Abstract
Background
Proactive recommendations for human papillomavirus (HPV) vaccines in Japan have been suspended for 5 years because of safety concerns. While no scientific evidence exists to substantiate these concerns, one reason given for not reinstating recommendations is the lack of reliable vaccine effectiveness (VE) data in a Japanese population. This study reports the VE of the bivalent HPV vaccine in Japanese women aged 20–22 years.
Methods
During cervical screening between 2014 and 2016, women had Papanicolaou smears and HPV tests performed and provided data about their sexual history. Estimates of VE for vaccine-targeted HPV type 16 (HPV16) and 18 and cross-protection against other types were calculated.
Results
Overall, 2197 women were tested, and 1814 were included in the analysis. Of these, 1355 (74.6%) were vaccinated, and 1295 (95.5%) completed the 3-dose schedule. In women sexually naive at vaccination, the pooled VEs against HPV16 and 18 and for HPV31, 45, and 52 were 95.5% (P < .01) and 71.9% (P < .01), respectively. When adjusted for number of sex partners and birth year, pooled VEs were 93.9% (P = .01) and 67.7% (P = .01) for HPV16 and 18 and HPV31, 45, and 52, respectively.
Conclusions
The bivalent HPV vaccine is highly effective against HPV16 and 18. Furthermore, significant cross-protection against HPV31, 45, and 52 was demonstrated and sustained up to 6 years after vaccination. These findings should reassure politicians about the VE of bivalent HPV vaccine in a Japanese population.
Keywords: HPV vaccine, HPV infection, cervical cancer, vaccine effectiveness, cross-protection, bivalent vaccine
Proactive recommendations for human papillomavirus (HPV) vaccines in Japan have been suspended. In this study, bivalent HPV vaccine is highly effective against HPV types 16 (HPV16) and 18, and significant cross-protection against HPV31, 45, and 52 was demonstrated. These findings should reassure politicians of vaccine effectiveness in a Japanese population.
Human papillomavirus (HPV) vaccines have been shown to be extremely effective in real-world prevention of infection with carcinogenic (ie, high-risk) HPV (HR-HPV), a necessary cause of cervical cancer [1–3]. The bivalent and quadrivalent vaccines contain virus-like particles that induce high-level antibody responses to HPV type 16 (HPV16) and 18, responsible for around 70% of cervical cancers globally [4]. In the bivalent vaccine clinical trials, some level of cross-protection was also shown against other HR-HPV types phylogenetically related to HPV16 and 18 [5]. These findings have been corroborated in 2 recent population-based studies, which showed sustained cross-protection against 4 other HR-HPV types: HPV31, 33, and 45 in Scotland [2] and HPV31, 45, and 52 in the Netherlands [6]. Together, these additional types could prevent a further 17% of cervical cancers globally [7].
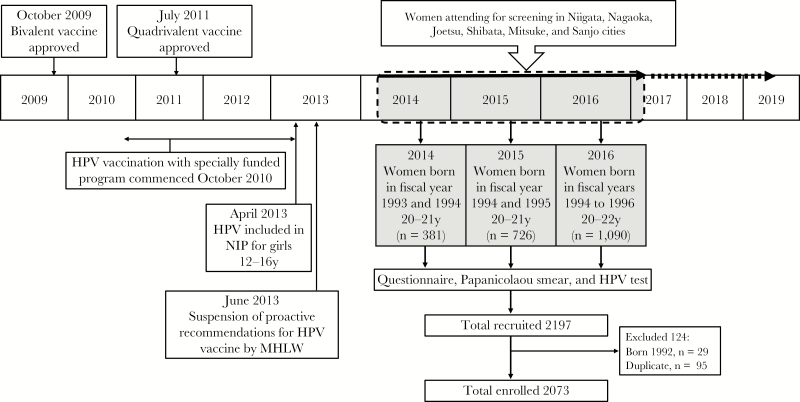
In Japan, the bivalent vaccine was licensed in October 2009 and the quadrivalent vaccine in July 2011 (Figure 1). From December 2010, a special fund was established whereby the national government paid 50% of the vaccine cost for girls aged 12–16 years if the local government contributed the remaining 50%. The initial uptake was so high that vaccination had to be partially suspended in girls who had not initiated the 3-dose course, because the government had not stockpiled sufficient doses of the vaccine [8]. This lack of foresight was sharply criticized in the Japanese mass media [9].
Figure 1.
Human papillomavirus (HPV) vaccination in Japan and the Niigata Study. In 2010, the Ministry of Health, Labor, and Welfare (MHLW) initiated an expedited promotion project for HPV vaccination, in which the national government would cover 50% of the total cost if local government also paid 50%. Public aid was gradually introduced in each municipality for girls aged 12–16 years. The cities of Niigata, Nagaoka, Joetsu, Shibata, and Mitsuke began providing public aid in 2010 for girls born in 1994 or later. Sanjo city began it in 2012 for girls born in 1996 or later. NIP, National Immunization Program.
Beginning in April 2013, both the bivalent and quadrivalent HPV vaccines were included in the Japanese National Immunization Program, which meant that the national government covered 100% of the cost for girls in the target age group of 12–16 years. Around the same time, one report of a junior high school student who was having difficulty walking and was unable to perform mathematical calculations after HPV vaccination was published in The Asahi Shimbun, one of the most influential newspapers in Japan [10, 11]. Following this, reports of adverse events were broadcast extensively on news programs despite no evidence that the vaccine had caused the symptoms [12]. In addition to motor disorders, reported symptoms included syncope, decreased level of consciousness, pyrexia, and widespread pain [13]. Then, in June 2013, only 2 months after the HPV vaccine had been introduced into the National Immunization Program, the Japanese Ministry of Health, Labor, and Welfare (MHLW) suspended proactive recommendations for the vaccine.
The Vaccine Adverse Reactions Review Committee (VARRC) investigating these adverse events continues to conclude there is no evidence to suggest a causal association between the HPV vaccine and reported symptoms. Furthermore, a recent study of 30000 vaccinated and unvaccinated women in Nagoya city reached the same conclusion [14]. However, although all data support the safety of the HPV vaccine, the Japanese government continues to suspend its proactive recommendations for it, and the suspension is now in its sixth year.
One immediate consequence of the suspension was that vaccination uptake plummeted from >70% in women eligible for free vaccination between October 2010 and March 2013, when the specially funded program was available, to <1% in women eligible for free vaccination after it was introduced into the National Immunization Program in April 2013 (Figure 1) [15, 16]. Since the incidence of and mortality from cervical cancer is increasing in Japanese women of reproductive age and because screening uptake is 30%–40% [17], the present situation puts Japanese women at risk of developing a highly preventable cancer [18].
A second consequence of the suspension has been a delay in the approval of licensing for the nonavalent HPV vaccine [19]. However, the most recent Japanese study on the distribution of HR-HPV genotypes in women with invasive cervical cancer (ICC) found the HPV16, 18, 31, 33, 45, and 52 were responsible for 47.7%, 23.5%, 2.0%, 2.7%, 0.7%, and 8.7% of cases, respectively, suggesting the bivalent vaccine could possibly prevent around 85% of invasive cervical cancers in Japan if cross-protection was confirmed [20].
Monitoring vaccine effectiveness (VE) in vaccination programs is essential to assess the impact of immunization. One of the reasons given by those opposing the HPV vaccine in Japan is that there are no VE data to show that the vaccines work in a Japanese population. Furthermore, apart from the United Kingdom and the Netherlands, Japan may be the only other country with substantial population-based coverage of the bivalent HPV vaccine to corroborate cross-protection of nonvaccine types, particularly HPV52, at the population level. Therefore, we investigated the VE of bivalent HPV vaccine against vaccine-targeted HPV types (ie, HPV16 and 18), as well as against HPV31, 33, 45, and 52, using data from the Niigata Clinical Group for Assessment after HPV Vaccination (NIIGATA) study.
MATERIALS AND METHODS
Study Design and Population
The Niigata study is an ongoing cross-sectional study recruiting women born after April 1993 who underwent cervical screening in 6 cities in Niigata Prefecture: Niigata, Nagaoka, Joetsu, Shibata, Mitsuke, and Sanjo. Niigata Prefecture is located on the northwest coast of Honshu, the largest island of Japan, and has a population of 2300000. The average annual income in Niigata is ¥5331000 ( $48500), similar to the average annual national income of ¥5382000 ($48000) [21]. The 6 cities included in the Niigata study account for around 70% of Niigata Prefecture’s total population and can be considered typical cities in Niigata Prefecture. Those born between April 1994 and March 2000 (fiscal years 1994–1999) were eligible for free HPV vaccination in the specially funded program that started in October 2010, where vaccine coverage was >70%. The cities of Niigata, Nagaoka, Joetsu, Shibata, and Mitsuke introduced the special funding in 2010 for girls born in 1994 or later, and Sanjo city began funding the program in 2012 for girls born in 1996 or later. This article presents findings of an interim analysis of data from women born between April 1993 and March 1997 and aged 20-22 years in fiscal years 1993–1996. Cervical cancer screening in Japan starts at age 20 years.
Immunization Status and Sexual History
Japan has no national vaccine registry, and official immunization records are managed independently by approximately 1700 municipalities [22]. Therefore, information about individual vaccination status, including date of immunization, number of doses received, and vaccine type, was obtained from municipal records archived at public health centers in each of the 6 cities in this study. Since it was hypothesized that municipal immunization records for some participants (ie, those who had been immunized in a different city or prefecture) would not be available, participants were also asked to self-report their HPV vaccine immunization status on a questionnaire. Additionally, in the questionnaire, information was also obtained on age at sexual debut and lifetime number of sex partners. For the latter, participants had to choose from the following 5 categories: 0, 1, 2–5, 6–9, and ≥10 sex partners.
HPV Testing
Residual samples from liquid-based cytologic analysis (SurePath BD Diagnostics, Sparks, MD) during cervical screening were collected. HPV genotyping was done with the Mebgen HPV kit (MBL, Nagoya, Japan) [23], using reverse sequence–specific oligonucleotide probe and Luminex xMAP (Austin, TX) technology. This assay detects 13 HR-HPV genotypes: HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59. All samples were also tested with Hybrid Capture 2 (HC2; Qiagen, Hilden, Germany), a nucleic acid hybridization assay with signal amplification that utilizes microplate chemiluminescent detection and screens for pooled infection with ≥1 of 13 HR-HPV genotypes (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59). For samples collected between April 2014 and December 2015, only those samples positive for the HC2 test underwent HPV genotyping. From January 2016 onward, all samples underwent HPV testing with both assays. When the Mebgen HPV assay result was equivocal, it was assigned to the HPV-negative group.
Statistical Analyses
Data were analyzed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria) [24]. Categorical data are presented as absolute numbers and percentages. Continuous data are presented as the mean values ± standard deviations. The Pearson χ2 test, the Fisher exact test, and the Student t test were used to evaluate differences in baseline characteristics between the vaccinated and unvaccinated population. Univariate and multivariate logistic regression analyses were conducted to assess VE. Models were adjusted for year of birth and lifetime number of sex partners. VE was calculated as 100 × [1 − odds ratio]; corresponding 95% confidence intervals (CIs) were also determine. A P value of <.05 was considered statistically significant.
Ethical Issues
Written, informed consent was obtained by all participants. HPV testing, which was not part of the Japanese screening program, was provided for free and participants were also given a ¥5000 ($50) gift card for taking part in the study. The study was approved by the Ethics Review Board at Niigata University Graduate School of Medical and Dental Science.
Role of the Funding Source
The sponsor of the present study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author (M. S.) had full access to all the data presented in the article and had final responsibility for the decision to submit the manuscript for publication. The present study is registered with the University Hospital Medical Information Network Clinical Trials Registry (trial number UMIN000026769).
RESULTS
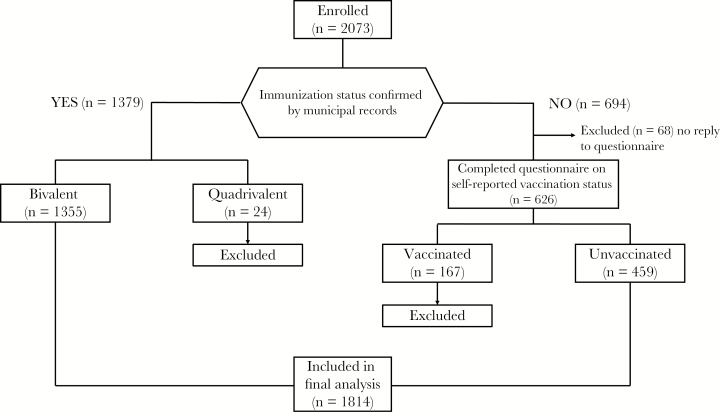
Enrollment of participants is shown in Figure 1. In total, 2197 women aged 20–22 years attending for screening and underwent a Pap smear, HPV testing, and completed the questionnaire. Of these, 124 women were excluded due to age or duplicate registration, leaving 2073 woman enrolled. Of those enrolled, immunization status was confirmed by municipal records in 1379 women and of these 1355 had received the bivalent vaccine (Figure 2). In the 694 women whose vaccination status could not be confirmed by official records, only those who reported they were unvaccinated (n = 459) were included. Therefore, 1814 women were included in the final analysis.
Figure 2.
Vaccination status of enrolled participants. This flow chart shows how vaccination status was classified in our study.
Basic characteristics of the population are shown in Table 1. The mean age (±SD) of participants was 20.5 ± 0.7 years and 20.7 ± 0.6 years in the vaccinated and unvaccinated groups, respectively. In the vaccinated group (1355 women), the mean age (±SD) at receipt of the first dose was 15.2 ± 0.9 years, and 95% of women (1294) had completed the 3-dose schedule. Furthermore, 82.6% of vaccinated women (1000) underwent vaccination before they were sexually active, and a further 7.0% were sexually active before they were received the first dose. Overall, a significantly higher proportion of women in the unvaccinated group were sexually active at the time of the survey, compared with those who were vaccinated (86.6% vs 79.2%; P < .01). Furthermore, women in the unvaccinated group were significantly more likely to have had a higher number of previous sex partners, with 20.3% in the unvaccinated group having ≥6 partners, compared with 12.9% in the vaccinated group (P < .01). Similarly, 16.1% of unvaccinated women were sexually active at ≤15 years of age, compared with 13.4% in the vaccinated group. The mean age (±SD) at sexual debut, however, was not statistically different between both groups (17.4 ± 2.0 years in the unvaccinated group and 17.9 ± 1.9 years in the vaccinated group).
Table 1.
Characteristics of the Sample Population
| Characteristic | Overall (n = 1814) | Vaccinated (n = 1355) | Unvaccinated (n = 459) | P |
|---|---|---|---|---|
| Age, y | 20.5 ± 0.7 | 20.5 ± 0.7 | 20.7 ± 0.6 | <.01c |
| Fiscal year of birth | ||||
| 1993 | 224 (12.3) | 0 (0.0) | 224 (48.8) | <.01d |
| 1994 | 686 (37.8) | 563 (41.5) | 123 (26.8) | |
| 1995 | 494 (27.2) | 418 (30.8) | 76 (16.6) | |
| 1996 | 410 (22.6) | 374 (27.6) | 36 (7.8) | |
| Age at first inoculation, y | 15.2 ± 0.9 | 15.2 ± 0.9 | NA | |
| Doses, no. | ||||
| 3 | 1294 (71.3) | 1294 (95.5) | NA | |
| 2 | 45 (2.5) | 45 (3.3) | NA | |
| 1 | 16 (0.9) | 16 (1.2) | NA | |
| Ever had intercoursea | ||||
| Yes | 1351 (81.2) | 958 (79.2) | 393 (86.6) | <.01e |
| No | 313 (18.8) | 252 (20.8) | 61 (13.4) | |
| Sex partners, lifetime no.a | ||||
| ≥10 | 118 (7.1) | 73 (6.0) | 45 (9.9) | <.01d |
| 6–9 | 131 (7.9) | 84 (6.9) | 47 (10.4) | |
| 2–5 | 645 (38.8) | 463 (38.3) | 182 (40.1) | |
| 1 | 457 (27.5) | 338 (27.9) | 119 (26.2) | |
| 0 | 313 (18.8) | 252 (20.8) | 61 (13.4) | |
| Age at sexual debut, ya | ||||
| ≤15 | 235 (14.1) | 162 (13.4) | 73 (16.1) | <.01d |
| 16–18 | 661 (39.7) | 468 (38.7) | 193 (42.5) | |
| ≥19 | 455 (27.3) | 328 (27.1) | 127 (28.0) | |
| NA | 313 (18.8) | 252 (20.8) | 61 (13.4) | |
| Overall | 17.4 ± 1.9 | 17.4 ± 1.9 | 17.3 ± 2.0 | .19c |
| Timing of sexual debuta | ||||
| Before vaccination | 85 (5.1) | 85 (7.0) | NA | |
| Same year as vaccination | 125 (7.5) | 125 (10.3) | NA | |
| After vaccinationb | 1000 (60.1) | 1000 (82.6) | NA |
Data are no. (%) of participants or mean ±SD
Abbreviation: NA, not applicable.
aData are for 1210 vaccinated subjects and 454 unvaccinated subjects who answered questions about sexual behavior.
bData include subjects who were sexually naive.
cBy the t test.
dBy the χ2 test.
eBy the Fisher exact test.
The crude prevalence of HPV genotypes is shown in Table 2 and Supplementary Figure 1. The vaccinated group includes all women who had received at least 1 dose of the bivalent vaccine. Overall, 226 participants (12.5%) were infected with a HR-HPV type. HPV52, 56, and 58 were the 3 mostly commonly detected HR-HPV types, at 2.8% (in 50 women), 2.4% (in 43), and 2.0% (in 36). Only 13 women (0.7%) had HPV16 or 18 infection. In the univariate model, the pooled VE against HPV16 and 18 infections was statistically significant, at 89.8% (95% CI, 63.9%–97.2%; P < .01); the VE against HPV16 alone was 87.3% (95% CI, 52.3%–96.6%; P < .01) and that for HPV31 alone was 79.7% (95% CI, 15.3%–95.1%; P = .03). While the VE was 100% for HPV18, it was not statistically significant (P = .06), owing to the low overall number of infections. In the multivariate model adjusted for year of birth, the pooled VE against HPV16 and 18 infections increased to 91.9% (95% CI, 66.8%–98.0%; P < .01) and was 88.8% for HPV16 alone (95% CI, 51.0%–97.5%; P < .01). In this model, the VE against HPV52 was 48.3%, (95% CI, −1.0%–73.6%; P = .05).
Table 2.
Crude Prevalence of and Vaccine Effectiveness (VE) Against Human Papillomavirus (HPV) Infection
| Variable | Overall, No. (%) (n = 1814) |
Vaccinated, No. (%) (n = 1355) |
Unvaccinated, No. (%) (n = 459) |
Univariate Analysis | Multivariate Analysisc | ||||
|---|---|---|---|---|---|---|---|---|---|
| PR (95% CI) | VE, % (95% CI) | P b | aOR (95% CI) | aVE, % (95% CI) | P | ||||
| High-risk HPVa | 226 (12.5) | 156 (11.5) | 70 (15.3) | 0.76 (.58–.98) | 24.5 (2.0–41.9) | .04d | 0.72 (.51–1.02) | 27.9 (−2.0–49.0) | .06 |
| HPV16/18 | 13 (0.7) | 3 (0.2) | 10 (2.2) | 0.10 (.03–.37) | 89.8 (63.9–97.2) | <.01d | 0.08 (.02–.33) | 91.9 (66.8–98.0) | <.01d |
| HPV16 | 11 (0.6) | 3 (0.2) | 8 (1.7) | 0.13 (.03–.48) | 87.3 (52.3–96.6) | <.01d | 0.11 (.03–.49) | 88.8 (51.0–97.5) | <.01d |
| HPV18 | 2 (0.1) | 0 (0.0) | 2 (0.4) | 0.00 … | 100.0 … | .06 | 0.00 … | 100.0 … | 1.00 |
| HPV31 | 8 (0.4) | 3 (0.2) | 5 (1.1) | 0.20 (.05–.85) | 79.7 (15.3–95.1) | .03d | 0.27 (.05–1.48) | 73.4 (−48.0–95.2) | .13 |
| HPV33 | 9 (0.5) | 7 (0.5) | 2 (0.4) | 1.19 (.25–5.69) | −18.6 (−468.7–75.3) | 1.00 | 1.30 (.22–7.86) | −30.0 (−686.0–78.4) | .77 |
| HPV35 | 5 (0.3) | 5 (0.4) | 0 (0.0) | ∞ … | −∞ … | .34 | >100 … | Less than −100.0 … | .99 |
| HPV39 | 22 (1.2) | 17 (1.3) | 5 (1.1) | 1.15 (.43–3.10) | −15.2 (−210.4–57.3) | 1.00 | 1.61 (.50–5.18) | −61.0 (−418.0–50.2) | .43 |
| HPV45 | 3 (0.2) | 1 (0.1) | 2 (0.4) | 0.17 (.02–1.86) | 83.1 (−86.4–98.5) | .16 | 0.14 (.01–1.95) | 86.5 (−95.0–99.1) | .14 |
| HPV51 | 21 (1.2) | 15 (1.1) | 6 (1.3) | 0.85 (.33–2.17) | 15.3 (−117.0–66.9) | .62 | 0.64 (.22–1.85) | 36.3 (−85.0–78.0) | .41 |
| HPV52 | 50 (2.8) | 33 (2.4) | 17 (3.7) | 0.66 (.37–1.17) | 34.2 (−16.9–63.0) | .14 | 0.52 (.26–1.01) | 48.3 (−1.0–73.6) | .05 |
| HPV56 | 43 (2.4) | 33 (2.4) | 10 (2.2) | 1.12 (.56–2.25) | −11.8 (−125.0–44.5) | 1.00 | 1.51 (.66–3.46) | −51.0 (−246.0–34.5) | .34 |
| HPV58 | 36 (2.0) | 27 (2.0) | 9 (2.0) | 1.02 (.48–2.15) | −1.6 (−114.5–51.8) | 1.00 | 0.73 (.32–1.70) | 26.8 (−70.0–68.5) | .47 |
| HPV59 | 24 (1.3) | 20 (1.5) | 4 (0.9) | 1.69 (.58–4.93) | −69.4 (−392.9–41.8) | .48 | 1.52 (.47–4.98) | −52.0 (−398.0–53.4) | .49 |
| HPV68 | 15 (0.8) | 12 (0.9) | 3 (0.7) | 1.36 (.38–4.78) | −35.5 (−378.0–61.6) | 1.00 | 1.60 (.38–6.80) | −60.0 (−580.0–62.1) | .52 |
Abbreviations: aOR, adjusted odds ratio; aVE, adjusted VE; CI, confidence interval; PR, prevalence ratio.
aDefined as HPV16/18/31/33/35/39/45/51/52/56/58/59/68 (detected by the HCII assay).
bBy the Fisher exact test.
cData are adjusted for fiscal year of birth.
d P <.05.
The VE among women who were sexually naive at initiation of the HPV vaccine schedule is shown in Table 3. The pooled VEs against HPV16 and 18 and against HPV 31, 45, and 52 were statistically significant, at 95.5% (95% CI, 64.6%–99.4%; P < .01) and 71.9% (95% CI, 44.4%–85.8%; P < .01), respectively. Similarly, the VE against HPV types 16, 31, and 52 individually was also measured, with values of 94.3% (95% CI, 54.8%–99.3%; P < .01), 100% (P = .01), and 63.1% (95% CI, 24.0%–82.1%; P = .01), respectively. The VEs against HPV 18 and HPV 45 individually were 100% but did not reach statistical significance owing to the small sample size.
Table 3.
Vaccine Effectiveness (VE) Against Human Papillomavirus (HPV) Infection in 1454 Study Participants Who Were Vaccinated Before Sexual Debut
| Variable | Vaccinated, No. (%) (n = 1000) |
Unvaccinated, No. (%) (n = 454) |
Univariate Analysis | ||
|---|---|---|---|---|---|
| PR (95% CI) | VE, % (95% CI) | P b | |||
| High-risk HPVa | 79 (7.9) | 69 (15.2) | 0.52 (.38–.70) | 48.0 (29.6–61.6) | <.01c |
| HPV16/18 | 1 (0.1) | 10 (2.2) | 0.05 (.01–.35) | 95.5 (64.6–99.4) | <.01c |
| HPV31/45/52 | 13 (1.3) | 21 (4.6) | 0.28 (.14–.56) | 71.9 (44.4–85.8) | <.01c |
| HPV16 | 1 (0.1) | 8 (1.8) | 0.06 (.01–.45) | 94.3 (54.8–99.3) | <.01c |
| HPV18 | 0 (0) | 2 (0.4) | 0.00 … | 100.0 … | .09 |
| HPV31 | 0 (0) | 4 (0.9) | 0.00 … | 100.0 … | .01c |
| HPV33 | 2 (0.2) | 2 (0.4) | 0.45 (.06–3.21) | 54.6 (−221.3– 93.6) | .59 |
| HPV35 | 2 (0.2) | 0 (0.0) | ∞ … | −∞ … | 1.00 |
| HPV39 | 8 (0.8) | 5 (1.1) | 0.73 (.24–2.21) | 27.4 (−120.8–76.1) | .55 |
| HPV45 | 0 (0) | 2 (0.4) | 0.00 … | 100.0 … | .09 |
| HPV51 | 6 (0.6) | 6 (1.3) | 0.45 (.15–1.40) | 54.6 (−40.0–85.3) | .20 |
| HPV52 | 13 (1.3) | 16 (3.5) | 0.37 (.18–.76) | 63.1 (24.0–82.1) | .01c |
| HPV56 | 17 (1.7) | 9 (2.0) | 0.86 (.39–1.91) | 14.2 (−90.9–61.5) | .67 |
| HPV58 | 14 (1.4) | 9 (2.0) | 0.71 (.31–1.62) | 29.4 (−62.0–69.2) | .36 |
| HPV59 | 12 (1.2) | 4 (0.9) | 1.36 (.44–4.20) | −36.2 (−320.0–55.8) | .79 |
| HPV68 | 7 (0.7) | 3 (0.7) | 1.06 (.28–4.08) | −5.9 (−307.8–72.5) | 1.00 |
Abbreviations: CI, confidence interval; PR, prevalence ratio.
aDefined as HPV16/18/31/33/35/39/45/51/52/56/58/59/68 (detected by the HCII assay).
bBy the Fisher exact test.
c P <.05.
Table 4 shows the VE among women sexually naive at initiation of the HPV vaccine schedule after adjustment for lifetime number of sex partners and birth year. The pooled adjusted VE against HPV16 and 18 was 93.9% (95% CI, 44.8%–99.3%; P = .01) and that against HPV 31, 45, and 52 was 67.7% (95% CI, 24.9%–86.1%; P = .01). The VE remained statistically significant.
Table 4.
Adjusted Vaccine Effectiveness (aVE) Against Human Papillomavirus (HPV) Infection in 1454 Study Participants Who Were Vaccinated Before Sexual Debut
| Variable | HPV Positive, No. (%) | Multivariate Analysisa | ||
|---|---|---|---|---|
| aOR (95% CI) | aVE, % (95% CI) | P | ||
| HPV16/18 | ||||
| Unvaccinated | 10 (2.2) | 1 (reference) | ||
| Vaccinated | 1 (0.1) | 0.06 (.01–.55) | 93.9 (44.8–99.3) | .01 |
| HPV31/45/52 | ||||
| Unvaccinated | 21 (4.6) | 1 (reference) | ||
| Vaccinated | 13 (1.3) | 0.32 (.14–.75) | 67.7 (24.9–86.1) | .01 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval.
aAdjusted for number of sex partners and fiscal year of birth.
DISCUSSION
To ascertain the impact of the Japanese HPV vaccination program on vaccine-type HPV infections, as well as to investigate any cross-protection for nontargeted vaccine types with the bivalent vaccine, we analyzed data from 1814 Japanese women born between 1 April 1993 and 31 March 1997 enrolled in the Niigata study.
Effect estimates from observational studies against nonvaccine HPV types are still limited. However, similar to reports from Scotland [2] and the Netherlands [6], we found that the bivalent HPV VE was not only extremely high against HPV16 and 18, but also conferred significant cross-protection against HPV31, 45, and 52. While HPV45, which is phylogenetically related to HPV18 and associated with adenocarcinoma, is quite rare in Japan, HPV52 is not. HPV52 is more commonly found in Asian populations and, after HPV16, the second most commonly detected genotype in high-grade cervical lesions in Japan. Azuma et al found that HPV52 was responsible for 27.4% and 26.0% of cervical intraepithelial neoplasia grade 2 (CIN2) and CIN3 lesions [20], respectively, while Onuki et al found it was responsible for 17.5% of CIN2/3 lesions [25]. In invasive cervical cancers (ICCs), it is the third most commonly detected HPV genotype after HPV16 and HPV18, accounting for around 8.5% of ICCs [20, 25]. In the study by Azuma et al, HPV16, 18, 31, 45, and 52 were found in around 75.6%, 91.6%, and 82.6% of CIN2, CIN3, and ICC cases, respectively, in Japan [20]. This suggests that, in addition to offering excellent protection against HPV16 and 18, the bivalent HPV vaccine may have a significant clinical role to play in reducing high-grade disease and cancerous lesions not associated with nonvaccine-targeted types in Japan, especially when the nonavalent vaccine, estimated to prevent around 90% of ICCs, as well as most cases of genital warts, is not available in Japan.
In the Patricia phase 3 trial of the bivalent vaccine, cross-protection was reported against persistent HPV31, 33, 45, 51, and 52 infections and against incident HPV35 infection [26]. A recent Scottish study also found significant cross-protection against HPV33 [2]. However, as with the Costa Rica vaccine trial and the previously cited Dutch study by Woestenberg et al [6, 27], we found no statistically significant protection against HPV33.
In the aforementioned Dutch study, a negative VE against HPV59, which was just statistically significant in a sensitivity analysis restricted to women who reported 3 doses versus no vaccination, was observed. The phenomenon of increased detection is referred to as unmasking. Another possible explanation for a negative VE is type replacement, which means that an HPV type is taking over the vacated ecological niche of the vaccine and cross-protective types [28]. In the present study, a negative VE against HPV59 was also observed, but it was not statistically significant in either the univariate analysis (VE, −69.4%; 95% CI, −392.9%–41.8%; P = .48) or the multivariate analysis (VE, −52.0%; 95% CI, −398.0%–53.4%; P = .49).
Compared with the bivalent vaccine, the quadrivalent vaccine has more-limited cross-protection [29], mainly seen against HPV31 [30]. In the women recruited for the present study, the number of women vaccinated with the quadrivalent vaccine was small (n = 24), so we were unable to perform any meaningful investigations for the quadrivalent vaccine. Furthermore, since only 4.5% of participants had received ≤2 doses of the vaccine, we were unable to investigate cross-protection associated with receipt of <3 doses. However, with a mean age at vaccination initiation of 15 years, we were able to demonstrate that the cross-protection seen in the present study lasts for at least 5–6 years.
Monitoring VE in vaccination programs is essential to assess the impact of immunization. One of the reasons given by those opposing the HPV vaccine in Japan is that there are no reliable VE data to show that the vaccines work in a Japanese population. Three studies to date, which reported a significant reduction in abnormal cytologic findings in vaccinated populations as compared to nonvaccinated populations, relied on self-reporting, which is subject to recall bias, and misclassification of vaccination status by self-report may influence the reported VE and safety [31–33]. When public confidence in a vaccine is low, it is essential that data on vaccination status are as accurate as possible. Japan has no national vaccine registry system and no national screening registry. Consequently, it is difficult to obtain accurate information on individual vaccination status and even more difficult to link vaccine status with screening results. In one recent study, comparing self-reported vaccination status and official municipal records, 20.6% of Japanese women aged 20–22 years incorrectly reported their HPV vaccination status [22].
One of the main strengths of the present study is the fact that we used official vaccination records to ascertain vaccination status, as well as the date of vaccination, type of vaccine administered, and number of doses administered. Furthermore, we were also able to perform subanalyses that included only women who reported being sexually naive at vaccination and to adjust for potential confounders, such as lifetime number of sex partners, albeit from self-reported information.
This study also has some limitations that must be addressed. The main limitation is that, for the first 20 months of the study, HPV genotyping was only performed on samples from women who had a positive result of an HC2 test. HC2 is a clinical, HPV-based assay used for cervical screening, and the cutoffs are set to detect only HPV infections that are clinically significant. The Mebgen HPV kit is a more sensitive assay and may have detected infections in women for whom the HC2 assay yielded negative results. Therefore, referring only HC2-positive women for genotyping may have led to an underestimation of the HR-HPV prevalence. A second limitation is that most women in the present study were vaccinated according to the 3-dose schedule, as this was and remains the guideline for HPV vaccination in Japan. Therefore, the results might not be generalizable to a schedule involving <3 doses. A third limitation is the small number of HPV-infected women in this study. However, as mentioned in Materials and Methods, this is an interim analysis, and we hope to have data on >3500 women in the final study. A final limitation is the fact that the VE against cervical abnormalities was not evaluated in the present study. However, studies from the United Kingdom and Australia, which started their national immunization programs 2–3 years before Japan, have shown that if you can reduce the infection burden, you will reduce the disease burden [34–36]. The woman in the present study are being followed up, and the results of cervical and histological analyses of all registrants will be presented in future studies.
In conclusion, this is the first report of the VE against vaccine-targeted infection in the Japanese national HPV immunization program that is based on verified immunization data. In line with international data, we have shown a high VE of the bivalent vaccine against vaccine-targeted HR-HPV types (ie, HPV16 and 18) and significant cross-protection against pooled HR-HPV types 31, 45, and 52, which are associated with an additional 10% of ICCs in Japan. This means that the bivalent vaccine may be able to prevent around 82% of ICCs in Japan. We hope the data presented in this article can convince Japanese politicians that the HPV vaccine is indeed effective in a Japanese population and encourage them to reinstate proactive recommendations for the vaccine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr Tomomi Egawa-Takata, Dr Akiko Morimoto, Dr Yusuke Tanaka, Ms Asami Yagi, Ms Yuka Watanabe, Ms Sachiko Ono, Ms Anna Ishida, and the administrators of Niigata, Nagaoka, Shibata, Sanjo, Joetsu, and Mitsuke cities for their support in conducting the survey.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Health and Labor Sciences Research Grant (26272001) and the Japanese Agency for Medical Research and Development (AMED) under grant number JP15ck0106103 and JP17ck0106369.
Potential conflicts of interest. T. E. received lecture fees from GlaxoSmithKline/Japan Vaccine and Merck Sharp and Dohme, as well as research funds from Merck Sharp and Dohme. Y. U. received lecture fees from GlaxoSmithKline/Japan Vaccine and Merck Sharp and Dohme, as well as research funds from Merck Sharp and Dohme. E. M. received honoraria and lecture fees from GlaxoSmithKline/Japan Vaccine and Merck Sharp and Dohme, as well as grants from Merck Sharp and Dohme. M. H. received lecture fees from GlaxoSmithKline/Japan Vaccine. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Drolet M, Bénard É, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017; 17:1293–302. [DOI] [PubMed] [Google Scholar]
- 3. Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus types among 18- to 35-year old Australian women 9 years following implementation of vaccination. J Infect Dis 2018; 217:1590–600. [DOI] [PubMed] [Google Scholar]
- 4. Muñoz N, Bosch FX, de Sanjosé S, et al. ; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–27. [DOI] [PubMed] [Google Scholar]
- 5. Wheeler CM, Castellsagué X, Garland SM, et al. ; HPV PATRICIA Study Group Cross-protective efficacy of HPV-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by non-vaccine oncogenic HPV types: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol 2012; 13:100–10. [DOI] [PubMed] [Google Scholar]
- 6. Woestenberg PJ, King AJ, van Benthem BHB, et al. Bivalent vaccine effectiveness against type-specific HPV positivity: evidence for cross-protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis 2018; 217:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arbyn M, Tommasino M, Depuydt C, Dillner J. Are 20 human papillomavirus types causing cervical cancer?J Pathol 2014; 234:431–5. [DOI] [PubMed] [Google Scholar]
- 8. Hanley SJ, Yoshioka E, Ito Y, et al. Acceptance of and attitudes towards human papillomavirus vaccination in Japanese mothers of adolescent girls. Vaccine 2012; 30:5740–7. [DOI] [PubMed] [Google Scholar]
- 9. The cervical cancer vaccine in short supply. Relaxation of the target-age group by the MHLM. The Nikkei 7 March 2011. https://www.nikkei.com/article/DGXNASDG07035_X00C11A3CR8000/. Accessed 30 July 2018. [Google Scholar]
- 10. The cervical cancer vaccine. A junior high school student showed serious adverse reactions. The Asahi Shimbun. 26 March 2013. http://www.asahi.com/area/tokyo/articles/MTW1303261300002.html. Accessed 30 July 2018. [Google Scholar]
- 11. Tsuda K, Yamamoto K, Leppold C, et al. Trends of media coverage on human papillomavirus vaccination in Japanese newspapers. Clin Infect Dis 2016; 63:1634–8. [DOI] [PubMed] [Google Scholar]
- 12. Larson HJ, Wilson R, Hanley S, Parys A, Paterson P. Tracking the global spread of vaccine sentiments: the global response to Japan’s suspension of its HPV vaccine recommendation. Hum Vaccin Immunother 2014; 10:2543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yagi A, Ueda Y, Kimura T. A behavioral economics approach to the failed HPV vaccination program in Japan. Vaccine 2017; 35:6931–3. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki S, Hosono A. No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: results of the Nagoya study. Papillomavirus Res 2018; 5:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanley SJ, Yoshioka E, Ito Y, Kishi R. HPV vaccination crisis in Japan. Lancet 2015; 385:2571. [DOI] [PubMed] [Google Scholar]
- 16. Sekine M, Kudo R, Adachi S, et al. Japanese crisis of HPV vaccination. Int Pathol Clin Res 2016; 2:039. [Google Scholar]
- 17. Motoki Y, Mizushima S, Taguri M, et al. Increasing trends in cervical cancer mortality among young Japanese women below the age of 50 years: an analysis using the Kanagawa population-based Cancer Registry, 1975–2012. Cancer Epidemiol 2015; 39:700–6. [DOI] [PubMed] [Google Scholar]
- 18. Yagi A, Ueda Y, Egawa-Takata T, et al. Realistic fear of cervical cancer risk in Japan depending on birth year. Hum Vaccin Immunother 2017; 13:1700–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chatterjee A. The next generation of HPV vaccines: nonavalent vaccine V503 on the horizon. Expert Rev Vaccines 2014; 13:1279–90. [DOI] [PubMed] [Google Scholar]
- 20. Azuma Y, Kusumoto-Matsuo R, Takeuchi F, et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grade 2/3 and invasive cervical cancer in Japanese women. Jpn J Clin Oncol 2014; 44:910–7. [DOI] [PubMed] [Google Scholar]
- 21. The 2014 National Survey of Family Income and Expenditure. http://www.stat.go.jp/data/zensho/2014/index.html. Accessed 30 July 2018. [Google Scholar]
- 22. Yamaguchi M, Sekine M, Kudo R, et al. Differential misclassification between self-reported status and official HPV vaccination records in Japan: implications for evaluating vaccine safety and effectiveness. Papillomavirus Res 2018; 6:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozaki S, Kato K, Abe Y, et al. Analytical performance of newly developed multiplex human papillomavirus genotyping assay using Luminex xMAP™ technology (Mebgen™ HPV Kit). J Virol Methods 2014; 204:73–80. [DOI] [PubMed] [Google Scholar]
- 24. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Onuki M, Matsumoto K, Satoh T, et al. Human papillomavirus infections among Japanese women: age-related prevalence and type-specific risk for cervical cancer. Cancer Sci 2009; 100:1312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Struyf F, Colau B, Wheeler CM, et al. ; HPV PATRICIA Study Group Post hoc analysis of the PATRICIA randomized trial of the efficacy of human papillomavirus type 16 (HPV-16)/HPV-18 AS04-adjuvanted vaccine against incident and persistent infection with nonvaccine oncogenic HPV types using an alternative multiplex type-specific PCR assay for HPV DNA. Clin Vaccine Immunol 2015; 22:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herrero R, Castle PE, Schiffman M, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis 2005; 191:1796–807. [DOI] [PubMed] [Google Scholar]
- 28. Tota JE, Struyf F, Merikukka M, et al. Evaluation of type replacement following HPV16/18 vaccination: pooled analysis of two randomized trials. J Natl Cancer Inst 2017; 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev 2018; 5:Cd009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown DR, Kjaer SK, Sigurdsson K, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis 2009; 199:926–35. [DOI] [PubMed] [Google Scholar]
- 31. Ozawa N, Ito K, Tase T, Metoki H, Yaegashi N. Beneficial effects of human papillomavirus vaccine for prevention of cervical abnormalities in Miyagi, Japan. Tohoku J Exp Med 2016; 240:147–51. [DOI] [PubMed] [Google Scholar]
- 32. Tanaka H, Shirasawa H, Shimizu D, et al. Preventive effect of human papillomavirus vaccination on the development of uterine cervical lesions in young Japanese women. J Obstet Gynaecol Res 2017; 43:1597–601. [DOI] [PubMed] [Google Scholar]
- 33. Konno R, Konishi H, Sauvaget C, Ohashi Y, Kakizoe T. Effectiveness of HPV vaccination against high grade cervical lesions in Japan. Vaccine 2018. [DOI] [PubMed] [Google Scholar]
- 34. Pollock KG, Kavanagh K, Potts A, et al. Reduction of low- and high-grade cervical abnormalities associated with high uptake of the HPV bivalent vaccine in Scotland. Br J Cancer 2014; 111:1824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377:2085–92. [DOI] [PubMed] [Google Scholar]
- 36. Gertig DM, Brotherton JM, Budd AC, Drennan K, Chappell G, Saville AM. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med 2013; 11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.