The activity of the siderophore cephalosporin cefiderocol is targeted against carbapenem-resistant Gram-negative bacteria. In this study, the activity of cefiderocol against characterized carbapenem-resistant Acinetobacter baumannii complex, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, and Enterobacteriaceae strains was determined by microdilution in iron-depleted Mueller-Hinton broth.
KEYWORDS: Enterobacteriaceae, Gram-negative bacteria, S-649266, cefiderocol, cephalosporin, siderophore
ABSTRACT
The activity of the siderophore cephalosporin cefiderocol is targeted against carbapenem-resistant Gram-negative bacteria. In this study, the activity of cefiderocol against characterized carbapenem-resistant Acinetobacter baumannii complex, Stenotrophomonas maltophilia, Pseudomonas aeruginosa, and Enterobacteriaceae strains was determined by microdilution in iron-depleted Mueller-Hinton broth. The MIC90s against A. baumannii, S. maltophilia, and P. aeruginosa were 1, 0.25, and 0.5 mg/liter, respectively. Against Enterobacteriaceae, the MIC90 was 1 mg/liter for the group harboring OXA-48-like, 2 mg/liter for the group harboring KPC-3, and 8 mg/liter for the group harboring TEM/SHV ESBL, NDM, and KPC-2.
INTRODUCTION
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) have designated that antibiotic resistance is a threat of enormous gravity to public health (1, 2). At least 2 million people in the United States acquire serious infections with bacteria that are resistant to the antibiotics that they are designed to treat, and more than 23,000 deaths from these infections occur each year. Novel agents and strategies are urgently required to combat this scourge.
This report is the first in a series of studies called ARGONAUT (Antibacterial Resistance Leadership Group [ARLG] Reference Group for the testing of Novel Therapeutics), supported by the ARLG (3). In this study, the in vitro activity of cefiderocol and comparator agents against reference collections of Gram-negative bacterial species (3–6), with an emphasis on carbapenem-resistant isolates, was determined to assess the spectrum of activity of this novel agent.
Cefiderocol is a siderophore cephalosporin under development and is targeted for activity against carbapenem and multidrug-resistant (MDR) Gram-negative species (7–9). This novel agent possesses a catechol moiety on the 3 position of the R2 side chain and binds primarily to PBP3 (10) (Fig. S1 in the supplemental material). The catechol moiety acts as a siderophore to form a chelating complex with ferric iron, which facilitates transport to the periplasmic space. Cefiderocol has been reported to be more stable against β-lactamases, such as KPC-3, VIM-2, L1 (the chromosomal metallo-type carbapenemase of Stenotrophomonas maltophilia), and NDM-1 carbapenemases than agents such as cefepime and meropenem (11). The human plasma protein binding of cefiderocol is 58% (12), and pharmacokinetic/pharmacodynamic (PK/PD) studies show that cefiderocol is an agent with time-dependent activity, with the fraction of the free drug concentration in plasma exceeding a MIC (fTMIC) target of 75% of the dosing interval (13, 14). At the proposed dosing regimen for cefiderocol of 2 g infused over 3 h every 8 h, PK/PD modeling showed that this 75% fTMIC target would be attained in >90% of patients for organisms with MICs of ≤4 mg/liter (15).
In this analysis, the MICs of cefiderocol and comparators were determined by broth microdilution according to the current Clinical and Laboratory Standards Institute (CLSI) guidelines (16, 17). Testing was performed using customized frozen 96-well trays provided by International Health Management Associates, Inc. (Schaumburg, IL). The range of concentrations of the agents tested and current MIC interpretative breakpoints are shown in Table S1 in the supplemental material. Cefiderocol was tested in iron-depleted cation-adjusted Mueller-Hinton (MH) broth, and MICs were read as the concentration in the first drug well in which the growth was significantly reduced (i.e., to a button of <1 mm or light/faint turbidity) relative to the growth observed in the growth control well containing the same medium; trailing endpoints were disregarded (16, 18). All other agents were tested in cation-adjusted MH broth. Appropriate quality control (QC) strains were tested on each day of testing using appropriate reference strains, including Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 for QC for cefiderocol (16).
A total of 1,086 Gram-negative clinical isolates were tested, including 834 Enterobacteriaceae isolates and 252 nonfermenters. The Enterobacteriaceae tested included Klebsiella pneumoniae (n = 794), E. coli (n = 35), Citrobacter freundii (n = 1), and Enterobacter species (n = 4); the resistance mechanisms present included blaKPC in 737 isolates, blaNDM in 28 isolates, blaOXA-48-like in 7 isolates, blaNDM and blaOXA-48-like in 1 isolate, and extended-spectrum β-lactamases (ESBLs) or AmpCs in 43 isolates; 18 isolates lacked ESBLs or carbapenemases. The Enterobacteriaceae group included 700 recent, carbapenem-resistant, clinical isolates of K. pneumoniae from the Great Lakes region (19). In addition, there were 116 clinical strains of various enterobacterial species selected from a reference collection with various β-lactamases and included the 18 isolates without ESBLs or carbapenemases. The nonfermenter isolates tested were clinical isolates from a worldwide collection of Acinetobacter baumannii complex isolates (n = 200; 101 were resistant to at least one carbapenem), S. maltophilia (n = 25 with blaL1), and P. aeruginosa (n = 27) isolates with blaVIM, blaPDC, and/or porin OprD deletions from hospitals in the Cleveland, OH, area (6, 20–22). All A. baumannii complex isolates and a subset of the K. pneumoniae isolates were sequenced by whole-genome sequencing (WGS) using paired-end NexteraXT libraries and an Illumina NextSeq sequencer (2× 150 bp) to ∼100-fold coverage. Reads were assembled using SPAdes software (23), annotated using NCBI’s PGAP pipeline (24), and deposited in the NCBI SRA and GenBank whole-genome sequencing (WGS) repositories (BioProject accession numbers PRJNA384060 and PRJNA384065). Resistance mechanisms in isolates whose whole genomes were not sequenced were characterized by PCR amplification and sequencing of the KPC β-lactamase genes, if present, as previously reported (4, 25). In addition, a modified carbapenemase multiplex PCR that detects blaIMP, blaNDM, blaOXA-48-like, and blaVIM was employed (26). The P. aeruginosa and S. maltophilia β-lactamase content was previously determined in other studies (20–22). In this manner, we identified the relevant β-lactamases for the entire collection of 1,086 Gram-negative clinical isolates. The results for each organism group are shown as MIC ranges, MIC50 and MIC90 values, and the percentage of isolates susceptible to each agent for cefiderocol and comparator agents in Tables 1 to 4.
TABLE 1.
Activity of antimicrobial agents tested against Enterobacteriaceaeb
| Agent | MIC (mg/liter) |
% susceptible | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Cefiderocola | ≤0.03 to >64 | 0.5 | 4 | 90.5 |
| Amikacin | ≤4 to >64 | 16 | 32 | 67.7 |
| Ciprofloxacin | ≤0.25 to >4 | >4 | >4 | 8.0 |
| Colistin | ≤0.5 to >8 | 0.5 | 4 | 89.3 |
| Tigecycline | ≤0.25 to >4 | 0.5 | 1 | 98.4 |
| Aztreonam | ≤0.5 to >32 | >32 | >32 | 3.2 |
| Ceftolozane-tazobactam | 0.06 to >64 | 64 | >64 | 5.5 |
| Cefepime | ≤0.5 to >16 | >16 | >16 | 4.2 |
| Ceftazidime | ≤0.03 to >64 | >64 | >64 | 3.7 |
| Ceftazidime-avibactam | ≤0.03 to >64 | 0.5 | 2 | 96.6 |
| Meropenem | ≤0.03 to >64 | 8 | >64 | 12.7 |
Percent susceptible based on a provisional breakpoint of 4 mg/liter.
A total of 834 Enterobacteriaceae were tested.
TABLE 4.
Activity of antimicrobial agents tested against carbapenem-resistant P. aeruginosaa and S. maltophiliab
| Agent | MIC (mg/liter) |
% susceptible | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| P. aeruginosa | ||||
| Cefiderocolc | ≤0.03 to 1 | 0.25 | 0.5 | 100 |
| Amikacin | ≤4 to >64 | 64 | >64 | 48 |
| Ciprofloxacin | 2 to >4 | >4 | >4 | 0 |
| Colistin | ≤0.5 to >8 | 1 | 1 | 96 |
| Tigecycline | 1 to >4 | >4 | >4 | 22 |
| Aztreonam | 4 to >32 | 32 | >32 | 22 |
| Ceftolozane-tazobactam | 1 to >64 | >64 | >64 | 26 |
| Cefepime | 8 to >16 | >16 | >16 | 7 |
| Ceftazidime | 16 to >64 | 64 | >64 | 0 |
| Ceftazidime-avibactam | 1 to >64 | 64 | >64 | 8 |
| Meropenem | 2 to >64 | 32 | 64 | 4 |
| S. maltophilia | ||||
| Cefiderocolc | ≤0.03 to 0.25 | 0.06 | 0.25 | 100 |
| Amikacin | ≤4 to >64 | >64 | >64 | —d |
| Ciprofloxacin | 0.5 to >4 | 2 | >4 | — |
| Colistin | ≤0.5 to >8 | 1 | 8 | 68 |
| Tigecycline | ≤0.25 to >4 | 0.5 | 2 | — |
| Aztreonam | >32 | >32 | >32 | — |
| Ceftolozane-tazobactam | 1 to >64 | >64 | >64 | — |
| Cefepime | 8 to >16 | >16 | >16 | — |
| Ceftazidime | 2 to >64 | >64 | >64 | 8 |
| Ceftazidime-avibactam | 0.25 to >64 | 64 | >64 | 16 |
Data are for 27 P. aeruginosa isolates. Resistance mechanisms included VIM plus PDC (n = 12) and PDC alone (n = 4).
Data are for 25 S. maltophilia isolates. All isolates contained L1 and L2 β-lactamases.
Percent susceptible based on a provisional breakpoint of 4 mg/liter.
—, no breakpoint is available.
Enterobacteriaceae.
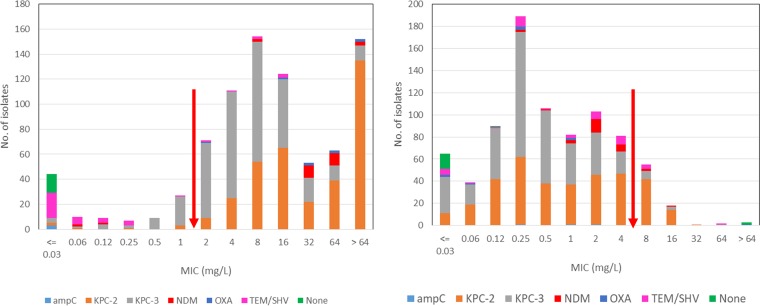
Cefiderocol MICs ranged from ≤0.03 to >64 mg/liter, with the MIC90 being 4 mg/liter (Table 1). The rates of susceptibility of the comparator agents ranged from 3.7% (ceftazidime; MIC90, >64 mg/liter) to 96.6% (ceftazidime-avibactam; MIC90, 2 mg/liter) and 98.4% (tigecycline; MIC90, 1 mg/liter). Analysis of these results based on β-lactam resistance mechanisms showed a cefiderocol MIC90 of ≤0.03 mg/liter for isolates without ESBLs or carbapenemases versus a cefiderocol MIC90 of from 1 to 8 mg/liter for isolates with ESBLs, carbapenemases, or AmpCs. There was no obvious association between the resistance mechanism and the MIC, although there was a bimodal MIC distribution, with peaks at 0.25 and 2 mg/liter (Table 2 and Fig. 1). In contrast, ceftazidime-avibactam susceptibility was related to β-lactam resistance mechanisms, with only 3/28 isolates (10.7%) with blaNDM being susceptible and 735/738 isolates (99.6%) with blaKPC being susceptible.
TABLE 2.
Activity of cefiderocol against Enterobacteriaceae with characterized ESBLs and carbapenemases
| β-Lactamase groupa | No. of isolates | MIC (mg/liter) |
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| None | 18 | ≤0.03 to 4 | ≤0.03 | ≤0.03 |
| KPC-2 | 355 | ≤0.03 to 32 | 1 | 8 |
| KPC-3 | 380 | ≤0.03 to 64 | 0.25 | 2 |
| KPC-4 or KPC-4-like | 2 | 0.5 to 16 | 0.5 | 16 |
| NDM | 28 | 0.25 to >64 | 2 | 8 |
| OXA-48-like | 7 | ≤0.03 to 1 | 0.25 | 1 |
| NDM and OXA-48-like | 1 | 1 | ||
| Other (TEM ESBL, SHV ESBL, CTX-M, PER, and/or AmpC) | 43 | ≤0.03 to >64 | 2 | 8 |
| All isolates | 834 | ≤0.03 to >64 | 0.5 | 4 |
The isolates included in the β-lactamase groups were as follows: none, E. coli (n = 10) and K. pneumoniae (n = 8); KPC-2, K. pneumoniae (n = 350), E. coli (n = 4), and Enterobacter cloacae (n = 1); KPC-3, K. pneumoniae (n = 378), C. freundii (n = 1), and Enterobacter aerogenes (n = 1); KPC-4 or KPC-4-like, K. pneumoniae (n = 2); NDM, K. pneumoniae (n = 19), E. coli (n = 7), and E. cloacae (n = 2); OXA-48-like, K. pneumoniae (n = 7); NDM and OXA-48-like, K. pneumoniae (n = 1); and other, E. coli (n = 14; 2 CMY, 1 TEM-5, 8 CTX-M, 3 not determined) and K. pneumoniae (n = 29; 1 CMY-2; 5 CTX-M; 1 CTX-M and SHV-2; 3 CTX-M and SHV-12; 2 PER; 1 SHV-2 and PER; 1 SHV-5 and PER; 10 SHV-2, -5, -7, or -12; 2 SHV-2 and TEM-10; 2 SHV-5 and TEM-10; 1 SHV-5; and TEM-26) (this group did not contain KPC, NDM, or OXA-48-like β-lactamases).
FIG 1.
MIC distributions of meropenem (left) and cefiderocol (right) against Enterobacteriaceae. Arrows indicate the upper limit of susceptible and proposed susceptible MIC ranges for meropenem and cefiderocol, respectively. AmpC, CMY; OXA, OXA-48-like; TEM/SHV, TEM ESBL and/or SHV ESBL.
A. baumannii complex.
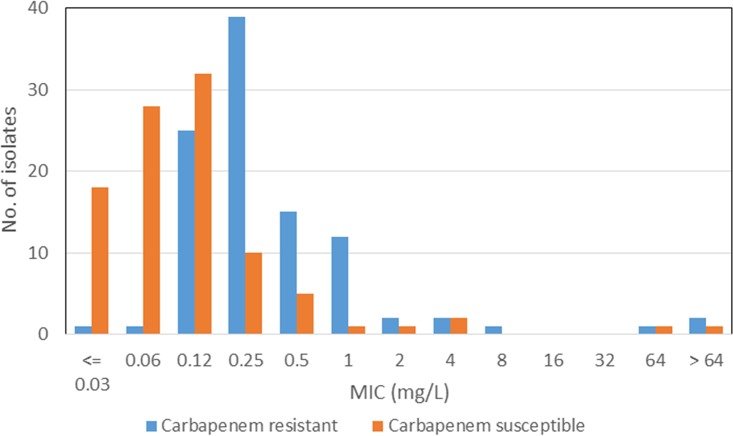
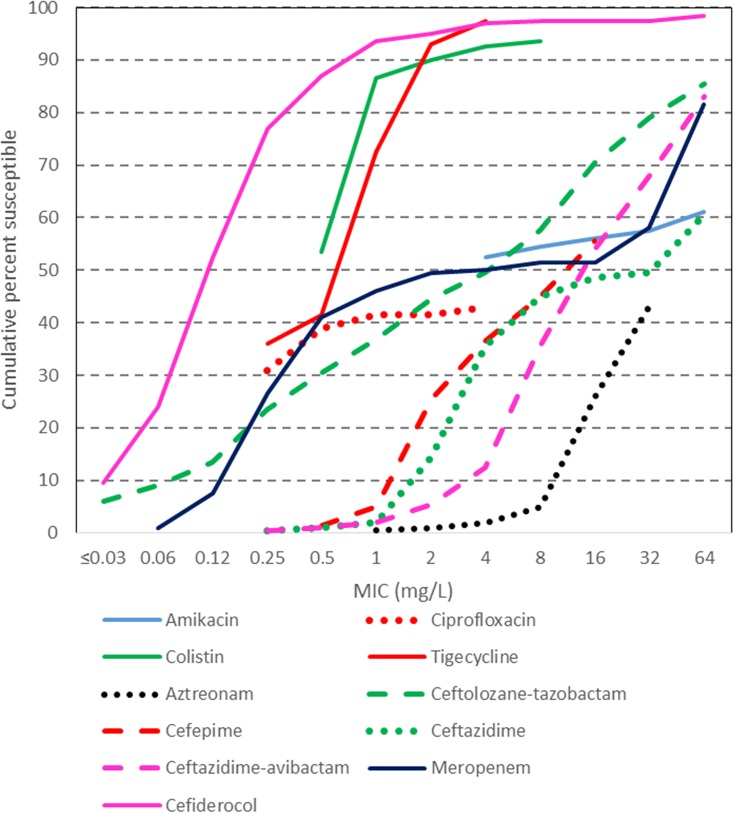
Cefiderocol MICs ranged from ≤0.03 to >64 mg/liter, with the overall MIC90 being 1 mg/liter, and there was little difference between the carbapenem-susceptible and -resistant groups (Table 3; Fig. 2 and 3). Other agents active against both groups were colistin (to which 90.0% of isolates were susceptible) and tigecycline (to which 93% of isolates were susceptible). Agents more active against the carbapenem-susceptible group than against the carbapenem-resistant group included amikacin (94.9% versus 17.8%), ciprofloxacin (83.7% versus 0%), ceftazidime (86.9% versus 4.0%), ceftazidime-avibactam (64.6% versus 6.9%), and meropenem (100% versus 0%).
TABLE 3.
Activity of antimicrobial agents tested against carbapenem-susceptible and -resistant Acinetobacter baumannii complex isolatesa
| Agent | Carbapenem-susceptible isolates (n = 99)b |
Carbapenem-resistant isolates (n = 101)c |
All isolates (n = 200) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/liter) |
% susceptible | % susceptible | % susceptible | |||||||||
| Range | 50% | 90% | Range (mg/liter) | 50% | 90% | Range | 50% | 90% | ||||
| Cefiderocold | ≤0.03 to >64 | 0.12 | 0.5 | 97.9 | ≤0.03 to >64 | 0.25 | 1 | 96.0 | ≤0.03 to >64 | 0.12 | 1 | 97.0 |
| Amikacin | ≤4 to >64 | ≤4 | 8 | 94.9 | ≤4 to >64 | >64 | >64 | 17.8 | ≤4 to >64 | ≤4 | >64 | 56.0 |
| Ciprofloxacin | ≤0.25 to >4 | ≤0.25 | >4 | 83.8 | 4 to >4 | >4 | >4 | 0.0 | ≤0.25 to >4 | >4 | >4 | 41.5 |
| Colistin | ≤0.5 to >8 | ≤0.5 | 1 | 97.0 | ≤0.5 to >8 | 1 | >8 | 83.2 | ≤0.5 to >8 | ≤0.5 | 2 | 90.0 |
| Tigecycline | ≤0.25 to >4 | ≤0.25 | 1 | 97.0 | 0.5 to >4 | 1 | 4 | 89.1 | ≤0.25 to >4 | 1 | 2 | 93.0 |
| Aztreonam | 2 to >32 | 32 | >32 | 1 to >32 | >32 | >32 | 1 to >32 | >32 | >32 | |||
| Ceftolozane-tazobactam | 0.06 to >64 | 0.25 | 4 | 0.25 to >64 | 32 | >64 | 0.06 to >64 | 8 | >64 | |||
| Cefepime | ≤0.5 to >16 | 2 | 16 | 1 to >16 | >16 | >16 | ≤0.5 to >16 | 16 | >16 | |||
| Ceftazidime | 0.5 to >64 | 4 | 16 | 86.9 | 4 to >64 | >64 | >64 | 4.0 | 0.25 to >64 | 64 | >64 | 45.0 |
| Ceftazidime-avibactam | 0.5 to >64 | 16 | 32 | 64.6 | 8 to >64 | 64 | >64 | 6.9 | 0.25 to >64 | 16 | >64 | 35.5 |
| Meropenem | 0.06 to >64 | 0.25 | 1 | 100.0 | 4 to >64 | 64 | >64 | 0.0 | 0.06 to >64 | 2 | >64 | 49.5 |
All carbapenem-resistant isolates were A. baumannii sensu stricto (n = 101). The carbapenem-susceptible group contained A. baumannii (n = 54), A. pittii (n = 37), A. nosocomialis (n = 5), and a few mixed cultures (n = 3). Species identification within the A. baumannii complex was determined by WGS and other molecular methods (i.e., gyrB sequencing and identification of the intrinsic oxacillinases).
OXA β-lactamases present in 99 carbapenem-susceptible isolates: OXA-100 or -100-like (n = 5), OXA-106 (n = 1), OXA-121 (n = 4), OXA-208 (n = 1), OXA-217 or -217-like (n = 2), OXA-223 (n = 1), OXA-263 (n = 1), OXA-270-like (n = 1), OXA-273 (n = 1), OXA-314 (n = 4), OXA-338-like (n = 2), OXA-340 (n = 1), OXA-421 or -421-like (n = 4), OXA-429-like (n = 1), OXA-500 or -500-like (n = 26), OXA-502-like (n = 1), OXA-506 (n = 5), OXA-51 or -51-like (n = 2), OXA-63 (n = 3), OXA-64 (n = 4), OXA-66 (n = 5), OXA-66 and -437-like (n = 1), OXA-68 (n = 2), OXA-69 (n = 1), OXA-71 (n = 1), OXA-78 (n = 1), OXA-90 (n = 4), OXA-94 (n = 3), OXA-98 (n = 4), and no OXA or unknown (n = 7).
OXA β-lactamase combinations present in 101 carbapenem-resistant isolates: OXA-23, -24/40, and -65 (n = 1); OXA-23 and -64 (n = 1); OXA-23 and -66 (n = 54); OXA-23, -66, and -72 (n = 2); OXA-23 and -68 (n = 2); OXA-23 and -69 (n = 1); OXA-23 and -71 (n = 2); OXA-23 and -82 (n = 12); OXA-23 and -407 (n = 3); OXA-24/40 and -65 (n = 4); OXA-24/40 and -71 (n = 1); OXA-58 and -66 (n = 1); OXA-58 and -100 (n = 1); OXA-66 (n = 1); OXA-72 and -66 (n = 4); OXA-82 (n = 5); OXA-82 and -167 (n = 1); OXA-172 (n = 2); OXA-223 (n = 1); OXA-223 and -72 (n = 1); and OXA-338-like (n = 1).
Percent susceptible based on a provisional breakpoint of 4 mg/liter.
FIG 2.
MIC distributions of cefiderocol against carbapenem-susceptible and -resistant A. baumannii complex isolates.
FIG 3.
Cumulative MICs of cefiderocol and comparator agents against A. baumannii complex isolates.
P. aeruginosa.
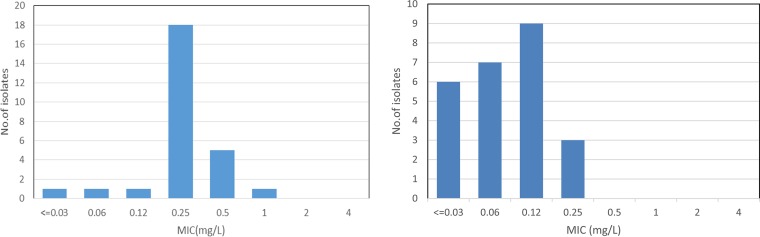
Cefiderocol MICs ranged from ≤0.03 to 1 mg/liter, with the MIC90 being 0.5 mg/liter (Table 4 and Fig. 4). Notably, the MICs against isolates with blaVIM ranged from 0.06 to 1 mg/liter, with the MIC90 being 0.5 mg/liter. Colistin was the only comparator tested with good activity (96.3% of isolates were susceptible), while amikacin was active against 48.1% of isolates, aztreonam 22.2%, and ceftolozane-tazobactam 25.9%, and <10% of isolates were susceptible to ciprofloxacin, cefepime, ceftazidime, ceftazidime-avibactam, or meropenem.
FIG 4.
MIC distribution of cefiderocol against P. aeruginosa (left) and S. maltophilia (right).
S. maltophilia.
Cefiderocol MICs ranged from ≤0.03 to 0.25 mg/liter, with the MIC90 being 0.25 mg/liter (Table 4 and Fig. 4). Colistin was active against 68.0% of isolates, while the MIC90 of tigecycline was 2 mg/liter. Amikacin and the other β-lactam agents tested had little activity, as expected, against this intrinsically aminoglycoside- and carbapenem-resistant species.
Several studies on the in vitro activity of cefiderocol against carbapenem-resistant species have recently been published, and our findings are in general agreement with the findings of these studies (10, 21–24, 28). However, overall, cefiderocol MIC90s against Enterobacteriaceae were lower (≤1 mg/liter) in two studies: (i) in the study of Kohira et al. against a 2009 to 2011 global collection of 617 Enterobacteriaceae isolates, although the MICs were up to 4 mg/liter against 226 of 233 strains (97.0%) with characterized β-lactamases, including 116 isolates with blaKPC, blaSME, or blaNDM (27), and (ii) in the study of Hackel et al. (29) against a U.S. and European collection of 6,087 Enterobacteriaceae isolates collected between 2014 and 2016, although the MIC90 was 4 mg/liter against 169 meropenem-nonsusceptible Enterobacteriaceae isolates.
Our findings on the in vitro activity of cefiderocol are in alignment with those in the publications discussed above for Enterobacteriaceae (MIC90, 4 mg/liter), P. aeruginosa (MIC90, 0.5 mg/liter), and S. maltophilia (MIC90, 0.25 mg/liter). Uniquely, our analysis also provides a correlation between the activity of cefiderocol and β-lactam resistance mechanisms; the activity against Enterobacteriaceae was affected by various β-lactamases, while the activity against nonfermenters was independent of the presence of β-lactamases, including carbapenemases. The wide distribution of MIC values for Enterobacteriaceae found here has been observed in other studies and is independent of the medium used, including iron-depleted-cation-adjusted (CA) MH broth (approved by CLSI), iron-depleted medium using the chelator ApoT, and non-iron-depleted CA MH broth (11, 12). This wide distribution is believed to be due to variations in iron transport channel expression, the primary mechanism for cell entry of siderophore antibiotic conjugates, which varies by species and within species (7, 11, 15).
For A. baumannii our MIC90 of 1 mg/liter was in agreement with the MIC90 values of 1 and 2 mg/liter, respectively, reported by Ito et al. (10) and Hackel et al. (29), while a later publication by Hackel et al. reported a higher MIC90 value of 8 mg/liter (30). The reason for this difference in activity against A. baumannii may be associated with regional differences in the resistance mechanisms of this species. Overall, our study showed the potent activity of cefiderocol against all isolates of P. aeruginosa and S. maltophilia tested and activity against most isolates of the Acinetobacter baumannii complex, with little difference between carbapenem-susceptible and -resistant isolates. Cefiderocol shows higher MICs against isolates with ESBLs (including the blaTEM ESBL, the blaSHV ESBL, and blaCTX-M) and carbapenemases (including blaKPC, blaNDM, and blaOXA-48-like) than against isolates of Enterobacteriaceae without ESBLs or carbapenemases.
Based on the proposed dosing regimen of cefiderocol and the PK/PD target attained at this dosing regimen, our findings support the in vivo activity of this agent against carbapenem-resistant Gram-negative nonfermenters and most carbapenem-resistant Enterobacteriaceae. Based on studies performed to date, cefiderocol may prove to be a particularly valuable addition to our limited armamentarium for combating infections caused by carbapenem-resistant Gram-negative nonfermenters.
Accession number(s).
The reads obtained in this study have been deposited in the NCBI Sequence Read Archive and GenBank WGS repositories under BioProject accession numbers PRJNA384060 and PRJNA384065.
Supplementary Material
ACKNOWLEDGMENTS
Susceptibility test panels were provided by International Health Management Associates, Inc.
The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) under award number UM1AI104681. Additional NIH support to R.A.B. includes R01AI100560, R01AI063517, and R01AI072219. This study was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs, award number 1I01BX001974 to R.A.B. from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development, and the Geriatric Research Education and Clinical Center VISN 10. In addition, research support was also received from Shionogi.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the U.S. Department of Veterans Affairs.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01801-18.
REFERENCES
- 1.World Health Organization. 2017. Antibacterial agents in clinical development. An analysis of the antibacterial clinical development pipeline, including tuberculosis. Report WHO/EMP/IAU/201711 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf.
- 3.Doi Y, Bonomo RA, Hooper DC, Kaye KS, Johnson JR, Clancy CJ, Thaden JT, Stryjewski ME, van Duin D, for the Gram-Negative Committee of the Antibacterial Resistance Leadership Group (ARLG). 2017. Gram-negative bacterial infections: research priorities, accomplishments, and future directions of the Antibacterial Resistance Leadership Group. Clin Infect Dis 64:S30–S35. doi: 10.1093/cid/ciw829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas LJ, Salim M, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Marshall S, Rudin SD, Domitrovic TN, Hujer AM, Hujer KM, Doi Y, Kaye KS, Evans S, Fowler VG Jr, Bonomo RA, van Duin D, Antibacterial Resistance Leadership Group. 2017. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis 64:711–718. doi: 10.1093/cid/ciw805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright MS, Iovleva A, Jacobs MR, Bonomo RA, Adams MD. 2016. Genome dynamics of multidrug-resistant Acinetobacter baumannii during infection and treatment. Genome Med 8:26. doi: 10.1186/s13073-016-0279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans SR, Hujer AM, Jiang H, Hill CB, Hujer KM, Mediavilla JR, Manca C, Tran TT, Domitrovic TN, Higgins PG, Seifert H, Kreiswirth BN, Patel R, Jacobs MR, Chen L, Sampath R, Hall T, Marzan C, Fowler VG Jr, Chambers HF, Bonomo RA. 2017. Informing antibiotic treatment decisions: evaluating rapid molecular diagnostics to identify susceptibility and resistance to carbapenems against Acinetobacter spp. in PRIMERS III. J Clin Microbiol 55:134–144. doi: 10.1128/JCM.01524-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. doi: 10.1128/AAC.01405-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsube T, Echols R, Arjona Ferreira JC, Krenz HK, Berg JK, Galloway C. 2017. Cefiderocol, a siderophore cephalosporin for Gram-negative bacterial infections: pharmacokinetics and safety in subjects with renal impairment. J Clin Pharmacol 57:584–591. doi: 10.1002/jcph.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright H, Bonomo RA, Paterson DL. 2017. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect 23:704–712. doi: 10.1016/j.cmi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Ito A, Kohira N, Bouchillon SK, West J, Rittenhouse S, Sader HS, Rhomberg PR, Jones RN, Yoshizawa H, Nakamura R, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother 71:670–677. doi: 10.1093/jac/dkv402. [DOI] [PubMed] [Google Scholar]
- 11.Ito A, Sato T, Ota M, Takemura M, Nishikawa T, Toba S, Kohira N, Miyagawa S, Ishibashi N, Matsumoto S, Nakamura R, Tsuji M, Yamano Y. 2018. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother 62:e01454-17. doi: 10.1128/AAC.01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto S, Singley CM, Hoover J, Nakamura R, Echols R, Rittenhouse S, Tsuji M, Yamano Y. 2017. Efficacy of cefiderocol against carbapenem-resistant Gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrob Agents Chemother 61:e00700-17. doi: 10.1128/AAC.00700-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghazi IM, Monogue ML, Tsuji M, Nicolau DP. 2018. Pharmacodynamics of cefiderocol, a novel siderophore cephalosporin, in a Pseudomonas aeruginosa neutropenic murine thigh model. Int J Antimicrob Agents 51:206–212. doi: 10.1016/j.ijantimicag.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi N, Katsube T, Echols R, Wajima T. 2018. Population pharmacokinetic analysis of cefiderocol, a parenteral siderophore cephalosporin, in healthy subjects, subjects with various degrees of renal function, and patients with complicated urinary tract infection or acute uncomplicated pyelonephritis. Antimicrob Agents Chemother 62:e01391-17. doi: 10.1128/AAC.01391-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsube T, Miyazaki S, Narukawa Y, Hernandez-Illas M, Wajima T. 2018. Drug-drug interaction of cefiderocol, a siderophore cephalosporin, via human drug transporters. Eur J Clin Pharmacol 74:931–938. doi: 10.1007/s00228-018-2458-9. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2018. M100-S28 Performance standards for antimicrobial susceptibility testing, 28th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2018. M07 Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Huband MD, Ito A, Tsuji M, Sader HS, Fedler KA, Flamm RK. 2017. Cefiderocol MIC quality control ranges in iron-depleted cation-adjusted Mueller-Hinton broth using a CLSI M23-A4 multi-laboratory study design. Diagn Microbiol Infect Dis 88:198–200. doi: 10.1016/j.diagmicrobio.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Henig O, Cober E, Richter SS, Perez F, Salata RA, Kalayjian RC, Watkins RR, Marshall S, Rudin SD, Domitrovic TN, Hujer AM, Hujer KM, Doi Y, Evans S, Fowler VG Jr, Bonomo RA, van Duin D, Kaye KS, Antibacterial Resistance Leadership Group. 2017. A prospective observational study of the epidemiology, management, and outcomes of skin and soft tissue infections due to carbapenem-resistant Enterobacteriaceae. Open Forum Infect Dis 4:ofx157. doi: 10.1093/ofid/ofx157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mojica MF, Papp-Wallace KM, Taracila MA, Barnes MD, Rutter JD, Jacobs MR, LiPuma JJ, Walsh TJ, Vila AJ, Bonomo RA. 2017. Avibactam restores the susceptibility of clinical isolates of Stenotrophomonas maltophilia to aztreonam. Antimicrob Agents Chemother 61:e00777-17. doi: 10.1128/AAC.00777-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans SR, Tran TTT, Hujer AM, Hill CB, Hujer KM, Mediavilla JR, Manca C, Domitrovic TN, Perez F, Farmer M, Pitzer KM, Wilson BM, Kreiswirth BN, Patel R, Jacobs MR, Chen L, Fowler VG Jr, Chambers HF, Bonomo RA, Antibacterial Resistance Leadership Group. 20 September 2018. Rapid molecular diagnostics to inform empiric use of ceftazidime/avibactam and ceftolozane/tazobactam against Pseudomonas aeruginosa: PRIMERS IV. Clin Infect Dis doi: 10.1093/cid/ciy801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez F, Hujer AM, Marshall SH, Ray AJ, Rather PN, Suwantarat N, Dumford D, O’Shea P, Domitrovic TNJ, Salata RA, Chavda KD, Chen L, Kreiswirth BN, Vila AJ, Haussler S, Jacobs MR, Bonomo RA. 2014. Extensively drug-resistant Pseudomonas aeruginosa isolates containing blaVIM-2 and elements of Salmonella genomic island 2: a new genetic resistance determinant in northeast Ohio. Antimicrob Agents Chemother 58:5929–5935. doi: 10.1128/AAC.02372-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J. 2016. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endimiani A, Hujer AM, Perez F, Bethel CR, Hujer KM, Kroeger J, Oethinger M, Paterson DL, Adams MD, Jacobs MR, Diekema DJ, Hall GS, Jenkins SG, Rice LB, Tenover FC, Bonomo RA. 2009. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the eastern USA. J Antimicrob Chemother 63:427–437. doi: 10.1093/jac/dkn547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, Rittenhouse S, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother 60:729–734. doi: 10.1128/AAC.01695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobias J, Denervaud-Tendon V, Poirel L, Nordmann P. 2017. Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis 36:2319–2327. doi: 10.1007/s10096-017-3063-z. [DOI] [PubMed] [Google Scholar]
- 29.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2017. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant Gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother 61:e00093-17. doi: 10.1128/AAC.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2018. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of Gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 62:e01968-17. doi: 10.1128/AAC.01968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.