This review focuses on immune‐mediated adverse events (IMAEs) reported during the treatment of relapsed or refractory classical Hodgkin lymphoma with the programmed death‐1 (PD‐1) immune checkpoint inhibitors nivolumab and pembrolizumab, describing strategies for promptly recognizing and managing IMAEs in these patients, with the aim of ensuring timely recovery and minimizing discontinuation of anti‐PD‐1 therapy to improve overall disease control.
Keywords: Hodgkin disease, Programmed cell death 1 receptor, Immunotherapy, Drug‐related side effects and adverse reactions
Abstract
The programmed death‐1 (PD‐1) receptor checkpoint inhibitors nivolumab and pembrolizumab represent an important therapeutic advance in the treatment of relapsed or refractory classical Hodgkin lymphoma (cHL). Clinical trials have shown substantial therapeutic activity and an acceptable safety profile in heavily pretreated patients, resulting in U.S. Food and Drug Administration approval of nivolumab for the treatment of cHL that has relapsed or progressed after either autologous hematopoietic cell transplantation (auto‐HCT) and brentuximab vedotin treatment or three or more lines of systemic therapy (including auto‐HCT), and of pembrolizumab for adult or pediatric patients with refractory cHL or cHL that has relapsed after three or more prior therapies. Mechanistically, anti‐PD‐1 therapy prevents inhibitory signaling through PD‐1 receptors on T cells, thereby releasing a ‘block’ to antitumor T‐cell responses. However, this disinhibition can also lead to inappropriate T‐cell activation and responses against healthy tissues, resulting in immune‐mediated adverse events (IMAEs) that affect a number of organ systems. The skin, gastrointestinal, hepatic, and endocrine systems are most commonly involved, typically resulting in rash, colitis, abnormal liver enzyme levels, and thyroiditis, respectively. Notably, pneumonitis is a potentially fatal complication of checkpoint inhibitor immunotherapy. Hematologic oncologists who treat cHL with PD‐1 immune checkpoint inhibitors should monitor patients for IMAEs, as early recognition and treatment can rapidly reduce morbidity and mortality. This review focuses on IMAEs during the treatment of relapsed or refractory cHL with nivolumab and pembrolizumab.
Implications for Practice.
This article highlights the importance of monitoring for immune‐mediated adverse events (IMAEs) in patients with Hodgkin lymphoma (HL) who receive anti‐programmed death‐1 (anti‐PD‐1) therapy, with particular attention given to the recognition and management of such events. The risk of individual IMAEs differs between patients with HL and those with solid tumors, as prior treatments may predispose certain organ systems to specific IMAEs. Accurate and prompt diagnosis of IMAEs is essential for optimal management, allowing PD‐1 inhibitor therapy to be restarted in order to maintain disease control. Potential difficulties, such as distinguishing disease progression from pneumonitis, or colitis from diarrhea, are highlighted to raise clinical awareness.
Introduction
Classical Hodgkin lymphoma (cHL) is characterized by scattered, tumor‐initiating Reed‐Sternberg cells surrounded by a dense rosette‐like pattern of infiltrating, dysfunctional T cells that are incapable of mediating productive antitumor responses [1]. Upregulation of ligands for the programmed death‐1 (PD‐1) immunoreceptor—PD‐L1 and PD‐L2—has been identified as a mechanism by which Reed‐Sternberg cells suppress T‐cell responses [2]. Normally, PD‐L1 and PD‐L2 are upregulated in tissues as a physiological response to inflammation [3], and engagement of PD‐1 on the T‐cell surface by PD‐L1 and PD‐L2 inhibits T‐cell signaling, thereby preventing excessive tissue damage [3], [4]. However, in cHL, Reed‐Sternberg cells mediate pathological suppression of antitumor immune responses [2], [5] via increased PD‐L1 and PD‐L2 expression because of copy number gains in the short arm of chromosome 9 (9p24.1) [5], [6]. The extent of 9p24.1 genetic alteration ranges from polysomy (median of one additional copy) to higher‐order copy number gains and amplifications (upwards of 6–10 additional copies) [5]. PD‐L1/PD‐L2 amplifications have been associated with advanced‐stage disease at presentation and poor progression‐free survival [5].
Nivolumab and pembrolizumab are immunoglobulin G4 monoclonal antibodies that act as checkpoint inhibitors by binding to the PD‐1 receptor and blocking the interaction between PD‐1 and PD‐L1 or PD‐L2 [7], [8]. As a result, the ‘brake’ on T‐cell activation is released, leading to disinhibition of the immune response and improved control of tumor growth. Clinical trials have shown these agents to have substantial therapeutic activity and an acceptable safety profile in patients with relapsed or refractory cHL after multiple prior lines of therapy (supplemental online Table 1) [9], [10], [11], [12]. However, such disinhibition may also lead to inappropriate T‐cell activation against normal tissues and immune‐mediated adverse events (IMAEs).
This review focuses on IMAEs reported during the treatment of cHL with nivolumab and pembrolizumab. Since these monoclonal antibodies are only used in the relapsed and/or refractory clinical settings, special consideration is given to the treatment history of patients with cHL, as the adverse events associated with earlier lines of therapy may overlap with IMAEs associated with checkpoint inhibitor therapy.
Overview of Nivolumab and Pembrolizumab in cHL
Nivolumab and pembrolizumab have demonstrated clinical activity in patients with cHL in phase I and phase II trials. Across cHL trials, patients with relapsed or refractory disease after multiple prior lines of therapy (median of three to five lines, including prior autologous hematopoietic cell transplantation [auto‐HCT] and brentuximab vedotin [BV] in certain cohorts) showed high objective response rates (65%–87%) [9], [11], [12], [13] and prolonged duration of response (overall median of 16.6 months after extended follow‐up in the phase II CheckMate 205 trial of nivolumab) [11] after anti‐PD‐1 checkpoint inhibitor therapy (supplemental online Table 1). Although treatment with PD‐1 inhibitors has not been shown to cause cumulative toxic effects similar to those reported with chemotherapeutic agents [14], patients remaining on immunotherapy for prolonged intervals could nevertheless be at increased risk of cumulative immune‐mediated specific toxicities (Table 1, supplemental online Table 1 [9], [10], [11], [12]). Clinicians must remain vigilant to the diverse clinical presentation and onset of IMAEs, as patients may present with IMAEs late in the course of treatment, and—in some cases—perhaps even after treatment discontinuation [11], [15]. Awareness of these toxicities may enable early identification and timely treatment, thereby reducing the risk of treatment discontinuation and improving overall morbidity and mortality outcomes.
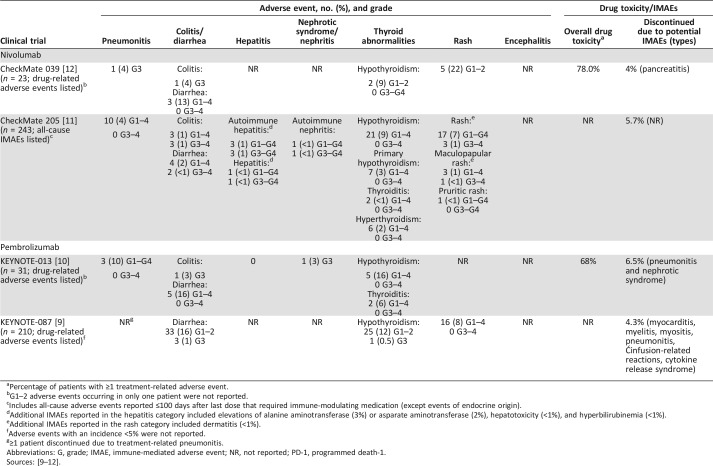
Table 1. Adverse events of potential immune‐related cause with PD‐1 inhibitors for classical Hodgkin lymphoma.
Percentage of patients with ≥1 treatment‐related adverse event.
G1–2 adverse events occurring in only one patient were not reported.
Includes all‐cause adverse events reported ≤100 days after last dose that required immune‐modulating medication (except events of endocrine origin).
Additional IMAEs reported in the hepatitis category included elevations of alanine aminotransferase (3%) or asparate aminotransferase (2%), hepatotoxicity (<1%), and hyperbilirubinemia (<1%).
Additional IMAEs reported in the rash category included dermatitis (<1%).
Adverse events with an incidence <5% were not reported.
≥1 patient discontinued due to treatment‐related pneumonitis.
Abbreviations: G, grade; IMAE, immune‐mediated adverse event; NR, not reported; PD‐1, programmed death‐1.
Treatment History
It is possible that prior treatments, such as bleomycin, carmustine, and BV, may predispose patients to certain IMAEs during checkpoint inhibitor therapy. In addition, patients treated with checkpoint inhibitors may have also been previously exposed to radiotherapy [16], further increasing the risk of IMAEs.
Although a causal link between treatment history and patient vulnerability to IMAEs with checkpoint inhibitors has not been established, it is helpful for the clinician to be mindful of adverse events that may be associated with previous therapies.
Bleomycin, a chemotherapeutic agent that induces double‐stranded DNA breaks, is used as part of all first‐line regimens for cHL, including ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine), Stanford V (doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, prednisone/prednisolone), and BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) [16]. The most serious adverse effects of bleomycin are pulmonary complications, which occur in approximately 10% of patients [17] and may lead to the development of pneumonitis. In approximately 1% of patients, pneumonitis progresses to fatal cases of pulmonary fibrosis [17]. Pulmonary toxicity is unpredictable, but appears to be related to age and dose, increasing in patients aged more than 70 years or with a total dose above 400 units [17].
Carmustine, a DNA alkylating agent, is frequently used as part of conditioning regimens prior to auto‐HCT for relapsed or refractory cHL [18]. Carmustine can adversely affect organs also affected by IMAEs, including the pulmonary system and, less frequently, the hepatic and renal systems [18], potentially predisposing patients to pneumonitis and hepatitis.
BV, an antibody‐drug conjugate consisting of an anti‐CD30 monoclonal antibody linked to an antineoplastic agent (monomethyl auristatin E), is approved in the U.S. for the treatment of cHL in the following indications: after failure of auto‐HCT, after failure of at least two prior multiagent chemotherapy regimens in patients who are not auto‐HCT candidates, or as consolidation after auto‐HCT for patients at high risk of relapse or progression [19]. BV may cause pulmonary, hepatic, and gastrointestinal adverse events, among others [19], therefore potentially predisposing patients to pneumonitis, hepatitis, and colitis/diarrhea. In the phase III AETHERA trial, pulmonary toxicity, including pneumonitis, was reported in 5% (8/167) of patients randomized to receive BV and in 3% (5/160) of those who received the placebo [19], [20]. Of note, the concomitant use of BV with bleomycin is contraindicated because of an increased incidence of pulmonary toxicity reported during combination studies [19].
Radiotherapy is associated with a variety of pulmonary complications, including both subclinical effects and pneumonitis [21], [22]. Thyroid abnormalities can also occur after radiotherapy to the neck (targeting the commonly involved cervical nodes in cHL) as a result of vascular damage to the thyroid gland [23]. Hypothyroidism is the most typical abnormality, with a dose‐related effect observed [24]. Furthermore, the delayed effects of irradiation may predispose patients to additional immunotherapy‐associated thyroid or pulmonary toxicity.
Recognition and Management of IMAEs
Across clinical trials of nivolumab and pembrolizumab, the most common IMAEs observed in patients with cHL have been rashes and hypothyroidism/thyroiditis (Table 1) [9], [10], [11], [12], [25]. Colitis has been reported with lower frequency, although the cases reported have been more severe (grade 3), and hepatitis has also been reported at low frequencies (Table 1) [7], [9], [10], [11], [12]. PD‐1 checkpoint inhibitors are also associated with an increased risk of immune‐mediated pneumonitis [26]. Infusion‐related reactions can occur in up to 14% of patients [9], [10], [11], although these are severe in <1% of patients [7], [8]. Infusion reactions requiring immune‐modulating medication were reported in 4% of patients with cHL who received nivolumab [11]. Patients given PD‐1 inhibitors generally have a relatively low disease burden at trial entry (Eastern Cooperative Oncology Group performance status 0 or 1), and caution should be applied when treating patients with a higher disease burden. In addition, the risk of infusion‐related reactions may be higher when PD‐1 inhibitors are given as part of certain combination therapies [27]. Notably, pulmonary dysfunction is a known complication of cHL treatment. It is therefore critical that hematologists who treat cHL with immuno‐oncology agents be able to recognize IMAEs, as prompt initiation of corticosteroid therapy can reduce toxicity and the likelihood of treatment discontinuation, thereby decreasing morbidity and mortality [7], [8].
Case Study
J.S. is a 33‐year‐old man with stage IV‐B cHL. His disease was refractory to multiple treatment regimens, including ABVD, auto‐HCT, and BV, and he subsequently progressed on all treatments. He started pembrolizumab on a phase I clinical trial and achieved a partial response after four cycles.
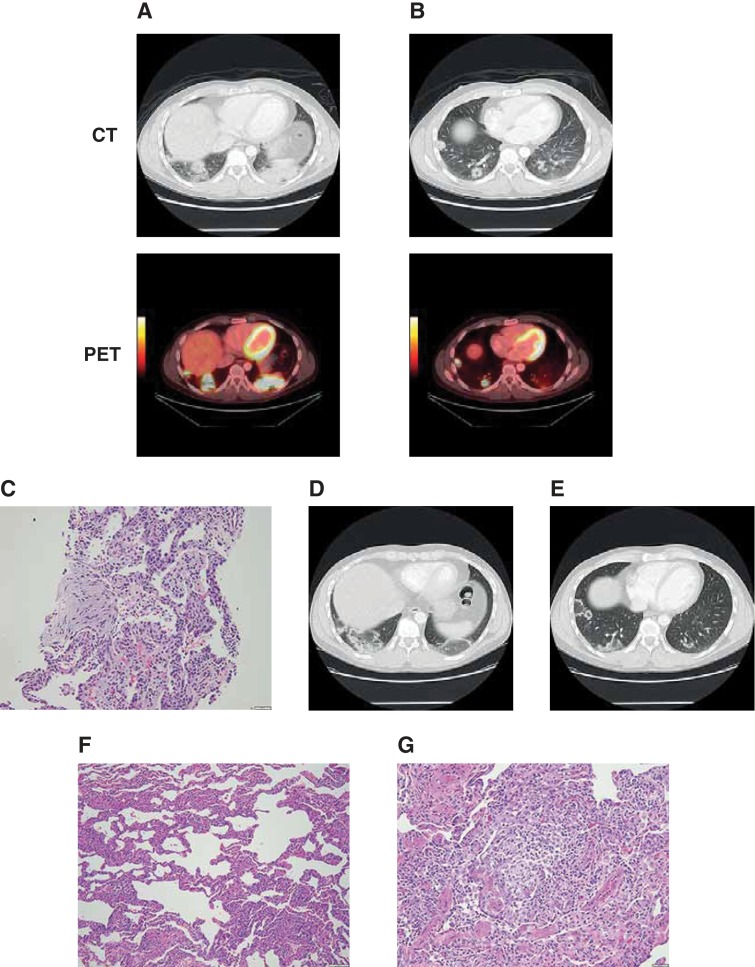
Positron emission tomography–computed tomography (PET‐CT) performed as part of routine restaging after cycle 28 showed new bilateral basilar lung consolidations with standard uptake values (SUVs) of up to 9.2 (Fig. 1A), as well as multiple pulmonary nodules, some of which were cavitary, with an SUV maximum of 7.0 (Fig. 1B). The patient was asymptomatic at this time, with normal exercise capacity. He was seen by the pulmonary service, and results from an extensive workup for infection were negative. He underwent an interventional radiographic‐guided right lung biopsy that showed cellular interstitial pneumonia and organizing pneumonia (Fig. 1C). A follow‐up CT scan revealed a centrifugal expansion and central clearing of both the bilateral consolidations and pulmonary nodules (Fig. 1D–E). Because of the expansion of lesions shown on CT, J.S. underwent a wedge resection of the involved right lower and upper lobe lung regions. This showed cellular and interstitial pneumonia (Fig. 1F) with multiple intra‐alveolar poorly formed granulomas and scattered eosinophils (Fig. 1G). Focal organizing pneumonia was identified. The pleura showed chronic inflammation and fibrous thickening. Immunohistochemical studies did not reveal Langerhans, Reed‐Sternberg, or Hodgkin cells.
Figure 1.
Patient with pneumonitis on programmed death‐1 inhibitor therapy. Bilateral basilar lung consolidations (A) and pulmonary nodules, including cavitary nodules (B) detected on routine PET‐CT at cycle 28; cellular interstitial pneumonia and organizing pneumonia detected on interventional radiographic‐guided right lung biopsy (C); centrifugal expansion and central clearing of both the bilateral consolidations and pulmonary nodules on follow‐up CT (D–E); cellular and interstitial pneumonia (F) with multiple intra‐alveolar poorly formed granulomas and scattered eosinophils (G) identified on wedge resection of the involved right lower and upper lobe lung regions. Abbreviations: CT, computed tomography; PET, positron emission tomography.
J.S. was treated for asymptomatic pneumonitis with oral steroids tapered over 4–6 weeks. Pembrolizumab was withheld, and he was observed while off all treatment. He remained asymptomatic, and a repeat PET‐CT scan 1 month after biopsy depicted ongoing improvement in right and left lung opacities but mild progression of hypermetabolic lymphadenopathy at all sites involved with disease. As he did not suffer any pneumonitis‐related symptoms (grade 1), he was restarted on pembrolizumab 10 weeks after his biopsy without further development of any pulmonary complications. Because of early detection of pneumonitis with prompt initiation of corticosteroids, he has completed 52 cycles of pembrolizumab as of January 2017, with ongoing disease control and continued gradual regression of his pulmonary infiltrates.
Immune‐Mediated Pneumonitis
Abdel‐Rahman and Fouad conducted a meta‐analysis of 11 randomized clinical trials to determine the overall risk of developing pneumonitis in patients treated with nivolumab, pembrolizumab, and/or ipilimumab for melanoma, renal cell carcinoma, prostate cancer, or non‐small cell lung cancer [26]. The use of immune checkpoint inhibitors was associated with an increased risk of all‐grade pneumonitis (odds ratio, 3.96; p < .0001) compared with standard chemotherapy or placebo controls.
In trials of PD‐1 inhibitor therapy across the indications, the incidence of pneumonitis is about 3%, with a median time to onset of 3.3–3.5 months. Pneumonitis led to discontinuation in ≤1.3% of all patients, whereas symptoms resolved completely in approximately 60% of affected patients [7], [8]. The incidences of pneumonitis in trials of PD‐1 inhibitor therapy for cHL have ranged from 3%–10%, with one case of grade 3 pneumonitis and no cases of grade 4 pneumonitis reported across the four trials (Table 1) [9], [10], [11], [12].
Diagnosis and Management of Pneumonitis
Pneumonitis after checkpoint inhibitor therapy can have variable clinical, radiologic, and pathologic presentations. Onset may occur early or late in the course of treatment, although onset is typically observed earlier with the use of combination therapy than with monotherapy [28], [29]. Patients may be asymptomatic at the onset or may present with dyspnea, cough, and, less frequently, fever and chest pain [30]. Standard treatment guidelines for mild‐to‐moderate pneumonitis recommend chest imaging (chest CT with contrast [preferred] or radiograph) every 3–4 weeks or as clinically indicated (mild cases) [29], [31]. Radiographic features may include the following: a cryptogenic organizing pneumonia‐like presentation with discrete patchy or confluent consolidation, the appearance of ‘ground glass’ opacities, interstitial markings with interlobular septal thickening, a centrilobular nodular presentation with a bronchiolitis appearance, or a combination of the above [30]. Given that the radiographic appearance of pneumonitis is highly varied and can resemble malignant lung infiltration or infection, diagnostic biopsy, via bronchoscopy or video‐assisted thorascopic surgery, is essential to distinguish drug‐induced pneumonitis from progression of disease or infectious pneumonitis [30]. Biopsy may reveal granulomatous inflammation, focal fibrin, diffuse alveolar damage, eosinophils, or vessels with recanalized thrombi [30]. In cases of grade 3 or higher pneumonitis, consultations from both pulmonary and infectious diseases physicians are recommended [31]. Potential infectious causes of pneumonitis involve assessment by a respirologist and bronchoscopy or video‐assisted thoracoscopic surgery as needed. Patients developing hypoxia or respiratory distress should have a low threshold for hospitalization. Pneumonitis should be promptly treated with corticosteroids, although steroid‐sparing immunosuppressants are used in patients with refractory pneumonitis. Checkpoint inhibitor therapy should be delayed or stopped entirely, depending on IMAE severity (Table 2) [7], [8].
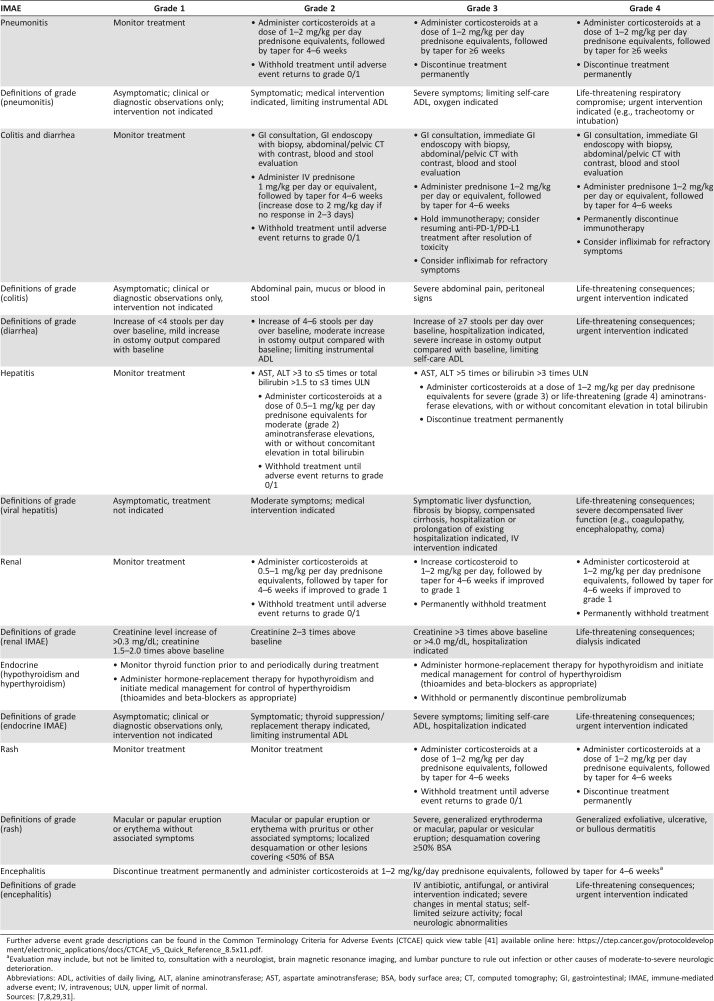
Table 2. Management of IMAEs, by gradea and organ system.
Further adverse event grade descriptions can be found in the Common Terminology Criteria for Adverse Events (CTCAE) quick view table [41] available online here: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf.
Evaluation may include, but not be limited to, consultation with a neurologist, brain magnetic resonance imaging, and lumbar puncture to rule out infection or other causes of moderate‐to‐severe neurologic deterioration.
Abbreviations: ADL, activities of daily living, ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSA, body surface area; CT, computed tomography; GI, gastrointestinal; IMAE, immune‐mediated adverse event; IV, intravenous; ULN, upper limit of normal.
Immune‐Mediated Colitis/Diarrhea
Colitis has been reported in 2%–3% of patients across approved indications of PD‐1 inhibitors [7], [8], with a median time to onset of 3.5–5.3 months. Colitis led to treatment discontinuation in ≤1% of all patients, whereas symptoms completely resolved in ≥74% of affected patients [7], [8]. Similarly, colitis has been reported in >1%–4% of patients with cHL who received nivolumab or pembrolizumab; none of these cases was reported as leading to discontinuation of checkpoint inhibitor therapy [9], [10], [11], [12]. Distinguishing diarrhea from colitis (radiographic or endoscopic fındings of colonic inflammation) is important to help prevent severe or potentially life‐threatening complications. Although similar, colitis can be distinguished from diarrhea by presentation of abdominal pain, blood and mucus in stools, and fever. When patients present with these specific colitis symptoms, it is considered an emergency and an indication for hospitalization. Colitis and diarrhea are treated by prompt administration of corticosteroids [28]. Furthermore, checkpoint inhibitor therapy should be withheld or discontinued depending on the severity of colitis (Table 2). Patients who experience four or more bowel movements above baseline frequency per day (grade 2 or higher) warrant a pause of treatment and subsequent referral for gastrointestinal evaluation, including colonoscopy and esophagogastroduodenoscopy with biopsy and possible gastrointestinal‐directed (e.g., budesonide) or systemic corticosteroid treatment [29], [31]. Antidiarrheals can be used in very mild cases or if gastrointestinal evaluation determines that diarrhea is not immune mediated. Careful attention to, and appropriate treatment of, the earliest symptoms associated with gastrointestinal adverse events can decrease the risk of severe toxicity.
Immune‐Mediated Endocrinopathies
In trials evaluating treatment of cHL with nivolumab or pembrolizumab, endocrine abnormalities, specifically consisting of hypothyroidism, hyperthyroidism, and thyroiditis, have been observed [7]. The incidence of hypothyroidism ranged from 9%–16% [9], [10], [11], [12], and thyroiditis ranged from <1%–6% (Table 1) [10], [11]. Treatment‐related immune‐mediated endocrinopathies reported in patients treated with pembrolizumab (Table 1) consisted of hypothyroidism (12%–16%) and thyroiditis (0%–6%) [9], [10]. In a phase II trial of nivolumab for cHL, all‐cause immune‐mediated endocrinopathies reported included hypothyroidism (9%; primary hypothyroidism was reported in 3% of patients), hyperthyroidism (2%), thyroiditis (<1%), adrenal insufficiency (<1%), and diabetes (<1%) [11].
In cases of symptomatic thyroiditis, patients may initially develop hyperthyroidism that can be treated with beta‐blockers. Hypothyroidism develops later and usually requires thyroid hormone replacement [28]. Typically, a high thyroid‐stimulating hormone (TSH) level with low free T4 indicates primary hypothyroidism, a low TSH level with low free T4 indicates hypophysitis, and high TSH and T3/T4 levels indicate hyperthyroidism [32]. Current treatment guidelines recommend measurement of TSH and free T4 levels every 4–6 weeks as part of routine clinical monitoring [29], [31]. Thyroid hormone replacement is effective for the management of hypothyroidism, and beta‐blockers, corticosteroids, and, in some cases, antithyroid therapy (such as methimazole, carbimazole, or propylthiouracil) or permanent radioiodine ablation [8] may be used to manage hyperthyroidism; however, this treatment should be administered by an endocrinologist [28].
Adrenal suppression and hypophysitis are clinically challenging to diagnose as they may present with symptoms such as fatigue, headache, photophobia, dizziness, nausea, or anorexia [28], [31]. Hypophysitis is diagnosed by biochemical testing of the pituitary‐hypothalamus (prolactin), pituitary‐thyroid (low or normal TSH with a low free T4), and pituitary‐gonadal axes (low luteinizing hormone, follicle‐stimulating hormone with low estradiol or testosterone). Patients presenting with these symptoms are referred to an endocrinologist regardless of IMAE grade [28], [29], [31].
Dermatologic, Hepatic, and Renal IMAEs
Rash has been reported in 1%–9% of patients across all approved indications with PD‐1 checkpoint inhibitors [7], [8]. Trials specific to cHL have reported a wider and higher range of incidence of treatment‐related rash: 12%–22% with nivolumab (immune‐mediated rash and maculopapular rash was reported for 7% and 1% of patients, respectively) [11], [12] and 8% with pembrolizumab [9], [10]. Hepatic adverse events associated with PD‐1 inhibitors consist mainly of elevations in aspartate aminotransferase and alanine aminotransferase levels [11], [32]. Autoimmune hepatitis has been reported in 0.7%–1.8% of patients across clinical trials of PD‐1 inhibitors, with a median time to onset of 1–3 months [7], [8]. Similarly, autoimmune hepatitis was reported in 3 (1%) patients with cHL who received nivolumab in the phase II CheckMate 205 study (all grade 3–4) [11]. Renal adverse events with PD‐1 inhibitors consist mainly of elevated serum creatinine levels. Nephritis has been reported in 0.3%–1.2% of patients across clinical trials [7], [8]. Among patients with cHL, one case of nephrotic syndrome was reported with pembrolizumab in a phase I study, and one case of autoimmune nephritis was reported in a phase II study of nivolumab (Table 1) [10], [11].
Additional Uncommon IMAEs
Clinicians should also be aware of other rare (<1% of patients) but potentially severe IMAEs during PD‐1 inhibitor therapy. Such IMAEs include pancreatic toxicities, cardiovascular disease (e.g., myocarditis), nervous system disorders (e.g., myasthenia gravis), ocular toxicity (e.g., episcleritis, blepharitis, uveitis), endocrine system disorders (e.g., type I diabetes mellitus), severe rashes (e.g., Stevens‐Johnson syndrome or toxic epidermal necrolysis), and renal (e.g., interstitial nephritis) and musculoskeletal disorders (e.g., myositis/polymyositis) [28], [29], [31]. Diagnosis and management of these rare IMAEs is covered in detail in the American Society of Clinical Oncology guidelines [29].
Allogeneic HCT After PD‐1 Inhibitor Therapy
Allogeneic (allo‐) HCT is frequently considered for patients whose disease has progressed after treatment with a checkpoint inhibitor. PD‐1 inhibition prior to allo‐HCT may enhance allogeneic T‐cell responses and augment the graft‐versus‐tumor effect. However, prior immunomodulation may also increase risk of graft‐versus‐host‐disease (GVHD). A small retrospective analysis examined outcomes in patients who had received allo‐HCT after treatment with a PD‐1 inhibitor. Among 39 patients treated with nivolumab or pembrolizumab prior to allo‐HCT, the 1‐year cumulative incidence of grade 2–4 and grade 3–4 acute GVHD was 44% and 23%, respectively, and transplant‐related mortality (death without disease progression) was 11% [33]. These outcomes are within the ranges of historically published values for patients with cHL undergoing allo‐HCT without prior checkpoint inhibitor therapy (100‐day incidences of 6%–28% and 26%–60% for transplant‐related mortality and grade 3–4 acute GVHD, respectively, for studies with varying conditioning regimens and donor characteristics) [34], [35], [36], [37], [38], suggesting that prior anti‐PD‐1 therapy does not preclude allo‐HCT. Patients should nonetheless still be monitored for hyperacute GVHD, grade 3–4 acute GVHD, steroid‐requiring febrile syndrome, hepatic veno‐occlusive disease, and other IMAEs [7], [8].
PD‐1 Inhibitor Therapy After Allo‐HCT
Although use of PD‐1 inhibitors typically precedes allo‐HCT, some patients with lymphoma may receive PD‐1 inhibitors after failure of allo‐HCT. Two recent multicenter, retrospective analyses have provided valuable information on the safety and efficacy of PD‐1 inhibitors after allo‐HCT [39], [40]. In both studies, prolonged survival and a high overall clinical response (overall response 77% and 95%) were observed, indicative of an activated graft‐versus‐tumor effect, but outcomes were frequently complicated by the onset of treatment‐related GVHD [39], [40]. Haverkos et al. [39] reported that 55% (17/31) of patients developed either acute or chronic GVHD after PD‐1 inhibition, with eight treatment‐related deaths; 94% of GVHD cases occurred after one to two doses of anti‐PD‐1 therapy. Herbaux et al. [40] reported that 30% (6/20) of patients experienced acute GVHD, with three patients dying from treatment‐related causes. However, in the Herbaux study, GVHD was observed only in patients with prior acute GVHD, whereas in the Haverkos report, 71% of patients who developed GVHD after PD‐1 blockade had prior acute or chronic GVHD [39], [40]. Additional long‐term data are needed to fully evaluate the risks and benefits of using PD‐1 inhibitor therapy after allo‐HCT. Caution and careful monitoring are warranted, particularly in patients who have a history of GVHD.
Conclusion
Recovery from IMAEs is generally expected if clinicians institute timely intervention with corticosteroid therapy; therefore, awareness and recognition of common IMAEs is of utmost importance. Prompt management, as in the pneumonitis case study detailed here, may allow patients to quickly recover from IMAEs, restart anti‐PD‐1 therapy, and maintain disease control. Emerging clinical experience with checkpoint inhibitors in cHL will help to guide future management of IMAEs. IMAEs reported in clinical trials of PD‐1 checkpoint inhibitors in cHL have so far been predominantly grade 1 or 2 and appear to be generally consistent with those reported in nonhematologic malignancies, albeit with some variability.
Consideration of treatment history, with particular attention to organ systems that may be predisposed to toxicities from prior therapies, may aid in early recognition of IMAEs related to checkpoint inhibitor therapy. Only limited data on the treatment of patients with cHL using PD‐1 checkpoint inhibitors are currently available. To optimize patient management, further improvements in the understanding and treatment of IMAEs are needed. In addition, further research on outcomes and side effects of allo‐HCT both before and after checkpoint inhibitor therapy is needed to clarify the relationship between anti‐PD‐1 therapy and transplant outcomes.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
Professional medical writing assistance was provided by Nicole Draghi, Ph.D., and Matthew Thomas, Ph.D., of Caudex, in the form of copyediting, editorial assistance, and production assistance, and was funded by Bristol‐Myers Squibb. The authors were free to submit without influence from the sponsor, Bristol‐Myers Squibb. M.J.V. is currently affiliated with Pathology and Laboratory Medicine, University of Rochester Medical Center, Rochester, New York, USA. C.H. Moskowitz is currently affiliated with the Sylvester Comprehensive Cancer Center, Miller School of Medicine, University of Miami Health System, Miami, FL, USA.
Author Contributions
Conception/design: Santosha Vardhana, Craig H. Moskowitz
Collection and/or assembly of data: Moises J. Velez, Craig H. Moskowitz
Data analysis and interpretation: Kara Cicero, Craig H. Moskowitz
Manuscript writing: Santosha Vardhana, Kara Cicero, Moises J. Velez, Craig H. Moskowitz
Final approval of manuscript: Santosha Vardhana, Kara Cicero, Moises J. Velez, Craig H. Moskowitz
Disclosures
Santosha Vardhana: Agios Pharmaceuticals (H); Craig H. Moskowitz: Bristol‐Myers Squibb, Merck, Seattle Genetics (C/A, RF), Pfizer (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Küppers R, Engert A, Hansmann ML. Hodgkin lymphoma. JClin Invest 2012;122:3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vardhana S, Younes A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica 2016;101:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keir ME, Butte MJ, Freeman GJ et al. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roemer MG, Advani RH, Ligon AH et al. PD‐L1 and PD‐L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. JClin Oncol 2016;34:2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green MR, Monti S, Rodig SJ et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD‐1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B‐cell lymphoma. Blood 2010;116:3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merck . Keytruda (pembrolizumab) prescribing information. July 2017. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125514s012lbl.pdf. Accessed August 3, 2017.
- 8.Bristol‐Myers Squibb . Opdivo (nivolumab) prescribing information. July 2017. http://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed August 3, 2017.
- 9.Chen R, Zinzani PL, Fanale MA et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. JClin Oncol 2017;35:2125–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armand P, Shipp MA, Ribrag V et al. Programmed death‐1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. JClin Oncol 2016;34:3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armand P, Engert A, Younes A et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow‐up of the multicohort single‐arm phase II CheckMate 205 trial. JClin Oncol 2018;36:1428–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansell SM, Lesokhin AM, Borrello I et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armand P, Shipp MA, Ribrag V et al. Pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: Long‐term efficacy from the phase 1b Keynote‐013 study. Blood 2016;128:1108A. [Google Scholar]
- 14.Topalian SL, Sznol M, McDermott DF et al. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. JClin Oncol 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luke JJ, Ott PA. PD‐1 pathway inhibitors: The next generation of immunotherapy for advanced melanoma. Oncotarget 2015;6:3479–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Hodgkin Lymphoma Version 1.2018. April 16, 2018. Available at https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. Accessed April 13, 2018.
- 17.Hospira . Bleomycin for injection [prescribing information]. January 2013. https://www.pfizerinjectables.com/sites/default/files/prod/child/uspi/43 4001.pdf. Accessed March 26, 2018.
- 18.Heritage Pharmaceuticals . BICNU (carmustine) prescribing information. March 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/01 7422s055lbl.pdf. Accessed May 8, 2017.
- 19.Seattle Genetics . Adcetris (brentuximab vedotin) prescribing information. September 2016. http://www.seattlegenetics.com/pdf/adcetris_USPI.pdf. Accessed May 8, 2017.
- 20.Moskowitz CH, Nademanee A, Masszi T et al. Brentuximab vedotin as consolidation therapy after autologous stem‐cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): A randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2015;385:1853–1862. [DOI] [PubMed] [Google Scholar]
- 21.Pinnix CC, Smith GL, Milgrom S et al. Predictors of radiation pneumonitis in patients receiving intensity‐modulated radiation therapy for Hodgkin and non‐Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 2015;92:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatramani R, Kamath S, Wong K et al. Pulmonary outcomes in patients with Hodgkin lymphoma treated with involved field radiation. Pediatr Blood Cancer 2014;61:1277–1281. [DOI] [PubMed] [Google Scholar]
- 23.Jereczek‐Fossa BA, Alterio D, Jassem J et al. Radiotherapy‐induced thyroid disorders. Cancer Treat Rev 2004;30:369–384. [DOI] [PubMed] [Google Scholar]
- 24.Ng AK, LaCasce A, Travis LB. Long‐term complications of lymphoma and its treatment. JClin Oncol 2011;29:1885–1892. [DOI] [PubMed] [Google Scholar]
- 25.Younes A, Santoro A, Shipp M et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem‐cell transplantation and brentuximab vedotin: A multicentre, multicohort, single‐arm phase 2 trial. Lancet Oncol 2016;17:1283–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdel‐Rahman O, Fouad M. Risk of pneumonitis in cancer patients treated with immune checkpoint inhibitors: A meta‐analysis. Ther Adv Respir Dis 2016;10:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera AF, Moskowitz AJ, Bartlett NL et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018;131:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naidoo J, Page DB, Li BT et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. JClin Oncol 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. Epub 2018 Feb 14. PubMed PMID: 29442540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naidoo J, Wang X, Woo KM et al. Pneumonitis in patients treated with anti‐programmed death‐1/programmed death ligand 1 therapy. JClin Oncol 2017;35:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Management of Immunotherapy‐Related Toxicities (Immune Checkpoint Inhibitor‐Related Toxicities). Version 1.2018. February 14, 2018. Available at https://www.nccn.org/store/login/login.aspx?Return URL=https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. Accessed April 6, 2018.
- 32.Postow MA. Managing immune checkpoint‐blocking antibody side effects. Am Soc Clin Oncol Educ Book 2015:76–83. [DOI] [PubMed] [Google Scholar]
- 33.Merryman RW, Kim HT, Zinzani PL et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD‐1 blockade in relapsed/refractory lymphoma. Blood 2017;129:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderlini P, Saliba RM, Ledesma C et al. Gemcitabine, fludarabine, and melphalan for reduced‐intensity conditioning and allogeneic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2016;22:1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devetten MP, Hari PN, Carreras J et al. Unrelated donor reduced‐intensity allogeneic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow Transplant 2009;15:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcais A, Porcher R, Robin M et al. Impact of disease status and stem cell source on the results of reduced intensity conditioning transplant for Hodgkin's lymphoma: A retrospective study from the French Society of Bone Marrow Transplantation and Cellular Therapy (SFGM‐TC). Haematologica 2013;98:1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson SP, Sureda A, Canals C et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin's lymphoma: Identification of prognostic factors predicting outcome. Haematologica 2009;94:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sureda A, Robinson S, Canals C et al. Reduced‐intensity conditioning compared with conventional allogeneic stem‐cell transplantation in relapsed or refractory Hodgkin's lymphoma: An analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. JClin Oncol 2008;26:455–462. [DOI] [PubMed] [Google Scholar]
- 39.Haverkos BM, Abbott D, Hamadani M et al. PD‐1 blockade for relapsed lymphoma post‐allogeneic hematopoietic cell transplant: High response rate but frequent GVHD. Blood 2017;130:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herbaux C, Gauthier J, Brice P et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood 2017;129:2471–2478. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Department of Health and Human Services . Common Terminology Criteria for Adverse Events. Version 5.0. Rockville, MD: U.S. Department of Health and Human Services, 2017. [Google Scholar]