Abstract
Ecdysteroid hormones influence the development and reproduction of arthropods by binding a heterodimeric complex of nuclear receptors, the ecdysone receptor (EcR) and the retinoid-X-receptor/ultraspiracle (RXR/USP). Here, we report on the in vivo role(s) of the ecdysone receptor complex, SchgrEcR/SchgrRXR, in the female reproductive physiology of a major phytophagous pest insect, i.e. the desert locust, Schistocerca gregaria. Tissue and temporal distribution profiles were analysed during the first gonadotrophic cycle of adult female locusts. RNA interference was used as a reverse genetics tool to investigate the in vivo role of the ecdysone receptor complex in ovarian maturation, oogenesis, fertility and fecundity. We discovered that silencing the ecdysone receptor complex in S. gregaria resulted in impaired ovulation and oviposition, indicative for a crucial role of this complex in chorion formation. We also found evidence for a feedback of SchgrEcR/SchgrRXR on juvenile hormone biosynthesis by the corpora allata. Furthermore, we observed a tissue-dependent effect of the SchgrEcR/SchgrRXR knockdown on the transcript levels of the insulin receptor and neuroparsin 3 and 4. The insulin receptor transcript levels were upregulated in the brain, but not the fat body and gonads. Neuroparsins 3 and 4 transcript levels were down regulated in the brain and fat body, but not in the gonads.
Introduction
Ecdysteroids and juvenile hormones (JHs) are two major insect hormone families famous for their roles in development, moulting and metamorphosis. However, these hormones are also crucial in the reproductive physiology of insects, as reviewed by Raikhel et al.1. Both hormones act via a nuclear receptor, thereby regulating the transcription of several response genes. JH acts via the methoprene-tolerant (Met) receptor2–7, while the active ecdysteroid, 20-hydroxyecdysone (20E), acts via the heterodimeric ecdysone receptor complex, ecdysone receptor/ultraspiracle (EcR/USP)8. The hemimetabolan orthologue of USP is called retinoid-X-receptor (RXR). The crucial role of the ecdysone receptor complex during moulting of the desert locust, Schistocerca gregaria, was recently described9. The precursor for ecdysteroid synthesis is cholesterol, which is converted to ecdysone and its active metabolite 20E through a series of oxidation and hydroxylation steps. These enzymatic conversions are catalysed by cytochrome P450 enzymes encoded by the Halloween genes spook (Spo), phantom (Phm), disembodied (Dib), shadow (Sad) and shade (Shd), as reviewed by Niwa and Niwa10. In Schistocerca gregaria, the Halloween genes spook (SchgrSpo), phantom (SchgrPhm) and shade (SchgrShd) were previously characterised in fifth nymphal locusts11,12.
In addition to their regulatory role in insect moulting, ecdysteroids are necessary in different aspects of female reproductive physiology. In all insects, ecdysteroids are incorporated in the oocytes as a maternal source of ecdysteroids during embryogenesis13. Most knowledge about the involvement of ecdysteroids in the regulation of ovarian maturation and oogenesis in insects was obtained in three holometabolous insects: the silk moth, Bombyx mori, the yellow fever mosquito, Aedes aegypti, and the fruit fly, Drosophila melanogaster. In D. melanogaster, ecdysteroid signalling is needed first in repression, and later in activation of niche and primordial cell differentiation in the last larval stage14. Furthermore, progression of oogenesis at the onset of vitellogenesis requires ecdysteroid signalling15. Mutants of the Halloween genes, Spo and Shd, display arrested oogenesis at the initiation of vitellogenesis16,17. Usp mutants on the other hand were not affected in the progression of oogenesis at the onset of vitellogenesis, but USP is required for chorion formation at the end of oogenesis18. In A. aegypti, a blood meal triggers the release of ovary ecdysteroidogenic hormone by the brain, which then induces the ovarian production of ecdysteroids that subsequently stimulate the fat body to produce vitellogenins that are packaged into the oocytes19–21. Furthermore, ecdysteroids are also involved in formation of the vitelline envelope in the primary follicle at the end of oogenesis22. In B. mori, ecdysteroids regulate the development of ovaries during pupal and pharate adult stages23. Ecdysteroids from the PG play a role in ovarian maturation and oogenesis, while ecdysteroids produced by the follicle cells are exclusively incorporated in the oocytes and have no autocrine/paracrine or endocrine function in the regulation of oogenesis. High ecdysteroid titres are necessary for the early events of oogenesis (previtellogenic and vitellogenic stage), while a decline of the ecdysteroid titre is required for choriogenesis24. Research on the regulation of ovarian maturation and oogenesis in more primitive insect orders with panoistic ovaries has seriously lagged behind on that in Holometabola with meroistic ovaries, with only a few studies performed in the cockroach species, Blattella germanica, the German cockroach, and Diploptera punctata, the Pacific beetle cockroach25–27, and in the house cricket, Acheta domesticus28. In both Orthoptera and Dictyoptera, JH acts as the primary regulator of ovarian development and ecdysteroid titres significantly increase at the end of oogenesis1,28–31. Moreover, it has been shown in B. germanica that 20E can induce precocious choriogenesis and that ecdysteroid signalling is needed for the proliferation and function of the follicle cells and normal choriogenesis26,27,32,33. Also in the cockroach Diploptera punctata, it has been shown that ecdysteroid signalling is crucial for the termination of vitellogenesis and proper chorion formation at the end of the gonadotrophic cycle25. For extensive literature reviews on the role of ecdysteroids in ovarian maturation and oogenesis, the reader is referred to Bellés and Piulachs34 and Swevers and Iatrou13. In the migratory locust, Locusta migratoria, ovarian development and vitellogenin synthesis in the fat body are dependent on JH, while large amounts of ecdysteroids are synthesized at the end of the gonadotrophic cycle35. Most ecdysteroids produced by the follicle cells surrounding the oocytes are incorporated into the developing eggs, while a small amount is released in the haemolymph, showing a peak in ecdysteroid titre at the end of the gonadotrophic cycle11,35,36. The exact role of these circulating ecdysteroids is still unclear.
In this study, we investigated the role of ecdysteroid signalling in the female reproductive physiology of the desert locust, S. gregaria. This swarm-forming phytophagous pest insect is a serious threat to agricultural production in some of the world’s poorest countries. Non-specific chemical insecticides are still the preferred method to combat locust plagues, but unfortunately they have a negative impact on the environment and non-target organisms. Therefore, the search for new, more biorational strategies to control locusts is crucial. Furthermore, given the differences between species and the limited knowledge in more primitive insect orders having the ancestral, panoistic type of ovaries, our results are a valuable addition to the current knowledge about the hormonal regulation of ovarian development and oogenesis. We report an in-depth transcript profiling of the heterodimeric receptor components, SchgrEcR and SchgrRXR, during the first gonadotrophic cycle of S. gregaria. Ovary and oocyte growth can be divided into four stages37: (1) Immature stage: ovaries and oocytes are small and white; (2) Previtellogenic stage: ovaries grow and fat body is well developed; (3) Vitellogenic stage: basal oocytes incorporate vitellogenins, ovaries and fat body are large; (4) Choriogenic stage: fat body is small, oocytes reach their maximum length, chorion is formed and ovulation can occur. The exact time frame of maturation is dependent on the environmental and food conditions. However, in our laboratory conditions the locust ovaries will enter the vitellogenic stage between days 8 and 10 after adult emergence and they reach the choriogenic stage between days 12 and 18. Using RNA interference (RNAi), we have shown a crucial role for the ecdysone receptor in ovarian maturation, ovulation and oviposition. We also investigated the effect of silencing the ecdysone receptor complex cross-talk with other hormonal pathways, namely JH, insulin signalling and neuroparsins. More specifically, we investigated the effect of the SchgrEcR/SchgrRXR knockdown on the transcript levels of different genes of interests, belonging to the previously mentioned hormonal pathways.
Materials and Methods
Rearing of animals
The animals were reared as previously described by Lenaerts et al.38. In the described experiments, locusts were synchronized on the day of ecdysis into the fifth larval and adult stage. Different experimental groups (distinctly labelled) were kept together in the same cage. For the temporal distribution profiles of the genes of interest, adults were synchronized on the day of ecdysis to the adult stage and dissected every other day until they were 18 days old. Moreover, adults were staged during dissection according to oocyte length, to make sure female animals within the same pool had the same physiological age. Upon dissection, the length of the basal oocytes was measured under the binocular microscope using millimetre paper. Female adult locusts were found to start vitellogenesis around day 8, to commence mating on day 12 and finally deposited their eggs around day 18.
Tissue collection
Tissues were collected as previously described by Lenaerts et al.38. Tissues for the tissue and temporal expression profiles of SchgrEcR and SchgrRXR were collected in three independent pools (10 animals/pool). For the RNA interference experiments, tissues were collected in five independent pools consisting of three animals each. Tissues were stored at −80 °C until further processing.
RNA extraction and cDNA synthesis
Depending on the tissue, different RNA extraction methods were used. Brain, thoracic ganglia, suboesophageal ganglion, salivary gland, fat body, midgut, muscle, ovaries, testes and accessory glands were transferred to MagNA Lyser Green Beads Tubes (Roche) and homogenized using a MagNA Lyser instrument (1 min, 6500 rpm; Roche). Subsequently, total RNA was extracted from these tissue homogenates using the RNeasy Lipid Tissue Kit (Qiagen) according to the manufacturer’s protocol. A DNase treatment (RNase-Free DNase set, Qiagen) was performed to eliminate potential genomic DNA contamination. Because of the relatively small size of the prothoracic glands (PG), corpora allata (CA) and corpora cardiaca (CC) total RNA from these tissues was extracted using the RNAqueous-Micro Kit (Ambion) according to the manufacturer’s protocol. The manufacturer’s recommended DNase step was subsequently performed. Purity and concentration of the resulting RNA samples were checked using a Nanodrop spectrophotometer (Nanodrop ND-1000, Thermo Fisher Scientific, Inc.). For each RNA sample, cDNA was synthesized by reverse transcription of 500 ng of RNA with the PrimeScript™ RT reagent Kit (Perfect Real Time) (Takara, Invitrogen Life Technologies), using both random hexamer primers and oligo(dT) primers, according to the manufacturer’s protocol. The 10 µL reaction was diluted sixteen-fold with Milli-Q water (Millipore).
Quantitative real-time PCR (qRT-PCR)
Primers used in the qRT-PCR profiling are given in Supplementary Table 1. Primer validation and subsequent qRT-PCR reactions were performed as previously described by Lenaerts et al.38. In brief, we used the Fast SYBR® Green Master Mix (Applied Biosystems) and a StepOne System (ABI Prism, Applied Biosystems). Amplification efficiency and specificity, as well as dissociation curves, were checked for validation of the primer pairs. The primers used in all experiments have an efficiency between 90% and 110%, they amplify only the target of interest and show only one melting peak in the dissociation curve. ‘No template control’ reactions confirmed there was no contamination. For each experiment suitable reference genes were selected from a pool of candidate reference genes by means of the geNorm software39,40. The relative transcript levels of each gene of interest (GOI) were calculated using the comparative Ct method (normalization against selected reference genes and relative to a calibrator sample)39. qRT-PCR was used to determine the tissue and temporal distributions of SchgrEcR and SchgrRXR in the adult stage. The cDNA samples were derived from adult female locusts, with exception of the male accessory glands and testes. The transcript levels of the GOIs were normalized to β-actin and elongation factor 1α (EF1α) transcript levels. Moreover, qRT-PCR was used to check the efficiency of the RNAi-mediated knockdown of the known ecdysone receptor complex (SchgrEcR/SchgrRXR) as well as to determine the effect of silencing these genes on the relative transcript levels of several GOIs. In these RNAi experiments the transcript levels of the GOIs were normalized to CG13220 and α-tubulin1A transcript levels for the ovaries, CG13220, ubiquitin conjugating enzyme 10 (Ubi) and ribosomal protein 49 (RP49) transcript levels for the fat body, and β-actin and EF1α transcript levels for the CA/CC complex. GraphPad Prism 6 (GraphPad Software Inc.) was used to test the statistical significance of the observed differences for the RNAi experiments. Normalized relative quantities were log-transformed to allow the use of parametric statistical tests.
RNA interference experiments
Production of dsRNA
dsRNA constructs for SchgrEcR and SchgrRXR were produced using the MEGAscript® RNAi Kit (Ambion) according to the manufacturer’s protocol and as previously described by Lenaerts et al.38. Primers used to produce the dsRNAs are given in Supplementary Table 2. The dsRNA construct against SchgrEcR targets a region of 250 bp, while the dsRNA against SchgrRXR targets a region of 253 bp. Using Blast, it was confirmed that the dsRNA constructs are specific against EcR and RXR. Moreover, the specificity of our dsRNA constructs against SchgrEcR and SchgrRXR was previously confirmed by testing a second set of dsRNA constructs targeting a different region of each gene of interest (GOI)9.
RNAi experiment
Virgin female locusts were injected with 400 ng (in 10 µL Ringer solution) of dsRNA against SchgrEcR and SchgrRXR or GFP (control) six days after moulting to the last nymphal stage, as well as one, five, nine and thirteen days after moulting to the adult stage. These female locusts were kept with males of the same age till day 8 in the adult stage, so the females mature more synchronously. As the males start to display copulation behaviour around day 10 of the adult stage, they were removed on day 8 of the adult stage to prevent copulation with the virgin female locusts. A first group of locusts (N = 15 to 20) was dissected 12 days after ecdysis to check the knockdown efficiency and the effect of the knockdown on ecdysteroid titres in the haemolymph, ecdysteroid levels in the ovaries, and the transcript levels of other genes of interest, as well as the effect on ovarian and oocyte growth and maturation. A second group of locusts (N = 12) was used to observe copulation behaviour and post-copulation events.
Ecdysteroid measurements using an enzyme immunoassay (EIA)
Ecdysteroid titres and levels were measured using the enzyme immunoassay (EIA), as previously described by Lenaerts et al.38. Both ecdysteroid titres in the haemolymph, as well as ecdysteroid levels in the ovaries were measured in 12-day-old adult virgin female locusts (N = 15–20).
Microscopy and histological analysis
Dissected ovaries were rinsed in locust Ringer solution. Images of the ovaries and ovarioles were obtained using a light microscope (Zeiss SteREO Discovery.V8) equipped with an AxioCam ICc3 camera using the AxioVision 4.7 (Carl Zeiss-Benelux). Oocyte sections were made according to Billen41. In short, the ovarioles were fixed in 2% glutaraldehyde in sodium cacodylate buffer and postfixed in 2% osmium tetroxide in the same buffer. Afterwards, samples were dehydrated in a graded acetone series and embedded in araldite. Semi-thin (1 µm) sections (Leica EM UC6 microtome) were stained with methylene blue and thionin. Images of the oocyte sections were obtained with a light microscope (Zeiss Axio Imager Z1) equipped with an AxioCam MRm camera (1388 × 1040 pixels) using the Imaging software program Zen 2012 (Blue Edition; Carl Zeiss-Benelux).
Copulation behaviour and post-copulation effects
To investigate the possible effects of the RNAi-mediated knockdown of the ecdysone receptor complex (SchgrEcR/SchgrRXR) on the first display of copulation behaviour and fertility, the locusts were injected as described earlier (§’RNA interference experiments’). Copulation behaviour, fecundity and fertility were observed as previously described by Lenaerts et al.38. The males used for these copulation experiments were mature virgin males.
Results
Tissue specificity and developmental transcript profiles during the first gonadotrophic cycle
Using qRT-PCR, the tissue-specific and temporal distribution profiles of the components of the ecdysone receptor complex, SchgrEcR and SchgrRXR, were determined. The tissue distribution profile has been studied in the thoracic ganglia (TG), corpora cardiaca (CC), suboesophageal ganglion (SOG), salivary gland (Salgl), prothoracic glands (PG), fat body (Fb), midgut (MG), and ovaries (Ov) of 10-day-old female adult locusts and the testes and accessory glands (AG) of 10-day-old male adult locusts (Fig. 1A,B). The temporal distribution profile has been studied every other day throughout the first female reproductive cycle, starting on the day of moulting to the adult stage (Fig. 1C–H). For the temporal distribution profile, the ecdysteroid titre was also determined in the same animals.
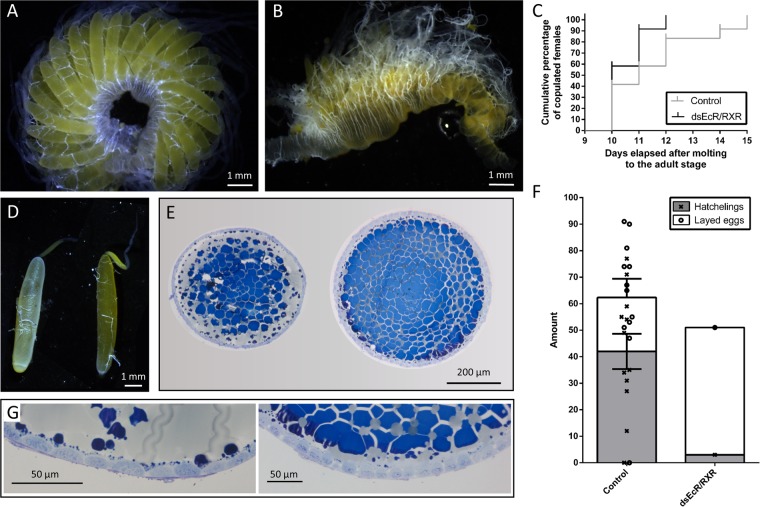
Figure 1.
Tissue and temporal distribution of SchgrEcR and SchgrRXR. Relative transcript levels of (A) SchgrEcR and (B) SchgrRXR were measured in different tissues of adult locusts, using qRT-PCR. All tissues were dissected from 10-day-old adult female locusts, with exception of the accessory glands (AG) and the testes of 10-day-old adult male locusts. The data represent mean ± S.E.M. of three independent pools (40, 10 and 10 animals/pool), run in duplicate and normalized to β-actin and elongation factor 1α (EF1α) transcript levels. Other abbreviations X-axis: TG: thoracic ganglia; CC: corpora cardiaca; SOG: suboesophageal ganglion; Salgl: salivary gland; PG: prothoracic glands; Fb: fat body; MG: midgut; Ov: ovaries. Temporal distribution profile of (C–E) SchgrEcR and (F–H) SchgrRXR in the ovaries, fat body and corpora allata during the first reproductive cycle. Using qRT-PCR, relative transcript levels of SchgrEcR and SchgrRXR were measured every other day, starting on the day of moulting to the adult stage (day 0). Data represent mean ± S.E.M. (bars) of three independent pools of ten animals each, run in duplicate and normalized to β-actin and EF1α transcript levels. The ecdysteroid titre in the hemolymph (red line), expressed in nM, throughout the first reproductive cycle was measured with an EIA. The data represent mean ± S.E.M. of 6–18 haemolymph samples taken from different animals.
Both components of the ecdysone receptor complex, SchgrEcR and SchgrRXR, show a wide tissue distribution profile (Fig. 1A,B). The highest transcript levels of SchgrEcR were found in the fat body of 10-day-old female adult locusts (Fig. 1A), while the highest transcript levels of SchgrRXR were found in the CA, the thoracic ganglia (TG) and the ovaries of 10-day-old female adult locusts, as well as in the testes of 10-day-old male adult locusts (Fig. 1B).
Since we wanted to investigate the role of the ecdysone receptor complex in female reproductive physiology, thereby looking at possible cross-talk with JH, the temporal distribution profiles of SchgrEcR and SchgrRXR were determined in the female ovary, fat body and corpora allata (CA) throughout the first reproductive cycle of female adult locusts (Fig. 1C–H). SchgrEcR transcript levels are high in the ovaries on the day of moulting to the adult stage, then decline and remain stable showing slightly higher transcript levels when ecdysteroid titres start rising (day 12) and as these decline again (day 18) (Fig. 1C). The SchgrEcR transcript levels in the fat body increase towards the end of the first reproductive cycle, which seems to coincide with the increase in ecdysteroid titre (Fig. 1D). In the CA the SchgrEcR transcript levels remain stable throughout the first reproductive cycle (Fig. 1E). As for SchgrEcR, SchgrRXR transcript levels in the ovaries are high at the day of moulting to the adult stage, after which they decline and remain stable throughout the rest of the first reproductive cycle (Fig. 1F). On the other hand, the temporal expression of SchgrRXR in the fat body and CA show a similar distribution profile, with high transcript levels at the day of moulting to the adult stage, a sudden decrease in 2-day-old female locusts and a peak right before the ecdysteroid titre rises (day 8–10) (Fig. 1G–H).
RNA interference of the ecdysone receptor complex
Knockdown efficiency
RNAi-mediated knockdown of SchgrEcR/SchgrRXR resulted in significantly reduced transcript levels of both genes in all three tissues of interest. SchgrEcR transcript levels were 72%, 78% and 75% lower compared to the control animals in the ovaries, fat body and CA/CC complex respectively (Suppl. Fig. 1A–C). SchgrRXR transcript levels were significantly reduced with 40%, 81% and 59% in respectively the ovaries, fat body and CA/CC complex of SchgrEcR/SchgrRXR knockdown locusts when compared to control locusts (Suppl. Fig. 1D–F). We can therefore conclude that the knockdown of both components of the receptor complex was successful.
Effect on the female reproductive physiology
To investigate the effect of the RNAi-mediated knockdown of the ecdysone receptor complex on the female reproductive physiology, one group of locusts was dissected on day 12. Silencing SchgrEcR/SchgrRXR did not result in smaller oocytes. As long as the basal oocytes of SchgrEcR/SchgrRXR knockdown locusts did not reach the end of the vitellogenic stage, the ovaries had a normal appearance, resembling those of control locusts as shown in Fig. 2A. However, when the oocytes reached their full length of 7 mm, it appeared that the SchgrEcR/SchgrRXR locusts were not able to start choriogenesis. During normal development, as the oocyte enters the choriogenic stage of maturation, the egg shell or chorion is formed between the oocyte and the surrounding follicular cells, thereby giving the oocytes a shiny appearance (Fig. 2D left ovariole). After choriogenesis, ovulation occurs and the basal oocytes will pass from the ovarioles to the lateral oviducts. In the SchgrEcR/SchgrRXR knockdown locusts, this shiny appearance was never observed, even though the basal oocytes of several locusts reached their maximal length and should have entered the choriogenic stage (Fig. 2D right ovariole). Moreover, a lot of resorbing basal oocytes were observed in the ovaries of the SchgrEcR/SchgrRXR knockdown locusts that should have entered the choriogenic stage. Furthermore, throughout several experimental repeats, we never detected the presence of intact oocytes in the oviduct. Instead, a yellow substance resembling the content of vitellogenic oocytes was found in the oviduct and accessory glands, which was never seen in control locusts (Fig. 2B).
Figure 2.
Effect of the RNAi-mediated knockdown of the ecdysone receptor complex (SchgrEcR/SchgrRXR) on the reproductive physiology of female S. gregaria. Locusts were injected as described in materials and methods. One part of the locusts (N = 15–20) was dissected on day 12 of the adult stage to assess ovary and oocyte growth (A,B,D,E,G), the other part of the locusts (N = 12) were kept alive to check copulation behaviour, fecundity and fertility (C and F). (A,B) Ovary of a representative (A) control locust (basal oocyte length = 3.1 mm) and (B) dsEcR/RXR-treated locusts (most basal oocytes resorbed, 2nd cycle oocytes just started vitellogenesis and were about 2 mm in length). (C) Starting from day 10 of the adult stage the females were assayed for displaying mating behaviour, by allowing them to mate with mature virgin males. The cumulative percentage of females that mated is presented (N = 12). No significant differences can be observed (Log-rank (Mantel-Cox) test). (D) Ovarioles from control (left, basal oocyte length = 7 mm) and dsEcR/RXR-treated locusts (right, basal oocyte length = 7 mm). (E) Histological sections of the basal oocytes from a control locust (left, length = 3 mm – width = 0.5 mm) and a dsEcR/RXR-treated locust (right, length = 4.5 mm – width = 0.7 mm). (F) The number of eggs per egg pod was counted, as well as the number of hatchlings per egg pod. The data represent mean ± S.E.M. (bars), as well as the individual number of eggs (Ο) or hatchlings (X) per egg pod (N control = 12; only 1 out of 12 dsEcR/RXR-treated locusts laid eggs). (G) Magnification of the follicular cell layer of the basal oocytes shown in E (same order). Scale bars: A, B & D = 1 mm; E = 200 µm; G = 50 µm.
Transverse sections of vitellogenic terminal oocytes were examined to further investigate the observed phenotypes (Fig. 2E,G). The oocytes used for the sections shown in Fig. 2E both had a similar length (indicated in figure legend). In both control and SchgrEcR/SchgrRXR knockdown sections yolk material is visible (blue matrix), but less lipid droplets (greyish droplets) were present in the oocytes of SchgrEcR/SchgrRXR knockdown locusts when compared to those of control locusts. From the magnification of the follicular cell layer of these oocytes (Fig. 2G), we observed that the follicle cells surrounding the oocyte of SchgrEcR/SchgrRXR knockdown locusts were rather small and cubical, when compared to those of a control locust. It thus seems that the nuclear receptor knockdown interferes with the shape of the follicle cells surrounding the oocyte.
We also examined whether the treatments affected the first display of copulation behaviour, fecundity and fertility. Therefore, the timing of actual copulation with a virgin male, egg laying and hatching, as well as the numbers of eggs and percentage of hatchlings was determined. SchgrEcR/SchgrRXR knockdowns did not affect the timing of the first display of copulation behaviour (Fig. 2C). However, oviposition was severely impaired in the knockdown animals (Fig. 2F). Only one out of twelve SchgrEcR/SchgrRXR knockdown locusts successfully deposited her eggs in the sand/turf mixture. Two other SchgrEcR/SchgrRXR knockdown locusts tried to lay eggs, but only deposited a white/yellow foam against the side of the cage. All three females that attempted to oviposit did this around the same time as the control locusts. From the one successfully deposited egg pod only three locusts hatched, which then died within two days after hatching. It can be concluded that silencing SchgrEcR/SchgrRXR severely affects post-copulation events, and particularly oviposition.
Effect on ecdysteroid synthesis
We investigated if RNAi-mediated knockdown of the ecdysone receptor complex affected the ecdysteroid synthesis by measuring both the transcript levels of the known Halloween genes (Suppl. Fig. 2A–E), as well as the ecdysteroid titres in the haemolymph and the ecdysteroid levels in the developing ovaries (Suppl. Fig. 1F–H). Most of the ecdysteroids produced by the folliclar cell layer are incorporated in the growing oocytes as conjugates, while only a small fraction of the ecdysteroids is stored in the oocytes as ‘free’ ecdysteroids42. Moreover, a small fraction of the produced ecdysteroids will reach the haemolymph. The transcript levels of the known Halloween genes were not affected. Noteworthy is the high variation in the transcript levels of SchgrSpo, SchgrPhm, SchgrSad and SchgrShd after silencing SchgrEcR/SchgrRXR. Moreover, ecdysteroid titres in the haemolymph and ecdysteroid levels in the ovaries were not affected. It therefore seems that in adult female S. gregaria the SchgrEcR/SchgrRXR receptors do not induce a feedback on ecdysteroid synthesis.
Effect on JH synthesis and signalling
To investigate the possible cross-talk between SchgrEcR/SchgrRXR and JH synthesis and signalling, we checked the transcript levels of the genes encoding the enzymes responsible for the last two steps in the JH biosynthetic pathway (SchgrJHAMT = juvenile hormone acid methyltransferase and SchgrCYP15A1 = methyl farnesoate epoxidase), the JH receptor methoprene-tolerant (SchgrMet), as well as the JH response gene Krüppel-homologue 1 (SchgrKr-h1) (Fig. 3). SchgrJHAMT and SchgrCYP15a1 transcript levels were respectively 5.1 and 2.7 fold higher in SchgrEcR/SchgrRXR knockdown locusts compared to control locusts (Fig. 3A,B). The transcript levels of the JH receptor gene, SchgrMet, were significantly reduced with 35% in the fat body of SchgrEcR/SchgrRXR knockdown locusts (Fig. 3D), while no effect could be observed on the transcript levels of Kr-h1 (Fig. 3G). SchgrKr-h1 transcript levels were 2.1 fold higher in the CA/CC complex of dsEcR/RXR-treated locusts compared to control locusts (Fig. 3F). Transcript levels of neither SchgrKr-h1 nor SchgrMet were affected in the ovaries of SchgrEcR/SchgrRXR knockdown locusts compared to control locusts (Fig. 3E,H). Also transcript levels of SchgrMet remained stable in the CA/CC complex upon treatment with dsRNA against SchgrEcR/SchgrRXR (Fig. 3C).
Figure 3.
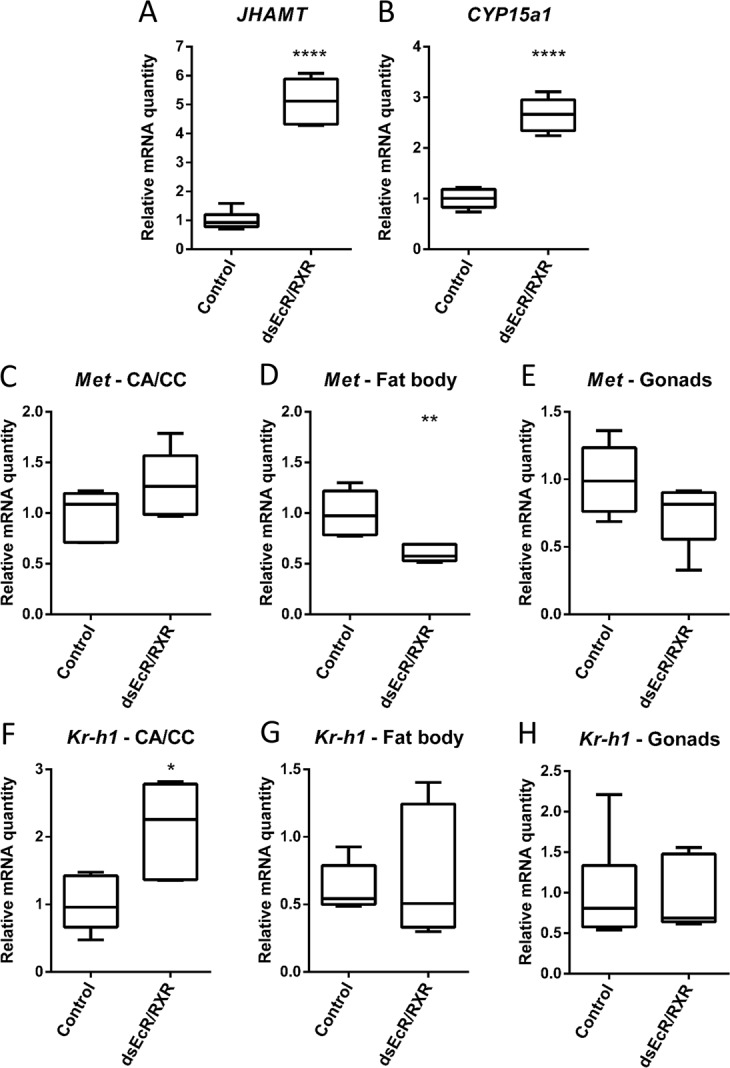
Effect of the RNAi-mediated knockdown of the ecdysone receptor complex (SchgrEcR/SchgrRXR) on JH synthesis and signalling in 12-day-old adult female S. gregaria. Locusts were injected as described in materials and methods and dissected on day 12 of the adult stage. Relative transcript levels of the last two enzymes involved in JH synthesis, SchgrJHAMT and SchgrCYP15a1, the JH receptor, SchgrMet, and a downstream response gene of JH, SchgrKr-h1, were measured in the CA/CC, fat body and ovaries of 12-day-old female locusts, using qRT-PCR. The data represent box plots (min to max) of five independent pools of three locusts, run in duplicate and normalized to CG13220 and α-tubulin1A transcript levels for the ovaries, CG13220, ubiquitin conjugating enzyme 10 (Ubi) and ribosomal protein 49 (RP49) transcript levels for the fat body, and β-actin and EF1α transcript levels for the CA/CC complex. Significant differences (p < 0.05, p < 0.01, and p < 0.0001) are indicated by (an) asterisk(s) (*, ** and **** respectively) (t-test or Mann-Withney U test on log-transformed data).
Effect on insulin-related peptide and neuroparsin signalling systems
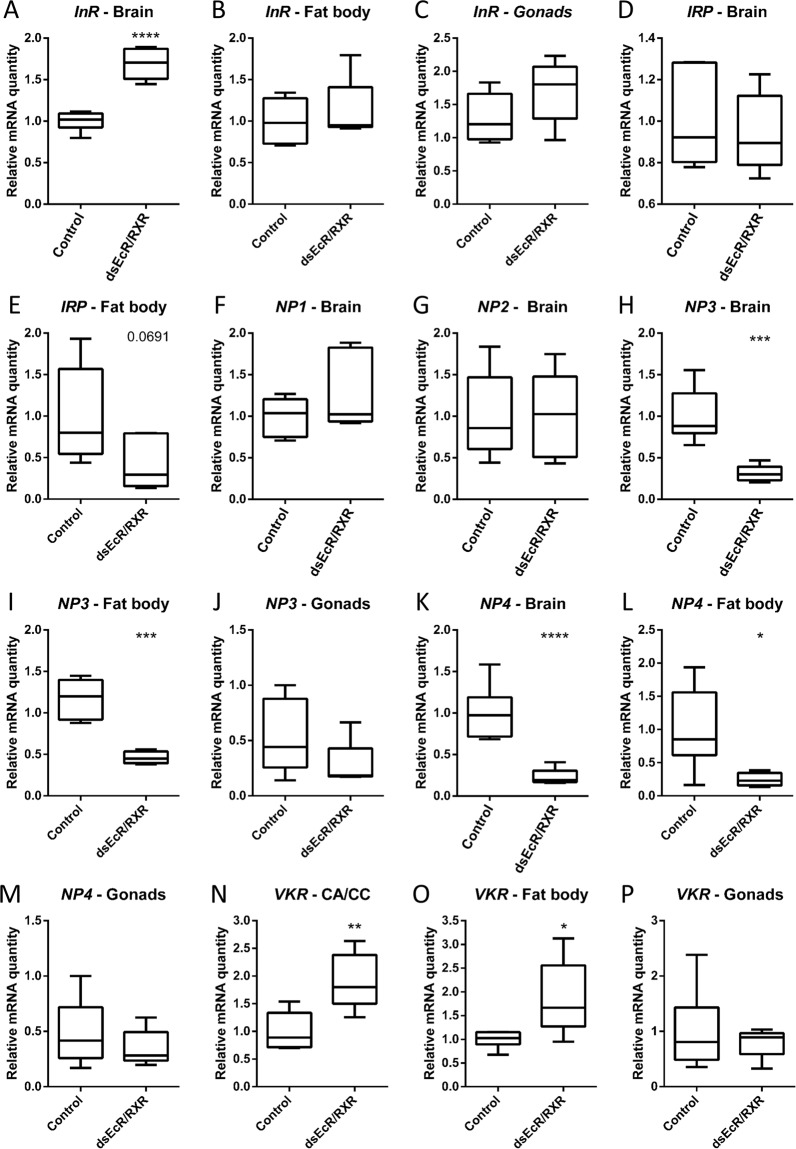
As insulin-related peptide (SchgrIRP) and neuroparsins (SchgrNPs) are known to have respectively gonadotropic and anti-gonadotropic roles in locusts, we investigated the possible cross-talk between SchgrEcR/SchgrRXR and these hormonal systems. To do so, we investigated the effect of the RNAi-mediated knockdown of SchgrEcR/SchgrRXR on the transcript levels of the insulin receptor (SchgrInR), SchgrIRP and the known neuroparsins (SchgrNP1-4). Furthermore, we also investigated the effect of the knockdown on the transcript levels of the venus kinase receptor (SchgrVKR), which is known in A. aegypti to act as the receptor for the neuroparsin-like ovary ecdysteroidogenic hormone19. If SchgrVKR acts as the receptor for NPs in locusts is still unknown. However, it does seem to play a role in the regulation of locust reproductive physiology38. The transcript levels of SchgrInR were significantly upregulated in the brain of SchgrEcR/SchgrRXR knockdown locusts when compared to control locusts (1.7 fold; Fig. 4A). However, SchgrInR transcript levels were not affected in the fat body and the ovaries (Fig. 4B,C). SchgrIRP, SchgrNP1 and SchgrNP2 transcript levels were also not affected (Fig. 4D–G). SchgrNP3 and SchgrNP4 transcript levels were, respectively, 69% and 77% lower in the brain of SchgrEcR/SchgrRXR locusts (Fig. 4H,K) and 31% and 36% lower in the fat body of SchgrEcR/SchgrRXR locusts, when compared to control locusts (Fig. 4I,L). No effect was observed on their transcript levels in the ovaries of SchgrEcR/SchgrRXR locusts (Fig. 4J,M). SchgrVKR transcript levels were 1.9 fold higher in both CA/CC and fat body of SchgrEcR/SchgrRXR locusts when compared to control locusts (Fig. 4N,O), while its transcript levels were not affected in the ovaries (Fig. 4P).
Figure 4.
Effect of the RNAi-mediated knockdown of the ecdysone receptor complex (SchgrEcR/SchgrRXR) on transcripts coding for the insulin receptor (InR), insulin-related peptide (IRP), neuroparsins (NP1–4) and the venus kinase receptor (VKR) in 12-day-old adult female S. gregaria. Locusts were injected as described in materials and methods and dissected on day 12 of the adult stage. Relative transcript levels of SchgrInR, SchgrIRP, SchgrNP1-4 and SchgrVKR in the brain, fat body and ovaries of 12-day-old female locusts, using qRT-PCR. The data represent box plots (min to max) of five independent pools of three locusts, run in duplicate and normalized to CG13220 and α-tubulin1A transcript levels for the ovaries, CG13220, ubiquitin conjugating enzyme 10 (Ubi) and ribosomal protein 49 (RP49) transcript levels for the fat body, and β-actin and EF1α transcript levels for the brain. Significant differences (p < 0.05, p < 0.01, p < 0.001 and p < 0.0001) are indicated by (an) asterisk(s) (*, **, *** and **** respectively) (t-test or Mann-Withney U test on log-transformed data).
Effect on vitellogenin synthesis
We also investigated whether interfering with ecdysteroid signalling affected vitellogenin synthesis (Suppl. Fig. 3). For both known vitellogenin genes (SchgrVg1 and SchgrVg2), the transcript levels were not significantly different from control locusts after silencing SchgrEcR/SchgrRXR.
Discussion
Expression patterns of SchgrEcR and SchgrRXR
The components of the ecdysone receptor complex, SchgrEcR and SchgrRXR, have previously been identified in S. gregaria9. The broad tissue distribution of SchgrEcR and SchgrRXR is consistent with findings in P. americana, B. germanica and D. punctata (Fig. 1A,B)25,32,43. The highest expression of SchgrEcR was observed in the fat body, a major player in energy storage and metabolism and the primary site of vitellogenin synthesis. An increase of SchgrEcR transcript levels was observed in fat body towards the end of the first reproductive cycle, which seemed to coincide with the observed peak of the ecdysteroid titre. The role of SchgrEcR in the female fat body remains unclear, since RNAi-mediated silencing of SchgrEcR/SchgrRXR did not affect vitellogenin transcript levels (Suppl. Fig. 3). This is contrary to D. punctata where ecdysteroid signalling was proven to be critical for the termination of vitellogenin synthesis25. It would be interesting to further investigate the role of SchgrEcR in the storage and release of energy reserves by the fat body, since it is known that in several insect species the nutritional status contributes at different levels to the control of the female reproductive physiology44. SchgrRXR was highly expressed in the ovaries, but the temporal distribution remained stable throughout the first reproductive cycle at the time points that were analysed. The significantly higher transcript levels in freshly moulted females compared to older females might be residual transcripts from the last nymphal stage. Both components of the ecdysone receptor complex were also highly expressed in the CA, indicating a possible role in the regulation of JH synthesis. We therefore determined the temporal distribution of both receptors in the CA (Fig. 1E,H). We observed a relatively stable expression of SchgrEcR, while the SchgrRXR transcript levels peak right before the ecdysteroid titre rises (day 8–10). This is contrary to D. punctata, where DippuEcR and DippuRXR transcript levels peak at the end of the first gonadotrophic cycle, coinciding with a drop in JH levels suggesting a feedback on JH biosynthesis25. The differences in expression profiles of SchgrEcR and SchgrRXR indicate possible distinct roles for both nuclear receptors, thereby possibly interacting with other, still unknown nuclear receptors. For instance, earlier studies have suggested that RXR/USP might function on its own, therefore binding different ligands45, such as retinoic acid and sesquiterpenoid ligands, like methyl farnesoate, a precursor of JH46,47.
Ecdysteroid signalling complex is crucial in female S. gregaria reproductive physiology
In insects, ecdysteroids are incorporated into the growing oocytes as a maternal source during embryonic development48. This is also the case for S. gregaria, where ecdysteroids are mostly incorporated as polar conjugates of ecdysone or 20E42. Previous research has shown that the involvement of ecdysteroids in oogenesis is species-dependent (as reviewed by Swevers and Iatrou, 2009)13. Moreover, most recent research on the role of ecdysteroids in the female reproductive physiology focussed on the ecdysone receptor and its downstream response genes in the meroistic ovaries of Holometabola13,49. From an evolutionary perspective, it is therefore of great interest to further explore the role of ecdysteroids in the more ancestral panoistic type of ovaries as well.
Silencing the ecdysone receptor complex, SchgrEcR/SchgrRXR, had a significant impact on the female reproductive physiology in locusts. Ovulation and subsequent oviposition were blocked and a lot of resorption of the oocytes was observed upon silencing of the ecdysone receptor complex (Fig. 2). Our observations suggest that the dsEcR/RXR-treated locusts were not able to enter the choriogenic stage of oocyte maturation, resulting in more resorption compared to control locusts and part of the oocyte’s content leaking into the oviduct. It might also have been that those ovaries reached the point at which oocytes are normally passed from the ovarioles to the oviduct, and that the basal oocytes that did not resorb were sucked into the oviduct, resulting in the leakage of the oocyte’s content into the oviduct, due to the lack of a firm chorion which normally protects the oocytes upon ovulation. This observation is in accordance with previous research in D. melanogaster, B. germanica and D. punctata. In D. melanogaster, EcR and USP are known to regulate the transcription of chorion genes50,51 and mutants targeting the ecdysone receptor components resulted in thin and fragile eggshells18,52,53. In B. germanica, treatment with 20E causes precocious depositions of choriogenic material27. RNAi-mediated knockdown of EcR/RXR in D. punctata resulted in a similar phenotype, in which the basal oocytes reached their maximal length and subsequently were resorbed. Moreover, this research has proven that the RNAi-mediated knockdown of EcR/RXR reduced the expression of FCP 3 C, a gene which is selectively expressed in follicle cells during choriogenesis25. Since at present no chorion genes are known in S. gregaria, we were not able to verify whether the expression of any genes involved in locust choriogenesis was also affected by the ecdysone receptor complex knockdown.
Cross-talk between ecdysteroids and other hormonal pathways
Silencing the ecdysone receptor complex did not influence ecdysteroid titres in the haemolymph or ecdysteroid levels in the ovaries. Remarkable, however, was the big variation in the transcript levels of SchgrSpo, SchgrPhm, SchgrSad and SchgrShd upon silencing of SchgrEcR/SchgrRXR. A possible explanation lies in the resorption of oocytes and big variation in the resulting oocyte sizes upon silencing of SchgrEcR/SchgrRXR. It is also important to keep in mind that we performed an RNAi-mediated knockdown of the receptor complex and as such, there might still be enough functional receptor present in order for ecdysteroids to feed back on their own synthesis. The observed results after silencing SchgrEcR/SchgrRXR in adult female locusts are also in contrast with previous research in D. punctata, where a knockdown of EcR/RXR resulted in significantly lower ecdysteroid titres in the haemolymph25.
We observed an upregulation of the last two JH synthesizing enzymes, as well as the JH response gene Kr-h1 in the CA/CC of SchgrEcR/SchgrRXR knockdown locusts when compared to control locusts (Fig. 3). So it seems that the SchgrEcR/SchgrRXR receptors also regulate JH biosynthesis in vitellogenic female S. gregaria. However, the exact nature (direct or indirect) of this regulation is still unclear. Our findings are conform to the research done by Liu and co-workers54, where a cross-talk between ecdysteroids and juvenile hormones was observed in D. melanogaster. However, as SchgrKr-h1 transcript levels were only higher in the CA/CC and not in the fat body and ovaries of SchgrEcR/SchgrRXR knockdown locusts, we hypothesize that those animals indeed synthesised more JH, but did not release it, or that JH was immediately metabolised upon release. The fact that SchgrKr-h1 transcript levels were not affected in the fat body might also be due to significantly lower SchgrMet transcript levels in this tissue upon silencing of the ecdysone receptor complex. It therefore also seems that SchgrMet expression is under the control of the ecdysteroid receptor complex, but the exact nature of this control remains unclear. When comparing our results with those found after silencing EcR/RXR in adult female D. punctata25, some similarities and some differences can be observed. For instance, DippuKr-h1 transcript levels were significantly lower in the CA/CC complex, but significantly higher in the fat body and ovary of EcR/RXR knockdown cockroaches. Moreover, DippuMet transcript levels were not affected in these three tissues. Furthermore, DippuCYP15a1 transcript levels were significantly lower in EcR/RXR cockroaches, while the transcript levels of several other JH synthesis enzymes were significantly higher in these females. This recent research suggested both direct and indirect feedback of ecdysteroids on JH synthesis in D. punctata25. However, the exact reasons for the discrepancies observed between hemimetabolous insect species, when comparing our current data with those obtained with D. punctata25, remain unknown. More research is required to further explain this phylogenetic divergence. Moreover, the sensitivity of the CA to 20E seemed to be age-dependent25. Also, in B. germanica, it has been shown that ecdysteroids are responsible for lowering JH synthesis, which is needed for the termination of the gonadotrophic cycle27. As silencing of SchgrEcR/SchgrRXR resulted in the upregulation of the last to JH synthesizing enzymes in S. gregaria, we hypothesise that, similarly to D. punctata and B. germanica, high ecdysteroid titres at the end of the gonadotrophic cycle are needed to lower JH synthesis, subsequently initiate proper chorion formation, and thus terminate the gonadotrophic cycle in S. gregaria. However, this hypothesis deserves further attention in future studies. For instance, it might be interesting to investigate whether a rise in ecdysteroid titre earlier in the gonadotrophic cycle would result in a reduction of JH synthesis and consequently induction of early choriogenesis.
Next, we also investigated the possible cross-talk between SchgrEcR/SchgrRXR and insulin-related peptide or neuroparsin signalling systems, as these are known to exert gonadotropic and anti-gonadotropic actions, respectively55. The insulin signalling pathway (ISP) acts as a systemic nutrient sensor, thereby linking the insect’s nutritional state and reproductive processes. For an extensive review on this, the reader is referred to Badisco et al.44. No effect could be observed on the transcript levels of SchgrIRP upon silencing of SchgrEcR/SchgrRXR (Fig. 4), while the transcript levels of SchgrInR were significantly higher in the brain of SchgrEcR/SchgrRXR knockdown locusts (Fig. 4). As the transcript levels of SchgrInR were not affected in the fat body and the gonads, the cross-talk between SchgrEcR/SchgrRXR and the ISP seems to be tissue dependent. Moreover, we hypothesize that the observed role of SchgrEcR/SchgrRXR in the regulation of the ISP is most likely indirect. Our findings are in contrast with previous research in B. mori and D. melanogaster, where ecdysteroids were found to regulate the ISP by acting on the expression of the insulin-like peptides by the fat body56–58. The evolutionary distance between these holometabolous insects and our hemimetabolous research model, might explain these contrasting findings. However, further research is needed to unravel the exact molecular mechanisms that link ecdysteroid signalling and insulin signalling. The RNAi-mediated knockdown of SchgrEcR/SchgrRXR significantly reduced the transcript levels of SchgrNP3 and SchgrNP4 in the brain and fat body, but not in the ovaries (Fig. 4), which is another suggestion that ecdysteroids are able to exert tissue-specific actions. As a knockdown of SchgrNPs is known to affect vitellogenin levels in S. gregaria55, one might expect that vitellogenin levels would also be affected upon knockdown of SchgrEcR/SchgrRXR since this knockdown lowered the transcript levels of SchgrNP3 and SchgrNP4. However, as this is not the case, SchgrNP3 and SchgrNP4 may not be the major neuroparsin variants that are involved in the control of vitellogenin expression. Alternatively, their effects may have been compensated by other changes that were directly or indirectly induced by SchgrEcR/SchgrRXR knockdown.
Conclusions
Ecdysteroid signalling is crucial for successful ovulation and oviposition in S. gregaria, since RNAi-mediated silencing of the ecdysone receptor complex affects ovarian maturation by affecting choriogenesis. We have also found evidence for a cross-talk between ecdysteroid signalling and other important hormonal pathways in adult female S. gregaria. For instance, SchgrEcR/SchgrRXR silencing influences the expression of JH biosynthesis and signalling components in the CA. However, the RNAi-mediated knockdown of the ecdysone receptor complex might not affect JH titres in the rest of the body, as SchgrKr-h1 transcript levels were not affected in the fat body and the ovaries. Furthermore, we have also shown an in vivo effect of the ecdysone receptor complex knockdown on the tissue specific regulation of SchgrInR, SchgrNP3 and SchgrNP4 gene expression.
Electronic supplementary material
Acknowledgements
The authors are very grateful to Prof. Dr. Johan Billen and An Vandoren for their help with the histological sections. A special thanks goes to Roger Jonckers and Evelien Herinckx for their technical assistance, Dr. Jornt Spit for the useful input, and Rik Verdonck for his work on the Schistocerca transcriptome database. The authors also acknowledge Prof. Jean-Paul Delbecque (Université de Bordeaux, France) for his kind gifts of antiserum and tracer. The authors gratefully acknowledge the Agency for Innovation by Science and Technology (IWT) and the Research Foundation of KU Leuven (GOA/11/02 and C14/15/050) for funding.
Author Contributions
Molecular cloning, dissections, transcript profiling and RNAi experiments were performed by C.L., E.M. and P.P. J.V. is the senior academic author responsible for funding acquisition and project administration. Design and supervision of the study, writing and correction of the manuscript were performed by C.L. and J.V. The authors declare no conflict of interest, financial or otherwise, that might potentially bias this work.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-36763-9.
References
- 1.Raikhel AS, Brown MR, Bellés X. Hormonal control of reproductive processes. Compr. Mol. insect Sci. 2005;3:433–491. doi: 10.1016/B0-44-451924-6/00040-5. [DOI] [Google Scholar]
- 2.Konopova B, Jindra M. Broad-Complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development. 2008;135:559–568. doi: 10.1242/dev.016097. [DOI] [PubMed] [Google Scholar]
- 3.Parthasarathy R, Tan A, Palli SR. bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval-pupal metamorphosis. Mech. Dev. 2008;125:601–616. doi: 10.1016/j.mod.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles J-P, et al. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. 2011;108:21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M, Mead Ea, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. USA. 2011;108:638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindra M, Uhlirova M, Charles J-P, Smykal V, Hill RJ. Genetic Evidence for Function of the bHLH-PAS Protein Gce/Met As a Juvenile Hormone Receptor. PLOS Genet. 2015;11:1–16. doi: 10.1371/journal.pgen.1005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jindra, M., Bellés, X. & Shinoda, T. Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. (2015). [DOI] [PubMed]
- 8.Yao T-P, et al. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- 9.Lenaerts C, Van Wielendaele P, Peeters P, Vanden Broeck J, Marchal E. Ecdysteroid signalling components in metamorphosis and development of the desert locust. Schistocerca gregaria. Insect Biochem. Mol. Biol. 2016;75:10–23. doi: 10.1016/j.ibmb.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Niwa R, Niwa YS. Enzymes for ecdysteroid biosynthesis: their biological functions in insects and beyond. Biosci. Biotechnol. Biochem. 2014;78:1283–1292. doi: 10.1080/09168451.2014.942250. [DOI] [PubMed] [Google Scholar]
- 11.Marchal E, et al. Role of the Halloween genes, Spook and Phantom in ecdysteroidogenesis in the desert locust, Schistocerca gregaria. J. Insect Physiol. 2011;57:1240–1248. doi: 10.1016/j.jinsphys.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Marchal E, Verlinden H, Badisco L, Van Wielendaele P, Vanden Broeck J. RNAi-mediated knockdown of Shade negatively affects ecdysone-20-hydroxylation in the desert locust, Schistocerca gregaria. J. Insect Physiol. 2012;58:890–896. doi: 10.1016/j.jinsphys.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Swevers, L. & Iatrou, K. In Ecdysones: Structures and Functions (ed. Smagghe, G.) 127–164 (Springer Science, 2009).
- 14.Gancz D, Lengil T, Gilboa L. Coordinated Regulation of Niche and Stem Cell Precursors by Hormonal Signaling. PLoS Biol. 2011;9:e1001202. doi: 10.1371/journal.pbio.1001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buszczak M, et al. Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development. 1999;126:4581–4589. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- 16.Petryk A, et al. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. USA. 2003;100:13773–13778. doi: 10.1073/pnas.2336088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ono H, et al. Spook and Spookier code for stage-specific components of the ecdysone biosynthetic pathway in Diptera. Dev. Biol. 2006;298:555–570. doi: 10.1016/j.ydbio.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Oro AE, McKeown M, Evans RM. The Drosophila retinoid X receptor homolog ultraspiracle functions in both female reproduction and eye morphogenesis. Development. 1992;115:449–462. doi: 10.1242/dev.115.2.449. [DOI] [PubMed] [Google Scholar]
- 19.Vogel KJ, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2015;112:9–10. doi: 10.1073/pnas.1501814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown MR, et al. Identification of a steroidogenic neurohormone in female mosquitoes. J. Biol. Chem. 1998;273:3967–3971. doi: 10.1074/jbc.273.7.3967. [DOI] [PubMed] [Google Scholar]
- 21.Sieglaff DH, Duncan KA, Brown MR. Expression of genes encoding proteins involved in ecdysteroidogenesis in the female mosquito. Aedes aegypti. Insect Biochem. Mol. Biol. 2005;35:471–490. doi: 10.1016/j.ibmb.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, et al. Structure, expression, and hormonal control of genes from the mosquito, Aedes aegypti, which encode proteins similar to the vitelline membrane proteins of Drosophila melanogaster. Dev. Biol. 1993;155:558–568. doi: 10.1006/dbio.1993.1052. [DOI] [PubMed] [Google Scholar]
- 23.Swevers L, Raikhel AS, Sappington TW, Shirk P, Iatrou K. Vitellogenesis and Post-Vitellogenic Maturation of the Insect Ovarian Follicle. Compr. Mol. Insect Sci. 2005;1:87–155. doi: 10.1016/B0-44-451924-6/00093-4. [DOI] [Google Scholar]
- 24.Swevers L. The ecdysone agonist tebufenozide (RH-5992) blocks the progression into the ecdysteroid-induced regulatory cascade and arrests silkmoth oogenesis at mid-vitellogenesis. Insect Biochem. Mol. Biol. 1999;29:955–963. doi: 10.1016/S0965-1748(99)00059-4. [DOI] [Google Scholar]
- 25.Hult EF, Huang J, Marchal E, Lam J, Tobe SS. RXR/USP and EcR are critical for the regulation of reproduction and the control of JH biosynthesis in Diploptera punctata. J. Insect Physiol. 2015;80:48–60. doi: 10.1016/j.jinsphys.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Cruz J, Mané-Padrós D, Bellés X, Martín D. Functions of the ecdysone receptor isoform-A in the hemimetabolous insect Blattella germanica revealed by systemic RNAi in vivo. Dev. Biol. 2006;297:158–171. doi: 10.1016/j.ydbio.2006.06.048. [DOI] [PubMed] [Google Scholar]
- 27.Bellés X, et al. Induction of choriogenesis by 20-hydroxyecdysone in the german cockroach. Tissue Cell. 1993;25:195–204. doi: 10.1016/0040-8166(93)90019-H. [DOI] [PubMed] [Google Scholar]
- 28.Whiting P, Sparks S, Dinan L. Endogenous ecdysteroid levels and rates of ecdysone acylation by intact ovaries in vitro in relation to ovarian development in adult female crickets, Acheta domesticus. Arch. Insect Biochem. Physiol. 1997;35:279–299. doi: 10.1002/(SICI)1520-6327(199705)35:3<279::AID-ARCH3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 29.Dinan L. Ecdysteroids in adults and eggs of the house cricket, Acheta domesticus (Orthoptera: Gryllidae) Comp. Biochem. Physiol. - Part B Biochem. Mol. Biol. 1997;116:129–135. doi: 10.1016/S0305-0491(96)00221-0. [DOI] [Google Scholar]
- 30.Engelmann F, Mala J. The interactions between juvenile hormone (JH), lipophorin, vitellogenin, and JH esterases in two cockroach species. Insect Biochem. Mol. Biol. 2000;30:793–803. doi: 10.1016/S0965-1748(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 31.Cruz J, et al. Quantity does matter. Juvenile hormone and the onset of vitellogenesis in the German cockroach. Insect Biochem. Mol. Biol. 2003;33:1219–1225. doi: 10.1016/j.ibmb.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Maestro O, Cruz J, Pascual N, Martín D, Bellés X. Differential expression of two RXR/ultraspiracle isoforms during the life cycle of the hemimetabolous insect Blattella germanica (Dictyoptera, Blattellidae) Mol. Cell. Endocrinol. 2005;238:27–37. doi: 10.1016/j.mce.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Martín D, Maestro O, Cruz J, Mané-Padrós D, Bellés X. RNAi studies reveal a conserved role for RXR in molting in the cockroach Blattella germanica. J. Insect Physiol. 2006;52:410–6. doi: 10.1016/j.jinsphys.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Bellés X, Piulachs MD. Ecdysone signalling and ovarian development in insects: From stem cells to ovarian follicle formation. Biochim. Biophys. Acta - Gene Regul. Mech. 2015;1849:181–186. doi: 10.1016/j.bbagrm.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 35.Lagueux M, Hirn M, Hoffmann JA. Ecdysone during ovarian development in Locusta migratoria. J. Insect Physiol. 1977;23:109–119. doi: 10.1016/0022-1910(77)90116-0. [DOI] [PubMed] [Google Scholar]
- 36.Glass H, Emmerich H, Spindler K-D. Immunohistochemical localisation of ecdysteroids in the follicular epithelium of locust oocytes. Cell Tissue Res. 1978;194:237–244. doi: 10.1007/BF00220391. [DOI] [PubMed] [Google Scholar]
- 37.Tobe S, Pratt G. Corpus allatum activity in vitro during ovarian maturation in the desert locust. Schistocerca gregaria. J. Exp. Biol. 1975;62:611–627. doi: 10.1242/jeb.62.3.611. [DOI] [PubMed] [Google Scholar]
- 38.Lenaerts C, Palmans J, Marchal E, Verdonck R, Vanden Broeck J. Role of the venus kinase receptor in the female reproductive physiology of the desert locust, Schistocerca gregaria. Sci. Rep. 2017;7:11730. doi: 10.1038/s41598-017-11434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.1–0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Hiel MB, et al. Identification and validation of housekeeping genes in brains of the desert locust Schistocerca gregaria under different developmental conditions. BMC Mol. Biol. 2009;10:56. doi: 10.1186/1471-2199-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Billen J. Morphology and ultrastructure of the Dufour gland in workers of social wasps (Hymenoptera, Vespidae) Arthropod Struct. Dev. 2006;35:77–84. doi: 10.1016/j.asd.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Gande AR, Morgan ED. Ecdysteroids in the developing eggs of the desert locust. Schistocerca gregaria. J. Insect Physiol. 1979;25:289–293. doi: 10.1016/0022-1910(79)90057-X. [DOI] [Google Scholar]
- 43.Elgendy AM, Elmogy M, Takeda M. Molecular Cloning, Characterization, and Expression Pattern of the Ultraspiracle Gene Homolog (RXR/USP) from the Hemimetabolous Insect Periplaneta americana (Dictyoptera, Blattidae) during Vitellogenesis. Mol. Biotechnol. 2014;56:126–135. doi: 10.1007/s12033-013-9688-y. [DOI] [PubMed] [Google Scholar]
- 44.Badisco L, Van Wielendaele P, Vanden Broeck J. Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front. Physiol. 2013;4:1–16. doi: 10.3389/fphys.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hult EF, Tobe SS, Chang BSW. Molecular Evolution of Ultraspiracle Protein (USP/RXR) in Insects. PLoS One. 2011;6:e23416. doi: 10.1371/journal.pone.0023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nowickyj SM, et al. Locust retinoid X receptors: 9-Cis-retinoic acid in embryos from a primitive insect. Dev. Biol. 2008;22:124–137. doi: 10.1073/pnas.0712132105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones G, et al. Ligand binding pocket function of Drosophila USP is necessary for metamorphosis. Gen. Comp. Endocrinol. 2013;182:73–82. doi: 10.1016/j.ygcen.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Chapman, R. F. The Insects: Structure and Function. Cambridge University Press (Cambridge University Press, 2012).
- 49.Roy S, Saha TT, Zou Z, Raikhel AS. Regulatory Pathways Controlling Female Insect Reproduction. Annu. Rev. Entomol. 2018;63:489–511. doi: 10.1146/annurev-ento-020117-043258. [DOI] [PubMed] [Google Scholar]
- 50.Bernardi F, Romani P, Tzertzinis G, Gargiulo G, Cavaliere V. EcR-B1 and Usp nuclear hormone receptors regulate expression of the VM32E eggshell gene during Drosophila oogenesis. Dev. Biol. 2009;328:541–551. doi: 10.1016/j.ydbio.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Shea MJ, King DL, Conboy MJ, Mariani BD, Kafatos FC. Proteins that bind to Drosophila chorion cis-regulatory elements: a new C2H2 zinc finger protein and a C2C2 steroid receptor-like component. Genes Dev. 1990;4:1128–1140. doi: 10.1101/gad.4.7.1128. [DOI] [PubMed] [Google Scholar]
- 52.Cherbas L, Hu X, Zhimulev I, Belyaeva E, Cherbas P. EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development. 2003;130:271–284. doi: 10.1242/dev.00205. [DOI] [PubMed] [Google Scholar]
- 53.Hackney JF, Pucci C, Naes E, Dobens L. Ras signaling modulates activity of the ecdysone receptor EcR during cell migration in the Drosophila ovary. Dev. Dyn. 2007;236:1213–1226. doi: 10.1002/dvdy.21140. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, et al. Antagonistic actions of juvenile hormone and 20-hydroxyecdysone within the ring gland determine developmental transitions in Drosophila. Proc. Natl. Acad. Sci. 2018;115:139–144. doi: 10.1073/pnas.1716897115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Badisco L, et al. RNA interference of insulin-related peptide and neuroparsins affects vitellogenesis in the desert locust Schistocerca gregaria. Peptides. 2011;32:573–580. doi: 10.1016/j.peptides.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Okamoto N, et al. A Fat Body-Derived IGF-like Peptide Regulates Postfeeding Growth in. Drosophila. Dev. Cell. 2009;17:885–891. doi: 10.1016/j.devcel.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slaidina M, Delanoue R, Gronke S, Partridge L, Léopold P. A Drosophila Insulin-like Peptide Promotes Growth during Nonfeeding States. Dev. Cell. 2009;17:874–884. doi: 10.1016/j.devcel.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okamoto N, et al. An ecdysteroid-inducible insulin-like growth factor-like peptide regulates adult development of the silkmoth Bombyx mori. FEBS J. 2009;276:1221–1232. doi: 10.1111/j.1742-4658.2008.06859.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.