Key Points
Question
Is positive psychology effective as a treatment for chronic arthritis pain and does it reduce race disparities in pain management?
Findings
In this randomized clinical trial involving 360 Veterans Affairs patients with chronic pain from osteoarthritis, a 6-week telephone-administered positive psychological intervention did not improve pain or functional difficulty vs a control program. No difference by race was found in the effect of the intervention.
Meaning
A telephone-administered positive psychological intervention was not associated with improvement in chronic pain or functional difficulty from osteoarthritis for either white or African American patients.
Abstract
Importance
Positive psychological interventions for improving health have received increasing attention recently. Evidence on the impact of such interventions on pain, and racial disparities in pain, is limited.
Objective
To assess the effects of a positive psychological intervention on pain and functional difficulty in veterans with knee osteoarthritis.
Design, Setting, and Participants
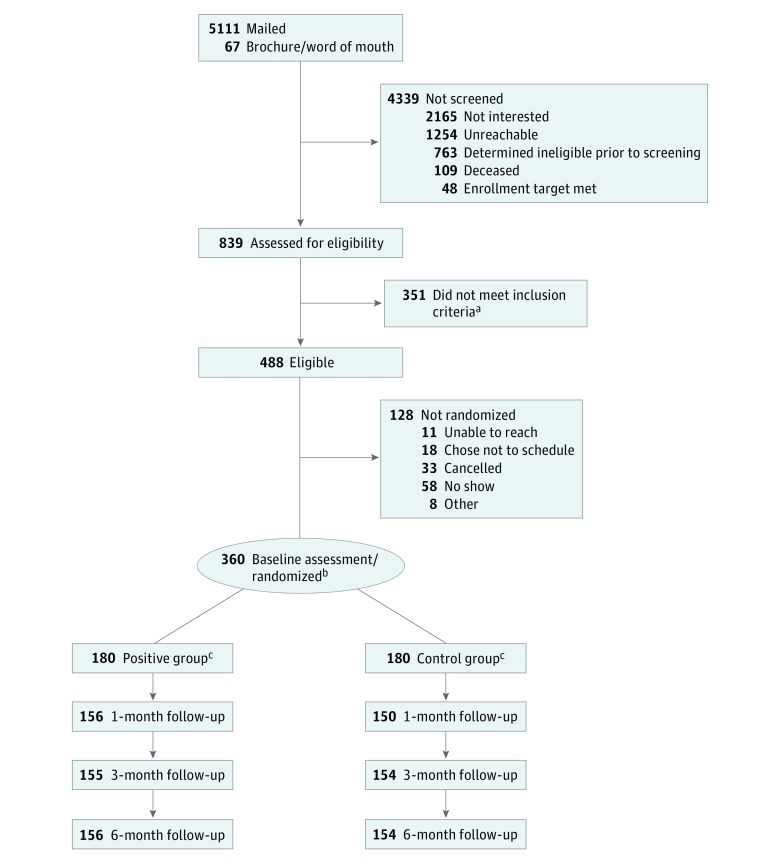
The Staying Positive With Arthritis Study is a large, double-blinded randomized clinical trial powered to detect race differences in self-reported pain in response to a positive psychological intervention compared with a neutral control intervention. Data were collected from 2 urban Veterans Affairs medical centers. Participants included non-Hispanic white and non-Hispanic African American patients aged 50 years or older with a diagnosis of osteoarthritis. Mailings were sent to 5111 patients meeting these criteria, of whom 839 were fully screened, 488 were eligible, and 360 were randomized. Enrollment lasted from July 8, 2015, to February 1, 2017, with follow-up through September 6, 2017.
Interventions
The intervention comprised a 6-week series of evidence-based activities to build positive psychological skills (eg, gratitude and kindness). The control program comprised similarly structured neutral activities. Programs were delivered via workbook and weekly telephone calls with interventionists.
Main Outcomes and Measures
The primary outcomes were self-reported pain and functional difficulty measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC; range 0-100). Secondary outcomes included affect balance and life satisfaction.
Results
The sample included 180 non-Hispanic white patients and 180 non-Hispanic African American patients (mean [SD] age, 64.2 [8.8] years; 76.4% were male). Mean (SD) baseline scores for WOMAC pain and functional difficulty were 48.8 (17.6) and 46.8 (18.1), respectively. Although both decreased significantly over time (pain: χ23 = 49.50, P < .001; functional difficulty: χ23 = 22.11, P < .001), differences were small and did not vary by treatment group or race. Exploratory analyses suggested that the intervention had counterintuitive effects on secondary outcomes.
Conclusions and Relevance
The results of this randomized clinical trial do not support the use of positive psychological interventions as a stand-alone treatment for pain among white or African American veterans with knee osteoarthritis. Adaptations are needed to identify intervention components that resonate with this population, and the additive effect of incorporating positive psychological interventions into more comprehensive pain treatment regimens should be considered.
Trial Registration
ClinicalTrials.gov Identifier: NCT02223858
This randomized clinical trial assesses the effects of a positive psychological intervention on pain and functional difficulty vs a control program in veterans with knee osteoarthritis and evaluates whether this management varies by race.
Introduction
With increasing acceptance of complementary and integrative health practices, there has been a surge of interest in using positive psychological interventions to improve the well-being of patients with chronic illness.1,2,3,4,5,6,7 Such interventions include activities that increase positive affect and cultivate qualities such as gratitude and kindness,1,2,8,9 and are based on theoretical and empirical work linking positive psychological skills and health.1,8,10 Evidence indicates that positive psychological interventions reduce depressive symptoms and increase overall well-being.1,2,3 Studies have begun testing the effects of positive psychological interventions in patient populations with chronic health conditions other than depression,11,12,13,14,15,16,17,18,19 and have started examining their effects on physical outcomes such as pain.18,20,21,22,23,24
The potential of positive psychological interventions to relieve chronic pain is supported by work demonstrating that positive affect can promote pain resiliency through neurobiological and cognitive pathways.25 Although some evidence suggests that participating in a positive psychological intervention decreases pain,18,21,24 reviews of extant research have concluded that large, well-controlled randomized trials are needed to delineate the benefits and limitations of positive psychological interventions for use in clinical care.3,4,5
This article reports findings of the Staying Positive With Arthritis Study, the largest randomized clinical trial, to our knowledge, testing the effects of a positive psychological intervention on self-reported pain and functional difficulty in patients with chronic pain from knee osteoarthritis (OA).26 The most common form of arthritis,27 OA is a condition for which positive psychological interventions have not previously been tested in a large trial. The objective of the Staying Positive With Arthritis Study was to evaluate the effect of a positive psychological intervention, compared with an active control program, on pain and functional difficulty in a predominantly male sample of non-Hispanic white and non-Hispanic African American patients with knee OA. We hypothesized that patients randomized to a 6-week positive psychological intervention (vs control program) would report greater improvements in the primary outcomes of self-reported pain and functional difficulty from baseline to 6 months, and that improvements would be larger for African American patients than for white patients. We powered the study to detect racial differences in response to the intervention because African American individuals (vs white individuals) tend to report worse OA-related pain and disability28,29,30 and express stronger preferences for nontraditional, nonpharmacological approaches to pain management.31,32,33,34,35 Affect balance36,37 and life satisfaction38 were secondary outcomes.
Method
Study Participants and Recruitment Strategy
The full study protocol and statistical analysis plan are published elsewhere26 and are available in Supplement 1. Briefly, patients with symptomatic knee OA from Veterans Affairs (VA) medical centers in Pittsburgh and Philadelphia, Pennsylvania, were recruited by mail and telephone. Mailings were sent to patients meeting basic eligibility criteria based on their VA medical records (Table 1). Patients who expressed interest or did not respond within 2 weeks were telephoned to be fully screened for eligibility (Table 1).39,40 Patients who learned about the study from flyers at participating sites were also screened. The VA Central institutional review board approved the study. We followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline
Table 1. Staying Positive With Arthritis Study Inclusion and Exclusion Criteria for Initial Mailing and Full Studya.
| Inclusion | Exclusion |
|---|---|
| Eligibility criteria for initial mailing (based on VA electronic medical record) | |
| Aged ≥50 y | Deceased |
| Non-Hispanic white or non-Hispanic African American race | Nonveteran |
| Had a primary care appointment at a participating site in the past 12 mo | Inflammatory arthritis (rheumatoid arthritis [ICD-9: 714.xx], lupus [ICD-9: 695.4, 710.0], psoriatic arthritis [ICD-9: 696.0], and ankylosing spondylitis [ICD-9: 720.0]) |
| Osteoarthritis (ICD-9: 715) | Alzheimer disease and dementia (ICD-9: 294.xx, 290.xx, 291.xx, 331.xx, 094.1) |
| Eligibility criteria for enrollment (based on telephone screen) | |
| Aged ≥50 y | Self-reported serious problems with hearing, eyesight, or memory |
| Non-Hispanic white or non-Hispanic African American race | Diagnosed with any type of arthritis other than osteoarthritis or degenerative arthritis |
| Receives primary care at a participating site | Treated for cancer in the last 3 y |
| Frequent pain characteristic of symptomatic knee osteoarthritis39 | Had a steroid injection for knee pain in the past 3 mo |
| Pain in worst knee during the past wk rated as ≥4 on a 0-10 scale | Had a knee replacement in the past 3 mo |
| Speak, read, and write in English | Plan to have a knee replacement in the next 6 mo |
| Self-reported inability to complete study-related telephone calls and program activities that involve reading and writing | |
| No reliable telephone number | |
| Answering ≥2 items incorrectly on a 6-item screener for cognitive impairment40 |
Abbreviations: ICD-9, International Classification of Diseases, Ninth Revision; VA, Veterans Affairs.
Eligibility was determined based on self-reported responses to a screening survey.
Study Protocol
Eligible patients attended an in-person baseline visit where they provided written informed consent, completed a staff-administered baseline assessment, and were randomized to a 6-week positive psychological intervention or neutral control program. Randomization was at the patient level, stratified by study site, and patient race (non-Hispanic white or non-Hispanic African American), with a 1 to 1 allocation using random block sizes of 2, 4, 6, or 8. The statistician placed positive and control program workbooks in sealed envelopes according to the randomization sequence and the staff took the next sealed envelope to each baseline visit. After collecting baseline measures, staff opened the envelope, oriented participants to their workbook, and reviewed the first activity, which participants completed over the next week. The staff called participants weekly for the next 6 weeks to assess adherence and review the next activity. Outcomes were collected via telephone surveys 1, 3, and 6 months after the final week. Participants were compensated up to $110. Patients and staff who collected baseline and outcome measures were blinded to the treatment group.
Intervention
The intervention was an individually based program, in which participants completed 1 new positive psychological activity for the first 5 weeks and repeated their favorite in week 6.26 Activities, which were adapted for the target population,24 included recalling and reflecting on positive events1,41; writing a letter of gratitude1,42; cultivating mindfulness43,44; practicing kindness45; and increasing engagement in activities that they enjoy, give them a sense of achievement, or bring them closer to others (a variant of behavioral activation).9
Control Program
The control program was identical to the intervention in terms of framing, reading level, format, duration, and delivery, but contained neutral control activities adapted from previous positive psychological intervention studies.1,42,46,47 Control activities asked participants to recall events that affected them each day, identify ways they could change their life circumstances, recall early memories, record things they did in the past week, plan their day, and repeat their favorite activity in week 6.
Intervention Delivery and Fidelity
Staff was trained to deliver the intervention and control program in a 1.5-day workshop co-led by the principal investigator and a positive psychologist. Prior to delivering the programs to participants, staff demonstrated proficiency in delivering both programs in calls with the positive psychologist. Staff participated in frequent cross-site calls to ensure uniform delivery of the program throughout enrollment (July 8, 2015, through February 1, 2017).
Study Measures
Primary Outcomes: Osteoarthritis Pain and Functional Difficulty
Primary outcomes included pain (5 items) and physical function (17 items) subscales of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC).48,49 Subscale scores were calculated as the sum of items, then transformed to a 0 to 100 scale (higher = worse).
Secondary Outcomes: Affect Balance and Life Satisfaction
Well-being measures commonly used in positive psychological intervention studies were included as secondary outcomes. Affect balance was assessed using the International Positive and Negative Affect Schedule Short Form, which asks how often participants felt 5 positive (eg, inspired) or 5 negative (eg, upset) emotions over the past week (1 = never; 5 = always).50 Affect balance was calculated by subtracting the sum of negative scores from the sum of positive scores.36,37 Life satisfaction was assessed using a 5-item scale that asked participants the extent to which they agreed with statements such as “In most ways, your life is close to your ideal” (1 = strongly disagree; 5 = strongly agree).38
Demographic and Clinical Characteristics
Race and ethnicity categorization was based on self-reported responses to the following questions: are you of Spanish, Hispanic, or Latino origin (including Mexican, Puerto Rican, Cuban, South or Central American, or other Spanish culture or origin)? (yes or no); and which category best describes your race: white, black or African American, Asian, Native Hawaiian or other Pacific Islander, American Indian or Alaska Native, or other (check all that apply)? Additional baseline characteristics included self-reported sex, age, income, education, employment, marital status, general health status, health literacy,51,52 physical comorbid medical conditions,53 and past diagnoses and current treatment of depression or anxiety.54 Patients were asked to report whether they were using several pharmacological and nonpharmacological OA treatments assessed in the Osteoarthritis Initiative.39 Body mass index and whether participants had radiography or magnetic resonance imaging reports documenting radiographic evidence of OA were ascertained from VA medical records.
Intervention Adherence and Engagement
In weekly telephone calls during the intervention period, participants were asked to recall the activity they were supposed to complete the previous week and to indicate whether they completed it entirely, partially, or not at all.6 Adherence was calculated as the number of weekly calls completed and the number of correctly identified activities that were partially or entirely completed.
Participants who reported at least partially completing an activity were also asked to rate the benefit, enjoyment, and difficulty of each exercise using a 7-point scale (1 = not at all; 7 = extremely).6 These ratings were treated as continuous indicators of intervention engagement.
Statistical Analysis
Analyses were performed using Stata, version 14 (Stata Corp).55 We checked outcome measures for normality and found no violations. Descriptive statistics were computed as means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Time was treated as a 4-level categorical variable based on plots showing a nonlinear change over time in the unadjusted primary outcomes. All models were adjusted for study site. Statistical significance was determined as P < .05 and all tests were 2-sided.
We compared intervention adherence across treatment and racial groups using separate logistic regression models testing the main effects of treatment group and participant race, and a model testing the treatment group × race interaction (including the main effects), on 2 binary adherence measures (completed ≥5 calls; entirely or partially completed ≥5 correct activities). We used linear mixed models to test the same effects for the repeated measures of intervention engagement.
We tested study hypotheses using linear mixed models that allowed the use of data from participants with missing data from 1 or more time points. In separate models for each outcome, we included fixed effects for treatment group, race, time, and all 2-way and 3-way interactions. We tested the statistical significance of the main effects and interaction terms using likelihood ratio tests via χ2 statistics. When the 3-way interaction was not significant, terms with race were removed and the treatment group × time interaction was examined. When this interaction was not significant, a model testing only the simple main effect of time was examined. We tested the study hypotheses using intention-to-treat analyses.56 We tested models adjusting only for site and models adjusting for all baseline characteristics. We also conducted exploratory subgroup analyses to elucidate our primary findings.
Missing Data and Power
For scales with 20% or fewer missing items, missing items were replaced with the mean of the remaining items. Scales with more than 20% missing items were treated as missing. A sample size of 360 patients (180 non-Hispanic white and 180 non-Hispanic African American) was chosen based on a priori power calculations to detect a 3-way interaction between treatment group, race, and time, assuming a 20% change in baseline, the WOMAC pain subscale scores, and 80% power.26
Results
Baseline Sample Characteristics
Of 5111 patients who were sent mailings and 67 who responded to study brochures, 839 completed the full telephone screening, 488 were eligible, and 360 were enrolled and randomized (Figure 1; eTables 1-3 in Supplement 2). Participants included 180 non-Hispanic white and 180 non-Hispanic African American patients (mean [SD] age, 64.2 [8.8] years; 76.4% were male) (Table 2). The mean (SD) pain rating at screening was 7.2 (1.7) on a scale of 0 to 10, and 63.6% of participants had an x-ray or MRI indicating OA in their VA medical record. There were several differences between African American and white patients (Table 2). For example, compared with white patients, African American patients were less likely to be married or living with a partner (99 [55.0%] vs 65 [36.1%]), more likely to be disabled or unemployed (50 [27.8%] vs 76 [42.2%]), and less likely to have a college degree (54 [30%] vs 36 [20%]).
Figure 1. CONSORT Flow Diagram for the Staying Positive With Arthritis Study.
aReasons for ineligibility are provided in eTable 1 in Supplement 2.
bRandomization was at the patient level, stratified by site and self-reported race.
cDetails on reasons for missing data points are provided in eTable 2 and eTable 3 in Supplement 2.
Table 2. Baseline Characteristics by Treatment Group and Race.
| Variable | No. (%) | ||||
|---|---|---|---|---|---|
| Total (N = 360) | Treatment Group | Participant Race | |||
| Positive (n = 180) | Control (n = 180) | White (n = 180) | African American (n = 180) | ||
| Age, mean (SD), y | 64.2 (8.8) | 64.4 (9.4) | 64.1 (8.1) | 65.9 (9.2) | 62.6 (8.0) |
| BMI, mean (SD) | 31.8 (6.5) | 31.8 (6.4) | 31.9 (6.6) | 32.4 (6.7) | 31.3 (6.3) |
| Female | 85 (23.6) | 44 (24.4) | 41 (22.8) | 42 (23.3) | 43 (23.9) |
| Site | |||||
| Site A | 180 (50.0) | 90 (50.0) | 90 (50.0) | 90 (50.0) | 90 (50.0) |
| Site B | 180 (50.0) | 90 (50.0) | 90 (50.0) | 90 (50.0) | 90 (50.0) |
| Married or living with partner | 164 (45.6) | 80 (44.4) | 84 (46.7) | 99 (55.0) | 65 (36.1) |
| Employment status | |||||
| Employed | 88 (24.4) | 46 (25.6) | 42 (23.3) | 38 (21.1) | 50 (27.8) |
| Retired | 146 (40.6) | 72 (40.0) | 74 (41.1) | 92 (51.1) | 54 (30.0) |
| Disabled/unemployed/other | 126 (35.0) | 62 (34.4) | 64 (35.6) | 50 (27.8) | 76 (42.2) |
| Income, $ | |||||
| <20 000 | 103 (28.6) | 48 (26.7) | 55 (30.6) | 36 (20.0) | 67 (37.2) |
| 20 000-39 999 | 100 (27.8) | 52 (28.9) | 48 (26.7) | 44 (24.4) | 56 (31.1) |
| ≥40 000 | 136 (37.8) | 67 (37.2) | 69 (38.3) | 88 (48.9) | 48 (26.7) |
| Do not know/refused | 21 (5.8) | 13 (7.2) | 8 (4.4) | 12 (6.7) | 9.0 (5.0) |
| Education | |||||
| ≤High school | 109 (30.3) | 56 (31.1) | 53 (29.4) | 49 (27.2) | 60 (33.3) |
| Some college | 161 (44.7) | 75 (41.7) | 86 (47.8) | 77 (42.8) | 84 (46.7) |
| ≥4 y degree | 90 (25.0) | 49 (27.2) | 41 (22.8) | 54 (30.0) | 36 (20.0) |
| Adequate health literacy | 284 (78.9) | 138 (76.7) | 146 (81.1) | 145 (80.6) | 139 (77.2) |
| Good, very good, or excellent self-rated health | 218 (60.6) | 108 (60.0) | 110 (61.1) | 124 (68.9) | 94 (52.2) |
| Charlson comorbidity index (self-report) | |||||
| 0-1 | 104 (28.9) | 52 (28.9) | 52 (28.9) | 49 (27.2) | 55 (30.6) |
| 2-3 | 121 (33.6) | 61 (33.9) | 60 (33.3) | 55 (30.6) | 66 (36.7) |
| ≥4 | 135 (37.5) | 67 (37.2) | 68 (37.8) | 76 (42.2) | 59 (32.8) |
| Pain rating on 0-10 scale, mean (SD) | 7.2 (1.7) | 7.2 (1.6) | 7.3 (1.7) | 6.9 (1.7) | 7.6 (1.5) |
| Anxiety disorder (self-report) | 141 (39.2) | 66 (36.7) | 75 (41.7) | 72 (40.0) | 69 (38.3) |
| Depressive disorder (self-report) | 166 (46.1) | 78 (43.3) | 88 (48.9) | 78 (43.3) | 88 (48.9) |
| Being treated for mental health or emotional condition (self-report) | 122 (33.9) | 51 (28.3) | 71 (39.4) | 64 (35.6) | 58 (32.2) |
| No. of treatments currently being used for joint paint or arthritis, mean (SD) | |||||
| Pharmacological (possible range: 0-6)a | 1.6 (1.0) | 1.5 (1.0) | 1.6 (1.1) | 1.6 (1.0) | 1.6 (1.1) |
| Nonpharmacological or alternative (possible range: 0-13)b | 3.1 (2.0) | 3.1 (2.0) | 3.1 (2.0) | 3.2 (2.1) | 3.1 (1.9) |
| Radiographic evidence of OAc | |||||
| No x-ray or MRI on file | 115 (31.9) | 57 (31.7) | 58 (32.2) | 64 (35.6) | 51 (28.3) |
| X-ray or MRI with no indication of OA | 16 (4.4) | 8 (4.4) | 8 (4.4) | 9 (5.0) | 7 (3.9) |
| X-ray or MRI on file with indication of OA | 229 (63.6) | 115 (63.9) | 114 (63.3) | 107 (59.4) | 122 (67.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MRI, magnetic resonance imaging; OA, osteoarthritis.
Count of the following treatments reportedly being used at baseline: acetaminophen, nonsteroidal anti-inflammatory drugs, topical nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 selective inhibitors, opioids, and hyaluronic acid or steroid injections.
Count of the following treatments reportedly being used at baseline: acupuncture, acupressure, or massage therapy; chiropractic care; homeopathy or naturopathy; physical therapy; water- or land-based exercise; health supplements for joint pain; vitamins; herbs; topical creams or oils; copper bracelets or magnets; yoga, tai chi, chi gong, pilates; relaxation or mind-body activities; and spiritual activities.
No x-ray or MRI on file and x-ray or MRI with no indication of OA were combined to create a dichotomous indicator of radiographic evidence of OA (no or yes) for analyses.
Adherence and Engagement
During the 6-week intervention, 287 participants (79.7%) completed 5 or more weekly calls, and 234 participants (65.0%) reported entirely or partially completing 5 or more correct activities. Adherence rates did not significantly differ by treatment group or race (P > .05; eTable 4 in Supplement 2). The positive (vs control) group rated the weekly activities as more beneficial (mean [SD]: 5.77 [1.32] vs 5.39 [1.68]; P = .001) and more enjoyable (mean [SD]: 5.91 [1.30] vs 5.33 [1.72]; P < .001), but as equally difficult (mean [SD]: 2.26 [1.80] vs 2.23 [1.87]; P = .95). Ratings did not differ by participant race (eTable 5 in Supplement 2).
Primary Outcomes: Pain and Functional Difficulty
Participants at baseline reported mean (SD) WOMAC pain and functional difficulty scores of 48.8 (17.6) and 46.8 (18.1), respectively. The hypothesized 3-way interaction between treatment group, race, and time was not significant for either outcome (Table 3). Models omitting nonsignificant interactions revealed no interactions between treatment group and time. Pain and functional difficulty both decreased significantly over time (pain, mean: 48.8 at baseline, 44.5 at 1 month, 43.6, at 3 months, and 42.4 at 6 months; overall test for time: χ23 = 49.50, P < .001; functional difficulty, mean: 46.8 at baseline, 43.9 at 1 month, 43.4 at 3 months, and 42.9 at 6 months; overall test for time: χ23 = 22.11, P < .001). Results were similar in models fully adjusting for all baseline characteristics.
Table 3. Change in Self-reported Pain and Functional Difficulty in White and African American Patients With Knee or Hip Osteoarthritis After Completing a 6-Week Positive Psychological Intervention or Neutral Control Programa.
| Outcomes | Positive Psychological Intervention | Neutral Control Program | Race × Program × Time Interactionb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 mo | 3 mo | 6 mo | Baseline | 1 mo | 3 mo | 6 mo | χ2 | P Value | |
| Pain (WOMAC)c | ||||||||||
| White | 1.03 | .79 | ||||||||
| No. | 89 | 82 | 79 | 79 | 88 | 73 | 79 | 82 | ||
| Mean (SD) | 45.2 (15.7) | 42.4 (15.8) | 40.1 (16.9) | 39.2 (18.0) | 45.1 (17.3) | 42.3 (21.8) | 40.8 (18.8) | 39.0 (18.7) | ||
| Change from baseline | −2.8 | −5.2 | −6.0 | −2.8 | −4.3 | −6.1 | ||||
| African American | ||||||||||
| No. | 90 | 74 | 76 | 77 | 90 | 76 | 74 | 71 | ||
| Mean (SD) | 55.2 (16.7) | 48.8 (20.8) | 47.8 (20.6) | 47.4 (23.0) | 49.7 (18.6) | 44.8 (20.1) | 46.0 (19.2) | 44.5 (20.9) | ||
| Change from baseline | −6.4 | −7.4 | −7.8 | −4.9 | −3.7 | −5.1 | ||||
| Functional difficulty (WOMAC)c | ||||||||||
| White | 3.09 | .38 | ||||||||
| No. | 89 | 82 | 78 | 77 | 85 | 68 | 73 | 79 | ||
| Mean (SD) | 43.6 (17.1) | 40.1 (16.3) | 39.9 (17.3) | 40.6 (17.5) | 44.2 (17.9) | 40.0 (22.0) | 40.5 (20.0) | 39.1 (18.7) | ||
| Change from baseline | −3.6 | −3.7 | −3.1 | −4.1 | −3.6 | −5.1 | ||||
| African American | ||||||||||
| No. | 88 | 74 | 75 | 75 | 89 | 74 | 74 | 69 | ||
| Mean (SD) | 52.3 (17.4) | 49.6 (22.1) | 47.0 (20.1) | 47.3 (21.9) | 47.1 (19.1) | 45.9 (19.7) | 46.1 (19.4) | 45.1 (21.0) | ||
| Change from baseline | −2.7 | −5.3 | −5.0 | −1.3 | −1.1 | −2.1 | ||||
Abbreviation: WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Means shown are unadjusted.
P values are based on χ2 tests of the 3-way interaction of program, race, and time from linear mixed models controlling for study site.
Higher scores indicate worse symptoms and negative change in scores indicates improvement.
Secondary Outcomes: Affect Balance and Life Satisfaction
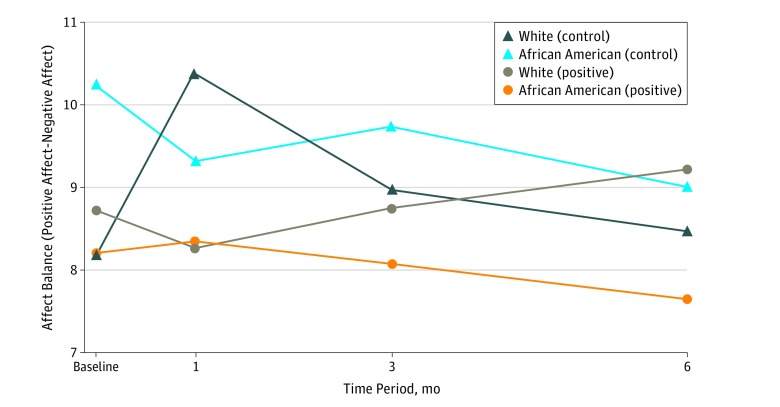
Mean (SD) affect balance at baseline was 8.8 (6.4), indicating more positive than negative affect overall. The 3-way interaction between treatment group, race, and time was significant (χ23 = 8.64; P = .03). Examining the means indicated that among white patients affect balance decreased from baseline to 1 month in the positive group, but increased over the same time in the control group (Figure 2). Among African American patients, affect balance decreased slightly at 1 month in the control group, and steadily declined over each time point in the positive group.
Figure 2. Mean Affect Balance by Race and Treatment Arm Over Time.
These means are provided to aid in interpretation of the significant interaction between treatment group, race, and time for affect balance (positive − negative affect scores), χ23 = 8.64; P = .03. Among white participants, affect balance decreased from baseline to 1 month in the positive group, but increased over the same time in the control group. Among African American participants, affect balance decreased slightly at 1 month in the control group, and steadily declined over each time point in the positive group.
Mean (SD) life satisfaction at baseline was 15.5 (5.1). The 3-way interaction between treatment group, race, and time was not significant (χ23 = 1.79; P = .62). When race was removed, neither the treatment group × time interaction nor main effect of time was significant.
Exploratory Subgroup Analyses
We explored 2 explanations for the lack of differences between treatment groups in the change in pain and functional difficulty—insufficient disease severity and nonadherence. For disease severity, we restricted analyses to participants with above-median baseline pain and functional difficulty, and to those with radiographic evidence of OA. Results in these subgroups were the same as those from the full sample.
For nonadherence, we tested the 3-way interaction between treatment group, race, and time, and the 2-way interaction between treatment group and time, in participants who reported completing 5 or more assigned activities. Results for pain and functional difficulty did not change. However, for life satisfaction the treatment group × time interaction was significant (χ23 = 8.01; P = .05). The means indicated that, compared with baseline, life satisfaction decreased at 1 month and then rebounded back toward baseline in the positive group, whereas life satisfaction increased slightly at 1 month and decreased at later time points in the control group (positive, mean: 16.9 vs 16.0 vs 16.4 vs 16.6; control, mean: 15.2 vs 15.6 vs 15.3 vs 14.7 at baseline and 1, 3, and 6 months, respectively).
Discussion
This randomized clinical trial was powered to detect racial differences in the effects of positive psychological interventions on chronic pain in older military veterans with knee OA. Although there were statistically significant reductions in pain and functional difficulty from baseline to 6 months, the differences were small and did not vary by treatment group or race. Affect balance and life satisfaction, core processes by which positive psychological interventions are thought to improve well-being, also did not show the predicted changes. In short, our study did not detect benefits of positive psychological interventions relative to a neutral control program for pain, functional difficulty, or measures of well-being in African American or white veterans with knee OA.
Our intervention may not have shown the hypothesized benefits owing to aspects of the patient population, study design, or the intervention itself. Our sample was older, more racially diverse, and more likely to be male compared with samples included in most prior positive psychological intervention studies. Our eligibility criteria allowed patients with a wide range of arthritis symptoms and those who were actively engaged in other pain treatments to enroll in the study. Although we erred on the side of inclusivity for pragmatic reasons and to increase generalizability, our broad criteria may have produced a sample with insufficient pain to show an effect of the intervention, or masked the response to the intervention by other treatments. These are unlikely explanations for our findings, however, given that the hypothesized effects did not occur in subgroups with more severe symptoms or radiologic evidence of OA, or after controlling for co-occurring pain treatments.
Additional pragmatic design choices make it difficult to know if the intervention would have had the expected effects under ideal circumstances. We did not assess outcomes during the intervention period to reduce patient burden and because interventions with only fleeting benefits are not sustainable in practice. We also compared our intervention with an active control condition to assess the active ingredients of positive psychological interventions and to account for alternative explanations such as motivation, placebo effects, or attention. Without a usual care control group, we do not know how the observed decreases in pain and functional difficulty compare with similar patients who did not participate in the study. Our measures also may not have been sufficiently sensitive to capture the effects of the intervention. There is wide within-person variability in patient-reported WOMAC pain and functional difficulty over time, especially among African American patients.57 Veterans may have derived benefit or satisfaction from the intervention that were not captured by our measures.
We conducted extensive pilot testing to adapt evidence-based positive psychological activities to the preferences and needs of veterans.24 The intervention showed acceptability and feasibility in pilot testing, and self-reported adherence to the program was reasonable in this trial. Nevertheless, the intervention did not show benefits for pain, functional difficulty, or well-being in this sample. Moreover, it showed subtle signs of backfiring on measures of well-being. For some patients, it is possible that activities in the intervention shed light on aspects of their lives that increased rather than decreased distress, such as reminding them of loved ones who have died, or the repetitiveness and isolation of their lives. Additional tailoring of individual activities, or including different activities that avoid such pitfalls, is needed for positive psychological interventions to be used effectively in this population.
The intervention also may have been ineffective because it did not focus explicitly on changing maladaptive pain-related emotions, thoughts, or behaviors, as do other psychological treatments for pain (eg, cognitive behavior therapy). Focusing on increasing positive affect, without addressing thoughts and behaviors that can worsen pain perception, may be insufficient to exert a meaningful shift in the central response to OA pain perception. For patients with chronic pain to reap the benefits of positive psychological interventions, it may be necessary to integrate principles from positive psychology into more comprehensive pain treatment regimens.
Our findings are surprising and disappointing in light of growing interest in applying positive psychological interventions in different populations with particular clinical conditions.3,4,5,7,11,13,15,16,18 Several studies describe how positive psychological interventions have been adapted for specific patient populations and delivery modalities, and demonstrate feasibility of such interventions in pilot studies.11,12,13,14,15,16,17,18,20,21,22,23,24 As one of the first completed large-scale randomized clinical trials with an active control group, this study does not demonstrate the benefits suggested by preliminary studies. Rather, it adds to a growing number of studies suggesting that effects of positive psychological interventions reported in early studies are smaller or nonexistent in later replications.58,59,60 In our pilot work, the positive psychological intervention showed medium to large effects on pain, difficulty functioning, and life satisfaction,24 none of which were maintained in the fully powered study. This underscores the imprecision of small pilot studies,61 and serves as a cautionary tale for moving forward with implementation of practices for which only preliminary evidence is available. Our study also aligns with a study showing that an active control program outperformed a positive psychological intervention among patients hospitalized with suicidal thoughts, suggesting that positive psychological interventions are not a panacea for all patient populations.62
This study was motivated by the need for effective, nonpharmacological treatments for alleviating OA pain and functional difficulties. Multiple psychological approaches to pain treatment (eg, cognitive behavioral therapy and mindfulness-based stress reduction) have been developed and tested for patients with chronic pain,63 but high quality evidence demonstrating the effectiveness of such approaches for patients with knee OA is lacking. A recent review examining evidence for the impact of psychological interventions on pain concluded that there is a dearth of strong empirical evidence that psychological treatments for pain management are effective.64 While the association of modifiable cognitions and behaviors with OA pain and functional difficulty (eg, pain catastrophizing, depression, and pain coping strategies) is well documented,65,66,67,68 interventions that target psychological or behavioral pain mechanisms and produce large improvements in pain outcomes remain elusive.
Limitations
Our study sample was limited to patients with knee OA from 2 VA medical centers, thereby limiting the generalizability of our findings to patients with other chronic pain conditions, nonveterans, or veterans being treated at other VA or non-VA facilities. Although we excluded patients with inflammatory arthritis conditions other than OA, we did not assess for pain conditions other than arthritis, so patients could have had pain from multiple illnesses. Our adherence and outcomes were self-reported and thus vulnerable to measurement bias. As noted, the omission of a usual care group makes it unclear how the changes we observed compare with similar patients not enrolled in the study.
Conclusions
This large 2-site randomized clinical trial of a positive psychological intervention for chronic pain fills important gaps in the literature by testing the use of such interventions in older veterans with chronic pain, testing for racial differences in response to such interventions, and comparing their long-term effects with those of a strong control group. Unfortunately, the results do not support the use of positive psychological interventions as a stand-alone psychological treatment for pain among white or African American veterans with knee OA. Adaptations are needed to identify specific positive psychological intervention components that resonate with this population, and the potential additive effect of incorporating positive psychological interventions into comprehensive pain treatment regimens should be considered.
Trial Protocol
eTable 1. Reasons for Ineligibility
eTable 2. Reasons for Missing Data Points
eTable 3. Reasons for Withdrawing From the Study
eTable 4. Adherence to Positive and Control Programs by Race
eTable 5. Ratings of Positive and Control Programs by Race
References
- 1.Seligman MEP, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol. 2005;60(5):-. doi: 10.1037/0003-066X.60.5.410 [DOI] [PubMed] [Google Scholar]
- 2.Sin NL, Lyubomirsky S. Enhancing well-being and alleviating depressive symptoms with positive psychology interventions: a practice-friendly meta-analysis. J Clin Psychol. 2009;65(5):467-487. doi: 10.1002/jclp.20593 [DOI] [PubMed] [Google Scholar]
- 3.Bolier L, Haverman M, Westerhof GJ, Riper H, Smit F, Bohlmeijer E. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health. 2013;13(1):119. doi: 10.1186/1471-2458-13-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casellas-Grau A, Font A, Vives J. Positive psychology interventions in breast cancer: a systematic review. Psychooncology. 2014;23(1):9-19. doi: 10.1002/pon.3353 [DOI] [PubMed] [Google Scholar]
- 5.Macaskill A. Review of positive psychology applications in clinical medical populations. Healthcare (Basel). 2016;4(3):66. doi: 10.3390/healthcare4030066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schueller SM, Parks AC. Disseminating self-help: positive psychology exercises in an online trial. J Med Internet Res. 2012;14(3):e63. doi: 10.2196/jmir.1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parks AC, Kleiman EM, Kashdan TB, et al. . Positive psychotherapeutic and behavioral interventions In: Jeste DV, Palmer BW, eds. Positive Psychiatry: A Clinical Handbook. Washington, DC: American Psychiatric Publishing; 2015:147-166. doi: 10.1176/appi.books.9781615370818.dj08 [DOI] [Google Scholar]
- 8.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131(6):925-971. doi: 10.1037/0033-2909.131.6.925 [DOI] [PubMed] [Google Scholar]
- 9.Mazzucchelli TG, Kane RT, Rees CS. Behavioral activation interventions for well-being: a meta-analysis. J Posit Psychol. 2010;5(2):105-121. doi: 10.1080/17439760903569154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fredrickson BL. The role of positive emotions in positive psychology: the broaden-and-build theory of positive emotions. Am Psychol. 2001;56(3):218-226. doi: 10.1037/0003-066X.56.3.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer PS, Johnson DP, Parks A, Iwanski C, Penn DL. Positive living: a pilot study of group positive psychotherapy for people with schizophrenia. J Posit Psychol. 2012;7(3):239-248. doi: 10.1080/17439760.2012.677467 [DOI] [Google Scholar]
- 12.Huffman JC, Mastromauro CA, Boehm JK, et al. . Development of a positive psychology intervention for patients with acute cardiovascular disease. Heart Int. 2011;6(2):e14. doi: 10.4081/hi.2011.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moskowitz JT, Hult JR, Duncan LG, et al. . A positive affect intervention for people experiencing health-related stress: development and non-randomized pilot test. J Health Psychol. 2012;17(5):676-692. doi: 10.1177/1359105311425275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redwine LS, Henry BL, Pung MA, et al. . Pilot randomized study of a gratitude journaling intervention on heart rate variability and inflammatory biomarkers in patients with stage B heart failure. Psychosom Med. 2016;78(6):667-676. doi: 10.1097/PSY.0000000000000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huffman JC, Millstein RA, Mastromauro CA, et al. . A positive psychology intervention for patients with an acute coronary syndrome: treatment development and proof-of-concept yrial. J Happiness Stud. 2016;17(5):1985-2006. doi: 10.1007/s10902-015-9681-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohn MA, Pietrucha ME, Saslow LR, Hult JR, Moskowitz JT. An online positive affect skills intervention reduces depression in adults with type 2 diabetes. J Posit Psychol. 2014;9(6):523-534. doi: 10.1080/17439760.2014.920410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DuBois CM, Millstein RA, Celano CM, Wexler DJ, Huffman JC. Feasibility and acceptability of a positive psychological intervention for patients with type 2 diabetes. Prim Care Companion CNS Disord. 2016;18(3). doi: 10.4088/PCC.15m01902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller R, Gertz KJ, Molton IR, et al. . Effects of a tailored positive psychology intervention on well-being and pain in individuals with chronic pain and a physical disability: a feasibility trial. Clin J Pain. 2016;32(1):32-44. doi: 10.1097/AJP.0000000000000225 [DOI] [PubMed] [Google Scholar]
- 19.Moskowitz JT, Carrico AW, Duncan LG, et al. . Randomized controlled trial of a positive affect intervention for people newly diagnosed with HIV. J Consult Clin Psychol. 2017;85(5):409-423. doi: 10.1037/ccp0000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanssen MM, Peters ML, Vlaeyen JW, Meevissen YM, Vancleef LM. Optimism lowers pain: evidence of the causal status and underlying mechanisms. Pain. 2013;154(1):53-58. doi: 10.1016/j.pain.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 21.Hausmann LRM, Parks A, Youk AO, Kwoh CK. Reduction of bodily pain in response to an online positive activities intervention. J Pain. 2014;15(5):560-567. doi: 10.1016/j.jpain.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 22.Goodin BR, Bulls HW. Optimism and the experience of pain: benefits of seeing the glass as half full. Curr Pain Headache Rep. 2013;17(5):329. doi: 10.1007/s11916-013-0329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodin BR, Kronfli T, King CD, Glover TL, Sibille K, Fillingim RB. Testing the relation between dispositional optimism and conditioned pain modulation: does ethnicity matter? J Behav Med. 2013;36(2):165-174. doi: 10.1007/s10865-012-9411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hausmann LRM, Youk A, Kwoh CK, et al. . Testing a positive psychological intervention for osteoarthritis. Pain Med. 2017;18(10):1908-1920. doi: 10.1093/pm/pnx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanssen MM, Peters ML, Boselie JJ, Meulders A. Can positive affect attenuate (persistent) pain? state of the art and clinical implications. Curr Rheumatol Rep. 2017;19(12):80. doi: 10.1007/s11926-017-0703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausmann LRM, Ibrahim SA, Kwoh CK, et al. . Rationale and design of the Staying Positive with Arthritis (SPA) Study: a randomized controlled trial testing the impact of a positive psychology intervention on racial disparities in pain. Contemp Clin Trials. 2018;64:243-253. doi: 10.1016/j.cct.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence RC, Felson DT, Helmick CG, et al. ; National Arthritis Data Workgroup . Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: part II. Arthritis Rheum. 2008;58(1):26-35. doi: 10.1002/art.23176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolen J, Schieb L, Hootman JM, et al. . Differences in the prevalence and severity of arthritis among racial/ethnic groups in the United States, National Health Interview Survey, 2002, 2003, and 2006. Prev Chronic Dis. 2010;7(3):A64. [PMC free article] [PubMed] [Google Scholar]
- 29.Allen KD, Oddone EZ, Coffman CJ, Keefe FJ, Lindquist JH, Bosworth HB. Racial differences in osteoarthritis pain and function: potential explanatory factors. Osteoarthritis Cartilage. 2010;18(2):160-167. doi: 10.1016/j.joca.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 30.Jordan JM, Helmick CG, Renner JB, et al. . Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172-180. [PubMed] [Google Scholar]
- 31.Ang DC, Ibrahim SA, Burant CJ, Siminoff LA, Kwoh CK. Ethnic differences in the perception of prayer and consideration of joint arthroplasty. Med Care. 2002;40(6):471-476. doi: 10.1097/00005650-200206000-00004 [DOI] [PubMed] [Google Scholar]
- 32.Jones AC, Kwoh CK, Groeneveld PW, Mor M, Geng M, Ibrahim SA. Investigating racial differences in coping with chronic osteoarthritis pain. J Cross Cult Gerontol. 2008;23(4):339-347. doi: 10.1007/s10823-008-9071-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Variation in perceptions of treatment and self-care practices in elderly with osteoarthritis: a comparison between African American and white patients. Arthritis Rheum. 2001;45(4):340-345. doi: [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim SA, Hanusa BH, Hannon MJ, Kresevic D, Long J, Kent Kwoh C. Willingness and access to joint replacement among African American patients with knee osteoarthritis: a randomized, controlled intervention. Arthritis Rheum. 2013;65(5):1253-1261. doi: 10.1002/art.37899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hausmann LRM, Mor M, Hanusa BH, et al. . The effect of patient race on total joint replacement recommendations and utilization in the orthopedic setting. J Gen Intern Med. 2010;25(9):982-988. doi: 10.1007/s11606-010-1399-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koydemir S, Schütz A. Emotional intelligence predicts components of subjective well-being beyond personality: a two-country study using self- and informant reports. J Posit Psychol. 2012;7(2):107-118. doi: 10.1080/17439760.2011.647050 [DOI] [Google Scholar]
- 37.Liu Y, Wang Z, Lü W. Resilience and affect balance as mediators between trait emotional intelligence and life satisfaction. Pers Individ Dif. 2013;54(7):850-855. doi: 10.1016/j.paid.2012.12.010 [DOI] [Google Scholar]
- 38.Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49(1):71-75. doi: 10.1207/s15327752jpa4901_13 [DOI] [PubMed] [Google Scholar]
- 39.Nevitt MC, Felson DT, Lester G. The Osteoarthritis Initiative: protocol for the cohort study. http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf. Accessed May 9, 2017.
- 40.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40(9):771-781. doi: 10.1097/00005650-200209000-00007 [DOI] [PubMed] [Google Scholar]
- 41.Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788. doi: 10.1037/0003-066X.61.8.774 [DOI] [PubMed] [Google Scholar]
- 42.Lyubomirsky S, Dickerhoof R, Boehm JK, Sheldon KM. Becoming happier takes both a will and a proper way: an experimental longitudinal intervention to boost well-being. Emotion. 2011;11(2):391-402. doi: 10.1037/a0022575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol. 2003;84(4):822-848. doi: 10.1037/0022-3514.84.4.822 [DOI] [PubMed] [Google Scholar]
- 44.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: a meta-analysis. J Psychosom Res. 2004;57(1):35-43. doi: 10.1016/S0022-3999(03)00573-7 [DOI] [PubMed] [Google Scholar]
- 45.Lyubomirsky S, Sheldon KM, Schkade D. Pursuing happiness: the architecture of sustainable change. Rev Gen Psychol. 2005;9(2):111-131. doi: 10.1037/1089-2680.9.2.111 [DOI] [Google Scholar]
- 46.Sheldon KM, Abad N, Ferguson Y, et al. . Persistent pursuit of need-satisfying goals leads to increased happiness: a 6-month experimental longitudinal study. Motiv Emot. 2010;34(1):39-48. doi: 10.1007/s11031-009-9153-1 [DOI] [Google Scholar]
- 47.Emmons RA, McCullough ME. Counting blessings versus burdens: an experimental investigation of gratitude and subjective well-being in daily life. J Pers Soc Psychol. 2003;84(2):377-389. doi: 10.1037/0022-3514.84.2.377 [DOI] [PubMed] [Google Scholar]
- 48.Bellamy N. WOMAC Osteoarthritis Index: User Guide XI. Atlanta, GA: American College of Rheumatology; 2015. [Google Scholar]
- 49.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840. [PubMed] [Google Scholar]
- 50.Thompson ER. Development and validation of an internationally reliable short-form of the Positive and Negative Affect Schedule (PANAS). J Cross Cult Psychol. 2007;38(2):227-242. doi: 10.1177/0022022106297301 [DOI] [Google Scholar]
- 51.Chew LD, Griffin JM, Partin MR, et al. . Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23(5):561-566. doi: 10.1007/s11606-008-0520-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powers BJ, Trinh JV, Bosworth HB. Can this patient read and understand written health information? JAMA. 2010;304(1):76-84. doi: 10.1001/jama.2010.896 [DOI] [PubMed] [Google Scholar]
- 53.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson comorbidity index for predicting mortality. Med Care. 2005;43(6):607-615. doi: 10.1097/01.mlr.0000163658.65008.ec [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention Behavioral risk factor surveillance system questionnaire. http://www.cdc.gov/brfss/questionnaires/pdf-ques/2010brfss.pdf. Accessed February 17, 2017.
- 55.StataCorp Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 56.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191-1194. doi: 10.1016/S0140-6736(00)04337-3 [DOI] [PubMed] [Google Scholar]
- 57.Vina ER, Ran D, Ashbeck EL, Kwoh CK. Natural history of pain and disability among African-Americans and Whites with or at risk for knee osteoarthritis: a longitudinal study. Osteoarthritis Cartilage. 2018;26(4):471-479. doi: 10.1016/j.joca.2018.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mongrain M, Anselmo-Matthews T. Do positive psychology exercises work? a replication of Seligman et al. (2005). J Clin Psychol. 2012;68(4):382-389. doi: 10.1002/jclp.21839 [DOI] [PubMed] [Google Scholar]
- 59.Woodworth RJ, O’Brien-Malone A, Diamond MR, Schüz B. Web-Based positive psychology interventions: a reexamination of effectiveness. J Clin Psychol. 2017;73(3):218-232. doi: 10.1002/jclp.22328 [DOI] [PubMed] [Google Scholar]
- 60.Woodworth RJ, O’Brien-Malone A, Diamond MR, Schüz B. Happy days: positive psychology interventions effects on affect in an N-of-1 trial. Int J Clin Health Psychol. 2016;16(1):21-29. doi: 10.1016/j.ijchp.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Arch Gen Psychiatry. 2006;63(5):484-489. doi: 10.1001/archpsyc.63.5.484 [DOI] [PubMed] [Google Scholar]
- 62.Celano CM, Beale EE, Mastromauro CA, et al. . Psychological interventions to reduce suicidality in high-risk patients with major depression: a randomized controlled trial. Psychol Med. 2017;47(5):810-821. doi: 10.1017/S0033291716002798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sturgeon JA. Psychological therapies for the management of chronic pain. Psychol Res Behav Manag. 2014;7:115-124. doi: 10.2147/PRBM.S44762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markozannes G, Aretouli E, Rintou E, et al. . An umbrella review of the literature on the effectiveness of psychological interventions for pain reduction. BMC Psychol. 2017;5(1):31. doi: 10.1186/s40359-017-0200-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Urquhart DM, Phyomaung PP, Dubowitz J, et al. . Are cognitive and behavioural factors associated with knee pain? a systematic review. Semin Arthritis Rheum. 2015;44(4):445-455. doi: 10.1016/j.semarthrit.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 66.Phyomaung PP, Dubowitz J, Cicuttini FM, et al. . Are depression, anxiety and poor mental health risk factors for knee pain? a systematic review. BMC Musculoskelet Disord. 2014;15:10. doi: 10.1186/1471-2474-15-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jia X, Jackson T. Pain beliefs and problems in functioning among people with arthritis: a meta-analytic review. J Behav Med. 2016;39(5):735-756. doi: 10.1007/s10865-016-9777-z [DOI] [PubMed] [Google Scholar]
- 68.Benyon K, Hill S, Zadurian N, Mallen C. Coping strategies and self-efficacy as predictors of outcome in osteoarthritis: a systematic review. Musculoskeletal Care. 2010;8(4):224-236. doi: 10.1002/msc.187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Reasons for Ineligibility
eTable 2. Reasons for Missing Data Points
eTable 3. Reasons for Withdrawing From the Study
eTable 4. Adherence to Positive and Control Programs by Race
eTable 5. Ratings of Positive and Control Programs by Race