Abstract
Vocal individuality is a prerequisite for individual recognition, especially when visual and chemical cues are not available or effective. Vocalizations encoding information of individual identity have been reported in many social animals and should be particularly adaptive for species living in large and complexly organized societies. Here, we examined the individuality in coo calls of adult male golden snub-nosed monkeys (Rhinopithecus roxellana) living in a large and multilevel society. Coo calls are one of the most frequently occurring call types in R. roxellana and likely serve as the signals for contact maintenance or advertisement in various contexts including group movement, foraging, and resting. From April to October 2016, April to July 2017, and September to October 2017, we recorded a total of 721 coo calls from six adult males in a provisioned, free-ranging group and one adult male in captivity in Shennongjia National Park, China. We selected 162 high-quality recordings to extract 14 acoustic parameters based on the source-filter theory. Results showed that each of all parameters significantly differed among individuals, while pairwise comparisons failed to detect any parameter that was different between all pairs. Furthermore, a discriminant function analysis indicated that the correct assignment rate was 80.2% (cross-validation: 67.3%), greater than expected by chance (14.3%). In conclusion, we found evidence that coo calls of adult male R. roxellana allowed the reliable accuracy of individual discrimination complementarily enhanced by multiple acoustic parameters. The results of our study point to the selective pressures acting on individual discrimination via vocal signals in a highly gregarious forest-living primate.
Electronic supplementary material
The online version of this article (10.1007/s10071-018-1222-y) contains supplementary material, which is available to authorized users.
Keywords: Rhinopithecus roxellana, Vocal individuality, Source-filter theory, Multilevel society
Introduction
Vocal cues play an important role in the instant communication of social animals, especially when visual and chemical signals are not available or effective (Kondo and Watanabe 2009; Rendall and Owren 2002). Many animals have evolved a variety of call types that differ acoustically and serve a number of functions, such as maintaining contact (Kondo and Watanabe 2009; Weiss et al. 2001), cultivating social relationships (Bolt and Tennenhouse 2017), and warning each other about predators (Seyfarth et al. 1980; Zuberbühler 2001). However, the acoustic structures of certain call types display graded within-type variation (Soltis et al. 2005). This acoustic variation usually conveys important function-related information about callers, such as group membership (Delgado 2007; Fan et al. 2011), age (Charlton et al. 2009a; Fischer et al. 2004), sex (Charlton et al. 2009a; Ey et al. 2007), body size (Pfefferle and Fischer 2006; Reby and McComb 2003), social rank (Bergman et al. 2003; Fischer et al. 2004), and estrus state (Charlton et al. 2010).
Individual distinctiveness in acoustic features of the same call type has also been reported in many species of social animals (e.g., Spheniscus demersus: Favaro et al. 2015; Pan troglodytes: Levréro and Mathevon 2013; Papio hamadryas ursinus: Rendall 2003; Dama dama: Vannoni and McElligott 2007). Vocal individuality provides a crucial prerequisite for individual recognition (Pollard and Blumstein 2012; Tibbetts and Dale 2007). Specifically, vocal signals encoding information of individual identity can be utilized to make decisions about whether to approach, avoid, or ignore particular individuals (Chapman and Weary 1990) and thus help to mediate social relationships within and between groups (Bolt and Tennenhouse 2017). For example, whinny calls of spider monkeys (Ateles geoffroyi) exhibit individual distinctiveness and are used to maintain appropriate cohesion and spacing when group members forage outside of each other’s visual range (Ramos-Fernández 2005). African penguins (Spheniscus demersus) produce contact calls containing information about individual identity, which are used by isolated individuals to rejoin companions (Favaro et al. 2015). In addition, vocal individuality is also essential for kin selection such as in the context of parental investment (Callorhinus ursinus: Charrier et al. 2003; Papio cynocephalus ursinus: Rendall et al. 2000), and for sexual selection, which involves male–male competition and female choice (Cervus elaphus: McComb 1991).
It has been suggested that individuality of acoustic signals is more important for species living in large and complex social systems, which include more interacting individuals, more diverse interactions, and/or more social structural levels (Freeberg et al. 2012; Pollard and Blumstein 2011, 2012; Tibbetts and Dale 2007). For example, some call types emitted by species with fission–fusion social dynamics exhibit clear individuality, such as contact calls of African elephants (McComb et al. 2003), whistles of bottlenose dolphins (Tursiops truncatus) (Janik and Slater 1998; Tyack 2000), and grunts of chacma baboons (Papio cynocephalus ursinus) (Owren et al. 1997; Rendall 2003). A comparative study of eight sciurid rodent species has shown that group size facilitates the evolution of individuality in alarm calls (Pollard and Blumstein 2011). However, evidence for vocal individuality in Asian colobine primates living in large and multilevel societies is still lacking.
The source-filter theory of vocal production, originated from human voice studies (Titze 1994), states that the fundamental frequencies and formant frequencies vary among individuals due to the differences in the length and shape of the callers’ vocal apparatus (Reby and McComb 2003), and one or both may provide robust individual distinctiveness. The source-filter theory has been widely applied to vocal studies in nonhuman animals (Charlton et al. 2010, 2017; Taylor and Reby 2010). For example, the fundamental frequencies of coo calls encode information of individual identity in Japanese macaques (Macaca fuscata) (Ceugniet and Izumi 2004). Formant frequencies play an important role in the individuality in contact calls of African elephants (McComb et al. 2003) and grunt calls of red-bellied lemurs (Eulemur rubriventer) (Gamba et al. 2012). Both fundamental frequency and formant parameters encode information of individual identity in contact calls of African penguins (Favaro et al. 2015).
The golden snub-nosed monkey (Rhinopithecus roxellana), a colobine species endemic to China, inhabits temperate forests in mountainous areas at high altitudes of 1000–4100 m and lives in large groups varying from 80 to more than 400 individuals (Kirkpatrick and Grueter 2010). Its social organization is described as a multilevel society, which comprises one breeding band consisting of several one-male multi-female units (OMUs) and one (occasionally more than one) peripherally attached all-male unit (AMU) (Qi et al. 2014; Yao et al. 2011; Zhang et al. 2006). The OMUs of the breeding band coordinate their activities on a day-to-day basis, while each of them is a relatively independent social entity maintained mainly by matrilineal kin-bonds (Wang et al. 2013; Zhang et al. 2012). The AMU comprises former OMU resident males who have been replaced, and subadult and juvenile males waiting for opportunities to take over the resident positions or to emigrate to other groups (Qi et al. 2017; Yao et al. 2011). Inter-individual interactions within and between units include both competitive and cooperative elements (Liu et al. 2016; Wada et al. 2015; Xiang et al. 2014; Zhang et al. 2010). The ecological and social settings of this primate (forest habitats with limited visibility and large groups with a multilevel structure) are expected to be conducive to the evolution of high levels of vocal individuality.
However, to date, it is not yet known whether and how vocal signals of R. roxellana (and the genus of snub-nosed monkeys in general) can convey information of individual identity. Coo calls are one of the most frequently occurring call types in adult R. roxellana and likely function to maintain contact in various contexts including group movement, foraging, and resting (Fan et al. 2018). The spectrogram of coo calls is characterized by few frequency modulations and rich harmonic patterns (Fan et al. 2018), and the dense harmonic structure should highlight the formants, making these vocalizations well suited for individual discrimination (Charlton et al. 2009b; Owren and Rendall 2001). Here, based on the source-filter theory, we investigated the individuality in coo calls of adult male R. roxellana. We chose adult males as our study subjects, because they play an important role in the maintenance of social cohesion and spacing at both levels within and between units (Huang et al. 2017; Qi et al. 2017; Xiang et al. 2014). We first examined whether coo calls had a sufficient degree of individual distinctiveness that would permit discrimination among callers. We then examined and identified the key acoustic parameters determining the distinctiveness among different individuals. The findings of this study will improve our understanding of social cognition in species living in large and multilevel societies.
Methods
Study site and subjects
This study was carried out at the Dalongtan Conservation Station (DCS) and the Golden Snub-nosed Monkey Reproduction Center (GRC) in Shennongjia National Park, Hubei Province, China. To facilitate ecotourism and research, a monkey group at DCS has been habituated and provisioned since 2006 (Yao et al. 2011). Food items including lichens, pine seeds, apples, carrots, oranges, and peaches are provisioned two or three times a day. When not provisioned, the monkeys range freely within an area of approximately 9 km2, characterized by a deciduous broadleaf and evergreen conifer mixed forest. We can identify all adult individuals based on their physical features (e.g., body size, hair coloration, scar, and face shape) in proximity (0.5–10 m). During the period from April to October 2016, the monkey group was composed of five OMUs (containing one adult male in each: GE, HH, NN, XB, and XZ) and one AMU (containing two adult males: DD and HT). In October 2016, an OMU male, NN, was replaced by an AMU male, DD. After being taken over, NN moved to the AMU and then emigrated entirely from the monkey group in November 2016. In December 2016, DD transferred back to the AMU since his unit members joined the OMU of XZ voluntarily. From that time to the end of this study, no change occurred in the unit memberships of adult males.
GRC, about 1200 m away from DCS, is responsible for rescuing and breeding injured monkeys from the wild. During the study period, an adult male, DW, was rescued and kept in captivity at GRC. Food items fed to the monkey are the same as those to the DCS group.
A total of seven adult males were selected as our study subjects, six (DD, GE, HH, NN, XB, and XZ) from DCS and one (DW) from GRC. The adult male from the DCS group, HT, was excluded because of the difficulties associated with approaching him to collect ample vocalization samples.
Vocalization recordings
We recorded vocalizations outside of the provisioning times and when there were not excessive human disturbances during the period from April to October 2016, April to July 2017, and September to October 2017. Vocalizations were collected at a sampling rate of 44.1 kHz (16 bit) using a Tascam DR44-WL digital recorder connected to a Sennheiser ME 66 directional microphone at distances within 10 m to the monkeys. For the DCS group, we selected one adult male as the subject on an observation day (08:00–18:00) and recorded his coo calls using 5-min focal animal sampling (Fan et al. 2018). We then rotated to another on the next day. Occasionally, we recorded calls of non-focal adult males opportunistically to increase the total amount of coo call samples using ad libitum sampling. For the adult male at GRC, we also used 5-min focal animal sampling to collect coo call samples. We recorded coo calls of GE over 15 days, HH over 21 days, NN over 17 days, XB over 24 days, XZ over 22 days, DD over 14 days, and DW over 7 days. The vocalization data were uploaded to a laptop computer for storage and analysis.
This study complied with the animal protection laws of the People’s Republic of China and was approved by the Committee of Animal Welfare and Ethic of the Beijing Normal University, the University of Chinese Academy of Sciences, and Shennongjia National Park. We made efforts to minimize potential disturbances to the monkeys during vocalization recording.
Acoustic parameter measurements
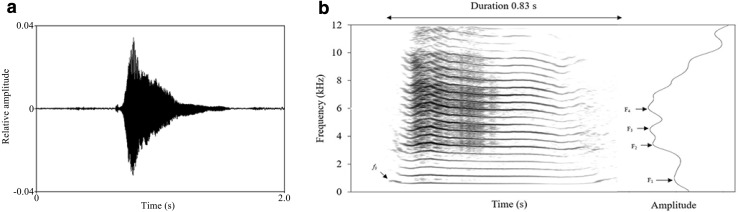
We used Adobe Audition CS6 (Adobe, USA) and Praat package 5.3.72 (P. Boersma and D. Weenink, University of Amsterdam, the Netherlands) for acoustic analyses. All vocalizations were standardized in Adobe Audition CS6. We then carried out visual and acoustical inspection of each coo call with narrow-band spectrograms generated by the Praat sound editor window (Gaussian window shape, view range = 0–12,000 Hz, window length = 0.03 s, dynamic range = 70 dB; Fig. 1. We excluded from further analysis poor-quality recordings with excessive background noise such as bird and stream sounds, and those that overlapped with other calls. For each high quality recording selected, we measured a series of acoustic parameters, including temporal (call duration), source-related (fundamental frequency: f0), and filter-related features (formant), and mean harmonics-to-noise ratio (HNR). We extracted the f0 contour of recordings using a cross-correlation method [Sound: To Pitch (cc) command; time step = 0.01 s, pitch floor = 75 Hz, pitch ceiling = 1200 Hz]. We measured temporal and source-related parameters including call duration, and the mean (mean f0), start (start f0), end (end f0), minimum (min f0), maximum (max f0), and standard deviation (SD f0) of fundamental frequency values from each extracted f0 contour. We calculated the range of fundamental frequency (range f0) as max f0 minus min f0. To measure formant parameters, we extracted the first four mean formants (F1–F4) of each recording using a Linear Predictive Coding analysis [Sound: To Formant (burg) command; time step = 0.01 s, maximum number of formants = 5, maximum formant = 8000 Hz]. We then used the method described by Reby and McComb (2003) to calculate the value of formant dispersion (ΔF). Finally, we measured the HNR value of each recording using the “To Harmonicity (cc) command” (time step = 0.01 s, minimum pitch = 75 Hz, silence threshold = 0.1, and periods per window = 1).
Fig. 1.
The waveform of a coo call from an adult male R. roxellana (a); Spectrogram (Gaussian window shape, view range = 0 − 12000 Hz, window length = 0.03 s, dynamic range = 70 dB, time step = 0.002 s, frequency step = 20 Hz) and LPC spectrum (Cepstral smoothing: 1200 Hz) of the coo call showing f0 and formants (F1–F4) (b)
Statistical analysis
We first calculated within-individual (CVw) and between-individual (CVb) coefficients of variation for each acoustic parameter as follows: CV = 100 (1 + 1/4n) (SD/) (Robisson et al. 1993). In this formula, n represents the sample size of vocalizations, SD the standard deviation of the sample, and the mean value. We calculated the potential for individual identity coding (PIC) using the ratio of the CVb to the mean CVw for all individuals (Gamba et al. 2012). For each acoustic parameter measured, a PIC value more than 1 indicates that this parameter has the potential for individual discrimination because of the lower variability within individuals than between individuals (Robisson et al. 1993). Furthermore, we performed a Kruskal–Wallis test to investigate which acoustic parameter was different among individuals. If the test yielded a significant result for a parameter, we carried out pairwise comparisons using Mann–Whitney U tests.
To quantify the individual distinctiveness of coo calls, we performed a principal component analysis (PCA) and subsequently a discriminant function analysis (DFA). PCA allowed us to obtain a reduced number of orthogonal variables (principal components: PCs) that accounted for the most amount of variance in the data set. We retained the PCs with eigenvalues greater than 0.6 (Kaiser’s criterion) using a varimax rotation method to improve component interpretation (Vannoni and Mcelligott 2007). These PCs were tested for normality (Kolmogorov–Smirnov test), and then used as input variables in the subsequent DFA. Based on the discriminant functions combined by the predictor variables that best describe the differences among groups, DFA assigns each vocalization to its appropriate group (correct) or another group (incorrect). Because the number of calls per individual was unbalanced, classification coefficients were adjusted according to the observed group sizes. For cross validation, we used the leave-one-out classification method, in which each case was classified by the functions derived from all cases except that one. All data were analysed with SPSS 21.0, and the tests were two-tailed with a significance level of 0.05 except the Mann–Whitney U tests, in which we used the Bonferroni adjusted significance level of 0.05/21 = 0.002.
Results
We recorded a total of 721 vocalization samples during the study period and selected 162 high quality recordings for further analysis (Table S1). We found that the CVb value of each acoustic parameter was higher than the mean CVw value, and thus all PIC values were greater than 1 (Table 1). The Kruskal–Wallis tests showed that each of all parameters was significantly different among individuals (Table 2). However, the pairwise comparisons failed to detect any parameter that was different between all pairs.
Table 1.
The coefficients of variation within (CVw) and between individuals (CVb), and the potential for individual identity coding (PIC) for each acoustic parameter of coo calls from adult male R. roxellana
| Parameter | Mean CVw (%) | CVb (%) | PIC |
|---|---|---|---|
| Duration | 15.80 | 18.67 | 1.18 |
| Mean f0 | 7.39 | 9.98 | 1.35 |
| SD f0 | 33.33 | 36.62 | 1.10 |
| Max f0 | 9.89 | 13.79 | 1.39 |
| Min f0 | 22.42 | 29.59 | 1.32 |
| Range f0 | 31.72 | 35.08 | 1.11 |
| Start f0 | 12.88 | 17.08 | 1.33 |
| End f0 | 25.55 | 30.38 | 1.19 |
| HNR | 18.79 | 28.10 | 1.50 |
| F 1 | 8.52 | 10.08 | 1.18 |
| F 2 | 3.56 | 5.32 | 1.49 |
| F 3 | 2.72 | 5.24 | 1.92 |
| F 4 | 3.16 | 3.64 | 1.15 |
| ΔF | 4.31 | 4.96 | 1.15 |
Table 2.
Inter-individual comparisons for each acoustic parameter of coo calls from adult male R. roxellana
| Parameter | Kruskal–Wallis test | Pairs for which differences were detected based on Mann–Whitney U tests* | |
|---|---|---|---|
| χ 2 | P value | ||
| Duration | 52.71 | < 0.001 | DD-GE, DD-XZ, DW-GE, GE-HH, GE-NN, GE-XB, HH-XB, HH-XZ, NN-XB, NN-XZ |
| Mean f0 | 74.34 | < 0.001 | DD-DW, DD-GE, DD-HH, DD-NN, DD-XB, DD-XZ, DW-HH, DW-XB, GE-HH, GE-XB, HH-XZ, NN-XZ XB-XZ |
| SD f0 | 23.65 | 0.001 | DW-GE, DW-XZ |
| Max f0 | 40.02 | < 0.001 | DD-DW, DD-GE, DD-NN, DD-XB, DD-XZ, DW-NN, DW-XB, GE-NN, NN-XZ, XB-XZ |
| Min f0 | 59.62 | < 0.001 | DD-DW, DD-GE, DD-HH, DD-NN, DD-XB, DD-XZ, DW-XB, GE-HH, GE-NN, GE-XB, HH-XZ, NN-XZ XB-XZ |
| Range f0 | 13.88 | 0.031 | GE-XB |
| Start f0 | 60.04 | < 0.001 | DD-GE, DD-NN, DD-XZ, DW-GE, DW-NN, DW-XZ, GE-HH, HH-NN, HH-XB, HH-XZ, XB-XZ |
| End f0 | 45.16 | < 0.001 | DD-GE, DD-XB, DD-XZ, DW-NN, GE-NN, NN-XB, NN-XZ |
| HNR | 69.65 | < 0.001 | DD-GE, DD-XB, DD-XZ, DW-GE, DW-XZ, GE-HH, GE-NN, GE-XB, HH-NN, NN-XB, NN-XZ |
| F 1 | 47.39 | < 0.001 | DD-DW, DW-GE, DW-NN, DW-XB, DW-XZ, HH-XB, HH-XZ |
| F 2 | 76.88 | < 0.001 | DD-GE, DD-HH, DD-NN, DD-XB, DD-XZ, DW-GE, DW-HH, DW-XB, DW-XZ, GE-NN, HH-NN, NN-XB NN-XZ |
| F 3 | 95.48 | < 0.001 | DD-DW, DD-NN, DW-GE, DW-HH, DW-NN, DW-XB, DW-XZ, GE-HH, GE-NN, HH-NN, HH-XB, NN-XB, NN-XZ |
| F 4 | 68.34 | < 0.001 | DD-DW, DD-GE, DD-NN, DW-HH, DW-XB, DW-XZ, GE-HH, GE-XB, GE-XZ, HH-NN, NN-XB, NN-XZ |
| ΔF | 64.49 | < 0.001 | DD-DW, DD-GE, DD-NN, DW-HH, DW-XB, DW-XZ, GE-HH, GE-XB, GE-XZ, HH-NN, NN-XB, NN-XZ |
*Adjusted significance level of 0.05/21 = 0.002
The first seven PCs explained 91.7% of the total variance in the data set (Table S2). Based on the seven PCs, DFA correctly assigned 80.2% of coo calls (Table 3). The classification accuracy of cross-validation was 67.3%, which was better than the 14.3% expected by chance (binomial test, P < 0.001). DFA generated six canonical discriminant functions, and the first three had eigenvalues > 0.5 (Function 1: 2.4, Function 2: 1.7, and Function 3: 0.6) and cumulatively explained 95.0% of the variance (Table S3). Function 1 explained 48.5% of the variance and was primarily related to PC3 and PC5. PC3 was mainly associated with Range f0, Min f0 and SD f0, while PC5 with F1 and F4. Function 2 explained 35.0% of the variance and was primarily associated with PC4, which was most strongly related to F1. Function 3 explained 11.5% of the variance and was mainly related to PC7, which was primarily associated with call duration.
Table 3.
The classification (cross-validation) of discriminant function analysis for seven individuals of adult male R. roxellana
| Individual | Predicted classification | |||||||
|---|---|---|---|---|---|---|---|---|
| DD | GE | HH | NN | XB | XZ | DW | Total | |
| DD | 7 (6) | 0 (0) | 0 (1) | 1 (1) | 4 (4) | 0 (0) | 0 (0) | 12 |
| GE | 0 (0) | 12 (11) | 0 (0) | 0 (0) | 0 (1) | 4 (4) | 0 (0) | 16 |
| HH | 3 (4) | 0 (0) | 25 (20) | 0 (0) | 4 (5) | 1 (2) | 0 (2) | 33 |
| NN | 0 (1) | 0 (0) | 0 (0) | 21 (18) | 0 (0) | 0 (1) | 2 (3) | 23 |
| XB | 0 (1) | 0 (0) | 2 (2) | 0 (0) | 33 (32) | 4 (4) | 1 (1) | 40 |
| XZ | 0 (0) | 2 (4) | 1 (2) | 1 (1) | 1 (3) | 18 (12) | 1 (2) | 24 |
| DW | 0 (0) | 0 (1) | 0 (3) | 0 (0) | 0 (0) | 0 (0) | 14 (10) | 14 |
Discussion
In the present study, we found that coo calls of adult male R. roxellana living in a large and multilevel social system could encode information of individual identity. Furthermore, all acoustic parameters analyzed in our study complementarily contributed to the differences in coo calls among individuals. This result supports the notion that subtle combinations of different acoustic features make up the call characteristics of an individual caller (Epsmark 1975). Similar findings that multiple parameters complementarily contribute to vocal individuality have also been reported in several other mammals and birds (Papio hamadryas ursinus: Rendall 2003; Presbytis thomasi: Wich et al. 2003; Dama dama: Vannoni and McElligott 2007; Pan troglodytes: Levréro and Mathevon 2013; Spheniscus demersus: Favaro et al. 2015; Bos taurus: Torre et al. 2015).
The acoustic parameters that contributed most to individuality were duration (temporal parameter), Range f0, Min f0, SD f0 (source-related parameters), and F1, F4 (filter-related parameters). This result suggests that three different parts of the respiratory apparatus, i.e., the lungs, vocal folds, and vocal tract, played important roles in producing and shaping the inter-individual differences in coo calls of adult male R. roxellana. Duration of acoustic waveform is determined by the airflows modulated by the chest muscles and the vital capacity of callers (Favaro et al. 2015). Therefore, call duration exhibits relatively stabilized variability within individuals (Favaro et al. 2015; Haimoff and Tilson 1985) and has the capacity to convey acoustically information about individual identity.
Differences in the source-related parameters are mainly determined by the length and stiffness (tension) of the vocal folds (Titze 1994). In general, the shorter and stiffer the vocal folds are, the higher the frequency is. Range f0 represents the difference between max f0 and min f0, while min f0 reflects the minimum rate of vibration of the vocal folds, which is physiologically constrained by its length (Titze 1994; Fitch 1997). SD f0, the standard deviation of fundamental frequency values, can be related to the stiffness of the vocal folds (Charlton et al. 2010). These characteristics of vocal folds may show some differences among individual callers of adult male R. roxellana (Charlton et al. 2009b). Individual distinctiveness in the source-related parameters of vocalizations have also been found in other animals, such as grunts of Guinea baboons (Papio papio) (Owren et al. 1997) and coo calls of Japanese macaques (Ceugniet and Izumi 2004).
Unlike the source-related features, the filter-related features of acoustic signals are determined by the shape and length of the vocal tract (Titze 1994). Specifically, lower formants are determined by the shape of the vocal tract, while higher formants are determined by the length (Reby and McComb 2003). The structure of the vocal tract is strongly related to body size (Fitch 1997; Reby and Mccomb 2003; Torre et al. 2015), and thus individual variation in formants is likely to reflect the differences in body size among callers (Pfefferle and Fischer 2006). In our study, both lower (F1) and higher (F4) formants were among the parameters most strongly related to individuality, suggesting that the shape and length of the vocal tract may vary among individuals of adult male R. roxellana. Several other studies have reported that the filter-related features are indicators of vocal individuality (Reby et al. 2006; Soltis et al. 2005), such as bleat calls of giant pandas (Ailuropoda melanoleuca) (Charlton et al. 2009b) and grunt calls of red-bellied lemurs (Gamba et al. 2012).
Interestingly, we found that HNR of coo calls had the potential for individual discrimination in adult male R. roxellana. HNR represents the ratio of harmonics to noise in spectrum resulting from turbulent airflows generated at the glottis during phonation (Hillenbrand 1987). Previous studies in humans have shown that the HNR values in elderly women are lower than those of juveniles and prime adults, suggesting that HNR may be a sensitive index of body aging, such as the ossification of cartilage and the degeneration of muscles and connective tissues in the larynx and vocal tract (Brown et al. 1996; Ferrand 2002). Similar findings that vocal structure can reflect age information have also been reported in some nonhuman animals, such as bleats of giant pandas (Charlton et al. 2009a) and loud calls of male chacma baboons (Fischer et al. 2004). Thus, HNR differences in coo calls among individuals of adult male R. roxellana may be a by-product of differences in age. Age information in vocal signals may advertise the callers’ physical quality indirectly (Fischer et al. 2004), which may further affect social relationships among different individuals.
Contact calls of social animals, such as coos of R. roxellana, serve as affiliative vocal signals that have evolved to coordinate group movement and establish and maintain social relationships with conspecifics (Bolt and Tennenhouse 2017; Kondo and Watanabe 2009). There is accumulating evidence that contact calls can be used for individual recognition (Sharpe et al. 2013), which is a critical precondition for successfully navigating a large and complex social landscape (Pollard and Blumstein 2012; Tibbetts and Dale 2007). Rhinopithecus roxellana lives in large and multilevel societies composed of several socio-spatially distinct units (Qi et al. 2014, 2017). The large group size and social complexity could constitute a strong selection force for the evolution of individuality in coo calls, facilitating individual discrimination (Pollard and Blumstein 2012), if individuals of a social unit are able or motivated to interact closely with or keep track of those of other units. While previous studies of some primates with multilevel societies noted the absence of such an ability or motivation (Bergman 2010; Maciej et al. 2013), studies of R. roxellana have shown that the social units of a group coordinate their activities on a daily basis (Liu et al. 2016; Wada et al. 2015; Zhang et al. 2010) and that the animals engage in particularly significant interactive events among units (Qi et al. 2017). For example, the resident males have been observed to collectively defend their OMUs against the bachelor males of the AMU (Huang et al. 2017; Xiang et al. 2014). The adult females of an OMU copulate with the males of other units and sire offspring (Guo et al. 2010; Zhao et al. 2005).
Although the capability of individual discrimination via coo calls in R. roxellana needs to be verified by playback experiments in further studies, the concurrent contexts of these vocalizations suggested that receivers could be able to recognize particular callers. Specifically, we observed that the resident males uttered coo calls towards the direction of their unit members that were out of sight during unit/group movement in the dense forest. Sometimes, the unit members responded vocally to these vocalizations (Fan et al. 2018). The resident males would continuously emit coo calls if their unit members did not catch up. Individual discrimination via vocal signals would allow the animals living in forest habitats to make adaptive decisions with regards to which individuals (and thus units/groups) to approach, avoid or ignore (Chapman and Weary 1990; Delgado 2007). For example, adult females may benefit from being able to recognize particular adult males based on vocal cues by reducing the risk of infanticide (Yao et al. 2016), as observed in Thomas langurs (Presbytis thomasi) (Wich 2002).
It is worth noting that the correct classification rate of DFA was not very high (67.3% by cross validation vs. 14.3% expected by chance), especially with respect to the large and complex social system of R. roxellana. It is very likely that the relatively small number of study subjects reduced the discriminant rate (Pfefferle et al. 2016). Alternatively, the vocalization samples occurred in various contexts, and the context-related variation in the acoustic structure may have partially masked the differences among individuals (Wich et al. 2003). Future studies are needed to address how vocal signals convey individuality information of the callers and contextual information of the calls.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate Jingyuan Yang, Shiping Zhu, Jinwen Yang, Zhonglin Yang, and Guangming Chen for their assistance in vocalization collection. We appreciate Prof. Ziyu Xiong from the Institute of Linguistics of the Chinese Academy of Social Sciences and Dr. Canwei Xia from the Beijing Normal University for their great suggestions on acoustic analysis. This work was financially supported by the Hubei Provincial Key Laboratory for Conservation Biology of Snub-nosed Monkeys, the National Key R & D Program of China (2016YFC0503202), and the Special Grant for Research and Education Integration (KJRH2015-016) and the Grant for Youth Scholars from the University of Chinese Academy of Sciences.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Dingzhen Liu, Phone: +86-10-58806699, Email: dzliu@bnu.edu.cn.
Xuecong Liu, Phone: +86-10-88256162, Email: xuecongliu@ucas.ac.cn.
References
- Bergman TJ. Experimental evidence for limited vocal recognition in a wild primate: implications for the social complexity hypothesis. Proc R Soc B Biol Sci. 2010;277:3045–3053. doi: 10.1098/rspb.2010.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. Hierarchical classification by rank and kinship in baboons. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. [DOI] [PubMed] [Google Scholar]
- Bolt LM, Tennenhouse E. Contact calling behaviour in the male ring-tailed lemur (Lemur catta) Ethology. 2017;123:614–626. doi: 10.1111/eth.12637. [DOI] [Google Scholar]
- Brown WS, Jr, Morris RJ, Murry T. Comfortable effort level revisited. J Voice. 1996;10:299–305. doi: 10.1016/S0892-1997(96)80011-7. [DOI] [PubMed] [Google Scholar]
- Ceugniet M, Izumi A. Individual vocal differences of the coo call in Japanese monkeys. Ethology. 2004;327:149–157. doi: 10.1016/j.crvi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Chapman AC, Weary DM. Variability in spider monkeys’ vocalizations may provide basis for individual recognition. Am J Primatol. 1990;22:279–284. doi: 10.1002/ajp.1350220407. [DOI] [PubMed] [Google Scholar]
- Charlton BD, Zhang Z, Snyder RJ. The information content of giant panda, Ailuropoda melanoleuca, bleats: acoustic cues to sex, age and size. Anim Behav. 2009;78:893–898. doi: 10.1016/j.anbehav.2009.06.029. [DOI] [Google Scholar]
- Charlton BD, Zhang Z, Snyder RJ. Vocal cues to identity and relatedness in giant pandas (Ailuropoda melanoleuca) J Acoust Soc Am. 2009;126:2721–2732. doi: 10.1121/1.3224720. [DOI] [PubMed] [Google Scholar]
- Charlton BD, Keating JL, Li R, Huang Y, Swaisgood RR. Female giant panda (Ailuropoda melanoleuca) chirps advertise the caller’s fertile phase. Proc R Soc B Biol Sci. 2010;277:1101–1106. doi: 10.1098/rspb.2009.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton BD, Taylor AM, Reby D. Function and evolution of vibrato-like frequency modulation in mammals. Curr Biol. 2017;27:2692–2697. doi: 10.1016/j.cub.2017.07.046. [DOI] [PubMed] [Google Scholar]
- Charrier I, Mathevon N, Jouventin P. Vocal signature recognition of mothers by fur seal pups. Anim Behav. 2003;65:543–550. doi: 10.1006/anbe.2003.2073. [DOI] [Google Scholar]
- Delgado RD., Jr Geographic variation in the long calls of male orangutans (Pongo spp.) Ethology. 2007;113:487–498. doi: 10.1111/j.1439-0310.2007.01345.x. [DOI] [Google Scholar]
- Espmark Y. Individual characteristics in the calls of reindeer calves. Appl Anim Ethol. 1975;1:50–59. doi: 10.1016/0304-3762(75)90094-2. [DOI] [Google Scholar]
- Ey E, Hammerschmidt K, Seyfarth RM, Fischer J. Age- and sex-related variations in clear calls of Papio ursinus. Int J Primatol. 2007;28:947–960. doi: 10.1007/s10764-007-9139-3. [DOI] [Google Scholar]
- Fan PF, Xiao W, Feng JJ, Scott MB. Population differences and acoustic stability in male songs of wild western black crested gibbons (Nomascus concolor) in Mt. Wuliang, Yunnan. Folia Primatol. 2011;82:83–93. doi: 10.1159/000329128. [DOI] [PubMed] [Google Scholar]
- Fan PL, Liu XC, Liu RS, Li F, Huang TP, Wu F, Yao H, Liu DZ. Vocal repertoire of free-ranging adult golden snub-nosed monkeys (Rhinopithecus roxellana) Am J Primatol. 2018 doi: 10.1002/ajp.22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaro L, Gamba M, Alfieri C, Pessani D, Mcelligott AG. Vocal individuality cues in the African penguin (Spheniscus demersus): a source-filter theory approach. Sci Rep. 2015;5:17255. doi: 10.1038/srep17255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand CT. Harmonics-to-noise ratio: an index of vocal aging. J Voice. 2002;16:480–487. doi: 10.1016/S0892-1997(02)00123-6. [DOI] [PubMed] [Google Scholar]
- Fischer J, Kitchen DM, Seyfarth RM, Cheney DL. Baboon loud calls advertise male quality: acoustic features and their relation to rank, age, and exhaustion. Behav Ecol Sociobiol. 2004;56:140–148. doi: 10.1007/s00265-003-0739-4. [DOI] [Google Scholar]
- Fitch WT. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J Acoust Soc Am. 1997;102:1213–1222. doi: 10.1121/1.421048. [DOI] [PubMed] [Google Scholar]
- Freeberg TM, Dunbar RI, Ord TJ. Social complexity as a proximate and ultimate factor in communicative complexity. Philos Trans R Soc Lond B Biol Sci. 2012;367:1785–1801. doi: 10.1098/rstb.2011.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamba M, Colombo C, Giacoma C. Acoustic cues to caller identity in lemurs: a case study. J Ethol. 2012;30:191–196. doi: 10.1007/s10164-011-0291-z. [DOI] [Google Scholar]
- Guo ST, Ji WH, Li M, Chang HL, Li BG. The mating system of the Sichuan snub-nosed monkey (Rhinopithecus roxellana) Am J Primatol. 2010;72:25–32. doi: 10.1002/ajp.20747. [DOI] [PubMed] [Google Scholar]
- Haimoff EH, Gittins SP. Individuality in the songs of wild agile gibbons (Hylobates agilis) of Peninsular Malaysia. Am J Primatol. 1985;8:239–247. doi: 10.1002/ajp.1350080306. [DOI] [PubMed] [Google Scholar]
- Hillenbrand J. A methodological study of perturbation and additive noise in synthetically generated voice signals. J Speech Lang Hear Res. 1987;30:448–461. doi: 10.1044/jshr.3004.448. [DOI] [PubMed] [Google Scholar]
- Huang ZP, Bian K, Liu Y, Pan RL, Qi XG, Li BG. Male dispersal pattern in golden snub-nosed monkey (Rhinopithecus roxellana) in Qinling Mountains and its conservation implication. Sci Rep. 2017;7:46217. doi: 10.1038/srep46217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik VM, Slater PJ. Context-specific use suggests that bottlenose dolphin signature whistles are cohesion calls. Anim Behav. 1998;56:829–838. doi: 10.1006/anbe.1998.0881. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick RC, Grueter CC. Snub-nosed monkeys: multilevel societies across varied environments. Evol Anthropol. 2010;19:98–113. doi: 10.1002/evan.20259. [DOI] [Google Scholar]
- Kondo N, Watanabe S. Contact calls: information and social function. Jpn Psychol Res. 2009;51:197–208. doi: 10.1111/j.1468-5884.2009.00399.x. [DOI] [Google Scholar]
- Levréro F, Mathevon N. Vocal signature in wild infant chimpanzees. Am J Primatol. 2013;75:324–332. doi: 10.1002/ajp.22108. [DOI] [PubMed] [Google Scholar]
- Liu RS, Yao H, Yang WJ, Xiang ZF. Motherinfant interaction in a provisioned group of golden snub-nosed monkeys Rhinopithecus roxellana in Shennongjia, China. Acta Theriol Sin. 2016;36:123–128. [Google Scholar]
- Maciej P, Patzelt A, Ndao I, Hammerschmidt K, Fischer J. Social monitoring in a multilevel society: a playback study with male Guinea baboons. Behav Ecol Sociobiol. 2013;67:61–68. doi: 10.1007/s00265-012-1425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb KE. Female choice for high roaring rates in red deer, Cervus elaphus. Anim Behav. 1991;41:79–88. doi: 10.1016/S0003-3472(05)80504-4. [DOI] [Google Scholar]
- McComb K, Reby D, Baker L, Moss C, Sayialel S. Long-distance communication of acoustic cues to social identity in African elephants. Anim Behav. 2003;65:317–329. doi: 10.1006/anbe.2003.2047. [DOI] [Google Scholar]
- Owren MJ, Rendall D. Sound on the rebound: bringing form and function back to the forefront in understanding nonhuman primate vocal signaling. Evol Anthropol. 2001;10:58–71. doi: 10.1002/evan.1014. [DOI] [Google Scholar]
- Owren MJ, Seyfarth RM, Cheney DL. The acoustic features of vowel-like grunt calls in chacma baboons (Papio cynocephalus ursinus): implications for production processes. J Acoust Soc Am. 1997;101:2951–2963. doi: 10.1121/1.418523. [DOI] [PubMed] [Google Scholar]
- Pfefferle D, Fischer J. Sounds and size: identification of acoustic variables that reflect body size in hamadryas baboons, Papio hamadryas. Anim Behav. 2006;72:43–51. doi: 10.1016/j.anbehav.2005.08.021. [DOI] [Google Scholar]
- Pfefferle D, Hammerschmidt K, Mundry R, Ruiz-Lambides AV, Fischer J, Widdig A. Does the structure of female rhesus macaque coo calls reflect relatedness and/or familiarity? PLoS One. 2016;11:e0161133. doi: 10.1371/journal.pone.0161133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KA, Blumstein DT. Social group size predicts the evolution of individuality. Curr Biol. 2011;21:413–417. doi: 10.1016/j.cub.2011.01.051. [DOI] [PubMed] [Google Scholar]
- Pollard KA, Blumstein DT. Evolving communicative complexity: insights from rodents and beyond. Philos Trans R Soc Lond B Biol Sci. 2012;367:1869–1878. doi: 10.1098/rstb.2011.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XG, Garber PA, Ji W, Huang ZP, Huang K, Zhang P, Guo ST, Wang XW, He G, Zhang P, Li BG. Satellite telemetry and social modeling offer new insights into the origin of primate multilevel societies. Nat Commun. 2014;5:5296–5305. doi: 10.1038/ncomms6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XG, Huang K, Fang G, Grueter CC, Dunn DW, Li YL, Ji WH, Wang XY, Wang RT, Garber PA, Li BG. Male cooperation for breeding opportunities contributes to the evolution of multilevel societies. Proc R Soc B Biol Sci. 2017;284:20171480. doi: 10.1098/rspb.2017.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Fernández G. Vocal communication in a fission-fusion society: do spider monkeys stay in touch with close associates? Int J Primatol. 2005;26:1077–1092. doi: 10.1007/s10764-005-6459-z. [DOI] [Google Scholar]
- Reby D, Mccomb K. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav. 2003;65:519–530. doi: 10.1006/anbe.2003.2078. [DOI] [Google Scholar]
- Reby D, Andréobrecht R, Galinier A, Farinas J, Cargnelutti B. Cepstral coefficients and hidden markov models reveal idiosyncratic voice characteristics in red deer (Cervus elaphus) stags. J Acoust Soc Am. 2006;120:4080–4089. doi: 10.1121/1.2358006. [DOI] [PubMed] [Google Scholar]
- Rendall D. Acoustic correlates of caller identity and affect intensity in the vowel-like grunt vocalizations of baboons. J Acoust Soc Am. 2003;113:3390–3402. doi: 10.1121/1.1568942. [DOI] [PubMed] [Google Scholar]
- Rendall D, Owren MJ. Animal vocal communication: say what? In: Bekoff M, Allen C, Burghardt GM, editors. The cognitive animal: empirical and theoretical perspectives on animal cognition. Cambridge: The MIT Press; 2002. pp. 307–313. [Google Scholar]
- Rendall D, Cheney DL, Seyfarth RM. Proximate factors mediating “contact” calls in adult female baboons (Papio cynocephalus ursinus) and their infants. J Comp Psychol. 2000;114:36–46. doi: 10.1037/0735-7036.114.1.36. [DOI] [PubMed] [Google Scholar]
- Robisson P, Aubin T, Bremond J. Individuality in the voice of emperor penguin Aptenodytes forsteri: adaptation to a noisy environment. Ethology. 1993;94:279–290. doi: 10.1111/j.1439-0310.1993.tb00445.x. [DOI] [Google Scholar]
- Seyfarth RM, Cheney DL, Marler P. Monkey responses to three different alarm calls: evidence of predator classification and semantic communication. Science. 1980;210:801–803. doi: 10.1126/science.7433999. [DOI] [PubMed] [Google Scholar]
- Sharpe LL, Hill A, Cherry MI. Individual recognition in a wild cooperative mammal using contact calls. Anim Behav. 2013;86:893–900. doi: 10.1016/j.anbehav.2013.07.023. [DOI] [Google Scholar]
- Soltis J, Leong K, Savage A. African elephant vocal communication II: rumble variation reflects the individual identity and emotional state of callers. Anim Behav. 2005;70:589–599. doi: 10.1016/j.anbehav.2004.11.016. [DOI] [Google Scholar]
- Taylor AM, Reby D. The contribution of source-filter theory to mammal vocal communication research. J Zool. 2010;280:221–236. doi: 10.1111/j.1469-7998.2009.00661.x. [DOI] [Google Scholar]
- Tibbetts EA, Dale J. Individual recognition: it is good to be different. Trends Ecol Evol. 2007;22:529–537. doi: 10.1016/j.tree.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Titze IR. Principles of voice production. Englewood Cliffs: Prentice-Hall; 1994. [Google Scholar]
- Torre MPDL, Briefer EF, Reader T, Mcelligott AG. Acoustic analysis of cattle (Bos taurus) mother—offspring contact calls from a source-filter theory perspective. Appl Anim Behav Sci. 2015;163:58–68. doi: 10.1016/j.applanim.2014.11.017. [DOI] [Google Scholar]
- Tyack PL. Dolphins whistle a signature tune. Science. 2000;289:1310–1311. doi: 10.1126/science.289.5483.1310. [DOI] [PubMed] [Google Scholar]
- Vannoni E, Mcelligott AG. Individual acoustic variation in fallow deer (Dama dama) common and harsh groans: a source-filter theory perspective. Ethology. 2007;113:223–234. doi: 10.1111/j.1439-0310.2006.01323.x. [DOI] [Google Scholar]
- Wada K, Li BG, Watanabe K. Affiliative interactions between one-male units in a band of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) living in the Qinling Mountains, China. Primates. 2015;56:327–337. doi: 10.1007/s10329-015-0475-1. [DOI] [PubMed] [Google Scholar]
- Wang XX, Wang CL, Qi XG, Guo ST, Zhao HT, Li BG. A newly-found pattern of social relationships among adults within one-male units of golden snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains, China. Integr Zool. 2013;8:400–409. doi: 10.1111/1749-4877.12026. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Garibaldi BT, Hauser MD. The production and perception of long calls by cotton-top tamarins (Saguinus oedipus): acoustic analyses and playback experiments. J Comp Psychol. 2001;115:258–271. doi: 10.1037/0735-7036.115.3.258. [DOI] [PubMed] [Google Scholar]
- Wich SA. Playbacks of loud calls to wild Thomas langurs (Primates; Presbytis thomasi): the effect of familiarity. Behaviour. 2002;139:79–87. doi: 10.1163/15685390252902283. [DOI] [Google Scholar]
- Wich SA, Koski S, Han DV, Schaik CPV. Individual and contextual variation in Thomas langur male loud calls. Ethology. 2003;109:1–13. doi: 10.1046/j.1439-0310.2003.00837.x. [DOI] [Google Scholar]
- Xiang ZF, Yang BH, Yu Y, Yao H, Grueter CC, Garber PA, Li M. Males collectively defend their one-male units against bachelor males in a multi-level primate society. Am J Primatol. 2014;76:609–617. doi: 10.1002/ajp.22254. [DOI] [PubMed] [Google Scholar]
- Yao H, Liu X, Stanford C, Yang J, Huang T, Wu F, Li YM. Male dispersal in a provisioned multilevel group of Rhinopithecus roxellana in Shennongjia Nature Reserve, China. Am J Primatol. 2011;73:1280–1288. doi: 10.1002/ajp.21000. [DOI] [PubMed] [Google Scholar]
- Yao H, Yu HL, Yang BH, Yang WJ, Xu H, Grueter CC, Li M, Xiang ZF. Male infanticide in the golden snub-nosed monkey (Rhinopithecus roxellana), a seasonally breeding primate. Int J Primatol. 2016;37:175–184. doi: 10.1007/s10764-016-9892-2. [DOI] [Google Scholar]
- Zhang P, Watanabe K, Li BG, Tan CL. Social organization of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains, Central China. Primates. 2006;47:374–382. doi: 10.1007/s10329-006-0178-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhao DP, Li BG. Postconflict behavior among female Sichuan snub-nosed monkeys Rhinopithecus roxellana within one-male units in the Qinling Mountains, China. Curr Zool. 2010;56:222–226. [Google Scholar]
- Zhang P, Li BG, Qi XG, Macintosh AJJ, Watanabe K. A proximity-based social network of a group of Sichuan snub-nosed monkeys (Rhinopithecus roxellana) Int J Primatol. 2012;33:1081–1095. doi: 10.1007/s10764-012-9608-1. [DOI] [Google Scholar]
- Zhao DP, Li BG, Li YH, Wada K. Extra-unit sexual behaviour among wild Sichuan snub-nosed monkeys (Rhinopithecus roxellana) in the Qinling Mountains of China. Folia Primatol. 2005;76:172–176. doi: 10.1159/000084379. [DOI] [PubMed] [Google Scholar]
- Zuberbühler K. Predator-specific alarm calls in Campbell’s monkeys, Cercopithecus campbelli. Behav Ecol Sociobiol. 2001;50:414–422. doi: 10.1007/s002650100383. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.