Abstract
Objective
Migraine with visual aura is associated with cardioembolic stroke risk. The aim of this study was to test association between migraine with visual aura and atrial fibrillation (AF), in the Atherosclerosis Risk in Communities study.
Methods
In the Atherosclerosis Risk in Communities study, a longitudinal, community-based cohort study, participants were interviewed for migraine history in 1993–1995 and were followed for incident AF through 2013. AF was adjudicated using ECGs, discharge codes, and death certificates. Multivariable Cox proportional hazards models were used to study the relation between migraine and its subtypes with incident AF, compared with controls without headaches. Mediation analysis was conducted to test whether AF was a mediator of migraine with visual aura-associated stroke risk.
Results
Of 11,939 participants assessed for headache and without prior AF or stroke, 426 reported migraines with visual aura, 1,090 migraine without visual aura, 1,018 nonmigraine headache, and 9,405 no headache. Over a 20-year follow-up period, incident AF was noted in 232 (15%) of 1,516 with migraine and 1,623 (17%) of 9,405 without headache. After adjustment for multiple confounders, migraine with visual aura was associated with increased risk of AF compared to no headache (hazard ratio 1.30, 95% confidence interval 1.03–1.62) as well as when compared to migraine without visual aura (hazard ratio 1.39, 95% confidence interval 1.05–1.83). The data suggest that AF may be a potential mediator of migraine with visual aura–stroke risk.
Conclusions
Migraine with aura was associated with increased risk of incident AF. This may potentially lead to ischemic strokes.
Epidemiologic studies show that migraine with aura is associated with increased risk of stroke based on meta-analysis of diverse cohorts of patients (risk ratio 2.14, 95% confidence interval [CI] 1.33–3.43).1 Migraine with visual aura is associated with an increased risk of stroke or TIA symptoms and ischemic stroke events in the Atherosclerosis Risk in Community (ARIC) study cohort.2 We recently reported that migraine with visual aura (not migraines without visual aura or migraine overall) was independently associated with ischemic stroke, when compared to those without history of headache.3 The results are concordant with the recent meta-analysis.1 Specifically, migraine with visual aura is independently associated with increased risk of cardioembolic stroke compared with migraine without aura.3 A Danish nationwide, population-based cohort study has reported association between migraine, stroke, myocardial infarction, atrial fibrillation (AF), and atrial flutter.4 They noted that the associations were stronger in patients with aura than in those without aura. Since AF is a common source of cardioembolic stroke, the question that begs to be answered is whether the association between migraine with visual aura and cardioembolic stroke may be explained by a higher rate of AF in this subgroup of the cohort.5 Therefore, we evaluated the potential association between migraine with visual aura and incident AF in a cohort based in the United States. If migraine with aura is associated with AF, the AF may lead to thromboembolism into the cerebral blood vessels leading to ischemic strokes.
Methods
Study cohort
In 1987, the ARIC study established its cohort to analyze atherosclerosis causes and clinical outcomes.6 A total of 15,792 participants aged 45 to 64 years were enrolled. Participants came from 4 different US communities (Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN; and Washington County, MD). The first visit occurred from 1987 to 1989 to obtain participant baseline information. Follow-up visits took place on the following timeline: visit 2 (1990–1992), visit 3 (1993–1995), visit 4 (1996–1998), and visit 5 (2011–2013). Vascular risk factors were assessed and hospital medical records were acquired, specifically if hospitalizations and new diagnoses occurred. In addition, the study team contacted the ARIC study participants on an annual basis to assess participant well-being and any adverse events, including stroke and AF.6 We have analyzed outcomes at follow-ups through the end of 2013.
We included all participants who completed visit 3 clinic procedures (1993–1995) in the ARIC study (n = 12,882). We excluded participants with known history of AF or those with inadequate AF information (n = 82), missing AF information (n = 158), those who reported AF prior to visit 3 (n = 579), those with prior ischemic stroke (n = 87), as well as any participants with missing headache information (n = 37).3 After excluding these, a total of 11,939 participants met our inclusion criteria and their data were included in our analysis.
Standard protocol approvals, registrations, and patient consents
The institutional review boards of all participating institutions approved the study, and all participants provided written informed consent.
Assessment of migraine
Trained interviewers administered a headache questionnaire to participants during visit 3. Modified ICHD-37 diagnostic criteria were applied to data collected in this questionnaire to differentiate migraine with visual aura and migraine without visual aura. Having a migraine was defined by participant responses endorsing headaches lasting at least 4 hours or longer in duration. Headaches were characterized with symptoms of throbbing or pulsating pain, and were mostly on one side. In addition to those symptoms, headaches were also accompanied by photophobia and phonophobia or nausea or vomiting. Headaches were differentiated into 2 categories: migraine with visual aura and migraine without visual aura. Participants with migraine with visual aura reported seeing spots, jagged lines, or heat waves in one or both eyes before onset of headache. Other types of auras (speech/language, motor sensory, and brainstem) that are less common were not assessed. Headaches lasting more than 4 hours that did not match requirements for either category were classified as nonmigraine headaches. Lastly, participants without a history of experiencing headaches more than 4 hours were categorized as the no-headache group. Those with nonmigraine headaches were not included in the analysis. Comparing this method of applying the modified ICHD-3 criteria to the headache data in ARIC to when the participant reported that a physician had made a migraine diagnosis (visit 3), we determined that the former had a positive predictive value of 95%.
AF ascertainment
Diagnoses of AF in ARIC study participants were sourced from study-performed ECGs, hospital discharge codes, and death certificates.8 ECGs were performed with participants in a supine position using standard, 10-second, 12-lead ECG devices at the baseline visit as well as at each follow-up examination. Recordings were performed using MAC PC Personal Cardiographs (Marquette Electronics, Inc.) and transferred for analysis to the ARIC ECG Reading Center (Epidemiological Cardiology Research Center, Wake Forest School of Medicine, Winston-Salem, NC). ECGs classified as AF were reviewed and confirmed by a cardiologist at the center. In addition, the ICD-9-CM code 427.31 or 427.32 was utilized in establishing incident AF in participants with hospitalizations and death certificates. Any incident AF cases that resulted as an outcome of cardiothoracic surgery were not categorized as participant having AF. These discharge codes have been shown to have a positive predictive value of 89% in ARIC for the diagnosis of AF.9 The above methodology has been validated within the ARIC cohort using chart abstraction, with a sensitivity of 80% to 85% and specificity of 97% to 99%.8
Stroke subtypes
Adjudication of stroke and stroke subtype has been previously described.3 Briefly, neuroimaging (CT or MRI) confirmation of ischemic stroke diagnosis was obtained from chart review. Stroke diagnosis was based on computer-derived diagnosis and physician medical record review, with differences adjudicated by a second physician reviewer. Ischemic strokes were further classified according to pathogenic subtype as thrombotic brain infarction, lacunar infarction, or cardioembolic stroke, according to criteria adopted from the National Survey of Stroke subtype classification. Specific cardiac sources of embolism that were assessed included valvular heart disease (including prosthetic heart valve), AF or atrial flutter, myocardial infarction, cardiac or arterial operation or procedure, cardiac myxoma, and bacterial endocarditis.
Covariates of interest
The following other variables were evaluated during our study: age, sex, race, and vascular risk factors including hypertension, body mass index (BMI), waist-to-hip ratio, and lipid profile.10 Date of birth, sex, and race were reported by participants during baseline. To test interaction, an age cutoff of 60 years was picked as prior reports from ARIC showed a 1.5 to 2.0 times or greater increase in incident AF in those 60 years and older compared with those younger than 60 years, across races as well as sex.8 Blood pressure readings were taken during each visit. Participants were determined as having hypertension from the average of 2 blood pressure readings at the visit. Systolic blood pressure equal to 140 mm Hg or higher and diastolic blood pressure equal to 90 mm Hg or higher served as boundaries to categorize hypertension. Participants who reported using hypertension medication were also categorized as having hypertension. Serum glucose measurements were used to determine diabetic status. Fasting blood glucose ≥3.26 mmol/L (126 mg/dL) or >5.17 mmol/L (200 mg/dL) if not fasting, self-reported physician diagnosis of diabetes, or use of diabetes medication were used to define diabetes. Fasting lipid profile and history of hyperlipidemia were recorded. Participant smoking status and alcohol use were also self-reported at each visit. Those with a history of myocardial infarction and a history of revascularization procedure were considered to have coronary artery disease. Those responding to questions related to congestive heart failure or evidence of prior heart failure hospitalization, up to visit 3, were considered to have medical history of congestive heart failure.11
Statistical analysis
Demographics including age, sex, and race as well as the investigated additional covariates including hypertension, diabetes, hypercholesterolemia, smoking status, current alcohol use, fasting lipid profile, coronary artery disease, and congestive heart failure were assessed. The mean age, BMI, and total and low-density lipoprotein cholesterol in migraine and no headache were compared using t test. All categorical variables were compared using χ2 test. Crude and adjusted hazard ratio (HR) and 95% CIs of AF by migraine subtype were calculated using Cox proportional hazards models, with those without headache as the reference category. Adjusted HR analysis was performed by adjusting for age and sex only, followed by adjusting for age, race, sex, hypertension, diabetes, hypercholesterolemia, smoking status, current alcohol use, fasting lipid profile, coronary artery disease, and congestive heart failure. Effect modification of the relation between migraine and AF by age (<60 and ≥60 years) and sex was evaluated because women have a lower risk of AF and stroke than men at younger ages but this tends to increase with age, and women are more likely to experience migraine than men. Crude and adjusted HR analyses were also performed comparing those with migraine with visual aura to those with migraine without visual aura. From the dataset, we have reported that migraine with visual aura is independently associated with increased risk of cardioembolic stroke.3 We adapted Baron and Kenny's causal-steps approach, and tested each of the 4 steps in the causal process12 to test the role of AF as a mediator of migraine with visual aura–associated cardioembolic stroke. All data analysis for this study were completed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Data availability
Anonymized data not published within this article will be made available by request through the ARIC Coordinating Center and payment of appropriate data processing fee from any qualified investigator.
Results
At visit 3, included participants were a mean age of 60 years (SD 5.7 years). This prospective follow-up visit included 56% women and 77% white individuals. Of the included participants during this visit, 12.6% had a history of migraine headaches. Migraine with visual aura accounted for 3.6% while migraine without visual aura contributed 9% of the participants. The mean duration of migraine history was 19 years in those with the reported condition. In examining demographics and stroke risk factors of the included 12,758 participants, migraine was shown to be more prevalent in women than in men (3:1).3 Of 11,939 assessed at visit 3 without history of AF or stroke, a total of 1,516 reported migraine and 9,405 reported no headache. There were some important significant differences in some of the baseline characteristics of these participants, as depicted in table 1. The nonheadache group was significantly (p < 0.0001) older (mean age 60.4 years) compared to the group with migraine headache (mean age 58.4 years). Other notable differences are that the nonheadache group had a significantly higher (p < 0.05) proportion of men, African American individuals, those with diabetes, smokers, alcohol users, and those with coronary artery disease compared with the migraine headache group. The migraine group had a higher (p < 0.0001) proportion of hypercholesterolemia and higher levels (p = 0.01) of total cholesterol compared to the no-headache group. Besides these differences, the BMI was significantly higher in the nonheadache group compared to the migraine group, as noted in table 1.
Table 1.
Baseline characteristics and risk factors of participants with migraine and those without headaches in the Atherosclerosis Risk in Communities study
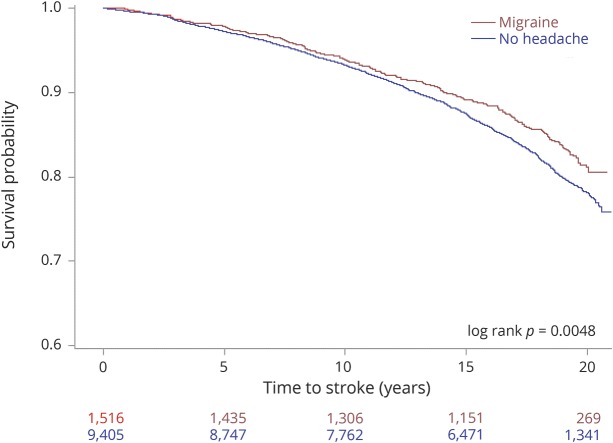
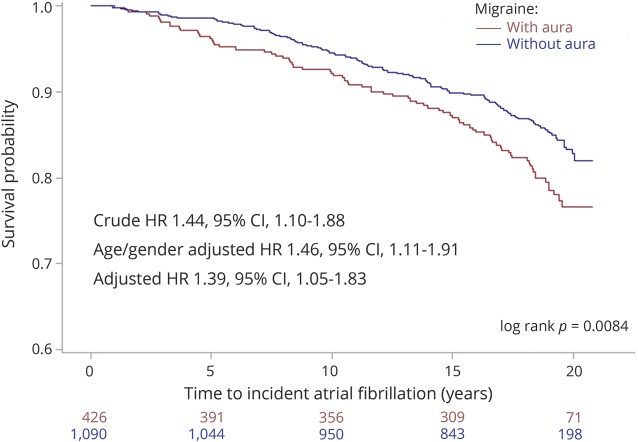
Over a 20-year follow-up period, incident AF was noted in 232 (15%) of 1,516 with migraine and 1,623 (17%) of 9,405 without headache (figure 1, log rank p = 0.0002). This translated to a crude incidence rate of AF of 7.7/1,000 person-years in the migraine group and 8.6/1,000 person-years in the no-headache group. These incidence estimates are similar to those reported from similar age groups in other population-based cohorts in the United States.8 Over the 20-year follow-up period, incident AF was noted in 80 (18%) of 440 with migraine with visual aura and 152 (14%) of 1,105 migraine without visual aura participants (as noted in the Kaplan-Meier plot in figure 2, log rank p = 0.008). The estimated crude incidence rates were 9.1/1,000 person-years in the migraine with visual aura group and 6.9/1,000 person-years in the migraine without visual aura group.
Figure 1. Kaplan-Meier curves depicting 20-year outcome of incident atrial fibrillation in participants with migraine headache and those without headache.
Inset: log rank p value.
Figure 2. Kaplan-Meier curves depicting 20-year outcome of incident atrial fibrillation in participants with migraine with visual aura and those with migraine without aura.
Inset: crude and adjusted hazard ratios (HRs) along with 95% confidence intervals (CIs) are indicated in the left bottom. Log rank p value is indicated in the right bottom.
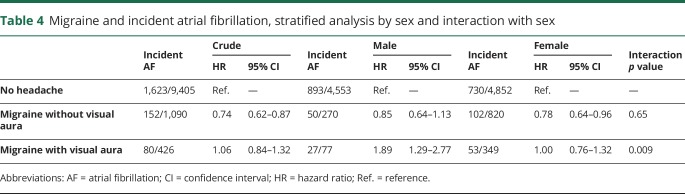
Using nonheadache as the reference group, participants with migraine overall (HR 0.82, 95% CI 0.72–0.94) and migraine without visual aura (HR 0.74, 95% CI 0.62–0.87) had a significantly lower risk of incident AF (table 2). Migraine with aura was not associated with incident AF (crude HR 1.06, 95% CI 0.84–1.32). After adjustment for age and sex, only migraine with aura was associated with a significantly higher risk of incident AF (HR 1.39, 95% CI 1.11–1.75). This change prompted testing for effect modification separately for sex (male vs female) and age (≥60 vs <60 years) (tables 3 and 4, respectively). In men (HR 1.89, 95% CI 1.29–2.77, interaction term p = 0.009) and those 60 years and older (HR 1.44, 95% CI 1.08–1.92, interaction term p = 0.07), migraine with aura was significantly associated with incident AF. After adjustment for age, sex, race, hypertension, diabetes, hypercholesterolemia, smoking, coronary artery disease, and congestive heart failure, only migraine with aura was associated with a significantly higher risk of incident AF (HR 1.30, 95% CI 1.03–1.62).
Table 2.
Hazard ratios (95% CIs) for the association of migraine overall, migraine with visual aura, and migraine without visual aura compared with no headache (reference group) and risk of AF
Table 3.
Migraine and incident atrial fibrillation, stratified analysis by age and interaction with age
Table 4.
Migraine and incident atrial fibrillation, stratified analysis by sex and interaction with sex
Migraine with aura was associated with incident AF (crude HR 1.44, 95% CI 1.10–1.88) compared with migraine without aura. After adjustment for age and sex, migraine with aura was associated with incident AF (HR 1.46, 95% CI 1.11–1.91). After adjustment for age, sex, race, hypertension, diabetes, hypercholesterolemia, smoking, coronary artery disease, and congestive heart failure, migraine with aura was associated with incident AF (HR 1.39, 95% CI 1.05–1.83). The HR and 95% CI are graphically depicted in figure 2.
Mediation analysis using the Baron and Kenny's causal-steps approach revealed that all 4 steps were satisfied, suggestive of a mediated model (table 5). The 4 steps included were:
The total effect of migraine with visual aura on cardioembolic stroke (C) was significant.
The effect of migraine aura on AF (A) was significant.
The effect of AF on cardioembolic stroke controlled for migraine (B) was significant.
The direct effect of migraine with visual aura on cardioembolic stroke adjusted for AF (C′) was nonsignificant.
Table 5.
Mediation analysis testing the role of atrial fibrillation in mediating the effect of migraine with visual aura on cardioembolic stroke
As previously noted, the average duration of migraine history was 19 years.3 Of 167 patients with incident cardioembolic strokes, in the majority (145, 87%), the strokes could be attributed to AF that preceded the stroke. The mean age of incident AF (±SD) of 71.6 (±8.7) was less (p = 0.03) compared to the mean age of incident cardioembolic ischemic stroke (±SD) of 73.7 (±7.8).
Discussion
The no-headache group in this cohort of older participants had a higher rate of incident AF compared to the migraine group (figure 1). This difference could be explained by the significant differences noted in the baseline characteristics (table 1). Most important, the no-headache participants were older, males, smokers, and those with coronary artery disease, and hence may be at cardiovascular risk predisposing them to AF. It is not surprising that the no-headache–AF association disappeared on adjustment to these risk factors (table 2). The migraine group was younger and predominantly women. Within the migraine subgroups, we found a significant association between migraine with visual aura and incident AF after adjustment for age and sex, as well as risk factors (table 2). The sex differences are further highlighted in women exhibiting a higher prevalence and a higher associated risk of thromboembolic events regarding a history of AF.13 Such epidemiologic differences have paved a way for the cultivation of risk scores for patients with AF. Women receive an additional point for their sex in the CHA2DS2-VASc category. CHA2DS2-VASc is defined as congestive heart failure/left ventricular dysfunction, hypertension, age >75 years, diabetes mellitus, stroke/TIA/thromboembolism, vascular disease, age 65 to 74 years, sex category score.14 Women are 4 times more likely than men to experience migraine headaches with the strongest association between migraine headache with aura and stroke shown to be in those younger than 55 years.15–17 The female cohort also shows a significant association between migraine with aura frequency and stroke events.18 Estrogen has been implicated in migraine pathophysiology19 and thromboembolic stroke in women with AF, but not with AF itself.20 Contrary to prior studies,15–17 we found that patients with migraine with aura were likely to have incident AF if they were male and aged 60 years or older. These results need to be verified in other studies but appear to be consistent with those reported in AF studies.21 The stroke incidence rate in migraine with aura group (4.1/1,000 person-years) was approximately twice that noted in migraine without aura (2.07/1,000 person-years) and higher than that noted in the no-headache group (3.02/1,000 person-years).
Several case reports as well as case series have reported the incidence of AF during a migraine attack. Autonomic dysfunction has a significant role in influencing pathophysiology of both migraine and AF.22,23 Cardiac arrhythmia recordings have been shown to be present in ECGs of patients while experiencing migraine headaches as compared to migraine-free phases.23 This hypothesis is further supported by AF ablation procedures that have shown tendencies to reduce migraine symptoms and frequencies.24 This hypothesis then begs the question of whether migraine with aura development is attributable to cardioembolic stroke arising from the AF. The mediation analysis suggests that the migraine with visual aura stroke risk factor may be mediated via AF. It is possible that those with migraine with visual aura are at higher risk of AF due to autonomic dysfunction. The AF predisposes to left atrial thrombus formation and embolism into the brain resulting in cardioembolic stroke. The findings are relevant to the present context in light of development of a wider range of testing to detect AF (e.g., internal loop recorder) and availability of oral thrombin inhibitors that prevent AF-related ischemic stroke.25
The strength of this study includes its large population cohort, longer follow-up period, and assessment of major covariates that may confound the study results. Our ascertainment of migraine, AF, and ischemic stroke is closely aligned with current diagnostic criteria. A limitation of the study is the possibility of unmeasured confounders. A potential unmeasured confounder is that patent foramen ovale (PFO), which is associated with paradoxical embolism, was not assessed in the ARIC study. Although PFO and AF have been implicated in embolic ischemic strokes, the paradoxical embolism in a stroke related to PFO is distinct from stroke arising from AF-related cardioembolism. Studies have shown that PFO presence is more prevalent in young patients with cryptogenic stroke and those with migraine.26–29 PFO prevalence tends to be higher in patients with migraine with aura.30 Because of this association, treatment of migraines by PFO closure devices has been a sought-out method for the population in focus. Yet, the Migraine Intervention with STARFlex Technology trial, a randomized, double-blind, sham-controlled trial, did not produce significant results in terminating migraine headaches or any secondary outcome.31 More recently, the PREMIUM (Prospective, Randomized Investigation to Evaluate Incidence of Headache Reduction in Subjects with Migraine and PFO Using the AMPLATZER PFO Occluder to Medical Management) trial did not meet the primary endpoint of reduction in responder rate in patients with frequent migraine.32 Based on these results,33 it is believed that there is no convincing evidence that excess risk of migraine is simply mediated by PFO through paradoxical embolism. A study conducted in the past reported atrial vulnerability a substrate for AF with atrial septal abnormalities PFO or atrial septal aneurysm (ASA) in patients with cryptogenic stroke.34 Whether such an association remains in migraine with visual aura remains to be determined. There is a significantly increased incidence of new-onset AF in post-PFO closure.35 It is possible that these patients had AF that was undetected before the closure and was likely the cause of the stroke. Another unmeasured confounder may be structural heart disease such as left atrial enlargement, which was not assessed in the entire ARIC cohort. Left atrial enlargement is associated with AF and cardioembolic stroke.36 However, these structural heart abnormalities have not been linked with migraine with visual aura. Participants may have also developed migraine with visual aura after the initial assessment on visit 3. In light of the cohort of older participants studied, the likelihood of developing new-onset migraine with visual aura is considered low. Since prevalent migraine, AF, and risk factors were assessed at visit 3 and incident AF was measured over a 20-year period, there is a possibility that the AF risk factors may have changed.
Other limitations of this study include a lower sensitivity of paroxysmal AF detection using the 3 methods: study ECGs, hospital discharge codes, and death certificates. Although validated with high sensitivity and specificity, it is likely that because of lack of long-term monitoring, paroxysmal AF may have gone undetected. A second limitation is that the migraine criteria used in ARIC are much stricter and more likely to have missed migraine diagnoses in patients who presented with bilateral headache or headache lasting less than 1 year, or had history of migraine at younger ages, but likely have included migraineurs with high-frequency migraine episodes in mid to later life. Finally, one limitation may also be the lack of details and ascertainment of migraine medications (e.g., propranolol and triptans) that influence heart rate.
Despite the limitations, this is one of few cohort studies to evaluate the association between migraine and AF in both men and women. The Danish, nationwide, population-based cohort study included younger adults (median age 35 years) and reported association between migraine and AF.4 They noted that the association was stronger in patients with aura than in those without aura and in women than in men. We believe this may be one of the first reports of migraine-AF association from the United States. In this large community-based cohort of African American or white adults without prior AF, we report a significant and independent association between migraine with visual aura and incident AF. This finding has important clinical implications and may help us better understand the AF mediation of the migraine-stroke link. A randomized clinical trial may help ascertain whether patients with migraine with visual aura may benefit from AF detection and subsequent anticoagulation or antiplatelet therapy as a primary stroke prevention strategy.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions.
Glossary
- AF
atrial fibrillation
- ARIC
Atherosclerosis Risk in Community
- BMI
body mass index
- CI
confidence interval
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICHD-3
International Classification of Headache Disorders, 3rd edition
- PFO
patent foramen ovale
Footnotes
Editorial page 1077
Author contributions
S. Sen: study concept and design, statistical analysis, interpretation, critical revision of the manuscript for important intellectual content. X.M. Androulakis: study concept and design, data analysis, interpretation, manuscript preparation, critical revision of the manuscript for important intellectual content. V. Duda: data analysis and interpretation, manuscript preparation, critical revision of the manuscript for important intellectual content. A. Alonso: study design, data analysis, interpretation, critical revision of the manuscript for important intellectual content. L.Y. Chen: study design, data analysis, interpretation, critical revision of the manuscript for important intellectual content. E.Z. Soliman: study design, data analysis, interpretation. J. Magnani: study concept and design, data analysis, interpretation, critical revision of the manuscript for important intellectual content. T. Trivedi: data analysis, interpretation, critical revision of the manuscript for important intellectual content. A.T. Merchant: data analysis, interpretation, critical revision of the manuscript for important intellectual content. R.F. Gottesman: study design, critical revision of the manuscript for important intellectual content. W.D. Rosamond: study design, critical revision of the manuscript for important intellectual content.
Study funding
The Atherosclerosis Risk in Communities Study is conducted as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Additional funding was provided by American Heart Association grant 16EIA26410001 (A. Alonso).
Disclosure
S. Sen is funded by NIH grants NS062754 and MD009738. X. Androulakis received research support from Tian Medical. V. Duda, A. Alonso, L. Chen, E. Soliman, J. Magnani, T. Trivedi, and A. Merchant report no disclosures relevant to the manuscript. R. Gottesman is Associate Editor for Neurology®. W. Rosamond reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
Publication history
Received by Neurology May 20, 2018. Accepted in final form August 23, 2018.
References
- 1.Hu X, Zhou Y, Zhao H, et al. Migraine and the risk of stroke: an updated meta-analysis of prospective cohort studies. Neurol Sci 2017;38:33–40. [DOI] [PubMed] [Google Scholar]
- 2.Stang PE, Carson AP, Rose KM, et al. Headache, cerebrovascular syndromes, and stroke: the Atherosclerosis Risk in Communities study. Neurology 2005;64:1573–1577. [DOI] [PubMed] [Google Scholar]
- 3.Androulakis XM, Kodumuri N, Giamberardino L, et al. Ischemic stroke subtypes and relationship with migraine with aura in the ARIC study. Neurology 2016;87:2527–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adelborg K, Szépligeti SK, Holland-Bill L, et al. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ 2018;360:k96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sposato LA, Peterlin BL. Cardioembolism as the unsuspected missing link between migraine and ischemic stroke. Neurology 2016;87:2504–2505. [DOI] [PubMed] [Google Scholar]
- 6.ARIC Investigators. The Atherosclerosis Risk in Community (ARIC) study: design and objectives. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 7.Headache Classification Committee of the International Headache Society (IHS): The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 8.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen PN, Johnson K, Floyd J, et al. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf 2012;21(suppl 1):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol 2003;41:47–55. [DOI] [PubMed] [Google Scholar]
- 11.Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychol Sci 2007;18:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang MC, Singer DE, Chang Y, et al. Sex differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Circulation 2005;112:1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 15.Merikangas KR. Contributions of epidemiology to our understanding of migraine. Headache 2013;53:230–246. [DOI] [PubMed] [Google Scholar]
- 16.Kurth T, Slomke MA, Kase CS, et al. Migraine, headache, and the risk of stroke in women: a prospective study. Neurology 2005;64:1020–1026. [DOI] [PubMed] [Google Scholar]
- 17.Schuürks M, Rist PM, Bigal ME, et al. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allais G, Chiarle G, Sinigaglia S, et al. Estrogen, migraine, and vascular risk. Neurol Sci 2018;39(suppl 1):11–20. [DOI] [PubMed] [Google Scholar]
- 19.Cheng EY, Kong MH. Gender differences of thromboembolic events in atrial fibrillation. Am J Cardiol 2016;117:1021–1027. [DOI] [PubMed] [Google Scholar]
- 20.Kurth T, Schuürks M, Logroscino G, et al. Migraine frequency and risk of cardiovascular disease in women. Neurology 2009;73:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staerk L, Sherer JA, Darae K, et al. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res 2017;120:1501–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Wasmund SL, Hamdan MH. Back to the future: the role of the autonomic nervous system in atrial fibrillation. Pacing Clin Electrophysiol 2006;29:413–421. [DOI] [PubMed] [Google Scholar]
- 23.Melek IM, Seyfeli E, Duru M, et al. Autonomic dysfunction and cardiac repolarization abnormalities in patients with migraine attacks. Med Sci Monit 2007;13:RA47–RA49. [PubMed] [Google Scholar]
- 24.Verma A, Saliba WI, Lakkireddy D, et al. Vagal responses induced by endocardial left atrial autonomic ganglion stimulation before and after pulmonary vein antrum isolation for atrial fibrillation. Heart Rhythm 2007;4:1177–1182. [DOI] [PubMed] [Google Scholar]
- 25.Spence JD. Cardioembolic stroke: everything has changed. Stroke Vasc Neurol 2018;3:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology 2000;55:1172–1179. [DOI] [PubMed] [Google Scholar]
- 27.Webster M, Chancellor A, Smith H, et al. Patent foramen ovale in young stroke patients. Lancet 1988;2:11–12. [DOI] [PubMed] [Google Scholar]
- 28.Lechat P, Mas J, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988;318:1148–1152. [DOI] [PubMed] [Google Scholar]
- 29.Consoli D, Paciaroni M, Galati F, et al. ; on behalf of SISIFO Group. Prevalence of patent foramen ovale in ischaemic stroke in Italy: results of SISIFO study. Cerebrovasc Dis 2015;39:162–169. [DOI] [PubMed] [Google Scholar]
- 30.Zeller J, Frahm K, Baron R, Stingele R, Deuschl G. Platelet-leukocyte interaction and platelet activation in migraine: a link to ischemic stroke? J Neurol Neurosurg Psychiatry 2004;75:984–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowson A, Mullen M, Peatfield R, et al. Migraine Intervention with STARFlex Technology (MIST) Trial. A prospective, multicenter, double-blinded, sham-controlled trial to evaluate the effectiveness of patent foramen ovale closure with STARFlex septal repair implant to resolve refractory migraine headache. Circulation 2008;117:1397–1140. [DOI] [PubMed] [Google Scholar]
- 32.Tobis JM, Charles A, Silberstein SD, et al. Percutaneous closure of patent foramen ovale in patients with migraine: the PREMIUM trial. J Am Coll Cardiol 2017;70:2766–2774. [DOI] [PubMed] [Google Scholar]
- 33.Finocchi C, Del Sette M. Migraine with aura and patent foramen ovale: myth or reality? Neurol Sci 2015;36(suppl 1):61–66. [DOI] [PubMed] [Google Scholar]
- 34.Berthet K, Lavergne T, Cohen A, et al. Significant association of atrial vulnerability with atrial septal abnormalities in young patients with ischemic stroke of unknown cause. Stroke 2000;31:398–403. [DOI] [PubMed] [Google Scholar]
- 35.Abdelaziz HK, Saad M, Abuomara HZ, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after cryptogenic stroke: a meta-analysis of randomized trials. Catheter Cardiovasc Interv Epub 2018 May 4. [DOI] [PubMed]
- 36.Di Tullio MR, Sacco RL, Sciacca RR, et al. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke 1999;30:2019–2024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request through the ARIC Coordinating Center and payment of appropriate data processing fee from any qualified investigator.