Abstract
Increasing number of patients with high body-mass index (BMI) are encountered in the orthodontic clinic with the growing prevalence of obesity and overweight worldwide. Some clinical studies found that the rate of orthodontic tooth movement (OTM) in obese patients decreased. However, how obesity can impact OTM has not been determined yet. Here, we used the high-fat diet (HFD) induced obese mouse model to translate this clinical problem to the basic research, and back to exploring the potential clinical applications. C57BL/6J mice were fed with high-fat diet (HFD) for 5 weeks to induce obesity and orthodontic nickel-titanium springs were applied to the upper first molars to establish OTM model. The serum level of leptin was tested by ELISA. Mouse macrophage cell line RAW264.7 cells were used as osteoclast progenitor cells stimulated by sRANKL with the presence or absence of letpin in vitro. TRAP staining was used to detect osteoclasts. Leptin was administrated intraperitoneally in mice to determine whether it can affect OTM in vivo. In obese mice, we found that OTM was attenuated and the number of osteoclasts decreased with the elevated serum level of leptin. Mechanically, we confirmed that leptin inhibited osteoclastogenesis and osteoclast functional genes expression. To translate our findings back to potential applications, we then revealed the administration of leptin could decrease OTM in wild type mice along with the decreased number of TRAP-positive osteoclasts. Taken together, these results demonstrated that the elevated level of leptin in obese mice was able to inhibit osteoclastogenesis and decrease OTM. Administration of leptin could inhibit molar mesial movement and possessed the potential to be a clinical anchorage reinforcement method.
Keywords: Obesity, leptin, orthodontic tooth movement, osteoclastogenesis, bone remodeling, mice
Introduction
The number of obese patients encountered in orthodontic treatment are becoming more common as the incidence of obesity and overweight is increasing worldwide [1,2]. High body mass index (BMI, the weight divided by the square of the height) is the main characteristic of obesity. Epidemiologic studies have indicated that high BMI as a risk factor for several systemic diseases, such as cardiovascular disease, diabetes mellitus, chronic kidney diseases, many cancers and several musculoskeletal disorders [3-9].
Moreover, recent studies have indicated that force-induced orthodontic tooth movement (OTM) was not only a local reaction within the periodontal tissue, but could also induce systemic reaction in this process [10-15]. Interestingly, several clinical studies have suggested that the OTM was influenced in patients with high BMI [16-18]. However, the precise effects and underlying mechanism has yet been determined. Therefore, the objective of this study is to translate this clinical problem to a basic study. By using high-fat diet (HFD) induced obese mouse model, we try to determine whether obesity can affect OTM, and then explore the possible mechanism. Moreover, since the level of circulating leptin, which is a cytokine-like hormone secreted by the adipocytes, was found elevated among population with high BMI [19], whether it can influence OTM also remains to be elucidated.
In this preliminary translational study, we found that obese mouse had decreased OTM rate with the elevated serum level of leptin. Mechanistically, we revealed that leptin could inhibit osteoclasts generation and function through the leptin receptor.
Materials and methods
Animals and treatment
Three-week-old male C57BL/6 mice (Weitong Lihua Experimental Animal Center, Beijing, China) were fed with high-fat diet (HFD, 45% kcal from fat) (Research Diets Inc., New Brunswick, NJ, USA) or normal diet (10% kcal from fat) for 5 weeks. Mice were housed in cages under controlled conditions of temperature (23°C ± 1°C), humidity (50% ± 10%), and a 12:12-hour light-dark cycle. They were given tap water and standard laboratory chow. Orthodontic nickel-titanium coiled springs (0.2 mm in thickness, 1 mm in diameter, 5 mm in length; Smart Technology, Beijing, China) were ligated between the left maxillary first molar and central incisors of mice. The springs were activated to deliver an orthodontic force of 30 g. The model was modified from previously reported methods and the reliability was evaluated [20] (Figure S1).
Leptin (40 μg/mouse, PeproTech, USA) or vehicle (PBS) were injected intraperitoneally every other day since one day before force application. Mice were sacrificed by pentobarbital sodium overdose. The animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Peking University (LA2013-92).
Measurement of OTM and MicroCT analysis
We used a stereo microscope (SWZ1000, Nikon, Japan) to record the occlusal surface of the maxillae. The OTM distance is measured between the midpoint of distal-marginal ridge of the first and second molar as previously reported [14,15]. Then, the measurement of OTM distance was done by using Image J software (1.37v, National Institutes of Health). The final result came from the average of 2 measurements.
A micro-computed tomography (MicroCT, Skyscan1174, Bruker micro CT, Belgium) was used to scan the maxillae of mice. To analyze the bone mass indicators, we choose the furcation of the first molar as the measurement area due to its reproducible morphological landmarks.
Tartrate-resistant acid phosphatase staining
Mice maxillae tissues were collected and fixed in 4% paraformaldehyde, then demineralized in 15% EDTA and embedded in paraffin. The transverse cutting method was employed to get serial sections from the corresponding group at a thickness of 4 μm. Then the sections were deparaffinized to perform tartrate-resistant acid phosphatase (TRAP) test using a leukocyte acid phosphatase kit (387A, Sigma, USA) according to the manufacturer’s protocol. TRAP-positive multinucleated (> 3 nuclei) cells that attached to the alveolar bone surface mesial to the distal buccal roots were counted.
Cell culture and treatment
The murine macrophage RAW264.7 cell line (National Infrastructure of Cell Line Resource, Beijing, China) were cultured in DMEM containing 10% FBS supplemented with antibiotics (100 U/ml of penicillin and 100 μg/ml of streptomycin). Cells were cultured at 37°C in a 5% CO2 incubator. Osteoclasts differentiation was induced by sRANKL (R&D Systems, USA) at concentration of 10 ng/ml with the presence or absence of leptin (100 ng/ml, PeproTech, USA) for 5 days. To investigate the influence to the osteoclast functional genes expression, RAW264.7 cells were treated with various doses of leptin (0-100 ng/ml) with the presence of sRANKL (10 ng/ml) for 24 h.
Immunofluorescence staining
RAW264.7 cells were incubated with the primary antibodies against leptin receptor (LepR, 1:100, Santa Cruz, USA) to detect the expression of leptin receptor, and then washed, incubated with FITC-conjugated secondary antibodies (Zhongshan Golden Bridge Biotechnology, Beijing, China). Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI). Confocal microscopic images were processed with LSM 5 Release 4.2 software after acquisition by a laser-scanning microscope (LSM 510; Zeiss, Germany).
Enzyme-linked immunosorbent assay (ELISA)
Mice serum samples obtained from the experimental and control group were centrifuged at 1000 r/min for 10 min to collect the supernatant and stored at -80°C. Concentration of leptin in mice serum was measured by using a mouse leptin ELISA kit (FineTest) according to the manufacturer’s instructions. The measurements were repeated three times.
Quantitative real-time polymerase chain reaction (q-PCR)
Total RNA was extracted from cell lysate the with TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. Reverse transcription and real-time PCR were performed following previously described protocol [21]. The sequences of primers were from previous reported studies [22,23] and commercially synthesized as listed below:
Mouse TRAP sence/antisence: 5’-CACTCCCAACCCTGAGATTTGT-3’/5’CATCGTCTGCACGGTTCTG-3’. Mouse CTSK sence/antisence: 5’-CAGCAGAACGGAGGCATTGA-3’/5’-CCTTTGCCGTGGCGTTATAC-3’. Mouse RANK sence/antisence: 5’-CAGCAGCCAAGGAGGACTAC-3’/5’-ACATAGCCCACACCGTTCTC-3’. Mouse β-actin sence/antisence: 5’-CACGATGGAGGGGCCGGACTCATC-3’/5’-TAAAGACCTCTATGCCAACACAGT-3’.
Statistical analysis
All data were presented as the means ± SD, and statistical analysis was carried out by Student’s T-test or one-way ANOVA followed by the least significant difference (LSD) multiple-comparison test. All statistical analyses were performed with GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA); P<0.05 was considered to be statistically significant.
Results
Force-induced OTM is attenuated in HFD induced obese mice
In order to evaluate the effect of obesity on OTM, we first established an obese mouse model by feeding 3-week old mice high-fat diet (HFD) for 5 weeks (Figure 1A). The body weight was significantly elevated in obese mice (Figure S2). After 3 days, 5 days and 7 days of force application, the mice were sacrificed for further evaluation (Figure 1A). The mouse model of OTM showed that the upper first molars were driven mesially by nickel-titanium open coil springs (Figure 1B).
Figure 1.
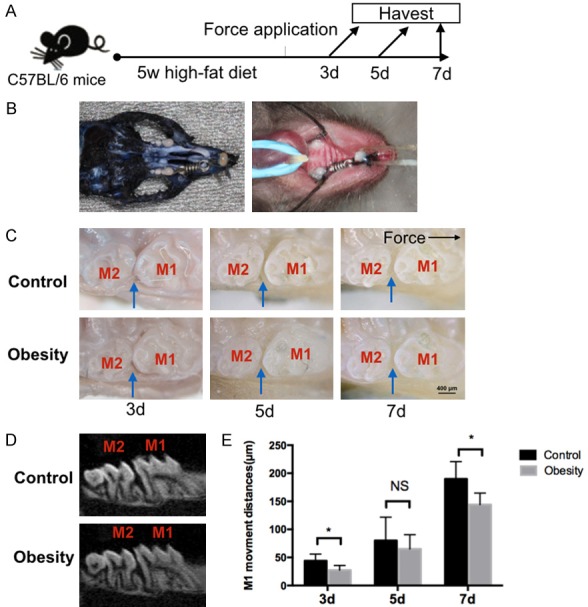
Obesity and orthodontic tooth movement models in mice were established. A. Schedule of the experiment. After 5 weeks of high-fat diet, orthodontic force was applied to mice. Mice were sacrificed at 3 day, 5 day and 7 day after force application. B. The orthodontic tooth movement model in mice. The maxillary left first molars were moved mesially by closed-coil springs. C. Representative intra-oral photograph of tooth movement model in obese and control mice after 3 days, 5 days and 7 days of force application. D. MicroCT representative image of tooth movement model in obese and control mice after 7 days of force application. E. Amount of orthodontic tooth movement on 3 day, 5 day and 7 day after force application in obese and control mice. The black arrow indicated direction of tooth movement. The blue arrows indicated tooth movement distances, M1: first molar. M2: second molar; n=5 in each group, error bars, means ± SD; scale bar: 400 μm; *P<0.05; NS: not statistically significant.
As shown in the intra-oral photograph, compared with the control group, we found that the amount of OTM was decreased in the obese mice group after 3 days and 7 days of force application, but no significant difference was noted after 5 days of force application. The MicroCT scan data also demonstrated that there was a reduced OTM in the obese mice after 7 days of force application when comparing to the control group (Figure 1C-E).
We next examined whether the force-induced osteoclastogenesis was influenced in obese mice during the process of OTM. Consistent with the amount of OTM results, the TRAP staining of histological sections showed that the number of osteoclasts was significantly reduced after 3 days and 7 days of force application in the obese mice group, but no significant difference was observed on day 5 when compared with the control group (Figure 2A and 2B). These results indicated that obesity may attenuate force-induced OTM via inhibiting osteoclastogenesis in mice.
Figure 2.
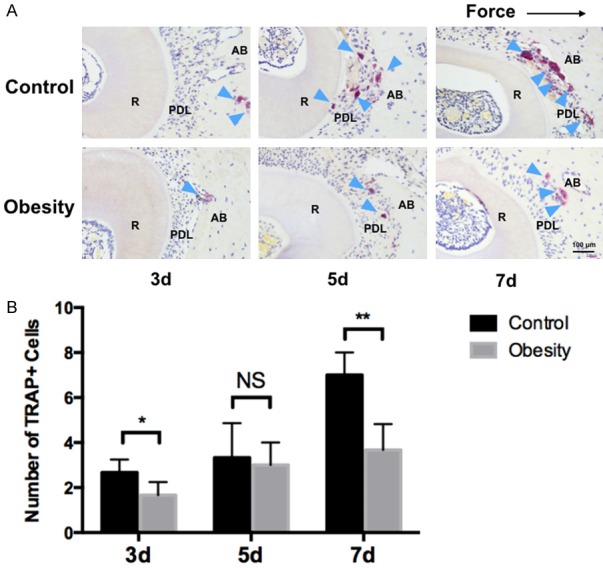
Effects of obesity on osteoclastogenesis in mice. A. Representative images of TRAP+ cells on the compression side of PDL and alveolar bone. B. Obese mice had significant reduced number of TRAP+ cells on 3 day and 7 day after force application. No significant difference was noted on day 5. R: root. PDL: periodontal ligament. AB: alveolar bone. The dark arrow indicated the force direction. The blue arrow indicated osteoclasts. Data represent as mean ± SD, n=5 in each group; **P<0.01, *P<0.05; NS: not statistically significant.
Leptin may participate in the attenuated tooth movement in obese mice and inhibit osteoclastogenesis
A previous study showed that obese patients displayed elevated serum level of leptin [19], thus we first verified this finding in the obese mice. We examined the serum leptin level in the obesity and control group before and after force application. We confirmed that there was significant elevated serum leptin level before and after 7 days of force application in the obese mice when compared to the control group (Figure 3A).
Figure 3.
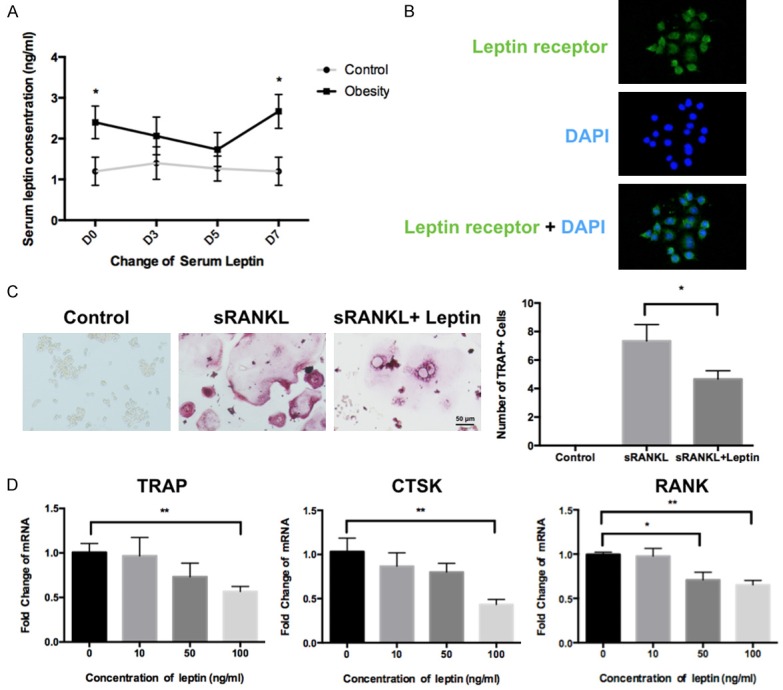
Serum leptin level was consistent with the tooth movement change during the experiment course and the effects of leptin on osteoclastogenesis in RAW264.7 cells. A. Serum leptin level was significantly elevated before and after 7 days of force application. No significant difference was noted on day 3 and day 5. B. Immunofluorescence staining confirmed that leptin receptor was expressed on RAW264.7 cells. C. TRAP staining showed that administration of leptin (100 ng/ml) inhibited osteoclatogenesis in RAW264.7 cells. D. Relative RNA expression of osteoclast-specific functional gene was determined by quantitative reverse-transcription polymerase chain reaction (q-PCR). β-actin served as a loading control; **P<0.01, *P<0.05; NS, not statistically significant.
Whether the elevated level of leptin can directly affect osteoclastogenesis so as to influence OTM is unknown. Thus, we hypothesized that leptin may contribute to the reduction of OTM in obese mice via inhibiting osteoclastogenesis. We first tested the expression of leptin receptor in murine osteoclasts cell line RAW264.7 cells, and confirmed that leptin receptor was expressed on RAW264.7 cells (Figure 3B). TRAP staining assay was used to evaluate the effect of leptin on osteoclastogenesis in RAW264.7 cells. The results showed that leptin treatment significantly inhibited the number of RANKL-induced TRAP+ osteoclasts when compared to the vehicle-treated group (Figure 3C).
Since TRAP, CTSK and RANK are typical osteoclast-specific functional genes, we next assessed whether the administration of leptin could affect osteoclast function [24]. The q-PCR assay showed that the expression of TRAP and CTSK was significantly inhibited by leptin at the concentration of 100 ng/ml, and expression of RANK was significantly inhibited by leptin at the concentration of 50 and 100 ng/ml (Figure 3D). These results indicated that leptin could directly inhibit osteoclasts generation and function.
Leptin attenuates OTM to reinforce anchorage in mice
Since inhibiting posterior tooth mesial movement to reinforce anchorage is a crucial issue in clinical applications, thus we next evaluate whether leptin can influence force-induced OTM in vivo. We injected leptin or vehicle intraperitoneally to each group of wild type mice respectively every other day from one day prior to force application (Figure 4A). Though there was a tendency of reduction in body weight for the leptin-treated group, no statistically significant difference was observed between the groups after 3 days, 5 days and 7 days of leptin or vehicle administration (Figure 4B). Comparing to the control group, the intra-oral photograph showed that the OTM in leptin-treated group was significantly reduced on day 5 and 7 after force application, but no significant difference was noted on day 3. MicroCT scan also revealed that the OTM was attenuated in leptin-treated mice after 7 days of force application when compared with the vehicle-treated group (Figure 4C-E).
Figure 4.
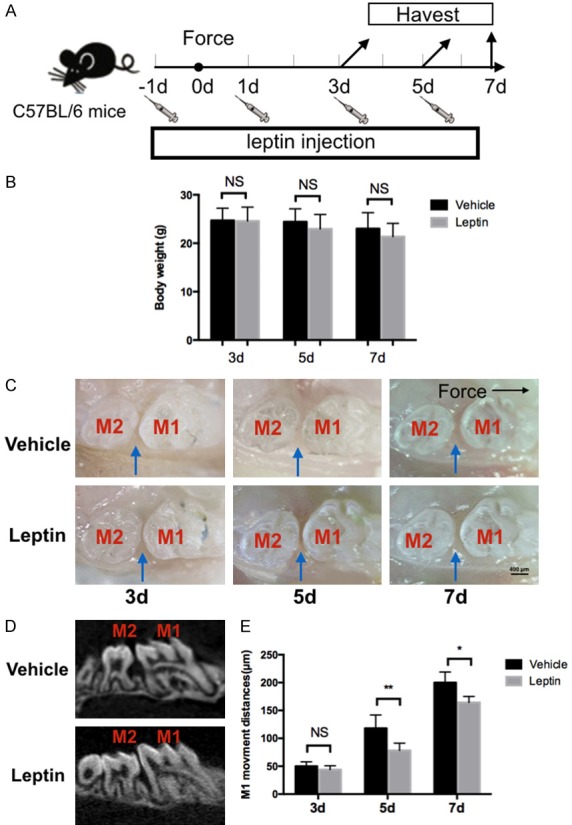
Effects of leptin on force induced orthodontic tooth movement in mice. A. Schedule of the experiment. Orthodontic force was applied to mice. Mice were sacrificed at 3 d, 5 d and 7 d after force application. Intraperitoneal injection of leptin or vehicle was performed every other day since one day prior to force application. B. Trends of decreased body weight was noted, while no statistically significant difference was found. C. Representative intra-oral photograph of tooth movement mice with injection of leptin or vehicle at 3 day, 5 day and 7 day of force application. D. MicroCT representative image of tooth movement in mice with injection of leptin or vehicle after 7 days of force application. E. Amount of orthodontic tooth movement on 3 day, 5 day and 7 day after force application in mice with leptin or vehicle administration. The blue arrows indicated tooth movement distances, M1: first molar. M2: second molar. n=5 in each group, error bars, means ± SD; scale bar: 400 μm; **P<0.01, *P<0.05; NS: not statistically significant.
To determine whether the administration of leptin can affect osteoclastogenesis in vivo, we then evaluated the number of osteoclasts in the leptin-treated and control group respectively through TRAP staining of histological sections. The number of TRAP+ osteoclasts was significantly reduced on day 3, day 5 and day 7 in the leptin-treated group when compared with the vehicle-treated group (Figure 5A and 5B). Taken together, these results indicated that leptin administration was able to attenuate force-induced OTM via inhibiting osteoclastogenesis in mice.
Figure 5.
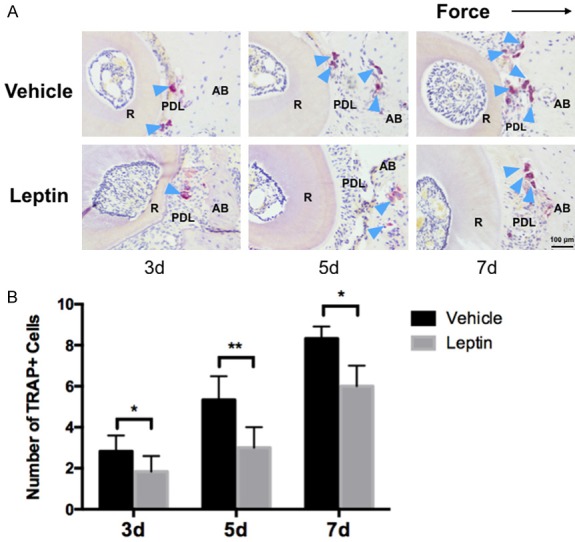
Effects of leptin on osteoclatogenesis after force application in mice. A. Representative images of TRAP+ cells on the compression side of PDL and alveolar bone. B. Leptin-treated mice had significant reduced number of TRAP+ cells on 3 day and 7 day after force application. No significant difference was noted on day 5. R: root. PDL: periodontal ligament. AB: alveolar bone. The dark arrow indicated the force direction. The blue arrow indicated osteoclasts. n=5 in each group, error bar: mean ± SD. scale bar: 400 μm; **P<0.01, *P<0.05.
Discussion
Obesity and overweight have been considered as a systemic disease with the fundamental basis of imbalance between energy intake and expenditure [9]. Globally, it is estimated that there are more than 2.1 billion people with high BMI and the prevalence is still increasing, which has already become a major public health problem worldwide [25,26]. The correlation between obesity and bone health has also attracted much attention and previous studies have suggested that obesity could exert influence on bone metabolism [27-29]. Most clinical studies indicated that obesity was a protective factor for bone from osteoporosis and could increase bone mass [30-32]. While alveolar bone remodeling is the essential biological process of OTM [33], during which constant exertion of mechanical force will induce alveolar bone remodeling [34-36]. Since osteoclasts play a critical role in the process of OTM [33,37,38], the inhibition of osteoclastogenesis is very likely to affect OTM. Though there is an increasing number of obese and overweight patients encountered in orthodontic clinic, only a few studies focused on how high BMI can affect OTM and the possible mechanisms still remain to be elucidated. Therefore, we translate the clinical problem into a basic research model and try to explore the underlying mechanisms.
In the present study, we first attempted to determine the effects of obesity on OTM in vivo. We fed 3-week old mice HFD for 5 weeks to induce obesity and evaluated the OTM in mice model. We demonstrated that the amount of OTM was reduced in obese mice group, which was consistent with previous clinical findings [16,17]. In addition, histological analysis revealed that there were less TRAP+ osteoclasts generation in force-induced OTM in obese mice group. We chose 3 d, 5 d and 7 d as the timepoints for it has been widely used in mice OTM model. Furthermore, we did the prolonged the follow-up as long as 12 d in the preliminary study, the results were similar to the 7 d data. However, the appliance dropout rate would be raised up after 7 days of force application.
As suggested in previous studies, the prolonged treatment duration was a risk factor for root resorption [39]. However, as far as we have observed, no significant root resorption was noted in both groups (Figure S3). Since previous studies also indicated that the inhibition of osteoclasts may reduce root resorption [40], we consider that the possible prolonged duration caused by obesity may not exert significant influence on root resorption, yet it demands for further investigation.
Though we confirmed that the OTM was reduced in obese mice, how this phenotype occurred was unknown, we then tried to determine the underlying mechanism of the reduced OTM in obese mice. We first confirmed that there was elevated serum level of leptin in obese mice as previously found in obese patients [19]. Interestingly, there was a trend of decreased serum level of leptin in the obese mice group. The possible cause of this phenotype could be attributed to the decrease of food intake as the appliances were installed in the obese mice, which may be more susceptible to the food intake reduction. With the adaption of appliances, the food intake in obese mice may recover and caused the rise of serum leptin level.
Previous studies have revealed that leptin was able to inhibit bone formation through the hypothalamus [41,42]. Yet, these results have been challenged in recent years, for leptin is mostly generated by adipose tissue peripherally and can pass through the blood brain barrier [43,44]. Also, recent studies indicated that the administration of leptin peripherally was able to enhance osteoblast activity in vitro, inhibit bone resorption and improve bone quantity and quality in various osteopenia animals [45-48]. Moreover, leptin was reported to be able to directly inhibit osteoclasts generation and function in vitro [23,49].
Here, we hypothesized that leptin may participate in the reduced OTM in obese mice by influencing osteoclastogenesis. We first confirmed that the expression of leptin receptor on RAW264.7 cells which suggested that leptin could act directly on osteoclasts. We then revealed that the administration of leptin was able to inhibit osteoclasts generation in RAW264.7 cells, along with the inhibited expression of osteoclasts functional genes. However, the in-depth mechanism of leptin affecting osteoclasts is still unknown. Previously, it was reported that leptin was able to inhibit PPAR-γ expression [50], which has been found to be able to promote osteoclast generation and function through the regulation of c-fos [51]. Thus, whether leptin can affect osteoclasts generation and function through the PPAR-γ pathway needs to be further investigated.
We further evaluated the effects of leptin on OTM in vivo. We confirmed that the administration of leptin was able to attenuate the mesial movement of molars through inhibiting osteoclasts generation in mice. Clinically, enforcing the stability of posterior teeth called anchorage reinforcement is very critical. Theoretically, local administration of leptin would be the best method for application. However, the local delivery was very difficult to control the dose for each mouse with confining space for operation. Therefore, we administrated leptin intraperitoneally for its possible application instead. The local administration of leptin to reinforce anchorage demands for further investigation in larger experiment animals. Furthermore, it is possible to increase tooth movement by blocking the leptin receptor, since previous studies reported that the bone mass of db/db mice (leptin receptor deficient mice) was reduced [52], which may result in the acceleration of tooth movement. For the potentials in medications, the acceleration of OTM and reinforcing anchorage in each patient may be done just by the leptin/leptin receptor pathway, which demands for further studies.
In conclusion, this study may serve as a preliminary example of translating orthodontic clinical problem to bench-top and back to clinical practice. We translated the clinical findings into basic a research model and confirmed that OTM was attenuated in obese mice. Mechanistically, it can be explained by the elevated level of leptin via the inhibition of osteoclastogenesis (Figure 6). Considering future clinical applications, our results suggest that when patients with high BMI undergo orthodontic treatment, the treatment duration may be prolonged and leptin has the potential to reinforce anchorage.
Figure 6.
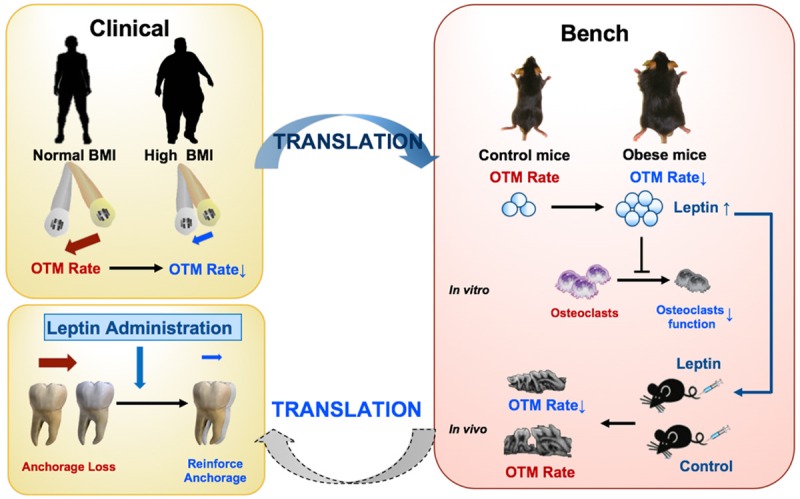
A schema of translating the clinical findings into basic research model and the possible implication for clinical application.
Acknowledgements
This work was supported by the Capital Health Research and Development of Special (No. 2016-1-4102, Y.Z.); the Young Scientists Fund of PKUSS (No. 20110203, No. 20170109, D.L.) and the New Clinical Technology Fund of PKUSS (No. PKUSSNCT-11A07, D.L.); Peking University Medicine Seed Fund for Interdisciplinary Research to D.L. the National Natural Science Foundation of China (No. 81470717, Y.Z.), (No. 81600893, D.H.), (No. 81600865, R.Y.), (No. 81671015, X.W.), (No. 81571815, Y.L.) and (No. 81871492, Y.L); the Beijing Natural Science Foundation (No. 7182182, R.Y.); the Beijing New-star Plan of Science and Technology (No. Z171100001117018, Y.L.); the Beijing Nova Programme Interdisciplinary Cooperation Project (No. Z181100006218135, Y.L.); the Young Elite Scientist Sponsorship Program by CAST (YESS, 2017QNRC001, R.Y); the Beijing Municipal Science & Technology Commission (No. Z171100001017128, X.W.).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Arnlov J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Furst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GB, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DA, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabares-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberto CA, Swinburn B, Hawkes C, Huang TT, Costa SA, Ashe M, Zwicker L, Cawley JH, Brownell KD. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385:2400–2409. doi: 10.1016/S0140-6736(14)61744-X. [DOI] [PubMed] [Google Scholar]
- 3.Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S, Di Angelantonio E, Vander Hoorn S, Lawes CM, Ali MK, Mozaffarian D, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group; Asia-Pacific Cohort Studies Collaboration (APCSC); Diabetes Epidemiology: Collaborative analysis of Diagnostic criteria in Europe (DECODE); Emerging Risk Factor Collaboration (ERFC); Prospective Studies Collaboration (PSC) The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer--viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, Zhao Y, Wang C. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2012;79:291–297. doi: 10.1016/j.jbspin.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Jiang L, Rong J, Wang Y, Hu F, Bao C, Li X, Zhao Y. The relationship between body mass index and hip osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2011;78:150–155. doi: 10.1016/j.jbspin.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Zhou W, Gu T, Zhu D, Bi Y. A retrospective study on association between obesity and cardiovascular risk diseases with aging in Chinese adults. Sci Rep. 2018;8:5806. doi: 10.1038/s41598-018-24161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 10.Zeng M, Kou X, Yang R, Liu D, Wang X, Song Y, Zhang J, Yan Y, Liu F, He D, Gan Y, Zhou Y. Orthodontic force induces systemic inflammatory monocyte responses. J Dent Res. 2015;94:1295–1302. doi: 10.1177/0022034515592868. [DOI] [PubMed] [Google Scholar]
- 11.Yan Y, Liu F, Kou X, Liu D, Yang R, Wang X, Song Y, He D, Gan Y, Zhou Y. T cells are required for orthodontic tooth movement. J Dent Res. 2015;94:1463–1470. doi: 10.1177/0022034515595003. [DOI] [PubMed] [Google Scholar]
- 12.Feng L, Yang R, Liu D, Wang X, Song Y, Cao H, He D, Gan Y, Kou X, Zhou Y. PDL progenitor-mediated PDL recovery contributes to orthodontic relapse. J Dent Res. 2016;95:1049–1056. doi: 10.1177/0022034516648604. [DOI] [PubMed] [Google Scholar]
- 13.Kirschneck C, Maurer M, Wolf M, Reicheneder C, Proff P. Regular nicotine intake increased tooth movement velocity, osteoclastogenesis and orthodontically induced dental root resorptions in a rat model. Int J Oral Sci. 2017;9:174–184. doi: 10.1038/ijos.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Wen F, He D, Liu D, Yang R, Wang X, Yan Y, Liu Y, Kou X, Zhou Y. Force-induced H2S by PDLSCs modifies osteoclastic activity during tooth movement. J Dent Res. 2017;96:694–702. doi: 10.1177/0022034517690388. [DOI] [PubMed] [Google Scholar]
- 15.Cao H, Kou X, Yang R, Liu D, Wang X, Song Y, Feng L, He D, Gan Y, Zhou Y. Force-induced Adrb2 in periodontal ligament cells promotes tooth movement. J Dent Res. 2014;93:1163–1169. doi: 10.1177/0022034514551769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayachandran T, Srinivasan B, Padmanabhan S. Salivary leptin levels in normal weight and overweight individuals and their correlation with orthodontic tooth movement. Angle Orthod. 2017;87:739–744. doi: 10.2319/120216-869.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Bremen J, Wagner J, Ruf S. Correlation between body mass index and orthodontic treatment outcome. Angle Orthod. 2013;83:371–375. doi: 10.2319/070612-555.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saloom HF, Papageorgiou SN, Carpenter GH, Cobourne MT. Impact of obesity on orthodontic tooth movement in adolescents: a prospective clinical cohort study. J Dent Res. 2017;96:547–554. doi: 10.1177/0022034516688448. [DOI] [PubMed] [Google Scholar]
- 19.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 20.Shi J, Liu Z, Kawai T, Zhou Y, Han X. Antibiotic administration alleviates the aggravating effect of orthodontic force on ligature-induced experimental periodontitis bone loss in mice. J Periodontal Res. 2017;52:725–733. doi: 10.1111/jre.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kou XX, Wu YW, Ding Y, Hao T, Bi RY, Gan YH, Ma X. 17beta-estradiol aggravates temporomandibular joint inflammation through the NF-kappaB pathway in ovariectomized rats. Arthritis Rheum. 2011;63:1888–1897. doi: 10.1002/art.30334. [DOI] [PubMed] [Google Scholar]
- 22.Kuroshima S, Al-Salihi Z, Yamashita J. Mouse anti-RANKL antibody delays oral wound healing and increases TRAP-positive mononuclear cells in bone marrow. Clin Oral Investig. 2016;20:727–736. doi: 10.1007/s00784-015-1550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Zhu Y, Yang X, Yang G, Zhu W, Ma T, Zhang J. Leptin inhibits the differentiation of RAW264.7 macrophages into osteoclasts via depressing the expression of PPARgamma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31:145–148. [PubMed] [Google Scholar]
- 24.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J Biol Chem. 2010;285:25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong ME, Spencer EA, Cairns BJ, Banks E, Pirie K, Green J, Wright FL, Reeves GK, Beral V. Body mass index and physical activity in relation to the incidence of hip fracture in postmenopausal women. J Bone Miner Res. 2011;26:1330–1338. doi: 10.1002/jbmr.315. [DOI] [PubMed] [Google Scholar]
- 28.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 29.Evans AL, Paggiosi MA, Eastell R, Walsh JS. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res. 2015;30:920–928. doi: 10.1002/jbmr.2407. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong ME, Cairns BJ, Banks E, Green J, Reeves GK, Beral V. Different effects of age, adiposity and physical activity on the risk of ankle, wrist and hip fractures in postmenopausal women. Bone. 2012;50:1394–1400. doi: 10.1016/j.bone.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Premaor MO, Compston JE, Fina Aviles F, Pages-Castella A, Nogues X, Diez-Perez A, Prieto-Alhambra D. The association between fracture site and obesity in men: a population-based cohort study. J Bone Miner Res. 2013;28:1771–1777. doi: 10.1002/jbmr.1878. [DOI] [PubMed] [Google Scholar]
- 32.Prieto-Alhambra D, Premaor MO, Fina Aviles F, Hermosilla E, Martinez-Laguna D, Carbonell-Abella C, Nogues X, Compston JE, Diez-Perez A. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012;27:294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- 33.Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 35.Huang H, Williams RC, Kyrkanides S. Accelerated orthodontic tooth movement: molecular mechanisms. Am J Orthod Dentofacial Orthop. 2014;146:620–632. doi: 10.1016/j.ajodo.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Xie R, Kuijpers-Jagtman AM, Maltha JC. Osteoclast differentiation during experimental tooth movement by a short-term force application: an immunohistochemical study in rats. Acta Odontol Scand. 2008;66:314–320. doi: 10.1080/00016350802317488. [DOI] [PubMed] [Google Scholar]
- 37.Masella RS, Meister M. Current concepts in the biology of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;129:458–468. doi: 10.1016/j.ajodo.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 38.Holliday LS, Ostrov DA, Wronski TJ, Dolce C. Osteoclast polarization and orthodontic tooth movement. Orthod Craniofac Res. 2009;12:105–112. doi: 10.1111/j.1601-6343.2009.01443.x. [DOI] [PubMed] [Google Scholar]
- 39.Lopatiene K, Dumbravaite A. Risk factors of root resorption after orthodontic treatment. Stomatologija. 2008;10:89–95. [PubMed] [Google Scholar]
- 40.Inubushi T, Tanaka E, Rego EB, Ohtani J, Kawazoe A, Tanne K, Miyauchi M, Takata T. Ultrasound stimulation attenuates resorption of tooth root induced by experimental force application. Bone. 2013;53:497–506. doi: 10.1016/j.bone.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 41.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 42.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 43.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 44.Wiedenhoft A, Muller C, Stenger R, Blum WF, Fusch C. Lack of sex difference in cerebrospinal fluid (CSF) leptin levels and contribution of CSF/plasma ratios to variations in body mass index in children. J Clin Endocrinol Metab. 1999;84:3021–3024. doi: 10.1210/jcem.84.9.5983. [DOI] [PubMed] [Google Scholar]
- 45.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, Grey AB, Broom N, Myers DE, Nicholson GC, Reid IR. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 46.Martin A, de Vittoris R, David V, Moraes R, Begeot M, Lafage-Proust MH, Alexandre C, Vico L, Thomas T. Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology. 2005;146:3652–3659. doi: 10.1210/en.2004-1509. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed HH, Morcos NY, Eskander EF, Seoudi DM, Shalby AB. Potential role of leptin against glucocorticoid-induced secondary osteoporosis in adult female rats. Eur Rev Med Pharmacol Sci. 2012;16:1446–1452. [PubMed] [Google Scholar]
- 48.Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, Iwaniec UT. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013;28:22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Q, Guan W, Qiao H, Cheng Y, Li Z, Zhai X, Zhou Y. GATA binding protein 2 mediates leptin inhibition of PPARgamma1 expression in hepatic stellate cells and contributes to hepatic stellate cell activation. Biochim Biophys Acta. 2014;1842:2367–2377. doi: 10.1016/j.bbadis.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 52.Ealey KN, Fonseca D, Archer MC, Ward WE. Bone abnormalities in adolescent leptin-deficient mice. Regul Pept. 2006;136:9–13. doi: 10.1016/j.regpep.2006.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


