Abstract
As part of an epidemiological survey for gastrointestinal parasites in school children across five primary schools on the shoreline of Lake Albert, the prevalence of giardiasis was 87.0% (n = 254) as determined by real-time PCR analysis of faecal samples with a genus-specific Giardia 18S rDNA probe. Faecal samples were further characterised with taxon assemblage-specific triose phosphate isomerase (TPI) Taqman® probes and by sequence characterisation of the β-giardin gene. While less sensitive than the 18S rDNA assay, general prevalence by TPI probes was 52.4%, with prevalence by taxon assemblage of 8.3% (assemblage A), 35.8% (assemblage B) and 8.3% co-infection (A & B assemblages). While assemblage B was dominant across the sample, proportions of assemblages A and B, and co-infections thereof, varied by school and by age of child; mixed infections were particularly common at Runga school (OR = 6.9 [95% CI; 2.5, 19.3]) and in children aged 6 and under (OR = 2.7 [95% CI; 1.0, 7.3]). Infection with assemblage B was associated with underweight children (OR = 2.0 [95% CI; 1.0, 3.9]). The presence of each assemblage was also confirmed by sequence analysis of the β-giardin gene finding sub-assemblage AII and further genetic diversity within assemblage B. To better explore the local epidemiology of giardiasis and its impact on child health, additional sampling of school children with assemblage typing would be worthwhile.
Keywords: Giardia duodenalis, Real-time PCR, Assemblage B, β-giardin, Wasting
1. Introduction
The binucleate flagellated protozoan Giardia duodenalis (syn. G. lamblia, G. intestinalis) is a common gastrointestinal parasite able to infect a variety of mammals (Adam, 2001; Helmy et al., 2014). Where sanitation and hygiene are poor, these parasites can cause acute and/or chronic giardiasis across all ages (Wegayehu et al., 2016; Muhsen and Levine, 2012; Rogawski et al., 2017; Tellevik et al., 2015). While levels of endemicity of giardiasis may vary across the world, it can be common in children living within low and middle income countries (Laishram et al., 2012; Muhsen and Levine, 2012); for example, in Uganda giardiasis can be particularly rife (Al-Shehri et al., 2016; Fuhrimann et al., 2016), but its effect on child health is not fully appreciated but in Rwanda nearby, the very high prevalence of G. duodenalis in children aged 5 and under, was associated with being underweight (Ignatius et al., 2012).
There are eight distinct groups or taxonomic assemblages (A to H) within Giardia currently recognised (Sprong et al., 2009; Almeida et al., 2010; Takumi et al., 2012). Assemblages A and B are typically held most responsible for human infections, with the latter assemblage associated with zoonotic transmission (Almeida et al., 2010; Feng and Xiao, 2011; Vanni et al., 2012; Asher et al., 2014; Thompson and Ash, 2016); each assemblage can be further divided into sub-assemblages, e.g. A: AI, AII & AIII and B: BIII and BIV on the basis of sequence variation within molecular markers e.g. glutamate dehydrogenase (GDH), β-giardin, small subunit ribosomal DNA (18S rDNA), and triose phosphate isomerase (TPI) (Durigan et al., 2014; Karim et al., 2015; Minetti et al., 2015). Despite efforts to investigate specific assemblages with disease symptoms and severity, there is no absolute association to date (Sprong et al., 2009; Thompson and Ash, 2016).
In Uganda, general investigations on the epidemiology of giardiasis are increasing (Nizeyi et al., 1999; Graczyk et al., 2002; Nizeyi et al., 2002; Johnston et al., 2010), although only a single study has employed molecular methods of characterisation (Ankarklev et al., 2012). Ankarklev et al. (2012) investigated associations between taxon assemblages and Helicobacter pylori infection in apparently healthy children aged 0–12 living in Kampala, the capital. Assemblage B was found dominant and a risk factor for H. pylori infection (Ankarklev et al., 2012) and like in other parts of the world, assemblage B was more associated with symptomatic infections (Pelayo et al., 2008; Puebla et al., 2014).
To shed light on the taxonomic assemblages of Giardia within school children living on the shoreline of Lake Albert, we undertook a molecular characterisation of previously characterised stool samples as reported by Al-Shehri et al. (Al-Shehri et al., 2016). Faecal samples were further characterised with assemblage-specific TaqMan® TPI probes and the presence of each taxon assemblage confirmed by sequence analysis of the β-giardin gene. Associations between taxon assemblage and collected epidemiological data were explored.
2. Materials and methods
2.1. Faecal material and epidemiological information
Faecal samples were available for further molecular analysis (see below) that were initially collected within the epidemiological survey of 254 school children from five primary schools (Bugoigo, Runga, Walakuba, Biiso and Busingiro) as reported by Al-Shehri et al. (Al-Shehri et al., 2016). Each sampled child underwent an epidemiological questionnaire and clinical examination; data on socio-demographical aspects and standard biometry were recorded (height with a clinical stadiometer, model 214; SECA, Hanover, MD and weight by weighing scales with a model 803; SECA, Hanover; MD). Heights and weights were used to assess stunting, height-for-age Z-score (HAZ), and wasting, weight-for-age Z-score (WAZ). Children were defined as stunted if their height-for-age Z score was −2 ≤ SD and underweight if their weight-for-age Z score was −2 ≤ SD (WHO, 2007). Finger-prick blood was collected from each child and tested for haemoglobin levels by HemoCue® portable haemoglobin photometer (HemoCue, CA 92630, USA). Children were considered anaemic if haemoglobin levels were below 115 g/L (WHO, 2011).
During the surveys, all sampled stools were tested for faecal occult blood (Mission Test, Acon Laboratories, San Diego, CA, USA) but owing to a limited supply of rapid diagnostic tests (RDTs), only stools collected from Bugiogo and Runga were tested in-field with Quik-Chek RDTs for giardiasis and cryptosporidiosis (GIADIA/CRYPTOSPORIDIUM Quik-Chek, Alere, Galway, Ireland). Stools were then stored in absolute ethanol for later DNA analysis.
2.2. Molecular profiling of G. duodenalis assemblages
After transfer to the UK and each faecal sample was spiked with Phocine Herpes Virus to act as an internal control for genomic DNA extraction and amplification performance of later real-time PCR assays. Genomic DNA was extracted, and detection of Giardia 18S rDNA was performed using TaqMan® assay following primers, probes and protocols of Verweij et al. (Verweij et al., 2004). These extractions were again retested with a duplex real-time PCR assay with assemblage-specific A and B probes using the TPI locus (Elwin et al., 2014). The real-time PCR analysis of faecal extractions from each school was completed in separate PCR plates that each contained negative and positive controls; a negative control (without genomic DNA template) of extraction elution buffer (10 mM Tris-HCl [pH 8], 1 mM EDTA) and a positive control (with reference genomic Giardia DNA template) from a heavily infected individual excreting approximately 1000 cysts per gram of faeces as estimated by microscopy. As a further quality control, reamplification of 10% of samples was undertaken to assess assemblage assay reliability. Assays were performed in a Chromo-4 with Opticon monitor™ version 3.1. (Bio-Rad, UK). The infection was determined according to Ct values; for the 18S rDNA TaqMan® assay no-infection was Ct ≥ 40 and positive infection Ct ≤ 39 while for assemblages-specific probes was Ct ≤ 45.
To further confirm assemblage A and B, the β-giardin gene was amplified from samples from six children using nested PCR following protocols of Minetti et al. (Minetti et al., 2015). PCR products were purified using the QIAquick® PCR purification kit (QIAGEN Ltd.) and were sequenced in both directions by Sanger sequencing. Nucleotide sequences and chromatograms were analysed and edited using Geneious software (Vejlsøvej55, 8600 Silkeborg, Denmark). Sequences from this study were aligned with each other and reference sequences downloaded from GenBank (listed below). The assemblages and sub-assemblages at each locus were identified by BLAST searches against the following reference sequences: β-giardin (accession nos. X14185.1–AI, AY072723.1–AII, DQ650649.1–AIII, AY072726.1–BIII, AY072725.1–BIV).
2.3. Statistical analyses
Statistical analysis was performed using Minitab Ltd.® (Brandon Court, Unit E1-E2 Coventry CV3 2TE UK). Binary logistic regression tests were performed to compare data from each school and as well as risk variables as an independent indicator to assess any associations with specific assemblages.
3. Results
Out of the 254 samples examined, 221 tested positive (87.0%) by targeting Giardia 18S rDNA assay while 133 (52.3%) tested positive with TPI assemblage-specific probes. Across Bugoigo and Runga schools, the prevalence of giardiasis by Quik-Chek RDT was 41.6%. Of the 133-tested positive by TPI probes, 21 samples were positive for assemblage A (15.8%) only, 91 positives for assemblage B (68.4%) only and 21 positives for both assemblage A and B (15.8%), mixed assemblage infections.
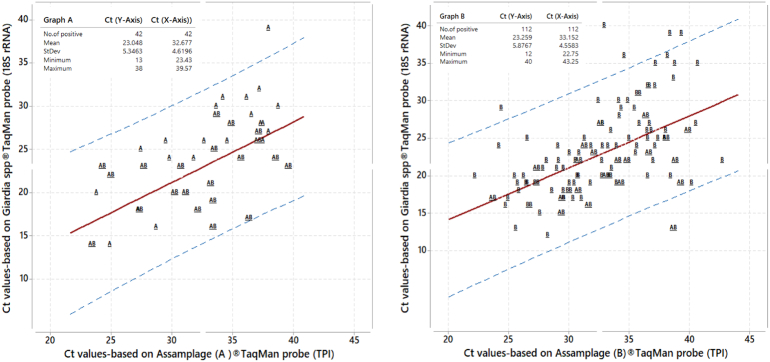
Across these samples assemblage, A was less common than assemblage B, an approximate ratio of 1: 2.7, with assemblage B dominant. To ascertain if there was any amplification bias in assemblage detection, Fig. 1A shows a bivariate plot of Ct values for Giardia 18S rDNA TaqMan® probe and the corresponding Ct value of assemblage A TPI TaqMan® probe (18S rDNA = 0.203 + 0.6991 TPI, with R-squared 34.91% (P < 0.005), and positive correlation (r = 0.60)); Fig. 1B shows bivariate plot for assemblage B (18S rDNA = 0.228 + 0.6947 TPI, with R-squared 28.39% (P < 0.005), and positive correlation (r = 0.54)). The performance of each TaqMan® assay appeared equivalent. Of note, however, is that mixed assemblage infections appear more common at Runga school where the local prevalence of assemblage A was also much higher.
Fig. 1.
Bivariate plot of Ct values obtained for each sample using Giardia TaqMan® 18S rDNA versus Ct value of assemblage-specific TaqMan® TPI probe with dashed lines showing the 95% prediction interval. Fig. 1A. Using assemblage A probe; Fig. 1B Using assemblage B probe.
Table 2 shows epidemiological associations cross-tabulated against available assemblage information. Most notable is the association of mixed assemblages in younger children (OR = 2.7 [95% CI; 1.0, 7.3]) that assemblage B was associated with the presence of faecal occult blood (OR = 2.2 [95% CI; 1.0, 5.2]). It appeared that there was also a significant association of infection with assemblage B and children of lower weight-for-age, i.e. wasting (OR = 2.0 [95% CI; 1.0, 3.9]).
Table 2.
Analysis of potential epidemiological associations by binary logistic regression with Giardia assemblages A, B or A/B co-infection.
| Epidemiological factors | Assemblage (A, B & AB)® TaqMan probe (TPI) |
|||||
|---|---|---|---|---|---|---|
| Infected with A | OR [95 CL] | Infected with B | OR [95 CL] | Infected with AB | OR [95 CL] | |
| Gender | ||||||
| Male | 10 | 1.0 [0.4, 2.7] | 44 | 1.0 [0.6, 1.9] | 12 | 1.5 [0.6, 3.9] |
| Female | 11 | 0.9 [0.4, 2.4] | 47 | 0.9 [0.5, 1.6] | 9 | 0.6 [0.3, 1.6] |
| Age group | ||||||
| 5 to 6 | 11 | 1.5 [0.6, 3.8] | 38 | 0.9 [0.6, 1.7] | 14 | 2.7 [1.0, 7.3] |
| 7 to 8 | 9 | 0.9 [0.4, 2.5] | 34 | 0.7 [0.5, 1.4] | 6 | 0.5 [0.2, 1.5] |
| 9 to 10 | 1 | 0.2 [0.0, 2.3] | 19 | 1.5 [0.7, 3.1] | 1 | 0.2 [0.0, 2.3] |
| Faecal occult blood (FOB) | ||||||
| Negative | 18 | 0.6 [0.2, 2.4] | 74 | 0.4 [0.2, 1.0] | 17 | 0.4 [0.1, 1.5] |
| Positive | 3 | 1.6 [0.4, 6.6] | 17 | 2.2 [1.0, 5.2] | 4 | 2.3 [0.7, 8.2] |
| Height-for-age Z score, mean | ||||||
| −2 > SD height-for-age Z score | 17 | 1.3 [0.4, 4.3] | 63 | 0.7 [0.4, 1.3] | 14 | 0.6 [0.2, 1.7] |
| −2 ≤ SD height-for-age Z score | 4 | 0.7 [0.2, 2.4] | 28 | 1.4 [0.8, 2.6] | 7 | 1.5 [0.6, 4.3] |
| Weight-for-age Z score, mean | ||||||
| −2 > SD weight-for-age Z score | 20 | 3.9 [0.5,31.2] | 65 | 0.4 [0.3, 1.0] | 16 | 0.6 [0.2, 1.9] |
| −2 ≤ SD weight-for-age Z score | 1 | 0.2 [0.0, 2.0] | 26 | 2.0 [1.0, 3.9] | 5 | 1.5 [0.5, 4.8] |
| Anaemia (<115 Hbg/L) | ||||||
| Negative | 9 | 0.4 [0.1, 1.4] | 46 | 0.5 [0.3, 1.2] | 9 | 0.3 [0.1, 1.0] |
| Positive | 6 | 2.3 [0.7, 7.5] | 23 | 1.7 [0.8, 3.7] | 8 | 3.1 [1.1, 9.4] |
| Not determined | 6 | – | 22 | – | 4 | – |
Table 3 details the point mutations with the six representative samples for the β-giardin gene, finding an exact match with sub-assemblage AII and no sequence within the three sample inspected. By contrast, each of the three samples for assemblage B was different and did not match either BIII and BIV precisely. The sequence from Sample 102 is particularly notable as there appeared to be allelic variation within the TPI gene as evidenced by split-peak chromatograms of A/G or T/C at three locations present within this region (see Annex Supplemental Fig. 1).
Table 3.
Single nucleotide polymorphisms within β-giardin of Giardia duodenalis.
| Assemblage | Isolate/Genbank number | Nucleotide position | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A isolates | Beta-giardin (bg) | 284 | 383 | 407 | 473 | 491 | 563 | 593 | 596 | 611 |
| AI | X14185.1 | C | T | T | T | A | G | T | C | A |
| AII | AY072723.1 | C | T | T | T | A | G | T | T | A |
| AIII | DQ650649.1 | T | C | C | C | G | A | C | C | G |
| Sample 9 | C | T | T | T | A | G | T | T | A | |
| Sample 22 | C | T | T | T | A | G | T | T | A | |
| Sample 103 | C | T | T | T | A | G | T | T | A | |
| B isolates | 170 | 176 | 188 | 233 | 287 | 314 | 317 | 398 | ||
| BIII | AY072726.1 | C | A | A | G | C | C | C | C | |
| BIV | AY072725.1 | T | A | A | A | T | T | T | T | |
| Sample 24 | C | A | A | A | C | T | T | C | ||
| Sample 104 | C | A | A | A | C | C | T | C | ||
| Sample 102 | C | A/G | A/G | A | C | T/C | T | C | ||
4. Discussion
The high prevalence of giardiasis reported here by real-time PCR with the 18S rDNA probe analysis (87.0%) demonstrates that children living on the shoreline of Lake Albert are at very high risk of both acute and more likely, chronic infections. The high burden of giardiasis was also corroborated in field by the Quik-Chek RDT at Runga and Bugoigo schools confirming that some 41.6% of children were patently shedding copious amounts of Giardia cysts within their stools. It is unsurprising perhaps that the levels are so high since this lakeshore environment has very poor local sanitation and water hygiene, as well as being hyperendemic for intestinal schistosomiasis, an another waterborne disease (Al-Shehri et al., 2016). Nonetheless the prevalence of giardiasis here is much elevated in comparison to other parts of the world (Thompson and Smith, 2001), although in Rwanda over 60% of rural children have been shown to be infected with Giardia by molecular typing methods (Ignatius et al., 2014). More broadly, the diagnostic sensitivity of real-time PCR methods is known to be superior to alternative diagnostic methods, often revealing giardiasis to be more pervasive (Gotfred-Rasmussen et al., 2016), and also creates opportunities for investigations of (sub)assemblage transmission dynamics (Thompson and Ash, 2016).
Given the multi-copy nature of the 18S rDNA against the lower copy number of TPI, the diagnostic sensitivity of TPI probes is lower, such that just under a half of the infected cases detected by 18S rDNA were missed. It has been stated previously that the detection limit of Giardia 18S rDNA probe assay is approaching 10 pg DNA/μL (Jaros et al., 2011), presumably that of TPI assay is much higher (Elwin et al., 2014) such that assemblage typing of ‘light’ intensity infections is not always possible. A similar level of diagnostic discordance has been observed elsewhere (Ignatius et al., 2014) which hopefully does not lead to a systematic bias in general reporting of each assemblage, as evidenced by Ct values in Fig. 1, but rather that typing parasites with assemblage-specific primers is not possible when shedding cysts are too few in number.
Nonetheless, in this sample assemblage B dominates upon comparison to assemblage A. Notably this 1:2.7 ratio varied by school with Runga having a greater proportion of assemblage A, as well as co-infection with assemblage B thereof, see Table 1, and more broadly, there appeared to be some interesting epidemiological associations by assemblage, see Table 2. Although there was no association with gender, younger children appeared to harbour a greater proportion of mixed assemblage infections than older counterparts (OR = 2.7 [95% CI; 1.0, 7.3]). There was also an indication that faecal occult blood was associated with assemblage B (OR = 2.2 [95% CI; 1.0, 5.2]) and in children being underweight (OR = 2.0 [95% CI; 1.0, 3.9]). These findings add to the general debate on the health consequences of giardiasis with particular emphasis on assemblage B, which also appears more genetically heterogeneous that assemblage A here (Thompson and Ash, 2016).
Table 1.
Prevalence (%) of G. duodenalis and assemblages across all five schools by real-time PCR; the odds ratio of assemblages A, B or A/B by school compared against the total given (with 95% confidence limits).
| School | Giardia TaqMan® 18S rDNA probe |
Assemblage (A & B) TaqMan® TPI probe |
|||||
|---|---|---|---|---|---|---|---|
| Number of positives % (x/y) |
95% CL | Number of positives % (x/y) |
95% CL | A % (x/y) OR [95% CI] |
B % (x/y) OR [95% CI] |
AB % (x/y) OR [95% CI] |
|
| Bugoigo | 94.5% (52/55) | [85.8–98.6] | 56.3% (31/55) | [43.1–69.0] | 5.4% (3/55) 0.6 [0.2, 2.5] |
43.6% (24/55) 1.4 [0.8, 2.8] |
7.2% (4/55) 0.9 [0.3, 3.1] |
| Runga | 94.1% (48/51) | [84.8–98.5] | 72.5% (37/51) | [59.2–83.4] | 15.6% (8/51) 4.7 [1.7, 13.3] |
37.2% (19/51) 2.0 [1.0, 4.3] |
19.6% (10/51) 6.9 [2.5, 19.3] |
| Walukuba | 88.0% (44/50) | [76.7–95.0] | 40.0% (20/50) | [27.2–54.0] | 2.0% (1/50) 0.1 [0.0, 1.2] |
32.0% (16/50) 0.6 [0.3, 1.8] |
6.0% (3/50) 0.5 [0.1, 1.8] |
| Biiso | 84.0% (42/50) | [71.9–92.3] | 54.0% (27/50) | [40.2–67.4] | 14.0% (7/50) 2.1 [0.8, 5.9] |
36.0% (18/50) 1.0 [0.5, 2.1] |
4.0% (2/50) 0.4 [0.1, 2.1] |
| Busingiro | 72.9% (35/48) | [59.1–84.0] | 37.5% (18/48) | [24.7–51.8] | 4.1% (2/48) 0.3 [0.1, 1.5] |
29.1% (14/48) 0.5 [0.3, 1.1] |
4.1% (2/48) 0.3 [0.1, 1.5] |
| All | 87.0% (221/254) | [82.4–90.7] | 52.4% (133/254) | [46.2–58.5] | 8.3% (21/254) —————— |
35.8% (91/254) —————— |
8.3% (21/254) —————— |
It is an interesting observation that of the six samples subjected to sequence analysis of β-giardin, the three samples selected from assemblage A were identical and could be further unequivocally assigned to sub-lineage AII, which has been reported in other studies (Cacciò and Ryan, 2008; Plutzer et al., 2010; Cacciò and Sprong, 2010; Ryan and Cacciò, 2013; Beck et al., 2012; Zhang et al., 2012). By contrast, of the three samples selected from assemblage B, there were each different, see Table 3, and none matched exactly either BIII or BIV sub-assemblages. Most notable are the point mutations at positions 176, 188 and 314, where split-peak chromatograms were observed (see Annex). This is indicative of mixed amplicon templates inferring putative allelic variation within the TPI locus. The genomic complexity of Giardia is complex, being binucleate and sometimes aneuploid (Aguiar et al., 2016) which might infer sample 102 was either a mixed co-infection of two independent B lineages or contains a single infection lineage with an unusual genomic TPI variant. Nonetheless, there is greater diversity within assemblage B and with further genetic profiling would reveal additional variants which might point towards currently unknown heterogeneities in local transmission cycles. For example, there is numerous livestock e.g. cattle and goats, that regularly enter into the lake and while drinking openly defecate into the water which may add to raised zoonotic potential in such domestic water directly drawn from the lake.
To conclude, additional sampling of school children would be worthwhile if putative associations between assemblage B and detrimental health outcomes reported here are to be fully verified statistically. Furthermore to better monitor local transmission cycles of Giardia, we encourage future studies that track each assemblage within local livestock and undertake environmental sampling of lake water where domestic water is drawn.
The following is the supplementary data related to this article.
DNA chromatograms illustrative of genetic variation at each variable position.
Acknowledgements
We wish to thank the VCD field team for their support during the fieldwork and Dr. Jaco Verweij for technical assistance in real-time PCR. HA is grateful to receive sponsored PhD studentship from the Ministry of Health, Kingdom of Saudi Arabia.
References
- Adam R.D. Biology of Giardia lamblia. Clin. Microbiol. Rev. 2001;14(3):447–475. doi: 10.1128/CMR.14.3.447-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar J.M. Evidence of heterozygosity and recombinant alleles in single cysts of Giardia duodenalis. Revista Brasileira De Parasitologia Veterinaria. 2016;25(2):187–195. doi: 10.1590/S1984-29612016031. [DOI] [PubMed] [Google Scholar]
- Almeida A., Pozio E., Cacciò S.M. Genotyping of Giardia duodenalis cysts by new real-time PCR assays for detection of mixed infections in human samples. Appl. Environ. Microbiol. 2010;76(6):1895–1901. doi: 10.1128/AEM.02305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shehri H. An extensive burden of giardiasis associated with intestinal schistosomiasis and anaemia in school children on the shoreline of Lake Albert, Uganda. Trans. R. Soc. Trop. Med. Hyg. 2016;110(10):597–603. doi: 10.1093/trstmh/trw072. [DOI] [PubMed] [Google Scholar]
- Ankarklev J. Common coinfections of Giardia intestinalis and Helicobacter pylori in non-symptomatic Ugandan children. PLoS Negl. Trop. Dis. 2012;6(8):e1780. doi: 10.1371/journal.pntd.0001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher A.J. Distribution of Giardia duodenalis assemblages A and B among children living in a remote indigenous community of the Northern Territory, Australia. PLoS One. 2014;9(11):e112058. doi: 10.1371/journal.pone.0112058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck R. Genotyping Giardia duodenalis isolates from dogs: lessons from a multilocus sequence typing study. Vector-Borne and Zoonotic Diseases. 2012;12(3):206–213. doi: 10.1089/vbz.2011.0751. [DOI] [PubMed] [Google Scholar]
- Cacciò S.M., Ryan U. Molecular epidemiology of giardiasis. Mol. Biochem. Parasitol. 2008;160(2):75–80. doi: 10.1016/j.molbiopara.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Cacciò S.M., Sprong H. Giardia duodenalis: genetic recombination and its implications for taxonomy and molecular epidemiology. Exp. Parasitol. 2010;124(1):107–112. doi: 10.1016/j.exppara.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Durigan M. Genetic diversity of Giardia duodenalis: multilocus genotyping reveals zoonotic potential between clinical and environmental sources in a metropolitan region of Brazil. PLoS One. 2014;9(12):e115489. doi: 10.1371/journal.pone.0115489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwin K. Giardia duodenalis typing from stools: a comparison of three approaches to extracting DNA, and validation of a probe-based real-time PCR typing assay. J. Med. Microbiol. 2014;63(1):38–44. doi: 10.1099/jmm.0.066050-0. [DOI] [PubMed] [Google Scholar]
- Feng Y., Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011;24(1):110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrimann S. Risk of intestinal parasitic infections in people with different exposures to waste water and fecal sludge in Kampala, Uganda: a cross-sectional study. PLoS Negl. Trop. Dis. 2016;10(3):e0004469. doi: 10.1371/journal.pntd.0004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotfred-Rasmussen H. Comparison of sensitivity and specificity of 4 methods for detection of Giardia duodenalis in feces: immunofluorescence and PCR are superior to microscopy of concentrated iodine-stained samples. Diagn. Microbiol. Infect. Dis. 2016;84(3):187–190. doi: 10.1016/j.diagmicrobio.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Graczyk T.K. Anthropozoonotic Giardia duodenalis genotype (assemblage) A infections in habitats of free-ranging human-habituated gorillas, Uganda. J. Parasitol. 2002;88(5):905–909. doi: 10.1645/0022-3395(2002)088[0905:AGDGAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Helmy Y.A. Epidemiology of Giardia duodenalis infection in ruminant livestock and children in the Ismailia province of Egypt: insights by genetic characterization. Parasit. Vectors. 2014;7(1):321. doi: 10.1186/1756-3305-7-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius R. High prevalence of Giardia duodenalis Assemblage B infection and association with underweight in Rwandan children. PLoS Negl. Trop. Dis. 2012;6(6):e1677. doi: 10.1371/journal.pntd.0001677. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius R. Detection of Giardia duodenalis assemblage A and B isolates by immunochromatography in stool samples from Rwandan children. Clin. Microbiol. Infect. 2014;20(10):O783–O785. doi: 10.1111/1469-0691.12596. [DOI] [PubMed] [Google Scholar]
- Jaros D. Detection of Giardia intestinalis assemblages A, B and D in domestic cats from Warsaw, Poland. Pol. J. Microbiol. 2011;60:259–263. [PubMed] [Google Scholar]
- Johnston A.R. Molecular epidemiology of cross-species Giardia duodenalis transmission in western Uganda. PLoS Negl. Trop. Dis. 2010;4(5):e683. doi: 10.1371/journal.pntd.0000683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M.R. Multi-locus analysis of Giardia duodenalis from non-human primates kept in zoos in China: geographical segregation and host-adaptation of assemblage B isolates. Infect. Genet. Evol. 2015;30:82–88. doi: 10.1016/j.meegid.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Laishram S., Kang G., Ajjampur S.S.R. Giardiasis: a review on assemblage distribution and epidemiology in India. Indian J. Gastroenterol. 2012;31(1):3–12. doi: 10.1007/s12664-012-0161-9. [DOI] [PubMed] [Google Scholar]
- Minetti C. Determination of Giardia duodenalis assemblages and multi-locus genotypes in patients with sporadic giardiasis from England. Parasit. Vectors. 2015;8(1):444. doi: 10.1186/s13071-015-1059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhsen K., Levine M.M. A systematic review and meta-analysis of the association between Giardia lamblia and endemic pediatric diarrhea in developing countries. Clin. Infect. Dis. 2012;55:S271–S293. doi: 10.1093/cid/cis762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizeyi J.B. Cryptosporidium sp. and Giardia sp. infections in mountain gorillas (Gorilla gorilla beringei) of the Bwindi Impenetrable National Park, Uganda. J. Parasitol. 1999;85:1084–1088. [PubMed] [Google Scholar]
- Nizeyi J., Cranfield M., Graczyk T. Cattle near the Bwindi Impenetrable National Park, Uganda, as a reservoir of Cryptosporidium parvum and Giardia duodenalis for local community and free-ranging gorillas. Parasitol. Res. 2002;88(4):380–385. doi: 10.1007/s00436-001-0543-x. [DOI] [PubMed] [Google Scholar]
- Pelayo L. Giardia infections in Cuban children: the genotypes circulating in a rural population. Ann. Trop. Med. Parasitol. 2008;102(7):585–595. doi: 10.1179/136485908X355247. [DOI] [PubMed] [Google Scholar]
- Plutzer J., Ongerth J., Karanis P. Giardia taxonomy, phylogeny and epidemiology: facts and open questions. Int. J. Hyg. Environ. Health. 2010;213(5):321–333. doi: 10.1016/j.ijheh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Puebla L.J. Correlation of Giardia duodenalis assemblages with clinical and epidemiological data in Cuban children. Infect. Genet. Evol. 2014;23:7–12. doi: 10.1016/j.meegid.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Rogawski E.T. Determinants and impact of Giardia infection in the first 2 years of life in the MAL-ED birth cohort. Journal of the Pediatric Infectious Diseases Society. 2017;6(2):153–160. doi: 10.1093/jpids/piw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U., Cacciò S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013;43(12−13):943–956. doi: 10.1016/j.ijpara.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Sprong H., Cacciò S.M., van der Giessen J.W. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl. Trop. Dis. 2009;3(12):e558. doi: 10.1371/journal.pntd.0000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi K. Population-based analyses of Giardia duodenalis is consistent with the clonal assemblage structure. Parasit. Vectors. 2012;5(1):168. doi: 10.1186/1756-3305-5-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellevik M.G. Prevalence of Cryptosporidium parvum/hominis, Entamoeba histolytica and Giardia lamblia among young children with and without diarrhea in Dar es Salaam, Tanzania. PLoS Negl. Trop. Dis. 2015;9(10) doi: 10.1371/journal.pntd.0004125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.C.A., Ash A. Molecular epidemiology of Giardia and Cryptosporidium infections. Infect. Genet. Evol. 2016;40:315–323. doi: 10.1016/j.meegid.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Thompson R., Smith A. Zoonotic enteric protozoa. Vet. Parasitol. 2001;182(1):70–78. doi: 10.1016/j.vetpar.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Vanni I. Detection of Giardia duodenalis assemblages A and B in human feces by simple, assemblage-specific PCR assays. PLoS Negl. Trop. Dis. 2012;6(8):e1776. doi: 10.1371/journal.pntd.0001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij J.J. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J. Clin. Microbiol. 2004;42(3):1220–1223. doi: 10.1128/JCM.42.3.1220-1223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegayehu T. Multilocus genotyping of Giardia duodenalis isolates from children in Oromia Special Zone, Central Ethiopia. BMC Microbiol. 2016;16(1):89. doi: 10.1186/s12866-016-0706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2007. Growth Reference Data for 5–19 Years. [Google Scholar]
- WHO Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. 2011. http://www.who.int/vmnis/indicators/haemoglobin.pdf Download from: (2015)
- Zhang W. Genetic characterizations of Giardia duodenalis in sheep and goats in Heilongjiang Province, China and possibility of zoonotic transmission. PLoS Negl. Trop. Dis. 2012;6(9):e1826. doi: 10.1371/journal.pntd.0001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DNA chromatograms illustrative of genetic variation at each variable position.