Abstract
The mandate of folic acid supplementation in grained products has reduced the occurrence of neural tube defects by one third in the U.S since its introduction by the Food and Drug Administration in 1998. However, the advantages and possible mechanisms of action of using folic acid for peripheral nerve engineering and neurological diseases still remain largely elusive. Herein, folic acid is described as an inexpensive and multifunctional niche component that modulates behaviors in different cells in the nervous system. The multiple benefits of modulation include: 1) generating chemotactic responses on glial cells, 2) inducing neurotrophin release, and 3) stimulating neuronal differentiation of a PC-12 cell system. For the first time, folic acid is also shown to enhance cellular force generation and global methylation in the PC-12 cells, thereby enabling both biomechanical and biochemical pathways to regulate neuron differentiation. These findings are evaluated in vivo for clinical translation. Our results suggest that folic acid-nerve guidance conduits may offer significant benefits as a low-cost, off-the-shelf product for reaching the functional recovery seen with autografts in large sciatic nerve defects. Consequently, folic acid holds great potential as a critical and convenient therapeutic intervention for neural engineering, regenerative medicine, medical prosthetics, and drug delivery.
Keywords: folic acid, neural engineering, regenerative medicine, medical prosthetics
1. Introduction
Folic acid (a synthetic form of folate) is an essential source of the single carbon group used in DNA methylation and plays a pivotal role in the development, function, regeneration, and repair of the central nervous system (CNS).[1–4] Folic acid deficiency has been associated with a variety of CNS disorders[5–8], such as neural tube defects (NTDs)[9–11], developmental delays, dementia, and seizures.[12,13] Supplementing the diet with folic acid can prevent some of these clinical problems, provided the supplementation occurs sufficiently early in embryonic development. For instance, periconceptional folic acid intake was shown to considerably reduce the occurrence of NTDs such as spina bifida and anencephaly in newborn babies[14]: the recommended daily dose of folic acid of 4 mg/day resulted in a remarkable 72% reduction in NTD recurrence.[15,16]
Notably, the systemic benefits of folic acid are not just limited to stimulating growth and differentiation during embryonic brain and spinal cord development. Iskandar et al. have shown that parenteral folic acid consumption produces up to 10-fold, dose-dependent improvement in axonal regrowth and functional recovery after injury to the adult CNS, an effect well in excess of other interventions.[7] Despite clinical efficacy of folic acid supplementation for preventing NTDs and other neurological disorders, and the potential benefits of folic acid on CNS nerve repairs reported in animal models[17], the exact pro-regenerative effects and underlying mechanisms of folic acid on the peripheral nervous system ( PNS) at the cellular and subcellular levels have not been revealed.
This paper aims to show how folic acid plays a role as a multifunctional, pro-regenerative niche component by affecting different cells of the PNS, their behavior (proliferation and migration of glial cells, neurotrophin release of Schwann cells, and cell survival and differentiation of a model neuronal PC-12 cell system), and their network with each other, ultimately leading to improved peripheral regeneration. The new findings will elaborate how certain doses of exogenous folic acid affect the PNS axon regeneration by exerting both chemical and mechanical stimuli to the neurons. These outcomes will serve as important benchmarks for designing innovative and advanced biomaterials that can provide microenvironments aimed at inducing specific cell behaviors in glial cells and neurons.
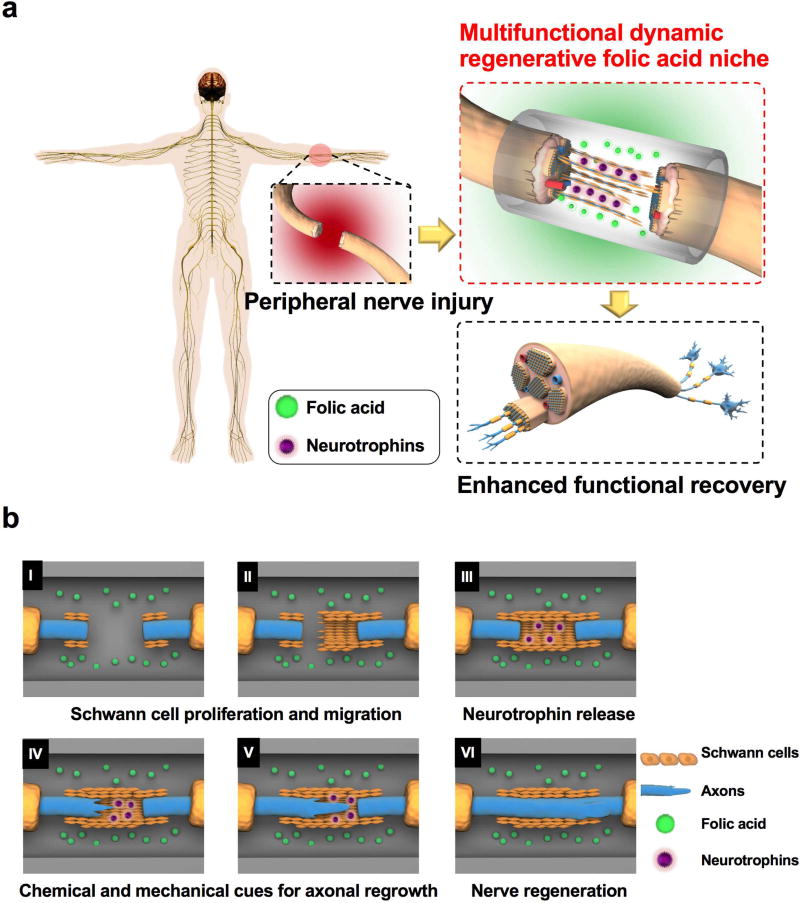
Furthermore, distinguishing it from all of the previous work involving systemic injections of folic acid into animals to aid in CNS regeneration, we designed nerve guidance conduits out of biodegradable crosslinked urethane-doped polyester (CUPE) that locally deliver folic acid at the injury site for the repair of critical-sized peripheral nerve gaps (>20 mm) in Wistar rats. CUPE was used here to fabricate NGC s due to its reported merits that include excellent biocompatibility, hemocompatibility, and soft and highly elastic property[18–20], making it suitable for nerve tissue engineering and other vascular applications. A folic acid-incorporated nerve guidance conduit (Figure 1a) is considered to create a chemical gradient in the surrounding tissue as a guide for Schwann cells to migrate into the device, and then serve as a suitable microenvironment that provides both chemical and mechanical cues to facilitate 1) Schwann cell proliferation, 2) the release of neurotrophins from the Schwann cells, and 3) axonal regeneration (Figure 1b). In conclusion, we propose that folic acid may offer multiple benefits to orchestrate the regeneration of peripheral nerves as a cost-effective regenerative niche component, and can be distinguished from other biological agents such as nerve growth factors or transplanted cells due to its known structure and chemical stability. The outcomes generate great insights into unveiling the regulatory effects of folic acid effects and establishing a folic acid niche model for future neuroregenerative medicine.
Figure 1.
A) Overview of the folic acid niche that provides chemical and mechanical cues to enhance functional recovery in a peripheral injury model. B) Cartoon illustrating the detailed mechanism of the multifunctional and dynamic neuroregenerative folic acid niche and how it allows peripheral nerve regeneration by modulating the proliferation and migration of Schwann cells (I, II), stimulating the Schwann cells to release more neurotrophins (III), and increasing mechanical forces in the neurons that in turn boost the axonal regeneration of the neurons (IV and V) and peripheral nerve regeneration (VI).
2. Materials and Methods
2.1 Materials
Folic acid (purity ≥ 97%), 1,6-hexamethyl diisocyanate (purity ≥ 99%), and 4 1,4-dioxane (purity 99.8%, anhydrous) were purchased from Sigma-Aldrich. Citric acid (purity ≥ 99.5%, anhydrous, ACS) and 1,8-octanediol (purity ≥ 98%) were purchased from Alfa Aesar.
2.2 Cell culture
Rat Schwann cells (ATCC, Manassas, VA, USA; catalog #: CRL-2768™) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and 1% antibiotic antimycotic 100× solution. The cells were maintained in a 37°C incubator with 5% CO2 and a relative humidity of 95%. The medium was changed every two or three days. Rat PC-12 Adh cells (ATCC, Catalog #: CRL-1721.1™) were seeded in 25cm2 CellBIND® flasks (Corning, Corning, NY, USA) and maintained in complete F-12K medium (Kaighn’s Modification of Ham’s F-12 Medium) (ATCC) supplemented with 15% horse serum (ATCC), 2.5% fetal bovine serum (ATLANTA biologics, Flowery Branch, GA, USA), and 1% antibiotic antimycotic 100× solution (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in a humidified incubator with 5% CO2. The medium was changed every two days. To induce differentiation, PC-12 cells were treated with 50 ng/mL of nerve growth factor (NGF)-β from rat (Sigma-Aldrich) dissolved in differentiation media (F-12K medium containing 2.5% horse serum and 1% antibiotic antimycotic 100× solution) for three consecutive days.
Human astrocytes or HAs (HA; ScienCell Research Laboratories, Corte Del Cedro, Carlsbad, CA, USA; catalog #: 1800) were received as a gift from Dr. Gong Chen (Pennsylvania State University, University Park, PA, USA) and maintained in astrocyte medium (ScienCell Research Laboratories) at 37°C in a humidified atmosphere containing 5% CO2. These HAs were isolated from human cerebral cortex.
2.3 Cell Cytotoxicity and Proliferation Assays
Both Schwann and PC-12 cells were seeded in 96-well plates (Corning) at a cell density of 10,000 cells/well for cytotoxicity assays and 500 cells/well for a proliferation assays, respectively. The cells were incubated in 100 µL of their complete growth media containing 5 different concentrations of folic acid (Sigma-Aldrich) at 37°C and 5% CO2. After the incubation for predetermined times (24 h for cytotoxicity assays and one, three, five, and seven days for proliferation assays, respectively), colorimetric cell counting kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was performed according to the manufacturer’s specifications using a plate reader (Tecan, Charlotte, NC, USA) in order to determine the number of viable cells in each sample. The experiment was repeated three times, independently.
2.4 Cell Migration Assay
Chemotactic property of folic acid on promoting the migration of Schwann cells and astrocytes was studied in the cell migration experiments. Migration assay was performed on Schwann cells and astrocytes using the transwells (Corning; 6.5 mm Diameter polycarbonate membrane inserts with 8 µm pore size). Before the migration assay, the cells were starved overnight in serum-free media and the bottom of each transwell insert was coated with fibronectin (Corning) and incubated overnight at 4°C in order to allow cells to adhere to the bottom side of the membrane during migration assays. A 100-µL serum-free medium containing resuspended Schwann cells or astrocytes (106 cells/mL) was transferred to the top chambers of each transwell and allowed to adhere for 1 h at 37°C in 5% CO2. After the cells adhered, 600 µL of serum-free medium was added into the lower chambers and the cells were allowed to migrate for 4 h. After the cells migrated, the upper surface of each membrane was cleaned with a cotton swab and the cells adhering to the bottom surface of each membrane were stained with 0.1% crystal violet and rinsed with DI water until it ran clear, imaged using a light microscope (Nikon USA, Melville, NY, USA), and counted using ImageJ. Assays were performed three times using triplicate wells.
2.5 Immunostaining for Neurite Outgrowth Assay
Undifferentiated PC-12 cells were plated in a density of 5 × 104 cells/well in their growth medium in 6-well plate and incubated at 37°C in a humidified atmosphere containing 5% CO2. After cell attachment overnight, each well was treated with different concentrations of folic acid and with or without 50 ng/mL NGF. After certain days of incubation, the cells in each well were fixed in 4.0% paraformaldehyde and stained with 4',6-Diamidino-2-Phenylindole or DAPI (Sigma Aldrich; catalog #: D9542) and Alexa Fluor® 555 Phalloidin (Thermo Fisher Scientific, Waltham, MA, USA; catalog #: A34055). Fluorescent images of stained PC-12 cells were taken with a florescence microscope (Nikon). Five random fields (200–300 cells/well) were examined in each well. The number of axon-like processes, defined as extensions longer than twice the cell body diameter, was recorded. Neurite lengths were quantified using ImageJ and data was expressed as the mean ± standard error of mean (SEM). Percentages of differentiation (percentages of cells bearing neurites in entire attached cells), average neurite lengths, and numbers of branches per neurite were quantified over more than 10 images and 100 non-overlapping cells for each sample. Only neurites longer than twice the diameter of original, round PC-12 cells were considered as positive neurite extension and cells with at least one neurite longer than 10 µm were counted as neurite-bearing cells. The mean number of neurite-bearing cells was quantified by scoring the total number of neurite-bearing cells over the total number of viable cells per field. At least three independent experiments were conducted. Average neurite length (µm) was determined by measuring >200 neurites for each folic acid concentration and for each time point. Branches per 100 µm of neurite are counted as a function of increasing folic acid concentration.
2.6 Neurotrophic Factor Release from Schwann Cells and Neurotrophin ELISA Assay
Rat Schwann cells were grown to confluency in T-25 flasks and once they became confluent, the media were changed to serum-free media containing low (4 mg/L) and high (50 mg/L) concentrations of folic acid. After three days in a 37°C incubator with 5% CO2, the cell culture supernatants were removed from flasks and particulates in the cell culture supernatants were removed via centrifugation at 10,000 × g for 5 min. The supernatant from each medium was collected and filtered before being stored at −20°C for long-term use if not used immediately. Each supernatant was then added to culture PC-12 cells. In the control group, PC-12 cells were cultured in the serum-free medium with 50 mg/L of folic acid. The images of the cells were taken every day to monitor the morphological changes of PC-12 cells in terms of neurite outgrowths. The supernatants along with a control of serum-free medium were analyzed for their neurotrophin contents using Rat Multi-Neurotrophin Rapid™ Screening ELISA Kit (Biosensis®, South Australia; catalog #: BEK-2232) based on its manual.
2.7 Traction Force Substrate Preparation
Polyacrylamide (PAA) hydrogels with one-plane embedded fluorescent beads were prepared for traction force assays by following the method published by Knoll et al.[21] with some modifications. Briefly, clean No. 1 coverslips (24 mm × 24 mm) were silanized with 2% 3-aminopropyl-trimethoxysilane (Sigma-Aldrich; catalog #: 281778) in isopropanol and then activated with 1% diluted glutaraldehyde solution (Alta Aesar; catalog #: 111-30-8) to become super hydrophilic. Then, Rain-X® Original Glass Water Repellent (Walmart) was used to vigorously wipe the glass slides to make them hydrophobic, followed by sequentially coating the glass slides with Poly-D-Lysine (Millipore; catalog #: A-003-E) and fluorescent carboxylate-modified beads (Thermo Scientific; product #: F8810). A drop of 35 µL pre-mixed acrylamide (EMD Millipore; catalog #: 1185-OP) and bis-acrylamide solution (EMD Millipore; catalog #: 2640-OP) with their final concentrations of 8% and 0.13%, respectively, was then sandwiched between the glass slide and the amino-activated coverslip. Polymerization was initiated by adding 2.5µl 10% APS (Promega; catalog #: V3131) and 0.8 µL TEMED (Teknova; catalog #: T0761) into the pre-mixing solution. After polymerization, the coverslip attached to the hydrogel was gently detached from the glass slide and quickly immersed in ddH2O for short-term storage. Collagen IV (Sigma-Aldrich; catalog #: C5533) at 4.5 µg/cm2 was cross-linked to the PAA gel surface overnight at 4°C after activating the crosslinker, Sulfo-SANPAH (Thermo Scientific; product #: 22589) under UV light at the wavelength of 365 nm. Prior to seeding PC-12 cells, the gel was rinsed thrice with DPBS and sterilized in a biological cabinet for 30 min.
2.8 Measurement of Traction Forces
Three images were taken in a series on a chosen area with an inverted fluorescent microscope (Nikon, Eclipse Ti-U): one was the phase contrast image of the selected PC-12 cell; the other two were the pair images of fluorescent beads, one with cells attached to the gel (deformed configuration) and the other with cells as attached (undeformed configuration). Derivation of cell traction force was conducted with the open source plugins in ImageJ developed by Tseng et al.2.
2.9 Western Blotting Assay
PC-12 cells were first cultured in 25cm2 CellBIND® culture flasks with differentiation media containing various concentrations of folic acid, either with or without 50 ng/mL NGF. After 14 days of culture, the cells were washed with ice-cold Tris-buffered saline (TBS; 20 mM Tris pH 7.5, 150 mM NaCl). Once the TBS was aspirated, RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1mM NaF, 1mM sodium orthovanadate, and protease inhibitor cocktail) was added to the cells in ice until the cells were lysed. The cells were then harvested with a scraper and agitated in ice for 30 min at 4°C. The cells were ultrasonicated in ice three times for 10–15 sec to completely lyse the cells, followed by centrifugation at 16,000 × g for 20 min. The supernatants were collected for downstream experiments. The protein concentration in each sample was measured using a Precision Red Advanced Protein Assay kit (Cytoskeleton, Inc., Denver, CO, USA). 20 µg of each protein sample was taken and added with an equal volume of 2× Laemmli sample buffer (Bid-Rad; Hercules, CA, USA; catalog #: 1610747) before being boiled for five min at 95°C. The samples were centrifuged at 16,000 × g for one min. Protein samples were run through the Criterion™ TGX Any kD Stain-Free™ precast gels (Bio-Rad) for 10 min at a constant voltage of 50 V, followed by 25 min at a constant voltage of 250 V. Proteins were then transferred onto a blotting membrane overnight using a wet tank at 4°C. Proteins were then detected using primary antibodies for MAP-2 (1:200 dilution; Abcam; Cambridge, MA, USA; catalog #: 32454) and β-actin (1:200 dilution; Abcam; catalog #: 8229) in a 3% BSA/TBST buffer overnight and incubation with a secondary goat anti-rabbit (1:5000 dilution; Santa Cruz Biotechnology, Dallas, TX, USA; catalog #: 2004) and a mouse anti-goat (1:5000 dilution; Santa Cruz Biotechnology; catalog #: 2354). Immunoblot detection was performed using a ChemiDoc MP Imager.
2.10 Quantification of Global DNA Methylation
Each Schwann cells and PC-12 cells were cultured for three days in DMEM medium and F-12 K medium containing different concentrations of folic acid, respectively and additional 50 ng/mL NGF for PC-12 cells. The DNA extraction was performed using a FitAmp Blood and Cultured Cell DNA Extraction kit (Epigentek, Farmingdale, NY, USA; catalog #: P-1018-100), followed by the quantification of DNA in each sample using NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). An equal amount of DNA was used in all experiments and the quantification of the global DNA methylation was performed with a MethylFlash Methylated DNA 5-mC Quantification Kit (Epigentek; catalog #: P-1034-96). The absolute amount and percentage of methylated DNA (5-mC) in total DNA of each sample were calculated according to the manufacturer’s manual and the percentage of methylated DNA (5-mC %) in each sample was reported in this work (Figure 5). The experiment was repeated three times, independently.
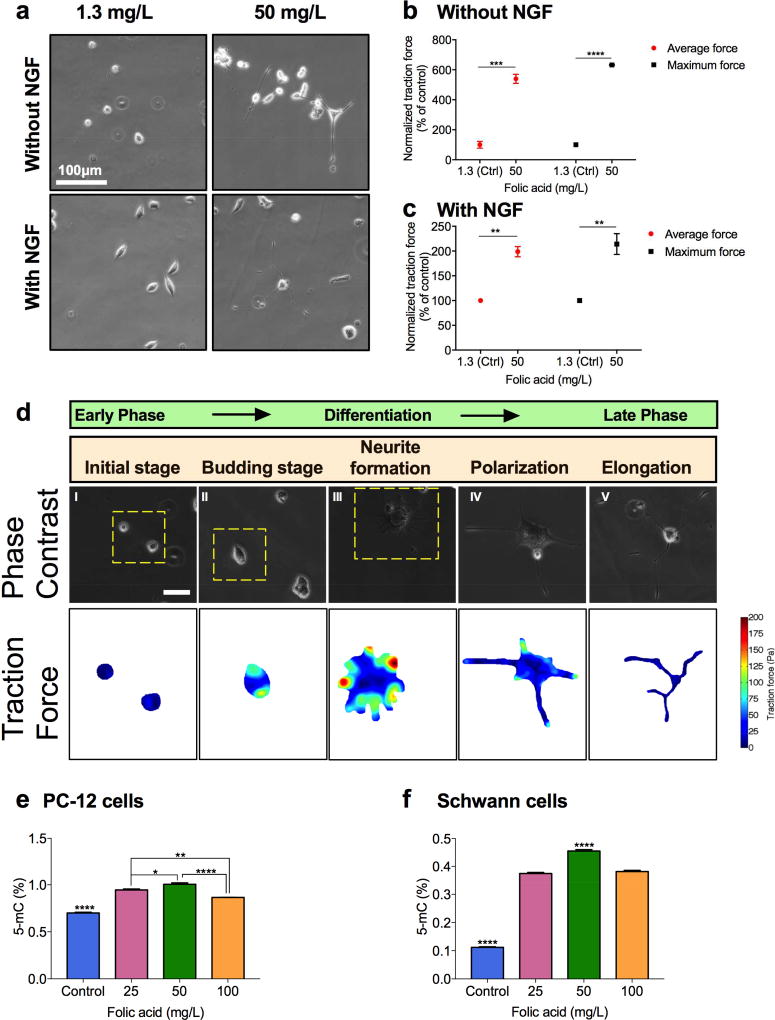
Figure 5.
Effects of folic acid on traction force and global DNA methylation levels of PC-12 cells. A) Representative phase contrast microscopic images of PC-12 cells stimulated with low (1.3 mg/L) and high (50 mg/L) concentrations of folic acid in the presence/absence of 50 ng/mL NGF after four days. B, C) Average and maximum traction forces on PC-12 cells stimulated with low (1.3 mg/L) and high (50 mg/L) concentrations of folic acid without (B) and with (C) 50 ng/mL NGF after four days. D) Phase contrast images and traction force maps of PC-12 cells stimulated with 50 mg/L of folic acid at different stages of differentiation. (****P<0.0001, ***P<0.001, **P<0.01, *P<0.05) (Scale bar: 100 µm). E) Global DNA methylation levels in PC-12 cells treated with different concentrations of folic acid in the presence of 50 ng/mL NGF. F) Global DNA methylation levels in Schwann cells treated with different concentrations of folic acid. (****P<0.0001, **P<0.01, *P<0.05).
2.11 Synthesis of Crosslinked Urethane-doped Pre-polymer (pre-CUPE) and Preparation of Folic acid-CUPE Nerve Guidance Conduits
Pre-CUPE was synthesized in two steps similar to previously published methods.[20] In the first step, a citric acid-based prepolymer, poly (octanediol citrate) (pre-POC) or Pre-POC, was synthesized by melting a 1.0:1.1 molar feeding ratio of citric acid and 1,8-octanediol, respectively, at 160°C in a three-necked round bottom flask. Once all the monomers had melted, the temperature of the system was reduced to 140°C, and the reaction mixture was allowed to proceed for 60 min. The resulting mixture was purified by drop-wise precipitation in deionized water, collected, and lyophilized for 24 h to obtain the final POC pre-polymer. In the second urethane-doping step, pre-POC chain extension was achieved by dissolving the purified pre-POC in 1,4-dioxane to form a 3.0% solution by weight, and reacted with 1,6-hexamethyl diisocyanate (HDI) (1:1 molar ratio of pre-POC:HDI) under constant stirring at 55°C. Complete diisocyanate reaction to pre-POC was confirmed upon the disappearance of the isocyanate peak located at 2267 cm−1 of Fourier-Transform Infrared (FT-IR) analysis using Bruker Vertex70 FTIR spectrometer (Bruker Corporation, Billerica, MA, USA). CUPE nerve guidance conduits (NGCs) were prepared by first 1) coating glass rods with a CUPE pre-polymer solution, 2) controlled, thermal post-polymerization of CUPE at 100°C for three days, 3) removing NGCs from glass rods by soaking the polymer-coated rods in DI water, 4) followed by freeze-drying. Folic acid coated-CUPE (fCUPE) NGCs were then prepared by dip-coating polymerized CUPE NGCs in a 100-mg/L folic acid solution. The average dimensions of NGCs measured with a caliper were 2.5 mm (inner diameter), 0.25 mm (wall thickness), and 3 mm (outer diameter). The release of folic acid was measured by using a high-performance liquid chromatography (Shimadzu) equipped with a photodiode array detector (Shimadzu) and a Phenomenex Kinetex C18 column. The mobile phase was a mixture of 95% PBS and 5% acetonitrile, and the flow rate was 1 mL/min. Folic acid concentration was determined by reading the absorbance at 280 nm. For drug release tests, fCUPE NGCs (approximate weight: 10 mg) were immersed in 5 mL PBS and incubated at 37 °C. At each time point, the film suspensions were centrifuged and then 0.5 mL of release solution was removed for HPLC measurement. After each collection, 0.5 mL of fresh PBS was added into each sample.
2.12 Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra were recorded with a computer controlled Bruker Vertex70 FTIR spectrometer (Bruker Corporation, Billerica, MA, USA) with a MCT detector at Penn State Material Characterization Laboratory. The samples were scanned 400 times in the wavenumber range of 400–4000 cm−1. The spectrum was collected using the software Opus version 7.0 and replotted using the OriginLab® Origin software.
2.13 Rat Sciatic Nerve Injury
A total of 12 adult male Wistar rats (180–220 g, three months old) were used in the experiments. The Animal Ethical Committee and Neurosurgical Institute of Beijing at the Capital Medical University approved all animal experiments. The rats were randomized into two groups: group A, which was treated with autografts and group B, which was treated with folic acid-CUPE nerve conduits (CUPE nerve guides dip-coated with 100 mg/L folic acid solution in PBS followed by freeze-drying method). The rats were anesthetized with 10% chloral hydrate (0.3 ml/100g). Using aseptic technique, the right sciatic nerve was exposed 22 mm distal to the sciatic notch, transected by an ophthalmic scissors, and bridged by fCUPE NGCs or sutured in situ reversed 180°. After the surgery, we evaluated the recovery based on walking track assessment, electrophysiological assessment, and post-mortem histological analysis.
2.14 Walking Track Assessment
To evaluate the functional restoration from sciatic nerve injury, the following parameters were measured during the walking track analysis: print length (PL) on both the experimental and normal sides (EPL and NPL), toe spread (TS) between the first and fifth digits on both sides (ETS, NTS), and the distance between the middle of the second and fourth toes on both sides (EIT, NIT). The following formula (Equation 1) was then used to calculate the sciatic function index (SFI) values:
| (1) |
The SFI value varies from 0 to −100, with 0 corresponding to normal function and −100 to total impairment of the sciatic nerve function. Walking track analysis was performed at 2, 4, 8, and 12 months post implantation. In detail, the animals were dipped in carbon ink and allowed to walk on a paper-lined walkway (radius: 10 cm), leaving their hind footprints on the paper. Four to five clear footprints of each hind were recorded on each side.
Electrophysiological Assessment
The electrophysiological assessment was performed at 4, 8 and 12 weeks post surgery. The nerve conduction velocity (NCV) and incubation period were recorded using myoelectricity-evoked potential equipment (Dantec Keypoint co., Denmark) at 4, 8, and 12 weeks, postoperatively. The rats were anesthetized through intraperitoneal injection of 10% chloral hydrate solution (0.3 ml/ 100g body weight). The stimulating double needle electrode was placed on the lower back of experimental rats (about L4–L5). The two recording electrodes were placed on the ankle where the sciatic nerve went through and gastrocnemius muscle; the reference electrode was placed on the tail. NCV (m/s) was calculating by dividing the distance between the stimulating electrode and the recording electrode (m) by the conducting time (s). The NCV and incubation period were assessed three times separately for each rat and the average value was used.
2.15 Statistical Analysis
All statistical analyses were performed using Prism 7 software (GraphPad Software, La Jolla, CA, USA). All data are presented as the mean ± standard error of the men (SEM). All the experiments were performed at least three times. Data for cell migration, differentiation, and traction force experiments, and global DNA methylation were analyzed using two-way repeated-measures analysis of variance (ANOVA). In case of neurite outgrowth analysis, the differences in various culture conditions were assessed by the means of one-way ANOVA followed by the Tukey’s test (multiple comparisons vs. control). For comparisons between two groups, the Tukey test was employed, and in all cases, a value of p<0.05 was considered to be statistically significant. P values less than 0.05 were considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001)
3. Results and Discussion
3.1 Folic acid supports survival and proliferation of Schwann cells and PC-12 cells and induces cell migration of Schwann cells and astrocytes
In vitro cytotoxicity assessment confirmed that folic acid is harmless on rat Schwann cells and adrenal pheochromocytoma cells, PC-12 cells when applied at concentrations up to 200 mg/L (Figure S1, Supporting Information). Both cell lines were then exposed to different concentrations of folic acid (basal folic acid concentration in each cell medium, 50 mg/L, and 100 mg/L of folic acid) for predetermined times (1–7 days). The basal folic acid concentrations in Schwann cell and PC-12 cell media were 4 mg/L and 1.3 mg/L, respectively. Folic acid moderately stimulated the proliferation of Schwann and PC-12 cells at low concentrations (25–100 mg/L) as compared to the controls (Figure 2a and 2b).
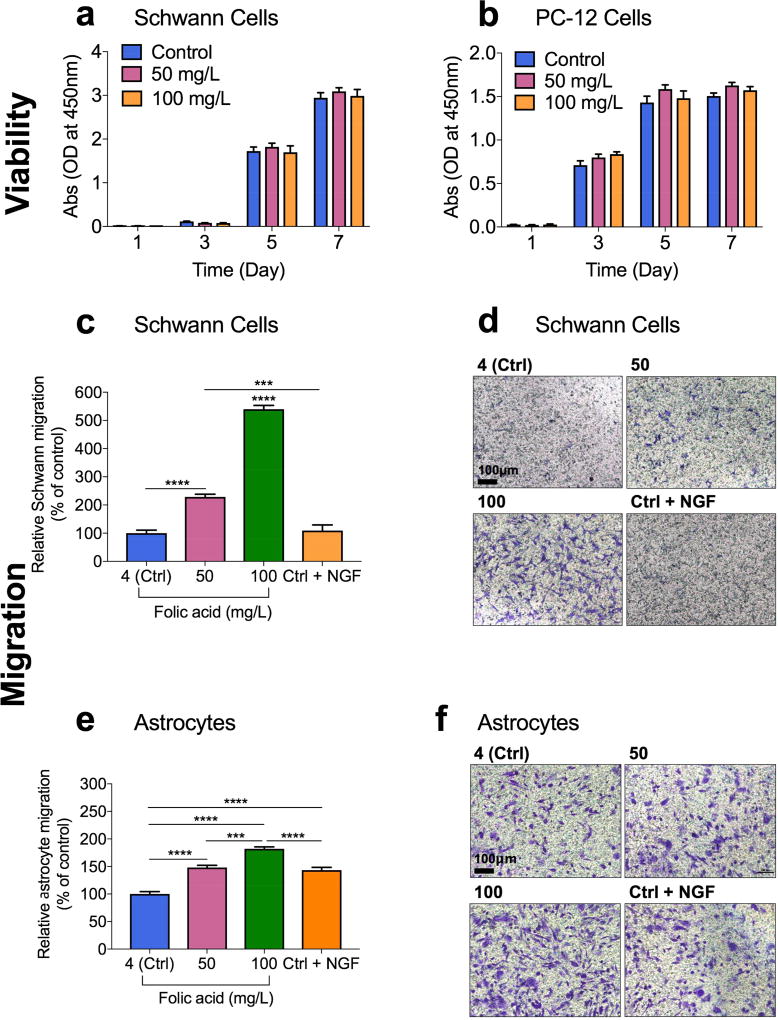
Figure 2.
Proliferative and chemotactic effects of folic acid. A, B) Cytocompatibility and proliferative effect of different concentrations of folic acid (mg/L) on Schwann cells (A) and on PC-12 cells (B) at various time points. Error bars indicate mean ± S.E.M. (n=8). C, E) Relative cell migration of Schwann cells (C) and human astrocytes (E) determined by the number of the migrated cells in each sample normalized to the total number of the migrated cells in the control sample where the folic acid concentration in the basolateral chamber was 4 mg/L (labeled as ctrl). Each folic acid concentration on x-axes indicates the folic acid concentration in the basolateral chamber when the folic acid concentration was consistent at 4 mg/L. Data are shown as mean ± S.E.M., n=5. (****P<0.0001; ***P<0.001). D, F) Bright field images of the migrated Schwann cells (D) and human astrocytes (F) stained with crystal violet in each sample. The number on top of each picture indicates the folic acid concentration in the basolateral chamber.
Next, we evaluated the ability of Schwann cells and astrocytes to directionally respond to different concentrations of folic acid using transwell migration assays and live-cell imaging. Cells were placed in the apical chamber and the cell medium containing each test agent (different concentrations of folic acid and NGF) was placed in the basolateral chamber. For comparison, a widely used neurotrophic factor and a chemotactic agent for Schwann cells[22], NGF (50 ng/mL) and a reverse folic acid gradient or lack of a folic acid gradient were tested as well.
Migrating cells (% of control) of both cell types are shown in Figure 2c and 2e. In all cases, the cells moved from apical chambers to basolateral chambers. As expected, the medium containing NGF showed a slightly higher percentage of Schwann cell migration than the control medium (Figure 2c and 2d). In astrocytes, however, the NGF-containing medium exhibited a considerably higher migration than the control medium (P<0.0001) (Figure 2e and 2f). The results also indicate that folic acid increased Schwann and astrocyte cell migration in a dose-dependent manner, with 100 mg/L folic acid being the concentration that induced the highest cell migration in both cell types. The cell medium with 100 mg/L of folic acid significantly increased the percentage of Schwann cell migration by 5.40 folds (P<0.0001) and astrocyte migration by 1.82 folds (P<0.0001), when compared to the control medium that contained 4 mg/L of folic acid (Figure 2c and 2e). It is remarkable that the magnitude of the increase induced by the medium of 100 mg/L folic acid was much higher than that observed with NGF, our positive control (P<0.0001). The chemotactic effect of folic acid was concentration-dependent up to 100 mg/L in both cell lines.
In addition, Schwann cell migration in response to folic acid gradient was also observed in polydimethylsiloxane (PDMS) micro-channels using live-cell imaging (Figure S2, Supporting Information). In the reservoirs left (not shown) to the micro-channels, we added a small amount of Dulbecco’s modified eagle’s medium (DMEM) containing 4 mg/L folic acid and in the reservoirs right (not shown) to the micro-channels, we added DMEM containing 100 mg/L folic acid. As measured in time-lapsed microscopy experiments, Schwann cells moved at a velocity of 0.12 ± 0.016 µm/min towards a higher concentration of folic acid. The sequential pictures of migrating Schwann cells showed a cycle of cell adhesion at a cell’s leading edge, whole cell contraction, and rear-end retraction.
3.2 Folic acid stimulates neurite outgrowth and elevates the expression of microtubule-associated protein 2 (MAP-2) in differentiating PC-12 cells
PC-12 cells were employed to study folic acid-induced neuritogenesis. To assess chemical effects of folic acid on differentiating PC-12 cells, neurite outgrowth assays and the quantification of a maker of neuronal differentiation, microtubule-associated protein 2 (MAP-2), were performed (Figure 3). PC-12 neurites were induced in differentiation media supplemented with 50 ng/mL NGF and different concentrations of folic acid for seven (for neurite outgrowth assay) and 14 days (for MAP-2 measurement). 50 ng/mL NGF alone was used as a positive control due to its well-known role in inducing neurite outgrowth from PC-12 cells by activating the receptor tyrosine kinase (TrkA).[23]
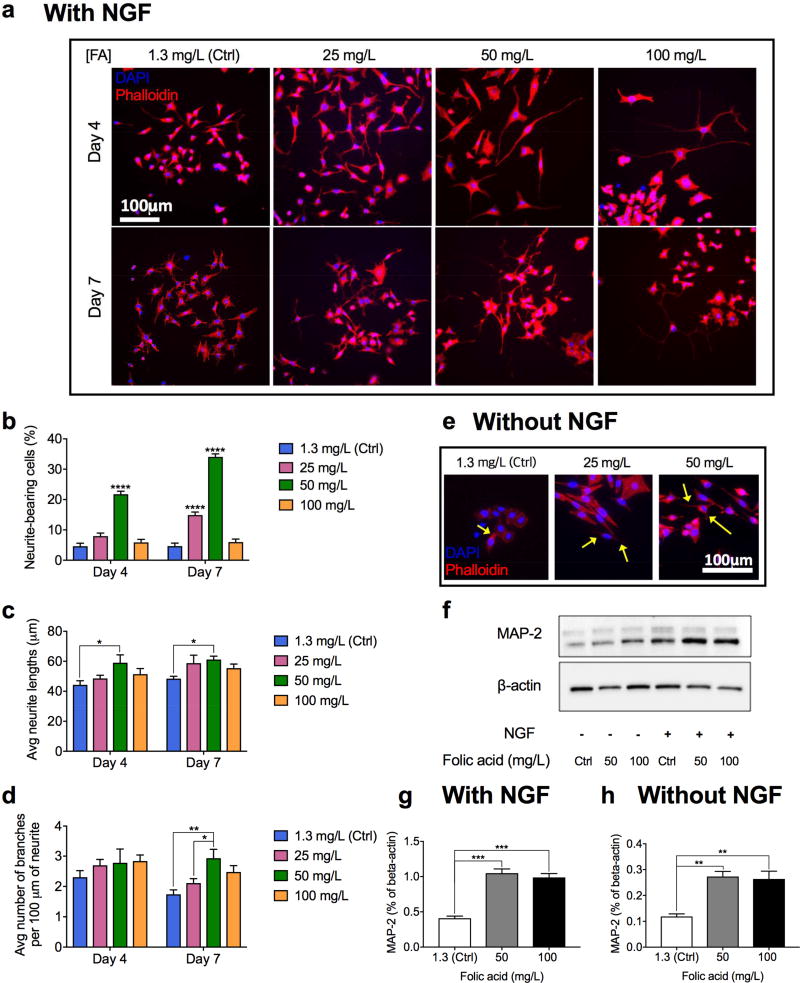
Figure 3.
In vitro effects of folic acid supplementation on differentiation of PC-12 cells. A) Representative immunohistochemical images of PC-12 cells treated with different concentrations of folic acid (mg/L) in the presence of NGF for four and seven days. B–D) Percentage of neurite-bearing cells (B), average neurite lengths (C), and average number of branches per 100 µm of a neurite (D) in PC-12 cells treated with different concentrations of folic acid (mg/L) in the presence of NGF for four and seven days. Data are shown as mean ± S.E.M., n=200. E) Representative immunohistochemical images of PC-12 cells stimulated solely with folic acid (mg/L) in the absence of NGF (arrows indicate extended neurites). F) Western blotting results showing protein expressions (MAP-2 and load control, β-actin) in PC-12 cells treated with various concentrations of folic acid (mg/L) in the absence or presence of 50 ng/mL NGF for 14 days. G, H) Quantitative comparison of MAP-2 expressions (F) in PC-12 cells treated with different concentrations of folic acid in the presence of NGF (G) and in the absence of NGF (H) for 14 days. (****P<0.0001, ***P<0.001, **P<0.01, *P<0.05).
Folic acid significantly improved PC-12 differentiation in terms of the number of neurite-bearing cells, mean neurite lengths, and number of branches per 100 µm of neurites. For the neurite outgrowth assay, cells displaying neurites two times longer than the length of the cell body were considered positive. The percentages of neurite-bearing cells in the PC-12 cells treated with 50 mg/L folic acid were 21.74% and 34.04% on day 4 and 7, respectively, both significantly higher (P<0.0001) the percentage of neurite-bearing cells cultured in the control medium containing 1.3 mg/L folic acid (4.61% on day 4 and 4.64% on day 7) (Figure 3b). 25-mg/L folic acid also induced higher percentage of neurite-bearing cells (P<0.0001) as compared to the control medium on day seven. Average neurite length increased progressively and in concert with percentage of neurite-bearing cells as the dose of folic acid increased from 1.3 mg/L to 50 mg/L (P<0.05) (Figure 3c). The number of branches on each neurite was counted per 100 µm of the neurite and 50 mg/L of folic acid induced the highest number of branches on day seven (P<0.05).
MAP-2 protein expressed in PC-12 cells was quantified in each sample to further investigate the folic acid effect. Following the supplementation of folic acid for 14 days, MAP-2 expression increased by about three folds (P<0.01) compared with the control group in the absence of NGF and two folds (P<0.001) in the presence of NGF (Figure 3f and 3g and 3h). It is remarkable to note that folic acid can facilitate neurite outgrowth even without any NGF stimulation (Figure 3e and 3f and 3h). In the absence of NGF, the PC-12 cells without additional folic acid are less spread and do not extend neurites whereas the PC-12 cells stimulated with additional folic acid are well spread and several of them extend neurites (Figure 3e). This morphological observation was verified by the western blotting results: both 50 and 100 mg/L folic acid increased the expression of the neuronal marker, MAP-2, in PC-12 cells (P<0.01) by more than two folds (Figure 3f and 3h).
3.3 Folic acid-supplemented Schwann cells accelerated neuronal outgrowths by producing NT3 and NT-4/5
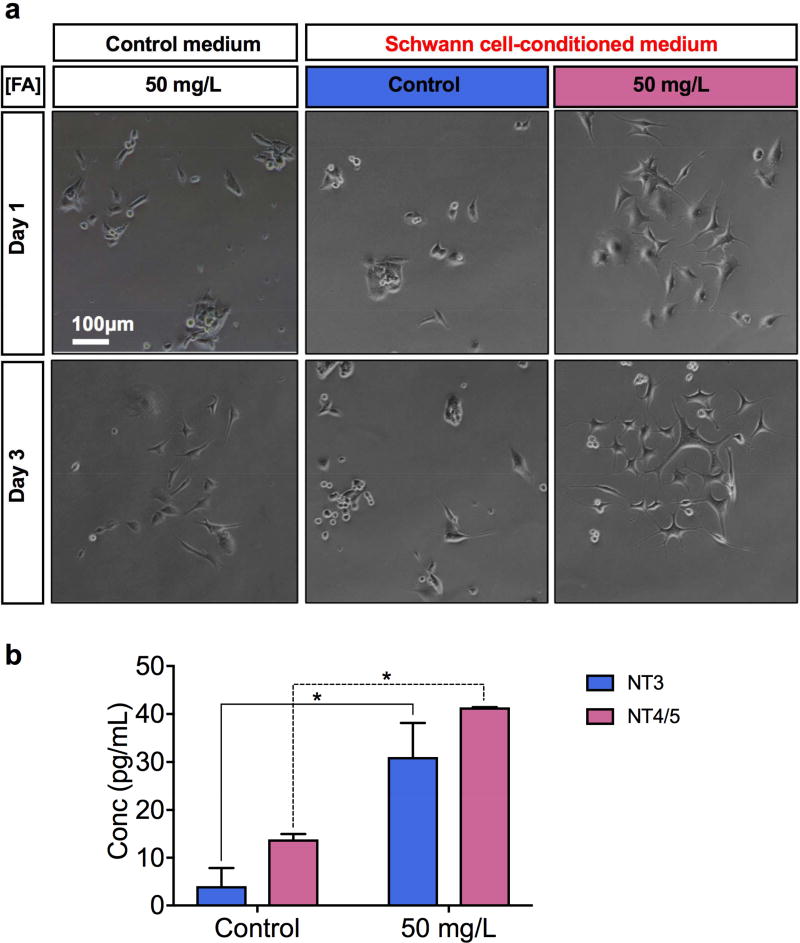
Neurotrophins are essential for neural development, maturation, and regeneration, particularly for cell differentiation, survival, death, and synaptic regulation. Schwann cells are known for their role in secreting neurotrophins so we investigated the possibility of using folic acid for inducing neurotrophin release from Schwann cells. First, confluent Schwann cells were incubated with a serum-free cell culture medium with 1) 4 mg/L of folic acid or 2) 50 mg/L of folic acid. Those cell supernatants were collected after three days and used to differentiate PC-12 cells. As a control, a serum-free, unconditioned cell culture medium with 4 mg/L of folic acid was also included in the study. The three types of cell media were used to induce the differentiation of PC-12 cells. The cell culture supernatant collected from the Schwann cells cultured with 50 mg/L of folic acid helped accelerate the differentiation of PC-12 cells the most, in comparison to the control media prepared at a lower folic acid concentration (4 mg/L) either with or without conditioning with Schwann cells (Figure 4a). A majority of the PC-12 cells cultured with the high folic acid medium conditioned with the Schwann cells already had a prominent neurite protrusion on day three.
Figure 4.
Effects of folic acid supplementation on neurotrophin release from Schwann cells. A) Representative microscopic images of PC-12 cells cultured for one day and three days with 1) control medium with 50 mg/L of folic acid, 2) cell culture supernatant from the Schwann cells primed with 4 mg/L of folic acid, and 3) cell culture supernatant from the Schwann cells primed with 50 mg/L of folic acid. B) Mean NT3 and NT4/5 levels measured in Schwann cultures incubated with control (4 mg/L) and high-folic acid (50 mg/L) cell media (*P<0.05).
Neurotrophin release was compared in each cell supernatant sample using ELISA. The Schwann cells that have been exposed to 50-mg/L folic acid medium induced 7.63 times higher neurotrophin-3 (NT3) release (P<0.05) and 2.98 times higher neurotrophin-4/5 (NT-4/5) release (P<0.05) than the Schwann cells incubated with 4-mg/L folic acid (Figure 4b). The results in Figure 4 clearly show that folic acid supplementation enabled Schwann cells to release more neurotro phins, specifically NT3 and NT-4/5, for the neurite outgrowth of PC-12 cells. Both NT3 and NT-4/5 are known to promote the growth, survival, and differentiation of neurons in the CNS and PNS.[24] In detail, NT3 has been found to enhance Schwann cell migration through Tropomyosin receptor kinase C (TrkC).[25] On the other hand NT-4/5 expression elevates by the cells in the proximal nerve segment and also participates in promoting axon regeneration after peripheral nerve injury.[26] The effect of folic acid in increasing the expression of NT3 and NT-4/5 from Schwann cells induces further Schwann cell migration and axonal regeneration. These findings complement and provide possible explanation to the results seen in Figure 2 and Figure 3 where folic acid induced higher Schwann cell migration, and higher PC-12 differentiation.
3.4 Folic acid induces traction force changes in PC-12 cells that lead to further differentiation
Neurons transduce environmental chemical cues into mechanical forces to achieve properly controlled cell polarization and differentiation.[27] As discussed earlier, additional folic acid increased the expression of a marker of neuronal differentiation, microtubule-associated protein 2 (MAP-2), in PC-12 cells. Here, we explored whether the chemical stimuli by folic acid would not only increase the protein expression of cytoskeletal filaments such as microtubules, but also lead to higher cytoskeletal tension, thus increasing cellular traction forces. The transduction of the chemical cues induced by folic acid into the mechanical cues that affected differentiation and neurogenesis of PC-12 cells was measured with traction force microscopy (TFM). Specifically, we measured the maximum and average forces generated along the cell bodies and neurites of PC-12 cells treated with either additional folic acid or NGF or both.
Our studies show that additional folic acid (50 mg/L) in the cell medium stimulated PC-12 cells to generate about 5.3 times higher average traction force and 6.3 times higher maximum traction force than the control group (1.3 mg/L folic acid) in the absence of NGF and about two times higher average traction force than the control group in the presence of 50 ng/mL NGF (Figure 5a and 5b and 5c). The effect of folic acid treatment on the traction forces in PC-12 cells at different stages of differentiation was also carefully examined through bright field images and traction force maps (Figure 5d). The PC-12 cells exposed to 50 mg/L folic acid followed sequential changes of surface morphology equivalent to those of cells responding to NGF, a neurotrophic factor commonly used for inducing the differentiation of PC-12 cells into sympathetic-like neurons. Additional folic acid alone initially increased the traction force generation gradually at the cell periphery of PC-12 cells (‘budding stage’), generated the highest maximum traction forces near the terminals of the neurites while multiple small neurites started to emerge (‘neurite formation’), and reduced the maximum traction force along the cells as the morphological polarization formed the axons and dendrites (‘polarization’). Based on these observations, such mechanical stresses exerted by folic acid are considered to be critical for neurite formation.
3.5 Folic acid changes global DNA methylation levels in PC-12 cells and Schwann cells
Suppressed global DNA methylation in spinal neurons was observed after spinal cord injury combined with peripheral nerve injury in rodents.[17] To demonstrate the effect of exogenous folic acid on restoring global DNA methylation, global DNA methylation levels were quantified in control and folic acid-treated cultures following three days of folic acid treatments on Schwann and PC-12 cells. Results show dose-dependent changes in global DNA methylation after the folic acid treatment in both cell lines (Figure 5e and 5f). The dose-dependent pattern of these changes (Figure 5e) was similar in peak folic acid doses and overall morphology to those observed with differentiated PC-12 cells stimulated by folic acid (Figure 3a). In Schwann cells treated by 50 mg/L folic acid, DNA methylation levels reached more than four times of that in control cultures (Figure 5f).
3.6 In vivo nerve regeneration using a folic acid-nerve guidance conduit in a critical-sized peripheral nerve gap
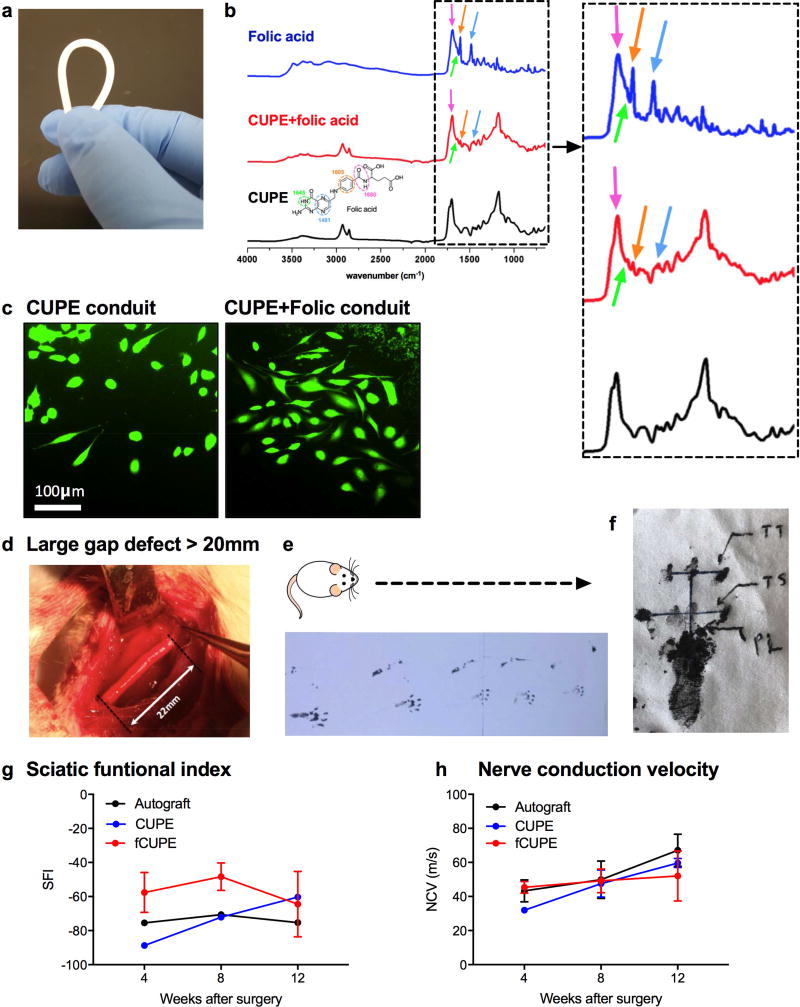
In order to confirm the in vitro findings above and to examine the in vivo efficacy of locally-delivered folic acid to promote peripheral nerve regeneration, we prepared kink-resistant, soft, elastic, and biodegradable crosslinked urethane-doped polyester (CUPE) NGCs[18,28] (Figure 6a) by first 1) coating glass rods with a CUPE pre-polymer solution, 2) controlled, thermal post-polymerization of CUPE, and 3) leaching of the CUPE NGCs in DI water followed by freeze-drying. Folic acid-modified CUPE (fCUPE) NGCs were made by dip-coating CUPE NGCs in PBS of 100-mg/L folic acid. During the dip-coating process, folic acid was absorbed in the NGCs. Although it moderately varies depending on the monomer ratios used and polymerization conditions, the average crosslink density of CUPE film materials ranges from 1.1717 ± 0.0211 to 1.2253 ± 0.0207[20]. The deposition of folic acid on fCUPE NGCs was confirmed by the Fourier-transform infrared spectroscopy (FTIR) as the FTIR spectra of folic acid, and CUPE and fCUPE materials are shown in Figure 6b. The in vitro release profile of folic acid from fCUPE NGCs is shown in Figure S3. It was found that about 70% of the incorporated folic acid was released after 8 hours. The characteristic IR absorption peaks of folic acid were found in the fCUPE samples at 1697 cm−1 (amide), 1605 cm−1 (benzene, conjugated double absorption), 1481 cm−1 (hetero-ring, conjugated double bond), and 1645 cm−1 (primary amine).[29] Prior to the in vivo study, PC-12 cells were cultured on the top of the control CUPE NGCs and fCUPE NGCs in the presence of 50 ng/mL NGF. The PC-12 cells grown on the fCUPE NGC samples and stained with the intracellular fluorescent dye, carboxyfluorescein succinimidyl ester (CFSE; green), showed more and longer neurites than the PC-12 cells grown on the control CUPE NGCs samples (Figure 6c).
Figure 6.
In vivo evaluation of folic acid-coated NGCs. A) A kink-resistant CUPE NGC. B) FTIR spectra of folic acid, CUPE and fCUPE conduits (dotted box: magnified spectra in range below 2000 cm−1). C) PC-12 cells grown on CUPE and fCUPE NGCs and stained with a green CFSE fluorescent cell staining dye. D) CUPE NGCs, fCUPE NGCs, and autografts were used to connect large nerve gaps in the length of 22 mm. E) A representative example of recorded footprints of a recovering animal. F) Representation of the values utilized in calculating the SFI after obtaining the animals’ footprints. G) SFI values of autogtafts, CUPE and fCUPE NGCs four, eight, and 12 weeks post-surgery. H) NCVs of autografts, CUPE and fCUPE NGCs four, eight, and 12 weeks post-surgery.
A commonly used rat sciatic nerve transection model was used for the evaluation of fCUPE NGCs in promoting peripheral nerve regeneration in large nerve defects (gap length: 22 mm) in vivo (Figure 6d). Autografts (n=6), CUPE NGCs (n=6), and fCUPE NGCs (n=6) were directly implanted into the lesion site immediately after the nerve transection. Nerve autografts were reserved 180° and implanted as a control. Functional, electrophysiological, and morphological analysis of peripheral nerve regeneration after the injury was evaluated by performing 1) walking track analysis (two, four, eight, 12 weeks post-surgery) (Figure 6e and 6f), 2) electrophysiological assessment (four, eight, 12 weeks post-surgery), and 3) histological evaluation (16 weeks post-surgery). Figure 6g and 6h show the sciatic functional index (SFI) and nerve conduction velocity (NCV) values of the autografts, CUPEs, and fCUPEs at four, eight, and 12 weeks post-implantation.
The walking patterns of fCUPE NGCs were similar to or better than the autograft group. The fCUPE group showed better SFI values than the autograft and CUPE groups throughout the first 8-week implantation (Figure 6g). After four weeks of implantation, the SFI values of the fCUPE NGCs were 24% improved than the autograft group and 35% improved than the CUPE group. After 8 weeks of implantation, the SFI values of the fCUPE NGCs were still 32% higher than the autograft group. After 12 weeks of implantation, the fCUPE NGCs showed 14% improved SFI values as compared to the autografts. A slight reduction in the SFI values of the fCUPE group at week 12 is likely due to the loose bonding between folic acid and the CUPE NGCs as a simple dipping method was applied to prepare the fCUPE NGCs. The folic acid effect probably dissipated slowly during a 12-week implantation. The motor nerve conductive velocity (NCV) in the fCUPE group was slightly faster than the autograft and CUPE groups at four weeks, which indicates that the addition of folic acid may have aided in the early-phase axonal regeneration in rats with transected sciatic nerves (Figure 6h). However, the improvement made by folic acid reduced over time and by eight weeks post-surgery, the fCUPE group had similar NCV results with the autograft group, slightly leading in the comparison. Figure S4 in the Supporting Information shows representative microscopic images of autogtafts and fCUPE NGCs, stained with hematoxylin and eosin (H&E) following 16 weeks of implantation. The cross-sections from the central region of the both groups (autografts and fCUPE NGCs) were normal in appearance consisting of myelinated axons, large amounts of axon bundles, and the presence of fascicles. A large number of Schwann cells and fibroblasts were also observed in ordered arrangement throughout the fCUPE NGCs. Tissue infiltration can be seen throughout the interior of all the conduits tested. Both groups, however, still had some void spaces, and it indicates that large defects might require a longer time than four months to be bridged completely.
4. Conclusions
Developing treatments for peripheral neuropathies and neurological disorders is critical, and folic acid may provide solutions to some of these issues. Therefore, a greater understanding of the important chemical and mechanical regulation of folic acid will be essential for 22 investigating folic acid as an effective therapeutic agent. Here, we report the dose-dependent effects of exogenous folic acid on supporting the proliferation of Schwann cells and, PC-12 cells, the migration of glial cells in the PNS and CNS (Schwann cells and astrocytes), and the differentiation of PC-12 cells. Glial cell migration is crucial for both peripheral nerve and spinal cord injuries as glial cells provide physical support and secrete growth factors to enhance axon regeneration following PNS and CNS injuries.[30] Folic acid also induced the release of NT3 and NT-4/5, which are known to enhance Schwann cell migration and peripheral nerve regeneration.
The dose-dependent effect of folic acid on stimulating the differentiation of PC-12 cells was closely correlated over its dose range with global DNA methylation levels. Aberrant DNA methylation leads to a reduction in neurogenesis and neural stem cell differentiation[31] and it was shown to be involved in the development of brain ischemia[7,32] and neurological disorders, such as the fragile X, ICF, and Rett syndromes.[33,34] The folic acid effect on global DNA methylation in the Schwann cells still needs more investigation on the methylation expression patterns to gain a better understanding on if the genes associated with folic acid-induced hypermethylation have any neuroregenerative effects. The traction force changes in the PC-12 cells exposed to different doses of folic acid were quantified using traction force microscopy (TFM) and they showed the effectiveness of folic acid as a mediator of early-stage neurite formation, which then leads to neuronal polarization that is critical for further differentiation. These findings suggest that folic acid could accelerate the differentiation of PC-12 cells in part through DNA methylation and chemical-to-mechanical force transduction on the PC-12 cells. This discovery adds an important aspect to the existing understanding of the biological actions of folic acid.
Based on the findings, for the first time, we incorporated folic acid as a multifunctional therapeutic agent in our previously reported cross-linked urethane-doped polyester (CUPE; derived from citric acid) NGCs[28] by a dip-coating method in order to deliver folic acid locally to help repair critical-sized 22 mm-long rat sciatic nerve gaps. CUPE NGCs have already shown successful morphological recovery in a 10-mm rat sciatic defect model[28] so folic acid was added to further enhance their performance in treating large nerve defects. Folic acid-coated CUPE NGCs resulted in the promising PNS regeneration and functional recovery that are comparable to those of the autografts. All these findings impart huge potential to folic acid-releasing scaffolds and/or biomaterials as a cell niche that enhances the repair of peripheral nerve injury after injury. In the future, we would like to develop a more controlled delivery system that can release folic acid for long periods of time by using a different incorporation method (i.e. particle incorporation or other conjugation chemistries). The investigated roles of folic acid plays in cell behaviors of glial cells and adult neurons, and peripheral nerve regeneration may further help us to develop potent strategies or biomaterials not only to treat peripheral nerve injuries, but also other PNS and CNS neurological disorders.[35]
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health (USA) awards (CA182670, HL118498) (to Jian Yang), National Science Foundation (USA) award (CMMI 1537008) (to Jian Yang), and High Levels of Health Technical Personnel in Beijing City Health System (Beijing, China, 2013-3-050) (to Jiazhi Yan). The authors also wish to thank Peter Kim and Alexandre Bourcier of the Pennsylvania State University for the assistance with cell culture experiments, Prof. Yong Wang of the Pennsylvania State University for access to the NanoDrop instrument in his laboratory, and Dr. Jeremy Chae of Georgia Institute of Technology for insightful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
Dr. Yang and the Pennsylvania State University have a financial interest in Aleo BME, Inc. These interests have been reviewed by the University’s Institutional and Individual Conflict of Interest Committees and are currently being managed by the University.
References
- 1.Couto MR, Goncalves P, Catarino T, Araujo JR, Correia-Branco A, Martel F. Cell Biol Toxicol. 2012;28:369. doi: 10.1007/s10565-012-9228-8. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X-M, Huang G-W, Tian Z-H, Ren D-L, Wilson JX. Nutr. Neurosci. 2009;12:226. doi: 10.1179/147683009X423418. [DOI] [PubMed] [Google Scholar]
- 3.Smith AD, Smith SM, de Jager CA, Whitbread P, Johnston C, Agacinski G, Oulhaj A, Bradley KM, Jacoby R, Refsum H. PLoS One. 2010;5:1. doi: 10.1371/journal.pone.0012244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faux NG, Ellis KA, Porter L, Fowler CJ, Laws SM, Martins RN, Pertile KK, Rembach A, Rowe CC, Rumble RL, Szoeke C, Taddei K, Taddei T, Trounson BO, Villemagne VL, Ward V, Ames D, Masters CL, Bush AI. J. Alzheimer’s Dis. 2011;27:909. doi: 10.3233/JAD-2011-110752. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds E. Lancet Neurol. 2006;5:949. doi: 10.1016/S1474-4422(06)70598-1. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds EH. J Neurol Neurosurg Psychiatry. 2002;72:567. doi: 10.1136/jnnp.72.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iskandar BJ, Nelson A, Resnick D, Skene JHP, Gao P, Johnson C, Cook TD, Hariharan N. Ann. Neurol. 2004;56:221. doi: 10.1002/ana.20174. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds EH. 2002;324 [Google Scholar]
- 9.American Academy of Pediatrics. Pediatrics. 1999;104:325. [Google Scholar]
- 10.Liu Q, Lu X, Li J, Yao X, Li J. Biosens. Bioelectron. 2007;22:3203. doi: 10.1016/j.bios.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 11.De Wals P, Tairou F, Van Allen MI, Uh S-H, Lowry RB, Sibbald B, Evans JA, Van den Hof MC, Zimmer P, Crowley M, Fernandez B, Lee NS, Niyonsenga T. N. Engl. J. Med. 2007;357:135. doi: 10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 12.Mattson MP, Shea TB. Trends Neurosci. 2003;26:137. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 13.Bailey LB, Rampersaud GC, Kauwell GPA. J. Nutr. 2003;133:1961S. doi: 10.1093/jn/133.6.1961S. [DOI] [PubMed] [Google Scholar]
- 14.Tinetti ME GS, Speechey M. N. Engl. J. Med. 1988;319 doi: 10.1056/nejm198811243192103. [DOI] [PubMed] [Google Scholar]
- 15.MRC VITAMIN STUDY RESEARCH GROUP. Lancet. 1991;338:131. [PubMed] [Google Scholar]
- 16.Simpson JL, Bailey LB, Pietrzik K, Shane B, Holzgreve W. J. Matern. Neonatal Med. 2010;23:1323. doi: 10.3109/14767051003678234. [DOI] [PubMed] [Google Scholar]
- 17.Iskandar BJ, Rizk E, Meier B, Hariharan N, Bottiglieri T, Finnell RH, Jarrard DF, Banerjee RV, Skene JHP, Nelson A, Patel N, Gherasim C, Simon K, Cook TD, Hogan KJ. J. Clin. Invest. 2010;120:1603. doi: 10.1172/JCI40000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dey J, Xu H, Nguyen KT, Yang J. J Biomed Mater Res A. 2010;95:361. doi: 10.1002/jbm.a.32846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dey JYJ, Tran R, Shen J, Tang L. 2011;296:1149. doi: 10.1002/mame.201100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dey J, Xu H, Shen J, Thevenot P, Gondi SR, Nguyen KT, Sumerlin BS, Tang L, Yang J. Biomaterials. 2008;29:4637. doi: 10.1016/j.biomaterials.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knoll SG, Ali MY, Saif MTA. J. Vis. Exp. 2014:1. doi: 10.3791/51873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taniuchi M, Clark HB, Schweitzer JB, Johnson EM. J. Neurosci. 1988;8:664. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sierra-fonseca JA, Najera O, Martinez-jurado J, Walker EM, Varela-ramirez A, Khan AM, Miranda M, Lamango NS, Roychowdhury S. 2014:1. doi: 10.1186/s12868-014-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang LF, Eric, Reichardt Annu Rev Neurosci. 2001:677. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamauchi J, Chan JR, Shooter EM. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14421. doi: 10.1073/pnas.2336152100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.English AW, Cucoranu D, Mulligan A, Rodriguez Ja, Manning J. Eur J Neurosci. 2011;33:2265. doi: 10.1111/j.1460-9568.2011.07724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubo Y, Baba K, Toriyama M, Minegishi T, Sugiura T, Kozawa S, Ikeda K, Inagaki N. J. Cell Biol. 2015;210:663. doi: 10.1083/jcb.201505011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran RT, Choy WM, Cao H, Qattan I, Chiao JC, Ip WY, Yeung KWK, Yang J. J. Biomed. Mater. Res. - Part A. 2014;102:2793. doi: 10.1002/jbm.a.34952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cipreste MF, Gonzalez I, Maria da Mata Martins T, Goes AM, Augusto de Almeida Macedo W, Barros de Sousa EM. RSC Adv. 2016;6:76390. [Google Scholar]
- 30.Zhang S-X, Huang F, Gates M, Holmberg EG. Neural Regen. Res. 2013;8:177. doi: 10.3969/j.issn.1673-5374.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marceau K, Ruttle PL, Shirtcliff EA, Essex MJ, Susman EJ, Studies A, Hospital RI, Studies F, Orleans N. 2015;57:742. doi: 10.1002/dev.21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endres M, Meisel A, Biniszkiewicz D, Namura S, Prass K, Ruscher K, Lipski A, Jaenisch R, Moskowitz MA, Dirnagl U. J. Neurosci. 2000;20:3175. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robertson KD, Wolffe AP. Nat. Rev. Genet. 2000;1:11. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Ueba T, Christie BR, Barkho B, McConnell MJ, Nakashima K, Lein ES, Eadie BD, Willhoite AR, Muotri AR, Summers RG, Chun J, Lee K-F, Gage FH. Proc. Natl. Acad. Sci. U. S. A. 2003;100:6777. doi: 10.1073/pnas.1131928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Correale J, Farez MF. Front. Neurol. 2015;6:1. doi: 10.3389/fneur.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.