Abstract
Plasmacytoid dendritic cells (PDCs) have perplexed pathologists for decades, undergoing multiple adjustments in nomenclature as their lineage and functions have been characterized. Although PDCs account for less than 0.1% of peripheral blood mononuclear cells, they serve as a principal source of interferon-α and are also known as interferon-I producing cells (IPCs). Upon activation in vitro, they can differentiate into dendritic cells, and recent studies have substantiated a potential role in antigen presentation. Thus, PDCs may act as a link between innate and adaptive immunity. Normally found in small quantities in primary and secondary lymphoid organs, PDCs accumulate in a variety of inflammatory conditions, including Kikuchi-Fujimoto lymphadenopathy, hyaline-vascular Castleman disease, and auto-immune diseases, and in certain malignancies such as classical Hodgkin lymphoma and carcinomas. Demonstrating potential for neoplastic transformation reflective of varying stages of maturation, clonal proliferations range from PDC nodules most commonly associated with chronic myelomonocytic leukemia to the rare but highly aggressive malignancy now known as blastic plasmacytoid dendritic cell neoplasm (BPDCN). Formerly called blastic natural killer cell lymphoma or CD4+ /CD56+ hematoder- mic neoplasm, BPDCN, unlike natural killer cell lymphomas, is not associated with Epstein-Barr virus infection and is generally not curable with treatment regimens for non-Hodgkin lymphomas. In fact, this entity is no longer considered to be a lymphoma and instead represents a unique precursor hematopoietic neoplasm. Acute leukemia therapy regimens may lead to sustained clinical remission of BPDCN, with bone marrow transplantation in first complete remission potentially curative in adult patients.
Keywords: plasmacytoid dendritic cell, interferon-α, blastic plasmacytoid dendritic cell neoplasm, CD4+/CD56+ hematodermic neoplasm
HISTORIC BACKGROUND AND IMMUNOLOGY
The enigmatic cell that is now known as a plasmacytoid dendritic cell (PDC) was described in 1958 by Lennert and Remmele1 as a “lymphoblast,” occurring in clusters in some cases of reactive lymphoid hyperplasia. On the basis of its localization to T-cell-rich areas of lymph nodes and the discovery of its well-developed rough endoplasmic reticulum, it was designated as “T-associated plasma cell” in 1973.2 Ten years later, based on its positivity for CD4, it was renamed as “plasmacytoid T cell” and proposed to act as the T-cell counterpart to plasma B cells, secreting lymphokines rather than immunoglobulins.3 However, CD4 may also mark myelomonocytic cells, and demonstration of expression of myelomonocytic markers such as CD15 (after neuraminidase treatment) and CD68, with lack of other T-lineage markers, prompted the term “plasmacy-toid monocyte” in 1988.4
Functional characterization of these cells became possible in the late 1990s with improved cell sorting and culture techniques, and a dichotomous paradigm emerged. Upon stimulation with CD40 ligand (CD40L) and interleukin (IL)-3, or a variety of microbial stimuli, plasmacy-toid cells develop long dendrites in culture to assume a typical mature dendritic-cell morphology and, functionally, can stimulate both naive CD4+ and CD8+ T cells.5,6 Thus, the term “plasmacytoid dendritic cell” was adopted, although the terms “plasmacytoid” and “dendritic” refer to 2 disparate states. CD4+ CD11c( —) plasmacytoid cells apparently produce large amounts of type I interferons (IFN-I), but upon differentiation into dendritic cells (DCs) in culture lose their capacity to do so.7,8 Furthermore, cells in the “plasmacytoid” state express low levels of major histocompatibility complex (MHC) class II, whereas cells in the “dendritic” state up-regulate MHC class I and II and a variety of T-cell costimulatory molecules, such as CD80 and CD86.5,9,10 To address these 2 morphologically and functionally distinct states described in culture, an alternative nomenclature proposes using the terms “plasmacy-toid predendritic cell” to describe “plasmacytoid” IFN-I producing cells (IPCs) and “plasmacytoid predendritic cell- derived dendritic cell” to describe the “dendritic” state.11 For the purposes of this review, the term PDC will encompass both putative states.
PDCs are defined in human blood as CD11c( —) CD123Hi MHC II + CD45RA + cells that are distinguished from conventional CD11c + CD123( — ) dendritic cells.12 The mouse counterpart of PDCs, as defined by the capacity to generate IFN-I, has been described as CD11cLoCD11b( — ) B220/CD45RA + CD19( — ) Ly-6C/ Gr-1+ CD43+ CD24Lo.13-16 Heterogeneity among PDC fractions has been described in both mouse and human blood.16,17 Thus, subsets expressing CD2, CD5, or CD7 may translate into phenotypic heterogeneity among blastic plasmacytoid dendritic cell neoplasm (BPDCN) cases.17,18
In response to a variety of DNA and RNA viruses or synthetic Toll-like receptor (TLR) 9 and TLR7 agonists, PDCs rapidly secrete copious amounts of IFN-α, accounting for their extensive rough endoplasmic reticulum, and to a lesser extent, other cytokines including IL-6, IL-8, and tumor necrosis factor-α.19 Through both IFN-dependent and IFN-independent mechanisms, PDCs are involved in the recruitment of natural killer (NK) cells and activated T cells, activation of NK cells and macrophages, differentiation of naive CD4+ T cells into effector cells that secrete IFN-γ and IL-10, development of IL-4 secreting T cells, and plasma cell differentiation.9,20-23 In addition to the direct antiviral effect of IFN-I, its secretion promotes the function of myeloid (conventional) DCs.10 Altogether, PDCs function as important immunomodulatory cells that exert a wide variety of effects on both innate and adaptive immunity, inducing either TH2 or TH1 polarization in a stimulus-specific manner.9,22,24,25 More recent data suggests that PDCs may have tolerogenic functions, particularly in inducing CD4 + CD25 + regulatory T cells.26,27
By virtue of their efficient production of IFN-I, PDCs are established as critical immunomodulatory cells, but their role in antigen presentation has been debated.28 Although the acquisition of DC morphology and markers has been described in culture, the morphology of PDCs in histologic sections of peripheral lymphoid tissue generally resembles that in bone marrow and circulation.29 However, recent studies suggest that the PDC counterpart in mice may function as antigen presenting cells; 1 report described direct and sustained interactions between ex vivo isolated PDCs and CD4 + T cells, with efficient induction of T cell activation.30 Furthermore, a graft-versus-host disease (GVHD) model system using MHC-deficient mice has indirectly demonstrated in vivo antigen presentation by PDCs in the absence of other DC subsets.31
PLASMACYTOID DENDRITIC CELL DISTRIBUTION, MORPHOLOGY, AND IMMUNOPHENOTYPE
PDCs account for approximately 0.01% to 0.05% of peripheral blood mononuclear cells and accumulate in inflammatory sites to contribute to the ongoing inflammatory response.8 In response to chemokine-driven signals, PDCs are recruited to tissues, where they occur as scattered elements, clusters, or aggregates (Figs. 1A, B). PDCs enter lymph nodes through high endothelial venules (HEVs) in a CXCL9 and E-selectin dependent manner, using multiple cell adhesion molecules.32,33 PDCs are relatively abundant in lymph nodes, where they are typically located in the vicinity of HEVs (Fig. 1B), and in tonsils, but rare in the spleen and mucosa-associated lymphoid tissue.34,35 PDC numbers in the peripheral blood and their frequency in lymph nodes decline with age, in association with decreased IFN-I production.36
FIGURE 1.
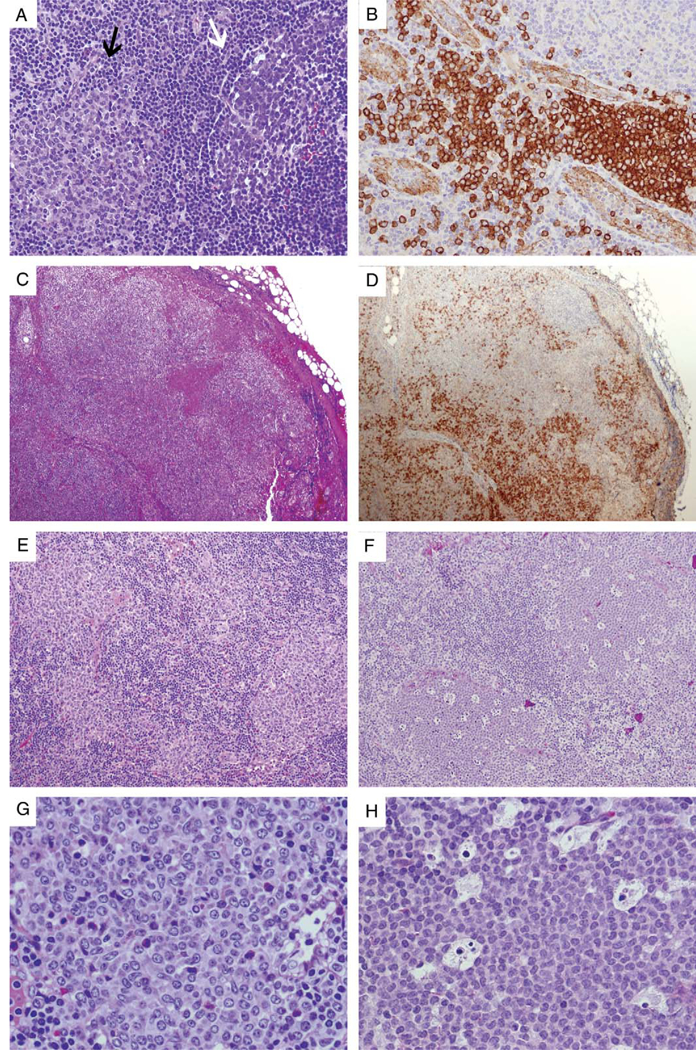
PDCs in reactive and neoplastic conditions. A, In this reactive lymph node, a PDC nodule (left, indicated by a black arrow) is compared with a neighboring germinal center (right, indicated by a white arrow), the latter having a heterogeneous cellular composition and surrounded by a discrete mantle zone. B, PDCs stain strongly positive for CD123 and are most prominent around high endothelial venules, which as a useful internal control are weakly positive for CD123. C (H&E) and D (CD123), Kikuchi-Fujimoto lymphadenopathy, which on H&E may mimic non-Hodgkin lymphoma, is often associated with loose sheets of PDCs (highlighted by CD123 staining), admixed with histiocytes and immunoblasts. E, Clusters of reactive PDCs in an exceptional case of classical Hodgkin lymphoma. F, PDC nodules in a child with juvenile myelomonocytic leukemia; the abundance of apoptotic bodies may result in a low power appearance resembling “naked” germinal centers. G, High-power view of (E) demonstrates intermediate-sized cells with distinct nuclear membranes and pinpoint nucleoli. H, High-power view of (F) demonstrates uniform intermediate-sized cells with finely dispersed chromatin and inconspicuous nucleoli, admixed with numerous apoptotic bodies. H&E indicates hematoxlin and eosin; PDC, Plasmacytoid dendritic cells.
The typical PDC is intermediate in size between that of a small lymphocyte and that of a monocyte, with a round- to-ovoid nucleus that may be slightly indented and eccentric, finely dispersed or reticular chromatin, inconspicuous nucleoli, and moderately abundant cytoplasm that is eosinophilic with hematoxylin and eosin staining but basophilic with Giemsa staining. PDCs are much more readily identified after immunostaining (Table 1), particularly with antisera against CD123 (IL-3 receptor α chain),22,37,38 CD68,4 the lymphoid protooncogene TCL1 (T-cell leukemia/lymphoma 1),39 cutaneous lymphocyte- associated antigen (CLA)/HECA-452,40 CD2-associated protein(CD2AP),29 and blood dendritic cell antigen (BDCA)-2/CD303.41 CD2AP and BDCA-2/CD303, a C-type lectin transmembrane glycoprotein that is important for antigen internalization and presentation to T cells,42 seem to be the most specific markers for PDCs. PDCs also express BDCA-4 (neuropilin-1/CD304), antibodies to which may be used for isolation of PDCs from blood by immunomagnetic selection.43 PDC expression of CLA, which binds to E-selectin on dermal endothelial cells, may enhance their recruitment to skin in certain inflammatory conditions.44
TABLE 1.
PDC Immunophenotype. Antigens Labeled With (F) or (P) are Particularly Helpful for PDC Indentification on Frozen or Paraffin Sections, Respectively.
| CD designation | |
| Positive | CD4, CD11a, CD31, CD32, CD36 (F), CD40, CD43, CD44, CD45RA, CD45RB, CD49e, |
| CD62L*,CD68 (P), CD71, CD74, CD123 (P), CD128, CD162 | |
| Negative | CD1, CD2†, CD3, CD5†, CD7†, CD8, CD10, CD11b, CD11c‡, CD13‡, CD14, CD15§, CD16, |
| CD19, CD20, CD21, CD23, CD25, CD27, CD28, CD30, CD33‡, CD34, CD38, CD45RO, | |
| CD56†, CD57, CD64, CD65, CD80, CD83, CD86, CD94, CD95, CD103, CD117, CD125, | |
| CD138, CDw150, CD161 | |
| Chemokine receptors | |
| Positive | CCR2, CCR5, CCR7‡, CXCR3, CXCR4, Chemerin Receptor |
| Negative | CCR1, CCR3, CCR4, CCR6, CXCR1, CXCR2, CXCR5 |
| Toll-like and pattern recognition receptors | |
| Positive | TLR1 (CD281)∥, TLR6 (CD286)∥, TLR7 (CD287), TLR9 (CD289), TLR10 (CD290)∥, BDCA-2 |
| (CD303)(P) | |
| Negative | TLR2 (CD282), TLR3 (CD283), TLR4 (CD284), TLR5 (CD285), TLR8 (CD288), Mannose |
| receptor (CD206), DC-SIGN (CD209) | |
| Miscellaneous | |
| Positive | BCL11a, BDCA-4 (Neuropilin-1/CD304), CD2AP (P), CLA/HECA-452(P), E-cadherin†, |
| Granzyme B(P), HLA-ABC, HLA-DP, HLA-DQ, HLA-DR, MxA, TCL1 (P) | |
| Negative | DC-LAMP (CD208), Elastase, FOXP3, Langerin (CD207), LAT, Lysozyme, MUM1/IRF4, |
| Myelopero xidase, Perforin, S-100, slg and clg, T-bet, T-cell receptor αβ and γ§, TdT, TIA-1, | |
| ZAP70 | |
Membranous expression on circulating PDCs and cytoplasmic on tissue PDCs.
Positivity reported in a subset of circulating PDCs
Expression induced upon in vitro differentiation or activation.
Positivity after neuraminidase digestion.
Low expression
BDCA indicates blood dendritic cell antigen; CLA, cutaneous lymphocyte-associated antigen; MxA, myxovirus A; PDC, plasmacytoid dendritic cell; TCL, T-cell leukemia/lymphoma; TdT, terminal deoxynucleotidyl transferase; TLR, Toll-like receptor.
At the protein level, human PDCs typically lack expression of lineage-specific markers for B cells (ie, CD19, CD20, PAX5 and surface and cytoplasmic immunoglobulin), T cells [CD3 and T-cell receptor (TCR)], and NK cells (CD16 and CD94). Expression of CD56 and certain T-lineage markers (CD2, CD5, and CD7) may be seen in small proportions of circulating PDCs.17,18,45 They also lack myeloid or monocytic markers such as myeloperoxidase, lysozyme, and cD14. CD11c, CD13, and CD33 are negative in circulating PDCs but can be up-regulated upon in vitro differentiation.5 Furthermore, PDCs are normally negative for stem/progenitor cell markers such as CD34 and CD117, and terminal deoxynucleotidyl transferase (TdT). Interestingly, PDCs lack perforin but may express granzyme B.18,46 The function of granzyme B in PDCs is not entirely clear but may be related to CD4 + CD25 + regulatory T-cell-mediated immunosuppression in the context of peripheral tolerance.47
PLASMACYTOID DENDRITIC CELL DEVELOPMENT
The presence of PDCs in fetal liver, thymus, and bone marrow is in accordance with their development from hematopoietic stem cells within primary lymphoid tissues,48,49 but lineage assignment of PDCs to either a myelomonocytic or lymphoid derivation has been problematic. Recent evidence points to developmental commonalities between PDCs and conventional dendritic cells (CDCs), both have thought to arise from a common DC precursor, which in turn is derived from a common macrophage and DC precursor.50 There is also evidence of plasticity between different subsets of DCs.51 Development of both PDCs and CDCs is controlled by Flt3L (Fms- like tyrosine kinase 3 ligand). Its receptor, Flt3, which is also known as Flk-2, is a receptor tyrosine kinase with homology to c-Kit/CD117 and highly expressed in hematopoietic progenitor cells. Flt3L injection leads to massive expansion of PDCs and CDCs in lymphoid and nonlymphoid organs.52,53 Furthermore, genetic deletion of Flt3L or treatment with Flt3 inhibitors in mice reportedly leads to dramatic reduction of PDCs and CDCs in lymphoid organs.12
Although PDCs can express a number of lymphoid transcripts, such as pre-T-cell antigen receptor alpha chain (pT-α), Spi-B, γ5, Pax5, and TdT,16,49,54 produce some B-lineage transcription factors such as BCL11A and FOXP1,29 and may demonstrate immunoglobulin H diversity-joining (IgH D-J) gene rearrangements,55 even in PDCs experimentally derived from myeloid progenitors,56 these findings may not necessarily indicate a common developmental program of PDCs and lymphocytes. Rather, some of these findings may be attributed to the activity of related transcription factors E2-2, a regulator of PDC development, and E2A/HEB, which is critical in lymphocyte development.57 Of note, PAX5 expression is not observed in purified PDCs at the protein level.29
PLASMACYTOID DENDRITIC CELLS IN HUMAN DISEASES
TLRs are microbial pattern recognition receptors highly conserved across species from Drosophila to humans, and distinct TLR expression patterns occur in different DC subsets. These receptors recognize various pathogen-associated molecular patterns (PAMPs), and their stimulation leads to NF-κB activation.58 In PDCs, engagement of TLR7 and TLR9 results in robust IFN-I secretion.59 TLR7 recognizes single-stranded viral RNA (eg, influenza), whereas TLR9 recognizes hypomethylated CpG sequences corresponding to bacterial DNA and viral DNA (eg, herpes simplex virus 1 and 2).59-61 Interestingly, these receptors may play a role in the detection of host RNA, host DNA, and RNA-associated or DNA-associated proteins in the setting of autoimmune diseases such as systemic lupus erythematosus (SLE).62 In SLE, serum immune complexes composed of double stranded DNA antibodies and DNA may stimulate PDCs to produce high levels of IFN-α.63,64
Although the pathogenesis of SLE is multifaceted, PDCs are likely to play a pivotal role in interacting with other immune cells.65 The paradoxical finding of decreased numbers of circulating PDCs in SLE is attributed to their recruitment to sites of tissue damage.66 Although other cytokines are also increased in lupus sera, IFN-α levels best correlate with disease activity, and an IFN-inducible gene expression signature as defined by microarray studies can serve as a marker for disease severity.67-69 Furthermore, SLE and SLE-like conditions may develop in patients who receive long-term IFN-α therapy for a variety of unrelated conditions such as viral infections or tumors.70Overall, these findings provide a strong rationale for the development of new therapies that block IFN pathways in SLE. Interestingly, BDCA-2/CD303 seems to be a potent inhibitor of IFN-I production by PDCs and is rapidly down-regulated upon PDC activation.71 A potential therapy for SLE may entail inhibition of IFN-I production by up-regulating BDCA-2, to halt irreversible tissue damage.
Although PDCs are normally very rare in skin, their accumulation has been described in inflammatory cutaneous conditions such as SLE, psoriasis, lichen planus, and contact dermatitis.72-74 In psoriasis, PDCs infiltrate skin and secrete IFN-α during early disease, and in a xenograft model, blocking IFN-α production and/or signaling prevented the development of psoriasis.75 Whereas PDCs normally do not respond to self-DNA, in autoimmune diseases such as psoriasis, self-DNA has the potential to trigger TLR9.76 In lichen planus, a pathogenetic role for human herpes virus 7 has been proposed, and PDCs seem to harbor human herpes virus 7 DNA and proteins in lichen planus lesions.77
In HIV-1 infection, PDC levels are markedly decreased in peripheral blood, inversely correlating with viral load.78 Thus, PDCs may suppress HIV-1 replication, and fusion of HIV with PDCs seems to induce apoptosis and necrosis.79,80 PDC counts may conceivably be used in clinical immunology to monitor treatment of HIV-1, and their depletion contributes to the immunodeficiency caused by HIV-1. The role of PDCs in other viral infections, such as chronic hepatitis C virus infection, is more controver-sial.81
In lymph nodes, PDCs are considerably increased in a wide range of inflammatory conditions, including infection (tuberculosis, toxoplasmosis) and sarcoidosis.35 They are particularly abundant in 2 rare forms of reactive lymphadenopathy, the hyaline-vascular subtype of Castle- man disease and Kikuchi-Fujimoto lymphadenopathy (Figs. 1C, D).82,83 Kikuchi-Fujimoto lymphadenopathy (histiocytic necrotizing lymphadenitis) is typically a self-limited disorder that most often presents as cervical lymphadenopathy, particularly in young adults, and morphologically may resemble non-Hodgkin lymphoma due to architectural effacement and the accumulation of immunoblasts. PDCs are most prominent around HEVs and in viable parenchyma surrounding the areas of necrosis.
In addition to their role in antimicrobial immunity, PDCs infiltrate a variety of malignancies and may be involved in antitumor immunity. In melanomas, there is evidence to suggest that PDCs elicit tumor-specific cytotoxic T-cell responses.84,85 Furthermore, the use of a synthetic TLR9 agonist, resulting in PDC activation, may have promise in treating patients with metastatic melanoma.86 Increased PDCs have also been described in squamous cell carcinoma,87 basal cell carcinoma, cutaneous T-cell lymphoma,88 breast carcinoma,89 and ovarian carcinoma.90 In fact, the use of imiquimod, a synthetic agonist of TLR7, in the topical treatment of basal cell carcinoma, melanoma in situ, and condyloma accuminata, seems to correlate with the recruitment of PDCs and up-regulation of tumor necrosis factor- related apoptosis-inducing ligand (TRAIL) activity, resulting in tumor cell death.88,91,92
These findings suggest that PDCs may be exploited in maximizing an antitumor immune response, with TLR7 and TLR9 agonists as an avenue for developing such therapeutic strategies. However, there is also evidence to suggest that PDCs may be involved in tolerogenic functions, with potent suppression of host antitumor T-cell responses, presumably through the activation of regulatory T cells.93,94 Upon TLR9 stimulation, PDCs express high quantities of indoleamine 2,3- dioxygenase, which was recently demonstrated through the use of pharmacologic agents to play an essential role in PDC-mediated generation of regulatory T cells from CD4+ CD25( - ) T cells.95
Increased PDCs have also been observed in association with some cases of classical Hodgkin lymphoma (Figs. 1E, G). They may occur in clusters or loose aggregates, or as scattered single cells in nodular sclerosis, mixed cellularity, and lymphocyte-rich subtypes.96 In affected nodes, PDCs and Reed-Sternberg cells seem topographically distinct, and there is no evidence to suggest clonal relatedness between the 2 cell populations. Instead, the cytokine milieu in classical Hodgkin lymphoma may lead to recruitment of PDCs, perhaps in relation to the generation of CD4+ CD25+ regulatory T cells.
“TUMOR FORMING” PLASMACYTOID DENDRITIC CELLS ASSOCIATED WITH MYELOID NEOPLASMS
Although experimental evidence had suggested derivation of PDCs from either myeloid or lymphoid progeni- tors,97-99 a compelling histopathologic argument in support of a closer relationship to the myeloid lineage is the occurrence of PDC accumulations in patients who have myeloid neoplasms, with cytogenetic evidence of clonal relatedness between the 2 processes.100-103 Nodules of cytologically bland, morphologically mature PDCs have been described in association with chronic myelomonocytic leukemia and other myeloid neoplasms with monocytic differentiation and may occur in lymph nodes, skin, spleen, or bone marrow.100-102,104-106 They are not associated with chronic myelogenous leukemia or atypical chronic myeloid leukemia.107 The nodules are often sharply demarcated (Figs. 1F, H) and express PDC markers including CD123, CLA, TCL1, and the IFN-α inducible protein myxovirus A (MxA).29,102
Although similar-appearing nodular accumulations of PDCs may be seen as part of the immune response and are not thought to be neoplastic (Figs. 1E, G), fluorescence in situ hybridization (FISH) studies in multiple cases of PDC nodules associated with myeloid neoplasms have provided evidence not only that the PDC nodules are clonal in nature but also that they may be clonally related to the associated myeloid disorder.100-103 In 1 case of a myelodysplastic syndrome described by Mongkonsri- tragoon et al,100 FISH studies demonstrated trisomy 9 and 11 in the presumed PDC lesions and in the surrounding marrow.106 Chen et al101 subsequently reported 2 additional cases with FISH evidence of clonal relatedness between the PDC nodular proliferation and associated myelodysplasia, with monosomy 7 in 1 case and loss of 20q12 in the other. Monosomy 7 was also observed in both populations in a case of acute myelomonocytic leukemia with associated PDC proliferation reported by Vermi et al.102 In 2 cases of intestinal myeloid sarcoma studied by Pileri et al,103 inversion 16 was noted in both the myeloid sarcoma component and in areas of PDC differentiation in spite of opposing immunophenotypes. Aberrant expression of markers such as CD2, CD5, CD7, CD10, CD14, or CD15 may also support the neoplastic nature of PDC prolifera- tions.102,108 Such PDC nodules may regress upon ther- apy,105 and in general, the prognosis reflects the patient’s underlying myeloid neoplasm rather than the expansion of PDCs, in contrast to BPDCN, which also may be associated with myeloid disorders (discussed in the next section).
PDCs in this setting usually form compact and well-demarcated nodules, but a diffuse pattern of infiltration may also be observed, with effacement of underlying lymph node architecture (Fig. 2, left column). Clinically, patients may present with generalized lympha- denopathy and/or hepatosplenomegaly.108,109 In contrast to BPDCN, these lesions have very low-proliferative rates (<10% Ki-67 proliferative index), are negative or weakly/focally positive for CD56, and lack TdT expres- sion.29,101,102 Thus, a panel of stains including CD56, TdT, and Ki-67, along with clinical correlation, are recommended in cases of diffuse PDC proliferations that may mimic BPDCN (Table 2).
FIGURE 2.
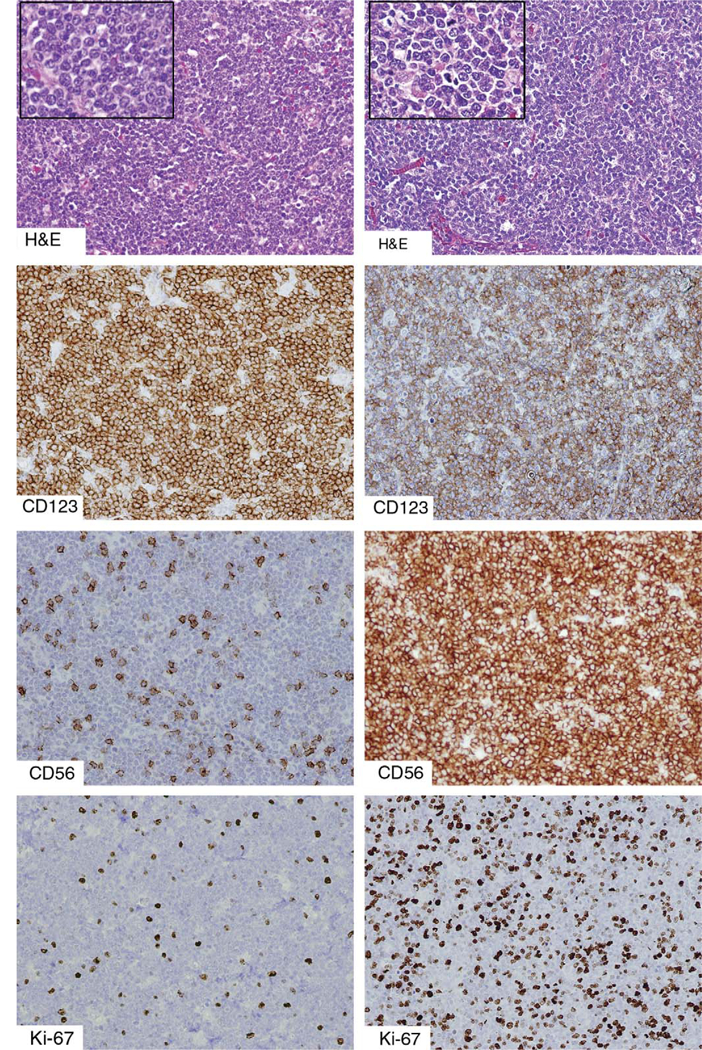
An unusual example of a “tumor forming” PDC proliferation associated with a myeloid neoplasm, compared with BPDCN. The left column depicts a PDC proliferation in a patient with underlying chronic myelomonocytic leukemia, and the right column depicts a case of BPDCN, both diffusely effacing the underlying lymph node architecture. The distinction can be difficult on histologic examination alone, although the tumor forming PDCs are more round and uniform than the cells of BPDCN. Both are positive for CD123. Both CD56 and TdT (not shown) are usually negative in mature PDC proliferations, although a small subpopulation of PDCs may be CD56+, as shown. In contrast, BPDCN is more uniformly positive for CD56 and in almost half of all cases demonstrates TdT positivity. The Ki-67 proliferative index is typically very low (<10%) in mature PDC proliferations and higher (>30%) in BPDCN. BPDCN indicates blastic plasmacytoid dendritic cell neoplasm; PDC, plasmacytoid dendritic cells; TdT, terminal deoxynucleotidyl transferase.
TABLE 2.
Immunophenotypic Profiles of Normal PDCs, “Tumor Forming” PDC Proliferations Associated With Myeloid Neoplasms, and BPDCN
| Antigen | Normal PDCs |
“Tumor forming” PDC Proliferations |
BPDCN |
|---|---|---|---|
| CD123 | + | + | + |
| TCL1 | + | + | + |
| CD4 | + | + | + |
| CD56 | −* | ±† | +‡ |
| TdT | − | − | ± |
| Ki-67 | <5% | < 10% | > 30% |
Positivity reported in a small subset of circulating PDCs.
Usually negative but may show focal positivity.
Negative cases extremely rare.
BPDCN indicates blastic plasmacytoid dendritic cell neoplasm; PDC, plasmacytoid dendritic cell; TCL, T-cell leukemia/lymphoma; TdT, terminal deoxynucleotidyl transferase.
BLASTIC PLASMACYTOID DENDRITIC CELL NEOPLASM
The entity that is listed as BPDCN in the 2008 World Health Organization classification of tumors of hematopoietic and lymphoid tissues110 historically has undergone multiple adjustments in nomenclature reflective of our evolving understanding of its histogenesis.111 On the basis of its blastic cytologic appearance and expression of CD56, it was known as blastic NK-cell lymphoma. However, CD4 positivity and lack of expression of other NK-lineage markers in most cases cast doubt on its derivation from NK precursors, and CD4+/CD56+ hematodermic neoplasm was adopted as an alternative, descriptive term for this disease, which has a striking predilection for cutaneous involvement.112,113
The cell of origin of this disease remained a mystery until recent phenotypic and functional characterization of both normal PDCs and neoplastic cells of CD4+ /CD56+ hematodermic neoplasm showed many features in common, suggesting derivation from PDC precursors.45,114‘115 One striking phenotypic commonality between normal circulating PDCs and BPDCN is strong expression of CD123 (IL-3 receptor α chain).24,37,114 Upon in vitro stimulation with IL-3 and CD40L, tumor cells up-regulate myeloid antigens and differentiate into cells with dendritic processes that are capable of inducing naive CD4+ T-cell proliferation, as described in normal PDCs.5,114,116 Furthermore, tumor cells, like non-neoplastic PDCs, can produce IFN-α in response to viral stimulation and express IFN-α inducible myxovirus A.114,117,118 Also, both normal PDCs and BPDCN express CLA/HECA-452, which may contribute to skin tropism by binding specifically to E-selectin on dermal endothelial cells and cutaneous T cells.40,119,120 Additional identification of specific markers, as described below, and gene expression profiling studies have further supported the notion that BPDCN arises from PDC precursors.29,39,119,121-123
Clinical Presentation
BPDCN primarily afflicts elderly adults, with a median age of approximately 65 years, although it can occur at any age, even in infants.111,124-130 The male/female ratio is 2.5 to 3:1. Approximately 85% of reported cases demonstrated cutaneous involvement at presentation, and Petrella et al126 described that in about half the cases with complete staging, disease seemed limited to the skin at the time of diagnosis. Through the hematopathology consultation service at the National Institutes of Health (NIH), Bethesda, MD, we have evaluated cases from 55 patients with BPDCN since 1996, confirming the findings reported by others (Table 3). Forty-six of the 55 patients (84%) initially presented with cutaneous disease, the overall male/female ratio was 2.7:1, and the median age was 67 years, although 8 patients (15% of total) were younger than 18-years-old at diagnosis.
TABLE 3.
Clinical and Immunophenotypic Features of BPDCN Cases Reviewed at the National Cancer Institute, Bethesda, MD
| Presenting With Cutaneous Involvement | Lacking Cutaneous Involvement at Presentation | |
|---|---|---|
| Number of patients | 46 (84% of total) | 9 (16% of total) |
| Age (y): median, range | 67; 4-87 | 68; 10-84 |
| Male/female, ratio | 34/12; 2.8: 1 | 6/3; 2:1 |
| Prior or concomitant myeloid neoplasia | 4/46 (9%) | 1/9 (11%) |
| Immunophenotype | ||
| CD4 | 39/39 (100%) | 8/8 (100%) |
| CD56 | 44/45 (98%) | 9/9 (100%) |
| CD123 | 32/32 (100%) | 7/7 (100%) |
| TdT | 22/41 (54%) | 6/7 (86%) |
| S-100 | 6/18 (33%) | 1/5 (20%) |
| CD3 | 0/38 (0%) | 0/9 (0%) |
| CD34 | 0/17 (0%) | 0/4 (0%) |
| CD117 | 0/16 (0%) | 0/4 (0%) |
BPDCN indicates blastic plasmacytoid dendritic cell neoplasm; TdT, terminal deoxynucleotidyl transferase.
The skin lesions may be either localized or widespread and range in appearance from small bruise-like areas to violaceous patches, nodules, and ulcerated masses, measuring up to several centimeters. Low-level bone marrow or peripheral blood involvement is likely in early disease, although overt leukemia is more characteristic of advanced cases or relapse after therapy.124 Leukemic variants without cutaneous involvement have also been documented, on the basis of rigorous immunophenotypic profiling.111,124,131 Extracutaneous and extramedullary sites of involvement at presentation, in order of decreasing frequency, include lymph nodes (about 40% of cases), spleen, liver, and tonsils. Cytopenias, particularly thrombocytopenia, are frequent at presentation, whereas B systemic symptoms are not common.124 Rare cases involve the central nervous system at presentation,124,132 and this is not an unusual site of relapse, possibly acting as a sanctuary site for BPDCN blasts.126,129,133
Morphology
The typical skin infiltrate is diffuse and dermal without epidermotropism, but early lesions may be patchy or nodular, with a perivascular and periadnexal distribution. Erythrocyte extravasation may be abundant in some cases, but an inflammatory component is usually minimal. The subcutis is often extensively involved. The neoplastic cells are usually intermediate-sized forms with fine chromatin and indistinct nucleoli, resembling lymphoblasts, although larger forms with multiple small nucleoli may sometimes be noted on cytologic preparations. Mitotic figures may be observed but are generally not prominent. The cytoplasm is variably abundant and lacks granules, and on cytologic preparations may demonstrate pseudopod-like extensions and/or multiple peripheral vacuoles of varying sizes, sometimes with a “pearl necklace” appearance.124 Bone marrow involvement may range from the presence of small nests, detection of which is greatly aided by immunohis- tochemistry, to more diffuse sheets. Lymph node involvement is typically in a “leukemic” pattern sparing the follicles, or may be extensive with effacement of the nodal architecture.
Immunophenotype
BPDCN is typically positive for CD4, CD56, CD123 (strong), and CD45 (dim, blast gate on flow cytometry), with CD68 immunoreactivity (punctate Golgi pattern) and/or TdT expression in a subset of cases, and lacks other myelomonocytic, NK, T, or B-lineage markers. Coexpression of CD7 or CD33, or less commonly CD2, may be observed.124,127,134-136 Expression of CD3 should raise suspicion for a T-lineage malignancy, although there have been rare cases of BPDCN reported with cytoplasmic CD3 expression.111,137 Furthermore, some cases may show expression of S-100 (zonal distribution; Table 3),138,139 CD10,135 or CD117.135 cD34, CD8, myeloperoxidase, lysozyme, PAX5, CD20, CD79a, Epstein Barr-Virus, and T-cell receptor protein are consistently negative, and expression of such markers should exclude the diagnosis of BPDCN.
Neither CD4 nor CD56 positivity may be absolutely necessary for diagnosis, and either marker can vary in intensity from weak and focal to diffuse and strong. CD4( —) cases are rarely observed, although this may be partly technical, given the decreased sensitivity of detection in formalin-fixed tissues.129,140 Lymphoblastic leukemia/ lymphoma should be considered in the differential diagnosis, although such cases typically do not express strong and uniform CD123.141 Rare CD56(−) BPDCN cases have been reported.29,127,142,143 Although CD56 expression is characteristic of BPDCN, only a very minute fraction of circulating PDCs isolated from healthy individuals expresses CD56.45 Thus, this small fraction may potentially give rise to BPDCN or, alternatively, CD56 acquisition may be associated with oncogenic transformation, analogous to CD56 expression in plasma cell myeloma but not in benign plasma cells.144 Unlike normal PDCs, BPDCN may express CD38, which was positive in 74% of BPDCN cases reported by Feuillard et al,124 or TdT, which may indicate a more immature phenotype and is variably positive in about one-third to one- half of BPDCN cases. Furthermore, the proliferative rate is greatly increased in BPDCN (30% to 90% Ki-67 proliferative index), compared with non-neoplastic PDCs.
Strong CD123 expression is not entirely specific for BPDCN, as basophils normally express high levels of CD123,145 which has also been reported in acute basophilic leukemia.146 CD123 expression in other forms of acute leukemia may also be observed, although quantitatively at lower levels through flow cytometric analysis, with little overlap seen between BPDCN and acute non-PDC leukemia.135 CD123 expression may also be detected in histiocytic sarcoma and Langerhans cell histiocytosis. Ultimately, accurate diagnosis of BPDCN may greatly be facilitated through the use of extensive antibody panels including more recently discovered PDC markers, namely TCL1, CD2AP, BDCA-2/CD303, and BDCA-4.
Given the morphologic and immunophenotypic overlap between BPDCN and myeloid sarcoma, both of which can coexpress CD4, CD56, and CD123, specific PDC markers have great diagnostic utility. TCL1 is more commonly expressed in BPDCN (90%) than in myeloid sarcoma (17%), and is negative in mature NK malignancies.39,119 Testing a large panel of candidate proteins, Marafioti et al29 determined that CD2AP, an adapter protein originally cloned from T cells, was specifically expressed in PDCs and also may be used to distinguish BpDCN from acute myeloid leukemia (AML).
The use of CD123, BDCA-4, and BDCA-2/CD303 in flow cytometric analysis may help reliably diagnose BPDCN. BDCA-4/neuropilin-1/CD304) is expressed in normal PDCs41-42 and in 85% (17/20) of BPDCN cases according to 1 study, but is not entirely specific for BPDCN, as 12% (14/113) of acute non-PDC leukemia cases in the same study also displayed BDCA-4 expression at comparable levels.135 In contrast, 0 of the 113 acute non-PDC leukemia cases expressed BDCA-2/CD303, whereas 70% of the BPDCN cases expressed BDCA-2/ CD303, serving as a highly specific marker for PDCs.135 BDCA-2/CD303, like CD123, may be tested through either flow cytometric analysis or immunohistochemistry on paraffin sections.147
Genetics
Karyotypic analysis often shows complex aberrations, and specific abnormalities have not been described. How-ever, 6 major recurrent chromosomal targets have been identified, namely 5q (72%), 12p (64%), 13q (64%), 6q (50%), 15q (43%), and 9 (28%).148 Furthermore, comparative genomic hybridization and gene expression profiling studies conducted by Dijkman et al123 demonstrated recurrent deletion of regions on 4q34, 9p13-p11, 9q12- q34, and 13q12-q31, resulting in loss of tumor suppressor genes such as RBI and LATS2. The same studies also identified a “PDC signature” in BPDCN samples, supporting its histogenesis and establishing the disease as genetically distinct from cutaneous myelomonocytic leukemias. Interestingly, BPDCN samples compared with normal PDCs showed increased expression levels of genes involved in Notch signaling (HES6, RUNX2), which may serve as oncogenes in the molecular pathogenesis of BPDCN.123 Also overexpressed was a set of genes not described in other hematopoietic cells but highly expressed in neuronal cells and implicated in neurogenesis.
More recently, Jardin et al133 performed high-resolution array comparative genomic hybridization and quantitative multiplex polymerase chain reaction of short fluorescent fragments (QMPSF) on nine BPDCN cases, showing losses of multiple genes involved in the G1 /S transition. Especially common were deletions of chromosomes 9 (harboring the CDKN2A and CDKN2B genes), 13q (containing the RB1 locus), 17p (containing TP53), or 12p (including the tumor suppressor genes CDKN1B and ETV6). In 2 cases, deletion breakpoints were located near the MYC gene, providing a mechanism for deregulated MYC expression. Furthermore, genomic aberrations were described involving miRNA coding genes. All in all, the genetic profile in BPDCN was distinct from that in AML and may provide a basis for the phenomenon of chemoresistance that frequently develops in BPDCN patients in spite of an initial favorable response to chemotherapy.
Association With Myeloid Disorders
A significant proportion of BPDCN cases, estimated at 10% to 20% depending on the study and more than can be accounted for by coincidence, is associated with prior, coexistent, or subsequent myelomonocytic leukemias, with or without an underlying myelodysplastic syndrome.39,111,117,124,126-128,139,141 These findings are in keeping with a common cell of origin with multilineage potential and propensity for PDC and myelomonocytic differentiation, providing an explanation for cases of “phenotypic conversion” of acute leukemia. Cytogenetic analysis has provided support for the notion of clonal relatedness in cases of tumorous PDC proliferations associated with myeloid disorders, as described above.100-103 Notably, cases of myelomonocytic leukemia arising in patients with BPDCN were much more likely to express TCL1 than de novo acute myelomonocytic leukemia.39 Although PDCs may be developmentally more closely related to histiocytes or DCs, composite cases of BPDCN with histiocytic or DC neoplasms have not been reported, to our knowledge.
Clinical Course and Therapy
The initial clinical presentation of BPDCN often seems deceptively indolent, and a variety of intensive chemotherapy regimens may achieve resolution of symptoms. However, at least in adults, this is almost invariably followed by rapid extracutaneous dissemination, with or without overt leukemia. In determining optimal treatment for this often rapidly fatal disease, Reimer et al149 evaluated 91 published cases and 6 additional patients, reporting a median survival of 13 months. The authors concluded that patients with disease initially restricted to skin fared no better than those with disseminated disease at presentation and identified age as an adverse prognostic factor. Sustained clinical remission or cure is uncommon, usually occurring in patients who received acute leukemia chemotherapy regimens and allogeneic stem cell transplantation in first complete remission.124,149 In addition to bone marrow transplantation, intrathecal chemoprophylaxis has been recommended for this disease, as 5 of 15 (33%) patients who relapsed after complete remission had relapse in the central nervous system according to the Feuillard et al study.124 Although allogeneic stem cell transplantation is often advocated for this disease, there have been cases of sustained clinical remission or apparent cure, particularly in the pediatric population, with high-risk acute leukemia therapy regimens without bone marrow trans- plantation.131,150 Of note, AML-type therapy may be associated with a worse outcome than acute lymphoblastic leukemia type therapy, in spite of evidence of closer biologic relatedness between PDCs and the myeloid lineage.124,126
Given the rarity of this disease and lack of standardized treatment regimens, optimal therapy has not yet been determined. Confounding matters further is disease heterogeneity based on immunophenotype and/or clinical presentation, particularly in comparing cases with cutaneous involvement to those lacking cutaneous involvement at presentation. In analyzing the BPDCN cases reviewed at the NIH, those that lacked cutaneous disease at presentation were more likely to be TdT positive, but no other significant differences were observed between the 2 groups (Table 3). Among those patients with available clinical follow-up, the prognosis was usually poor, although the outcome was overall more favorable in the pediatric population (manuscript in preparation).
Identification of prognostic markers in BPDCN may potentially contribute to therapeutic strategies. For example, Jaye et al147 described an inverse correlation between TdT and BDCA-2/CD303 levels, postulating that TdT+ BDCA-2/ CD303( —) cells correspond to earlier PDC precursors, whereas TdT( —) BDCA-2/CD303+ cells correspond to more mature PDC precursors. Interestingly, BDCA-2/ CD303+ cases were associated with reduced survival compared with BDCA-2/CD303( —) cases. Furthermore, high TdT expression in cases that had been called blastic NK-cell lymphoma was associated with a more favorable prognosis.151 These findings are consistent with derivation of BPDCN from PDC precursors at varying stages of maturation, which may translate into varying degrees of therapeutic responsiveness.
CONCLUSIONS
PDCs, through their secretion of type I IFNs and, to a lesser extent, other cytokines, are implicated in a wide variety of immune functions, including antiviral immunity, antitumor immunity, and peripheral tolerance. Therapeutic manipulations of PDCs, as a main source of IFN-I, may provide a viable approach for treating a variety of diseases. The precise developmental pathway of PDCs awaits determination but likely represents a unique lineage, and BPDCN is a highly aggressive hematopoietic precursor neoplasm with commitment to the PDC lineage. BPDCN, although exceedingly rare (constituting well under 1% of acute leukemia cases), may encompass multiple variants as evidenced by some phenotypic and clinical heterogeneity. Comprehensive review of historical cases is problematic and may warrant reclassification based on more specific PDC markers that were not previously available for testing. Indeed, the former category of blastic NK-cell lymphoma would also include examples of lymphoblastic leukemias/ lymphomas or primitive hematopoietic malignancies of uncertain lineage, which may biologically behave differently. Owing to its rarity and only recent recognition as a distinct clinicopathologic entity, no standardized therapeutic approach has been established for BPDCN, and multicenter prospective trials are required to determine optimal therapy.
REFERENCES
- 1.Lennert K, Remmele W. Karyometrische Untersuchungen an Lymphknotenzellen des Menschen: i mitt germinoblasten, lymphoblasten und lymphozyten. Acta Haematol (Basel) 1958;19:99–113. [DOI] [PubMed] [Google Scholar]
- 2.Müller-Hermelink HK, Kaiserling E, Lennert K. Pseudofolli- kuläre Nester von Plasmazellen (eines besonderen Typs?) in der paracorticalen Pulpa menschlicher Lymphknoten. Virchows Arch (Cell Pathol). 1973;14:47–56. [PubMed] [Google Scholar]
- 3.Feller AC, Lennert K, Stein H, et al. Immunohistology and etiology of histiocytic necrotizing lymphadenitis. Report of three instructive cases. Histopathology. 1983;7:825–839. [DOI] [PubMed] [Google Scholar]
- 4.Facchetti F, de Wolf-Peeters C, Mason DY, et al. Plasma- cytoid T cells. Immunohistochemical evidence for their monocyte/macrophage origin. Am J Pathol. 1988;133:15–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Grouard G, Rissoan MC, Filgueira L, et al. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalod M, Hamilton T, Salomon R, et al. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med. 2003;197:885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 8.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. [DOI] [PubMed] [Google Scholar]
- 9.Kadowaki N, Antonenko S, Lau JY, et al. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. [DOI] [PubMed] [Google Scholar]
- 11.Soumelis V, Liu YJ. From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre- dendritic cell differentiation. Eur J Immunol. 2006;36:2286–2292. [DOI] [PubMed] [Google Scholar]
- 12.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakano H, Yanagita M, Gunn MD. CD11c( + )B220( + )Gr- 1(+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Keeffe M, Hochrein H, Vremec D, et al. Mouse plasma- cytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8( + ) dendritic cells only after microbial stimulus. J Exp Med. 2002;196:1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nikolic T, Dingjan GM, Leenen PJ, et al. A subfraction of B220( + ) cells in murine bone marrow and spleen does not belong to the B cell lineage but has dendritic cell characteristics. Eur J Immunol. 2002;32:686–692. [DOI] [PubMed] [Google Scholar]
- 16.Pelayo R, Hirose J, Huang J, et al. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comeau MR, Van der Vuurst de Vries AR, Maliszewski CR, et al. CD123bright plasmacytoid predendritic cells: progenitors undergoing cell fate conversion? J Immunol. 2002; 169:75–83. [DOI] [PubMed] [Google Scholar]
- 18.Matsui T, Connolly JE, Michnevitz M, et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009; 182:6815–6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type IIFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19:3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Megjugorac NJ, Young HA, Amrute SB, et al. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J Leukoc Biol. 2004;75:504–514. [DOI] [PubMed] [Google Scholar]
- 21.Boehm U, Klamp T, Groot M, et al. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. [DOI] [PubMed] [Google Scholar]
- 22.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. [DOI] [PubMed] [Google Scholar]
- 23.Jego G, Palucka AK, Blanck JP, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. [DOI] [PubMed] [Google Scholar]
- 24.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. [DOI] [PubMed] [Google Scholar]
- 25.Cella M, Facchetti F, Lanzavecchia A, et al. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat Immunol. 2000;1:305–310. [DOI] [PubMed] [Google Scholar]
- 26.Moseman EA, Liang X, Dawson AJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynu- cleotides induce the generation of CD4+ CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. [DOI] [PubMed] [Google Scholar]
- 27.Ochando JC, Homma C, Yang Y, et al. Alloantigen- presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. [DOI] [PubMed] [Google Scholar]
- 28.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. [DOI] [PubMed] [Google Scholar]
- 29.Marafioti T, Paterson JC, Ballabio E, et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008;111:3778–3792. [DOI] [PubMed] [Google Scholar]
- 30.Mittelbrunn M, Martinez del Hoyo G, Lopez-Bravo M, et al. Imaging of plasmacytoid dendritic cell interactions with T cells. Blood. 2009;113:75–84. [DOI] [PubMed] [Google Scholar]
- 31.Koyama M, Hashimoto D, Aoyama K, et al. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft- versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. [DOI] [PubMed] [Google Scholar]
- 32.Yoneyama H, Matsuno K, Zhang Y, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16:915–928. [DOI] [PubMed] [Google Scholar]
- 33.Matsutani T, Tanaka T, Tohya K, et al. Plasmacytoid dendritic cells employ multiple cell adhesion molecules sequentially to interact with high endothelial venule cells- molecular basis of their trafficking to lymph nodes. Int Immunol. 2007;19:1031–1037. [DOI] [PubMed] [Google Scholar]
- 34.Facchetti F, De Wolf-Peeters C, van den Oord JJ, et al. Plasmacytoid T cells: a cell population normally present in the reactive lymph node. An immunohistochemical and electro- nmicroscopic study. Hum Pathol. 1988;19:1085–1092. [DOI] [PubMed] [Google Scholar]
- 35.Facchetti F, Vermi W, Mason D, et al. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 2003; 443:703–717. [DOI] [PubMed] [Google Scholar]
- 36.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–521. [DOI] [PubMed] [Google Scholar]
- 37.Facchetti F, Candiago E, Vermi W. Plasmacytoid monocytes express IL3-receptor alpha and differentiate into dendritic cells. Histopathology. 1999;35:88–89. [DOI] [PubMed] [Google Scholar]
- 38.Olweus J, BitMansour A, Warnke R, et al. Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proc Natl Acad Sci USA. 1997;94:12551–12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herling M, Teitell MA, Shen RR, et al. TCL1 expression in plasmacytoid dendritic cells (DC2s) and the related CD4+ Cd56+ blastic tumors of skin. Blood. 2003;101: 5007–5009. [DOI] [PubMed] [Google Scholar]
- 40.Facchetti F, de Wolf-Peeters C, van den Oord JJ, et al. Antihigh endothelial venule monoclonal antibody HECA-452 recognizes plasmacytoid T cells and delineates an “extranodular” compartment in the reactive lymph node. Immunol Lett. 1989;20:277–281. [DOI] [PubMed] [Google Scholar]
- 41.Dzionek A, Fuchs A, Schmidt P, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165: 6037–6046. [DOI] [PubMed] [Google Scholar]
- 42.Dzionek A, Inagaki Y, Okawa K, et al. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum Immunol. 2002;63:1133–1148. [DOI] [PubMed] [Google Scholar]
- 43.Boor PP, Ijzermans JN, van der Molen RG, et al. Immunomagnetic selection of functional dendritic cells from human lymph nodes. Immunol Lett. 2005;99:162–168. [DOI] [PubMed] [Google Scholar]
- 44.Bangert C, Friedl J, Stary G, et al. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J Invest Dermatol. 2003;121:1409–1418. [DOI] [PubMed] [Google Scholar]
- 45.Petrella T, Comeau MR, Maynadie M, et al. “Agranular CD4+ CD56 + hematodermic neoplasm” (blastic NK-cell lymphoma) originates from a population of CD56+ precursor cells related to plasmacytoid monocytes. Am J Surg Pathol. 2002;26:852–862. [DOI] [PubMed] [Google Scholar]
- 46.Rissoan MC, Duhen T, Bridon JM, et al. Subtractive hybridization reveals the expression of immunoglobulinlike transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood. 2002;100: 3295–3303. [DOI] [PubMed] [Google Scholar]
- 47.Gondek DC, Lu LF, Quezada SA, et al. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783–1786. [DOI] [PubMed] [Google Scholar]
- 48.Blom B, Ho S, Antonenko S, et al. Generation of interferon alpha-producing predendritic cell (Pre-DC)2 from human CD34( + ) hematopoietic stem cells. J Exp Med. 2000; 192:1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bendriss-Vermare N, Barthelemy C, Durand I, et al. Human thymus contains IFN-alpha-producing CD11c( — ), myeloid CD11c( + ), and mature interdigitating dendritic cells. J Clin Invest. 2001;107:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu K, Victora GD, Schwickert TA, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009; 324:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuniga EI, McGavern DB, Pruneda-Paz JL, et al. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karsunky H, Merad M, Cozzio A, et al. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Res PC, Couwenberg F, Vyth-Dreese FA, et al. Expression of pTalpha mRNA in a committed dendritic cell precursor in the human thymus. Blood. 1999;94:2647–2657. [PubMed] [Google Scholar]
- 55.Corcoran L, Ferrero I, Vremec D, et al. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J Immunol. 2003;170:4926–4932. [DOI] [PubMed] [Google Scholar]
- 56.Shigematsu H, Reizis B, Iwasaki H, et al. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 2004;21: 43–53. [DOI] [PubMed] [Google Scholar]
- 57.Cisse B, Caton ML, Lehner M, et al. Transcription factor E2- 2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito T, Wang YH, Liu YJ. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin Immunopathol. 2005;26:221–229. [DOI] [PubMed] [Google Scholar]
- 59.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diebold SS, Kaisho T, Hemmi H, et al. Innate antiviral responses by means of TLR7-mediated recognition of singlestranded RNA. Science. 2004;303:1529–1531. [DOI] [PubMed] [Google Scholar]
- 61.Lund JM, Alexopoulou L, Sato A, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598–5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Landenberg P, Bauer S. Nucleic acid recognizing Toll-like receptors and autoimmunity. Curr Opin Immunol. 2007; 19:606–610. [DOI] [PubMed] [Google Scholar]
- 63.Vallin H, Blomberg S, Alm GV, et al. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-alpha) production acting on leukocytes resembling immature dendritic cells. Clin Exp Immunol. 1999;115:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. [DOI] [PubMed] [Google Scholar]
- 65.Theofilopoulos AN, Baccala R, Beutler B, et al. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. [DOI] [PubMed] [Google Scholar]
- 66.Cederblad B, Blomberg S, Vallin H, et al. Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-alpha producing cells. J Autoimmun. 1998;11:465–470. [DOI] [PubMed] [Google Scholar]
- 67.Bengtsson AA, Sturfelt G, Truedsson L, et al. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9:664–671. [DOI] [PubMed] [Google Scholar]
- 68.Baechler EC, Batliwalla FM, Karypis G, et al. Interferon- inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gota C, Calabrese L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity. 2003;36:511–518. [DOI] [PubMed] [Google Scholar]
- 71.Dzionek A, Sohma Y, Nagafune J, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194: 1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farkas L, Beiske K, Lund-Johansen F, et al. Plasmacytoid dendritic cells (natural interferon-alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wollenberg A, Wagner M, Gunther S, et al. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol. 2002;119:1096–1102. [DOI] [PubMed] [Google Scholar]
- 74.Santoro A, Majorana A, Roversi L, et al. Recruitment of dendritic cells in oral lichen planus. J Pathol. 2005; 205:426–434. [DOI] [PubMed] [Google Scholar]
- 75.Nestle FO, Conrad C, Tun-Kyi A, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202:135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. [DOI] [PubMed] [Google Scholar]
- 77.de Vries HJ, Teunissen MB, Zorgdrager F, et al. Lichen planus remission is associated with a decrease of human herpes virus type 7 protein expression in plasmacytoid dendritic cells. Arch Dermatol Res. 2007;299:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Soumelis V, Scott I, Gheyas F, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. [DOI] [PubMed] [Google Scholar]
- 79.Groot F, van Capel TM, Kapsenberg ML, et al. Opposing roles of blood myeloid and plasmacytoid dendritic cells in HIV-1 infection of T cells: transmission facilitation versus replication inhibition. Blood. 2006;108: 1957–1964. [DOI] [PubMed] [Google Scholar]
- 80.Meyers JH, Justement JS, Hallahan CW, et al. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS ONE. 2007;2:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Albert ML, Decalf J, Pol S. Plasmacytoid dendritic cells move down on the list of suspects: in search of the immune pathogenesis of chronic hepatitis C. J Hepatol. 2008;49: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 82.Harris NL, Bhan AK. “Plasmacytoid T cells” in Castleman’s disease. Immunohistologic phenotype. Am J Surg Pathol. 1987;11:109–113. [DOI] [PubMed] [Google Scholar]
- 83.Facchetti F, de Wolf-Peeters C, van den Oord JJ, et al. Plasmacytoid monocytes (so-called plasmacytoid T-cells) in Kikuchi’s lymphadenitis. An immunohistologic study. Am J Clin Pathol. 1989;92:42–50. [DOI] [PubMed] [Google Scholar]
- 84.Vermi W, Bonecchi R, Facchetti F, et al. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol. 2003;200:255–268. [DOI] [PubMed] [Google Scholar]
- 85.Salio M, Cella M, Vermi W, et al. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur J Immunol. 2003;33:1052–1062. [DOI] [PubMed] [Google Scholar]
- 86.Pashenkov M, Goess G, Wagner C, et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol 2006;24:5716–5724. [DOI] [PubMed] [Google Scholar]
- 87.Hartmann E, Wollenberg B, Rothenfusser S, et al. Identification and functional analysis of tumor-infiltrating plasmacy-toid dendritic cells in head and neck cancer. Cancer Res. 2003;63:6478–6487. [PubMed] [Google Scholar]
- 88.Urosevic M, Dummer R, Conrad C, et al. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst. 2005;97:1143–1153. [DOI] [PubMed] [Google Scholar]
- 89.Treilleux I, Blay JY, Bendriss-Vermare N, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res. 2004;10:7466–7474. [DOI] [PubMed] [Google Scholar]
- 90.Zou W, Machelon V, Coulomb-L’Hermin A, et al. Stromal- derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. [DOI] [PubMed] [Google Scholar]
- 91.Chaperot L, Blum A, Manches O, et al. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plas- macytoid dendritic cells. J Immunol. 2006;176:248–255. [DOI] [PubMed] [Google Scholar]
- 92.Stary G, Bangert C, Tauber M, et al. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114: 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma MD, Baban B, Chandler P, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen W, Liang X, Peterson AJ, et al. The indoleamine 2,3- dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Facchetti F, De Wolf-Peeters C, van den Oord JJ, et al. Plasmacytoid monocytes (so-called plasmacytoid T cells) in Hodgkin’s disease. J Pathol. 1989;158:57–65. [DOI] [PubMed] [Google Scholar]
- 97.Karsunky H, Merad M, Mende I, et al. Developmental origin of interferon-alpha-producing dendritic cells from hematopoietic precursors. Exp Hematol. 2005;33:173–181. [DOI] [PubMed] [Google Scholar]
- 98.Yang GX, Lian ZX, Kikuchi K, et al. Plasmacytoid dendritic cells of different origins have distinct characteristics and function: studies of lymphoid progenitors versus myeloid progenitors. J Immunol. 2005;175:7281–7287. [DOI] [PubMed] [Google Scholar]
- 99.Ishikawa F, Niiro H, Iino T, et al. The developmental program of human dendritic cells is operated independently of conventional myeloid and lymphoid pathways. Blood. 2007;110:3591–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mongkonsritragoon W, Letendre L, Qian J, et al. Nodular lesions of monocytic component in myelodysplastic syndrome. Am J Clin Pathol. 1998;110:154–162. [DOI] [PubMed] [Google Scholar]
- 101.Chen YC, Chou JM, Ketterling RP, et al. Histologic and immunohistochemical study of bone marrow monocytic nodules in 21 cases with myelodysplasia. Am J Clin Pathol. 2003;120:874–881. [DOI] [PubMed] [Google Scholar]
- 102.Vermi W, Facchetti F, Rosati S, et al. Nodal and extranodal tumor-forming accumulation of plasmacytoid monocytes/interferon-producing cells associated with myeloid disorders. Am J Surg Pathol. 2004;28:585–595. [DOI] [PubMed] [Google Scholar]
- 103.Pileri SA, Ascani S, Cox MC, et al. Myeloid sarcoma: clinico- pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340–350. [DOI] [PubMed] [Google Scholar]
- 104.Müller-Hermelink HK, Steinmann G, Stein H, et al. Malignant lymphoma of plasmacytoid T cells. Morphologic and immunologic studies characterizing a special type of T- cell. Am J Surg Pathol. 1983;7:849–862. [PubMed] [Google Scholar]
- 105.Harris NL, Demirjian Z. Plasmacytoid T-zone cell proliferation in a patient with chronic myelomonocytic leukemia: histologic and immunohistologic characterization. Am J Surg Pathol. 1991;15:87–95. [DOI] [PubMed] [Google Scholar]
- 106.Ferry JA, Harris NL. Plasmacytoid monocytes? Am J Clin Pathol. 1999;111:569. [DOI] [PubMed] [Google Scholar]
- 107.Orazi A, Chiu R, O’Malley DP, et al. Chronic myelomono- cytic leukemia: the role of bone marrow biopsy immunohis- tology. Mod Pathol. 2006;19:1536–1545. [DOI] [PubMed] [Google Scholar]
- 108.Facchetti F, De Wolf-Peeters C, Kennes C, et al. Leukemia- associated lymph node infiltrates of plasmacytoid monocytes (so-called plasmacytoid T-cells). Evidence for two distinct histological and immunophenotypical patterns. Am J Surg Pathol. 1990;14:101–112. [DOI] [PubMed] [Google Scholar]
- 109.Baddoura FK, Hanson C, Chan WC. Plasmacytoid monocyte proliferation associated with myeloproliferative disorders. Cancer. 1992;69:1457–1467. [DOI] [PubMed] [Google Scholar]
- 110.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4th ed Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 111.Herling M, Jones D. CD4+/CD56+ hematodermic tumor: the features of an evolving entity and its relationship to dendritic cells. Am J Clin Pathol. 2007;127:687–700. [DOI] [PubMed] [Google Scholar]
- 112.Petrella T, Dalac S, Maynadie M, et al. CD4+ CD56+ cutaneous neoplasms: a distinct hematological entity? Groupe Francais d’Etude des Lymphomes Cutanes (GFELC). Am J Surg Pathol. 1999;23:137–146. [DOI] [PubMed] [Google Scholar]
- 113.Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. [DOI] [PubMed] [Google Scholar]
- 114.Chaperot L, Bendriss N, Manches O, et al. Identification of a leukemic counterpart of the plasmacytoid dendritic cells. Blood. 2001;97:3210–3217. [DOI] [PubMed] [Google Scholar]
- 115.Chaperot L, Perrot I, Jacob MC, et al. Leukemic plasmacy-toid dendritic cells share phenotypic and functional features with their normal counterparts. Eur J Immunol. 2004;34: 418–426. [DOI] [PubMed] [Google Scholar]
- 116.Maeda T, Murata K, Fukushima T, et al. A novel plasmacytoid dendritic cell line, CAL-1, established from a patient with blastic natural killer cell lymphoma. Int J Hematol. 2005;81:148–154. [DOI] [PubMed] [Google Scholar]
- 117.Urosevic M, Conrad C, Kamarashev J, et al. CD4+CD56 + hematodermic neoplasms bear a plasmacytoid dendritic cell phenotype. Hum Pathol. 2005;36:1020–1024. [DOI] [PubMed] [Google Scholar]
- 118.Pilichowska ME, Fleming MD, Pinkus JL, et al. CD4 + / CD56+ hematodermic neoplasm (“blastic natural killer cell lymphoma”): neoplastic cells express the immature dendritic cell marker BDCA-2 and produce interferon. Am J Clin Pathol. 2007;128:445–453. [DOI] [PubMed] [Google Scholar]
- 119.Petrella T, Meijer CJ, Dalac S, et al. TCL1 and CLA expression in agranular CD4/CD56 hematodermic neoplasms (blastic NK-cell lymphomas) and leukemia cutis. Am J Clin Pathol. 2004;122:307–313. [DOI] [PubMed] [Google Scholar]
- 120.Hunger RE, Yawalkar N, Braathen LR, et al. The HECA-452 epitope is highly expressed on lymph cells derived from human skin. Br J Dermatol. 1999;141:565–569. [DOI] [PubMed] [Google Scholar]
- 121.Bendriss-Vermare N, Chaperot L, Peoc’h M, et al. In situ leukemic plasmacytoid dendritic cells pattern of chemokine receptors expression and in vitro migratory response. Leukemia. 2004;18:1491–1498. [DOI] [PubMed] [Google Scholar]
- 122.Gopcsa L, Banyai A, Jakab K, et al. Extensive flow cytometric characterization of plasmacytoid dendritic cell leukemia cells. Eur J Haematol. 2005;75:346–351. [DOI] [PubMed] [Google Scholar]
- 123.Dijkman R, van Doorn R, Szuhai K, et al. Gene-expression profiling and array-based CGH classify CD4+CD56+ hematodermic neoplasm and cutaneous myelomonocytic leukemia as distinct disease entities. Blood. 2007;109: 1720–1727. [DOI] [PubMed] [Google Scholar]
- 124.Feuillard J, Jacob MC, Valensi F, et al. Clinical and biologic features of CD4( + )CD56( + ) malignancies. Blood. 2002;99: 1556–1563. [DOI] [PubMed] [Google Scholar]
- 125.Jacob MC, Chaperot L, Mossuz P, et al. CD4+ CD56+ lineage negative malignancies: a new entity developed from malignant early plasmacytoid dendritic cells. Haematologica. 2003;88:941–955. [PubMed] [Google Scholar]
- 126.Petrella T, Bagot M, Willemze R, et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123:662–675. [PubMed] [Google Scholar]
- 127.Reichard KK, Burks EJ, Foucar MK, et al. CD4( + ) CD56( + ) lineage-negative malignancies are rare tumors of plasmacytoid dendritic cells. Am J Surg Pathol. 2005;29: 1274–1283. [DOI] [PubMed] [Google Scholar]
- 128.Khoury JD, Medeiros LJ, Manning JT, et al. CD56( + ) TdT( + ) blastic natural killer cell tumor of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer. 2002;94:2401–2408. [DOI] [PubMed] [Google Scholar]
- 129.Ng AP, Lade S, Rutherford T, et al. Primary cutaneous CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma): a report of five cases. Haematologica. 2006; 91:143–144. [PubMed] [Google Scholar]
- 130.Hu SC, Tsai KB, Chen GS, et al. Infantile CD4+/CD56+ hematodermic neoplasm. Haematologica. 2007;92:e91–e93. [DOI] [PubMed] [Google Scholar]
- 131.Rossi JG, Felice MS, Bernasconi AR, et al. Acute leukemia of dendritic cell lineage in childhood: incidence, biological characteristics and outcome. Leuk Lymphoma. 2006;47:715–725. [DOI] [PubMed] [Google Scholar]
- 132.Hamadani M, Magro CM, Porcu P. CD4+ CD56+ haematodermic tumor (plasmacytoid dendritic cell neoplasm). Br J Haematol. 2008;140:122. [DOI] [PubMed] [Google Scholar]
- 133.Jardin F, Callanan M, Penther D, et al. Recurrent genomic aberrations combined with deletions of various tumor suppressor genes may deregulate the G1/S transition in CD4+CD56+ haematodermic neoplasms and contribute to the aggressiveness of the disease. Leukemia. 2009;23:698–707. [DOI] [PubMed] [Google Scholar]
- 134.Garnache-Ottou F, Chaperot L, Biichle S, et al. Expression of the myeloid-associated marker CD33 is not an exclusive factor for leukemic plasmacytoid dendritic cells. Blood. 2005;105:1256–1264. [DOI] [PubMed] [Google Scholar]
- 135.Garnache-Ottou F, Feuillard J, Ferrand C, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukemia. Br J Haematol. 2009;145:624–636. [DOI] [PubMed] [Google Scholar]
- 136.Narita M, Kuroha T, Watanabe N, et al. Plasmacytoid dendritic cell leukemia with potent antigen-presenting ability. Acta Haematol. 2008;120:91–99. [DOI] [PubMed] [Google Scholar]
- 137.Pina-Oviedo S, Herrera-Medina H, Coronado H, et al. CD4+/CD56+ hematodermic neoplasm: presentation of 2 cases and review of the concept of an uncommon tumor originated in plasmacytoid dendritic cells expressing CD123 (IL-3 receptor alpha). Appl Immunohistochem Mol Morphol. 2007;15:481–486. [DOI] [PubMed] [Google Scholar]
- 138.Bilbao EA, Chirife AM, Florio D, et al. Hematodermic CD4+ CD56 + neoplasm in childhood. Medicina (B Aires). 2008;68:147–150. [PubMed] [Google Scholar]
- 139.Kazakov DV, Mentzel T, Burg G, et al. Blastic natural killercell lymphoma of the skin associated with myelodysplastic syndrome or myelogenous leukaemia: a coincidence or more? Br J Dermatol. 2003;149:869–876. [DOI] [PubMed] [Google Scholar]
- 140.Ascani S, Massone C, Ferrara G, et al. CD4-negative variant of CD4+/CD56+ hematodermic neoplasm: description of three cases. J Cutan Pathol. 2008;35:911–915. [DOI] [PubMed] [Google Scholar]
- 141.Karube K, Ohshima K, Tsuchiya T, et al. Non-B, non- T neoplasms with lymphoblast morphology: further clarification and classification. Am J Surg Pathol. 2003;27:1366–1374. [DOI] [PubMed] [Google Scholar]
- 142.Momoi A, Toba K, Kawai K, et al. Cutaneous lymphoblastic lymphoma of putative plasmacytoid dendritic cell-precursor origin: two cases. Leuk Res. 2002;26:693–698. [DOI] [PubMed] [Google Scholar]
- 143.Petrella T, Teitell MA, Spiekermann C, et al. A CD56- negative case of blastic natural killer-cell lymphoma (agranular CD4+/CD56+ haematodermic neoplasm). Br J Dermatol. 2004;150:174–176. [DOI] [PubMed] [Google Scholar]
- 144.Kaiser U, Auerbach B, Oldenburg M. The neural cell adhesion molecule NCAM in multiple myeloma. Leuk Lymphoma. 1996;20:389–395. [DOI] [PubMed] [Google Scholar]
- 145.Toba K, Koike T, Shibata A, et al. Novel technique for the direct flow cytofluorometric analysis of human basophils in unseparated blood and bone marrow, and the characterization of phenotype and peroxidase of human basophils. Cytometry. 1999;35:249–259. [PubMed] [Google Scholar]
- 146.Yokohama A, Tsukamoto N, Hatsumi N, et al. Acute basophilic leukemia lacking basophil-specific antigens: the importance of cytokine receptor expression in differential diagnosis. Int J Hematol. 2002;75:309–313. [DOI] [PubMed] [Google Scholar]
- 147.Jaye DL, Geigerman CM, Herling M, et al. Expression of the plasmacytoid dendritic cell marker BDCA-2 supports a spectrum of maturation among CD4+ CD56+ hematodermic neoplasms. Mod Pathol. 2006;19:1555–1562. [DOI] [PubMed] [Google Scholar]
- 148.Leroux D, Mugneret F, Callanan M, et al. CD4( + ), CD56(+) DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: a study of 21 cases by the Groupe Francais de Cytogenetique Hematologique. Blood. 2002;99:4154–4159. [DOI] [PubMed] [Google Scholar]
- 149.Reimer P, Rudiger T, Kraemer D, et al. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transplant. 2003;32:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Falcao RP, Garcia AB, Marques MG, et al. Blastic CD4 NK cell leukemia/lymphoma: a distinct clinical entity. Leuk Res. 2002;26:803–807. [DOI] [PubMed] [Google Scholar]
- 151.Bekkenk MW, Jansen PM, Meijer CJ, et al. CD56+ hematological neoplasms presenting in the skin: a retrospective analysis of 23 new cases and 130 cases from the literature. Ann Oncol. 2004;15:1097–1108. [DOI] [PubMed] [Google Scholar]


