Abstract
Objective
To report 3 patients with relapsing-remitting multiple sclerosis (RRMS) showing vitiligo after treatment with alemtuzumab.
Methods
Retrospective case series including flow cytometric analyses and T-cell receptor (TCR) sequencing of peripheral blood mononuclear cells.
Results
We describe 3 cases of alemtuzumab-treated patients with RRMS developing vitiligo 52, 18, and 14 months after alemtuzumab initiation. Histopathology shows loss of epidermal pigmentation with absence of melanocytes and interface dermatitis with CD8+ T-cell infiltration. Also compatible with pathophysiologic concepts of vitiligo, peripheral blood mononuclear cells of one patient showed high proportions of CD8+ T cells with an activated (human leukocyte antigen–DR+), memory (CD45RO+), and type 1 cytokine (interferon-γ + tumor necrosis factor–α) phenotype at vitiligo onset compared to a control cohort of alemtuzumab-treated patients with RRMS (n = 30). Of note, analysis of CD8 TCR repertoire in this patient revealed a highly increased clonality and reduced repertoire diversity compared to healthy controls and treatment-naive patients with RRMS. We observed a predominance of single clones at baseline in this patient and alemtuzumab treatment did not substantially affect the proportions of most abundant clones over time.
Conclusion
The 3 cases represent a detailed description of vitiligo as a T-cell-mediated secondary autoimmune disease following alemtuzumab treatment. The prevailing concept of unleashed B-cell responses might therefore not cover all facets of alemtuzumab-related secondary autoimmunity. Mechanistic studies, especially on TCR repertoire, might help clarify the underlying mechanisms.
Alemtuzumab is an anti-CD52 antibody leading to rapid depletion followed by differential repopulation of B and T lymphocytes approved for the treatment of active relapsing-remitting multiple sclerosis (RRMS). Secondary autoimmunity following alemtuzumab treatment represents the most relevant risk. Seven-year data from the Cambridge cohort demonstrated 41.0% of patients develop autoimmune thyroid disorders and 3.5% immune thrombocytopenia (ITP); moreover, cases of nephropathies and other autoimmune disorders have been described.1,2 Here, we present a retrospective case series of 3 patients developing vitiligo after alemtuzumab treatment.
Methods
Patients and biomaterials
Patients were recruited at the Department of Neurology of the University Hospitals Münster and Essen, Germany. Thirty patients with RRMS prior to and under alemtuzumab (Lemtrada®, Genzyme) treatment (mean number of relapses was 2.2 ± 1.1 and mean Expanded Disability Status Scale [EDSS] progression was 1.2 ± 1.1 2 years prior to alemtuzumab initiation), 11 sex- and age-matched, treatment-naive patients with RRMS (mean number of relapses was 1.8 ± 0.7 and mean EDSS progression was 1.1 ± 0.7 in the last 2 years), and 10 sex- and age-matched healthy controls were included in the current study. Alemtuzumab patients received pretreatments including azathioprine, β-interferons (IFNs), glatiramer acetate, teriflunomide, fingolimod, natalizumab, mitoxantrone, and siponimod (within a clinical trial). Peripheral blood mononuclear cells (PBMCs) were isolated from ethylenediaminetetraacetic acid blood drawn from alemtuzumab-treated patients at baseline, 6, 12, and 18 months after standard treatment regimen and cryopreserved as previously described.3
Standard protocol approvals, registrations, and patient consents
This study was performed according to the Declaration of Helsinki and approved by the local ethics committees (Münster: 2014-398-f-S, Essen: 16-7290-BO). All patients gave written informed consent.
Flow cytometry
Flow cytometry of thawed PBMCs was performed as previously described3 using fluorochrome-conjugated antibodies for CD3, CD4, CD8, CD14, CD19, CD45RO, CD56, human leukocyte antigen (HLA)–DR, IFN-γ, tumor necrosis factor–α (TNF-α), and perforin (all purchased from BioLegend [San Diego, CA]). Intracellular staining for cytokines (IFN-γ and TNF-α) and perforin was performed using the intracellular staining kit (eBioscience [San Diego, CA]) following the manufacturer's instructions. Samples were acquired on a 10-color Navios (Beckman Coulter [Sharon Hill, PA]) or FACSCanto II (BD Biosciences [East Rutherford, NJ]) flow cytometer and analyzed by FlowJo v10 and Kaluza 1.3.
T-cell receptor sequencing
T-cell receptor (TCR) sequencing and analysis was performed as previously described.4 TCRβ chain sequencing of magnetic-activated cell sorted CD8+ T cells (CD8+ T Cell Isolation Kit, human; Miltenyi Biotec [Bergisch Gladbach, Germany]) was performed at Adaptive Biotechnologies (Seattle, WA) using the ImmunoSEQ platform with primers specific for all 54 known expressed Vβ and all 13 Jβ regions.
Data availability statement
Any data not published within the article will be shared anonymized upon request from any qualified investigator.
Results
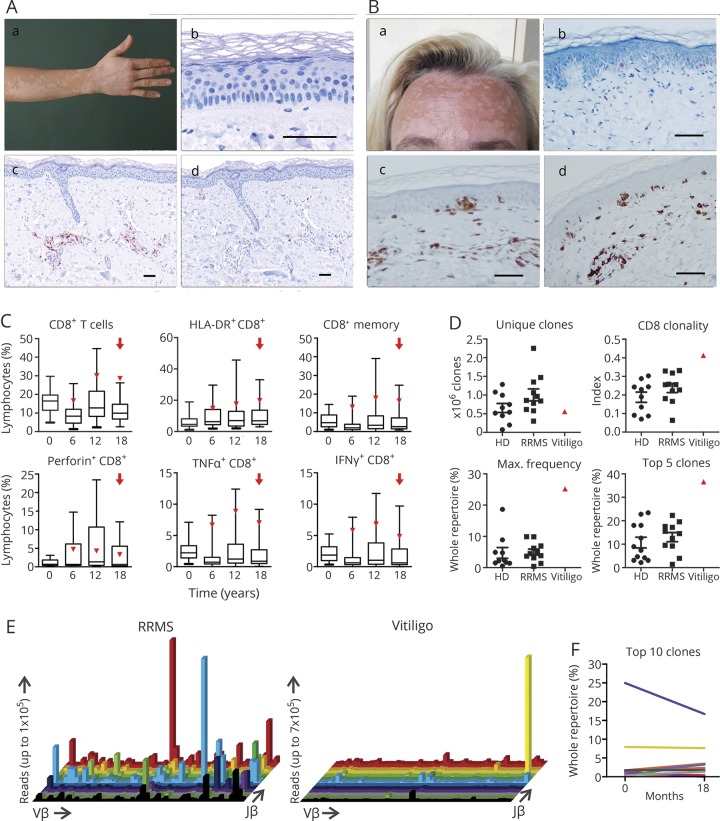
In September 2016, a 31-year-old woman presented with depigmentation of her skin following therapy with alemtuzumab. She had been diagnosed with RRMS in 2004 with typical findings on brain MRI and positive oligoclonal bands in the CSF. Despite receiving different immunomodulatory treatments including IFN-β-1a and natalizumab, she demonstrated ongoing disease activity on MRI and several relapses with an EDSS progression to 3.5. In terms of compassionate use, a first course of alemtuzumab was given in June 2012. In March 2013, the patient presented with a worsening of preexisting right-sided sensorimotor hemiparesis responsive to IV methylprednisolone. Since alemtuzumab was retained from the market at this time, she received 2 courses of rituximab 1,000 mg in August 2013 and April 2014. However, she experienced 3 further relapses within 1 year and was therefore switched back to alemtuzumab, receiving the second course in October 2015. Since then, clinical and MRI disease course remained stable. Skin depigmentation started in September 2016 with circumscribed, symmetrical white macules and patches typical for vitiligo (figure, A), which slowly progressed, now involving 30% of the body surface. Skin biopsy confirmed diagnosis showing complete loss of epidermal pigmentation with absence of melanocytes at the basal layer and a subtle perivascular dermatitis consisting of CD3+ and CD8+ T cells (figure, A), the latter expressing T-cell-restricted intracellular antigen-1, while CD20+ B cells were almost absent (data not shown).
Figure. CD8+ T-cell-driven pathology in alemtuzumab-treated patients with relapsing-remitting multiple sclerosis (RRMS) developing vitiligo.
(A) Patient 1: circumscribed, symmetrical white macules and patches typical for vitiligo (A.a). Exemplary skin biopsy from the same patient: melanocyte staining (A.b), panMel antibody cocktail directed to tyrosine, melanA, HMB45. Positive stainings for CD3 (A.c) and CD8 (A.d). (B) Patient 3: typical skin lesions for vitiligo (B.a). Exemplary skin biopsy from the same patient: melanocyte staining (B.b). Positive stainings for CD3 (B.c) and CD8 (B.d). Scale bars represent 100 μM in A and B. (C) Proportions of CD8+ T cells and CD8+ T-cell subsets (defined by the displayed markers) within peripheral blood mononuclear cells (PBMC) isolated from alemtuzumab-treated patients with RRMS (n = 30 for baseline, n = 29 at 6 months, n = 25 for 12 months, n = 17 for 18 months) and patient 2 (red triangle) were determined by flow cytometry. For the analysis of cytokine production, cells were treated with leukocyte activation cocktail (BD) for 4 hours. The red arrow indicates the timepoint of vitiligo onset. Boxplots show median, 25% and 75% percentile, whiskers represent 5% and 95% percentile. (D) Unique clones, clonality, as well as maximum frequency of distinct clones and top 5 clones in the CD8 T-cell receptor (TCR) repertoire was analyzed by deep sequencing. Prior to analysis, CD8+ T cells were magnetic-activated cell sorted from PBMC of healthy controls (ctrl, n = 10), treatment-naive patients with RRMS (MS naive, n = 11), and patient 2 at baseline (vitiligo). RNA of at least 2 × 106 CD8+ T cells from patient 2 at baseline was purified and cDNA was sequenced by Adaptive Biotechnologies, resulting in a range from 1.5 to 2.5 × 106 total reads in each analyzed sample. (E) 3D histograms show expansion of single clones in patient 2 in comparison to a representative treatment-naive patient with RRMS (i.e., sex- and age-matched and exhibiting a similar mean number of relapses and mean EDSS progression in the last 2 years as compared to patient 2). The x axis lists all Vβ genes, the z axis the Jβ genes, and the column height indicates the total reads of this specific V/J combination. (F) Clone tracking of the top 10 expanded clones (highest number of total reads) of patient 2 before and 18 months after alemtuzumab treatment. The proportion of clones in the whole TCR repertoire is depicted. HLA = human leukocyte antigen; IFN-γ = interferon-γ; TNF-α = tumor necrosis factor–α.
A second patient, a 34-year-old man, was referred to our clinic with skin lesions suspicious for vitiligo in February 2017 after having received courses of alemtuzumab in August 2015 and 2016. He was diagnosed with MS in 2001, fulfilling McDonald criteria with typical findings on MRI and in the CSF. Several immunomodulatory treatments including IFN-β-1a, natalizumab, fingolimod, and dimethyl fumarate were ineffective. Since alemtuzumab initiation the patient has demonstrated clinical and radiologic stable disease. However, in February 2017, he reported patchy, well-defined depigmentation of the skin progressively affecting the whole integument in September 2017. Dermatologic examination established the diagnosis of vitiligo due to characteristic clinical presentation and disease course; the patient refused a skin biopsy.
A third patient, a 42-year-old woman diagnosed with MS in August 2015, was treated with alemtuzumab in the University Hospital Essen in February 2017 and 2018 due to ongoing disease activity with dimethyl fumarate and fingolimod. Fourteen months after treatment initiation, she reported vitiligo-characteristic depigmentation of the skin starting from the extremities and then affecting the whole body including the face (figure, B). Skin biopsy confirmed the diagnosis, showing interface dermatitis with predominant CD3+CD8+ lymphocyte infiltration along the basal lamina and missing melanocytes in central parts of the biopsy specimen (figure, B).
The pathophysiologic mechanisms underlying autoimmunity secondary to alemtuzumab remain insufficiently understood. However, currently it is assumed that B cells are the central drivers of these autoimmune conditions due to predominance of antibody-mediated autoimmune disorders.5,6 In contrast, vitiligo is a T-cell-driven autoimmune disorder, where autoreactive, melanocyte-specific CD8+ T cells are recruited to the skin and target melanocytes.7
As patient 2 is part of a prospective biobanking cohort, we were able to analyze PBMCs for vitiligo-related immune patterns. Interestingly, he showed high proportions of CD8+ T cells with an activated (HLA-DR+), memory (CD45RO+), and type 1 cytokine (IFN-γ+ and TNF-α+) phenotype at vitiligo onset compared to a control cohort of alemtuzumab-treated patients with RRMS (figure, C). Analysis of CD8 TCR repertoire in patient 2 at baseline revealed a highly increased clonality and reduced repertoire diversity (figure, D) as compared to healthy controls and treatment-naive patients with RRMS. Furthermore, a predominance of single clones could be observed at baseline in this patient (figure, E), illustrated in a 3D histogram, which provides an overview of the clonal distribution within the TCR repertoire. Of note, alemtuzumab treatment did not substantially affect the proportions of the top 10 clones over time (figure, F).
Discussion
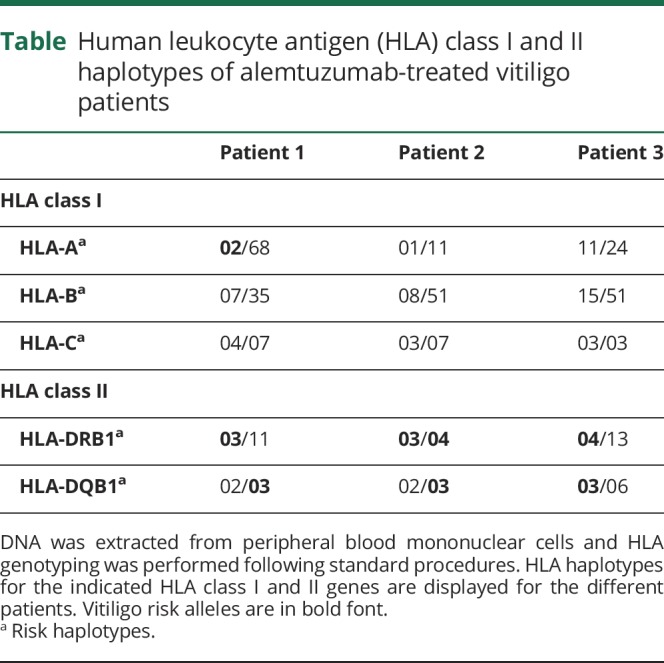
Previous mechanistic studies support a pivotal role of interleukin (IL)–21-driven homeostatic proliferation of chronically activated, oligoclonal, effector memory T cells in autoimmunity following alemtuzumab.8 Of note, IL-21 has also been implicated in the pathogenesis of vitiligo, therefore it can be speculated that increased IL-21 levels might contribute to vitiligo development in these patients.9 Shared HLA haplotypes between MS and vitiligo conferring increased disease risk such as HLA-DRB1 or HLA-DQB1 might further facilitate coincidence of both diseases in susceptible individuals.7,10 Interestingly, all 3 patients expressed at least 2 vitiligo risk alleles (HLA-A*02, HLA-DRB1*03, HLA-DRB1*04, and HLA-DQB1*03) (table).
Table.
Human leukocyte antigen (HLA) class I and II haplotypes of alemtuzumab-treated vitiligo patients
The 3 cases represent a detailed description of vitiligo as an autoimmune complication after alemtuzumab; however, they teach something more important: T cells also can be drivers of secondary autoimmunity after alemtuzumab. Hence, the prevailing concept of unleashed B-cell autoimmunity due to faster reconstitution kinetics might not cover all facets of secondary autoimmunity in this context. More mechanistic studies, especially on TCR repertoire, as well as comprehensive monitoring for various autoimmune conditions are warranted to improve our knowledge of autoimmunity in the context of alemtuzumab treatment in MS.
Acknowledgment
The authors thank Julia Sundermeier and Lena Schünemann for technical assistance.
Glossary
- EDSS
Expanded Disability Status Scale
- HLA
human leukocyte antigen
- IFN-γ
interferon-γ
- IL
interleukin
- PBMC
peripheral blood mononuclear cell
- RRMS
relapsing-remitting multiple sclerosis
- TCR
T-cell receptor
- TNF-α
tumor necrosis factor–α
Author contributions
T. Ruck: design and concept of study, analysis and interpretation of data, writing of the manuscript. S. Pfeuffer: analysis and interpretation of data, critical revision of manuscript for important intellectual content. A. Schulte-Mecklenbeck: acquisition of data, critical revision of manuscript for intellectual important content. C.C. Gross: analysis and interpretation of data, critical revision of manuscript for intellectual important content. M. Lindner: acquisition, analysis, and interpretation of data, critical revision of manuscript for intellectual important content. J. Ehrchen: acquisition, analysis, and interpretation of data, critical revision of manuscript for intellectual important content. D. Metze, W. Sondermann: acquisition and analysis of data, critical revision of manuscript for intellectual important content. R. Pul, C. Kleinschnitz: acquisition and analysis of data, critical revision of manuscript for intellectual important content. H. Wiendl: critical revision of manuscript for intellectual important content. S.G. Meuth, L. Klotz: study concept and design, study supervision and critical revision of manuscript for intellectual content.
Study funding
Supported by the German Ministry of Education, Science, Research and Technology (01GI1603D, to T.R. and S.G.M.; FKZ01FI1603A to H.W., L.K., and C.C.G.), the Collaborative Research Centre CRC TR128 “Initiating/effector vs regulatory mechanisms in multiple sclerosis: progress towards tackling the disease” (Project Z2 to H.W.), and Genzyme Therapeutics Ltd., UK (Alemtuzumab in autoimmune inflammatory neurodegeneration: mechanisms of action and neuroprotective potential, 2014-000709-10, ALAIN to T.R., H.W., and S.G.M.).
Disclosure
T. Ruck received travel expenses and financial research support from Genzyme and Novartis and received honoraria for lecturing from Roche, Merck, Genzyme, Biogen, and Teva. S. Pfeuffer received travel reimbursements and lecturing honoraria from Sanofi Genzyme, Biogen, Merck, and Mylan. He received research support from Diamed. A. Schulte-Mecklenbeck reports no disclosures relevant to the manuscript. C. Gross received speaker honoraria and travel expenses for attending meetings from Biogen, Euroimmun, Genzyme, Novartis Pharma GmbH, and Bayer Health Care. Her work is funded by the German Ministry for Education and Research (BMBF; 01GI1603A), the German Research Foundation (DFG; GR3946/3-1 and SFB/Transregio 128 A09), and the European Union (Horizon2020, RESTORE). M. Lindner and D. Metze report no disclosures relevant to the manuscript. J. Ehrchen received research grants from Pfizer and Actelion, was investigator for Boehringer Ingelheim, and received speaker honoraria from Actelion, Pfizer, and Chugai. W. Sondermann received travel expenses for attending meetings from MSD, Bristol-Myers Squibb, Novartis, Celgene, LEO Pharma, Janssen, AbbVie, and Lilly, and received speaker honoraria from Novartis, Roche, Almirall, AbbVie, LEO Pharma, and Lilly. R. Pul received travel expenses and financial research support from Merck, Genzyme, Biogen, and Novartis. He received honoraria for lecturing from Merck, Genzyme, Roche, Biogen, Teva, and Mylan. C. Kleinschnitz reports no disclosures relevant to the manuscript. H. Wiendl receives honoraria for acting as a member of Scientific Advisory Boards and as consultant for Biogen, Evgen, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG, and Sanofi-Genzyme, as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Sanofi-Genzyme, TEVA, and WebMD Global. H.W. is acting as a paid consultant for AbbVie, Actelion, Biogen, IGES, Novartis, Roche, Sanofi-Genzyme, and the Swiss Multiple Sclerosis Society. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgesellschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Münster and RE Children's Foundation, Biogen GmbH, GlaxoSmithKline GmbH, Roche Pharma AG, and Sanofi-Genzyme. S. Meuth has received honoraria for lecturing, travel expenses for attending meetings, and financial research support from Almirall, Bayer Health Care, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, and Teva. L. Klotz received compensation for serving on Scientific Advisory Boards for Sanofi Genzyme, Roche, and Novartis. She received speaker honoraria and travel support from Novartis, Merck Serono, Biogen, Sanofi Genzyme, and Merck. She receives research support from Novartis and Biogen. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology May 9, 2018. Accepted in final form August 28, 2018.
References
- 1.Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry 2015;86:208–215. [DOI] [PubMed] [Google Scholar]
- 2.Ruck T, Bittner S, Wiendl H, Meuth SG. Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Int J Mol Sci 2015;16:16414–16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gross CC, Ahmetspahic D, Ruck T, et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016;3:e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muraro PA, Robins H, Malhotra S, et al. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J Clin Invest 2014;124:1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willis M, Harding K, Pickersgill T, et al. Alemtuzumab for multiple sclerosis: long term follow-up in a multi-centre cohort. Mult Scler J 2016;22:1215–1223. [DOI] [PubMed] [Google Scholar]
- 6.Wiendl H, Kieseier B. Multiple sclerosis: reprogramming the immune repertoire with alemtuzumab in MS. Nat Rev Neurol 2013;9:125–126. [DOI] [PubMed] [Google Scholar]
- 7.Strassner JP, Harris JE. Understanding mechanisms of autoimmunity through translational research in vitiligo. Curr Opin Immunol 2016;43:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones JL, Phuah CL, Cox AL, et al. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest 2009;119:2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, Shi YL, Li K, et al. Increased circulating Th17 cells and elevated serum levels of TGF-beta and IL-21 are correlated with human non-segmental vitiligo development. Pigment Cell Melanoma Res 2015;28:324–329. [DOI] [PubMed] [Google Scholar]
- 10.Hollenbach JA, Oksenberg JR. The immunogenetics of multiple sclerosis: a comprehensive review. J Autoimmun 2015;64:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within the article will be shared anonymized upon request from any qualified investigator.