidant enzyme GPX1.
Conclusions
Our data provide evidence that moderate weight loss attenuates systemic inflammation and IR and promotes the amelioration of ER stress and mitochondrial dysfunction, increasing the expression of chaperones, SIRT1 and antioxidant GPX1.
Keywords: Diet, Inflammation, Endoplasmic reticulum, Oxidative stress, Mitochondria
Abbreviations: ATF6, activating transcription factor 6; BMI, body mass index; CHOP, CCAAT/enhancer binding protein [C/EBP] homologous protein; C3c, complement component 3; eIF2α-P, phosphorylated eukaryotic translation initiation factor 2 subunit 1; ER, endoplasmic reticulum; GPX1, glutathione peroxidase 1; GRP78, 78-kDa glucose regulated protein; IR, insulin resistance; IRE1α, inositol requiring enzyme 1 α; JNK, cJun NH2-terminal kinase; LCD, low-calorie diet; PERK, protein kinase RNA-like endoplasmic reticulum kinase; ROS, reactive oxygen species; sXBP1, spliced X-box binding protein 1; SIRT1, Sirtuin 1; T2D, type 2 diabetes; UPR, unfolded protein response; VLCD, very-low-calorie diet
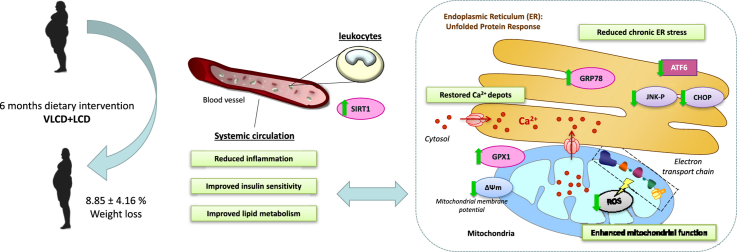
Graphical abstract
Highlights
-
•
Caloric restriction is prescribed to obese patients in order to improve their metabolic outcomes.
-
•
Chronic ER stress was significantly reduced and Ca2+ depots restored after diet.
-
•
Mitochondrial ROS decreased in parallel with the upregulation of antioxidant enzyme GPX1.
-
•
Adaptive GRP78 and SIRT1 responses were enhanced after intervention.
1. Introduction
Obesity is a multifactorial disease associated with the appearance of several comorbidities, such as dyslipidemia, hypertension, and type 2 diabetes (T2D), the prevalence of which has risen significantly in the past decades in parallel with the rise in the obesity rate worldwide [1]. Metabolic overload and increase in fat accumulation during obesity favors the release of several adipokines and cytokines, contributing to systemic chronic low-grade inflammation, which is closely related to the development of insulin resistance (IR) and other metabolic abnormalities [2]. Despite the emerging body of evidence supporting the role of inflammatory and stress responses in the context of obesity, the molecular pathways and mechanisms underlying these processes remain unclear.
It is known that the endoplasmic reticulum (ER) acts as a systemic nutrient sensor in peripheral tissues during obesity, in which elevated circulating levels of free fatty acids, glucose, or inflammatory cytokines may act as stress signals for the organelle [3]. The accumulation of misfolded proteins during ER stress triggers the activation of the unfolded protein response (UPR) through three different leaders: the inositol requiring enzyme 1 α (IRE1α), activating transcription factor 6 (ATF6), and protein kinase RNA-like endoplasmic reticulum kinase (PERK). However, the failure of the adaptive response and the chronicity of the stress lead the UPR to generate the expression of proapoptotic factors such as CCAAT/enhancer binding protein [C/EBP] homologous protein (CHOP). Previous findings have described the role of CHOP in the cytokine-ER-stress-mediated apoptosis of pancreatic β-cells [4]. On the other hand, IRE1α kinase activity has been associated with IR through the cJun NH2-terminal kinase (JNK) inflammatory pathway, partly as a result of serine phosphorylation of insulin receptor substrates (IRS1) [5]. In addition, JNK activation in macrophages has been related to increased tissue infiltration [6] and is known to play a key role in chronic inflammation in obesity [7]. In contrast, it has been reported that chemical chaperones that reduce ER stress improve insulin sensitivity in ob/ob mice [8] and β-cell function in humans [9], and we have recently described an improvement in ER stress and inflammatory markers in subcutaneous adipose tissue that was mediated by an insulin sensitizer [10]. This accumulated evidence of the adaptive capacity of ER supports a role for ER stress in human metabolic disease and points to potential novel therapeutic targets for the treatment of obesity and related disorders. However, how ER stress is modulated in vivo is a question yet to be answered.
Sirtuin 1 (SIRT1), a NAD+-dependent protein deacetylase, is an important regulator of energy homeostasis in response to nutrient availability; its expression is down-regulated in adipose tissue [11] and peripheral blood mononuclear cells in obese populations and has been related with IR and metabolic syndrome [12]. Accumulating evidence shows that SIRT1 helps to regulate inflammatory [13] and ER stress responses in obesity, since both endogenous induction and overexpression of SIRT1 exert a protective role by alleviating ER stress and inflammatory markers in the liver [14], [15] and adipose tissue [10], [16].
Furthermore, an excess of energy substrates in obesity is believed to lead to increased mitochondrial dysfunction and reactive oxygen species (ROS) signaling, which may underlie IR [17], [18], metabolic syndrome [19] and impaired endothelium function [20]. In fact, enhanced oxidative stress is reported to be increased in leukocytes and adipose tissue from obese patients and has been correlated with body mass index (BMI) [20], [21].
Caloric restriction displays several metabolic benefits in the obese population, improving insulin signaling and reducing cardiovascular risk [22]. The molecular mechanisms implicated in these effects could be targeted to decelerate the progressive deterioration in the health of obese subjects, but, unfortunately, they are poorly understood. Since nutrient overload has been related to ER stress and mitochondrial dysfunction [23], the aim of the present study was to explore whether caloric restriction modulates UPR pathways during ER stress and improves redox status and mitochondrial function in human obesity, and to determine the role of inflammatory mediators such as SIRT1 and JNK.
2. Materials and methods
2.1. Subjects
Patients attending the Endocrinology and Nutrition Department at the University Hospital Dr. Peset (Valencia, Spain) were consecutively recruited as they were referred for treatment for their obesity.
Eligible participants were obese patients between 18 and 60 years of age that had maintained a stable weight (±2 kg) over the 3 months prior to the study and whose disease duration was at least five years. The inclusion criteria were BMI ≥35 kg/m2, with or without associated comorbidities, including T2D diagnosed according to the American Diabetes Association Guidelines [24]. Exclusion criteria were pregnancy or lactation, severe disease, history of cardiovascular disease or chronic inflammatory disease and secondary obesity (hypothyroidism, Cushing's syndrome).
The study protocol was approved by the Ethics Committee of the Hospital (Code: 96/16) and was conducted according to the guidelines laid down in the Declaration of Helsinki. The dietary weight loss intervention was designed in accordance with the guidelines of the Spanish Society for the Study of Obesity (SEEDO) [25]. Written informed consent was signed by all the participants.
After an initial evaluation, patients underwent treatment consisting of a 6-week very-low-calorie diet (VLCD) in liquid formula (Optisource Plus®, Nestlé S.A., Vevey, Switzerland), containing 52.8 g protein, 75.0 g carbohydrates, 13.5 g fat and 11.4 g of fiber and the vitamins, minerals and trace elements that are essential according to Recommended Dietary Allowances (RDA). The energy provided by this formula was 2738 kJ/day (654 kcal/day). Participants replaced their usual 3 meals a day with the commercially available meal replacement provided by the National Healthcare System, under prescription from the endocrinologist. After this period, patients met the dietician for dietary counseling. During the appointment, the patient was interviewed, weighed, and prescribed a further 18 weeks of low-calorie diet (LCD) following an estimate of the caloric requirements of each individual according to sex, height, weight, and physical activity level. This diet consisted of an average daily energy intake of 5023–7535 kJ (1200–1800 kcal) in accordance with the recommended caloric requirement: 15–20% proteins, 50–55% carbohydrates and 28–33% fats. After the experimental period, patients were re-evaluated by the dietician.
Throughout the study, subjects were given detailed written and oral instructions about their diet, including precise amounts to be eaten, and cooking methods. A daily ingestion of more than two litres of calorie-free liquids was recommended. Patients were encouraged to maintain their normal pattern of activity and to ask for dietary counseling if necessary. No modifications were made to drug prescriptions during the course of the study.
Anthropometrical parameters, including weight (kg), height (m), BMI (kg/m2), waist circumference, and systolic and diastolic blood pressure (SBP and DBP) (mmHg) were measured in all the participants both at baseline and 6 months after dietary weight loss intervention. Blood samples of the patients were drawn from the antecubital vein during both appointments, after a 12 h fasting period.
2.2. Biochemical parameters
Biochemical determinations were performed at the Hospital's Clinical Analysis Service. An enzymatic method was used to determine serum levels of glucose, total cholesterol (TC) and triglycerides (TG). HDL cholesterol (HDLc) levels were obtained with a Beckman LX20 analyzer (Beckman Corp., Brea, CA, US) using a direct method. The intra-serial variation coefficient was <3.5% for all determinations. The Friedewald method was used to calculate levels of LDL cholesterol (LDLc) when triglycerides were under 300 mg/dl. Insulin was measured by a chemiluminescence immunoassay, and IR was estimated with the Homeostasis Model of Assessment (HOMA-IR = (fasting insulin (μU/ml) × fasting glucose (mg/dl)/405)). Percentage of glycated hemoglobin (A1c) was measured using an automatic glycohemoglobin analyzer. Levels of apolipoprotein (Apo) AI and B, high-sensitive C-reactive protein (hsCRP) and complement component 3 (C3c) were determined with an immunonephelometric assay whose intra-assay variation coefficient was <5.5%. Serum retinol binding protein 4 (RBP4) concentrations were measured by nephelometry with intra- and inter-assay coefficients of variation of 3.1% and 2.2%, respectively.
2.3. TNFα levels
Levels of TNFα in the serum were measured with a Luminex 200 analyzer system (Austin, TX, USA) by means of a Milliplex® MAP Kit (Merck Millipore, Burlington, MA, USA). The intra-serial and inter-serial variation coefficients were <5.0% and <15.0% respectively.
2.4. Cell isolation
Blood samples were incubated with dextran 3% for 45 min and subjected to centrifugation (650 g for 25 min at room temperature) in a Ficoll-Hypaque density gradient to isolate leukocyte fraction. After centrifugation, remnant erythrocytes were lysed and the pellet was washed with HBSS (Capricorn Scientific, Ebsdorfergrund, Germany).
2.5. Protein expression
Proteins were extracted by incubating leukocytes on ice for 15 min with lysis buffer (20 mM HEPES pH 7.5, 400 mM NaCl, 20% Glycerol, 0.1 mM EDTA, 10 μM Na2MoO4, 0.5% NP-40, 10 mM NaF, 1 mM NaVO3, 10 mM PNP, 10 mM β-glycerolphosphate, 1 mM dithiothreitol). BCA protein Assay Kit (Thermo Scientific, Waltham, MA, USA) was used to quantify total protein content of the samples. 25 μg of protein were resolved in a SDS-PAGE, transferred onto nitrocellulose membranes and blotted with the following primary antibodies: monoclonal anti-SAPK/JNK-P (Thr183/Tyr185) from Cell Signaling (Danvers, MA, USA), polyclonal anti-SIRT1 from Merck Millipore (Burlington, MA, USA), monoclonal anti-GPX1, polyclonal anti-eIF2α-P (Ser52) and monoclonal anti-CHOP from Thermo Scientific (Waltham, MA, USA), monoclonal anti-ATF6 and polyclonal anti-GRP78 (BiP) from Abcam (Cambridge, UK). HRP-goat anti-mouse (Thermo Scientific, Waltham, MA, USA) and HRP-goat anti-rabbit (Sigma Aldrich, Kawasaki, Kanagawa, Japan) were used as secondary antibodies. A chemiluminescence signal was detected after incubation of the membrane with ECL plus reagent (GE Healthcare, Little Chalfont, UK) or Supersignal West Femto (Thermo Scientific, Waltham, MA, USA). Images were acquired and bands densitometrically analyzed with the Fusion FX5 system coupled to the Bio1D software (VilbertLourmat, Marne LaVallée, France).
2.6. Fluorescence microscopy
Leukocytes were seeded in duplicate in 48-well plates and incubated for 30 min at 37 °C with the following fluorogenic dyes: MitoSOX Red (5 μM) was used to assess mitochondrial ROS (mROS) production, Fluo-4 (1 μM) indicated levels of cytosolic Ca2+, and tetramethylrhodamine methyl ester (TMRM, 5 μM) was used to evaluate changes in mitochondrial membrane potential. All the fluorescent probes were purchased from Invitrogen (Life Technologies, Carlsbad, CA, USA). Imaging was performed with an IX81 Olympus inverted fluorescence microscope and 20 images/well were analyzed with the static cytometry ScanR software 2.03.2 (Olympus, Hamburg, Germany).
2.7. Gene expression
Total RNA was extracted from leukocytes using the GeneAllR RibospinTM total RNA extraction kit (Geneall Biotechnology, Seoul, Korea) according to the manufacturer's instructions. A total of 1 μg of RNA samples were reverse-transcribed using the RevertAid first-strand cDNA synthesis kit (Thermo Scientific, Waltham, MA, USA). Quantitative RT-PCR analysis was then performed using the FastStart Universal SYBR Green Master (Roche Applied Science, Penzberg, Germany) and a 7500 Fast RT-qPCR system (Life technologies, Carlsbad, CA, USA), as described previously [26]. Spliced X-box binding protein 1 gene (s-xbp1; 103 pb) was amplified using the following primers: Forward 5′-CTGAGTCCGCAGCAGGTG-3′ and Reverse 5′-AACAGGATATCAGACTCTGAATCTGAA-3′. The internal control gene gapdh (168 pb) was amplified using the following primers: Forward 5′-CGCATCTTCTTTTGCGTCG-3′ and Reverse 5′-TTGAGGTCAATGAAGGGGTCA-3′.
2.8. Statistical analysis
The study was designed based on preliminary data [22] in order to have a power of 80% and to detect differences between two paired means in relation to the primary efficacy criterion (minimum expected difference in mROS) ≥50 relative fluorescence units (RFU), assuming a common standard deviation of 100 units. Under these premises, at least 32 subjects were considered.
Statistical differences between variables before and after the dietary treatment were analyzed using the paired Student's t-test or the Mann Whitney U-test for non-parametric variables with SPSS 19.0 statistics software (SPSS Statistics Inc., Chicago, IL, USA). The strength of the association between variables was measured by means of Pearson's correlation coefficient. Continuous variables in tables are expressed as mean ± standard deviation (SD) for parametric data or as median and 25th and 75th percentiles for non-parametric data. Qualitative data are expressed as percentages. In the figures, data are represented as mean +standard error (SE). All the tests used a confidence interval of 95% and the threshold of significance for all the analyses was set at p < 0.05.
3. Results
In the present study, we analyzed a total of 64 obese patients of middle age (43.5 ± 9.9 years) – mainly females of reproductive-age (n = 14), pre-menopausal (n = 16) and menopausal status (n = 16) – with an average BMI of 44.0 ± 5.7 kg/m2. The 6-month VLCD + LCD treatment resulted in a significant reduction of body weight and BMI (p < 0.001), with an average weight loss of 8.85 ± 4.16%. Waist circumference, SBP and DBP (p < 0.01), as well as hydrocarbonated metabolic parameters such as insulin, HOMA-IR and A1c, decreased significantly (p < 0.05), whereas fasting glucose levels did not change. Regarding blood lipid profile, triglycerides were significantly decreased and HDLc increased after weight loss (p < 0.01), although we did not observe changes in either total cholesterol or LDLc (Table 1).
Table 1.
Anthropometric and biochemical parameters of the study population before and after dietary weight loss intervention.
| Before | After | |
|---|---|---|
| n (females %) | 64 (71.9) | 64 (71.9) |
| Age (years) | 43.5 ± 9.9 | – |
| Weight (kg) | 120 ± 18 | 109 ± 15 |
| BMI (Kg/m2) | 44.0 ± 5.7 | 40.0 ± 4.8*** |
| Waist (cm) | 122 ± 14 | 114 ± 13*** |
| SBP (mmHg) | 134 ± 17 | 127 ± 15** |
| DBP (mmHg) | 85 ± 11 | 78 ± 10*** |
| Glucose (mg/dl) | 100 ± 21 | 97 ± 22 |
| Insulin (μU/ml) | 17.5 ± 10.2 | 15.3 ± 9.1* |
| HOMA-IR | 4.43 ± 3.02 | 3.79 ± 2.95* |
| A1c (%) | 5.73 ± 0.70 | 5.60 ± 0.75* |
| TC (mg/dl) | 184 ± 34 | 183 ± 41 |
| HDLc (mg/dl) | 41.4 ± 9.4 | 43.7 ± 10.8** |
| LDLc (mg/dl) | 115 ± 30 | 116 ± 36 |
| TG (mg/dl) | 120 (89,174) | 103 (83,143)** |
| Apo AI (mg/dl) | 139 ± 25 | 143 ± 28 |
| Apo B (mg/dl) | 99 ± 25 | 96 ± 26 |
| Treatment | ||
| Oral antidiabetic drugs (%) | 14.1 | |
| Antihypertensive drugs (%) | 29.7 | |
| Lipid-lowering drugs (%) | 26.6 |
Data are presented as mean ± SD or percentage (n). For TG are represented as median and IQ range. *p < 0.05; **p < 0.01; ***p < 0.001 when compared with a paired Student's t-test or Wilcoxon test.
Apo, Apolipoprotein; A1c, glycated hemoglobin; DBP, diastolic blood pressure; HDLc, HDL cholesterol; LDLc, LDL cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
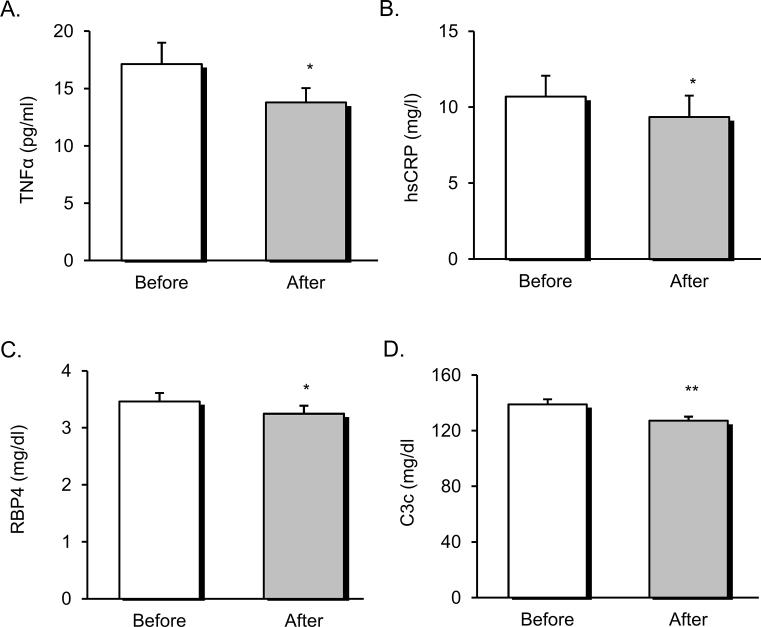
The dietary weight loss intervention induced significant changes in systemic inflammatory markers and adipokines. Serum levels of TNFα (Figure 1A) and hsCRP (Figure 1B) were lower after weight loss (p < 0.05). In addition, reductions in the adipokine RBP4 (Figure 1C) (p < 0.05) and in the cardiovascular risk marker C3c (Figure 1D) (p < 0.01) were detected at the end of the experimental period (p < 0.05).
Figure 1.
Systemic inflammatory markers and adipokines of obese patients before and after dietary weight loss intervention. Serum levels of TNFα (A), hsCRP (B), RBP4 (C), and C3c (D). Data are represented as mean +standard error. *p < 0.05; **p < 0.01, when compared using a paired Student's t-test. TNFα, tumor necrosis factor α; hsCRP, high sensitivity C-reactive protein; RBP4, retinol binding protein 4; C3c, complement component 3.
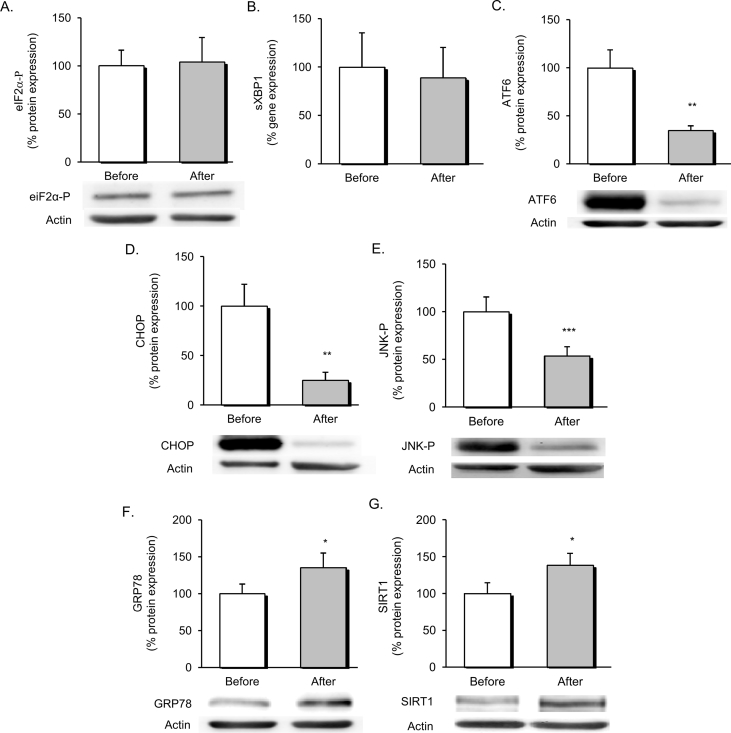
The effect of dietary intervention on ER stress was evaluated by assessing differential expression of markers among the three branches of the UPR, as represented in Figure 2. No changes were observed in the activity of the PERK-eIF2α-P branch (Figure 2A) or in the endoribonuclease activity of IRE1α, determined as mRNA levels of spliced XBP1 (Figure 2B). On the contrary, the dietary weight loss intervention seemed to have a profound effect on the ATF6-UPR branch, since we observed a marked decrease of p50/cleaved ATF6 levels (Figure 2C) that was associated with a down-regulation of the proapoptotic molecule CHOP (Figure 2D). In addition, we detected a drop in levels of phosphorylated JNK (Figure 2E), a major regulator of the inflammatory process in leukocytes, which can be activated through IRE1α kinase activity during ER stress.
Figure 2.
Evaluation of UPR markers and ER stress modulators in obese patients before and after dietary weight loss intervention. Relative protein expression of eIF2α-P (n = 14) (A) in the PERK-UPR pathway and representative western blot images. Endoribonuclease activity of IRE1α expressed in mRNA levels of sXBP1 (n = 11) (B). Protein levels of p50/activated ATF6 (n = 21) (C) and representative western blot images. Regulation of chronic downstream targets of the UPR, proapoptotic molecule CHOP (n = 21) (D) and phosphorylated JNK (n = 28) (E). Protein levels of major UPR chaperone GRP78 (n = 21) (F) and SIRT1 (n = 23) (G) and representative western blot images. Data are represented as mean +standard error. **p < 0.01; ***p < 0.001 when compared using a paired Student's t-test. UPR, unfolded protein response; ER, endoplasmic reticulum; eIF2α-P, phosphorylated eukaryotic translation initiation factor 2 subunit 1; PERK, protein kinase RNA-like endoplasmic reticulum kinase; IRE1α, inositol requiring enzyme 1 α; sXBP1, spliced X-box binding protein 1; ATF6, activating transcription factor 6; JNK, cJun NH2-terminal kinase; CHOP, CCAAT/enhancer binding protein [C/EBP] homologous protein; GRP78, 78-kDa glucose regulated protein; SIRT1, Sirtuin 1.
Based on the enhanced UPR expression profile, we decided to assess changes in ER stress modulators. Chaperones are major regulators of protein trafficking and processing in the ER. In line with this, protein expression of the chaperone 78-kDa glucose regulated protein (GRP78) was significantly up-regulated after dietary weight loss intervention (Figure 2F). In addition, increased expression of the anti-inflammatory mediator SIRT1 was observed in parallel with ER stress alleviation (Figure 2G).
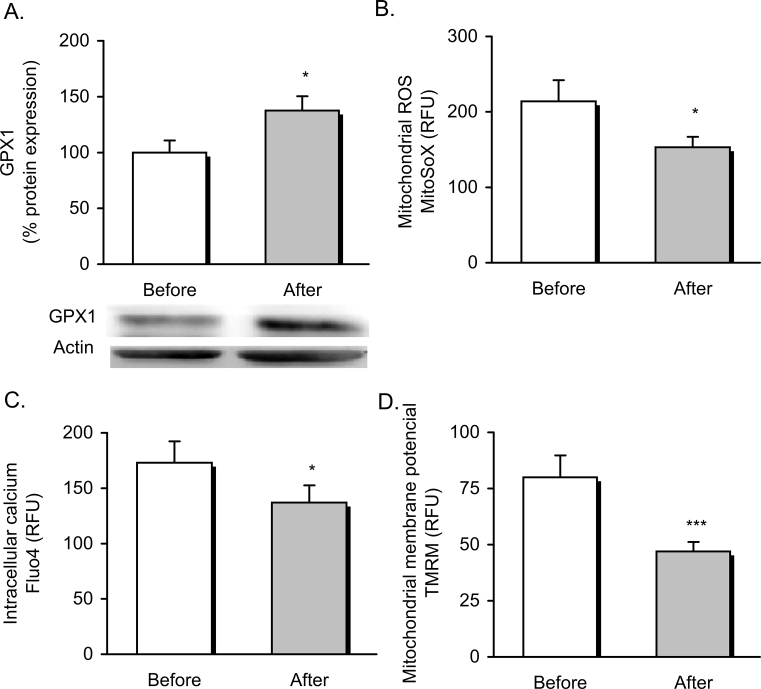
The known link between ER and mitochondrial function led us to explore whether the dietary weight loss intervention modulated mitochondrial function in our obese population. We found that ER stress relief was associated with an improvement in oxidative stress and mitochondrial function parameters. In fact, glutathione peroxidase 1 (GPX1) expression was induced after dietary treatment (Figure 3A) (p < 0.05), and was accompanied by a significant decrease in mROS production of leukocytes after dietary treatment (Figure 3B). Simultaneously, leukocytes showed a significant drop off in cytosolic Ca2+ content (p < 0.05) (Figure 3C), which was indicative of reduced ER Ca2+ depletion and a marked decrease of mitochondrial membrane potential (p < 0.001) (Figure 3D), pointing to a restoration of mitochondrial function and cellular homeostasis following the dietary weight loss intervention.
Figure 3.
Oxidative stress and mitochondrial function parameters in obese patients before and after dietary weight loss intervention. Expression of the antioxidant enzyme GPX1 (n = 16) and protein representative images (A) and levels of mROS production measured as arbitrary units of MitoSOX fluorescence dye (n = 31) (B), cytosolic Ca2+ measured as arbitrary units of Fluo-4 fluorescence dye (n = 18) (C) and mitochondrial membrane potential measured as arbitrary units of TMRM fluorescence dye (n = 30) (D). Data are represented as mean +standard error. *p < 0.05; ***p < 0.001 when compared using a paired Student's t-test. RFU, relative fluorescence units; GPX1, glutathione peroxidase 1; mROS, mitochondrial reactive oxygen species; TMRM, tetramethylrhodamine methyl ester.
Finally, when we explored possible associations among variations in molecular markers and clinical metabolic outcomes after dietary weight loss intervention, we found that percentage of change of HOMA-IR was correlated significantly with that of ATF6 (r = 0.478, p = 0.018, n = 24), JNK-P (r = 0.442, p = 0.016, n = 24) and CHOP – although in this latter case it did not reach statistical significance (r = 0.371, p = 0.075, n = 24) – pointing out to a relationship between changes in IR, ER stress and proinflammatory signals. There were also correlations among ER stress markers: percentage of change of GRP78 correlated positively with that of spliced XBP1 (r = 0.883, p = 0.001, n = 10); percentage of change of eIF2α-P correlated with that of ATF6 (r = 0.656, p = 0.003, n = 18) and JNK-P (r = 0.666, p = 0.003, n = 18); and percentage of change of CHOP correlated with that of ATF6 (r = 0.963, p < 0.001, n = 26), eIF2α-P (r = 0.691, p = 0.001, n = 18) and JNK-P (r = 0.850, p < 0.001, n = 26). In addition, a positive correlation between the percentages of change of GRP78 and SIRT1 was observed (r = 0.548, p = 0.018, n = 18), suggesting a relationship between the UPR adaptive response and SIRT1 expression (Table 2).
Table 2.
Pearson correlation coefficients of percentage of changes between insulin resistance and UPR markers and ER stress modulators in obese patients.
| HOMA-IR | GRP78 | eIF2α-P | ATF6 | sXBP1 | CHOP | JNK-P | SIRT1 | |
|---|---|---|---|---|---|---|---|---|
| HOMA-IR | – | n.s | n.s. | 0.478* | n.s. | n.s. | 0.442* | n.s. |
| GRP78 | – | – | n.s. | n.s | 0.883*** | n.s | n.s | 0.548* |
| eIF2α-P | – | – | – | 0.656** | n.s. | 0.691*** | 0.666** | n.s. |
| ATF6 | – | – | – | – | n.s. | 0.963*** | 0.842*** | n.s. |
| sXBP1 | – | – | – | – | – | n.s. | n.s. | n.s. |
| CHOP | – | – | – | – | – | – | 0.850*** | n.s. |
| JNK-P | – | – | – | – | – | – | – | n.s. |
| SIRT1 | – | – | – | – | – | – | – | – |
Data are expressed as Pearson's correlation and statistical significance *p < 0.05; **p < 0.01; ***p < 0.001 for each pair of variables. When correlation is not significant, it is represented as n.s.
Percentage of change was calculated following the formula: ((after-before)/before)*100.
GRP78, 78-kDa glucose regulated protein; eIF2α-P, phosphorylated eukaryotic translation initiation factor 2 subunit 1; ATF6, activating transcription factor 6; sXBP1, spliced X-box binding protein 1; CHOP, CCAAT/enhancer binding protein [C/EBP] homologous protein; JNK, cJun NH2-terminal kinase; SIRT1, Sirtuin 1.
4. Discussion
In this interventional study a population of middle-aged obese subjects underwent a dietary weight loss intervention consisting of 6 weeks of VLCD diet followed by 18 weeks of LCD. After this dietary program, we confirmed a moderate weight loss, which was associated with the improvement of anthropometric and cardiometabolic parameters and was accompanied by a reduction in the inflammatory response. When we examined the effect of the intervention on ER homeostasis we found that apoptotic pathways of the UPR were ameliorated and chaperone expression up-regulated. In parallel to this, we observed an improvement in oxidative stress and mitochondrial function in leukocytes. Altogether, these results suggest that the dietary weight loss intervention induced a partial recovery of cellular homeostasis mediated by better ER function and mitochondrial redox status, which were associated with an enhanced metabolic profile.
Several studies have described the benefits of caloric restriction and moderate weight loss for the metabolic profile of patients with obesity and related disorders. In fact, both obese and T2D patients have been shown to display improved insulin sensitivity and cardiovascular risk factor profiles when weight loss of 5–10% is achieved [22], [27]. In line with this, VLCDs have been shown to be an effective strategy for weight loss in obese patients, although the reported long-term effect of malnutrition has led to them being replaced by LCDs [28]. In our study population, the dietary weight loss intervention reduced BMI and abdominal fat accumulation, which was associated with the reduction of classic cardiovascular risk factors and circulating C3c levels. An association between C3c and metabolic syndrome [29] and IR in obesity has previously been described, and weight loss is reported to reduce levels of this adipokine, which is in accordance with the results published by our group and other researchers [30], [31]. In parallel, caloric restriction improved the lipid profile of our patients, including increased HDLc and lower circulating triglycerides.
Increased levels of adiposity in obesity are known to be responsible for the aberrant profile of circulating inflammatory markers and adipokines that may underlie IR in these patients. TNFα is overproduced by adipocytes and macrophages during obesity [32], triggering an impairment in insulin signaling at a systemic level. In the present study, reduced circulating levels of TNFα and hsCRP were detected after dietary weight loss intervention. In addition, lower levels of RBP4, an adipokine contributing to systemic IR and recently associated with hsCRP in the progression of metabolic syndrome [33], were detected. As a whole, inflammatory parameters were reduced after weight loss, suggesting a reduction in systemic inflammation mediated by caloric restriction.
It is interesting to speculate about the molecular mechanisms associated with metabolic improvements in obese populations following caloric restriction and moderate weight loss. In this context, ER stress has been reported to be activated in several tissues under conditions related to obesity and T2D, contributing to the development of IR and inflammation. In response to this, the UPR, a highly dynamic pathway, is activated to align ER functional capacity with demand according to external and intrinsic stress signals, such as alterations in metabolism and body weight. In the present study, our findings highlight a decrease in CHOP expression and in JNK activation in leukocytes from obese patients after weight loss, pointing to an alleviation of chronic ER stress, in accordance with previous findings [34].
It is known that all three UPR-branches, IRE1α, PERK, and ATF6, trigger adaptive and apoptotic responses and are involved in CHOP regulation. Our results suggest that dietary weight loss intervention modulates this apoptotic pathway, mainly by a decrease in ATF6 activation, since we found lower levels of p50-activated ATF6 to be correlated with a drop in CHOP expression. However, despite no significant changes in PERK-eIF2α-P being detected after the intervention, a positive correlation was observed between alterations in CHOP and eIF2α-P, suggesting a role of this branch in the regulation of CHOP. Finally, bearing in mind the role of the IRE1α-JNK axis in obesity-induced ER stress [7], [35], [36], it is likely that changes in JNK-P after dietary weight loss are mediated by a reduction in IRE1α kinase activity, although further analyses are required to confirm this hypothesis. Previous studies have shown reduced ER stress upon weight loss in murine models [34] and patients undergoing bariatric surgery [36], thus highlighting the relevance of body weight in ER homeostasis. However, to our knowledge, this is the first report of UPR modulation by dietary weight loss intervention in humans with obesity.
When exploring the mechanisms involved in ER restoration we have observed increased levels of GRP78, a chaperone that is a major regulator of the UPR. In previous studies, GRP78 upregulation was associated with a decrease in hepatic UPR markers and the IRE1α-JNK activation axis, and an improvement of insulin action and lipid profile in a murine model of obesity [37] and in hepatic cells [38], which is in line with the results of the present study. On the other hand, a growing body of evidence suggests an important role of SIRT1 in ER stress regulation and IR in metabolic disorders. Moreover, several authors have shown that caloric restriction and weight loss are powerful inducers of SIRT1 [39], [40] and have demonstrated a role for SIRT1 as an anti-inflammatory molecule in obesity [10], [41], which once again is compatible with our results. Interestingly, we found a positive correlation between changes in SIRT1 and GRP78 after the dietary weight loss intervention. These findings provide new insights into the association between ER stress adaptive response and SIRT1. However, since causality cannot be inferred from our data, further analyses are required to elucidate how these two intracellular signaling pathways are interrelated in the context of dietary weight loss in obesity.
Recent studies have provided new insight into the contribution of leukocyte-ER homeostasis to metabolic disease. In this sense, we have previously reported higher ER stress levels in leukocytes from obese subjects with metabolic disturbances when compared with healthy counterparts [19] and also in immune cells from T2D patients, especially in those with poor glycemic control [26]. In line with this, Sage et al., 2012 [42] demonstrated that induced UPR markers in mononuclear cells correlated with indicators of impaired glucose tolerance in metabolic syndrome. In accordance, we show here that changes in ATF6 and JNK-P in leukocytes from obese patients after dietary intervention correlate with changes in HOMA-IR, supporting a connection between ER homeostasis, glucose management and development of IR.
UPR pathways in immune cells have also been implicated in the progression of cardiovascular disease. Increased ER stress markers have been found in peripheral blood mononuclear cells, as well as in smooth muscle cells and infiltrated macrophages isolated from atherosclerotic plaques of patients with coronary disease [43], [44]. In another study, treatment with chaperones that reduce ER stress in macrophages was associated with a delay in the progression of atherosclerosis [45]. In line with this, when we previously explored the association between UPR activation and leukocyte-endothelium cell interactions, an enhancement of the GRP78 adaptive response in leukocytes was found to correlate with a lower level of interaction with the endothelium, whereas increased expression of CHOP seemed to promote adherence [26], which is the first step of the atherosclerotic process. In the present study, increasing levels of GRP78 and a drop in CHOP expression were observed in leukocytes of obese patients after dietary intervention, in parallel with the improvement of some cardiovascular risk factors. Nevertheless, how these changes are related to the interaction of these cells with the endothelium and the subsequent risk of developing cardiovascular disease remains to be confirmed.
Mitochondria are closely linked to the ER by physical contact and Ca2+ interchange, and accumulating evidence suggests a converging role of the two organelles in the progression of metabolic disorders [46]. During ER stress, mitochondrial Ca2+ overload, among other stress signals, disturbs mitochondrial membrane potential and causes excess ROS production. The imbalance between pro- and anti-oxidants leads to oxidative stress and mitochondrial dysfunction, a mechanism that has been related to the appearance of obesity-related comorbidities and IR [17], [47]. In fact, decreased levels of GPX1, an antioxidant enzyme located both in the cytosol and the mitochondria, have been reported in adipose tissue from a genetic murine model and related to impaired insulin signaling [48] and endothelial dysfunction [49]. Our present data demonstrate that caloric restriction reduces abnormal Ca2+ distribution and mitochondrial membrane potential, pointing to a restoration of cell homeostasis and mitochondrial function. Furthermore, redox balance was improved in our patients, since lower levels of mROS molecules and higher GPX1 expression were found in their leukocytes after dietary treatment. These results demonstrate that dietary weight loss intervention can modulate mitochondrial function and oxidative stress. However, further analyses are required to assess the degree of implication of these changes in the enhancement of metabolic status in obese patients under caloric restriction.
Finally, most of the literature supports an orchestrated response among leukocytes, adipocytes, and muscle cells in obesity. In fact, we have recently published results showing that total ROS, total superoxide, and mitochondrial membrane potential are selectively higher in obese patients [20], which is in line with impaired mitochondrial activity and enhanced ROS production in subcutaneous adipocytes and white adipose tissue [21], [50], [51]. In reference to oxidative stress and mitochondrial function in human skeletal muscle, considerable debate exists about whether alterations in mitochondrial respiratory capacity and/or content play a causal role in the development of IR during obesity. Previous reports have shown that mitochondrial content is significantly lower in muscle samples of obese individuals [52], [53], [54], whereas others have not reported such results [55], despite that elevations in H2O2 emission rates and reductions in cellular glutathione [52], [55], [56] are correlate with those measured in leukocytes and adipocytes.
This study presents some limitations. Firstly, it does not clarify whether the main findings were mediated by weight loss or caloric restriction per se, since no period of eucaloric stability was programmed after weight loss intervention. In addition, further analyses are required to elucidate the directionality of changes in ER and mitochondrial function and metabolic improvements in obese patients after dietary weight loss intervention. Nevertheless, the present results provide vital new insight into the modulation of ER stress and mitochondrial function in vivo that could have important implications for the treatment or prevention of obesity and T2D.
5. Conclusions
In summary, the results of the present study extend our understanding of the molecular changes and metabolic improvements that obese patients display when moderate weight loss is achieved by caloric restriction. Interestingly, the improvement in systemic inflammation and glucose tolerance was mirrored by an attenuation of chronic ER stress and mitochondrial dysfunction after dietary weight loss intervention, and was accompanied by enhanced expression of chaperones, SIRT1 and antioxidants. These findings highlight the relevance of restoration of ER homeostasis and mitochondrial function as potential targets for treating metabolic complications in obesity.
Authors contribution
The authors’ responsibilities were as follows. MR and VMV contributed to the conception and design of the study. CB and SR-L assisted in the design of the experiments and provided support throughout the course of the study. CM carried out the recruitment and diagnosis of the patients in the study. SL-D, ZA-J, FI, and AM performed the laboratory analyses and collected data. SL-D and MR analyzed the data, performed the statistical analysis and drafted the manuscript. MR and VMV critically revised the manuscript and were responsible for its final content. All authors have read and approved the final version of the manuscript.
Acknowledgements
The authors acknowledge the editorial assistance of Brian Normanly (CIBERehd) and the technical support of Rosa Falcón. This study was supported by grant PI16/00301 and PI16/01083 from Carlos III Health Institute and has been co-funded by the European Regional Development Fund (ERDF “A way to build Europe”) and PROMETEOII 2014/035 from the Regional Ministry Education of Valencian Community. SL-D, ZA-J and AM are recipients of predoctoral fellowship from Carlos III Health Institute (FI14/00350, FI17/00144, FI17/00126). FI is recipient of a predoctoral fellowship from the Regional Ministry Education of Valencian Community (GRISOLIAP/2016/015). SR-L is recipient of a Juan de la Cierva-Formación contract from the Spanish Ministry of Economy and Competitiveness (FJCI-2015-25040). MR is recipient of a Miguel Servet (CPII16/0037) contract from Carlos III Health Institute. Unrestricted grant from Menarini S.A. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Contributor Information
Víctor Manuel Víctor, Email: victor.victor@uv.es.
Milagros Rocha, Email: milagros.rocha@uv.es.
Conflict of interest
The authors have no conflict of interest to disclose.
References
- 1.Williams E.P., Mesidor M., Winters K., Dubbert P.M., Wyatt S.B. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Current Obesity Reports. 2015;4(3):363–370. doi: 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- 2.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nature Reviews Immunology. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allagnat F., Fukaya M., Nogueira T.C., Delaroche D., Welsh N., Marselli L. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in β-cells. Cell Death & Differentiation. 2012;19(11):1836–1846. doi: 10.1038/cdd.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 6.Han M.S., Jung D.Y., Morel C., Lakhani S.A., Kim J.K., Flavell R.A. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339(6116):218–222. doi: 10.1126/science.1227568. https://doi.org/0.1126/science.1227568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirosumi J., Tuncman G., Chang L., Gorgun C.Z., Uysal K.T., Maeda K. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 8.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R.O. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao C., Giacca A., Lewis G.F. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes. 2011;60(3):918–924. doi: 10.2337/db10-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Domenech S., Banuls C., de Maranon A.M., Abab-Jimenez Z., Morillas C., Gomez-Abril S.A. 2017. Pinitol alleviates systemic inflammatory cytokines in human obesity by a mechanism involving unfolded protein response and sirtuin 1. Oct 3. pii: S0261-5614(17)31347-X. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen S.B., Olholm J., Paulsen S.K., Bennetzen M.F., Richelsen B. Low Sirt1 expression, which is upregulated by fasting, in human adipose tissue from obese women. International Journal of Obesity. 2008;32(8):1250–1255. doi: 10.1038/ijo.2008.78. [DOI] [PubMed] [Google Scholar]
- 12.de Kreutzenberg S.V., Ceolotto G., Papparella I., Bortoluzzi A., Semplicini A., Dalla Man C. Downregulation of the longevity-associated protein sirtuin 1 in insulin resistance and metabolic syndrome: potential biochemical mechanisms. Diabetes. 2010;59(4):1006–1015. doi: 10.2337/db09-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshizaki T., Milne J.C., Imamura T., Schenk S., Sonoda N., Babendure J.L. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Molecular and Cellular Biology. 2009;29(5):1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Xu S., Giles A., Nakamura K., Lee J.W., Hou X. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. The FASEB Journal – Official Publication of the Federation of American Societies for Experimental Biology. 2011;25(5):1664–1679. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng X., Xu F., Liang H., Cao H., Cai M., Xu W. SIRT1/HSF1/HSP pathway is essential for exenatide-alleviated, lipid-induced hepatic endoplasmic reticulum stress. Hepatology. 2017;66(3):809–824. doi: 10.1002/hep.29238. [DOI] [PubMed] [Google Scholar]
- 16.Liu Z., Gu H., Gan L., Xu Y., Feng F., Saeed M. Reducing Smad3/ATF4 was essential for Sirt1 inhibiting ER stress-induced apoptosis in mice brown adipose tissue. Oncotarget. 2017;8(6):9267–9279. doi: 10.18632/oncotarget.14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 18.Rocha M., Rovira-Llopis S., Banuls C., Bellod L., Falcon R., Castello R. Mitochondrial dysfunction and oxidative stress in insulin resistance. Current Pharmaceutical Design. 2013;19(32):5730–5741. doi: 10.2174/13816128113199990373. [DOI] [PubMed] [Google Scholar]
- 19.Bañuls C., Rovira-Llopis S., Lopez-Domenech S., Diaz-Morales N., Blas-Garcia A., Veses S. Oxidative and endoplasmic reticulum stress is impaired in leukocytes from metabolically unhealthy vs healthy obese individuals. International Journal of Obesity. 2017;41(10):1556–1563. doi: 10.1038/ijo.2017.147. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Domenech S., Banuls C., Diaz-Morales N., Escribano-Lopez I., Morillas C., Veses S. Obesity impairs leukocyte-endothelium cell interactions and oxidative stress in humans. European Journal of Clinical Investigation. 2018;48(8):e12985. doi: 10.1111/eci.12985. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay M., Khemka V.K., Chatterjee G., Ganguly A., Mukhopadhyay S., Chakrabarti S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Molecular and Cellular Biochemistry. 2015;399(1–2):95–103. doi: 10.1007/s11010-014-2236-7. [DOI] [PubMed] [Google Scholar]
- 22.Wing R.R., Lang W., Wadden T.A., Safford M., Knowler W.C., Bertoni A.G. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu H., Schlegel V. Impact of nutrient overload on metabolic homeostasis. Nutrition Reviews. 2018;76(9):693–707. doi: 10.1093/nutrit/nuy023. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care. 2017;40:S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 25.Fernández G., Marset M., Basulto-Lesmes J., Breton-Izquierdo I., Quiles-Sala J., Formiguera-Salas X. Summary of the consensus FESNAD-SEEDO: evidence-based nutritional recommendations for the prevention and treatment of overweight and obesity in adults. Endocrinología y Nutrición. 2012;59(7):429–437. doi: 10.1016/j.endonu.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Rovira-Llopis S., Banuls C., Apostolova N., Morillas C., Hernandez-Mijares A., Rocha M. Is glycemic control modulating endoplasmic reticulum stress in leukocytes of type 2 diabetic patients? Antioxidants and Redox Signaling. 2014;21(12):1759–1765. doi: 10.1089/ars.2014.6030. [DOI] [PubMed] [Google Scholar]
- 27.Magkos F., Fraterrigo G., Yoshino J., Luecking C., Kirbach K., Kelly S.C. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metabolism. 2016;23(4):591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez Mijares A., Morillas Ariño C., Royo Taberner R., Sola Izquierdo E., Garzon Pastor S., Martinez Triguero M.L. Malnutrition evaluation in obese patients of both sexes on a very low caloric content diet. Revista Clínica Española. 2004;204(8):410–414. doi: 10.1157/13064314. [DOI] [PubMed] [Google Scholar]
- 29.Karkhaneh M., Qorbani M., Mohajeri-Tehrani M.R., Hoseini S. Association of serum complement C3 with metabolic syndrome components in normal weight obese women. Journal of Diabetes and Metabolic Disorders. 2017;16:49. doi: 10.1186/s40200-017-0330-6. 4017-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez-Mijares A., Banuls C., Bellod L., Jover A., Sola E., Morillas C. Effect of weight loss on C3 and C4 components of complement in obese patients. European Journal of Clinical Investigation. 2012;42(5):503–509. doi: 10.1111/j.1365-2362.2011.02606.x. [DOI] [PubMed] [Google Scholar]
- 31.Bratti L.O.S., do Carmo I.A.R., Vilela T.F., Wopereis S., de Moraes A.C.R., Borba B.G.M. Complement component 3 (C3) as a biomarker for insulin resistance after bariatric surgery. Clinical Biochemistry. 2017;50(9):529–532. doi: 10.1016/j.clinbiochem.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annual Review of Physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 33.Tabak O., Simsek G., Erdenen F., Sozer V., Hasoglu T., Gelisgen R. The relationship between circulating irisin, retinol binding protein-4, adiponectin and inflammatory mediators in patients with metabolic syndrome. Archives of Endocrinology and Metabolism. 2017;61(6):515–523. doi: 10.1590/2359-3997000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsutsumi A., Motoshima H., Kondo T., Kawasaki S., Matsumura T., Hanatani S. Caloric restriction decreases ER stress in liver and adipose tissue in ob/ob mice. Biochemical and Biophysical Research Communications. 2011;404(1):339–344. doi: 10.1016/j.bbrc.2010.11.120. [DOI] [PubMed] [Google Scholar]
- 35.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 36.Gregor M.F., Yang L., Fabbrini E., Mohammed B.S., Eagon J.C., Hotamisligil G.S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58(3):693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kammoun H., Chabanon H., Hainault I., Luquet S., Magnan C., Koike T. GRP78 expression inhibits insulin and ER stress–induced SREBP-1c activation and reduces hepatic steatosis in mice. Journal of Clinical Investigation. 2009;119(5):1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L., Xu S., Liu L., Wen X., Xu Y., Chen J. Cab45S inhibits the ER stress-induced IRE1-JNK pathway and apoptosis via GRP78/BiP. Cell Death & Disease. 2014;5:e1219. doi: 10.1038/cddis.2014.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding S., Jiang J., Zhang G., Bu Y., Zhang G., Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PloS One. 2017;12(8) doi: 10.1371/journal.pone.0183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rappou E., Jukarainen S., Rinnankoski-Tuikka R., Kaye S., Heinonen S., Hakkarainen A. Weight loss is associated with increased NAD(+)/SIRT1 expression but reduced PARP activity in white adipose tissue. The Journal of Clinical Endocrinology and Metabolism. 2016;101(3):1263–1273. doi: 10.1210/jc.2015-3054. [DOI] [PubMed] [Google Scholar]
- 41.Gillum M.P., Kotas M.E., Erion D.M., Kursawe R., Chatterjee P., Nead K.T. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60(12):3235–3245. doi: 10.2337/db11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sage A.T., Holtby-Ottenhof S., Shi Y., Damjanovic S., Sharma A.M., Werstuck G.H. Metabolic syndrome and acute hyperglycemia are associated with endoplasmic reticulum stress in human mononuclear cells. Obesity. 2012;20:748–755. doi: 10.1038/oby.2011.144. https://doi:10.1038/oby.2011.144 [DOI] [PubMed] [Google Scholar]
- 43.Mozzini C., Fratta Passini A., Garbin U., Stranieri C., Pasini A., Vallerio P. Increased endoplasmic reticulum stress and Nrf2 repression in peripheral blood mononuclear cells of patients with stable coronary artery disease. Free Radical Biology and Medicine. 2014;68:178–185. doi: 10.1016/j.freeradbiomed.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Myoishi M., Hao H., Minamino T., Watanabe K., Nishihira K., Hatakeyama K. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116(11):1226–1233. doi: 10.1161/circulationaha.106.682054. [DOI] [PubMed] [Google Scholar]
- 45.Erbay E., Babaev V.R., Mayers J.R., Makowski L., Charles K.N., Snitow M. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nature Medicine. 2009;15(12):1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arruda A.P., Hotamisligil G. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metabolism. 2015;22(3):381–397. doi: 10.1016/j.cmet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yubero-Serrano E.M., Delgado-Lista J., Pena-Orihuela P., Perez-Martinez P., Fuentes F., Marin C. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Experimental & Molecular Medicine. 2013;45:e28. doi: 10.1038/emm.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi H., Matsuda M., Fukuhara A., Komuro R., Shimomura I. Dysregulated glutathione metabolism links to impaired insulin action in adipocytes. Endocrinology and Metabolism. 2009;296(6):E1326–E1334. doi: 10.1152/ajpendo.90921.2008. [DOI] [PubMed] [Google Scholar]
- 49.Espinola-Klein C., Rupprecht H.J., Bickel C., Schnabel R., Genth-Zotz S., Torzewski M. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. The American Journal of Cardiology. 2007;99(6):808–812. doi: 10.1016/j.amjcard.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 50.Yin X., Lanza I.R., Swain J.M., Sarr M.G., Nair K.S., Jensen M.D. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. The Journal of Clinical Endocrinology and Metabolism. 2014;99(2):E209–E216. doi: 10.1210/jc.2013-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinonen S., Muniandy M., Buzkova J., Mardinoglu A., Rodríguez A., Frühbeck G. Mitochondria-related transcriptional signature is downregulated in adipocytes in obesity: a study of young healthy MZ twins. Diabetologia. 2017;60(1):169–181. doi: 10.1007/s00125-016-4121-2. [DOI] [PubMed] [Google Scholar]
- 52.Lefort N., Glancy B., Bowen B., Willis W.T., Bailowitz Z., De Filippis E.A. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes. 2010;59(10):2444–2452. doi: 10.2337/db10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ritov V.B., Menshikova E.V., He J., Ferrell R.E., Goodpaster B.E., Kelley D.E. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54(1):8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 54.Morino K., Petersen K.F., Dufour S., Befroy D., Frattini J., Shatzkes N. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. Journal of Clinical Investigation. 2005;115(12):3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher-Wellman K.H., Weber T.M., Cathey B.L., Brophy P.M., Gilliam L.A.A., Kane C.L. Mitochondrial respiratory capacity and content are normal in young insulin-resistant obese humans. Diabetes. 2014;63(1):132–141. doi: 10.2337/db13-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson E.J., Lustig M.E., Boyle K.E., Woodlief T.L., Kane D.A., Lin C.T. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Journal of Clinical Investigation. 2009;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]