Abstract
Objectives
Microorganisms in environmental samples are identified by sequential screening, isolation, and culture steps, followed by the verification of physiological characteristics and morphological classification. Isolation and purification of Amoebae from soil samples is extremely complex, laborious, and time-consuming and require considerable expertise for morphological evaluation. PCR testing of soil DNA seems to be an effective means for protozoa habitat screening. In this study, we added Acanthamoeba sp. (MK strain) to soil and developed a method of extracting protozoan DNA from the soil.
Methods
Soil allophane is a known DNA adsorbing substance that inhibits the PCR reaction. After comparing the soil properties and allophane contents of 7 soil samples, we attempted to combine multiple cell disruption and DNA purification methods to design an optimal soil DNA extraction method that can be used for downstream PCR analysis.
Results
We compared five different crushing/refining methods. Amplification of the gene was confirmed by Acanthamoeba specific PCR in protocol V where the concentration of Acanthamoeba in soil (1.0 × 102/g) was the detection limit of PCR.
Conclusion
The soil DNA extraction method following protocol V allows DNA amplification of protozoa, including Amoeba, which is difficult to cultivate, thus simplifying the investigation of protozoa habitats and genetic analyses.
Keywords: Soil, DNA, Protozoan, PCR inhibitor, Allophane, Epidemiological survey
1. Introduction
Microorganisms in environmental samples are identified by sequential screening, isolation, and culture steps, followed by verification of their physiological characteristics and morphological classification (Garland and Mills, 1991; Shi-Ying et al., 2018; Yamanouchi et al., 2018a; Dahal et al., 2018). Isolation of the unicellular protozoan, Amoeba, from soil is particularly challenging, time-consuming, labor-intensive, and requires complex culture purification techniques and significant expertise for morphological evaluation (Denet et al., 2017; Shokri et al., 2016; Yamanouchi et al., 2018b). Some soil amoebae are pathogenic to humans (Yamanouchi et al., 2018b; Pinna et al., 2017), and their isolation by culture methods is necessary for evaluation of their habitat and ecology. However, the generation time of amoeba is much longer than that of bacteria, and it is very difficult to avoid bacterial or fungal contamination in isolates using such methods (Yamanouchi et al., 2018b; Niyyati et al., 2016). Additionally, off-target events may occur frequently when amoeba isolated from soil is selected based on morphology and used for species-specific PCR identification (Yamanouchi et al., 2018b; Niyyati et al., 2016; Saburi et al., 2017). In this study, we collected DNA of soil amoebae and attempted to design a DNA extraction method for soil screening.
In recent years, DNA extraction from soil and comprehensive analysis of soil microbial environment by next generation sequence analysis has been actively researched and utilized in agricultural, ecological, and environmental studies (Shokralla et al., 2012; Rodrigues et al., 2013; Jacobsen and Hjelmsø, 2014). As a result, extracting environmental DNA (eDNA), particularly in bacteriological studies, has gained special focus and a large number of kits for extracting soil DNA are offered by manufacturers worldwide. Soil DNA extraction methods using such kits are classified into direct and indirect extraction methods (Gabor et al., 2003; Delmont et al., 2011; Courtois et al., 2001). The indirect method consists of recovering microbial fractions from soil followed by DNA extraction unlike the direct method that, as the name suggests, involves extracting DNA directly from the soil. The indirect extraction methods typically have lower soil contamination but also reduced yield of extractable DNA. In contrast, the direct extraction method has a higher yield of DNA but with significant soil contaminants. Although several physical, enzymatic, and chemical strategies have been adopted for cell disruption, issues such as DNA yield, length, and purity need to be resolved. Furthermore, soil in Japan contains rare volcanic ash (Aomine and Yoshinaga, 1955). Volcanic ash black soil has a global distribution of less than 1%. In contrast, more than 40% of the fields in Japan contain volcanic ash black soil (Takahashi and Shoji, 2001). Studies show that black soil contains large amounts of activated aluminum (allophane) (Zhang et al., 2017; Parfitt and Kimble, 1988), which adsorbs the extracted DNA, making it difficult to collect DNA and use in an experimental system such as PCR (Saeki et al., 2011; Yu-Tuan et al., 2014).
In this study, we attempted to design an optimal protocol for extracting large protozoan DNA in soil habitats using ISOIL (Nippon Gene, Japan), a DNA collection kit suitable for black soil typical to Japan.
2. Material and methods
2.1. Soil collection and soil specific gravity measurement
Six soil specimens were collected in Japan from fields, nature park and 7 specimens including 1 commercially available soil for gardening were included in the experiment. In the soil, 100 ml (Vt) of soil was collected by removing the vegetation and dead leaves of the surface layer. The collected samples were thoroughly dried at 60 °C, overnight, followed by measurement of the mass (Ms), and the dry bulk density (ρd)/ml was calculated from ‘ρd = Ms ÷ Vt’. The dry bulk density of volcanic ash soil (black soil) is smaller than 1/ml, because of the high organic matter content (Soane, 1990).
2.2. Determination of soil properties based on feel
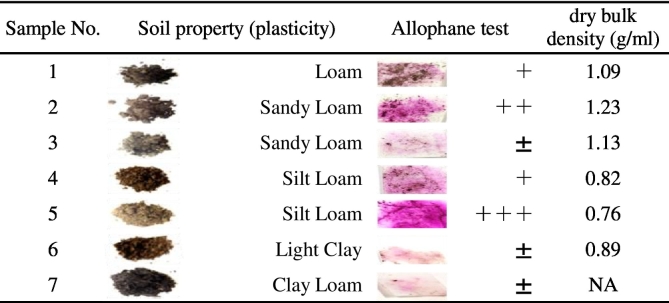
Soil properties were determined using 7 categories of international classification method based on soil texture and feel (Table 1) (Gabor et al., 2003).
Table 1.
Soil seven taxonomy by soil plasticity.
| Soil classification (international law) | Judgment methods |
|---|---|
| Sand: s | Almost sandy, no feel sticky at all. |
| Sandy loam: SL | The feeling of sand is strong, there is only a little stickiness. |
| Loam: L | Feel somewhat sandy and sticky. Sand and clay are felt to the same extent. |
| Silt loam: SiL | Not feel the sand very much, but feel like a flaky flour. |
| Clay loam: CL | Feel a bit of sand, but it is sticky. |
| Light clay: LiC | Hardly feel the sand and it sticks well. |
| Heavy clay: HC | Not feel the sand and it is sticky very well. |
2.3. Measurement of allophane degree of soil specimens
Allophane test is a method for measuring active aluminum (allophane), and it is often used for characterizing black soil (Japanese Society of Pedology, 2010; Bracewell et al., 1970). A small amount of soil was rubbed vigorously with the fingertips on a phenolphthalein paper and a sodium fluoride solution was added dropwise and observed for color change.
2.4. Construction of DNA extraction protocol
Soil sample #7 was used for designing the protocol. A mixture of Acanthamoeba MK strain with 1.0 × 105/g was added to the sample followed by DNA extraction. Protocols I and II involved removal of DNA adsorption factor by skimmed milk and protocols III and IV involved the use of glass beads (φ0.35 mm) to increase DNA yield by the indirect extraction method. Protocol V was created from the results of protocols I–IV.
In protocol V, physical disintegration with glass beads (φ0.35 mm) was attempted in addition to SDS Lysis Buffer for the chemical lysis of cells in the direct DNA extraction method. For DNA precipitation method, polyethylene glycol (PEG) and isopropanol, which are reported to have a lower coprecipitation rate of allophane than ethanol, were used. Protocols I–V are described in Table 2. The extracted DNA was used for Acanthamoeba-specific-PCR.
Table 2.
Protocol list for soil DNA extraction.
| Extraction steps | Protocols |
||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| 1 | 10 g of soil was placed in a 50 ml conical tube, Acanthamoeba MK strain (1.0 × 10 6 amoeba) was added, physiological saline was added and well agitated. | 10 g of soil was placed in a 50 ml conical tube, Acanthamoeba MK strain (1.0 × 10 6 amoeba) was added, and then 400 mg of skim milk and 4g of glass beads were added. | |||
| 2 | The soil solution was filtered with 2 pieces of gauze and centrifuged at 2300g rpm for 10 min at room temperature. | Soil solution was allowed to stand for 5 min, and 30 ml of supernatant was recovered. | 10 ml of pH 8.0 PBS and 8 ml of SDS Lysis Buffer were added and after 45 s vortexing, incubation was carried out at 60 °C. for 1 h. | ||
| 3 | The soil sediment was taken in medicine packaging paper and dried for about 2 h. | The supernatant was centrifuged at 2300g for 5 min, and the precipitate was recovered. The precipitate was dried for 2 h. | 45 s vortexed and centrifuged at 9000g for 20 min, room temperature. | ||
| 4 | Soil dried sediment 0.5 g | Soil dried sediment 0.5 g + Skim milk 20 mg | Soil dried sediment 0.5 g + Skim milk 20 mg | Soil dried sediment 0.5 g + Skim milk 20 mg + Glass beads 20 mg | The supernatant was transferred to a new 50 ml conical tube, 1/2 amount of 30% PEG-1.6 M NaCl was added and the mixture was vortexed and then allowed to stand overnight at room temperature. |
| 5 | |||||
| 6 | After centrifugation at 9000g for 20 min, room temperature, the supernatant was discarded. | ||||
| 7 | 8 ml of pH 8.0 TE Buffer was added to the sediment and dissolved. An equal volume of PCI was added to the solution, vortexed to milky white, and centrifuged at 9000g for 25 min, 4 °C. | ||||
| 8 | The upper aqueous layer was recovered and left at −20 °C. for 20 min at a ratio of aqueous layer: isopropanol: 3 M acetic acid Na = 1: 0.7: 0.1, and it was left standing at 9000 g for 15 min, 4 °C. | ||||
| 9 | DNA extraction (dissolved in 600 μl of pH 8.0 TE Buffer) with QIAamp DNA Mini | Dry the DNA precipitate and dissolve in 600 μl pH 8.0 TE Buffer. | |||
| 10 | |||||
| 11 | DNA extraction with ISOIL | ||||
2.5. Qualitative and quantitative evaluation of extracted DNA using protocol (I–V)
DNA quantity for each protocol was measured using a NanoDrop® 1000 (Thermo Fisher Scientific, USA), and agarose gel (1.2%) electrophoresis. The purity was determined using the 260/230 and 260/280 absorbance ratios.
2.6. Investigation of the usefulness of protocol V to Japanese soil
We investigated whether the DNA, extracted using protocol V was compatible with PCR. Acanthamoeba MK strain with 10 × 105/g was added to the 7 soil samples, followed by DNA extracted using protocol V. Subsequently, the extracted DNA was used for Acanthamoeba specific PCR.
2.7. Detection sensitivity of DNA extracted with protocol V
A spike sample containing 1.0 × 101 to 105 with Acanthamoeba MK Strain added to soil No. 7 (10 g) was prepared. DNA extraction was performed using protocol V for spike samples followed by Acanthamoeba-specific-PCR.
2.8. Conditions for Acanthamoeba-specific-PCR
JDP1/JDP2 primer sets and AmpliTaq Gold DNA Polymerase with Buffer II (Thermo Fisher Scientific, USA) were used for Acanthamoeba-specific-PCR in this study. PCR conditions were as follows: 35 cycles of thermal denaturation at 95 °C for 10 s; annealing at 55 °C for 30 s; and elongation at 72 °C for 60 s (Dyková et al., 1999). PCR products were identified with gel electrophoresis on a 1.2% agarose gel (Takara Bio, Japan) performed with 5 μl of the reaction solution.
3. Results
3.1. Determination of soil property and allophane degree
Table 3 shows result of the soil temporary specific gravity, soil property and allophane measurement. Allophane detection was high in No. 2 and No. 5 soil.
Table 3.
Results of judgment of soil property, soil type and allophane degree.
±: After a while it is weakly colored. +: Immediate coloring, but its extent is weak. ++: Color immediately and vividly. +++: Instantly color very clearly. NA: Not Available.
3.2. Comparison of DNA quantity and purity by soil DNA extraction protocol
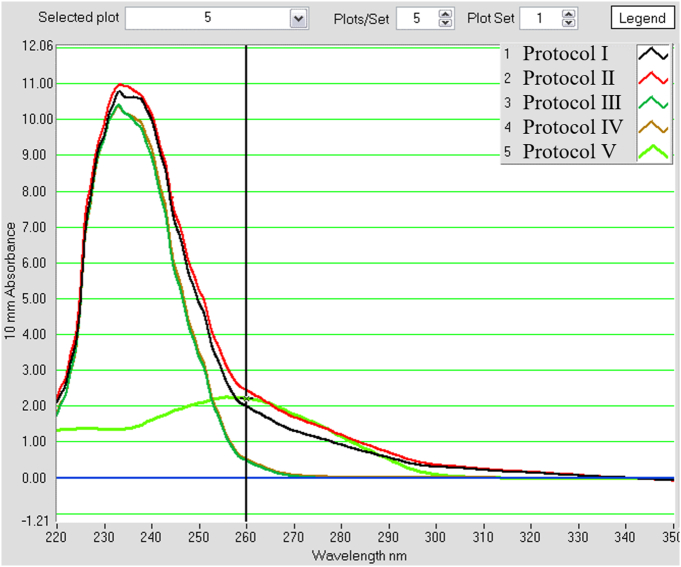
The amount of DNA for each extraction protocol was shown in Table 2. We observed an increase in DNA yield in protocols I and II using the direct extraction method, compared to indirect extraction methods used in protocols III and IV. DNA yield in protocol II, to which skimmed milk was added, was greater than that in protocol I. Protocol IV, using glass beads increased the DNA yield moderately compared to III (Table 4). Although DNA yield could be increased by skimmed milk and glass beads, amplification of Acanthamoeba gene was impossible in protocols I–IV. In protocol V, DNA was extracted by direct extraction method where skimmed milk and glass beads were also added. PEG and isopropanol were used to precipitate DNA. DNA yield in protocol V showed an improved A260/230 ratio. The peak absorbance was at 260 nm in protocol V in contrast with protocols I to IV where the absorbance peaked at 230 nm, implying higher purity of DNA extracted in protocol V (Fig. 1).
Table 4.
Comparison of DNA yield and purity by protocol I–V.
| Protocol no. | DNA yield (ng/μl) | A260/280 | A260/230 |
|---|---|---|---|
| I | 99.78 | 2.17 | 0.21 |
| II | 122.2 | 2.04 | 0.25 |
| III | 23.93 | NA | 0.05 |
| IV | 26.12 | NA | 0.06 |
| V | 110.9 | 1.92 | 1.63 |
NA: Not Available.
Fig. 1.
Comparison of DNA quantity and purity of soil DNA extraction protocol by Nono drop 1000.
3.3. PCR results of DNA extraction protocol (I–V)
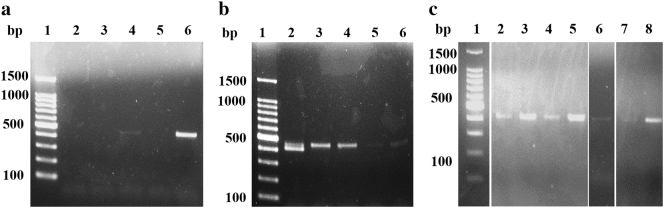
DNA extracted in protocols I, II and IV failed to produce any PCR signal. In protocol III, we observed a thin band. In protocol V, we were able to confirm the amplification of the gene most clearly (Fig. 2-A).
Fig. 2.
Agarose electrophoresis of Acanthamoeba-specific-PCR.
(A) PCR of extracted DNA with protocol I–V.
Lane 1) 100 bp ladder (Nippon gene, Japan), Lane 2) Protocol I, Lane 3) Protocol II, Lane 4) Protocol III, Lane 5) Protocol IV, Lane 6) Protocol V.
(B) PCR of spike sample DNA extracted with protocol V.
Lane 1) 100 bp ladder (Nippon gene, Japan), Lane 2) 1.0 × 105 amebas/10 g soil, Lane 3) 1.0 × 104 amebas/10 g soil, Lane 4) 1.0 × 103 amebas/10 g soil, Lane 5) 1.0 × 102 amebas/10 g soil, Lane 6) 1.0 × 101 amebas/10 g soil.
(C) PCR of soil No. 1-7 DNA extracted with protocol V.
Lane 1) 100 bp ladder (Nippon gene, Japan), Lane 2) No. 1, Lane 3) No. 2, Lane 4) 1 No. 3, Lane 5) No. 4, Lane 6) No. 5, Lane 7) No. 6, Lane 8) No. 7.
3.4. PCR sensitivity of DNA extracted with protocol V, PCR of 7 soil samples using protocol V
Gene amplification was confirmed in all spiked samples (soil # 7 to which Acanthamoeba MK strain was added). However, the band was difficult to identify when the added amount was less than 1.0 × 102/g (Fig. 2-B).
We examined the effectiveness of protocol V in soil with varying properties and allophane content. In soil # 1 through 7, Acanthamoeba MK strain was added at 1.0 × 103/g and soil DNA was extracted with protocol V. Acanthamoeba gene amplification was confirmed in DNA from all seven soil samples (Fig. 2-C).
4. Discussion
In this study, we extracted PCR-compatible DNA using protocol V from all 7 soil specimens. To create protocol V, we first verified protocols I–IV individually for the yield and purity of DNA. Between protocols I and II, an increase in DNA yield was observed with protocol II supplemented with skimmed milk. Although the mechanism is unknown, skimmed milk is adsorbed as a substitute by the DNA adsorbing substance contained in the soil resulting in increased DNA yield (Takada and Matsumoto, 2005; Volossiouk et al., 1995). In both protocols I and II, the absorbance of the DNA solution showed a value of A260/280 (standard value: 1.8 or more) of 2.0 or greater indicating that the samples did not contain any significant protein impurities. However, we did not observe any gene amplification signal from the soil DNA of protocols I and II using the JDP 1/2 primer set. This may be due to low A260/230 (reference value: 1.8 or more) ratios of the samples implying the presence of contaminants such as phenols and allophane that could have inhibited PCR in the DNA obtained with protocols I and II (Labarca and Paigen, 1980). This result emphasized on the requirement of a new protocol to remove contaminants. Therefore, in order to increase the degree of DNA purification, we created protocol III consisting of a two-step purification strategy using QIAamp DNA Mini kit and ISOIL. Amplification of DNA was observed in protocol III, but since the yield of extracted DNA was low, protocol IV was conducted to perform a strong disruption with glass beads. In protocol IV, DNA yield increased but the PCR amplification was negative. Our results from protocols III and IV suggested that although glass bead-based disruption is an effective strategy to increase DNA yield, indirect extraction using soil sediment or a two-step purification method using two kits reduces DNA yield and increases costs. This may not be feasible in epidemiological research where typically larger number of samples require to be tested.
Based on the results from protocols I–IV, we created a new protocol V, in which skimmed milk and glass beads were added to the soil and the final refining was done with ISOIL. In addition, isopropanol and PEG are reported to be superior to alcohols because they do not coprecipitate allophane (Takada and Matsumoto, 2005). Protocols I–IV use indirect extraction with filtered soil sediment. And when comparing protocols I and II with III and IV, the purification step increased to two stages, in III and IV and approximately 90% of the DNA was lost. In protocol V with a three-step DNA precipitation, it was predicted that the DNA loss would be even higher. Therefore, we decided to adopt a direct extraction method using all soil components. As a result, although the DNA yield in protocol V was inferior to that obtained in protocols I and II, we managed to obtain highly purified DNA with A260/280 of 1.92 and A260/230 of 1.63. Furthermore, by applying this protocol V, DNA, extracted from all seven soil samples yielded positive signal from Acanthamoeba-specific-PCR. The variable allophane content and soil properties implied that it is possible to acquire PCR-compatible DNA purification and yield from multiple soil sources using protocol V. Although this protocol uses a DNA extraction kit suitable for Japanese domestic soil, it is possible that other kits can be applied. In this report, we do not investigate soil microorganisms using protocol V. However, we have already successfully applied this protocol to the habitat survey of Balamuthia mandrillaris, a microorganism reported to be present in the soil (Yamanouchi et al., 2018b). Thus, we believe that the DNA collected by this method can be used for biological surveys in multiple soil types. Also, this protocol does not require high-speed centrifugation steps at the production stage using the 10 g soil prior to using the kit, and thus can be easily used for epidemiological research in developing countries where facilities are not sufficient.
Acknowledgments
Acknowledgements
This work was supported by a Grant-in-aid for Scientific Research for Young Scientists (JSPS KAKENHI Grant Number 18K17349) and Interdisciplinary Collaborative Research Grant for Young Scientists, Hirosaki University.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Kanako Yamanouchi, Email: kanako.8@hirosaki-u.ac.jp.
Hiroaki Arima, Email: bb55417001@ms.nagasaki-u.ac.jpb.
Takakiyo Tsujiguchi, Email: r.tsuji@hirosaki-u.ac.jp.
References
- Aomine S., Yoshinaga N. Clay minerals of some well-drained volcanic ash soils in Japan. Soil Sci. 1955;79(5):349–358. [Google Scholar]
- Bracewell J.M., Campbell A.S., Mitchell B.D. An assessment of some thermal and chemical techniques used in the study of the poorly-ordered aluminosilicates in soil clays. Clay Miner. 1970;8(3):325–335. [Google Scholar]
- Courtois S., Frostegård A., Göransson P., Depret G., Jeannin P., Simonet P. Quantification of bacterial subgroups in soil: comparison of DNA extracted directly from soil or from cells previously released by density gradient centrifugation. Environ. Microbiol. 2001;3(7):431–439. doi: 10.1046/j.1462-2920.2001.00208.x. [DOI] [PubMed] [Google Scholar]
- Dahal R.H., Chaudhary D.K., Kim J. Rhodanobacter hydrolyticus sp. nov., a novel DNA- and tyrosine-hydrolysing gammaproteobacterium isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2018;68(8):2580–2586. doi: 10.1099/ijsem.0.002881. [DOI] [PubMed] [Google Scholar]
- Delmont T.O., Robe P., Clark I., Simonet P., Vogel T.M. Metagenomic comparison of direct and indirect soil DNA extraction approaches. J. Microbiol. Methods. 2011;86(3):397–400. doi: 10.1016/j.mimet.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Denet E., Coupat-Goutaland B., Nazaret S., Pélandakis M., Favre-Bonté S. Diversity of free-living amoebae in soils and their associated human opportunistic bacteria. Parasitol. Res. 2017;116(11):3151–3162. doi: 10.1007/s00436-017-5632-6. [DOI] [PubMed] [Google Scholar]
- Dyková I., Lom J., Schroeder-Diedrich J.M., Booton G.C., Byers T.J. Acanthamoeba strains isolated from organs of freshwater fishes. J. Parasitol. 1999;85(6):1106–1113. [PubMed] [Google Scholar]
- Gabor E.M., de Vries E.J., Janssen D.B. Efficient recovery of environmental DNA for expression cloning by indirect extraction methods. FEMS Microbiol. Ecol. 2003;44(2):153–163. doi: 10.1016/S0168-6496(02)00462-2. 1. [DOI] [PubMed] [Google Scholar]
- Garland J.L., Mills A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991;57(8):2351–2359. doi: 10.1128/aem.57.8.2351-2359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen C.S., Hjelmsø M.H. Agricultural soils, pesticides and microbial diversity. Curr. Opin. Biotechnol. 2014;27:15–20. doi: 10.1016/j.copbio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Japanese Society of Pedology . Hakubunkan Shinsha Publishers; 2010. Soil Survey Handbook. (Chapter 4) [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal. Biochem. 1980;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Niyyati M., Karamati S.A., Morales J.L., Lasjerdi Z. Isolation of Balamuthia mandrillaris from soil samples in North-Western Iran. Parasitol. Res. 2016;115(2):541–545. doi: 10.1007/s00436-015-4770-y. [DOI] [PubMed] [Google Scholar]
- Parfitt R.L., Kimble J.M. Conditions for formation of allophane in soils. Soil Sci. Soc. Am. J. 1988;53(3):971–977. [Google Scholar]
- Pinna A., Porcu T., Boscia F., Cano A., Erre G., Mattana A. Free-living amoebae keratitis. Cornea. 2017;36(7):785–790. doi: 10.1097/ICO.0000000000001226. [DOI] [PubMed] [Google Scholar]
- Rodrigues J.L., Pellizari V.H., Mueller R., Baek K., Jesus J.E., Paula F.S., Mirza B., Hamaoui G.S., Tsai S.M., Feigl B., Tiedje J.M., Bohannan B.J., Nüsslein K. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc. Natl. Acad. Sci. U. S. A. 2013;110(3):988–993. doi: 10.1073/pnas.1220608110. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saburi E., Rajaii T., Behdari A., Kohansal M.H., Vazini H. Free-living amoebae in the water resources of Iran: a systematic review. J. Parasit. Dis. 2017;41(4):919–928. doi: 10.1007/s12639-017-0950-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki K., Kunito T., Sakai M. Effect of Tris-HCl buffer on DNA adsorption by a variety of soil constituents. Microbes Environ. 2011;26(1):88–91. doi: 10.1264/jsme2.me10172. [DOI] [PubMed] [Google Scholar]
- Shi-Ying Z., Cong F., Yong-Xia W., Yun-Sheng X., Wei X., Xiao-Long C. Salt-tolerant and plant growth-promoting bacteria isolated from high-yield paddy soil. Can. J. Microbiol. 2018;(27) doi: 10.1139/cjm-2017-0571. [DOI] [PubMed] [Google Scholar]
- Shokralla S., Spall J.L., Gibson J.F., Hajibabaei M. Next-generation sequencing technologies for environmental DNA research. Mol. Ecol. 2012;21(8):1794–1805. doi: 10.1111/j.1365-294X.2012.05538.x. [DOI] [PubMed] [Google Scholar]
- Shokri A., Sarvi S., Daryani A., Sharif M. Isolation and genotyping of Acanthamoeba spp. as neglected parasites in north of Iran. Korean J. Parasitol. 2016;54(4):447–453. doi: 10.3347/kjp.2016.54.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soane B.D. The role of organic matter in soil compactibility: a review of some practical aspects. Soil Tillage Res. 1990;16(1):179–201. [Google Scholar]
- Takada Y., Matsumoto N. Skim milk drastically improves the efficacy of DNA extraction from andisol, a volcanic ash soil. Agric. Environ. 2005;39(4):247–252. [Google Scholar]
- Takahashi T., Shoji S. Distribution and classification of volcanic ash soil. Glob. Environ. Res. 2001;10:83–95. [Google Scholar]
- Volossiouk T., Robb E.J., Nazar R.N. Direct DNA extraction for PCR-mediated assays of soil organisms. Appl. Environ. Microbiol. 1995;61(11):3972–3976. doi: 10.1128/aem.61.11.3972-3976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi K., Arima H., Sakamoto Y., Kanto K., Kasai K., Ito K., Inaba T. First report of the isolation of Balamuthia mandrillaris in the northern region of Japan. Parasitol. Res. 2018;117(9):2895–2900. doi: 10.1007/s00436-018-5980-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi K., Sakamoto Y., Fujioka M., Kasai K., Tsujiguchi T., Kimura T., Shimazu M., Kato A., Ito K. The effects of Pantoea and Kosakonia isolated from buckwheat sprouts on obese mice. Appl. Microbiol.: Open Access. 2018;4(1) [Google Scholar]
- Yu-Tuan H., David J.L., Churchman G.J., Schipper A. Louis, Rawlence Nicolas J., Cooper Alan. Carbon storage and DNA adsorption in allophanic soils and paleosols. Soil Carbon. 2014:163–172. [Google Scholar]
- Zhang J., Xiao J., Li S., Ran W. Manure amendment increases the content of nanomineral allophane in an acid arable soil. Sci. Rep. 2017;7(1):14256. doi: 10.1038/s41598-017-14445-2. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]