Abstract
Background
Budesonide is an oral glucocorticoid designed for the treatment of inflammatory bowel disease (IBD) that may reduce systemic adverse events (AEs). This review examined the efficacy and safety of budesonide for the induction and maintenance of clinical remission in Crohn’s disease (CD).
Methods
MEDLINE, EMBASE, other electronic databases, reference lists and conference proceedings were searched to November 2017 to identify randomized controlled trials of budesonide. Outcomes were the induction and maintenance of remission at eight weeks and one year, respectively, as well as corticosteroid-related AEs and abnormal adrenocorticotropic hormone (ACTH) tests. Pooled relative risks (RRs) and 95% confidence intervals (CIs) were estimated using random effects models.
Results
Thirteen induction and 10 maintenance trials were included. Budesonide 9 mg/day was more effective than placebo (RR 1.93; 95% CI, 1.37–2.73; GRADE: moderate) but less effective than conventional steroids (RR 0.85; 95% CI, 0.75–0.97; GRADE: moderate) to induce remission. Corticosteroid-related AEs occurred less often with induction doses of budesonide than steroids (RR 0.64; 95% CI, 0.54–0.76; GRADE: moderate); budesonide did not increase AEs relative to placebo (RR 0.97; 95% CI, 0.76–1.23; GRADE: moderate). Budesonide 6 mg/day was not different from placebo for maintaining remission (RR 1.13; 95% CI, 0.94–1.35; GRADE: moderate). Both induction (GRADE: low for 3 mg/day, moderate for 9 mg/day) and maintenance budesonide treatment (GRADE: very low for 3 mg/day, low for 6 mg/day) increased the risk of an abnormal ACTH test compared with placebo, but less than conventional steroids (GRADE: very low for both induction and maintenance).
Conclusion
For induction of clinical remission, budesonide was more effective than placebo, but less effective than conventional steroids. Budesonide was not effective for the maintenance of remission. Budesonide was safer than conventional steroids, but the long-term effects on the adrenal axis and bone health remain unknown.
Keywords: Budesonide, Corticosteroids, Crohn’s disease, Induction and maintenance of remission, Meta-analysis
INTRODUCTION
Crohn’s disease (CD) is characterized by chronic transmural inflammation of the gastrointestinal tract. Patients experience abdominal pain, diarrhea and fatigue. CD typically follows a relapsing and remitting disease course. Medications used in the management of CD suppress the inflammatory response.
Corticosteroids are a mainstay of treatment for acute flares of CD (1, 2). However, systemic corticosteroids are associated with adverse effects such as moon facies, acne, infection (including an increased risk of abdominal and pelvic abscess in CD patients), ecchymoses, hypertension, diabetes mellitus, osteoporosis, cataracts, glaucoma and growth failure in children (1). More importantly, the use of systemic corticosteroids has been independently associated with mortality in patients with CD (3).
Budesonide is a glucocorticoid with limited systemic bioavailability, due to extensive (90%) first-pass hepatic metabolism by the cytochrome p-450 enzyme system. These properties limit systemic adverse effects. Budesonide is commercially available in two forms: an oral controlled ileal release (CIR) preparation designed to deliver the drug to the distal small intestine (Entocort®, Astra Zeneca, London, UK; Entocir®, Sofar S.p.A, Trezzano Rosa, Italy; Budecol®, AstraZeneca A&D, Lund, Sweden) and a pH-dependent release formulation (Budenofalk® or Budeson®, Dr Falk Pharma, Freiburg, Germany). The controlled ileal release medication is in the form of a gelatin capsule containing acid-stable microgranules composed of an inner sugar core surrounded by a layer of budesonide in ethylcellulose and an outer acrylic-based resin coating (Eudragit L 100-55) that dissolves at a pH higher than 5.5. The pH-dependent release formulation is available as a capsule containing 400 pellets of budesonide coated with Eudragit resistant to a pH of less than six (4).
This systematic review and meta-analysis provides a summary of the evidence from randomized controlled trials (RCTs) with regard to the safety and efficacy of budesonide for the induction and maintenance of remission in CD. This systematic review and meta-analysis is based on two recent reviews published by the Cochrane collaboration (5, 6) and is updated to November 2017.
MATERIALS AND METHODS
This systematic review and meta-analysis was conducted based on a previously published protocol (5–10) and in accordance with the PRISMA guidelines (11).
Study identification and selection
RCTs of oral budesonide therapy (CIR or pH-dependent release formulations) for the induction or maintenance of remission published in any language were included. Participants were patients of any age with CD defined by conventional clinical, radiological and endoscopic criteria. Studies comparing budesonide to placebo or another active agent were considered for inclusion in this review. Studies comparing different doses of budesonide were excluded if they did not also include a non-budesonide comparison arm. Concomitant therapy was permitted, provided it was balanced between treatment and control groups.
We searched PubMed, MEDLINE (2014-November 2017), EMBASE (2014-November 2017) and the Cochrane Central Register of Controlled Trials (to November 2017). RCTs published before 2014 were identified from Cochrane reviews on the efficacy of budesonide in Crohn’s disease by Kuenzig et al. (maintenance, 2014) (5) and Rezaie et al. (induction, 2015) (6). The search strategy is outlined in Table S1 of the supplementary materials. Ongoing and unpublished trials were identified using clinicaltrials.gov. Reference lists of trials and review articles were reviewed to identify additional studies. Relevant pharmaceutical companies were contacted for ongoing studies. Abstracts were screened for eligibility independently by two study authors (MEK and AR). Full-text articles were independently reviewed by two authors (MEK and AR). Disagreements were resolved by consensus and consultation with EIB and CHS.
Outcomes
Induction of remission was defined by a Crohn’s Disease Activity Index (CDAI) <150 or a Pediatric Crohn’s Disease Activity Index (PCDAI) <10 by eight weeks of therapy. Maintenance of remission was defined as the proportion of patients in continued remission at 12 months, as defined by each trial. If patients were followed beyond these predetermined time points, only eight-week and 12-month data were pooled for induction and maintenance trials, respectively. Inductions studies with less than eight weeks of follow-up and maintenance studies with less than 12 months of follow-up were excluded. Corticosteroid-related adverse events (AEs) and abnormal ACTH stimulation tests were also assessed.
Data extraction
Two authors (MEK and AR) independently extracted data from each eligible study, including the following elements: study design and quality; formulation and dose of budesonide; comparator; study inclusion/exclusion criteria; age of participants; trial duration; method used to induce remission (maintenance trials); and all study outcomes including definition of remission (induction trials), definition of relapse (maintenance trials), corticosteroid-related AEs and abnormal ACTH stimulation tests. Prespecified subgroup analyses were conducted based on the dose and formulation of budesonide (CIR versus pH-dependent), disease location, the method used to induce remission (e.g., medical versus surgical induction, maintenance trials) and the age of trial participants (pediatric versus adult).
Risk of bias
The risk of bias of included studies was assessed independently by two reviewers (MEK and AR) using the Cochrane Collaboration’s tool (http://methods.cochrane.org/bias/assessing-risk-bias-included-studies) (12). Disagreements were resolved by consensus. The overall quality of evidence was assessed using the GRADE approach, incorporating risk of bias (methodological quality), indirectness of evidence, unexplained heterogeneity, imprecision (sparse data) and publication bias (12, 13).
Statistical analysis
Data were analyzed using Review Manager (RevMan 5.3.5, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Data from individual studies were pooled for meta-analysis if the interventions, patient groups and outcomes were sufficiently similar (determined by consensus). Relative risks (RR) and their corresponding 95% confidence intervals (CI) were calculated using random-effects models to allow for expected clinical and statistical heterogeneity across studies (14). Heterogeneity was assessed by calculating the I2 measure, interpreted as low heterogeneity (25%), moderate heterogeneity (50%) and high heterogeneity (75%) (15). Cochran’s χ2test for homogeneity (Q test) was also calculated, with P < 0.10 considered statistically significant. Publication bias was assessed using a visual inspection of funnel plots.
RESULTS
Description of studies
The published Cochrane reviews (5, 6) included 14 induction and 12 maintenance trials. The updated literature search yielded 243 new records; 181 remained after removing duplicates. None of these were eligible for inclusion; therefore, no additional studies were included in the updated systematic review and meta-analysis. One induction study (16) that had been included in the last Cochrane review (6) was excluded because the authors of this study did not define remission. Two maintenance studies that had been included in the last Cochrane review (5) were excluded. One did not have sufficient follow-up, reporting relapse rates at 13 weeks (17), and the other did not include a nonbudesonide treatment group (18). This left 13 induction and 10 maintenance trials for inclusion in the updated meta-analysis (Figure 1). There was 100% agreement among reviewers regarding the eligibility of the included studies. The characteristics of included induction and maintenance studies are provided in Tables 1 and 2, respectively. Table S2 (see supplementary materials) outlines the reasons for study exclusion.
Figure 1.
Study flow diagram depicting results of electronic database search from 2014–2017. Trials published before 2014 were identified from two previous Cochrane reviews on the efficacy of budesonide, published in 2014 and 2015 (5, 6).
Table 1.
Characteristics of included induction trials
| Study | Country (number of centres) | Years of recruitment | Number of Patients (ITT) | Interventions (Number of patients in each arm, ITT) | Formulation | Age of Participants (mean*) | Definition of Active Disease | Definition of Remission | Duration of Therapy | Disease Duration (mean*) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bar-Meir 1998 (35) | Israel (14) | Not reported | 201 | Budesonide 3 mg tid (n=100) Prednisone 40 mg od for two weeks, then tapered (n=101) |
pH-dependent | Adults (both arms: 33 y†) | CDAI 150–350 | CDAI ≤ 150 | 8 weeks | Both arms: 5 y† |
| Campieri 1997 (36) | Europe, New Zealand, Australia (26) | Not reported | 178‡ | Budesonide 9 mg od§ (n=58) Budesonide 4.5 mg bid§ (n=61) Prednisolone 40 mg od for two weeks, then tapered (n=58) |
CIR | Adults (37 y) | CDAI ≥200 | CDAI ≤ 150 | 12 weeks¶ | 7 y |
| Escher 2004 (37) | Europe (36) | 1998–2000 | 48 | Budesonide 9 mg daily§ (n=22) Prednisolone 1 mg/kg daily for four weeks, then tapered (n=26) |
CIR | Pediatric (13 y) | CDAI ≥200 | CDAI ≤ 150 | 12 weeks¶ | 0.7 y |
| Greenberg 1994 (38) | Canada (27) | 1991–1992 | 258 | Budesonide 3 mg daily (n=67) Budesonide 9 mg daily (n=61) Budesonide 15 mg daily (n=64) Placebo (n=66) |
CIR | Adults (32 y) | CDAI >200 | CDAI ≤ 150 | 8 weeks | 6 y |
| Gross 1996 (39) | Europe (16) | Not reported | 67 | Budesonide 3 mg tid (n=34) Methylprednisolone 48 mg for 1 week, then tapered (n=33) |
pH-dependent | Adults (31 y) | CDAI >150 | CDAI ≤ 150 or decrease ≥60 if baseline CDAI > 200 | 8 weeks | 68 months (6 y) |
| Levine 2003 (19) | Israel (13) | Not reported | 35 | Budesonide 3 mg tid (n=20) Prednisone 40 mg daily, then tapered (n=15) |
pH-dependent | Pediatric (14 y) | PCDAI 12.5–40 | PCDAI ≤ 10 | 10 weeks¶ | Not reported |
| Rutgeerts 1994 (40) | Europe (11) | Not reported | 176 | Budesonide 9 mg daily§ (n=88) Prednisolone 40 mg daily for two weeks, then tapered (n=88) |
CIR | Adults (34 y) | CDAI >200 | CDAI ≤ 150 or decrease in CDAI > 100 | 10 weeks¶ | 7 y |
| Suzuki 2013 (41) | Japan (21) | 2006–2008 | 77 | Budesonide 9 mg daily§ (n=26) Budesonide 15 mg daily§ (n=25) Placebo (n=26) |
CIR | Adults (37 y) | CDAI > 200 | CDAI ≤ 150 | 10 weeks¶ | <10 y: 63/77 (82%) ≥10 y: 14/77 (18%) |
| Thomsen 1998 (28) | Europe, South Africa, Australia (25) | 1994–1996 | 182 | Budesonide 9 mg daily (n=93) Mesalamine 2 mg bid (n=89) |
CIR | Adults (Budesonide: 34 y‖; Mesalamine: 31 y‖) | CDAI 200–400 | CDAI ≤ 150 | 16 weeks‡ | Budesonide: 6 y‖; Mesalamine: 5 y‖ |
| Tremaine 2002 (42) | USA (24) | 1995–1997 | 200 | Budesonide 9 mg od (n=80) Budesonide 4.5 mg bid (n=79) Placebo (n=41) |
CIR | Adults (39 y) | CDAI 200–400 | CDAI ≤ 150 | 10 weeks‡ | 11 y |
| Tromm 2011 (29) | Europe and Israel (46) | 2004–2008 | 311 | Budesonide 9 mg od (n=81) Budesonide 3 mg tid (n=77) Mesalamine 4.5 mg daily (n=153) |
pH-dependent | Adults (37 y) | CDAI 200–400 | CDAI ≤ 150 | 8 weeks | 6 y |
| Tursi 2006 (20) | Italy (multicentre; number of centres not reported) | 2004 | 30 | Budesonide 9 mg od (n=15) Beclomethasone dipropionate 10 mg daily (n=15) |
CIR | Adults (36 y) | CDAI 150–250 | CDAI ≤ 150 | 8 weeks | Not reported |
| Van Ierssel 1995 (21) | Netherlands (1) | Not reported | 18 | Budesonide 9 mg od§ (n=9) Prednisolone 40 mg daily for two weeks, then tapered (n=9) |
CIR | Adults (35 y**) | CDAI ≥200 | CDAI ≤ 150 | 10 weeks¶ | Not reported |
ABBREVIATIONS: od, once daily; bid, twice daily; tid, three times daily; CIR, controlled ileal release; CDAI, Crohn’s Disease Activity Index; y, years.
*Weighted average of all study arms.
†Unclear if study reported mean or median age and disease duration of trial participants.
‡Campieri 1997 did not provide intention-to-treat numbers for each treatment arm. One patient was randomized but did not receive treatment, but it is not known which treatment arm this patient was randomized to.
§Dose of budesonide was tapered after eight weeks.
¶When trials followed patients beyond the primary endpoint of eight weeks, only remission data at eight weeks were pooled.
‖Median.
**Average age for the full cohort was reported in the study.
Table 2.
Characteristics of included maintenance trials
| Study | Country (number of centres) | Years of recruitment | Number of Patients | Interventions | Formulation | Age of Participants (mean*) | Method to Induce Remission | Definition of Disease Relapse | Duration of Therapy | Disease Duration (mean*) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ewe 1999 (43) | Germany (3) | 1992–1994 | 83 | Budesonide 1 mg tid (n=43) Placebo (n=40) |
pH-dependent | Adults (Budesonide: 35 y†; Placebo: 33 y†) | Surgically | Endoscopic recurrence or increase in CDAI from 60 up to 200 from first follow-up or CDAI > 200 | 1 year | Budesonide: 100 months† (8 y) Placebo: 81 months† (7 y) |
| Ferguson 1998 (44) | Europe, Australia (20) | Not reported | 75 | Budesonide 6 mg bid (n=22) Budesonide 3 mg od (n=26) Placebo |
CIR | Adults (36 y) | Medically induced (budesonide clinical trial) | CDAI ≥ 150 | 1 year | 7 y |
| Greenberg 1996 (45) | Canada (23) | 1992–1994 | 105 | Budesonide 6 mg od (n=36) Budesonide 3 mg od (n=33) Placebo (n=36) |
CIR | Adults (35.6 y) | Medically induced (budesonide clinical trial) | CDAI ≥ 150 | 1 year | 8 y |
| Gross 1998 (46) | Germany (multicentre; number of centres not reported) | Not reported | 179 | Budesonide 1 mg tid (n=84) Placebo (n=95) |
pH-dependent | Adults (32 y) | Medically induced (corticosteroids) | CDAI > 150 for >2 subsequent weeks or CDAI > 150 at last visit | 1 year | 63 months (5 y) |
| Hanauer 2005 (47) | United States (22) | Not reported | 110 | Budesonide 6 mg od (n=55) Placebo (n=55) |
CIR | Adults (40 y) | Medically induced (budesonide clinical trial) | CDAI ≥ 150 | 1 year | Not reported |
| Hellers 1999 (48) | Europe (13) | 1992–1993 | 130 | Budesonide 6 mg od (n=63) Placebo (n=67) |
CIR | Adults (35 y) | Surgically | CDAI ≥ 150 | 1 year | Not reported |
| Lofberg 1996 (49) | Europe (11) | Not reported | 90 | Budesonide 6 mg od (n=32) Budesonide 3 mg od (n=31) Placebo (n=27) |
CIR | Adults (30 y) | Medically induced (budesonide clinical trial) | CDAI ≥ 150 | 1 year | 7 y |
| Mantzaris 2003 (23) | Greece (1) | 1994–1998 | 57 | Budesonide 6 mg daily (n=29) pH-dependent mesalamine (Salofalk) 1 g tid (n=28) |
CIR | Adults (33 y) | Medically induced (steroid-dependent) | CDAI ≥ 150 and increase of ≥100 points from baseline | 1 year | 3 y |
| Mantzaris 2009 (22) | Greece (1) | 1998–2001 | 77 | Budesonide 6–9 mg od (n=39) Azathioprine 2.0– 2.5 mg/kg daily (n=38) |
CIR | Adults (budesonide: 35 y‡; azathioprine: 34 y‡) | Medically (steroid- dependent) | Increase in CDAI ≥ 100 points from baseline and CDAI ≥ 150 | 1 year | 2 y |
| Schoon 2005 (24) | Europe (34) | 1996–1999 | 90 | Budesonide 9 mg daily with tapering prednisolone or prednisone (n=46) Continuation of pre-existing prednisolone (n=44) |
CIR | Adults (39 y) | Medically (corticosteroid-free and corticosteroid- dependent)§ | CDAI ≥ 200 | 2 years | 7 y‡ |
ABBREVIATIONS: od, once daily; bid, twice daily; tid, three times daily; CIR, controlled ileal release; CDAI, Crohn’s Disease Activity Index; y, years.
*Weighted average of all study arms, unless otherwise specified.
†Unclear if study reported mean or medina age and disase duration of trial participants.
‡Median.
§Efficacy data were only available for study participants who were steroid-dependent. Thus, only the steroid-dependent patients were included in the review.
Risk of bias in included studies
Three induction trials had a high risk of bias (Table S3 of the supplementary materials) (19–21). Two failed to ensure appropriate blinding (open label studies) (19, 20). One selectively reported study outcomes, failing to outline AEs for each study group (21). Three maintenance studies had a high risk of bias (Table S4 of the supplementary materials) due to failure to blind participants (22, 23) and outcome assessors (24). In addition, allocation was not adequately concealed in one maintenance trial (22).
Budesonide to induce remission
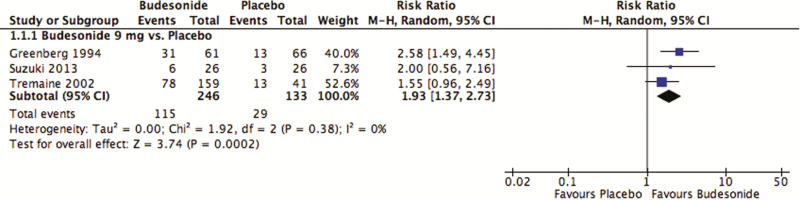
At eight weeks, 47% (115 of 246) of those receiving a daily dose of budesonide 9 mg/day entered remission compared with 22% (29/133) of those receiving placebo (Figure 2). This difference was statistically significant (pooled RR 1.93; 95% CI, 1.37–2.73, P = 0.00018; I2 = 0%; three studies; 379 participants). The 15 mg/day dose of budesonide was similarly superior to placebo (two studies), but there was no difference between budesonide 3 mg/day and placebo (one study; Figure S1 of the supplementary materials). All studies comparing budesonide to placebo used the CIR formulation of budesonide and excluded individuals with distal colonic disease. No study provided subgroup analyses based on disease severity, and all studies were limited to adult participants. As assessed with the GRADE approach, there was moderate quality of evidence for budesonide 9 mg/day and 15 mg/day to induce remission and low quality of evidence for budesonide 3 mg/day. The 9 mg/day and 15 mg/day doses were downgraded due to sparse data, and the 3 mg/day dose was downgraded due to very spare data. No evidence of publication bias was detected upon visual assessment of studies comparing budesonide 9 mg/day with placebo (Figure S2).
Figure 2.
Budesonide 9 mg versus placebo: induction of clinical remission.
Two studies compared budesonide with mesalamine. However, these studies could not be pooled due to significant heterogeneity (P = 0.002, I2 = 81%). Budesonide was superior to mesalamine in the trial by Thomsen et al. (27) (RR 1.63; 95% CI, 1.23–2.16) but there was no significant difference between the two medications in the trial by Tromm et al. (28) (RR 1.12; 95% CI, 0.95–1.32). A similar proportion of patients receiving budesonide entered clinical remission at eight weeks in both studies (68% and 69% for Thomsen et al. (27) and Tromm et al. (28), respectively). However, a greater proportion of patients receiving mesalamine entered clinical remission in the study by Tromm et al. (28) (62%) as compared with the study by Thomsen et al. (27) (42%). Subgroup analysis based on disease severity failed to explain between-study heterogeneity (I2 = 88% for mild-to-moderate disease as defined by a CDAI <300; I2 = 68% for severe disease as defined by CDAI ≥300). Children were not included in either study. Using the GRADE approach, the quality of evidence from each study was rated as moderate. Both studies were downgraded due to sparse data.
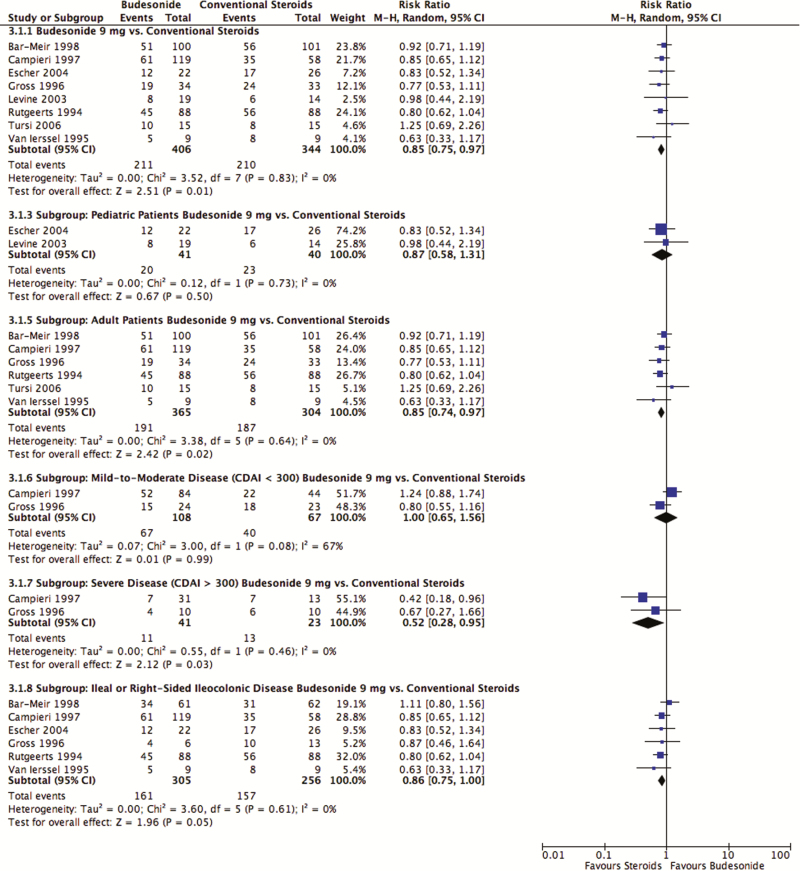
Conventional steroids induced remission in 61% (210 of 344) of patients, whereas budesonide 9 mg/day induced remission in 52% (211 of 406; Figure 3). Budesonide was inferior to conventional steroids (pooled RR 0.85; 95% CI, 0.75–0.97; P = 0.012; I2 = 0%; eight studies; 750 participants). Subgroup analyses yielded similar findings for adult patients (pooled RR 0.85; 95% CI, 0.74–0.97; P = 0.02; I2 = 0%; six studies; 669 participants) and patients with severe disease as defined by CDAI ≥300 (pooled RR 0.52; 95% CI, 0.28–0.95; P = 0.03; I2 = 0%; two studies; 64 participants). Conventional steroids were no longer superior to budesonide when limiting the analyses to the pediatric population (pooled RR 0.87; 95% CI, 0.58–1.31; P = 0.5; I2 = 0%; two studies; 81 participants), those with mild-to-moderate disease as defined by CDAI <300 (pooled RR 1.00; 95% CI, 0.65–1.56; P = 0.99; I2 = 67%; two studies; 175 participants) or those with ileal or right-sided ileocolonic disease (pooled RR 0.86; 95% CI, 0.75–1.00, P = 0.05; I2 = 0%; six studies; 561 participants). Conventional steroids were superior to the CIR formulation of budesonide, but not the pH-dependent formulation for the induction of remission (Table S5 of the supplementary materials). According to the GRADE approach, the evidence comparing budesonide with conventional steroids was of moderate quality and was downgraded due to the inclusion of studies at a high risk of bias. No evidence of publication bias was detected on the funnel plot (Figure S3 of the supplementary materials).
Figure 3.
Budesonide versus conventional steroids: induction of clinical remission.
Budesonide to maintain remission
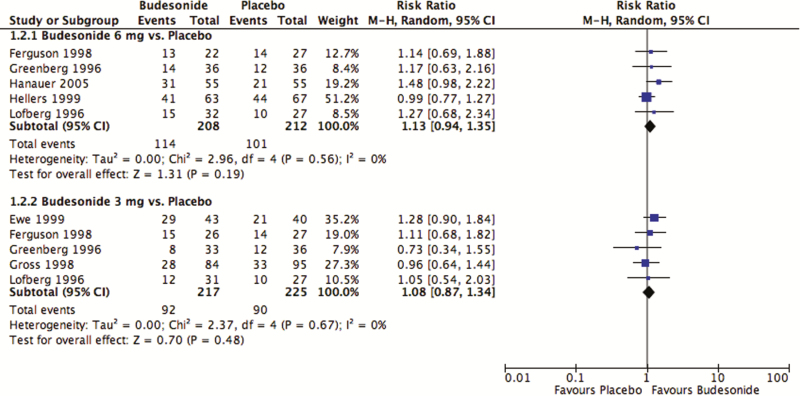
Neither the 3 mg/day nor the 6 mg/day doses of budesonide were more effective than placebo to maintain remission at 12 months (Figure 4). Fifty-five percent (114 of 208) of those receiving budesonide 6 mg/day remained in remission compared with 48% (101 of 212) of those receiving placebo (pooled RR 1.13; 95% CI, 0.94–1.35; P = 0.19; I2 = 0%; five studies; 420 participants). Among those receiving budesonide 3 mg/day, 42% (92 of 217) remained in remission compared with 40% (90 of 225) of participants receiving placebo (pooled RR 1.08; 95% CI, 0.87–1.34; P = 0.48; I2 = 0%; five studies; 442 participants). Based on the GRADE approach, there was evidence for both the 3 mg/day and 6 mg/day doses of budesonide compared with placebo was moderate. Both were downgraded due to sparse data. Of the five studies comparing budesonide 3 mg/day with placebo, three used the CIR formulation and two used the pH-dependent formulation: there were no significant differences between budesonide and placebo with either formulation (Table S5 of the supplementary materials). All studies with the 6 mg/day dose used the CIR formulation. Two studies evaluated the efficacy of budesonide to prevent postoperative recurrence (one with a dose of 3 mg/day and the other with a dose of 6 mg/day); the remainder of studies induced remission medically either with budesonide or conventional steroids. Budesonide was not significantly different from placebo with either mode of remission (Table S6 of the supplementary materials). Budesonide was superior to mesalamine, but was not significantly different from either conventional steroids or azathioprine (Table S7 of the supplementary materials). No evidence of publication bias was detected upon visual inspection of the funnel plot (Figure S4 of the supplementary materials). Based on the GRADE approach, there was very low quality of evidence comparing budesonide to mesalamine (very sparse data, high risk of bias due to lack of blinding) and azathioprine (sparse data; high risk of bias due to a single-blinded design and a lack of allocation concealment). There was low-quality evidence comparing budesonide with conventional steroids (sparse data; high risk of bias due to lack of blinding).
Figure 4.
Budesonide versus placebo: maintenance of clinical remission.
Safety of budesonide
Corticosteroid-related adverse events.
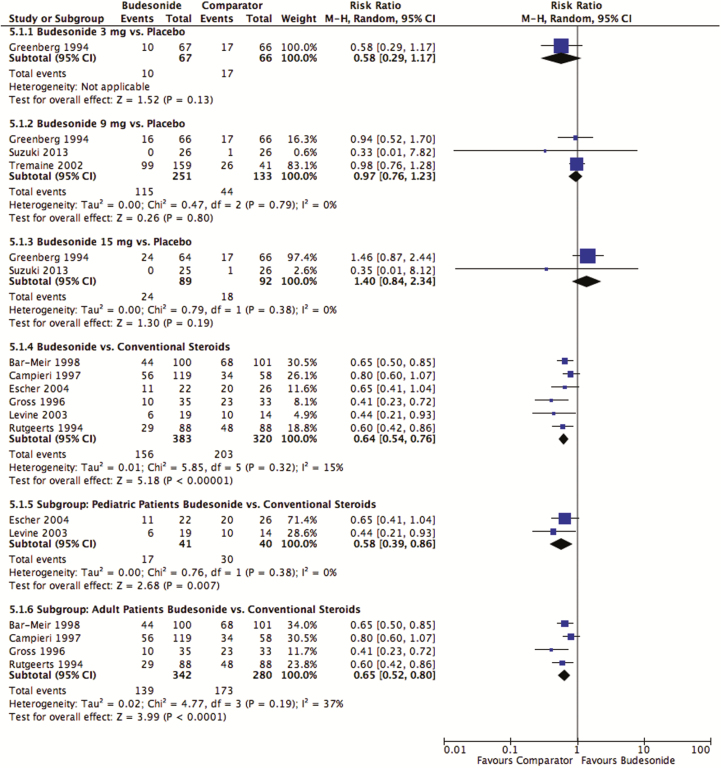
Induction treatment with budesonide did not increase either the risk of corticosteroid-related adverse events relative to placebo (3 mg/day: pooled RR 0.58; 95% CI, 0.29–1.17; P = 0.13; 1 study; 27 participants) (9 mg/day: RR 0.97; 95% CI, 0.76–1.13; P = 0.80; I2 = 0%; three studies; 384 participants) (15 mg/day: pooled RR 1.40; 95% CI, 0.84–2.34; P = 0.19; I2 = 0%; two studies; 181 participants) (Figure 5). Using a GRADE approach, the quality of evidence was moderate when comparing budesonide 9 mg/day and 15 mg/day with placebo, with both being downgraded due to sparse data. There was low quality of evidence for the comparison of budesonide 3 mg/day versus placebo due to very sparse data.
Figure 5.
Corticosteroid-related adverse events after induction treatment with budesonide compared with placebo and conventional corticosteroids.
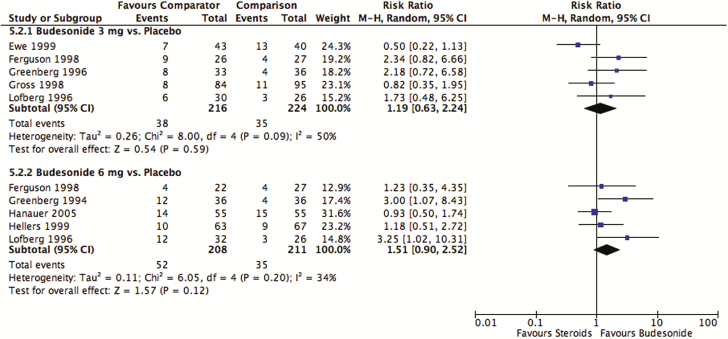
Likewise, there were no differences between budesonide and placebo in terms of corticosteroid-related adverse events following maintenance treatment (3 mg/day: pooled RR 1.19; 95% CI, 0.63–2.24; P = 0.59; I2 = 50%; five studies; 440 participants) (6 mg/day: pooled RR 1.51; 95% CI, 0.90–2.52; P = 0.12; I2 = 34%; five studies; 419 participants; Figure 6). Using the GRADE approach, there was moderate-quality evidence when comparing budesonide 3 mg/day and 6 mg/day with placebo due to sparse data in both cases. Findings remained consistent when pooling across doses of budesonide for both induction and maintenance treatment (Figure S5 of the supplementary materials). Using the GRADE approach, the quality of evidence for the pooled doses of budesonide compared with placebo was moderate for induction and maintenance treatment. Both were downgraded due to sparse data.
Figure 6.
Corticosteroid-related adverse events after maintenance treatment with budesonide compared with placebo.
Budesonide decreased the risk of corticosteroid-related adverse events compared with conventional steroids when used to induce remission (pooled RR 0.64; 95% CI, 0.54–0.76; P < 0.00001; I2 = 15%; Figure 5). Using a GRADE approach, there was high-quality evidence. This decreased risk of corticosteroid-related adverse events was seen in both children (pooled RR 0.58; 95% CI, 0.39–0.86; P = 0.007; I2 = 0%) and adults (pooled RR 0.65; 95% CI, 0.52–0.80; P = 0.19; I2 = 37%). The risk of corticosteroid-related adverse events was not assessed in induction trials comparing budesonide with mesalamine; this was also the case for maintenance trials comparing budesonide to conventional steroids, mesalamine and azathioprine.
Abnormal ACTH stimulation tests.
An induction dose of budesonide 9 mg/day increased the risk of an abnormal ACTH test relative to placebo (pooled RR 2.15; 95% CI, 1.41–3.29; P = 0.00040; I2 = 24%; three studies; 356 participants; Figure S5 of the supplementary materials). However, abnormal ACTH tests were less common for those receiving budesonide 9 mg/day than conventional steroids (pooled RR 0.65; 95% CI, 0.55–0.78; P < 0.0001; I2 = 0%; three studies; 244 participants) and remained consistent when limiting to studies including adult patients (RR 0.65; 95% CI, 0.53–0.79; 1 study; 177 participants), but not pediatric patients (pooled RR 0.69; 95% CI, 0.46–1.04; P = 0.49; I2 = 0%; two studies; 67 participants). There was no difference in the risk of an abnormal ACTH test when comparing budesonide 3 mg/day with placebo (RR 1.29; 95% CI, 0.68–2.44; P = 0.44; one study; 133 participants). Using a GRADE approach, the quality of evidence was low when comparing 3 mg/day with placebo (due to very sparse data) and moderate when comparing 9 mg/day with placebo (due to sparse data). There was very low-quality evidence when comparing budesonide to conventional steroids due to selective outcome reporting, sparse data and one study being at high risk of bias due to a lack of blinding. Comparisons between budesonide 15 mg/day with placebo and budesonide 9 mg/day with mesalamine could not be made due to significant heterogeneity; I2 values were 79% and 85%, respectively.
Maintenance doses of budesonide 6 mg/day also increased the likelihood of an abnormal ACTH test relative to placebo (pooled RR 2.72; 95% CI, 1.62–4.58; P = 0.0002; I2 = 0%; four studies; 297 participants; Figure S7 of the supplementary materials). However, abnormal ACTH tests were not more common among participants receiving maintenance doses of budesonide 3 mg/ day as compared with placebo (pooled RR 1.89; 95% CI, 0.76–4.69; P = 0.17; I2 = 27%; three studies; 165 participants). Budesonide resulted in significantly fewer abnormal ACTH tests than conventional steroids (RR 0.60; 95% CI, 0.36–1.00; P = 0.048; 1 study; 69 participants). Using a GRADE approach, there was very low-quality evidence when comparing budesonide 3 mg/day with placebo (due to very sparse data and selective outcome reporting) and budesonide to conventional steroids (due to very sparse data and high risk of bias due to lack of blinding). There was low quality evidence when comparing budesonide 6 mg/day with placebo (due to sparse data and selective outcome reporting).
DISCUSSION
Oral budesonide is a corticosteroid designed for release in the small intestine with high first-pass hepatic metabolism, limiting the systemic adverse events caused by conventional corticosteroids. This review summarizes available controlled clinical trials for the efficacy and safety of budesonide, compared with other active agents, for both the induction and maintenance of remission in CD.
Budesonide was more effective than placebo, but was less effective than conventional steroids for the induction of remission. Remission rates were 15% higher among those receiving conventional steroids as compared with those receiving budesonide. These results are in agreement with previous meta-analyses (6, 8, 9, 25–27). Subgroup analyses suggest the superiority of conventional steroids over budesonide to induce remission is specific to adults. However, the proportion of children achieving remission was almost 10% higher among those receiving conventional steroids than budesonide. We were underpowered to detect a difference between these two medications, as only two studies (81 patients) compared these two medications in children (19, 37).
Current data do not allow for a firm conclusion on the relative efficacy of budesonide in comparison to mesalamine. Although the study by Thomsen et al. (28) suggested that budesonide was superior to mesalamine. Another study by Tromm et al. (29) found no difference in the proportion of patients entering clinical remission at eight weeks. An editorial (30) accompanying Tromm et al. (29) highlighted that the remission rate in the mesalamine arm of that trial was higher than other RCTs of mesalamine: 62% compared with 42% in the Thomsen et al. (28) RCT. Methodological criticisms of that trial included its switch from superiority to noninferiority design, inclusion of individuals with low levels of inflammatory markers (i.e., erythrocyte sedimentation rate and C-reactive protein) and a lack of power to detect noninferiority between the two treatments. Unlike previous traditional meta-analyses (9, 25), our updated analysis included the RCT by Tromm et al. (29) Additionally, two recent network meta-analyses, which included the Tromm et al. study (29), are contradictory regarding the efficacy of budesonide relative to mesalamine: one found budesonide to be superior to mesalamine, while the other found no difference between the two treatments (31, 32). Further, prior systematic reviews and meta-analyses have concluded that 5-ASA agents are not more effective than placebo in the induction of remission in CD (33). Overall, our systematic review highlights uncertainty in the evidence comparing budesonide to mesalamine.
In contrast, budesonide was not more effective than placebo for maintaining remission in patients with CD. Similarly, neither weaning doses of conventional steroids nor azathioprine were found to be significantly different than budesonide for the maintenance of remission. Subgroup analyses of drug formulations (CIR and pH-dependent), varying doses and method used to induce remission consistently demonstrated lack of superiority for budesonide in maintaining remission. Subgroup analyses need to be interpreted with caution as several of the comparisons were made in a single RCT with a small number of patients, and several RCTs were associated with high risk of bias due to lack of blinding and allocation concealment. Overall, current evidence does not support the use of budesonide in maintenance of remission in CD.
Corticosteroid-related AEs were not elevated among patients receiving budesonide as compared with placebo, either when budesonide was used in the short-term to induce remission or in the long-term to maintain remission. As expected, conventional steroids were associated with statistically and clinically more corticosteroid-related AEs including moon face, acne, mood changes and muscle weakness.
Prolonged exposure to steroids is known to have detrimental effects on bone metabolism, leading to diminished growth, osteopenia or osteoporosis. The maintenance trial comparing budesonide with conventional steroids was specifically designed to compare bone mineral density among the two treatment groups (24). Among corticosteroid-naïve patients, decreases in bone mineral density were less pronounced after treatment with budesonide than prednisolone. However, this differential reduction in bone mineral density has not been consistently observed (34). No randomized clinical trial has compared changes in bone mineral density between budesonide and placebo. Considering the finding that adrenocortical axis suppression was more prominent in those treated with both induction and maintenance doses of budesonide, compared with placebo, bone health deterioration may be of significant concern in patients taking budesonide—particularly for long periods of time.
Our systematic review was limited by the availability and quality of data evaluating the efficacy of budesonide to induce and maintain remission. Three of the eight studies comparing budesonide with conventional steroids to induce remission were at high risk of bias, while studies comparing budesonide to mesalamine were highly heterogeneous and do not allow for a firm conclusion as to the relative efficacy of these two medications. Further, comparisons of budesonide to both mesalamine and azathioprine were limited to single studies, both of which were at a high risk of bias. While the safety of budesonide precludes its usefulness as a maintenance agent in CD, further research is needed to evaluate the roles of budesonide and mesalamine to induce remission with a focus on the specific phenotype each medication is designed to target (i.e., disease in the terminal ileum and proximal colon for budesonide and left-sided colonic disease with mesalamine).
In conclusion, budesonide is more effective than placebo for the induction of remission in active ileocecal CD. A dose of 9 mg daily for eight weeks, followed by weaning the dose to discontinuation, is considered the optimal dosing regimen. Budesonide was less effective than conventional steroids, particularly in patients with severe disease or those with extensive colonic involvement. However, the likelihood of adverse events with budesonide was significantly lower than with conventional steroids and was no higher than in patients receiving placebo. Budesonide was not found to be effective for maintenance of remission at 12 months in CD. While budesonide did not increase the risk of corticosteroid-related adverse events, the long-term implications of budesonide on bone metabolism and adrenal axis suppression remain uncertain (34). Thus, given the weak efficacy and the potential for long-term consequences, the use of budesonide for maintenance of remission in CD is difficult to justify.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of the Canadian Association of Gastroenterology online.
CONFLICTS OF INTEREST
AR and MEK have no known conflicts of interest to report.
ANM has received the following: fee(s) from Johnson and Johnson for Board membership; fee(s) from Janssen, Abbvie and Ferring for consultancy; grants or grants pending from Johnson and Johnson and Abbvie; lecture fee(s) from Abbvie and Merck and payment for development of educational presentations from Ferring. All of these activities are outside the current work.
ARO’s centre was a participating site in an AstraZeneca-funded, FDA-approved induction and maintenance clinical trial studying Entocort safety in pediatric Crohn’s disease. Funds were paid to the institution, and the site PI (ARO) does not receive payment directly. ARO did not participate in the initial review of potentially eligible studies to determine whether they should be included or excluded. ARO has received fee(s) from Janssen, AbbVie for Advisory Board membership, grants or grants pending from Janssen and AbbVie, is site-investigator for studies sponsored by Shire, Takeda, AbbVie and Janssen. All of these activities are outside the current work.
AHS has received fee(s) from Janssen, Abbvie, Shire, Pendopharm, Pfizer and Takeda for consultancy and lecture fee(s) from Janssen, Abbvie, Shire,Warner Chilcott, Aptalis and Takeda. His institution has received grants or grants pending from Janssen, Abbvie, Pfizer, Amgen, Takeda and Actavis. All of these activities are outside the current work.
GGK is an associate editor at the Journal of the Canadian Association of Gastroenterology. He has served as a speaker for Pfizer, Jansen, Merck, Schering-Plough and Abbvie. He has participated in advisory board meetings for Jansen, Abbvie, Merck and Schering-Plough. GGK has received research support from Merck, Abbvie, GlaxoSmith Kline and Shire. All of these activities are outside the current work.
EIB is an associate editor at the Journal of the Canadian Association of Gastroenterology.
CHS has served on advisory boards for Ferring, Actavis, Janssen Pharmaceuticals, Abbvie, Shire, Pfizer and Takeda. CHS has also provided lectures for Janssen Pharmaceuticals, Abbvie and Takeda. All of these activities are outside the current work.
ACKNOWLEDGEMENTS
The authors would like to thank John MacDonald, the managing editor at the Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders Group for his assistance with the cited Cochrane reviews. Funding for the IBD/FBD Review Group (September 1, 2010–August 31, 2015) was provided by the following: Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON-105529), the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD) and Infection and Immunity (III), and the Ontario Ministry of Health and Long-Term Care (HLTC3968FL-2010-2235). Eric Benchimol was supported by a New Investigator Award from the Canadian Institutes of Health Research, Canadian Association of Gastroenterology and Crohn’s and Colitis Canada. He was also supported by a career development award from the Canadian Child Health Clinician Scientist Program. Ellen Kuenzig was supported by a postdoctoral fellowship from the CIHR, Canadian Association of Gastroenterology and Crohn’s and Colitis Canada. Author contributions: MEK wrote the manuscript. MEK and AR performed the research, collected, analyzed and interpreted the data. GGK, EIB and CHS designed the study and reviewed the analysis and interpretation of the data. ARO, AHS and AMG were involved in the study design and data analysis of earlier versions of the Cochrane reviews on the efficacy of budesonide in Crohn’s disease and edited more recent versions of the Cochrane reviews and edited this manuscript. All authors approved the final version of the manuscript, including the authorship list.
References
- 1. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641–57. [DOI] [PubMed] [Google Scholar]
- 2. Hyams JS. Inflammatory bowel disease. Pediatr Rev. 2005;26(9):314–20. [DOI] [PubMed] [Google Scholar]
- 3. Lewis JD, Gelfand JM, Troxel AB, et al. Immunosuppressant medications and mortality in inflammatory bowel disease. Am J Gastroenterol. 2008;103(6):1428–35. [DOI] [PubMed] [Google Scholar]
- 4. Fedorak RN, Bistritz L. Targeted delivery, safety, and efficacy of oral enteric-coated formulations of budesonide. Advanced Drug Delivery Reviews. 2005;57(2):303–16. [DOI] [PubMed] [Google Scholar]
- 5. Kuenzig ME, Rezaie A, Seow CH, et al. Budesonide for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014;(8):CD002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rezaie A, Kuenzig ME, Benchimol EI, et al. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015;(6):CD000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Modigliani R, Steinhart AH, Ewe K, et al. Crohn’s disease: Budesonide for induction of remission. Cochrane Database Syst Rev. 1996;(3):CD000296. [DOI] [PubMed] [Google Scholar]
- 8. Otley A, Steinhart AH. Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2005;(4):CD000296. [DOI] [PubMed] [Google Scholar]
- 9. Seow CH, Benchimol EI, Griffiths AM, et al. Budesonide for induction of remission in Crohn’s disease (Review). Cochrane Database Syst Rev. 2008;(3):CD000296. [DOI] [PubMed] [Google Scholar]
- 10. Benchimol EI, Seow CH, Otley AR, et al. Budesonide for maintenance of remission in Crohn’s disease (Review). Cochrane Database Syst Rev. 2009;(1):CD002913. [DOI] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine. 2009;151(4):W65–94. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, Ltd, 2008. [Google Scholar]
- 13. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Haens G, Verstraete A, Cheyns K, Aerden I, Bouillon R, Rutgeerts P. Bone turnover during short-term therapy with methylprednisolone or budesonide in Crohn’s disease. Aliment Pharmacol Ther. 1998;12(5):419–24. [DOI] [PubMed] [Google Scholar]
- 17. Cortot A, Colombel JF, Rutgeerts P, et al. Switch from systemic steroids to budesonide in steroid dependent patients with inactive Crohn’s disease. Gut. 2001;48(2):186–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Jong DJ, Bac DJ, Tan G, et al. Maintenance treatment with budesonide 6 mg versus 9 mg once daily in patients with Crohn’s disease in remission. Neth J Med. 2007;65(9):339–45. [PubMed] [Google Scholar]
- 19. Levine A, Weizman Z, Broide E, et al. A comparison of budesonide and prednisone for the treatment of active pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2003;36(2):248–52. [DOI] [PubMed] [Google Scholar]
- 20. Tursi A, Giorgetti GM, Brandimarte G, et al. Beclomethasone dipropionate for the treatment of mild-to-moderate Crohn’s disease: An open-label, budesonide-controlled, randomized study. Med Sci Monit. 2006;12(6):PI29–32. [PubMed] [Google Scholar]
- 21. Van Ierssel AJ, Van der Sluys Veer A, Verspaget HW, et al. Budesonide and prednisolone suppress peripheral blood natural killer cells in Crohn’s disease. Aliment Pharmacol Ther. 1995;9(2):173–8. [DOI] [PubMed] [Google Scholar]
- 22. Mantzaris GJ, Christidou A, Sfakianakis M, et al. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohnʼs disease. Inflamm Bowel Dis. 2009;15(3):375–82. [DOI] [PubMed] [Google Scholar]
- 23. Mantzaris GJ, Petraki K, Sfakianakis M, et al. Budesonide versus mesalamine for maintaining remission in patients refusing other immunomodulators for steroid-dependent Crohn’s disease. Clin Gastroenterol Hepatol. 2003;1(2):122–8. [DOI] [PubMed] [Google Scholar]
- 24. Schoon EJ, Bollani S, Mills PR, et al. Bone mineral density in relation to efficacy and side effects of budesonide and prednisolone in Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3(2):113–21. [DOI] [PubMed] [Google Scholar]
- 25. Papi C, Luchetti R, Gili L, et al. Budesonide in the treatment of Crohn’s disease: A meta-analysis. Aliment Pharmacol Ther. 2000;14(11):1419–28. [DOI] [PubMed] [Google Scholar]
- 26. Kane SV, Schoenfeld P, Sandborn WJ, et al. Systematic review: The effectiveness of budesonide therapy for Crohn’s disease. Aliment Pharmacol Ther. 2002;16(8):1509–17. [DOI] [PubMed] [Google Scholar]
- 27. Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: Systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):590–9. [DOI] [PubMed] [Google Scholar]
- 28. Thomsen OØ, Cortot A, Jewell D, et al. A comparison of budesonide and mesalamine for active Crohn’s disease. N Engl J Med. 1998;339(6):370–4. [DOI] [PubMed] [Google Scholar]
- 29. Tromm A, Bunganič I, Tomsová E, et al. Budesonide 9 mg is at least as effective as mesalamine 4.5 g in patients with mildly to moderately active Crohn’s disease. Gastroenterology. 2011;140(2):425–34.e1. [DOI] [PubMed] [Google Scholar]
- 30. Levesque BG, Sandborn WJ. Setting a high threshold for noninferiority: Mesalamine and budesonide in Crohnʼs disease. Inflamm Bowel Dis. 2012;18(4):795–6. [DOI] [PubMed] [Google Scholar]
- 31. Moja L, Danese S, Fiorino G, et al. Systematic review with network meta-analysis: Comparative efficacy and safety of budesonide and mesalazine (mesalamine) for Crohn’s disease. Aliment Pharmacol Ther. 2015;41(11):1055–65. [DOI] [PubMed] [Google Scholar]
- 32. Coward S, Kuenzig ME, Hazlewood G, et al. Comparative effectiveness of mesalamine, sulfasalazine, corticosteroids, and budesonide for the induction of remission in Crohn’s disease: A Bayesian network meta-analysis. Inflamm Bowel Dis. 2017;23(3):461–72. [DOI] [PubMed] [Google Scholar]
- 33. Lim WC, Wang Y, MacDonald JK, et al. Aminosalicylates for induction of remission or response in Crohn’s disease. Cochrane Database Syst Rev. 2016;7:CD008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cino M, Greenberg GR. Bone mineral density in Crohn’s disease: A longitudinal study of budesonide, prednisone, and nonsteroid therapy. Am J Gastroenterol. 2002;97(4):915–21. [DOI] [PubMed] [Google Scholar]
- 35. Bar-Meir S, Chowers Y, Lavy A, et al. Budesonide versus prednisone in the treatment of active Crohn’s disease. Gastroenterology. 1998;115(4):835–40. [DOI] [PubMed] [Google Scholar]
- 36. Campieri M, Ferguson A, Doe W, et al. Oral budesonide is as effective as oral prednisolone in active Crohn’s disease. Gut. 1997;41(2):209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Escher JC. Budesonide versus prednisolone for the treatment of active Crohn’s disease in children. Eur J Gastroenterol Hepatol. 2004;16(1):47–54. [DOI] [PubMed] [Google Scholar]
- 38. Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide for active Crohn’s disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med. 1994;331(13):836–41. [DOI] [PubMed] [Google Scholar]
- 39. Gross V, Andus T, Caesar I, et al. Oral pH-modified release budesonide versus 6-methylprednisolone in active Crohn’s disease. German/Austrian Budesonide Study Group. Eur J Gastroenterol Hepatol. 1996;8(9):905–9. [PubMed] [Google Scholar]
- 40. Rutgeerts P, Lofberg R, Malchow H, et al. A comparison of budesonide with prednisolone for active Crohn’s disease. N Engl J Med. 1994;331(13):842–5. [DOI] [PubMed] [Google Scholar]
- 41. Suzuki Y, Motoya S, Takazoe M, et al. Efficacy and tolerability of oral budesonide in Japanese patients with active Crohn’s disease: A multicentre, double-blind, randomized, parallel-group Phase II study. J Crohn’s Colitis. 2013;7(3):239–47. [DOI] [PubMed] [Google Scholar]
- 42. Tremaine WJ, Hanauer SB, Katz S, et al. Budesonide CIR capsules (once or twice daily divided-dose) in active Crohn’s disease: A randomized placebo-controlled study in the United States. Am J Gastroenterol. 2002;97(7):1748–54. [DOI] [PubMed] [Google Scholar]
- 43. Ewe K, Böttger T, Buhr HJ, et al. Low-dose budesonide treatment for prevention of postoperative recurrence of Crohn’s disease: A multicentre randomized placebo-controlled trial. German Budesonide Study Group. Eur J Gastroenterol Hepatol. 1999;11(3):277–82. [DOI] [PubMed] [Google Scholar]
- 44. Ferguson A, Campieri M, Doe W, et al. Oral budesonide as maintenance therapy in Crohn’s disease—results of a 12-month study. Aliment Pharmacol Ther. 1998;12(2):175–83. [DOI] [PubMed] [Google Scholar]
- 45. Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide as maintenance treatment for Crohn’s disease: A placebo-controlled, dose-ranging study. Canadian Inflammatory Bowel Disease Study Group. Gastroenterology. 1996;110(1):45–51. [DOI] [PubMed] [Google Scholar]
- 46. Gross V, Andus T, Ecker KW, et al. Low dose oral pH modified release budesonide for maintenance of steroid induced remission in Crohn’s disease. Gut. 1998;42(4):493–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hanauer S, Sandborn WJ, Persson A, et al. Budesonide as maintenance treatment in Crohn’s disease: A placebo-controlled trial. Aliment Pharmacol Ther. 2005;21(4):363–71. [DOI] [PubMed] [Google Scholar]
- 48. Hellers G, Cortot A, Jewell D, et al. Oral budesonide for prevention of postsurgical recurrence in Crohn’s disease. Gastroenterology. 1999;116(2):294–300. [DOI] [PubMed] [Google Scholar]
- 49. Lofberg R, Rutgeerts P, Malchow H, et al. Budesonide prolongs time to relapse in ileal and ileocaecal Crohn’s disease: A placebo controlled one year study. Gut. 1996;39(1):82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.