Abstract
Resveratrol, a polyphenol found in a number of plant-based foods such as red wine, has received a great deal of attention for its diverse array of healthful effects. Beneficial effects of resveratrol are diverse; they include improvement of mitochondrial function, protection against obesity and obesity-related diseases such as type-2 diabetes, suppression of inflammation and cancer cell growth and protection against cardiovascular dysfunction, just to name a few. Investigations into the metabolic effects of resveratrol are furthest along and now include a number of clinical trials, which have yielded mixed results. There are a number of controversies surrounding resveratrol that have not been resolved. Here, we will review these controversies with particular emphasis on its mechanism of metabolic action and how lessons from resveratrol may help develop therapies that harness the effects of resveratrol but without the undesirable properties of resveratrol.
Keywords: Resveratrol, Sirt1, PDE4, AMPK
Introduction
As the chronic diseases of aging such as cancer, diabetes, and neurodegenerative diseases have become an increasing burden on society, we have continued to search for drugs that can solve these problems. Finding a drug that could reduce the overall effects of aging could increase both the health span and lifespan of humans. One method that has consistently been shown to increase the lifespan of organisms from single-celled organisms to mammals is caloric restriction. This concept was originally discovered when McCay et al. [1] showed that caloric restriction could extend the lifespan of rats. More recently, caloric restriction has been shown to extend the lifespan of a range of species from yeast to mammals [2]. With the increasing epidemic of obesity, it has become clear that attempts at calorie restriction in humans are likely to fail. Therefore, many have searched for compounds that could act as calorie restriction mimetics. Focus on the pathways involved in the effect of calorie restriction on lifespan in yeast discovered the sirtuin enzyme SIR2 to be a key mediator [3]. A high throughput screen for activators of SIRT1, the human homolog of the Saccharomyces cerevisiae enzyme discovered the small molecule resveratrol (3,5,4′-trihydroxystilbene) [4]. Resveratrol is a natural product that can be found in several plant species including red grapes. In the 1990s resveratrol first received attention as a potential explanation of the “French Paradox,” then later was characterized as a cyclooxygenase inhibitor and potential chemopreventive molecule [5]. Since its discovery as a potential calorie restriction mimetic, resveratrol has been shown to have beneficial effects in cardiovascular disease, metabolic disease, cancer and neurodegeneration. Much work has focused on how resveratrol is capable of having such wide-ranging effects, what the molecular targets are, and whether resveratrol treatment will be beneficial in humans. In this review, we will discuss the current research on the direct targets of resveratrol, the downstream effects of resveratrol in animals, and the current state of human clinical trials.
Molecular targets of resveratrol
Amidst much confusion, it has become clear that resveratrol potentially has several direct targets in the cell. Although the original discovery was as a cyclooxygenase inhibitor, it has subsequently been identified as an activator of Sirt1 [4]; an inhibitor of cAMP phosphodiesterases [6]; an inhibitor of the F1-ATPase [7]; an inhibitor of the estrogen receptor [8], and a modulator of numerous other targets. The poly-pharmacologic nature of resveratrol has sparked much debate about the most relevant targets for its downstream effects, much of this surrounding whether it truly is an activator of Sirt1, and whether Sirt1 is responsible for the downstream effects of resveratrol in vivo.
Sirtuins
The sirtuin family of proteins began receiving attention due to the ability of the S. cerevisiae SIR2 gene to modulate lifespan in yeast. It was shown that the extension of replicative lifespan due to caloric restriction depended on the presence of SIR2 [3], although a recent paper calls into question the magnitude of the lifespan expansion due to caloric restriction in this species [9]. It was further shown that deletion of Sir2 orthologs in other organisms ablated the effects of caloric restriction on lifespan [10, 11], although not in all systems tested [12, 13]. Increasing the expression of Sirt1 has extended lifespan in yeast [14], worms [15], and flies [10]. One group could not repeat these effects in C. elegans or Drosophila [16], thereafter the original group has repeated their result in C. elegans although with a smaller effect [17]. In drosophila, it has now been shown that Sir2 overexpression in the fat body can increase lifespan [18]. In mammals, sirtuins compose a family of seven proteins called Sirt1–Sirt7. Sirt1 is the closest homolog to the yeast SIR2, and has been most extensively studied for the effects of caloric restriction and lifespan extension. Overexpression of Sirt1 in mice partially phenocopies the effects of caloric restriction [19, 20], and overexpression of Sirt1 in the brain can extend lifespan [21]. However, some effects of Sirt1 overexpression in mice seem to contradict the effects of resveratrol, including an increase in atherosclerosis when the mice are placed on an atherogenic diet [22] and reduced mitochondrial and cardiac function [23].
Due to evidence showing the potential of increasing sirtuin activity to mimic the effects of caloric restriction, Howitz et al. [4] performed a high throughput screen for activators of human Sirt1 and identified resveratrol as the most potent activator. It was proposed that resveratrol activated Sirt1 by lowering the K m for both the peptide and NAD+ substrates of the enzyme. This screen involved the use of a fluorescently labeled peptide substrate, called Fluor-de-Lys, mimicking a short sequence from p53 that had been shown to be deacetylated by Sirt1 [24]. The direct activation of Sirt1 by resveratrol later became controversial, with evidence showing that the activation in the screen was due to interaction between resveratrol and the fluorescent moiety on the Fluor-de-Lys substrate [25–27]. Due to the proposed biological effects of resveratrol being mediated through Sirt1, further screens were performed to find novel sirtuin activating compounds (STACs). Several structurally distinct compounds, including SRT1720, were found by a screen using a TAMRA tagged substrate. However, the direct activation of Sirt1 by several of these compounds was also called into question [27, 28]. Recent experiments have shown possible explanations for the variety of results obtained for the activation of Sirt1 by resveratrol and STACs. One group showed that activation was possible with resveratrol if the fluorescent group on the peptide was replaced with large hydrophobic amino acids [29]. Further evidence for the dependence of activation on nearby hydrophobic amino acids came from Gertz and colleagues. First, through crystal structures of Sirt3 and Sirt5 co-complexed with resveratrol, they showed direct interaction between resveratrol and fluorescently labeled peptides in sirtuin active sites [30]. They then went on to show that resveratrol activated Sirt5 deacetylase activity towards longer unmodified peptides in a sequence-dependent manner [30]. In a later study, the same group screened a peptide library of 6,802 physiological acetylation sites for the resveratrol effect on Sirt1 deacetylation, showing both activation and inhibition for subsets of acetylation sites but the majority of acetylation sites unaffected [31]. There was a tendency for the activated peptides to have large hydrophobic residues C-terminal to the deacetylation site [31]. In another study, Hubbard et al. [32] showed that resveratrol activated deacetylase activity towards native substrate peptides using in vitro coupling and mass spectrometry-based assays. The common theme among the peptides that showed resveratrol activation was a hydrophobic residue located one or six positions C-terminal to the acetylated lysine. The recent data on the resveratrol activity on Sirt1 clearly show that in vitro, resveratrol can activate Sirt1 activity towards peptide substrates with hydrophobic residues C-terminal to the acetylated lysine on the peptide. Unfortunately, very little data has been produced on Sirt1 activity towards native full-length proteins. It remains to be seen if the peptide substrates that are activated in vitro are relevant (or the most relevant) to the biological effects of resveratrol in vivo.
AMPK and PDEs
While the controversy over whether resveratrol was a direct activator of Sirt1 unfolded, other pathways for indirect activation of Sirt1 were explored. Several groups have shown that resveratrol activates AMP-activated kinase (AMPK), albeit indirectly [33–38]. Subsequently, it was shown that AMPK was also required for the metabolic effects of resveratrol in mice [39]. AMPK is a nutrient-sensing enzyme that is activated by depletion in energy as reflected by an increased AMP/ATP ratio [40]. Several mechanisms of this activation include caloric restriction [41, 42], exercise and glucose deprivation [40], and pharmacologic compounds such as metformin and AICAR. In AMPK knockout mice, the effects of resveratrol on weight gain, glucose tolerance, insulin sensitivity, and mitochondrial biogenesis are all ablated [39]. AMPK activation could indirectly activate Sirt1 activity, as AMPK activation is known to increase the intracellular NAD+ pool [39, 43]. Questions still remain about how resveratrol could activate AMPK, and how the activation of AMPK and Sirt1 are related to each other.
Although AMPK is activated by an increase of the AMP/ATP ratio, there are other proteins that play a major role in the activation of AMPK. Under most circumstances, two well-known kinases phosphorylate AMPK on the T172 residue necessary for activation: LKB1 and calcium/calmodulin-dependent kinase kinase β (CamKKβ) [40]. LKB1 can modulate the activation of AMPK by energy depletion, and intracellular Ca2+ can activate CamKKβ, which in turn activates AMPK. It is possible that resveratrol could activate AMPK via depletion of ATP levels, or by activation of either LKB1 or CamKKβ. Although some evidence has shown that resveratrol can decrease ATP levels [44, 45], it seems to be dependent on doses of 50 μM or higher [46] and possibly the cell type used for the assay, and other studies have shown no effect of resveratrol on ATP levels [47]. Resveratrol has been shown to activate AMPK at doses 10 μM or lower, which are lower than those where ATP depletion has ever been measured [33, 38, 48]. One study showed that in an AMPK mutant that is insensitive to activation by AMP, resveratrol is no longer able to activate AMPK, arguing that activation is dependent on depletion of ATP [44]. However, this study only saw effects at doses >100 μM, calling into question the validity of this effect at lower concentrations [44]. In fact, several studies have shown that resveratrol leads to increased levels of ATP along with AMPK activation [32, 49].
An alternative pathway for activation of AMPK via inhibition of cyclic nucleotide phosphodiesterases (PDEs) was recently shown by Park et al. [6]. PDEs modulate the levels of intracellular cAMP by degrading it to AMP, counteracting the production of cAMP by adenylyl cyclases (ACs). The PDE family of proteins consists of 11 members and a large number of splice variants, capable of hydrolyzing either cAMP and/or cGMP (depending on the isoform) to AMP and GMP, respectively [50]. Park et al. [6] showed that resveratrol had no effect on the production of cAMP by ACs, but inhibited the breakdown of cAMP by PDE1, PDE3, and PDE4. They also tested PDE2 and PDE5 and showed no effect, while not studying the remaining PDE family members.
In cells, cAMP affects downstream processes through three major groups of effector proteins: protein kinase A (PKA), exchange proteins activated by cAMP (EPAC), and cyclic nucleotide regulated ion channels. It is possible for cAMP to activate AMPK through both a PKA-LKB1 pathway, and a pathway involving EPAC1 and CamKKβ [6, 51, 52]. In HeLa cells, which lack LKB1, resveratrol works through the EPAC1-dependent cascade [6]. In myotubes, which express both CamKKβ and LKB1, the activation of AMPK is also dependent on the EPAC1 cascade, but may also contain a contribution from PKA-LKB1 signaling. The actions of PKA on AMPK activation have been shown to be complicated. It has been shown that PKA can directly phosphorylate LKB1 on the S431 residue and increase its activity in neurons [53]. However, activation of AMPK by transiently overexpressed LKB1 is not dependent on phosphorylation at the S431 site [54]. Whether S431 in LKB1 is required for resveratrol to activate AMPK when LKB1 is expressed at physiological levels is not known. Activation of PKA by isoproterenol can inhibit AMPK [55], but activation of PKA by adiponectin can activate AMPK [56].Several other studies have found a relationship between cAMP-PDE signaling and AMPK activation in adipose tissue and muscle [57–59]. Some studies have also shown that PKA activation can lead to an increase in Sirt1 activity [60, 61].
Over the past two years, there has been an increasing amount of evidence that inhibition of PDE4 via rolipram or roflumilast can recapitulate some of the phenotypes of resveratrol treatment such as extension of lifespan in a C. elegans model of a neurodegenerative disease [62], chemoprevention by elimination of tetraploid cells in cancer cell lines [63] and protection against diabetic nephropathy in streptozotocin-treated rats [64].
Other molecular targets of resveratrol
Part of the reason there has been so much controversy and debate over the direct target of resveratrol is the fact that it is poly-pharmacologic. A number of proposed direct targets have been shown, at least in vitro, since it first came to prominence. As mentioned above, before the studies showing resveratrol action on Sirt1 in the early 2000s, resveratrol first received attention as a cyclooxygenase (COX) inhibitor in 1997 [5]. It has been shown to be an inhibitor of COX-1 but not COX-2 [65], and to have cancer chemopreventive activity [5]. Also mentioned above was the discovery that resveratrol has an inhibitory effect on the F0F1 ATPase in mitochondria, potentially decreasing ATP production [7, 66]. A similar model has been looked at with resveratrol inhibition of complex I of the mitochondrial oxidative phosphorylation machinery [67, 68]. However, another group found that resveratrol worked by activating complex I resulting in increased NAD+ levels in the cell and Sirt1 activation [69]. Also before the discovery of resveratrol as a sirtuin activator, resveratrol was studied as an estrogen receptor (ER) modulator [70, 71]. Originally, resveratrol was proposed as a strong ER agonist, but subsequent studies found it did not cause proliferation of mammary or uterine tissues [72]. A recent study by Nwachukwu et al. [8] shows a co-crystal structure of the ERα ligand binding domain with resveratrol, and proposes mechanisms by which resveratrol can mediate selective downstream effects through ERα. Resveratrol activates pathway specific effects on ERα by affecting recruitment of specific co-regulators. Another recent paper from Lee et al. [73] shows that resveratrol can be a direct activator of the Ataxia-Telangiectasia Mutated kinase (ATM). They show that resveratrol can stimulate ATM directly in the presence of oxidizers in an in vitro activity model. They also show that resveratrol activates ATM autophosphorylation and phosphorylation of substrates, such as p53, in vitro and in cell lines. However, because resveratrol can generate reactive oxygen species [74], and ATM is activated by oxidative stress [75], one cannot rule out the possibility that the effect of resveratrol on ATM activity in cell lines may, at least in part, be indirect. It is clear that resveratrol potentially interacts with a number of molecular targets both in vitro and in cell lines. Many of these targets converge on pathways related to metabolism and inflammation that are responsible for the phenotypes of resveratrol treatment in cells and animals.
Effects of resveratrol in animal models
Lifespan
Inspired by the research on the effects of caloric restriction and sirtuin genes on lifespan, the effects of resveratrol on organismal lifespan has been studied in species from yeast to non-human primates. Resveratrol has been shown to increase the lifespan of the yeast S. cerevisiae [4], the nematode C. elegans and the fruit fly D. melanogaster [76]. Lifespan extension has also been shown in the fish N. furzeri [77], another fish N. guentheri [78], and the bee A. mellifera [79]. In mammals, resveratrol did not extend the lifespan of healthy mice [80–82], but partially prevented premature death of mice fed a high fat diet [35, 80]. In rats, resveratrol also failed to extend the lifespan of animals on a normal diet [83]. In non-human primates, no lifespan studies have been performed with resveratrol. However, lifespan studies on caloric restriction have provided conflicting results [84, 85]. The lifespan studies across species appear to show that the effects of resveratrol are very dependent on the specific diet and conditions of the animals [86]. In mice, it appears that resveratrol is able to extend their lifespan when they are under metabolic stress. Below, we will discuss various ways in which resveratrol improves health span by reducing the effects of the diseases of aging.
Metabolism
The most robust effect that has been seen repeatedly with resveratrol treatment in vivo is its effect on metabolism. Resveratrol has been shown to relieve many of the negative effects of a high-fat diet in mice. As discussed above, resveratrol increased the lifespan of mice fed a high fat diet. In the same mice, resveratrol reduced body weight at a high dose, and reduced insulin resistance at a lower dose that did not cause reduction in body weight [35, 87]. Later, it was shown that the weight loss and improvements in the glucose tolerance test were dependent on the presence of AMPK [39]. Overexpression of Sirt1 is able to improve glucose tolerance in mice without changes in body weight or fat content [19, 88, 89]. With much of the focus of the resveratrol field on studying how resveratrol could work as a calorie restriction mimetic, the questions turned to how resveratrol could produce such profound metabolic benefits. One reproducible effect of resveratrol was an increase in mitochondrial content (Fig. 1) [6, 35, 39, 87]. Many of these studies pointed to the ability of resveratrol to up-regulate the activity of peroxisome proliferator-activated receptor γ co-activator (PGC-1α), a so-called “master regulator” of mitochondrial biogenesis [90]. PGC-1α controls the expression of many downstream regulators of mitochondrial content in the cell, and can lead to switching of oxidative fiber types in muscle [91] an effect observed under resveratrol treatment [87]. In mice, overexpression of PGC-1α also leads to a decrease in the metabolic disorders that occur during aging [92]. It has been well-established that Sirt1 can deacetylate and activate PGC-1α [93]. AMPK activation can lead to increased Sirt1 deacetylation and activation of PGC-1α [94]. Treatment with resveratrol has been shown to increase both Sirt1 and PGC-1α activity in mice fed a high-fat diet [6, 39, 87]. Although several papers have shown that resveratrol treatment leads to increased mitochondrial biogenesis via Sirt1 deacetylation and activation of PGC-1α, other papers have produced conflicting results. The first paper to demonstrate an interaction between Sirt1 and PGC-1α actually demonstrated that overexpression of Sirt1 decreased PGC-1α activity in PC12 cells [95]. A recent paper from Higashida et al. [96] showed that this was also true in C2C12 myotubes [96]. They were unable to demonstrate an increase in mitochondrial biogenesis upon resveratrol treatment of rats, but could recapitulate the increase in mitochondrial biogenesis in C2C12 myotubes overexpressing PGC-1α. This effect was dependent on activation of AMPK, but not dependent on Sirt1 activity. Currently, the mechanism by which resveratrol leads to this increase in PGC-1α activity continues to be under debate in the literature.
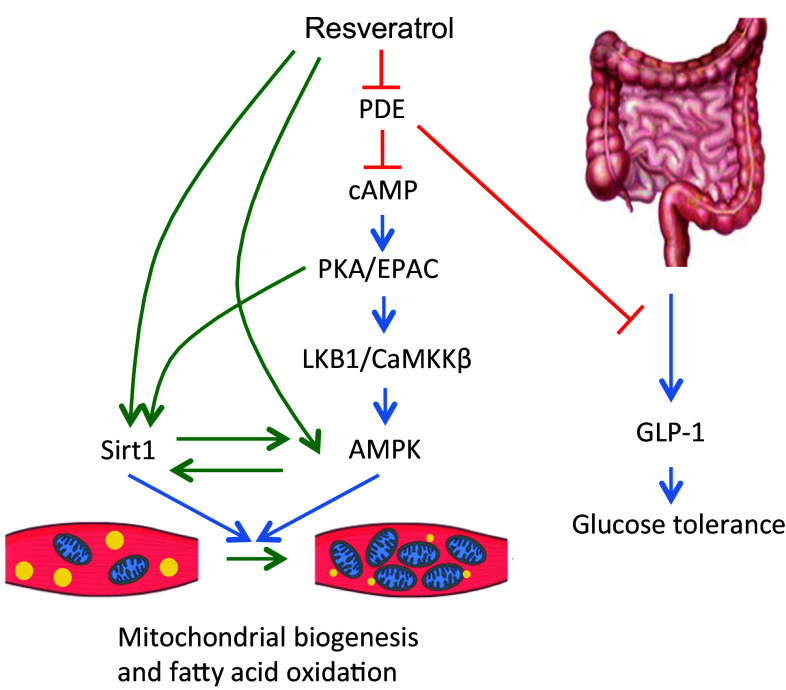
Fig. 1.
Schematic of the molecular targets of resveratrol related to metabolism. Resveratrol potentially activates multiple targets, converging on pathways that lead to mitochondrial biogenesis and fatty acid oxidation. In the gut (right), where resveratrol concentrations are likely higher than plasma in animals, resveratrol leads to secretion of GLP-1 and an improvement in glucose tolerance. Green and blue arrows represent activation and red bars represent inhibition
As stated above, many of the downstream effects of resveratrol on metabolism do not occur in AMPK knockout mice [39]. The effects of resveratrol on Sirt1 and AMPK involve a complicated series of interactions. Pathways have been shown for both Sirt1 to activate AMPK, and for AMPK to activate Sirt1, complicating the debate over the true mechanism in vivo [94, 97–99]. Activation of AMPK leads to an increase in cellular NAD+ levels through stimulation of nicotinamide phosphoribosyltransferase (NAMPT) activity [97]. Although increased NAD+ levels in vivo have not been shown to directly stimulate Sirt1 activity, NAD+ is a necessary cofactor for Sirt1 and increased NAD+ has correlated with increased Sirt1 and PGC-1α activity [39, 94, 97]. One study demonstrated an increase in Sirt1 activity by decreasing the consumption of NAD+ by another NAD+ utilizing enzyme [100]. Sirt1 could possibly stimulate AMPK activity through the deacetylation of LKB1, an upstream regulator of AMPK activity [98, 99]. To further complicate this interplay, Sirt1 stimulation by resveratrol has been shown to be independent of AMPK activation [46], and AMPK stimulation by resveratrol has been shown to be independent of Sirt1 activation [6, 33, 39].
As mentioned above, resveratrol was shown to be a direct inhibitor of phosphodiesterase activity, increasing cAMP levels in cells. Amongst the PDE isoforms inhibited by resveratrol is PDE4, the most abundant PDE in skeletal muscle [6]. Inhibition of phosphodiesterases leads to an increase in cAMP levels and downstream activation of the cAMP effectors PKA and EPAC. In myotubes, it was shown that treatment with resveratrol stimulated a signaling cascade via cAMP and EPAC that led to the activation of AMPK and subsequently Sirt1 (Fig. 1). Importantly, many of the downstream effects of resveratrol were recapitulated by treatment with rolipram, a specific inhibitor of PDE4 [6]. In fact, several other studies have found metabolic effects due to PDE inhibition or knockout. Phosphodiesterases are important signaling regulators in pancreatic β-cells, regulating both Ca2+ levels and insulin secretion [101]. In PDE3B knockout mouse islets, both glucose and incretin-stimulated insulin secretion are greater than the secretion in wild-type islets [102]. The PDE4B knockout mouse also has metabolic phenotypes, including reduced fat content and serum leptin levels [103]. These previous data on PDE inhibition combined with the Park et al. study demonstrate that resveratrol may mediate some of its metabolic effects through the PDE-cAMP-AMPK pathway.
Price et al. published a study exploring the role that Sirt1 and AMPK play in the downstream effects of resveratrol using conditional knockout mice. Studies of full-body Sirt1 knockout mice have been challenging because the mice have limited viability and many developmental defects [104, 105]. To address these problems, Price et al. [46] knocked out Sirt1 specifically in adult animals using a tamoxifen inducible knockout system, avoiding the developmental defects. The adult conditional knockout mice appeared largely normal. However, treatment with low doses of resveratrol led to an increase in mitochondrial content and function in wild type but not Sirt1 knockout mice. With the low-dose treatment, AMPK activation appeared to be dependent on Sirt1, whereas at a higher dose AMPK activation was Sirt1 independent. Interestingly, a previous study in Sirt1 knockout MEFs showed that AMPK activation by resveratrol was not Sirt1 dependent [39]. Despite the effects of resveratrol on mitochondrial biogenesis being Sirt1-dependent in the conditional knockout mice, there were several systematic effects in the mice that were Sirt1 independent. After being fed a high-fat diet, resveratrol improved glucose tolerance and reduced the amount of hepatic triglycerides in a Sirt1 independent manner [46]. A Sirt1 overexpressing mouse is also capable of reducing hepatic triglycerides and glucose intolerance [46], further displaying the complicated relationship between Sirt1, AMPK and metabolic effects in mice.
Despite the unclear picture of the direct molecular targets responsible for the metabolic effects of resveratrol, many of the effects have become clear in mammals from rodents to non-human primates. As discussed above, resveratrol treatment improves the glucose homeostasis and insulin sensitivity of mice fed a high-fat diet [35, 39, 46, 87, 106]. Resveratrol has also been shown consistently to reduce hepatic triglyceride content [46, 107]. Using a proposed Sirt1 activator, SRT1720, many of these same effects have been seen in mice fed a high fat diet including improved glucose tolerance, insulin sensitivity, mitochondrial content, and lifespan [108, 109]. In Zucker diabetic fatty rats, resveratrol lead to improved glucose and insulin tolerance. Subcutaneous adipose tissue from resveratrol-treated rats showed increased incorporation of pyruvate into triglycerides and increased adiponectin secretion. In subcutaneous and retroperitoneal adipose tissue from resveratrol-treated rats, there was an increase in mitochondrial respiration and cytochrome c oxidase IV protein, showing a potential increase in mitochondrial biogenesis in adipose tissue with resveratrol treatment [110]. In rhesus monkeys, resveratrol added to a high fat/high sugar diet did not prevent the development of insulin resistance, but was able to protect against β-cell loss in the pancreatic islets [111]. In visceral fat from rhesus monkeys fed a high fat/high sugar diet, resveratrol reduced adipocyte size and increased insulin sensitivity [112]. In Microcebus murinus, 33 months of treatment with resveratrol led to improvements on the homeostatic model assessment-insulin resistance (HOMA-IR) index and improved glucose tolerance [113]. The translation of these effects to human studies will be discussed below.
Inflammation
Chronic inflammation is increasingly accepted to play a major role in the diseases of aging [114] including metabolic disorders such as Type 2 diabetes [115]. One of the reasons that resveratrol has drawn so much attention as an anti-aging therapeutic is its ability to reduce inflammation. Even before studies showing resveratrol inhibition of COX2 or activation of sirtuins, resveratrol was shown to inhibit 5-lipoxygenase and cyclooxygenase products from rat peritoneal polymorphonuclear leukocytes [116]. Resveratrol treatment has also been shown to inhibit signaling through interleukin-10 and interferon-γ [117–119]. In human peripheral blood mononuclear cells, resveratrol has been shown to inhibit IL-17 production [120]. One important mediator of resveratrol’s effect on inflammation is NF-κB. Activation of Sirt1 by resveratrol treatment can lead to downstream inhibition of NF-κB by the direct deacetylation of the p65 subunit [121]. Resveratrol has been shown to reduce NF-κB activation in leukocytes [122]. Sirt1 activation can also modulate several stress response transcription factors including hypoxia-inducible factors and FOXO1, FOXO3, and FOXO4 [123–127]. In rhesus monkeys, resveratrol reduced inflammation in the white adipose tissue of animals fed a high fat/high sugar diet [112]. In a mouse model of Crohn’s disease, resveratrol reduced inflammatory cytokines [128]. It is clear that the anti-inflammatory properties of resveratrol can aid in its benefits to the health of aging animals.
Cardiovascular disease
The beneficial effects of resveratrol on the cardiovascular system have been reviewed extensively elsewhere [123, 129–131]. These effects are multifactorial, affecting various components of cardiovascular disease. In vascular function, resveratrol has been shown to promote vasodilation through effects on the expression and activity of endothelial nitric oxide synthase (eNOS) [132–136]. It has been proposed that resveratrol exerts these effects on eNOS via a direct Sirt1 deacetylation [137] or by transcriptional activation downstream of Sirt1 [138], and knockdown of Sirt1 expression blocked the induction of eNOS expression by resveratrol [139, 140]. AMPK is also capable of activating eNOS activity via a direct phosphorylation [141], and one group has shown that inhibition of AMPK blocks the effect of resveratrol on vasodilation in aortic rings [135]. There is also evidence that resveratrol can cause vasodilation by inhibiting vasoconstriction by angiotensin II [142] and its receptor [143].
Resveratrol has also been shown to have an effect on blood pressure in various model systems. In several rat models of hypertension, chronic treatment with resveratrol decreased blood pressure [142, 144–148]. In some other rat model systems, resveratrol has not had an effect on blood pressure [149–152]. Recent data has reaffirmed that resveratrol can lower blood pressure in the spontaneously hypertensive rat model of hypertension, and that this effect coincides with the activation of AMPK both in vitro and in vivo [153]. The beneficial effects of resveratrol on cardiac hypertrophy also coincide with the activation of AMPK [153, 154]. Resveratrol has also been shown to lower blood pressure in a porcine model of hypertension [155]. Most recently, resveratrol has been shown to reduce the amount of arterial wall inflammation in high fat/high sugar fed rhesus monkeys [156].
Resveratrol likely mediates many of its cardioprotective effects through its ability to reduce oxidative damage and inflammation. Resveratrol is known to be a direct antioxidant [157], which was an early theory for how it mediated cardioprotection. Resveratrol is also capable of inducing the expression of many antioxidant enzymes including superoxide dismutase, glutathione peroxidase, heme oxygenase, and catalase [158–163]. The induction of these antioxidant genes may be mediated by nuclear factor erythroid 2- related factor 2 (Nrf2) [164]. In addition, resveratrol can down-regulate NADPH-oxidase leading to decreased production of reactive oxygen species [165–167]. Oxidative stress is known to be an important mechanism for cardiovascular disease during aging [168, 169]. Resveratrol is capable of reducing mitochondrial oxidative stress in the cardiovascular system [170] and has also been shown to reduce the oxidation of LDL [171]. In the cardiovascular system, resveratrol shows many of the anti-inflammatory effects discussed in the previous section. Treatment with resveratrol reduces the expression of NF-κB, IL-6 and IL-8, and TNF-α [134, 172, 173]. Resveratrol also has benefits in ischemia–reperfusion injuries [130, 174], possibly assisting in the protective effects during myocardial infarction.
Neuroprotection
Resveratrol has been shown to have some benefit in animal models of several neurodegenerative diseases. In Alzheimer’s disease, resveratrol can reduce the accumulation of amyloid β (Aβ) peptides in various cell line models [175], and the accumulation of Aβ plaques [176]. An AMPK-mediated pathway was shown to be involved in the reduction of Aβ levels in cell-based models including in primary mouse neurons [177]. There is also evidence for a PDE-Sirt1-mediated pathway affecting the production of Aβ in cell lines [178]. Recently, studies have found that resveratrol can reduce microglial activation in Alzheimer’s model via a NF-κB/STAT/TLR based anti-inflammatory effect [179]. The combination of resveratrol’s antioxidant, anti-inflammatory, and amyloid lowering effects could combine to make it a potential treatment for Alzheimer’s disease.
Resveratrol has also shown some promise in other neurodegenerative diseases including Parkinson’s disease [180, 181], Huntington’s disease [182], and multiple sclerosis [183]. One major mechanism of neuroprotection may be resveratrol’s ability to prevent axonal degeneration through blocking the association between Sirt1 and its inhibitor DBC1 [184]. A recent paper showed that resveratrol could extend the lifespan of a C. elegans model of adult onset neuronal lipofuscinosis (ANCL). Mutation of the dnj-14 gene in C. elegans leads to a decrease in lifespan for the nematodes. Resveratrol was able to rescue the lifespan reduction in the dnj-14 mutants. This rescue was shown to be sirtuin independent, as deletion of the sir-2.1 gene did not remove the lifespan extending effect of resveratrol on the dnj-14. The lifespan extension effect of resveratrol was however, recapitulated by the PDE4 inhibitor rolipram, suggesting that neuroprotection in this model by resveratrol may be mediated by a PDE-cAMP pathway [62].
Resveratrol in humans
When resveratrol began to be studied in the late 1990s, its presence in red wine led some to hypothesize that resveratrol could explain the “French paradox,” that those who consume a diet high in fatty foods and wine seem to have a lower incidence of coronary heart disease [185]. Although this idea was enticing in the early days of resveratrol research, it became clear that the amounts of resveratrol consumed in the diet would not lead to sufficient concentrations of resveratrol in the body to achieve the effects seen in cellular models of disease [186]. In fact, a recent epidemiological study has shown that urinary resveratrol concentrations solely from dietary consumption are not correlated with a reduction in any cardiovascular disease or cancer incidence [187]. When consumed, resveratrol becomes modified by glucuronidation and sulfation [188] thereby reducing its bioavailability. It is unlikely that resveratrol reaches serum concentrations above 1 μM from dietary consumption, or 10 μM from direct resveratrol supplement consumption [189]. It is possible that despite the low serum concentrations of resveratrol, the lipophilicity of the compound allows it to have higher concentrations within the relevant cells and tissues for its effects. Also, some tissues express glucuronidases capable of removing these groups from resveratrol and enhancing the intracellular concentration [190]. The glucose-lowering effect of resveratrol may in part be occurring in the gut, where its concentrations are likely much higher than in serum. Glucose-lowering incretin glucagon-like peptide-1 (GLP-1), which is secreted by the gut, has numerous physiological effects that make it an attractive type 2 diabetes therapy: increased insulin secretion and insulin sensitivity and decreased glucagon secretion and appetite [191]. Indeed, GLP-1 receptor agonists or GLP-1 mimetics are one of the most widely utilized classes of drugs used to treat type-2 diabetes. One possible mechanism by which resveratrol lowers serum glucose levels may be via GLP-1. Secretion of GLP-1 is inhibited by PDE4D [192], and both resveratrol [6, 193] and the PDE4 inhibitor rolipram [6, 192] increase GLP-1 production (Fig. 1).
With the enticing health benefits in animal models, several clinical trials have focused on the use of resveratrol supplements to treat cardiovascular or metabolic diseases. In a series of Phase I dose escalation studies of resveratrol, it has been shown that resveratrol is generally safe in healthy subjects [194–197]. Higher doses were correlated with mild adverse effects such as diarrhea and nausea [195], but doses at 1 g/day or lower presented minimal side effects [195, 197]. Given the safety profile of resveratrol supplementation, clinical studies began to explore the effects of resveratrol on health. Brasnyo et al. [198] performed a trial with type 2 diabetic male patients receiving 10 mg resveratrol or a placebo for one month. The resveratrol treated group had improved HOMA-IR index, reduced glucose spike after a meal, and an increased pAKT:AKT ratio in platelets. Another trial treated 34 metabolic syndrome patients in a 6-month crossover trial receiving 100 mg of a resveratrol supplement [199]. The patients saw an improvement in flow-mediated dilation at the end of the 3-month treatment period with resveratrol that disappeared after removal of the treatment. Blood pressure and metabolic parameters tested were not affected. Type 2 diabetics receiving 250 mg resveratrol daily for 3 months had significantly improved fasting blood glucose, blood pressure, triglycerides and LDL cholesterol in another trial, compared to a control group receiving only standard of care for diabetes [200]. To examine the effects in cardiovascular disease, Tome-Carneiro et al. [201, 202] performed a trial with 75 subjects using placebo, grape extract lacking resveratrol, or grape extract supplemented with 8 mg of resveratrol over a full year. This trial explored the use of resveratrol treatment for pre-clinical benefits in subjects at high risk of cardiovascular disease. The trial found reduced ApoB and oxidized LDL levels in the resveratrol treated group compared to the no resveratrol grape extract or placebo groups. There also was a reduction in reactive oxygen species and the inflammatory markers CRP, TNF-α and IL-1β. To explore the effects of resveratrol on flow-mediated dilation, Wong et al. [203] performed a trial with 19 subjects including overweight men and post-menopausal women with high blood pressure. After treatment with varying doses of a resveratrol capsule or a placebo once per week, the investigators found that resveratrol caused an acute increase in flow-mediated dilation 45 min after treatment. Similarly, a trial of 40 patients after a myocardial infarction treated with 10 mg resveratrol for 3 months found some improvement in flow-mediated dilation and a decrease in LDL levels [204]. Two small trials exploring the effects of resveratrol on metabolic parameters found that resveratrol improved post-meal glucose levels in patients with impaired glucose tolerance, and reduced markers of oxidative stress in healthy subjects [205, 206]. The most recent small clinical trial reported that resveratrol had no positive effects on non-alcoholic fatty liver disease subjects given 3 g resveratrol daily for 8 weeks [207].
Three recent small controlled trials examined closely the effects of resveratrol on metabolic parameters in healthy and obese humans. Timmers et al. [208] treated 11 obese male patients with 150 mg/day resveratrol for 30 days in a crossover trial. During the treatment period, resveratrol improved insulin sensitivity on the HOMA-IR index, reduced systolic blood pressure, and reduced intrahepatic lipid content. A reduction in circulating levels of glucose and alanine aminotransferase was found in addition to markers of inflammation such as TNF-α. Microarray samples from muscle biopsies showed that resveratrol treatment up-regulated genes from pathways involved in mitochondrial oxidative phosphorylation, in line with the studies done in rodents. The muscle biopsies also showed that there was an increase in AMPK activity, Sirt1 level, and PGC-1α level in tissues from the resveratrol-treated group. A follow-up from this study examining adipose tissues from the subjects was recently reported [209]. This study found that the resveratrol treated subjects had lower mean adipocyte size and upregulation of expression of genes involved in adipogenesis [209]. Two subsequent studies found no effect of resveratrol on metabolic parameters. Yoshino et al. [210] performed a double-blind placebo-controlled trial with 12-week resveratrol supplementation of 75 mg/day. The patients in this study were 29 non-obese postmenopausal women. This trial found no effect of resveratrol on glucose homeostasis, insulin resistance, blood pressure, or markers of inflammation. A subset of the trial subjects had a skeletal muscle and adipose tissue biopsy taken. The biopsy results showed no increase in the expression of Sirt1, AMPK, NAMPT, or PGC-1α. Another randomized double-blinded placebo-controlled trial was done on 24 healthy obese male subjects. After treatment with 500 mg resveratrol daily for 4 weeks resveratrol-treated subjects showed no change in HOMA-IR, HbA1c, cholesterol or triglyceride levels, blood pressure, or body fat composition. They also showed no changes in inflammatory markers or liver function. Similar to the Yoshino et al. trial, a hyperinsulinemic euglycemic clamp study showed no significant changes with resveratrol treatment. This trial also found that resveratrol treatment did not affect levels of phosphorylated AMPK, or expression levels of PGC-1α, GLUT4, TNFα, or NF-κB [211].
Overall, the results of clinical trials in human present a very cloudy picture. Despite positive results for metabolic parameters and cardiovascular function in some trials, several other trials have shown no effects of resveratrol. These differences could potentially be due to the widely varying doses selected in the trials. The trials have also explored a diverse set of subjects with different clinical backgrounds, including differing degrees of impairment in glucose homeostasis. To better understand the role of resveratrol in humans, future trials will need to be well-designed and include larger patient populations.
Conclusions
As research into the diseases of aging became more prominent, the allure of caloric restriction and its ability to extend organismal lifespan increased. Resveratrol first came to the forefront as a potential chemopreventive molecule, then re-emerged as a potential calorie restriction mimetic, and continues to be studied for therapeutic potential in diseases ranging from cancer to metabolic disease to neurodegeneration. In laboratory models of these diseases of aging, resveratrol has shown an impressive ability to alleviate the symptoms. Unfortunately, like many other drugs that work well in animal models, resveratrol has not translated well to treatment in humans. Resveratrol has a low bioavailability in humans, as it is rapidly glucuronidated and sulfated as it is cleared through the body. So far, clinical trials have shown mixed results for metabolic and cardiovascular diseases. Unfortunately, since resveratrol is a natural substance it is not easily patent-protected, so it is unlikely any company will undertake the investment to perform any large-scale clinical trials that would clarify the therapeutic potential of resveratrol in human disease. Instead, work continues to focus on the molecular mechanisms underlying the beneficial effects of resveratrol in disease models. Two potential targets have come to the forefront as mediators of the metabolic effects of resveratrol: phosphodiesterases, whose inhibition leads to AMPK activation and Sirt1, which is thought to be activated directly. Although there is ongoing discussion in the scientific literature about what the direct target of resveratrol is, there is clear consensus that resveratrol’s metabolic action converges on pathways involving AMPK, Sirt1, and PGC-1α. With the lack of results for resveratrol in humans, many have shifted focus to other compounds that more specifically target these pathways. There have been a series of structurally distinct sirtuin activating compounds developed by Sirtris that have been explored for therapeutic potential in diseases of aging [32, 212]. More recently, evidence is mounting that increasing NAD+ levels in cells may work as an alternative pathway to increase sirtuin activity and improve health [213–216]. As that work is ongoing, we and other groups have begun to explore the potential of phosphodiesterase inhibitors, and more specifically PDE4 inhibitors to treat metabolic diseases. Indeed, the PDE4 inhibitor roflumilast, which is already an FDA-approved treatment for COPD, has been shown to lower blood glucose in individuals with Type 2 diabetes [217]. Other studies have also shown that PDE4 inhibition can recapitulate the effects of resveratrol in diabetic nephropathy and chemoprevention [63, 64]. In the future, work will continue to find molecules that can reproduce the therapeutic potential of resveratrol with a more favorable pharmaceutical profile.
Acknowledgments
This work was supported by the Intramural Research Program, National Heart Lung and Blood Institute, National Institutes of Health. We thank Alexandra Brown for manuscript preparation.
Abbreviations
- AMPK
Adenosine monophosphate activated kinase
- Sirt1
Sirtuin 1
- SIR2
Silent information regulator 2
- cAMP
Cyclic adenosine monophosphate
- CAMKKβ
Calcium/calmodulin-dependent kinase kinase β
- PDE
Phosphodiesterases
- PKA
Protein kinase A
- EPAC
Exchange protein activated by cAMP
- COX
Cyclooxygenase
- HOMA-IR
Homeostatic model assessment-insulin resistance
- eNOS
Endothelial nitric oxide synthase
- Nrf2
Nuclear factor erythroid 2- related factor 2
References
- 1.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 2.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 3.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae . Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 4.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 5.Jang MS, Cai EN, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, Fong HHS, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 6.Park S-J, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gledhill JR, Montgomery MG, Leslie AGW, Walker JE. Mechanism of inhibition of bovine F-1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci USA. 2007;104:13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nwachukwu JC, Srinivasan S, Bruno NE, Parent AA, Hughes TS, Pollock JA, Gjyshi O, Cavett V, Nowak J, Garcia-Ordonez RD, Houtman R, Griffin PR, Kojetin DJ, Katzenellenbogen JA, Conkright MD, Nettles KW. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. Elife (Cambridge) 2014;3:e02057. doi: 10.7554/eLife.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huberts DH, Gonzalez J, Lee SS, Litsios A, Hubmann G, Wit EC, Heinemann M. Calorie restriction does not elicit a robust extension of replicative lifespan in Saccharomyces cerevisiae . Proc Natl Acad Sci USA. 2014;111:11727–11731. doi: 10.1073/pnas.1410024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YM, Tissenbaum HA. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech Ageing Dev. 2006;127:48–56. doi: 10.1016/j.mad.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:1381–1387. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans . Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans . Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 16.Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, Orfila A-M, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477:E1–E2. doi: 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee KK, Ayyub C, Ali SZ, Mandot V, Prasad NG, Kolthur-Seetharam U. dSir2 in the adult fat body, but not in muscles, regulates life span in a diet-dependent manner. Cell Rep. 2012;2:1485–1491. doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 20.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiang L, Lin HV, Kim-Muller JY, Welch CL, Gu W, Accili D. Proatherogenic abnormalities of lipid metabolism in SirT1 transgenic mice are mediated through Creb deacetylation. Cell Metab. 2011;14:758–767. doi: 10.1016/j.cmet.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawashima T, Inuzuka Y, Okuda J, Kato T, Niizuma S, Tamaki Y, Iwanaga Y, Kawamoto A, Narazaki M, Matsuda T, Adachi S, Takemura G, Kita T, Kimura T, Shioi T. Constitutive SIRT1 overexpression impairs mitochondria and reduces cardiac function in mice. J Mol Cell Cardiol. 2011;51:1026–1036. doi: 10.1016/j.yjmcc.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- 25.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 26.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 27.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber JL, McBurney MW, DiStefano PS, McDonagh T. SIRT1-independent mechanisms of the putative sirtuin enzyme activators SRT1720 and SRT2183. Future Med Chem. 2010;2:1751–1759. doi: 10.4155/fmc.10.257. [DOI] [PubMed] [Google Scholar]
- 29.Dai H, Kustigian L, Carney D, Case A, Considine T, Hubbard BP, Perni RB, Riera TV, Szczepankiewicz B, Vlasuk GP, Stein RL. SIRT1 Activation by small molecules kinetic and biophysical evidence for direct interaction of enzyme and activator. J Biol Chem. 2010;285:32695–32703. doi: 10.1074/jbc.M110.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gertz M, Nguyen GT, Fischer F, Suenkel B, Schlicker C, Franzel B, Tomaschewski J, Aladini F, Becker C, Wolters D, Steegborn C. A molecular mechanism for direct sirtuin activation by resveratrol. PLoS One. 2012;7:e49761. doi: 10.1371/journal.pone.0049761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakshminarasimhan M, Rauh D, Schutkowski M, Steegborn C. Sirt1 activation by resveratrol is substrate sequence-selective. Aging (Albany NY) 2013;5:151–154. doi: 10.18632/aging.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 35.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM, Park OJ. Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of AMPK signaling pathway. Ann N Y Acad Sci. 2007;1095:441–448. doi: 10.1196/annals.1397.047. [DOI] [PubMed] [Google Scholar]
- 38.Park CE, Kim MJ, Lee JH, Min BI, Bae H, Choe W, Kim SS, Ha J. Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp Mol Med. 2007;39:222–229. doi: 10.1038/emm.2007.25. [DOI] [PubMed] [Google Scholar]
- 39.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, III, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1 alpha in skeletal muscle. Aging-Us. 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards AG, Donato AJ, Lesniewski LA, Gioscia RA, Seals DR, Moore RL. Life-long caloric restriction elicits pronounced protection of the aged myocardium: a role for AMPK. Mech Ageing Dev. 2010;131:739–742. doi: 10.1016/j.mad.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun. 2009;378:836–841. doi: 10.1016/j.bbrc.2008.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 51.Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung JH, Manganiello V, Dyck JR. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol. 2012;22:546–554. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell. 2007;129:565–577. doi: 10.1016/j.cell.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Fogarty S, Hardie DG. C-terminal phosphorylation of LKB1 is not required for regulation of AMP-activated protein kinase, BRSK1, BRSK2, or cell cycle arrest. J Biol Chem. 2009;284:77–84. doi: 10.1074/jbc.M806152200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Djouder N, Tuerk RD, Suter M, Salvioni P, Thali RF, Scholz R, Vaahtomeri K, Auchli Y, Rechsteiner H, Brunisholz RA, Viollet B, Makela TP, Wallimann T, Neumann D, Krek W. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 2010;29:469–481. doi: 10.1038/emboj.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medina EA, Oberheu K, Polusani SR, Ortega V, Velagaleti GV, Oyajobi BO (2014) PKA/AMPK signaling in relation to adiponectin’s antiproliferative effect on multiple myeloma cells. Leukemia [DOI] [PubMed]
- 57.Omar B, Zmuda-Trzebiatowska E, Manganiello V, Goransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal. 2009;21:760–766. doi: 10.1016/j.cellsig.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pulinilkunnil T, He H, Kong D, Asakura K, Peroni OD, Lee A, Kahn BB. Adrenergic regulation of AMP-activated protein kinase in brown adipose tissue in vivo. J Biol Chem. 2011;286:8798–8809. doi: 10.1074/jbc.M111.218719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelly M, Gauthier MS, Saha AK, Ruderman NB. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes. 2009;58:1953–1960. doi: 10.2337/db08-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerhart-Hines Z, Dominy JE, Jr, Blattler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+) Mol Cell. 2011;44:851–863. doi: 10.1016/j.molcel.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nin V, Escande C, Chini CC, Giri S, Camacho-Pereira J, Matalonga J, Lou Z, Chini EN. Role of deleted in breast cancer 1 (DBC1) protein in SIRT1 deacetylase activation induced by protein kinase A and AMP-activated protein kinase. J Biol Chem. 2012;287:23489–23501. doi: 10.1074/jbc.M112.365874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kashyap SS, Johnson JR, McCue HV, Chen X, Edmonds MJ, Ayala M, Graham ME, Jenn RC, Barclay JW, Burgoyne RD, Morgan A (2014) Caenorhabditis elegans dnj-14, the orthologue of the DNAJC5 gene mutated in adult onset neuronal ceroid lipofuscinosis, provides a new platform for neuroprotective drug screening and identifies a SIR-2.1-independent action of resveratrol. Hum Mol Genet [DOI] [PMC free article] [PubMed]
- 63.Lissa D, Senovilla L, Rello-Varona S, Vitale I, Michaud M, Pietrocola F, Boileve A, Obrist F, Bordenave C, Garcia P, Michels J, Jemaa M, Kepp O, Castedo M, Kroemer G. Resveratrol and aspirin eliminate tetraploid cells for anticancer chemoprevention. Proc Natl Acad Sci USA. 2014;111:3020–3025. doi: 10.1073/pnas.1318440111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tikoo K, Lodea S, Karpe PA, Kumar S (2014) Calorie restriction mimicking effects of roflumilast prevents diabetic nephropathy. Biochem Biophys Res Commun [DOI] [PubMed]
- 65.Szewczuk LM, Forti L, Stivala LA, Penning TM. Resveratrol is a peroxidase-mediated inactivator of COX-1 but not COX-2: a mechanistic approach to the design of COX-1 selective agents. J Biol Chem. 2004;279:22727–22737. doi: 10.1074/jbc.M314302200. [DOI] [PubMed] [Google Scholar]
- 66.Agarwal B, Baur JA. Resveratrol and life extension. Ann N Y Acad Sci. 2011;1215:138–143. doi: 10.1111/j.1749-6632.2010.05850.x. [DOI] [PubMed] [Google Scholar]
- 67.Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25:87–97. [PubMed] [Google Scholar]
- 68.Moreira AC, Silva AM, Santos MS, Sardao VA. Resveratrol affects differently rat liver and brain mitochondrial bioenergetics and oxidative stress in vitro: investigation of the role of gender. Food Chem Toxicol. 2013;53:18–26. doi: 10.1016/j.fct.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 69.Desquiret-Dumas V, Gueguen N, Leman G, Baron S, Nivet-Antoine V, Chupin S, Chevrollier A, Vessieres E, Ayer A, Ferre M, Bonneau D, Henrion D, Reynier P, Procaccio V. Resveratrol induces a mitochondrial complex I-dependent increase in NADH oxidation responsible for sirtuin activation in liver cells. J Biol Chem. 2013;288:36662–36675. doi: 10.1074/jbc.M113.466490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 72.Turner RT, Evans GL, Zhang M, Maran A, Sibonga JD. Is resveratrol an estrogen agonist in growing rats? Endocrinology. 1999;140:50–54. doi: 10.1210/endo.140.1.6460. [DOI] [PubMed] [Google Scholar]
- 73.Lee JH, Guo Z, Myler LR, Zheng S, Paull TT. Direct activation of ATM by resveratrol under oxidizing conditions. PLoS One. 2014;9:e97969. doi: 10.1371/journal.pone.0097969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hussain AR, Uddin S, Bu R, Khan OS, Ahmed SO, Ahmed M, Al-Kuraya KS. Resveratrol suppresses constitutive activation of AKT via generation of ROS and induces apoptosis in diffuse large B cell lymphoma cell lines. PLoS One. 2011;6:e24703. doi: 10.1371/journal.pone.0024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 76.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 77.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 78.Yu X, Li G. Effects of resveratrol on longevity, cognitive ability and aging-related histological markers in the annual fish Nothobranchius guentheri . Exp Gerontol. 2012;47:940–949. doi: 10.1016/j.exger.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 79.Rascon B, Hubbard BP, Sinclair DA, Amdam GV. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging (Albany NY) 2012;4:499–508. doi: 10.18632/aging.100474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68:6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.da Luz PL, Tanaka L, Brum PC, Dourado PM, Favarato D, Krieger JE, Laurindo FR. Red wine and equivalent oral pharmacological doses of resveratrol delay vascular aging but do not extend life span in rats. Atherosclerosis. 2012;224:136–142. doi: 10.1016/j.atherosclerosis.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang C, Wheeler CT, Alberico T, Sun X, Seeberger J, Laslo M, Spangler E, Kern B, de Cabo R, Zou S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster . Age (Dordr) 2013;35:69–81. doi: 10.1007/s11357-011-9332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 88.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 91.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 92.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1 alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci USA. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1 alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 94.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 96.Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11:e1001603. doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1—possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bai P, Canto C, Oudart H, Brunyanszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, Schoonjans K, Schreiber V, Sauve AA, Menissier-de Murcia J, Auwerx J. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walz HA, Wierup N, Vikman J, Manganiello VC, Degerman E, Eliasson L, Holst LS. Beta-cell PDE3B regulates Ca2+ -stimulated exocytosis of insulin. Cell Signal. 2007;19:1505–1513. doi: 10.1016/j.cellsig.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 102.Choi YH, Park S, Hockman S, Zmuda-Trzebiatowska E, Svennelid F, Haluzik M, Gavrilova O, Ahmad F, Pepin L, Napolitano M, Taira M, Sundler F, Stenson Holst L, Degerman E, Manganiello VC. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest. 2006;116:3240–3251. doi: 10.1172/JCI24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang R, Maratos-Flier E, Flier JS. Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B. Endocrinology. 2009;150:3076–3082. doi: 10.1210/en.2009-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McBurney MW, Yang XF, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2 alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu YS, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang W, Hong HJ, Guan J, Kim DG, Yang EJ, Koh G, Park D, Han CH, Lee YJ, Lee DH. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2012;61:424–433. doi: 10.1016/j.metabol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 107.Poulsen MM, Larsen JO, Hamilton-Dutoit S, Clasen BF, Jessen N, Paulsen SK, Kjaer TN, Richelsen B, Pedersen SB. Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutr Res. 2012;32:701–708. doi: 10.1016/j.nutres.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 108.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin Y-K, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martin-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R. SRT1720 improves survival and healthspan of obese mice. Sci Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beaudoin MS, Snook LA, Arkell AM, Simpson JA, Holloway GP, Wright DC. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R542–R551. doi: 10.1152/ajpregu.00200.2013. [DOI] [PubMed] [Google Scholar]
- 111.Fiori JL, Shin YK, Kim W, Krzysik-Walker SM, Gonzalez-Mariscal I, Carlson OD, Sanghvi M, Moaddel R, Farhang K, Gadkaree SK, Doyle ME, Pearson KJ, Mattison JA, de Cabo R, Egan JM. Resveratrol prevents beta-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes. 2013;62:3500–3513. doi: 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, Ward TM, Younts CM, Lewis K, Allard JS, Longo DL, Belman JP, Malagon MM, Navas P, Sanghvi M, Moaddel R, Tilmont EM, Herbert RL, Morrell CH, Egan JM, Baur JA, Ferrucci L, Bogan JS, Bernier M, de Cabo R. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18:533–545. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marchal J, Blanc S, Epelbaum J, Aujard F, Pifferi F. Effects of chronic calorie restriction or dietary resveratrol supplementation on insulin sensitivity markers in a primate. Microcebus murinus. PLoS One. 2012;7:e34289. doi: 10.1371/journal.pone.0034289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 116.Kimura Y, Okuda H, Arichi S. Effects of stilbenes on arachidonate metabolism in leukocytes. Biochim Biophys Acta. 1985;834:275–278. [PubMed] [Google Scholar]
- 117.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 118.Wirleitner B, Schroecksnadel K, Winkler C, Schennach HS, Fuchs D. Resveratrol suppresses interferon-gamma-induced biochemical pathways in human peripheral blood mononuclear cells in vitro. Immunol Lett. 2005;100:159–163. doi: 10.1016/j.imlet.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 119.Rachon D, Rimoldi G, Wuttke W. In vitro effects of genistein and resveratrol on the production of interferon-gamma (IFNgamma) and interleukin-10 (IL-10) by stimulated murine splenocytes. Phytomedicine. 2006;13:419–424. doi: 10.1016/j.phymed.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 120.Lanzilli G, Cottarelli A, Nicotera G, Guida S, Ravagnan G, Fuggetta MP. Anti-inflammatory effect of resveratrol and polydatin by in vitro IL-17 modulation. Inflammation. 2012;35:240–248. doi: 10.1007/s10753-011-9310-z. [DOI] [PubMed] [Google Scholar]
- 121.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappa B-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tao HY, Wu CF, Zhou Y, Gong WH, Zhang X, Iribarren P, Zhao YQ, Le YY, Wang JM. The grape component resveratrol interferes with the function of chemoattractant receptors on phagocytic leukocytes. Cell Mol Immunol. 2004;1:50–56. [PubMed] [Google Scholar]
- 123.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov. 2012;11:443–461. doi: 10.1038/nrd3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2 alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 125.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin YX, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 126.van der Horst A, Tertoolen LGJ, de Vries-Smits LMM, Frye RA, Medema RH, Burgering BMT. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2(SIRT1) J Biol Chem. 2004;279:28873–28879. doi: 10.1074/jbc.M401138200. [DOI] [PubMed] [Google Scholar]
- 127.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 128.Rahal K, Schmiedlin-Ren P, Adler J, Dhanani M, Sultani V, Rittershaus AC, Reingold L, Zhu J, McKenna BJ, Christman GM, Zimmermann EM. Resveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn’s disease. Inflamm Bowel Dis. 2012;18:613–623. doi: 10.1002/ibd.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 130.Dolinsky VW, Dyck JR. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim Biophys Acta. 2011;1812:1477–1489. doi: 10.1016/j.bbadis.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 131.Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Resveratrol in primary and secondary prevention of cardiovascular disease: a dietary and clinical perspective. Ann N Y Acad Sci. 2013;1290:37–51. doi: 10.1111/nyas.12150. [DOI] [PubMed] [Google Scholar]
- 132.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 133.Taubert D, Berkels R. Upregulation and activation of eNOS by resveratrol. Circulation. 2003;107:E78–E79. doi: 10.1161/01.cir.0000060819.46705.ee. [DOI] [PubMed] [Google Scholar]
- 134.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu Q, Hao X, Yang Q, Si L. Resveratrol prevents hyperglycemia-induced endothelial dysfunction via activation of adenosine monophosphate-activated protein kinase. Biochem Biophys Res Commun. 2009;388:389–394. doi: 10.1016/j.bbrc.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 136.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 137.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xia N, Strand S, Schlufter F, Siuda D, Reifenberg G, Kleinert H, Forstermann U, Li H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 139.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang J, Wang N, Li J, Zhang J, Feng P. Effects of resveratrol on NO secretion stimulated by insulin and its dependence on SIRT1 in high glucose cultured endothelial cells. Endocrine. 2010;37:365–372. doi: 10.1007/s12020-010-9314-8. [DOI] [PubMed] [Google Scholar]
- 141.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 142.Liu Z, Song Y, Zhang X, Liu Z, Zhang W, Mao W, Wang W, Cui W, Zhang X, Jia X, Li N, Han C, Liu C. Effects of trans-resveratrol on hypertension-induced cardiac hypertrophy using the partially nephrectomized rat model. Clin Exp Pharmacol Physiol. 2005;32:1049–1054. doi: 10.1111/j.1440-1681.2005.04303.x. [DOI] [PubMed] [Google Scholar]
- 143.Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J, Sunagawa K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 144.Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 145.Miatello R, Vazquez M, Renna N, Cruzado M, Zumino AP, Risler N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am J Hypertens. 2005;18:864–870. doi: 10.1016/j.amjhyper.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 146.Chan V, Fenning A, Iyer A, Hoey A, Brown L. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr Pharm Biotechnol. 2011;12:429–436. doi: 10.2174/138920111794480552. [DOI] [PubMed] [Google Scholar]
- 147.Toklu HZ, Sehirli O, Ersahin M, Suleymanoglu S, Yiginer O, Emekli-Alturfan E, Yarat A, Yegen BC, Sener G. Resveratrol improves cardiovascular function and reduces oxidative organ damage in the renal, cardiovascular and cerebral tissues of two-kidney, one-clip hypertensive rats. J Pharm Pharmacol. 2010;62:1784–1793. doi: 10.1111/j.2042-7158.2010.01197.x. [DOI] [PubMed] [Google Scholar]
- 148.Csiszar A, Labinskyy N, Olson S, Pinto JT, Gupte S, Wu JM, Hu F, Ballabh P, Podlutsky A, Losonczy G, de Cabo R, Mathew R, Wolin MS, Ungvari Z. Resveratrol prevents monocrotaline-induced pulmonary hypertension in rats. Hypertension. 2009;54:668–675. doi: 10.1161/HYPERTENSIONAHA.109.133397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rush JW, Quadrilatero J, Levy AS, Ford RJ. Chronic resveratrol enhances endothelium-dependent relaxation but does not alter eNOS levels in aorta of spontaneously hypertensive rats. Exp Biol Med (Maywood) 2007;232:814–822. [PubMed] [Google Scholar]
- 150.Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, Yu L, Juric D, Kopilas MA, Anderson HD, Netticadan T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens. 2010;23:192–196. doi: 10.1038/ajh.2009.228. [DOI] [PubMed] [Google Scholar]
- 151.Dolinsky VW, Chan AY, Robillard Frayne I, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- 152.Mizutani K, Ikeda K, Kawai Y, Yamori Y. Protective effect of resveratrol on oxidative damage in male and female stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2001;28:55–59. doi: 10.1046/j.1440-1681.2001.03415.x. [DOI] [PubMed] [Google Scholar]
- 153.Dolinsky VW, Chakrabarti S, Pereira TJ, Oka T, Levasseur J, Beker D, Zordoky BN, Morton JS, Nagendran J, Lopaschuk GD, Davidge ST, Dyck JR. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim Biophys Acta. 2013;1832:1723–1733. doi: 10.1016/j.bbadis.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 154.Thandapilly SJ, Louis XL, Yang T, Stringer DM, Yu L, Zhang S, Wigle J, Kardami E, Zahradka P, Taylor C, Anderson HD, Netticadan T. Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur J Pharmacol. 2011;668:217–224. doi: 10.1016/j.ejphar.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 155.Robich MP, Chu LM, Chaudray M, Nezafat R, Han Y, Clements RT, Laham RJ, Manning WJ, Coady MA, Sellke FW. Anti-angiogenic effect of high-dose resveratrol in a swine model of metabolic syndrome. Surgery. 2010;148:453–462. doi: 10.1016/j.surg.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 158.Spanier G, Xu H, Xia N, Tobias S, Deng S, Wojnowski L, Forstermann U, Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J Physiol Pharmacol. 2009;60(Suppl 4):111–116. [PubMed] [Google Scholar]
- 159.Kim JW, Lim SC, Lee MY, Lee JW, Oh WK, Kim SK, Kang KW. Inhibition of neointimal formation by trans-resveratrol: role of phosphatidyl inositol 3-kinase-dependent Nrf2 activation in heme oxygenase-1 induction. Mol Nutr Food Res. 2010;54:1497–1505. doi: 10.1002/mnfr.201000016. [DOI] [PubMed] [Google Scholar]
- 160.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Zhao X, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 161.Xia N, Daiber A, Habermeier A, Closs EI, Thum T, Spanier G, Lu Q, Oelze M, Torzewski M, Lackner KJ, Munzel T, Forstermann U, Li H. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J Pharmacol Exp Ther. 2010;335:149–154. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 162.Kaneko H, Anzai T, Morisawa M, Kohno T, Nagai T, Anzai A, Takahashi T, Shimoda M, Sasaki A, Maekawa Y, Yoshimura K, Aoki H, Tsubota K, Yoshikawa T, Okada Y, Ogawa S, Fukuda K. Resveratrol prevents the development of abdominal aortic aneurysm through attenuation of inflammation, oxidative stress, and neovascularization. Atherosclerosis. 2011;217:350–357. doi: 10.1016/j.atherosclerosis.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 163.Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chow SE, Hshu YC, Wang JS. Chen JK (2007) Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damages. J Appl Physiol. 1985;102:1520–1527. doi: 10.1152/japplphysiol.00881.2006. [DOI] [PubMed] [Google Scholar]
- 166.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Csiszar A, Labinskyy N, Orosz Z, Ungvari Z. Altered mitochondrial energy metabolism may play a role in vascular aging. Med Hypotheses. 2006;67:904–908. doi: 10.1016/j.mehy.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 169.Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- 170.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 172.Wang MJ, Huang HM, Hsieh SJ, Jeng KC, Kuo JS. Resveratrol inhibits interleukin-6 production in cortical mixed glial cells under hypoxia/hypoglycemia followed by reoxygenation. J Neuroimmunol. 2001;112:28–34. doi: 10.1016/s0165-5728(00)00374-x. [DOI] [PubMed] [Google Scholar]
- 173.Culpitt SV, Rogers DF, Fenwick PS, Shah P, De Matos C, Russell RE, Barnes PJ, Donnelly LE. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 2003;58:942–946. doi: 10.1136/thorax.58.11.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Goh SS, Woodman OL, Pepe S, Cao AH, Qin C, Ritchie RH. The red wine antioxidant resveratrol prevents cardiomyocyte injury following ischemia-reperfusion via multiple sites and mechanisms. Antioxid Redox Signal. 2007;9:101–113. doi: 10.1089/ars.2007.9.101. [DOI] [PubMed] [Google Scholar]
- 175.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 176.Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Albani D, Polito L, Forloni G. Sirtuins as novel targets for Alzheimer’s disease and other neurodegenerative disorders: experimental and genetic evidence. J Alzheimers Dis. 2010;19:11–26. doi: 10.3233/JAD-2010-1215. [DOI] [PubMed] [Google Scholar]
- 178.Lee HR, Shin HK, Park SY, Kim HY, Lee WS, Rhim BY, Hong KW, Kim CD. Cilostazol suppresses beta-amyloid production by activating a disintegrin and metalloproteinase 10 via the upregulation of SIRT1-coupled retinoic acid receptor-beta. J Neurosci Res. 2014;92:1581–1590. doi: 10.1002/jnr.23421. [DOI] [PubMed] [Google Scholar]
- 179.Capiralla H, Vingtdeux V, Zhao H, Sankowski R, Al-Abed Y, Davies P, Marambaud P. Resveratrol mitigates lipopolysaccharide- and A ss-mediated microglial inflammation by inhibiting the TLR4/NF-κB/STAT signaling cascade. J Neurochem. 2012;120:461–472. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mudo G, Makela J, Di Liberto V, Tselykh TV, Olivieri M, Piepponen P, Eriksson O, Malkia A, Bonomo A, Kairisalo M, Aguirre JA, Korhonen L, Belluardo N, Lindholm D. Transgenic expression and activation of PGC-1 alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol Life Sci. 2012;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ferretta A, Gaballo A, Tanzarella P, Piccoli C, Capitanio N, Nico B, Annese T, Di Paola M, Dell’aquila C, De Mari M, Ferranini E, Bonifati V, Pacelli C, Cocco T (2014) Effect of resveratrol on mitochondrial function: Implications in parkin-associated familiar Parkinson’s disease. Biochim Biophys Acta [DOI] [PubMed]
- 182.Gerhardt E, Graber S, Szego EM, Moisoi N, Martins LM, Outeiro TF, Kermer P. Idebenone and resveratrol extend lifespan and improve motor function of HtrA2 knockout mice. PLoS One. 2011;6:e28855. doi: 10.1371/journal.pone.0028855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shindler KS, Ventura E, Rex TS, Elliott P, Rostami A. SIRT1 activation confers neuroprotection in experimental optic neuritis. Invest Ophthalmol Vis Sci. 2007;48:3602–3609. doi: 10.1167/iovs.07-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Calliari A, Bobba N, Escande C, Chini EN. Resveratrol delays Wallerian degeneration in a NAD(+) and DBC1 dependent manner. Exp Neurol. 2014;251:91–100. doi: 10.1016/j.expneurol.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 185.Renaud S, Delorgeril M. Wine, alcohol, platelets, and the french paradox for coronary heart-disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 186.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 187.Semba RD, Ferrucci L, Bartali B, Urpi-Sarda M, Zamora-Ros R, Sun K, Cherubini A, Bandinelli S, Andres-Lacueva C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA Intern Med. 2014;174:1077–1084. doi: 10.1001/jamainternmed.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Meng X, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J Agric Food Chem. 2004;52:935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 189.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 190.Vihko V, Hirsimaki Y, Ajiduah AO. beta-Glucuronidase activity in trained red and white skeletal muscle of mice. Eur J Appl Physiol Occup Physiol. 1978;39:255–261. doi: 10.1007/BF00421449. [DOI] [PubMed] [Google Scholar]
- 191.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 192.Ong WK, Gribble FM, Reimann F, Lynch MJ, Houslay MD, Baillie GS, Furman BL, Pyne NJ. The role of the PDE4D cAMP phosphodiesterase in the regulation of glucagon-like peptide-1 release. Br J Pharmacol. 2009;157:633–644. doi: 10.1111/j.1476-5381.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dao TM, Waget A, Klopp P, Serino M, Vachoux C, Pechere L, Drucker DJ, Champion S, Barthelemy S, Barra Y, Burcelin R, Seree E. Resveratrol increases glucose induced GLP-1 secretion in mice: a mechanism which contributes to the glycemic control. PLoS One. 2011;6:e20700. doi: 10.1371/journal.pone.0020700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 195.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha JF, Nunes T, Wright L, Soares-da-Silva P. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(Suppl 1):S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 197.Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, Alberts DS. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila) 2010;3:1168–1175. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, Meszaros LG, Suemegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 199.Fujitaka K, Otani H, Jo F, Jo H, Nomura E, Iwasaki M, Nishikawa M, Iwasaka T, Das DK. Modified resveratrol Longevinex improves endothelial function in adults with metabolic syndrome receiving standard treatment. Nutr Res. 2011;31:842–847. doi: 10.1016/j.nutres.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 200.Bhatt JK, Thomas S, Nanjan MJ. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr Res. 2012;32:537–541. doi: 10.1016/j.nutres.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 201.Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Tomas-Barberan FA, Garcia-Conesa MT, Espin JC. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: a triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc Drugs Ther. 2013;27:37–48. doi: 10.1007/s10557-012-6427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tome-Carneiro J, Gonzalvez M, Larrosa M, Yanez-Gascon MJ, Garcia-Almagro FJ, Ruiz-Ros JA, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am J Cardiol. 2012;110:356–363. doi: 10.1016/j.amjcard.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 203.Wong YT, Gruber J, Jenner AM, Tay FE, Ruan R. Chronic resveratrol intake reverses pro-inflammatory cytokine profile and oxidative DNA damage in ageing hybrid mice. Age (Dordr) 2011;33:229–246. doi: 10.1007/s11357-010-9174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, Battyany I, Sumegi B, Toth K, Szabados E. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. Clinical Hemorheol Microcirc. 2012;50:179–187. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]
- 205.Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J Gerontol A Biol Sci Med Sci. 2012;67:1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ghanim H, Sia CL, Abuaysheh S, Korzeniewski K, Patnaik P, Marumganti A, Chaudhuri A, Dandona P. An antiinflammatory and reactive oxygen species suppressive effects of an extract of Polygonum cuspidatum containing resveratrol. J Clin Endocrinol Metab. 2010;95:E1–E8. doi: 10.1210/jc.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O’Moore-Sullivan TM, Lee P, Franklin M, Klein K, Taylor PJ, Ferguson M, Coombes JS, Thomas GP, Cowin GJ, Kirkpatrick CM, Prins JB, Hickman IJ (2014) Resveratrol does not benefit patients with non-alcoholic fatty liver disease. Clin Gastroenterol Hepatol [DOI] [PubMed]
- 208.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MKC, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, Muller M, Schrauwen P, Mariman EC, Blaak EE. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes (Lond) 2014;38:470–473. doi: 10.1038/ijo.2013.155. [DOI] [PubMed] [Google Scholar]
- 210.Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658–664. doi: 10.1016/j.cmet.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, Moller N, Jessen N, Pedersen SB, Jorgensen JO. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, Mercken EM, Palmeira CM, de Cabo R, Rolo AP, Turner N, Bell EL, Sinclair DA. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, Gademann K, Rinsch C, Schoonjans K, Sauve AA, Auwerx J. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, Viscomi C, Zeviani M. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19:1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wouters EF, Bredenbroker D, Teichmann P, Brose M, Rabe KF, Fabbri LM, Goke B. Effect of the phosphodiesterase 4 inhibitor roflumilast on glucose metabolism in patients with treatment-naive, newly diagnosed type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:E1720–E1725. doi: 10.1210/jc.2011-2886. [DOI] [PubMed] [Google Scholar]