Abstract
Purpose of Review:
Agriculture remains a major economic sector globally, and workers experience high rates of chronic inflammatory lung and musculoskeletal diseases. Whereas obstructive pulmonary diseases are known risk factors for bone loss, the underlying relationship between lung inflammation and bone health is not well-known.
Recent Findings:
An agriculture organic dust extract inhalation animal model has recently linked lung injury-induced inflammation to systemic bone loss. This process is dependent upon lipopolysaccharide and the Toll-like receptor 4 (TLR4) signaling pathway. Downstream systemic interleukin-6 is a key mediator that subsequently activates osteoclastogenesis. Age is a host factor that impacted bone disease with younger mice demonstrating increased susceptibility to bone loss following inhalant exposures as compared to older mice. Supplemental dietary vitamin D was shown to prevent organic dust-induced bone loss, but not lung disease, in animals.
Summary:
Recent animal studies provide new mechanistic insight into the lung-bone inflammatory axis. Host factors, diet, and lipopolysaccharide/TLR4 signaling pathways play a significant role in explaining how inhalant organic dust exposures impact bone health. These investigations might lead to specific targeted therapeutic approaches.
Keywords: Organic Dust, Bone, endotoxin, COPD, interleukin (IL)-6, Toll-like receptor 4/TLR4
Introduction
Agriculture work is associated with adverse health effects, with the most prevalent issues of chronic inflammatory lung and musculoskeletal diseases. Development of chronic obstructive pulmonary disease (COPD), chronic bronchitis, asthma, and hypersensitivity pneumonitis have all been associated with agricultural exposure (1,2,3,4). Exposed workers also have an increased life-time risk (90%) of developing musculoskeletal diseases (5). There is a high prevalence of osteoarthritis of upper and lower extremities, low back pain, and rheumatoid arthritis (RA) with subsequent increase in morbidity and mortality from hip fractures. (5,6,7). There is a similar association of bone loss and osteoporosis in persons with chronic inflammatory respiratory diseases like COPD, asthma, cystic fibrosis, and post-lung transplantation (8,9,10,11). Furthermore, it has been shown that having COPD is an independent risk factor for developing bone mineral density (BMD) loss, irrespective of body-mass index (BMI), sex, age, steroid use, smoking, or severity of COPD (12). Recognizing these similarities, it is reasonable to suggest that exposure to agriculture-related occupational inhalant insults triggers an airway inflammatory process that could impact systemic bone health.
More than two decades ago, associations between occupational exposure and bone disease were described for Ewing’s sarcoma (13). Analysis of medical and occupational family history of children with this bone disease revealed an increased risk (RR: 8.8, CI: 1.8–42.7) with fathers who were agricultural workers (13). Expounding on this initial observation, a later case-controlled study of 196 children from 64 U.S. institutions demonstrated that mothers or fathers who had perinatal or postnatal exposure to occupational organic dusts lead to an increased odds ratio (OR: 2.1, CI: 1.0–4.2) of their offspring developing Ewing’s sarcoma (14). The odds ratio was further increased (OR: 3.5, CI: 0.4–33.0) if exposure was primarily from the mother’s occupational exposure (14). Ewing’s sarcoma is a rare form of bone malignancy that is primarily found in adolescents (15). The primary mechanism associated with Ewing’s sarcoma is through overexpression of receptor activator of nuclear factor kappa-β ligand (RANKL), which leads to enhanced osteoclastogenesis (16).
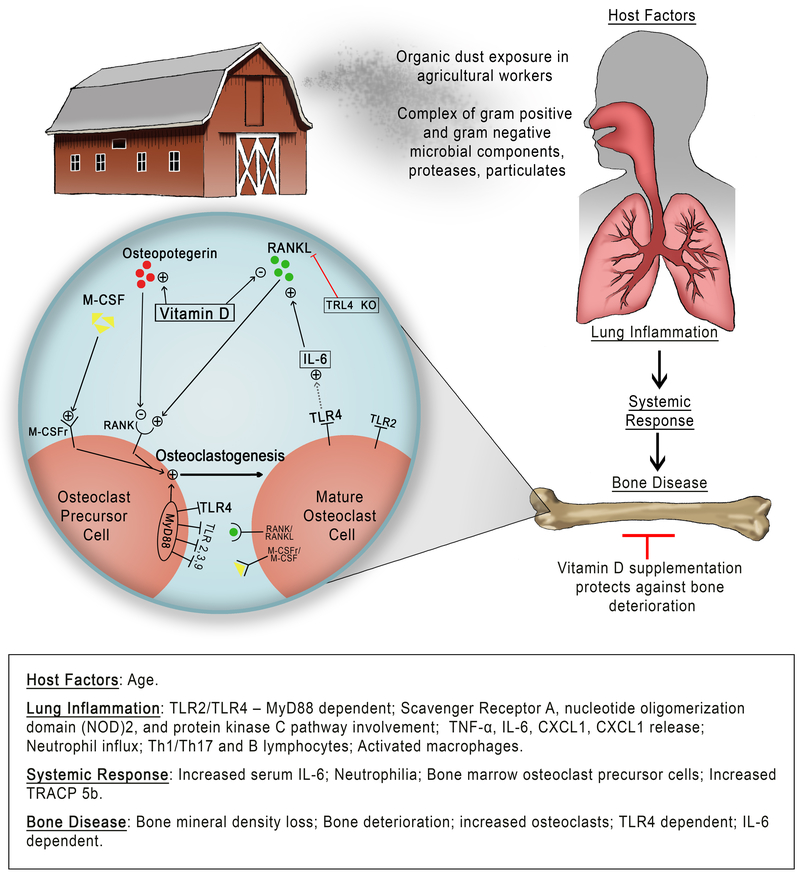
Balance of osteoclast and osteoblast activity is central to the maintenance of bone homeostasis (17). Osteoclasts are bone-specific, bone-resorbing macrophages derived from bone marrow osteoclast precursor cells that express membrane bound RANK. Osteoclast maturation and activation occurs in the presence of macrophage colony-stimulating factor (M-CSF) and RANKL (17). Following osteoclast activation and bone resorption, bone morphogenetic proteins, fibroblast growth factors, and transforming growth factor β are released from the bone matrix (18). These factors stimulate osteoblasts, which in turn produce new bone matrix and promote mineralization. Several factors stimulate lining cells to balance RANK/RANKL expression and bone homeostasis. These include mechanical stress, tumor necrosis factor (TNF)-α, insulin growth factor-I, parathyroid hormone, and interleukins (18). The inflammatory cytokines, interleukin (IL)-17, IL-1β, IL-6, and TNF-α, have all been shown to play a role in RANKL expression and osteoclastogenesis (18). Understanding these mechanisms regulating bone homeostasis could provide explanation linking airway inflammatory injury and bone disease. An overview schematic of known mechanisms involved in the lung-bone inflammatory axis are depicted in Figure 1.
Figure 1. Overview schematic of the proposed relationship of inhalant organic dust exposures induction of bone loss.
Inhalation of complex agriculture organic dust induces an airway inflammatory response that leads to a systemic response and bone deterioration findings. The “+” sign = positive activator of the pathway. The “-” sign = negative influence or blockade of the pathway. M-CSF = macrophage colony-stimulating factor. M-CSFr = macrophage colony-stimulating factor receptor. RANK = receptor activator of nuclear factor kappa-β. RANKL = receptor activator of nuclear factor kappa-β ligand. TLR = Toll-like receptor. MyD88 = myeloid differentiation factor 88. IL-6 = Interleukin-6.
Agriculture organic dust exposure and airway inflammatory disease
Agricultural workers have an increased risk of COPD and asthma-like disease (19). Following exposure to organic dust, human airways develop an inflammatory response marked by Th1/Th17 activation and neutrophil airway influx (20,21,22,23). Genetic polymorphisms in Toll-like receptors and neutrophil adhesion-molecule expression have been associated with agriculture respiratory disease. (25,26,27). Toll-like receptor activation is highly dependent on the adaptor protein, myeloid differentiation factor 88 (MyD88) (28). Repetitive exposure leads to an attenuated response in lung inflammation (29) but a sustained systemic effect (30). Animal models resembling human disease including approaches of intranasal instillations and nebulizations of agriculture organic dusts and extracts or direct facility exposure in mice and rats have all demonstrated similar patterns of inflammatory markers (21,23,28,31,32). Repetitive organic dust inhalation induces neutrophil influx, release of inflammatory cytokine/chemokine (i.e. TNF-α, IL-6, neutrophil chemoattractants), Th1/Th17 microenvironment, activation of macrophages, and lung pathology marked by lymphoid aggregates and peribronchial and perivascular inflammation (21,22,23,31,32,). It was recently reported that there is also a role for B cells in ODE-induced airway inflammation with further evidence for an autoreactive response marked by increased levels of IgG anti-citrullinated protein antibody and anti-malondialdehyde-acetaldehyde autoantibodies (24). Homeostatic regulators of the ODE-induced airway inflammatory process include protein kinase C epsilon (PKCε) (33), nucleotide-binding oligomerization domain 2 (NOD2) (34,35), and scavenger receptor class A (SRA) (36). Organic dusts obtained from swine confinements are commonly utilized due to their strong inflammatory consequences, and these dusts consists of a complex mixture of particulates containing gram negative and gram positive bacterial components such as lipopolysaccharide (LPS) and peptidoglycan (PGN) (37,38).
Animal model of inhalant organic dust-induced bone loss
To investigate the lung-bone inflammatory axis following organic dust exposures, a murine model has been developed and characterized (39). Namely, C57BL/6 mice were intranasally instilled with swine confinement organic dust extract (ODE), LPS, PGN, or saline control daily for 3 weeks whereupon hind limbs were collected 5 hours following final exposure. Bone images as well as bone quantity and quality analysis was conducted by micro-computed tomography (CT) imaging technology and software analysis with comparisons to bone histology. Initial studies focused on the distal calcaneus bone and found that as compared to saline inhalation, inhalant ODE resulted in significant loss (−24%) in bone mineral density and a −25% decrease in the trabecular bone volume fraction, which are markers of bone demineralization. ODE exposure also increased bone surface area (marker of bone resorption) by +20%, and resulted in deterioration in morphological bone structure as noted by decreased trabecular thickness (−12%), decreased trabecular number (−15%), increased trabecular separation (+18%), and disconnected trabecular pattern factor (+325%). ODE exposure induced a loss of torsional resistance (i.e. mean polar moment of inertia) by −43%. Similarly, bone histology findings demonstrated reduction in collagen and proteoglycan. Intranasal inhalation of LPS and PGN daily for 3 weeks also resulted in increased bone deterioration as compared to control. LPS demonstrated the most significant bone loss and deterioration as compared to ODE and PGN. This observation was in contrast to lung inflammation because PGN and ODE exposures induced enhanced lung tissue pathology as compared to LPS, which the authors interpreted as a compartmental response to inhalant ODE exposure (19).
Organic dust mediated lung-bone inflammatory axis is dependent on TLR4
To further understand the role of specific components driving the bone loss to inhalation of this complex organic dust, investigators employed Toll-like receptor 2 (TLR2) and TLR4 knockout mice. TLR2 is a key signaling pathway in cellular response to gram positive bacterial components, while TLR4 recognizes and responds to LPS from gram negative bacteria. TLR2 and TLR4 are also expressed on bone osteoclasts and play roles in osteoclastogenesis (40). Moreover, both TLR2 and TLR4 signaling pathways have been implicated in mediating airway inflammatory disease in agriculture workers, particularly swine confinement workers (25,26). Animals deficient in TLR2 or TLR4 demonstrate an approximate 50% reduction in airway inflammatory makers following organic dust challenge (28,41,42).
Using same exposure model and micro-CT analysis as already described (39), Staab, et al. (2016) investigated the inhalant ODE-induced bone consequences of the proximal tibia in TLR2 and TLR4 knockout mice to wild type animals (43). As compared to WT animals, ODE exposed TLR4 knockout mice were protected from adverse changes in bone mineral density, trabecular separation, bone surface area, trabecular pattern factor, and polar moment of inertia. However, the bone quantity and quality measurements with the TLR2 knockout mice were no different than the wild type mice exposed to ODE. Both wild type and TLR2 knockout animals instilled with ODE had significant changes in bone mineral density (−16%), polar moment of inertia (−41%), trabecular separation (+12%), bone surface area (+13%), and trabecular pattern factor (+13%) when compared to saline instilled animals. These studies demonstrated that TLR4 is necessary for mediating the bone loss consequences following respiratory injury with ODE.
In this same study (43), ODE-induced airway neutrophil influx was reduced by approximately 50% in both TLR2 and TLR4 knock out mice compared to wild type animals. Similar reductions were demonstrated with ODE-induced cytokines/chemokine in the lavage fluid. Moreover, inhalant ODE-induced systemic levels of were reduced by 51% in TLR2 KO and 64% in TLR4 KO mice as compared to wild type animals. These studies would suggest that the reduction in lung inflammation alone is not the explanation for the bone protective effect observed in TLR4 KO mice, but suggest the involvement of TLR4 signaling pathway in bone pathology.
Interleukin 6 Involvement in Osteoclastogenesis
IL-6 has been shown to play important roles in the pro-inflammatory state of COPD (44), heart disease (45), rheumatoid arthritis (46), and osteoclastogenesis (18). This suggests that systemic IL-6 may be important for explaining the lung-bone inflammatory axis. Ant-IL-6 therapies, such as tocilizumab, have been successful in the treatment of rheumatoid arthritis (47,48). IL-6 has also been shown to impact RANKL expression and influence osteoclastogenesis (18). In addition, IL-6 is a key cytokine that is consistently associated with organic dust induced airway injury (31,49,50,51), and murine serum levels of IL-6 have been detected following inhalant ODE exposure (30,52). Therefore, it was hypothesized that systemic IL-6 could be responsible for mediating inhalant-ODE induced bone loss.
In a study of 3 week inhalant exposure to ODE, airway inflammatory and bone outcomes in wild type mice were compared to IL-6 knockout animals (30). Unexpectedly, there was no difference in inflammatory cell influx, cytokine/chemokine release or lung pathology between ODE-treated wild type and IL-6 knockout animals, suggesting that the airway inflammatory response to ODE is not dependent on IL-6 (30). However, micro-CT analysis of the distal tibia demonstrated that IL-6 knockout mice were protected from inhalant ODE-induced bone loss. Specifically, there was no difference in bone mineral density, polar moment of inertia, trabecular separation, bone surface area, trabecular pattern factor, or bone volume ratio between saline and ODE-treated IL-6 knockout animals as there was in the ODE-treated wild type mice (30).
Bone marrow cells collected from these same saline and ODE-treated wild type and IL-6 knockout mice were investigated for osteoclast precursor cell (OCP) populations. Whereas wild type animals expressed increased OCP populations following inhalant ODE exposure compared to saline controls, there was no significant increase in OCP populations in the IL-6 knockout mice. These studies highlight the important role systemic IL-6 plays in the lung-bone inflammatory axis.
Organic dust-induced osteoclastogenesis
Osteoclasts and osteoclast precursor (OCP) cell count and activity correlate well with arthritis severity and bone loss (53,54). Osteoclasts are myeloid-derived cells, and osteoclast precursor cells express CD115, CD117, and CD27. CD115 and CD117 are receptors for M-CSF and stem-cell factor respectively; expression of both markers is necessary for osteoclast differentiation and proliferation (53). CD27 expression has also been shown to identify OCPs with high proliferation potential (55). CD70 is the ligand responsible for activation of CD27 and in chronic inflammatory states such as rheumatoid arthritis; CD70 is overexpressed and promotes arthritic affects (55). Using flow cytometry, OCPs can be isolated from lineage negative (CD3-/CD45R-/CD11b-) cells with expression of CD115/CD117/CD27. In the context of ODE inhalation, it has been demonstrated that osteoclast frequency as well as bone marrow derived osteoclast precursor cell populations are increased (30,43,56).
Tartrate-resistant acid phosphatase (TRACP 5b) is a marker of osteoclast number and bone resorption activity (57). Serum levels of TRACP 5b has been measured in animal studies. Serum TRACP 5b levels have been shown to be increased in wild type mice treated with ODE as compared to saline control animals (43). However, in TLR4 knockout animals, there is no difference in serum TRACP 5b levels with ODE exposure as compared to saline control, which further implicates the TLR4 signaling pathway in mediating ODE-induced osteoclastogenesis.
Toll-like receptors are activated by either pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) (58). TLRs 2, 3, 4, and 9 have been identified on osteoclast precursor cells and are key to stimulating maturation, while mature osteoclasts express TLR 2 and 4 (58,59). All ten functional human TLRs, except for TLR3, use the MyD88-dependant signaling pathway once activated (58). Each TLR has specific agonist that it responds to, such as PGN (TLR2), viral ds-RNA (TLR3), LPS (TLR4), and bacterial DNA (TLR9) (58,59). These TLRs are present on osteoclasts and act synergistically (59). When osteoclast precursor cells are committed to become mature osteoclasts by binding RANK-ligand with membrane bound RANK, TLR 2, 3, 4, and 9 all promote osteoclast maturation (59). Furthermore, TLR 4 and 9 stimulate an increase in IL-6 and TNF-α which feedback to bone marrow stromal cells to produce more RANK-ligand (59). However, if an osteoclast precursor cell is not yet committed with RANK/RANKL, TLR 9 activation will inhibit osteoclastogenesis by decreasing expression of membrane bound RANK and M-CSF receptor (59).
In vitro osteoclastogenesis assays using RANKL and M-CSF (43) pretreated bone marrow cells demonstrated that further stimulation with ODE, LPS, or peptidoglycan promoted osteoclast maturation as assessed by cell surface mRANKL expression (43). In comparison to WT, cells derived from TLR4 knockout mice demonstrated reduced expression of mRANKL following ODE treatment whereas TLR2 knockout cells demonstrated a normative response (43). Consistent with these in vitro studies, in vivo studies of TLR4 knockout mice treated with ODE inhalation, OCP populations were not increased. However, in both wild type and TLR2 knockout animals treated with ODE, these OCP populations were significantly increased compared to saline controls (43). Collectively, these studies suggest that TLR4 is sufficient to promote osteoclastogenesis following ODE exposure.
Host Factors
Host factors influence disease development and severity. In COPD, morbidity and mortality worsens with age (60,61). COPD is more common with male gender, history of frequent respiratory infections, smoking history, and alpha-1-anti-trypsin genotypes (62). Bone disease is also dependent upon several host factors. Gender, age, and body mass index (BMI) are associated with lower vitamin D levels and increase systemic TNF-α (63). Lower estrogen levels increase bone resorption and osteoporosis (64). Increased BMI and increased estrogen levels have been shown to improve bone regeneration following maxillary bone regeneration procedures (65). Host factors likely effect the lung-bone inflammatory axis.
American farmers are an aging population, with approximately 30% being older than 65 years of age (66). The impact of age in ODE inhalation-induced bone loss was investigated in a study of mice that were 7 to 9 weeks old (young mice) as compared to 12 to 14 month old mice (older mice) (52). This age difference approximately correlates to human ages of 20’s and 50’s, respectively. Older mice were noted to have less lung neutrophil influx and cytokine release following acute (one-time) ODE exposure and an increased lymphocyte and macrophage influx following repetitive (3 week) ODE exposures as compared to younger animals. Histological examination of the lungs also revealed an increase in alveolar inflammatory infiltrates in the older mice after ODE repetitive exposure, but no difference in the bronchiolar infiltrates. Older mice also had an increase in lung CD8+ T lymphocytes compared to the younger animals. As compared to the older animals, the younger mice demonstrated an increase in peripheral blood neutrophils and serum IL-6 following acute ODE inhalant exposure. Interestingly, the serum IL-6 response lessened with repetitive ODE exposures in the younger mice, but remained elevated in the older mice. Although the older animals had a blunted airway inflammatory response to ODE, they had higher inflammatory markers at baseline and a sustained systemic response with repetitive exposure.
Given the sustained systemic IL-6 response in the older animals, it was postulated that these older mice would have worse bone deterioration following repetitive ODE exposure. However, the younger, but not older, animals were susceptible to ODE-induced bone deterioration as assessed by micro-CT analysis of the distal tibia. Not surprisingly, in comparing saline control young and older mice, the older mice had considerably more bone loss and features of bone deterioration than the younger animals. This suggests that older age alone is a risk factor for bone deterioration, but that inhalant ODE exposure could have a greater impact on the young.
Current and Future Therapies
A mainstay treatment of osteoporosis has included bisphosphonates, which incorporate into bone structure to prevent resorption and cause general osteoclast apoptosis (67). With improved understanding of bone homeostasis, newer therapies have emerged that target specific pathways. Hormone based therapies include the selective estrogen receptor modulators (SERMs), such as Raloxifene, that are estrogen agonists which promote bone homeostasis (68). Teriparatide is a recombinant DNA which mimics parathyroid hormone and promotes osteoblast activity (68). Both of these therapies target specific bone-hormone interactions. Although this has improved treatment, these medications still carries potential side effects from altered hormone homeostasis such as thromboembolism, headaches, muscle cramping, nausea, or diarrhea (68). Targeting osteoclastogenesis directly could render potentially more effective treatment options with fewer side effects. One such pathway is the RANK/RANKL osteoclast interaction that stimulates osteoclastogenesis. Denosumab is a human monoclonal antibody that targets RANKL and inhibits osteoclastogenesis (69). Osteoprotegerin is a receptor protein that binds RANKL and inhibits osteoclastogenesis (70). Although these both have promise in osteoporosis treatment, denosumab use has an association with increased risk of infections (71) and osteoprotegerin use has an association with increased heart disease (72). Tocilizumab is an antibody that binds soluble and membrane bound IL-6 and has been approved for treatment of rheumatoid arthritis (73), but it might also increase the risk of upper airway infections (74). The IL-6 signaling pathway is comprised of two pathways called the classic and the trans pathways, which has been generally considered anti-inflammatory and pro-inflammatory, respectively (75). The trans IL-6-signaling pathway is dependent on a transmembrane protein named gp130, and current research is investigating the benefit of trans-signaling blockade via anti-gp130 recombinant protein use in several diseases (76,77,78).
Vitamin D is a low cost and low side-effect profile hormone that has been shown to directly inhibit osteoclastogenesis without compromising other systems (56). Vitamin D is an active hormone that is synthesized in the skin via sunlight exposure or can be absorbed from dietary intake. In a murine study, animals were fed either a low vitamin D (1 IU/g) or a high vitamin D (10 IU/g) diet for 5 weeks, followed by 3 weeks of daily intranasal inhalation of either saline, ODE, or LPS (56). The high vitamin D treatment group demonstrated protection against ODE and LPS-induced bone mineral density loss, bone volume loss, and bone micro-architecture deterioration (56). Furthermore, TRAP+ osteoclasts were frequently present in the low vitamin D mice but were nearly absent in the high vitamin D treatment group exposed to either ODE or LPS (56). There was no difference in lung pathology, airway neutrophil influx, lavage fluid levels of TNF-α, IL-6, IL-1β, IL-17A, and IFN-γ, or serum IL-6 levels between the ODE exposed low and high vitamin D treatment groups (56). The only detectable difference was a slight increase in serum IL-10 levels in the high vitamin D treatment group (56). This suggested that vitamin D plays a selective role in preventing osteoclast associated bone reabsorption without altering the airway inflammatory response. Other studies have confirmed that active vitamin D can indirectly inhibit osteoclastogenesis via decreased expression of RANKL from bone marrow stromal cells (79) or increase expression of osteoprotegerin (80). Vitamin D has also been shown to suppress c-fos (81) and nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) (82), which are both transcription factors required for osteoclast maturation.
Conclusion
Occupational agricultural work exposure and the development of musculoskeletal diseases have been well-recognized for more than two decades. With studies linking bone mineral density loss with COPD and asthma, recent studies have emerged investigating bone loss and mechanisms mediating bone loss following agriculture-related organic dust-induced airway injury in animal models. TLR4- and IL-6 signaling pathway have been shown to be important in explaining bone consequences following organic dust extract induced bone deterioration. As osteoclastogenesis is recognized as a primary mechanism of inflammatory disease related bone loss, it has been demonstrated that agriculture organic dusts induce osteoclasts/osteoclastogenesis. These events are also dependent upon TLR4 and IL-6 signaling pathways. The agricultural workforce is an aging population and whereas older age alone is an independent risk factor for bone loss, studies have shown that younger animals exposed to organic dusts appear to have increased susceptibility to bone loss following airway injury. Thus, attention to younger workers’ risk of potentially developing bone disease from occupational exposures is warranted. Vitamin D has been shown to prevent bone loss induced by inhalant organic dust exposure, which was associated with inhibition of osteoclastogenesis. Vitamin D supplementation is an inexpensive intervention that could be promoted among the agricultural workforce for bone loss/fracture risk protection.
Acknowledgements:
The authors wish to thank Melissa Carrington for graphical assistance with the figure design.
Declaration of all sources of funding for the research reported in the manuscript: Study supported by grants from the National Institute of Environmental Health Sciences (R01: ES019325 to JAP) and National Institute of Occupational Safety and Health (U54OH010162 to JAP). This work was supported in part by the Central States Center for Agricultural Safety and Health (CS-CASH).
References
- 1.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: The adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 2003; 9(3): 185–196. [DOI] [PubMed] [Google Scholar]
- 2.Eduard W, Pearce N, Douwes J. Chronic bronchitis, COPD, and the lung function in farmers: The role of biological agents. Chest. 2009; 136(3): 716–725. [DOI] [PubMed] [Google Scholar]
- 3.Poole JA. Farming-associated environmental exposures and the effect on atopic diseases. Ann Allergy Asthma Immunol 2012; 109(2): 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkhorn SR, Garry VF. Agricultural lung diseases. Environmental Health Perspectives. 2000;108(Suppl 4):705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborne A, Blake C, Fullen BM, Meredith D, Phelan J, McNamara J, et al. Prevalence of musculoskeletal disorders among farmers: A systematic review. Am J Ind Med 2012; 55: 143–158. doi: 10.1002/ajim.21033. [DOI] [PubMed] [Google Scholar]; * Complete review of the various musculoskeletal disorders found within a farming population.
- 6.Osborne A, Blake C, Fullen BM, Meredith D, Phelan J, McNamara J, et al. Risk factors for musculoskeletal disorders among farm owners and farm workers: a systematic review. Am J Ind Med 2012; 55: 376–389. doi: 10.1002/ajim.22001. [DOI] [PubMed] [Google Scholar]
- 7.Murphy D, Hutchinson D. Is Male Rheumatoid Arthritis an Occupational Disease? A Review. The Open Rheumatology Journal. 2017; 11:88–105. doi: 10.2174/1874312901711010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehouck A, Boonen S, Decramer M, Janssens W. COPD, bone metabolism, and osteoporosis. Chest 2011;139: 648–657. doi: 10.1378/chest.10-1427. [DOI] [PubMed] [Google Scholar]; ** Comprehensive review article on COPD and bone loss disease associations.
- 9.Graat-Verboom L, Smeenk FW, van den Borne BE, Spruit MA, Donkers-van Rossum AB, Aarts RP, et al. Risk factors for osteoporosis in Caucasian patients with moderate chronic obstructive pulmonary disease: a case control study. Bone. 2012; 50: 1234–1239. doi: 10.1016/j.bone.2012.02.638. [DOI] [PubMed] [Google Scholar]; * Important and well-designed case control study demonstrating risk factors for osteoporosis in subjects with COPD.
- 10.Robertson J, Macdonald K. Prevalence of bone loss in a population with cystic fibrosis. Br J Nurs 2010; 19: 636–639. [DOI] [PubMed] [Google Scholar]
- 11.Chauhan V, Ranganna KM, Chauhan N, Vaid M, Kelepouris E. Bone disease in organ transplant patients: pathogenesis and management. Postgrad Med 2012; 124: 80–90. doi: 10.3810/pgm.2012.05.2551. [DOI] [PubMed] [Google Scholar]
- 12.Jung JW, Kang HR, Kim JY, Lee SH, Kim SS, Cho SH. Are asthmatic patients prone to bone loss? Ann Allergy Asthma Immunol 2014; 112: 426–431. doi: 10.1016/j.anai.2014.02.013. [DOI] [PubMed] [Google Scholar]; ** Clinical research study demonstrating a positive relationship between airway hyper-responsivness and asthma to bone mineral density loss that is independent of corticosteroid use.
- 13.Holly EA, Aston DA, Ahn DK, Kristiansen JJ. Ewing’s Bone Sarcoma, Paternal Occupational Exposure, and other Factors. Am J Epidemiol 1992; 135: 122–9. [DOI] [PubMed] [Google Scholar]
- 14.Moore LE, Gold L, Stewart PA, Gridley G, Prince JR, Zahm SH. Parental occupational exposures and ewing’s sarcoma. International Journal of Cancer. 2005; 114(3): 472–478. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins RM, Pritchard DJ, Omer EB, Unni KK. Ewing’s sarcoma of bone. experience with 140 patients. Cancer. 1986; 58(11): 2551–2555. [DOI] [PubMed] [Google Scholar]
- 16.Taylor R, Knowles HJ, Athanasou NA. Ewing sarcoma cells express RANKL and support osteoclastogenesis. The Journal of Pathology. 2001; 225(2): 195–202. [DOI] [PubMed] [Google Scholar]
- 17.Rucci N, Teti A. Osteoclasts: Essentials and Methods In: Pietschmann P (eds) Principles of Bone and Joint Research. Learning Materials in Biosciences. 2017. Springer, Cham: Doi: 10.1007/978-3-319-58955-8_3. [DOI] [Google Scholar]
- 18.Rucci N Molecular biology of bone remodelling. Clinical Cases in Mineral and Bone Metabolism. 2008; 5(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- 19.Poole JA, Romberger DJ. Immunological and inflammatory responses to organic dust in agriculture. Curr Opin Allergy Clin Immunol 2012; 12:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivanov S, Palmberg L, Venge P, Larsson K, Linden A. Interleukin-17A mRNA and protein expression within cells from the human bronchoalveolar space after exposure to organic dust. Respir Res. 2005. May 25;6:44,9921–6–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole JA, Gleason AM, Bauer C, West WW, Alexis N, van Rooijen N, et al. CD11c+/CD11b+ cells are critical for organic dust-elicited Murine lung inflammation. Am J Respir Cell Mol Biol 2012;47(5):652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbe P, Draijer C, Borg TR, Luinge M, Timens W, Wouters IM, et al. Distinct macrophage phenotypes in allergic and nonallergic lung inflammation. Am J Physiol Lung Cell Mol Physiol 2015;308(4):L358–67. [DOI] [PubMed] [Google Scholar]
- 23.Poole JA, Gleason AM, Bauer C, West WW, Alexis N, Reynolds SJ, et al. Alphabeta T cells and a mixed Th1/Th17 response are important in organic dust-induced airway disease. Ann Allergy Asthma Immunol 2012;109(4):266–273.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole JA, Mikuls TR, Duryee MJ, Warren KJ, Wyatt TA, Nelson AJ, et al. A role for B cells in organic dust induced lung inflammation. Respir Res 2017. December 22;18(1):214,017–0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z, Dosman JA, Rennie DC, Schwartz DA, Yang IV, Beach J, et al. Association of Toll-like receptor 2 gene polymorphisms with lung function in workers in swine operations. Ann Allergy Asthma Immunol. 2013. January;110(1):44,50.e1. [DOI] [PubMed] [Google Scholar]; * First clinical research study to link genetic polymorphisms in Toll-like receptor 2 to lung function in swine workers.
- 26.Senthilselvan A, Chenard L, Kirychuk S, Predicala B, Schwartz DA, Burch LH, et al. Gender-related tumor necrosis factor-alpha responses in naive volunteers with Toll-like receptor 4 polymorphisms exposed in a swine confinement facility. J Interferon Cytokine Res 2009. December;29(12):781–90. [DOI] [PubMed] [Google Scholar]
- 27.Sahlander K, Larsson K, Palmberg L. Daily exposure to dust alters innate immunity. PLoS One. 2012;7(2):e31646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer C, Kielian T, Wyatt TA, Romberger DJ, West WW, Gleason AM, et al. Myeloid differentiation factor 88-dependent signaling is critical for acute organic dust-induced airway inflammation in mice. Am J Respir Cell Mol Biol 2013. June;48(6):781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 2003; 9:185–196. [DOI] [PubMed] [Google Scholar]
- 30.Wells A, Romberger DJ, Thiele GM, Wyatt TA, Staab E, Heires AJ, et al. Systemic IL-6 effector response in mediating systemic bone loss following inhalation of organic dust. Journal of Interferon & Cytokine Research : The Official Journal of the International Society for Interferon and Cytokine Research. 2017; 37(1), 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure–induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 2009; 296:L1085–L1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbe P, Spierenburg EA, Draijer C, Brandsma CA, Telenga E, van Oosterhout AJ, et al. Shifted T-cell polarisation after agricultural dust exposure in mice and men. Thorax. 2014; 69(7):630–7. [DOI] [PubMed] [Google Scholar]
- 33.Poole JA, Romberger DJ, Bauer C, Gleason AM, Sisson JH, Oldenburg PJ, et al. Protein kinase C epsilon is important in modulating organic-dust-induced airway inflammation. Exp Lung Res 2012. October;38(8):383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knetter SM, Tuggle CK, Wannemuehler MJ, Ramer-Tait AE. Organic barn dust extract exposure impairs porcine macrophage function in vitro: implications for respiratory health. Vet Immunol Immunopathol 2014. January 15;157(1–2):20–30. [DOI] [PubMed] [Google Scholar]
- 35.Poole JA, Kielian T, Wyatt TA, Gleason AM, Stone J, Palm K, et al. Organic dust augments nucleotide-binding oligomerization domain expression via an NF-{kappa}B pathway to negatively regulate inflammatory responses. Am J Physiol Lung Cell Mol Physiol 2011. September;301(3):L296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole JA, Anderson L, Gleason AM, West WW, Romberger DJ, Wyatt TA. Pattern recognition scavenger receptor A/CD204 regulates airway inflammatory homeostasis following organic dust extract exposures. J Immunotoxicol 2015. Jan-Mar;12(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boissy RJ, Romberger DJ, Roughead WA, Weissenburger-Moser L, Poole JA, LeVan TD. Shotgun pyrosequencing metagenomic analyses of dusts from swine confinement and grain facilities. PLoS One. 2014. April 18;9(4):e95578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poole JA, Dooley GP, Saito R, Burrell AM, Bailey KL, Romberger DJ, et al. Muramic acid, endotoxin, 3-hydroxy fatty acids, and ergosterol content explain monocyte and epithelial cell inflammatory responses to agricultural dusts. J Toxicol Environ Health A 2010;73(10):684–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dusad A, Thiele GM, Klassen LW, Gleason AM, Bauer C, Mikuls TR, et al. Organic dust, lipopolysaccharide, and peptidoglycan inhalant exposures result in bone loss/disease. American Journal of Respiratory Cell and Molecular Biology. 2013; 49(5): 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]; * First animal study to demonstrate that repetitive environmental inflammatory agents causing lung inflammation result in bone mineral density loss.
- 40.Krisher T, Bar-Shavit Z. Regulation of osteoclastogenesis by integrated signals from toll-like receptors. J Cell Biochem 2014;115(12):2146–54. doi: 10.1002/jcb.24891. [DOI] [PubMed] [Google Scholar]
- 41.Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, et al. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol 2011; 45: 711–719. doi: 10.1165/rcmb.2010-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Singh B. Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res 2008; 34(1): 19–35. doi: 10.1080/01902140701807779. [DOI] [PubMed] [Google Scholar]
- 43.Staab E, Thiele GM, Clarey D, Wyatt TA, Romberger DJ, Wells AD, et al. Toll-like receptor 4 signaling pathway mediates inhalant organic dust-induced bone loss. PloS One. 2016; 11(8). doi: 10.1371/journal.pone.0158735. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Animal study highlighting an important role for endotoxin and the Toll-like receptor 4 signaling pathway in mediating bone loss consequences following complex agriculture organic dust exposures.
- 44.Ferrari R, Tanni SE, Caram LM, Correa C, Correa CR, Godoy I. Three-year follow-up of interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respiratory Research. 2013; 14(1): 24–9921–14–24. doi: 10.1186/1465-9921-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sin DD, Macnee W. Chronic Obstructive Pulmonary Disease and Cardiovascular Diseases: A “Vulnerable” Relationship. American journal of respiratory and critical care medicine. 2013; 187: 2–4. 10.1164/rccm.201210-1953ED. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida Y, Tanaka T. Interleukin 6 and rheumatoid arthritis. BioMed Research International. 2014; doi: 10.1155/2014/698313. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** A comprehensive review article on the mechanistic role of IL-6 and bone disease.
- 47.Briot K, Rouanet S, Schaeverbeke T, et al. The effect of tocilizumab on bone mineral density, serum levels of dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis. Joint Bone Spine. 2015; 82(2): 109–115. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T, Narazaki M, Ogata A, Kishimoto T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin Immunol 2014; 26(1): 88–96. [DOI] [PubMed] [Google Scholar]
- 49.Caramori G, Adcock IM, Di Stefano A, Chung KF. Cytokine inhibition in the treatment of COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2014;9:397–412. doi: 10.2147/COPD.S42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wedzicha JA, Seemungal TAR, MacCallum PK, Paul EA, Donaldson GC, Bhowmik A, et al. Acute Exacerbations of Chronic Obstructive Pulmonary Disease Are Accompanied by Elevations of Plasma Fibrinogen and Serum IL-6 Levels. Thromb Haemost. 2000;84(2):210–5. [PubMed] [Google Scholar]
- 51.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol. 2002;93(1):289–96. [DOI] [PubMed] [Google Scholar]
- 52.Poole JA, Romberger DJ, Wyatt TA, Staab E, VanDeGraaff J, Thiele GM, et al. Age impacts pulmonary inflammation and systemic bone response to inhaled organic dust exposure. Journal of Toxicology and Environmental Health.Part A. 2015; 78(19): 1201–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. [DOI] [PubMed] [Google Scholar]
- 54.Sucur A, Katavic V, Kelava T, Jajic Z, Kovacic N, Grcevic D Induction of osteoclast progenitors in inflammatory conditions: Key to bone destruction in arthritis. International Orthopaedics. 2014; 38(9): 1893–1903. [DOI] [PubMed] [Google Scholar]; * Informative review article outlining the mechanisms of the role of osteoclast precursor cells induced by inflammatory conditions relating to bone health.
- 55.Xiao Y, Song JY, de Vries TJ, Fatmawati C, Parreira DB, Langenbach GE, et al. Osteoclast precursors in murine bone marrow express CD27 and are impeded in osteoclast development by CD70 on activated immune cells. Proc Natl Acad Sci U S A 2013; 110: 12385–12390. doi: 10.1073/pnas.1216082110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dusad A, Thiele GM, Klassen LW, Wang D, Duryee MJ, Mikuls TR, et al. Vitamin D supplementation protects against bone loss following inhalant organic dust and lipopolysaccharide exposures in mice. Immunologic Research. 2015; 62(1): 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y, Lee JW, Uy L, Abosaleem B, Gunn H, Ma M, et al. Tartrate-resistant acid phosphatase (TRACP 5b): A biomarker of bone resorption rate in support of drug development: Modification, validation and application of the BoneTRAP kit assay. Journal of Pharmaceutical and Biomedical Analysis. 2009; 49(5): 1203–1212. [DOI] [PubMed] [Google Scholar]
- 58.Shirjang S, Mansoori B, Solali S, Hagh MF, Shamsasenjan K. Toll-like receptors as a key regulator of mesenchymal stem cell function: An up-to-date review. Cell Immunol 2017;315:1–10. [DOI] [PubMed] [Google Scholar]
- 59.Krisher T, Bar-Shavit Z. Regulation of osteoclastogenesis by integrated signals from toll-like receptors. J Cell Biochem 2014;115(12):2146–54. [DOI] [PubMed] [Google Scholar]
- 60.Fletcher C, Peto R, Tinker CM, Speizer FE. The natural history of chronic bronchitis and emphysema, Oxford University Press, Oxford: (1976). [Google Scholar]
- 61.DM Mannino KJ Davis. Lung function decline and outcomes in an elderly population. Thorax. 2006;61: 472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller RD, Hepper NG, Kueppers F, Gordon H, Offord KP. Host factors in chronic obstructive pulmonary disease in an upper Midwest rural community. Design, case selection, and clinical characteristics in a matched-pair study. Mayo Clin Proc 1976;51(11):709–15. [PubMed] [Google Scholar]
- 63.Elizondo-Montemayor L, Castillo EC, Rodríguez-López C, et al. Seasonal Variation in Vitamin D in Association with Age, Inflammatory Cytokines, Anthropometric Parameters, and Lifestyle Factors in Older Adults. Mediators of Inflammation. 2017. doi: 10.1155/2017/5719461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ettinger B, Pressman A, Sklarin P, Bauer DC, Cauley JA, Cummings SR. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures J. Clin. Endocrinol. Metab 1998; 83: 2239–2243. [DOI] [PubMed] [Google Scholar]
- 65.Knabe C, Mele A, Kann PH, Peleska B, Adel-Khattab D, Renz H, Reuss A, Bohner M, Stiller M, Effect of sex-hormone levels, sex, body mass index and other host factors on human craniofacial bone regeneration with bioactive tricalcium phosphate grafts. Biomaterials. 2017; 123: 48–62. doi: 10.1016/j.biomaterials.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 66.Reed DB, Rayens MK, Conley CK, Westneat S, Adkins SM. Farm elders define health as the ability to work. Workplace Health Saf. 2017;60(8):345–51. [DOI] [PubMed] [Google Scholar]; * Study that outlines the impact of specific health conditions in older farm workers in the ability to function.
- 67.Drake MT, Clarke BL, Khosla S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clinic proceedings Mayo Clinic. 2008;83(9):1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rizzoli R, Reginster J-Y, Boonen S, et al. Adverse Reactions and Drug–Drug Interactions in the Management of Women with Postmenopausal Osteoporosis. Calcified Tissue International. 2011;89(2):91–104. doi: 10.1007/s00223-011-9499-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Josse R, Khan A, Ngui D, Shapiro M. Denosumab, a new pharmacotherapy option for postmenopausal osteoporosis. Current medical research and opinion. 2013; 29(3): 205–16. doi: 10.1185/03007995.2013.763779. [DOI] [PubMed] [Google Scholar]
- 70.Krakauer T Nuclear factor-kappaB: fine-tuning a central integrator of diverse biologic stimuli. International Reviews of Immunology. 2008; 27(5): 286–92. doi: 10.1080/08830180802317957. [DOI] [PubMed] [Google Scholar]
- 71.Khosla S Increasing options for the treatment of osteoporosis. N Engl J Med 2009; 361(8): 818–820. doi: 10.1056/NEJMe0905480. [DOI] [PMC free article] [PubMed] [Google Scholar]; * A relevant and complete review of the newer options available for the treatment of osteoporosis.
- 72.Venuraju SM, Yerramasu A, Corder R, Lahiri A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. Journal of the American College of Cardiology. 2010; 55(19): 2049–61. doi: 10.1016/j.jacc.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, Siri DA, Tomsic M, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION study. Annals of the rheumatic diseases. 2010; 69(1): 88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Genovese MC, McKay JD, Nasonov EL, Mysler EF, Da Silva NA, Alecock E, Woodworth T, Gomez-Reino JJ. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: The tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis & Rheumatism. 2008; 58(10): 2968–80. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 75.Schaper F, Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev 2015. October;26(5):475–87. [DOI] [PubMed] [Google Scholar]
- 76.Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Current Opinion in Immunology. 2015; 34: 75–82. doi: 10.1016/j.coi.2015.02.008 [DOI] [PubMed] [Google Scholar]; * A review article focused on the novel approaches and advances of targeting the IL-6 signaling pathway to reduce disease.
- 77.Xiao H, Bid HK, Chen X, Wu X, Wei J, Bian Y, et al. Repositioning Bazedoxifene as a novel IL-6/GP130 signaling antagonist for human rhabdomyosarcoma therapy. PLoS ONE. 2017; 12(7). doi: 10.1371/journal.pone.0180297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. The Journal of Clinical Investigation. 2011; 121(9): 3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harada S, Mizoguchi T, Kobayashi Y, Nakamichi Y, Takeda S, Sakai S, et al. Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone. J Bone Miner Res 2012; 27(2):461–73. [DOI] [PubMed] [Google Scholar]
- 80.Baldock PA, Thomas GP, Hodge JM, Baker SU, Dressel U, O’Loughlin PD, et al. Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res 2006; 21(10):1618–26. [DOI] [PubMed] [Google Scholar]
- 81.Takasu H, Sugita A, Uchiyama Y, Katagiri N, Okazaki M, Ogata E, et al. c-fos protein as a target of anti-osteoclastogenic action of vitamin D, and synthesis of new analogs. J Clin Invest 2006; 116(2):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakai S, Takaishi H, Matsuzaki K, Kaneko H, Furukawa M, Miyauchi Y, et al. 1-alpha, 25-dihydroxy vitamin D3 inhibits osteoclastogenesis through IFN-beta-dependent NFATc1 suppression. J Bone Miner Metab 2009; 27(6):643–52. [DOI] [PubMed] [Google Scholar]