Abstract
Inflammation activates indoleamine 2,3-dioxygenase (IDO) which metabolizes tryptophan into kynurenine. Circulating kynurenine is transported into the brain by the large amino transporter LAT1 at the level of the blood-brain barrier. We hypothesized that administration of leucine that has a high affinity for LAT1 should prevent the entry of kynurenine into the brain and attenuate the formation of neurotoxic kynurenine metabolites. To test whether leucine could prevent inflammation-induced depression-like behavior mice were treated with lipopolysaccharide (LPS, 0.83 mg/kg IP) or saline and treated with L-leucine (50 mg/kg, IP) or vehicle administered before and 6 h after LPS. Depression-like behavior was measured by increased duration of immobility in the forced swim test and decreased sucrose preference. Leucine decreased brain kynurenine levels, blocked LPS-induced depression-like behavior and had antidepressant-like effects in control mice. Leucine had no effect of its own on sickness behavior and neuroinflammation. To confirm that leucine acts by interfering with the transport of kynurenine into the brain, mice were injected with L-leucine (300 mg/kg, IP) immediately before kynurenine (33 mg/kg IP) and brain kynurenine and depression-like behavior were measured 3 hours later. Leucine did prevent the entry of exogenous kynurenine into the brain and abrogated depression-like behavior measured by increased duration of immobility in the forced swim test. Additional experiments using an in vitro model of the blood-brain barrier confirmed that kynurenine competes with leucine at the level of the amino acid transporter LAT1 for brain uptake. These experiments also revealed that efflux was the dominant direction of kynurenine transport and was largely independent of LAT1 and leucine, which explains why leucine could block brain uptake of kynurenine without affecting brain clearance. These findings demonstrate that leucine has antidepressant properties vis-à-vis inflammation-induced depression and one mechanism for this is by blocking the ability of kynurenine to enter the brain.
INTRODUCTION
Activation of the tryptophan degrading enzyme indoleamine 2,3-dioxygenase (IDO) is implicated in the onset of inflammation-induced depression. IDO metabolizes tryptophan into kynurenine that is metabolized further into neurotoxic kynurenine metabolites including quinolinic acid, a N-methyl-D-aspartate (NMDA) receptor agonist (1, 2). Clinical studies have shown increases in kynurenine:tryptophan ratios measured in the plasma and cerebrospinal fluid of malignant melanoma and hepatitis C patients receiving interferon-alpha therapy, which correlate with the intensity of depressive symptoms in these patients (3–6). Preclinical studies have demonstrated that both genetic and pharmacologic blockade of IDO abrogates depression-like behavior in mice induced by lipopolysaccharide (LPS) or Bacillus Calmette-Guerin (7, 8). The metabolism of kynurenine in the brain of mice exposed to LPS is biased toward the formation of neurotoxic kynurenine metabolites largely because of the increased activity of the microglial enzyme kynurenine monooxygenase (2). However other branches of the kynurenine pathway may also be involved given that genetic blockade of KMO does not rescue all depression-like behaviors (9). In addition, pre-treatment with the NMDA receptor antagonist ketamine blocks the development of LPS-induced depression-like behavior.
Importantly, almost all of the kynurenine in the brain is derived from the periphery. In the absence of inflammation, roughly 80% of brain kynurenine is transported from the blood to the brain; this value rises to 98% or higher in response to LPS (10). Both tryptophan and kynurenine are transported from the blood into the brain by the transport system L, also known as LAT1, which transports several aromatic and branched-chain amino acids, including L-leucine (11, 12). We sought to determine if it is possible to leverage blood-to-brain kynurenine transport to prevent inflammation-induced depression by competitively inhibiting kynurenine transport by LAT1 using L-leucine (hereafter referred to as leucine). Leucine was chosen because of its high affinity for LAT1 and the fact that it is not metabolized by IDO in contrast to tryptophan. Here, we show that leucine can reduce depression-like behavior in mice with and without inflammation by inhibiting kynurenine transport into the brain via LAT1.
MATERIALS AND METHODS
Animals and Treatments
CD1 and C57Bl/6J male mice (12-16 weeks old) (Charles River Laboratories) were used in these experiments. Mice were housed individually in standard shoebox cages in a temperature (23°C) and humidity (45–55%) controlled environment with a 12/12-h modified dark-light cycle (lights on at 2200 hours). Food and water were available ad libitum. Mice were handled daily for 1 week prior to experimentation.
On the day of injection, fresh solutions of LPS (L-020M4062, serotype 0127:B8; Sigma, St Louis, MO) and leucine were prepared and administered intraperitoneally. LPS was prepared with sterile endotoxin-free isotonic phosphate-buffered saline (PBS). Leucine (BP385-100, Fisher, USA) was prepared with 6% HCl in PBS. Appropriate vehicles were administered for each solution. Mice received 0.83 mg/kg LPS and 50 mg/kg leucine for each injection or vehicle (equivolume) for inflammation-induced depression studies. This dose of LPS was chosen for its ability to reliably induce the acute sickness behavior response and subsequent depression-like behaviors across the time points examined here (2). The dose of 50 mg/kg leucine was selected based on dose-response pilot studies. Leucine was administered at 0 and 6 h for behavioural assays and mice were randomly allocated into treatment groups for these studies: PBS + vehicle (n = 17); PBS + leucine (n = 15); LPS + vehicle (n = 21); LPS + leucine (n = 19). Biochemical analyses were conducted 27 h after LPS to ensure an appropriate concentration of leucine was maintained to block kynurenine transport to the brain at the point of peak IDO activation (24 h). To examine the impact of leucine on the early peak inflammatory response, mice were injected once at 0 h and tissue harvested at 6 h, before what would have normally been the time point of the second injection. Mice were randomly allocated into treatment groups for these studies: PBS + vehicle (n = 7); PBS + leucine (n = 8); LPS + vehicle (n = 8); LPS + leucine (n = 8). We examined the kynurenine:tryptophan levels in these animals 27 hours after LPS administration.
To confirm that leucine acts by blocking kynurenine entry into the brain, mice were injected intraperitoneally with leucine or vehicle immediately before kynurenine (equivolume) and tested 2–3 hours later. Independent groups of mice were used for measurement of brain leucine and for depression-like behaviour. Kynurenine was injected at the dose of 33 mg/kg as this dose reliably increased duration of immobility in the forced swim test. Leucine was injected at the dose of 330 mg/kg for the biochemistry experiment (n = 6 per group, randomly allocated) and 300 mg/kg for the behavior experiment. Mice were randomly allocated to groups. Vehicle + vehicle (n = 10); vehicle + kynurenine (n = 9); leucine + kynurenine (n = 10).
At the end of the experiment, mice were euthanized with CO2. Blood was collected by cardiac puncture and tissues were removed after perfusion with sterile PBS. All protocols in this study were approved either by the Institutional Animal Care and Use Committees (IACUC) of MD Anderson Cancer Center, Texas A&M University and Monash University.
Behavioral Assays
All behavioral experiments were performed during the first 5 h of the dark phase of the light cycle. Sickness (body weight loss, reduced food intake and locomotor activity) and depression-like behavior (decreased sucrose preference, increased immobility in the forced swim test (FST) were measured as described previously (2). We have previously shown that LPS-induced depression-like behavior can be dissociated from sickness from 24 h after LPS (2). Sickness was measured by LPS-induced decreases in body weight and food consumption measured 24 and 27h after LPS and in locomotor activity measured 2 and 24 h after LPS. Depression-like behavior was measured by reduced sucrose preference and increased duration of immobility in the forced swim test. For the sucrose preference test, mice were trained for optimal sucrose preference (1% solution) prior to testing. A two-bottle sucrose preference test was conducted immediately after locomotor assessment 24 h post-treatment for 3 h. Forced swim testing was conducted at 2 h post-kynurenine or 27 h post-LPS. The experimenter was blinded to treatment groups during behavioural testing sessions.
Assessment of kynurenine:tryptophan ratios in brains and plasma
To decrease variability in biochemical assays of brain tryptophan and kynurenine, only mice with frank behavioral responses to LPS were selected for plasma and brain levels of kynurenine and tryptophan (n =5 for all groups except LPS + vehicle where n = 7) using a kynurenine:tryptophan ratio ELISA kit (Immusmol, France). Limits of detections for kynurenine and tryptophan were below 47.5 ng/ml and 1.2 μg/ml respectively. Use of ELISAs to measure mouse brain kynurenine and tryptophan were optimized in pilot studies. Brain tissue was sonicated 3 times for 5 s at 65% amplitude in ice cold PBS at a 1:1 w:v ratio or 500 μl/sample. Samples were then centrifuged at 4°C at 13,000 rpm for 15 minutes and the supernatant stored at −80°C until assayed. In the experiment on the effects of leucine on exogenous kynurenine entry into the brain, mice were euthanized 3 hours after the administration of kynurenine and leucine and brain samples were processed in the same manner as described above.
Gene Expression Analyses
Total RNA from liver and brain samples were extracted in TRIzol® reagent (Life Technologies Corporation, Carlsbad, CA). All reverse transcription reactions used an Ambion® PureLink® RNA Mini reverse transcriptase kit (Life Technologies Corporation, Carlsbad, CA; cat # 12183018A) according to manufacturer’s instructions, with random decamer primers for each reaction. Real-time RT-PCR was carried out using TaqMan® gene expression assays for interleukin (Il)6, Il1b, Il10, tumor necrosis factor (Tnf)a, indoleamine 2,3-dioxygenase (IDO)1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as previously described (13). Assessment of SLC3A2 and SLC7A5 gene expression in the prefrontal cortex of mice in Experiment 2 was also determined using qRT-PCR. SLC3A2 and SLC7A5 respectively encode for the 4F2hc heavy subunit and CD98 light subunit proteins of the LAT1 transporter.
In vitro model of kynurenine, tryptophan, and leucine transport across the blood-brain barrier (BBB)
Primary brain microvascular endothelial cells (BMECs) were isolated from CD1 mice, grown according to previously published protocols (14) and seeded on the bottom of a polyester transwell insert coated with fibronectin and collagen type IV to form BMEC monolayers with tight junctions between cells. Cultures were used 3 days after initiation. Some wells were treated with lipopolysaccharide from Salmonella typhimurium (10 ng/ml; Sigma Aldrich) and interferon gamma (1 ng/ml; PeproTech) for 6 hours in the luminal chamber prior to the start of permeability experiments. Transwell inserts were then washed with warmed 1% bovine serum albumin in Lactated Ringer’s solution (1% BSA/LR) and placed into a new 24 well plate. For efflux studies, 100 μl fresh 1% BSA/LR were added to the inside of the Transwell insert (the “luminal” side). The radioactivity was added to the well (600 μl), and samples (50 μl) were collected from the luminal chamber. For influx studies, 600 μl of fresh 1% BSA/LR were added to the well (the “abluminal” side) and radioactivity added to the luminal chamber. Samples (500 ul) were collected from the abluminal chamber. For efflux studies, radioactive kynurenine was placed on the abluminal side and samples collected from the luminal side. The radioactivity solution consisted of 1% BSA/LR with 14C-Sucrose (Perkin Elmer), one of three tritiated amino acids (tryptophan, leucine, or kynurenine; Perkin Elmer) and a cold amino acid (Tryptophan, Leucine, Kynurenine, or the LAT1 inhibitor 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid [BCH]; Sigma Aldrich). Tryptophan, leucine, kynurenine and BCH were used at 1mM concentration. Samples were collected at 10, 20, 30, and 45 min after the initiation of permeability experiments. After taking samples, a volume equal to the sample of fresh 1% BSA/LR was replaced into the collecting chamber. Liquid scintillation fluid was added to each sample and counted in a beta counter. The permeability coefficient was calculated according to previous methods (14). Briefly, correction for variations in permeability of the monolayer were made by measuring the entry of 14C sucrose from the luminal into the abluminal chamber. The endothelial permeability coefficient Pe was calculated as the slope of the clearance curve according to time and expressed in microliters per minute per square centimetre. All isotopes were purchased from Perkin Elmer, USA, and used at a concentration of 1,000,000 dpm/ml.
Statistical analysis
Two-way analyses of variances (ANOVAs) (LPS vs. PBS × leucine vs. vehicle) were performed for LPS-induced depression-like behavior and all measures of biochemistry from tissues. Three-way repeated measures ANOVAs (LPS vs PBS × leucine vs vehicle × time as a repeated factor) were performed for measures of sickness (body weight loss, food consumption, locomotor activity). One-way ANOVAs and unpaired t-tests were performed for in vitro BBB permeability studies and kynurenine-induced depression-like behavior. Planned comparisons were performed to assess change from the control group, and were determined using Newman-Keuls multiple comparisons tests or t-tests with alpha adjusted for the number of comparisons. For the LPS × leucine interactions observed for sickness endpoints, planned comparisons for the effect of leucine were conducted against the presence or absence of LPS. Statistical tests were two-sided and variance between groups was assessed. In cases of heteroscedasticity t tests of unequal variance were applied. All experiments were replicated at least twice. Outliers were excluded when greater than 2 standard deviations from the mean. Sample size estimates were conducted based on previous studies investigating in vivo effects of LPS on behaviour and kynurenine, and in vitro blood-brain barrier modelling based on an expected effect size of 0.8 standardised unit with 80% power and 5% significance level.
RESULTS
Leucine prevents depression-like behavior (Figure 1A,B)
Figure 1. Leucine reduces brain kynurenine:tryptophan ratios and blocks depression-like behavior.
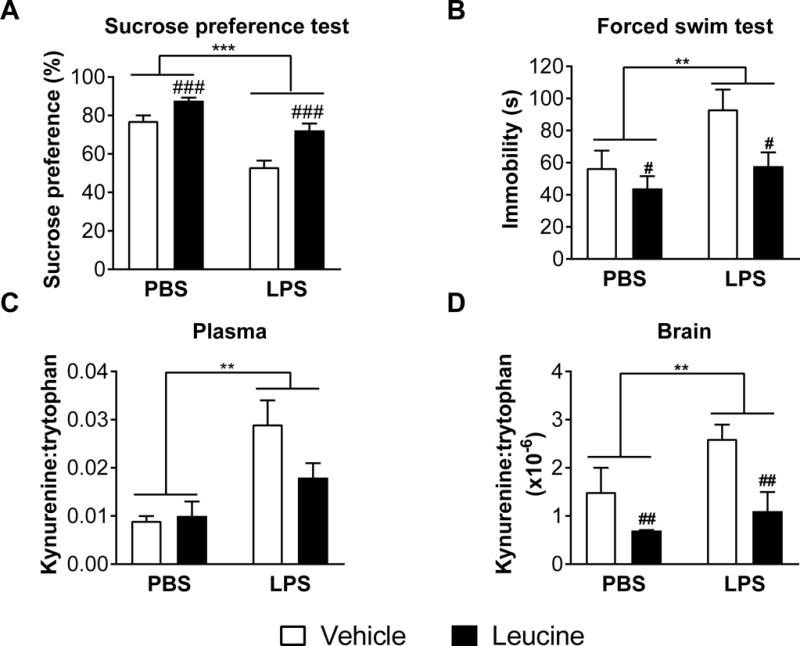
A,B Mean sucrose preference (%) and time spent immobile in the forced swim test (s) (± SEM) of mice treated with leucine or vehicle immediately before and 6 h after LPS or PBS (n ≥ 14 per group). C,D Mean kynurenine:tryptophan ratios in plasma and brains (± SEM) of mice treated with 50 mg/kg leucine or vehicle immediately before and 6 h after LPS or phosphate-buffered saline (PBS) (n = 5 per group). **p ≤ 0.01, ***p ≤ 0.0001 for LPS vs PBS main effects. #p ≤ 0.05, ##p ≤ 0.01, ###p ≤ 0.0001 for leucine vs vehicle main effects.
To determine whether leucine can block the development of LPS-induced depression-like behaviour we treated mice with 50 mg/kg IP leucine immediately prior to and 6 h after 0.83mg/kg LPS and assessed depression-like behavior from 24–27 h after LPS. LPS reduced sucrose preference (F(1,63) = 38.9, p < 0.0001) and increased FST immobility (F(1,66) = 5.14, p < 0.05). Conversely, treatment with leucine reversed these effects in both assays for mice treated with or without LPS (F(1,63) = 5.14, p < 0.0001 for sucrose preference, and F(1,66) = 5.95, p < 0.05 for FST immobility), indicating antidepressant properties of leucine (Figure 1A,B).
Leucine decreases kynurenine:tryptophan ratios in the brain (Figure 1C,D)
To determine if the depression-like behavior induced by LPS corresponded with increased plasma and brain kynurenine levels, and the antidepressant-like effects of leucine corresponded with decreased brain concentrations of kynurenine, we assessed kynurenine and tryptophan in brain and plasma of mice treated with LPS and leucine vs. controls. As expected, the kynurenine:tryptophan ratios in brain and plasma of mice treated with LPS and leucine vs. controls. As expected, LPS administered 27 h before increased kynurenine:tryptophan ratios in both plasma and brain independently of leucine treatment (F(1,20) = 17.6, p < 0.001 and F(1,17) = 5.96, p < 0.05 respectively). More importantly, leucine reduced brain kynurenine:tryptophan ratios in both LPS-treated and control mice (F(1,17) = 4.53, p < 0.05) (Figure 1C,D). No differences in plasma and brain tryptophan were observed. LPS increased plasma and brain kynurenine (F(1,20) = 16.4, p < 0.001 and F(1,18) = 4.83, p < 0.05 respectively). Leucine reduced brain kynurenine levels (F(1,18) = 10.46, p < 0.01) (Supplementary Figure 1). No interactions between LPS and leucine were observed.
To confirm that the reduced brain concentrations of kynurenine and the antidepressant-like effects of leucine were not confounded by off-target effects of leucine on inflammation, we assessed sickness and measured cytokines and IDO mRNAs in the livers, prefrontal cortices (PFC) and hippocampi collected 6 h (after one injection of leucine) and 27 h (after 2 injections of leucine). As expected, we observed that LPS increased the expression of proinflammatory cytokines, IL-10, and IDO in the liver, prefrontal cortex and hippocampus with some variation between 6 and 27 h and between the liver and brain areas. Leucine had in most cases no effect on cytokine and IDO response to LPS (see Supplementary Figures 2–5 and Supplementary Table 1).
Leucine prevents the entry of exogenous kynurenine into the brain and blocks kynurenine-induced depression-like behaviour (Figure 2)
Figure 2. Leucine blocks the entry of kynurenine into the brain and the depression-like behaviour induced by exogenous administration of kynurenine.
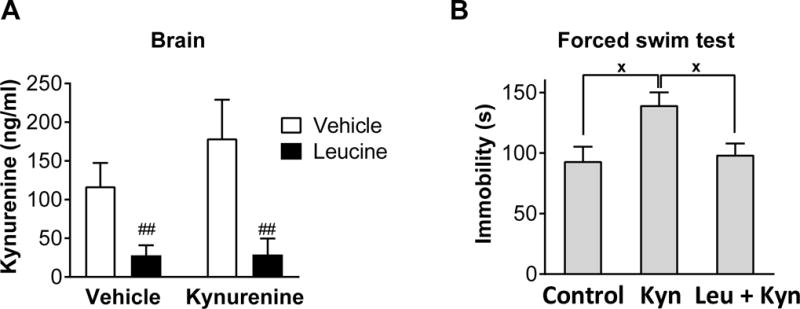
A Mean brain kynurenine concentrations (ng/ml) (± SEM) of mice treated with leucine or vehicle immediately before kynurenine or vehicle (n = 6 per group). B Mean time spent immobile in the forced swim test (s) (± SEM) of mice treated with leucine or vehicle immediately before kynurenine or vehicle (n ≥ 9). ##p ≤ 0.01 for leucine vs vehicle main effects. xp ≤ 0.05 for significant differences in Figure B.
To confirm that leucine abrogates LPS-induced depression-like behavior by blocking the entry of kynurenine into the brain, we treated mice with 330 mg/kg IP leucine immediately prior to 33 mg/kg IP kynurenine and assessed brain kynurenine concentrations 3 h after kynurenine (Figure 2A). As expected, leucine reduced brain kynurenine levels in response to exogenous kynurenine (F(1,20) = 14.24, p < 0.01).
To confirm that a dose of leucine that prevents the entry of exogenous kynurenine into the brain can also block kynurenine-induced depression-like behaviour in vivo, we treated mice with 300 mg/kg IP leucine immediately prior to 33 mg/kg IP kynurenine, and assessed depression-like behavior using the FST from 2–3 h after kynurenine. Kynurenine significantly increased FST immobility compared to vehicle-treated control mice which was reversed with pre-treatment with leucine (F(2,26) = 5.5, p = 0.01) (Figure 2B).
Kynurenine shares LAT1 influx with leucine but has an independent efflux system (Figure 3)
Figure 3. Leucine competes with kynurenine brain influx but not efflux via LAT1 in vitro.
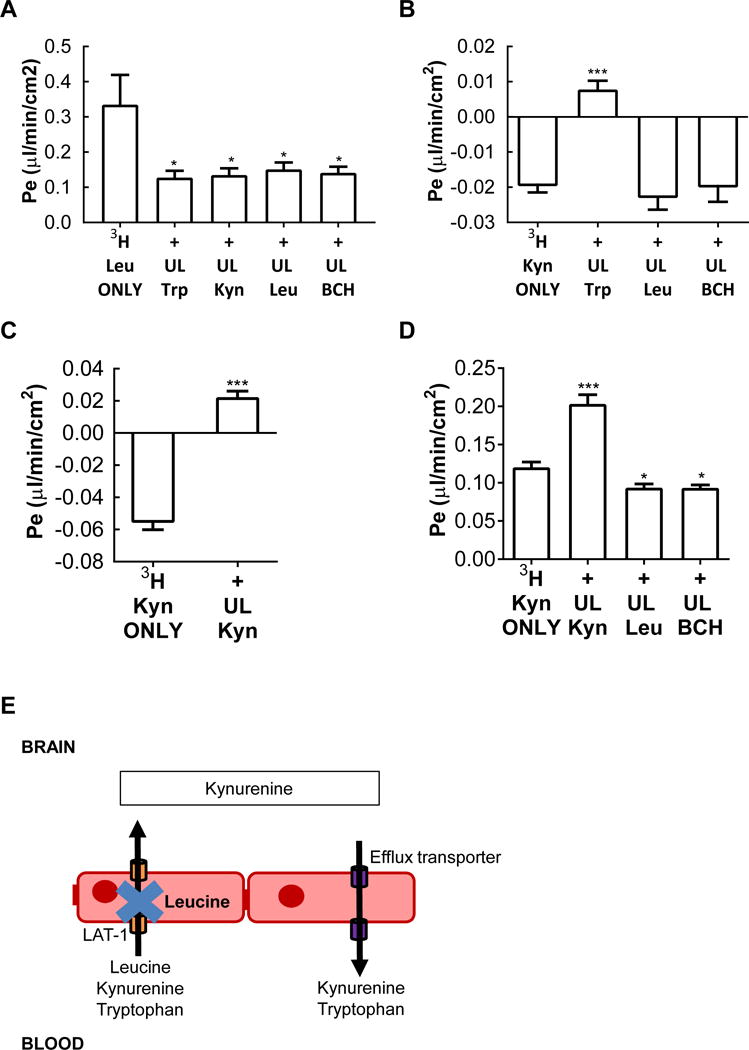
Tritiated (3H) materials were placed in the luminal chamber and influx into the abluminal chamber measured. A Mean permeability (μl/min/cm2) (± SEM) of tritiated leucine across brain microvascular endothelial cells (BMECs) from the luminal-to-abluminal chamber of a transwell. Unlabelled (cold) leucine (10 mg/ml), BCH (1 mM) and kynurenine (1 mM) inhibited leucine permeability. B Mean permeability (μl/min/cm2) (± SEM) of tritiated (3H) kynurenine across BMECs from the luminal-to-abluminal chamber of a transwell, which indicated the predominance of an efflux system that was unmasked by self-inhibition (C) and by unlabelled (cold) tryptophan (1 mM) but not unlabelled (cold) Leucine or BCH. D Efflux of kynurenine is mainly independent of LAT1. Tritiated (3H) kynurenine was placed in the abluminal chamber and efflux into the luminal chamber measured. Efflux was easily measured for tritiated (3H) kynurenine with modest inhibition by leucine or BCH. Enhanced efflux by unlabeled (cold) Kynurenine is consistent with an influx arm, but one more easily inhibited even by abluminal kynurenine than the efflux arm. E Diagrammatic summary of the findings of Figure 2, showing that leucine is able to inhibit transport of leucine, kynurenine and tryptophan into the brain via LAT1 but does not interfere with transport of tryptophan or excess kynurenine from leaving the brain. 3H = tritiated; UL = unlabelled. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.0001
The L-type amino acid transporter LAT1 mediates the entry of kynurenine and leucine at the level of the blood-brain barrier. Before analysing the interactions between leucine and kynurenine transport at the level of the endothelial cells of the blood-brain barrier we first determined if leucine or LPS altered expression of brain LAT1 transporter. Because of its richness in blood vessels we assessed mRNA levels of the heavy and light LAT1 transporter subunits in the prefrontal cortex at 6 h after LPS (n = 4–5 per group). LPS significantly increased SLC3A2 by 1.9 fold indicating an increase for the 4F2hc heavy subunit, but there was no change in the CD98 light subunit (F(1,15) = 4.58, p < 0.05). Leucine alone increased the expression of transcripts for both chains of LAT1 but this effect was smaller than that of LPS and did not reach significance (Supplementary Figure 6).
We used an in vitro monolayer model of the blood-brain barrier to determine the relations between leucine and kynurenine transport. This model is polarized, having luminal (blood side) and abluminal (brain side) surfaces, and so we were able to assess the directionality of transport as well. Values were corrected for residual leakiness as measured by 14C sucrose. Figure 3A shows that the blood-to-brain transport of tritiated leucine was self-inhibited by unlabeled leucine, by unlabeled tryptophan (which is also transported into brain by LAT1), by the selective LAT1 inhibitor BCH, and by kynurenine (F(4,25) = 3.86, p < 0.05). These results are consistent with LAT1 being the blood-to-brain transporter for kynurenine. Figure 3B shows that when tritiated kynurenine was placed in the luminal chamber, the resulting sucrose-corrected values were negative. A common cause for negative values is the presence of a robust brain-to-blood efflux transporter that is dominant to influx. Figure 3B shows that tryptophan, but not leucine or BCH, competed with this efflux system. The lack of effect of BCH shows the efflux is not mediated by LAT1 (F(3,28) = 15.01, p < 0.0001). Leucine also did not affect tritiated kynurenine, suggesting that it may block LAT1-dependent influx of kynurenine without interfering with its efflux. It is unclear whether tryptophan directly inhibited tritiated kynurenine efflux or whether it was kynurenine converted from tryptophan by IDO present in the brain endothelial cells. A test of the presence of an efflux system is whether adding unlabeled materials produces a paradoxical increase in influx values; indeed, we found that unlabeled kynurenine produced a paradoxical increase in influx of tritiated kynurenine (Figure 3C) (t(14) = 10.66, p < 0.0001).
We then formally assessed efflux by placing tritiated kynurenine in the abluminal chamber and collecting samples from the luminal chamber (Figure 3D). We found a positive value, confirming the presence of the efflux system. Efflux was not inhibited by leucine and BCH suggesting that LAT1 is not involved in efflux. Unlabeled kynurenine produced a paradoxical increase in efflux, confirming that there is an influx arm to the blood-brain barrier transport of kynurenine.
DISCUSSION
These results show that administration of the LAT1 competitive amino acid leucine blocks endogenous as well as exogenous kynurenine transport into the brain and abrogates the development of depression-like behaviour in response to LPS. These findings demonstrate for the first time that competitive inhibition of blood-to-brain kynurenine transport may represent an effective way of treating inflammation-induced depression.
Using an already validated model of inflammation-induced depression and two different behavioural measures of depression-like behavior, we were able to show that leucine blocks the development of depression-like behaviour in response to LPS. Surprisingly, we found that leucine could induce an antidepressant-like effect in mice independently of LPS treatment as well. These behavioral findings were supported by the ability of leucine to reduce brain kynurenine in both control and LPS-treated mice. This reduction is possible because the circulating levels of kynurenine are 100–1000 times lower than those of circulating tryptophan during inflammation (2), making it much easier to block kynurenine than tryptophan transport to the brain. Our findings are also consistent with the observation that the vast majority of the brain pool of kynurenine derives from the blood at baseline and in response to systemic inflammation (10). A recent study confirms the ability of leucine to leverage kynurenine transport across membranes by demonstrating that leucine can inhibit rat cortical kynurenic acid production in vitro (15). However, our study, based on an in vivo model of systemic inflammation and depression, is the first to show that leveraging blood-to-brain transport mechanisms may be a therapeutic tool.
To confirm that leucine was not affecting peripheral or central inflammation in our model, we examined sickness and inflammatory responses in our mice. In our behavioral experiments which focussed on examining depression-like behavior there was some evidence that leucine may have attenuated sickness responses to LPS and some inflammatory markers measured at 27 h after LPS. To directly examine the impact of leucine on sickness and inflammation, we injected leucine immediately before LPS and measured sickness responses and behavior at 6 h during the peak period of sickness. We found no evidence for leucine to ameliorate the proinflammatory response in LPS-treated mice at a time point that immediately precedes the activation of IDO. Although leucine decreased mRNA levels of IL-10 in the liver at 6 and 27 h post-LPS, with an effect more pronounced in LPS-than in PBS-treated mice, this effect had no consequence on proinflammatory cytokines expression in the liver and was not found in the brain. This is possibly because there was no change in IL-10 protein levels, which remains to be examined. Furthermore, we observed no differences in LPS-induced IDO activation between mice treated with leucine vs. vehicle, indicating that leucine did not alter the activation of IDO in response to LPS. This is further supported by the reductions in kynurenine:tryptophan ratios of control mice. It should be noted that in our depression-like behavioral experiments, mice were treated with leucine at baseline and at 6 h after LPS, whereas in our experiments to examine peak inflammatory responses, mice were only treated with leucine at baseline. A recent study demonstrated that leucine may be protective in fish against inflammatory responses to LPS (16), however the fish were supplemented with leucine for 8 weeks prior to LPS exposure which is much longer than the acute exposure of leucine used in this study.
To confirm that leucine acts on kynurenine entry to the brain we administered leucine to mice treated with exogenous kynurenine and showed that as expected, leucine did block the increase in kynurenine levels and the depression-like behaviour induced by exogenously administered kynurenine. In the behavioral experiment we did not include a leucine/placebo group as the objective was not to assess once more the effects of leucine per se but just to confirm that leucine did block the kynurenine-induced increase in immobility in the forced swim test. Of note, while exogenous kynurenine increased brain levels of kynurenine it did not lead to an increase in plasma levels of kynurenine. This is likely due to exogenous kynurenine being rapidly metabolised in the periphery and the brain. A previous study showed that kynurenine administered to mice was rapidly metabolised in the brain and liver within 30 minutes (17) - 2.5 h earlier than brain kynurenine assessment in the current study.
It appears that leucine inhibited blood-to-brain kynurenine transport, which reduced LPS-induced depression-like behavior. To identify the mechanism responsible for the reductions in kynurenine:tryptophan ratios of leucine-treated mice, we undertook in vitro modelling of tryptophan, kynurenine, and leucine transport across the BBB. BMECs form tight junctions when cultivated on transwell membranes and represent a validated in vitro model of the BBB (18). As such, this model is ideally suited to answering key proof-of-principle questions regarding leveraging transport of kynurenine across the BBB to treat depression. We first confirmed that leucine can compete with kynurenine for entry into the brain via LAT1 working as a common influx transporter by assessing the rate of transport of tritiated leucine across the BMEC layer. Tritiated leucine influx (movement from the luminal to the abluminal transwell chamber) was equally inhibited by unlabeled leucine. This is consistent with kynurenine influx being mediated by LAT1, thus providing a mechanism by which leucine inhibits its entry into brain.
We then examined influx directly in the in vitro BBB model by placing tritiated kynurenine in the luminal chamber and found not only a paradoxical negative value for influx, but also that unlabeled kynurenine paradoxically increased influx. The usual explanation for these finding is the presence of a robust efflux system. Efflux returns any tritiated kynurenine entering the abluminal chamber to the luminal chamber; as this includes material that has leaked across, uptake is even lower than that of 14C-sucrose and so is negative when corrected. Unlabeled material also crosses and can accumulate to levels that inhibit the efflux system, thus unmasking influx. Tryptophan also produced the paradoxical increase in tritiated kyurenine influx, but it is unclear whether this is because it is also a substrate for the transporter or because tryptophan was metabolized into kynurenine by brain endothelial cell IDO. By placing tritiated kynurenine in the abluminal chamber, we were able to formally demonstrate the presence of an efflux system for kynurenine. The ability of unlabeled kynurenine to paradoxically increase efflux of tritiated kynurenine indicates that the transporter is bidirectional, but with efflux being the dominant direction of transport. While the presence of this efflux transporter limits our ability to show in vitro that unlabeled leucine directly blocks tritiated kynurenine influx to the abluminal chamber, it indicates that previously reported brain-to-blood transport of kynurenine produced in the brain in response to systemic inflammation likely occurs through this newly discovered efflux mechanism (19). The nature of this transporter is still unknown despite its potential importance as its functioning in in vivo conditions could help decreasing the brain levels of kynrenine especially in the context of brain IDO activation as a consequence of direct brain injury or inflammation. We found that BCH and leucine did not affect the movement of tritiated kynurenine placed in the luminal chamber and had no significant effect for tritiated kynurenine placed in the abluminal chamber, showing that LAT1 does not play a major role in kynurenine efflux. Whatever the case, these findings confirm that leucine is a good choice for competitively inhibiting kynurenine transport into the brain and blocking depression-like behavior because it inhibits kynurenine transport to the brain but not its removal from the brain. In theory leucine could be replaced by other branched chain amino acids which share LAT-1 as their predominant transporter, and therefore may be equally efficacious in competing with kynurenine blood-to-brain transport and preventing inflammation-induced depression. This possibility was not tested as the primary objective of the present study was to demonstrate that interfering with blood-to-brain transport of kynurenine is sufficient to abrogate inflammation-induced depression.
Other limitations of our study are mainly due to the nature of the acute inflammatory stimulus that was used to induce inflammation-induced depression. LPS-induced depression-like behavior recapitulates the main features of inflammation-induced depression but lacks the chronicity that is characteristic of major depressive disorder. It still remains to be determined whether repeated administration of leucine can abrogate depression-like behavior associated with chronic inflammatory conditions and to ensure no toxicity arises from the use of leucine. There are currently, many NIH approved studies investigating the use of leucine in combating frailty and sarcopenia in aged individuals (ClinicalTrials.gov), which have shown no toxicity and may be informative in regards to the efficacy of leucine in individuals with chronic inflammation. In addition, it could be objected that chronic treatment with leucine or other branched chain amino acids might increase the risk for type 2 diabetes as circulating levels of branched chain amino acids tend to be elevated in individuals with obesity and insulin resistance. However, this association does not appear to be causal and more likely reflects the decreased activity of catabolic enzymes (20).
Conclusion
A summary of our findings on the mechanisms of leucine competitive inhibition of kynurenine blood-to-brain transport and blockade of depression are provided in Figure 4. Taken together these findings provide the first evidence that leveraging blood-brain barrier transport mechanisms can be used in the prevention and treatment of inflammation-induced depression-like symptoms. Specifically, here we demonstrated that blockade of kynurenine transport from the blood to the brain is effective in preventing inflammation-induced depression-like symptoms and potentially treating depression-like symptoms in the absence of inflammation. We confirmed that leucine is an optimal choice as a competitive antagonist of kynurenine entry to the brain using an in vitro BMEC model of blood-to-brain transport as it competes with kynurenine to bind to LAT1 for influx to the brain but does not compete with kynurenine for efflux from the brain. Supplementary information is available at Molecular Psychiatry’s website.
Figure 4. Diagrammatic representation of our major finding on leucine blockade of depression-like behavior in mice with or without inflammation.
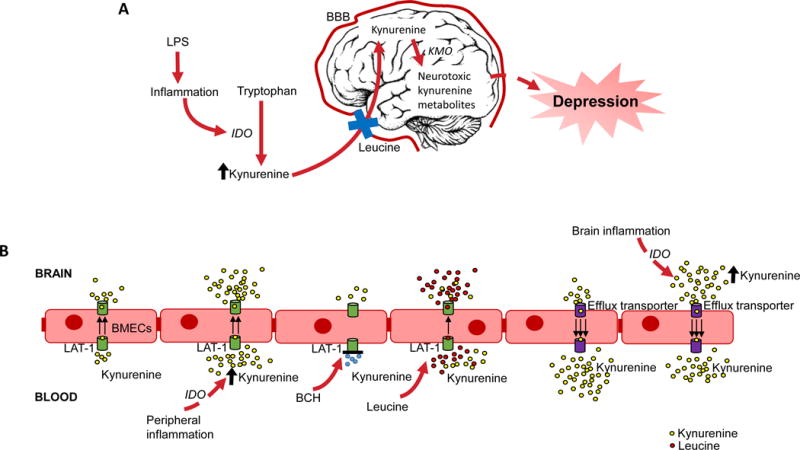
A Overview of pathway of inflammation-induced depression and the anti-depressant effects of leucine. B Overview of kynurenine blood-to-brain transport across BMECs. Inflammation increases blood kynurenine resulting in more kynurenine binding to LAT1 and being transported into the brain, which is blocked by the LAT1 specific antagonist BCH. Leucine competitively inhibits kynurenine binding to LAT1 and thus reduces blood-to-brain transport of kynurenine. An efflux transporter predominates kynurenine brain-to-blood transport, which is not LAT1 and is not bound by leucine. This efflux transporter extrudes kynurenine produced locally by inflammation.
Supplementary Material
Acknowledgments
This work was supported by the University of Texas MD Anderson Cancer Center [Support Grant CA016672], National Institutes of Health (R21MH104694) and National Institutes of Health National Institute of Neurological Disorders and Stroke [R01-NS073939]. RD has received honorarium from Danone Nutricia Research for work not related to this study. AKW is supported by a National Breast Cancer Foundation fellowship (PF-15-014). We thank Dr. Subhashree Kumaravel and Ms. Darlene Estrada for their help with the determination of the effects of leucine on kynurenine transport in the brain. We thank Jasmin Walker for her artistic input.
Footnotes
CONFLICT OF INTEREST:
Dr. Dantzer has received honoraria from Danone Nutricia Research, France, that are not related to the work presented in this article. All other authors declare no conflict of interest.
References
- 1.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013;38(9):1609–16. doi: 10.1038/npp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. Journal of clinical psychopharmacology. 2002;22(1):86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biological psychiatry. 2003;54(9):906–14. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 5.Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, et al. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Molecular psychiatry. 2001;6(4):475–80. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 6.Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Molecular psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. Journal of immunology (Baltimore, Md: 1950) 2009;182(5):3202–12. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Molecular psychiatry. 2009;14(5):511–22. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry. 2016;6:e918. doi: 10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kita T, Morrison PF, Heyes MP, Markey SP. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L-kynurenine and quinolinic acid pools in brain. Journal of neurochemistry. 2002;82(2):258–68. doi: 10.1046/j.1471-4159.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 11.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(21):12079–84. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omidi Y, Barar J, Ahmadian S, Heidari HR, Gumbleton M. Characterization and astrocytic modulation of system L transporters in brain microvasculature endothelial cells. Cell biochemistry and function. 2008;26(3):381–91. doi: 10.1002/cbf.1455. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor JC, Andre C, Wang Y, Lawson MA, Szegedi SS, Lestage J, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(13):4200–9. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ni Y, Teng T, Li R, Simonyi A, Sun GY, Lee JC. TNFα alters occludin and cerebral endothelial permeability: Role of p38MAPK. PloS one. 2017;12(2):e0170346. doi: 10.1371/journal.pone.0170346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekine A, Okamoto M, Kanatani Y, Sano M, Shibata K, Fukuwatari T. Amino acids inhibit kynurenic acid formation via suppression of kynurenine uptake or kynurenic acid synthesis in rat brain in vitro. SpringerPlus. 2015;4:48. doi: 10.1186/s40064-015-0826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giri SS, Sen SS, Jun JW, Sukumaran V, Park SC. Protective effects of leucine against lipopolysaccharide-induced inflammatory response in Labeo rohita fingerlings. Fish & shellfish immunology. 2016;52:239–47. doi: 10.1016/j.fsi.2016.03.148. [DOI] [PubMed] [Google Scholar]
- 17.Wang XD, Notarangelo FM, Wang JZ, Schwarcz R. Kynurenic acid and 3-hydroxykynurenine production from D-kynurenine in mice. Brain research. 2012;1455:1–9. doi: 10.1016/j.brainres.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks WA. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain, behavior, and immunity. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kita T, Morrison PF, Heyes MP, Markey S. Effects of systemic and central nervous system localized inflammation on the contributions of metabolic precursors to the L kynurenine and quinolinic acid pools in brain. Journal of neurochemistry. 2002;82(2):258–68. doi: 10.1046/j.1471-4159.2002.00955.x. [DOI] [PubMed] [Google Scholar]
- 20.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and ‐ insulin resistance. Nature reviews Endocrinology. 2014;10(12):723–36. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


