Abstract
Introduction
Hospital-acquired infections are common in neurointensive care units. We sought to review interventions which may reduce infection rates in neurocritically ill populations.
Methods
We conducted a systematic review of studies targeting adult patients in neurointensive care units (Neuro ICUs) with an intervention designed to prevent ICU-acquired infections. Our outcome of interest was change in the prevalence or rates of infection between active and control arms of these studies. We exclude studies based on the following criteria: no English full text version available; pediatric population; non-neurosciences ICU population; pre- or intraoperative methods to prevent infection; lack of discrete data for infection rates/prevalence; studies that were purely observational in nature and did not test an intervention; and studies performed in resource limited settings.
Results
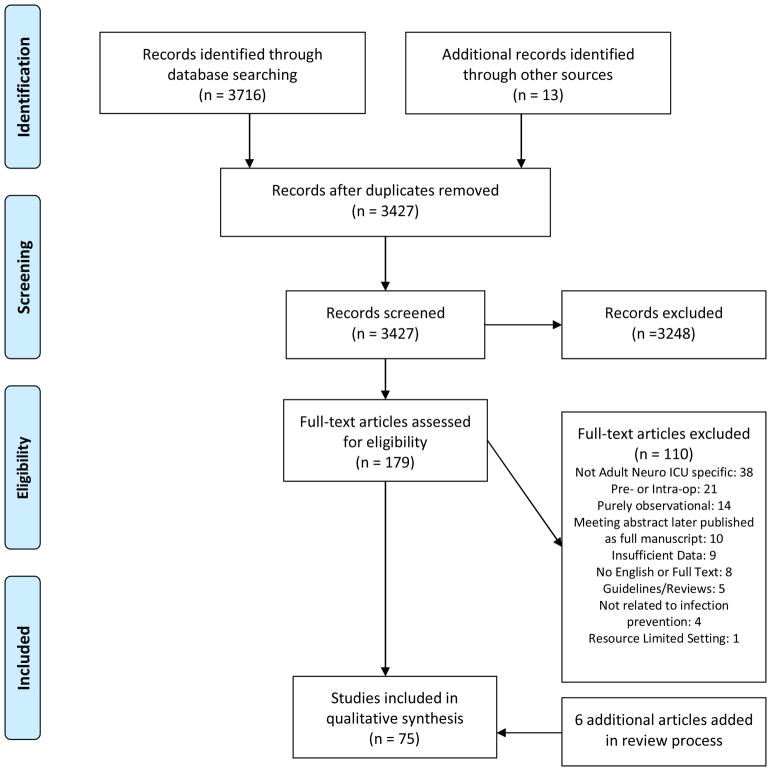
We initially retrieved 3716 results by searching the following databases: PubMed/MEDLINE, Embase via Ovid, and Cochrane CENTRAL via Ovid. No date or language limits were used in the search. Computerized deduplication was conducted using EndNote followed by a confirmatory manual review resulting in 3,414 citations. An additional 13 manuscripts were identified through review of references. The screening process followed a standard protocol, using two screeners at the title/abstract level to determine relevance and at the full-text level to determine eligibility for inclusion. The 3,427 titles/abstracts were independently screened by two board-certified neurointensivists to determine relevance for full-text review and 3,248 were rejected. The remaining 179 abstracts were reviewed in full-text using pre-determined inclusion/exclusion criteria. Ultimately, 69 articles met our inclusion criteria and were utilized in the final analysis.
Conclusion
The reviewed literature highlights the need for collaborative, multi-disciplinary, and multi-pronged approaches. Rates of VRI, SSI, VAP, CAUTI, and CLABSI can approach zero with persistence and a team-based approach.
Introduction
Hospital-acquired infections are common in intensive care units (ICUs) including those dedicated to the care of neurological patients. There is an extensive literature on preventing general ICU infections such as catheter-associated urinary tract infections (CAUTIs), central-line associated blood stream infections (CLABSIs), surgical site infections (SSIs) and ventilator-associated pneumonias in mixed medical and surgical ICU patients [1–4]. However, patients in neurointensive care units may have distinct characteristics that predispose them to infections when compared to a general ICU patient population including high utilization of external ventricular drains (EVDs), high rates of dysphagia and urinary retention, and presence of stroke and brain injury induced immunosuppression. Given the increasing focus on reducing rates of hospital-acquired infections and the growing use of these metrics as proxies for quality of care, we sought to review current interventions which may reduce rates of these infections in neurocritically ill populations.
Methods
This systematic review was conducted and reported in accordance with PRISMA statement[5]. This systematic review protocol was registered in PROSPERO (Registration Number: CRD42017057281) in February 2017. Search strategies were developed in collaboration with a medical librarian. The research question was framed using the PICO format and searches were developed using a combination of keywords and subject headings specific to population and intervention terms (see Supplemental Material for search terms). The population of interest was adult patients in neurosciences ICUs in developed countries. The studied interventions were either a) randomized-controlled trials or b) cohort studies with measurements before and after a protocol change. We only selected studies with primary or secondary outcomes of prevention of ICU-acquired infections. Exclusion criteria for studies included: no English full text version available; pediatric population; non-neurosciences ICU population; pre- or intraoperative methods to prevent infection; lack of discrete data for infection rates/prevalence; studies that were purely observational in nature (ie, did not test a protocol change) ; and resource limited settings.
We initially retrieved 3716 results by searching the following databases: PubMed/MEDLINE, Embase via Ovid, and Cochrane CENTRAL via Ovid. No date or language limits were used in the search. Computerized deduplication was conducted using EndNote followed by a confirmatory manual review resulting in 3,414 citations. An additional 13 manuscripts were identified through review of references. The screening process followed a standard protocol, using two screeners at the title/abstract level to determine relevance and at the full-text level to determine eligibility for inclusion. The 3,427 titles/abstracts were independently screened by two board-certified neurointensivists to determine relevance for full-text review and 3,248 were rejected. The remaining 179 abstracts were reviewed in full-text using pre-determined inclusion/exclusion criteria. Ultimately, 69 articles met our inclusion criteria and were utilized in the final analysis. An additional 6 articles on glycemic control were added during the review process of this paper bringing total number of articles to 75. See Figure 1 for PRISMA flowchart [5]. GRADE criteria were used to determine quality of evidence at the study level. After full-text review, studies were grouped based on type of intervention[6].
Figure 1. PRISMA 2009 Flow Diagram.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
RESULTS
Methods to Reduce Surgical Site Infections
The following studies demonstrated that attempts to decrease surgical site infections (SSI) via vigilant adoptions of care bundles can dramatically decrease SSI rate. Le et al. reported a pre/post study on the effect of a perioperative care bundle to reduce SSI and other complications after cranioplasty [7]. The bundle included 4 dose of perioperative vancomycin, a barrier dressing through post-operative day 3, and decolonization of the surgical incision using topical chlorhexidine from post-operative day 4 to 7.
In development of their bundle, the authors noted the high rate of Methicillin-resistant Staphylococcus aureus colonization compared to other patients in the neurosciences ICU (19% vs 6%). The care bundle led to decreased rates of SSI (24% to 3%, p=0.02) and eliminated the need for redo cranioplasty (19% vs 0%, p=0.02). Hale et al. report on a pre- and post-operative bundle to reduce SSI [8]. They introduced a “Craniotomy Checklist” which included pre-operative chlorhexidine gluconate (CHG) shampoo, preoperative CHG-alcohol skin prep, postoperative incision care orders, and a new glycemic control protocol. Craniotomy SSI decreased from 4.4% to 1.2% (p=0.03). As with other care bundles, it is difficult to assess the efficacy of individual components, but the effectiveness of the bundles demonstrated here denotes that very low rates of SSI are possible.
In a slightly difference approach, Adogwa et al. reported on use of negative pressure wound therapy on patients undergoing long-segment thoracolumbar fusion [9]. In 46 patients, the device was left in place until post-operative day 3 compared with 114 controls. All patients had subfascial drains. The authors demonstrated an relative decrease of 30% in rates of SSI (11% vs 15%, p=0.04) as well as decreased rates of wound dehiscence (6% vs 12%, p=0.02).
Infection Prevention for Patients with External Ventricular Drains (EVDs)
EVD Bundles
Table 1 describes the protocol details of 18 different EVD bundles extracted from 17 manuscripts describing 15 unique cohorts [10–26]. All of the manuscripts are quasi-experimental studies assessing the association between implementation of an EVD protocol and the rate of ventriculostomy related infections (VRIs). The protocols focus on a number of key categories related to EVD placement and maintenance including: a)use of a checklist; b) insertion setting (OR vs. ICU); c) strictness of sterility during the procedure; d) hair removal; e) catheter-type; f) tunneling; g) use of occlusive dressings; h) post-procedure prophylactic antibiotics; i) reduction in frequency of cerebrospinal fluid (CSF) sampling; j) aseptic technique for sampling or manipulation of drain; and k) routine EVD exchanges for extended dwell times. All protocols were developed, implemented and published by the multidisciplinary stakeholders in care for patients with EVDs. All protocols involved staff education and many included implementation of periodic infection control rounds.
Table 1.
Characteristics of External Ventricular Brain Bundles
| Study | Study Quality |
Insertion Checklist |
OR placement preferred |
Full barrier precautions |
Second pair of gloves |
Hair Removal | Iodine/Chlor -hexidine |
Antiobiotic/ Silver-EVDs |
Tunneling | Occlusive dressing |
Routine Dressing Change |
Post- Procedure Antibiotics |
No Daily CSF Sampling |
Routine EVD Exchange |
Other | Pre-protocol infection rate |
Post-protocol infection rate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bader et al. 1995 | very low | N | Y | Y | N | Shave | Y | N | N | Y | N | Y | Y | N | No irrigation or tubing changes; In lieu of CSF draws, send entire drainage bag using aspetic technique; Aspetic technique for zeroing/manipulation | 5/9 (55.5%) | 0/10 (0%) |
| Korinek et al. 2004 | low | N | Y | N | N | Use clippers on full scalp | Y | N | Y (3cm) | N | Y (q72H) | N | Y | N | Send drainage bag for infectious work-up and if positive do an aseptic draw from tubing | 16/161 (9.9%) | 10/216 (4.6%) |
| Dasic et al. 2009 | low | N | Y | Y | Y | Shave | Y | N | Y (minimum 10cm) | Y | N | N | Y | N | 10mg IT vanco after placement | 14/51 (27%) | 7/59 (12%) |
| Harrop et al. Part 1 2010 | low | N | N | Y | N | Use clippers | Y | N | N | Y | N | N | Y | N | 22/327 (6.7%) | 23/281 (8.2%) | |
| Harrop et al. Part 2 2010 | low | N | N | Y | N | Use clippers | Y | Y | N | Y | N | N | Y | N | 22/327 (6.7%) | 2/195 (1.0%) | |
| Honda et al. 2010 Part 1 | low | N | N | Y | N | Use clippers | Y | N | N | Y | Y (q48H) | N | N | N | Cap and mask for everyone in room; Sterile gauze/tegaderm at insertion site | 3.56/1000 EVD Days | 2/1000 EVD Days |
| Honda et al. 2010 Part 2 | low | N | N | Y | N | Use clippers | Y | Y | N | Y | Y (q48H) | N | N | N | Cap and mask for everyone in room; Sterile gauze/tegaderm at insertion site | 3.56/1000 EVD Days | 0.87/1000 EVD Days |
| Leverstein-Van Hall et al. 2010 | very low | N | Y | N | N | Use clippers on full scalp | N | N | Y (>5cm) | N | N | N | Y | N | Use aseptic technique for sampling; Used algorithim for diagnosis of suspected VRI | 20–37% | 9% |
| Amini et al. 2011 Rahman et al. 2012 Kubilay et al. 2013 |
low | Y | N | Y | Y | Use clippers | Y | Y | N | N | Y (q72H) | N | N | N | Checklist developed; RN can stop procedure if not adhering to checklist; developed Neuroscience Infection Prevention Team | 9.20% | 0.46% |
| Hill et al. 2012 | low | Y | N | Y | Y | Use clippers | Y | N | Y | Y | N | N | N | N | Weekly infection control rounds; Aspetic technique for zeroing/manipulation; Improved documentation; Increased surveillance | 16/1000 EVD Days | 1st year: 4.5/1000 EVD Days; 2nd Year: 1.3/1000 EVD Days; 3rd year 0/1000 EVD Days |
| Lwin et al. 2012 Part 1 | low | N | Y | N | N | Not indicated | N | N | N | N | N | N | Y | Y (Day 10) | Strict aspetic technique for all manipulations | 5/82 (6.1%) | 3/79 (3.8%) |
| Lwin et al. 2012 Part 2 | low | N | Y | N | N | Not indicated | N | Y | N | N | N | N | Y | Y (Day 10) | Strict aspetic technique for all manipulations | 5/82 (6.1%) | 0/73% (0%) |
| Flint et al. 2013 | low | N | N | Y | N | Use clippers on large area | Y | Y | Y (3–5cm) | Y | N | N | Y | N | Strict aspetic technique for all manipulations; chlorhexidine biopatch | 9/143 (6.3%) 7.35/1,000 EVD Days |
1/119 (0.8%) 0.79/1000 EVD Days |
| Camacho et al. 2013 | low | N | Y | Y | N | Use clippers on full scalp | Y | N | Y (5cm) | Y | Y (q24) | Y | Y | N | CSF draws only from distal point | 9.5% 14/1000 EVD Days |
4.8% 7/1000 EVD Days |
| Zakaria et al. 2013 | low | N | Y | N | N | Not indicated | Y | Mix | Y | N | N | N | Y | N | Weekly infection control rounds; Aspetic technique for manipulation | 54/234 (23.1%) 21.5/1000 EVD Days |
18/132 (13.6%) 13.7/1000 EVD Days |
| Chatzi et al. 2014 | low | Y | Y | Y | N | Not indicated | N | N | N | N | N | N | Y | Y (Day 7) | Unit-based reeducation twice monthly; Full barrier precautions/asepsis when accessing the drain | 28% 18/1000 EVD Days |
10.6% 7.1/1000 EVD Days |
| Angulo et al. 2015 | very low | N | N | N | N | Not indicated | N | Y | N | N | N | N | Y | N | 2.6/1000 EVD Days | 0.6/1000 EVD Days | |
| Phan et al. 2016 | very low | N | Y | N | N | Not indicated | N | N | N | N | N | N | Y | N | CSF draws ever 2–3 days; stopped prior routine EVD changes | 20.9% | 11.5% |
The large number of interventions that occur with implementation of each bundle makes it difficult to assess the effectiveness and necessity of each component of the bundle. Additionally, the heterogeneous patient populations, variations in surveillance techniques, and unique definitions of VRI complicate comparisons of the prevalence of VRI across institutions. However, these bundles demonstrate that implementation of a comprehensive EVD bundle that emphasizes aseptic technique throughout the entire lifespan of the EVD has the ability to significantly decrease rates of VRI and that rates approaching 0 are possible. The incremental improvements seen in the studies which introduced aspects of their bundles over different phases provides evidence that a multi-pronged approach is likely necessary to achieve very low rates of VRI.
Silver and Antibiotic Impregnated EVDs
A number of studies evaluated the benefits of antibiotic impregnated EVDs as compared with standard catheters. Wong et al. conducted a prospective RCT comparing the infection rate in patients with standard EVDs (who received dual prophylactic systemic antibiotics) and patients with EVDs impregnated with clindamycin and rifampicin (who received only periprocedural systemic antibiotics)[27]. They found no ventriculostomy-related infections in either group and no significant difference in the rates of extracranial infections (51% vs. 46%, p=0.617). In a 2003 prospective RCT of 288 patients at six medical centers, Zabramski et al. compared standard EVDs with minocycline/rifampin-impregnated EVDs and found that the standard EVDs were twice as likely to become colonized (37% vs. 18%, p<0.0012)[28]. Many studies by other authors confirmed Zabramski et al.’s findings [29–31], but two retrospective observational cohort studies found no significant difference in the rate of infections for patients with standard EVDs and those with antibiotic impregnated EVDs [32, 33]. Although Mikhaylov et al. found that antibiotic impregnated EVDs were associated with lower 3-year mortality rate than standard EVDs (15.8% vs. 24.6%, p=0.21), Shekhar et al. found that 20% of patients with antibiotic impregnated EVDs died and 12% of patients with standard EVDs died. The types of antibiotics in antibiotic impregnated EVDs vary, but in a study that compared the rates of infection in patients with minocycline/rifampin-impregnated catheters and clindamycin/rifampin-impregnated catheters, Abla et al. found no positive cerebrospinal fluid cultures in either cohort [34].
Silver-bearing catheters have also been used to prevent meningitis. One study of 164 patients that compared standard EVDs with silver-bearing EVDs showed that the occurrence of a positive cerebrospinal fluid culture, colonization of the catheter tip, or cerebrospinal fluid white blood cell count >4cells/ul was significantly less in patients with silver-bearing catheters (18.9% vs. 33.7%, p=0.04)[35].
While there is insufficient data to state whether silver bearing or antibiotic impregnated catheters are superior to one another, the above evidence suggests that both are superior to standard EVDs with respect to infection prevention. Silver or antibiotic impregnated catheters are included in many EVD bundles in neurosciences ICUs where the VRI rate approached zero. Additionally, use of silver or antibiotic impregnated catheters may reduce need for prolonged prophylactic systemic antibiotics.
Prolonged Prophylactic Antibiotics for EVDs
The data as to whether prolonged prophylactic antibiotics improve VRI rates is mixed. In one RCT of 255 patients comparing single (cefepime) and dual (ampicillin/sulbactam and aztreonam) antibiotics for the duration of EVD presence, the authors found 12% of patients in the single group and 6% of patients in the dual group developed VRI (p=0.7) [36]. In a separate RCT, Poon et al. found rates of VRI and extracranial infections were lower in patients who received ampicillin/sulbactam and aztreonam until EVD removal as compared with patients who received periprocedural ampicillin/sulbactam (3% vs. 11%, p=0.01 for VRI and 20% vs. 42%, p=0.002 for extracranial infections)[37].
While these studies showed superiority of aggressive antibiotic prophylaxis of EVDs, other studies found results questioning their efficacy [26, 38, 39]. One retrospective study comparing 209 patients who received cefuroxime for the duration of EVD placement and 99 patients who received it periprocedurally (for three or less doses) found that the infection rates were nearly identical (3.8% vs. 4.0%). The authors estimated that discontinuation of prolonged prophylactic antibiotics would lead to a savings of $80,000 per year in direct drug costs [38]. Additionally, in a prospective observational study of 866 patients with antibiotic impregnated catheters, Murphy et al. found that there was no significant difference in the rate of infection between patients who were given cefazolin or vancomycin for the duration of EVD placement versus those who were given antibiotics periprocedurally (1.1% vs. 0.4%, p=0.22). The authors noted that use of periprocedural antibiotics led to cost savings of $162,516 in drug costs and reduction in nosocomial infections [39]. Dellit et al. also found that limiting prophylactic antibiotic use to periprocedural administration decreased the incidence of Clostridium difficile infections [40]. Of note, they used antibiotic impregnated EVDs and there was no change in number of positive CSF cultures after discontinuation of prolonged prophylactic antibiotics.
These studies suggest that the utility of prolonged prophylactic antibiotics is dependent on a number of factors including background rate of VRI, concurrent use of other infection prevention methods or “bundles” and use of silver or antibiotic impregnated EVDs. Notably, decreased antibiotic use may be associated with lower rates of Clostridium difficile and growth of resistant bacteria.
Frequency of CSF Sampling
One study directly addressed the effect of decreasing the frequency of CSF sampling on rates on frequency of VRIs [41]. In this quasi-experimental study of mostly subarachnoid hemorrhage and traumatic brain injury (TBI) patients with EVDs, the authors looked at the effect of daily CSF sampling versus every 3 day sampling on frequency of “suspected” VRI (defined as those who were treated with antibiotics) and “proven” VRI (defined as those who were treated and had positive CSF cultures). The incidence of “suspected” VRI decreased from 17% to 11% and that of “proven” VRI from 10% to 3%. After controlling for other factors associated with VRI, the authors found the OR for VRI was 0.44 in the every 3 days sampling group compared to daily.
EVD Exchanges
Only one study provides experimental evidence on the effect of routine EVD exchange on rates of VRI. This small, randomized-controlled trial (RCT) of 103 patients compared regular EVD exchange every 5 days to exchange only when clinically indicated [42]. Patients requiring a new catheter had it placed contralateral to existing catheter. The authors found VRI in 8% of patients active arm vs. 4% in the control arm (p=0.44). The mean number of EVDs in the active group was 2.4, while only one patient needed a catheter exchange in the control group (for occlusion). All infections occurred after day 10 in both groups.
A small study of 32 patients investigated the effect of decreasing the frequency of changing the EVD drainage set [43]. They demonstrated a reduction in “evidence of ventriculitis” from 69% to 37% by changing the drainage set less frequently, from 3 days to 7 days. However, the high rate of infection in this study makes generalization difficult.
Methods to Prevent VRI at the EVD Insertion Site
In an effort to reduce infections with typical skin commensals, Schodel et al. examined the effect of using a bolt-kit to isolate the EVD as it traverses the skin upon skull entry [44]. In this pre/post study, the authors found a small but significant decrease in VRI using the bolt-kit system compared to traditional twist-drill hole only (4.9% vs. 6.8%, p=0.034). The results remained significant after controlling for patient age, drainage time, and number of punctures.
Drawing on parallel efforts to reduce central-line associated blood stream infections, one study examined the isolated effect of chlorhexidine-containing dressings on rates of VRI [45]. The authors saw a reduction from 6.98 to 1.70 VRI per 1000 EVD days, almost exclusively due to decreased infection with staphylococcus species.
Similarly, another quasi-experimental study examined the effect of a cyanoacrylate adhesive (Dermabond) on rates of VRI in patients mostly suffering from non-traumatic intracranial hemorrhage [46]. Dermabond, according to the authors, is a “water-catalyzed adhesive that is especially formulated for use on skin and indicated for the primary closure of small, clean surgical wounds....and as a barrier against common bacterial microbes.” The authors showed a reduction in VRI frequency from 15% to 4% (p=0.002), mostly due to reduction in rates of staphylococcus infections. This reduction supports the authors’ conclusions that Dermabond reduces VRI by providing a barrier to the entry of gram-positive skin flora along the EVD tract.
Infection Prevention for Patients with Intracranial Pressure Monitors
Only one study examined the effect of prolonged prophylactic antibiotics in patients with parenchymal intracranial pressure (ICP) monitors [47]. This study of 279 patients with TBI evaluated the effect of a protocol change from use of a broad-spectrum antibiotic (ceftriaxone) to a narrow-spectrum antibiotic (cefazolin) with the hypothesis that tailoring coverage would not change overall central nervous system (CNS) infection rates but would result in less resistant infections when they occurred both in the CNS and systemically. The authors found an insignificant difference in CNS infections rate between the narrow-spectrum group (1.7%) and the broad-spectrum group (4.4%). Of note, 19 out of 119 patients in the narrow-spectrum group received no antibiotics for reasons not described. There was a higher proportion of systemic infections arising from resistant gram negative species in the broad-spectrum group, especially in patients that developed infections later in the hospital course. There were no experimental or quasi-experimental studies investigating infection rates with and without antibiotic prophylaxis for patients with ICP monitors.
Infection Prevention for Patients with Subdural or Subgaleal Drains
One study from our group evaluated the effect of a protocol change which eliminated the use of prolonged prophylactic antibiotics (cefazolin or vancomycin) in postoperative neurosurgical patients with subdural and subgaleal drains [48]. The 105 patients in the antibiotic group received 513 doses of cefazolin and 77 doses of vancomycin postoperatively until the time of drain removal compared to 6 doses of cefazolin and 1 dose of vancomycin postoperatively for the 80 patients in the no antibiotic group. The rates of SSIs in both groups were similar (1 superficial and 1 deep in the antibiotics group) versus 1 infection in the no-antibiotics group. There were 2 cases of Clostridium difficile in the antibiotics group versus none in the no antibiotics group, but this was insignificant (p=0.5). We also noted a cost savings of $887.50 per patient in the no antibiotics group, mostly due to increased costs of treating three patients with resistant infections in the prolonged antibiotics group. There were no resistant organisms grown in the no-antibiotics group.
Glycemic Control to Reduce ICU-Acquired Infections
Six studies reported the results of RCTs on intensive insulin therapy versus more liberal glycemic control strategies and its effect on hospital-acquired infections in mixed groups of neurocritically ill patients [49–54]. Four of the six studies showed no difference in rates of ICU-acquired infections, while two showed overall lower rates of infections (in SAH and TBI populations). However, the authors of the study that showed decreased rates of infection with intensive insulin management in SAH patients were unable to replicate their findings in a follow-up study which on patients with TBI [53]. A recent meta-analysis pooled the rates of ICU-acquired pneumonia and found no difference in pooled rates of pneumonia between different arms of glucose control across studies (RR 1.04, 95% CI 0.82 to 1.32, P = 0.73)[49].
Infection Prevention after CNS Trauma
Five studies reported the results of RCTs on the use of prophylactic antibiotics in patients with basilar skull fractures and evidence of CSF leak [50–54]. Of note, these 5 studies were the subject of a 2015 Cochrane systematic review and meta-analysis which found no benefit to prophylactic antibiotics for reducing the rates of meningitis or meningitis-related mortality in this population [55]. The studies are summarized in Table 2. None of the 5 studies found a significant reduction in risk of meningitis with prophylactic antibiotic usage, although one study did show a decreased rate of combined meningitis and SSI. With one exception, the rates of meningitis in this clinical setting were low, and most authors recommended against use of prophylaxis based on their findings.
Table 2.
Studies of Prophylactic Antibiotics in Basilar Skill Fractures with CSF Leak
| Study | Study Quality | N | Antibiotic | Antibiotic Duration (days) | Rates of Meningitis | Comments |
|---|---|---|---|---|---|---|
| Ignelzi et al. 1975 | moderate | 10 | Ampicillin or Cephalothin 1g IV q6H | 10 | OR 1.0 (no infections in either group) | Nasopharyngeal flora changed towards more invasive, resistant Gram negative species |
| Hoff et al. 1976 | moderate | 160 | Treatment Group 1: Penicillin G 1.2M units IV daily Treatment Group 2: Penicillin G 20M IV units daily | 3 | OR 1.0 (no infections in the three groups) | None |
| Klastersky et al. 1976 | high | 52 | Penicillin G 5M units IV Q6H | Not Specified; Median 7.0 days (range 4–21 days) | 1/26 in placebo; 0/26 in treatment | 3 patients in placebo group and one patient in the penicillin group were treated for suspected meningitis but had negative cultures |
| Demetriades et al. 1992 | moderate | 157 | Treatment Group 1: Ceftriaxone 1g IV daily Treatment Group 2: Ampicillin/Sulphadiazine 1g/0.5g Q6H |
3 | 2.1% in placebo / 0.0% in treatment | If including scalp wounds, rates of meningitis/SSI was 8.7% in placebo group and 0.9% in prophylaxis group |
| Eftekhar et al. 2004 | moderate | 109 | Ceftriaxone 1gm IV daily | At least 5 | 21.5% in placebo / 18.9% in treatment | Overall high rate of meningitis; Inclusion criteria required pneumocephalus |
Prophylactic Antibiotics to Prevent Hospital Acquired Infections in the Neurosciences ICU
Prophylactic Antibiotics for Comatose Patients
We identified three studies (2 RCTs, 1 quasi-experimental) that examined whether prophylactic antibiotics were effective in lowering rates of ventilator-associated pneumonia (VAP) in patients presenting with coma [56–58]. Sirvent et al. published an open-label RCT of 100 patients in coma (mostly due to TBI (86%) with some stroke and post-neurosurgery patients) with GCS ≤12 and expected mechanical ventilation > 72 hours [56]. The experimental arm (n=50) received 2 doses of high-dose cefuroxime (1500mg) 12 hours apart compared to usual care for the control arm. The incidence of pneumonia in the experimental arm was 24% compared to 50% in the control group (p=0.007) with most pneumonia occurring within the first four days of mechanical ventilation. Despite the reduction in incidence of pneumonia, there was no difference in morbidity or mortality between groups. Acquarolo et al. performed a small randomized, open-label study using 3 days of ampicillin-sulbactam (3g every 6 hours) in 38 comatose patients (GCS≤8) on mechanical ventilation in a mixed TBI, stroke, and cardiac arrest population [57]. A reduction in early-onset (within first 4 days) pneumonia was seen (58% control vs. 21% prophylaxis, p=0.02) without an effect on functional outcome, but the study was underpowered for this outcome. A quasi-experimental study of 129 ventilated patients (71 in prophylaxis group, 58 in control) with coma (GCS≤8) compared a single dose of ceftriaxone (2g) to control in reduction of early-onset (within first four days) pneumonia [58]. The groups had similar age (56 vs 59), APACHE (17 vs. 18) and GCS (5 for both groups) scores. The incidence of early-onset pneumonia was lower in the prophylaxis arm compared to control (4.4 vs 23.1 /1000 days of mechanical ventilation, p=0.02). No mortality benefit was seen, though the prophylaxis group had lower mechanical ventilation and ICU days (10 and 15 day control vs. 6 and 10 prophylaxis, p=0.01 and 0.02).
Prophylactic Antibiotics for Patients with Severe Ischemic Stroke
While the majority of patients included in the seven published RCTs on use of prophylactic antibiotics after stroke had mild strokes and thus would not be admitted to a neurosciences ICU, two studies evaluating the role of prophylactic antibiotics in patients with severe stroke met our inclusion criteria [59, 60]. The Mannheim Infection in Stroke Study (MISS) was a RCT evaluating the efficacy of prophylactic mezlocillin/sulbactam for four days compared to usual care in patients with stroke who had modified Rankin scores > 3 [59]. The study enrolled 30 patients in each group within 4 hours of symptom onset. Infections developed in 50% of patients with prophylaxis and in 90% of patients with usual care in the first 10 days (p=0.002). The reduction was mostly due to decreased rates of urinary tract infections (UTIs). The authors also note that while the infection rate in the prophylactic group was high, most of these infections occurred after prophylactic antibiotics were stopped, with a mean day of infection of 5.1 compared to day 3.3 in the control arm (p=0.003). Functional outcomes at 90 days were improved in patients in the prophylaxis arm, with 18 patients with mRS 3–4 vs 9 in the control group P<0.05).
The PANTHERIS trial randomized 80 patients with severe middle cerebral artery infarcts to either 5 days of prophylactic intravenous moxifloxacin (ppx) or placebo within 36 hours of symptom onset. Both intention-to-treat (ITT) and per-protocol (PP) analyses were performed due to early deaths, withdrawal of consent, and protocol violations. The primary outcome was total infection rate at day 11. In the ITT analysis (n=79), the rate of infection was 15% in the treated group and 33% in the placebo group (p=0.1). In the PP analysis (n=66), the infection rate was 17% in the treated group and 42% in the control group (p=0.03). In contrast to the MISS study, the rate of UTIs was similar between groups (ITT: 7.7% ppx vs 12.5% control, p=0.7; PP 8.6% ppx vs 16.1% control, p=0.5). The rates of pneumonia were reduced to a greater extent but did not reach statistical significance (ITT: 7.7% ppx vs 20% control, p=0.2; PP: 8.6% ppx vs 25.8% control, p=0.1). Of note, only 9% of patients in the trial received mechanical ventilation (1 ppx, 6 control). Survival curve analysis over the first 180 days showed no survival difference between groups (p=0.6).
Prevention of VAP in the Neurosciences ICU
Ventilator Associated Pneumonia Bundles
Weireter et al. performed a quasi-experimental study evaluating the efficacy of a VAP bundle in reducing rates of VAP in a neurotrauma ICU [61]. The intervention instituted a weekly multidisciplinary team meeting, standardized weaning protocols (including empowerment of respiratory therapists and nursing to proceed with extubation if all protocol criteria were met), an oral care regimen, protocols for patient positioning, subglottic suctioning for patients with endotracheal tubes, and aggressive antibiotic stewardship. VAP rates dropped from 21/1000 patient days to <1/1000 patient days. Mechanical ventilation time also decreased from 5.8 to 4.75 days over a 10 year period.
Two additional conference abstracts reported on the efficacy of multi-intervention performance improvement projects in reducing rates of VAP in the neurosciences ICU [62, 63]. Both reports showed improvement in VAP. Interventions included use of multidisciplinary work groups, VAP bundles, hand hygiene, oral care, and reduction in transport of patients.
Selective Decontamination of the Digestive Tract (SDD)
In 1993, two trials reported conflicting results on the effect of SDD to prevent pneumonia in mechanically ventilated neurosciences ICU patients.
Korinek et al. performed a double-blinded RCT of SDD on 123 comatose patients intubated for at least 5 days after emergent admission to a neurosurgical ICU for mostly trauma and vascular insults [64]. The study period consisted of up to 15 days of a suspension of polymyxin E 100mg, tobramycin 80mg, and amphotericin B 500mg via nasogastric tube as well as oral application of a paste consisting of a 2% mixture of the above antimicrobials and 4% vancomycin. The primary outcome was incidence of bronchopneumonia. Patients were excluded for infection, extubation, or death prior to day 5. The authors found a decrease in the rates of both bronchopneumonia (42% vs. 24%, p<0.04) and total infections (82% vs. 46%, p<0.001). The authors found the reduction of infection to be higher in head trauma versus vascular patients. There was no difference in mortality between groups.
Hammond and Potgieter conducted a smaller RCT of SDD on 33 patients with mostly non-surgical neurological issues (AIDP, meningoencephalitis, status epilepticus, and myasthenia gravis) who were expected to remain intubated for at least 48 hours and remain in ICU for at least 5 days [65]. The SDD consisted of the same agents as in the Korinek study, however both SDD and placebo arms received three days of intravenous cefotaxime. There was an overall higher infection rate in the SDD group (46% vs. 32%, no p-value reported). The authors did not publish data on mortality or functional outcomes. The study is flawed by the use of intravenous cefotaxime in both arms, which the authors state was added because “the use of an intravenous placebo might have resulted in the withdrawal of an excessive number of cases from the study because the need for antibiotics would have led to unblinding.” However, given that prophylactic intravenous antibiotics for mechanical ventilation are not standard of care, the authors dilute the effect of SDD by their design.
A brief conference abstract reported the results of a quasi-experimental study on the effect of oral and enteral SDD with intravenous cefotaxime on rates of nosocomial pneumonia in a neurotrauma ICU [66]. They demonstrated a significant decrease in rates of pneumonia, but additional details were not available in the abstract.
Oral Hygiene
Three articles discussed the use oropharyngeal decontamination to prevent VAP in patients with neurotrauma or severe stroke.
Seguin et al. performed a single-center RCT with 110 patients to evaluate the efficacy of regular oropharyngeal application of povidone-iodine in closed head trauma patients with GCS≤8 after initial resuscitation and expected need for mechanical ventilation for ≥2 days [67]. The incidence of VAP in the decontamination group was 8% vs. 39% in the active control (saline, p=0.003) and 42% in the negative control (no oral application, p=0.001). Pneumonias occurred earlier (median day 3) in the povidone-iodine group compared to the control group (median day 8). There was no difference in length-of-stay or mortality between groups.
Based on these results, a multicenter, placebo-controlled randomized double-blind trial was performed to evaluate the same research question in an expanded patient population that included patients with hemorrhagic stroke in addition to neurotrauma patients [68]. In the 150 patients in the intention-to-treat analysis, they found VAP rates of 31% in the povidone-iodine group versus 28% in the control group (RR 1.11, CI 0.67–1.82). Of note, there were 5 cases of acute respiratory distress syndrome in the povidone-iodine group and none in the control arm. There were no differences in ICU and Hospital length-of-stay or ICU and 90 day mortality.
Wagner et al. performed a quasi-experimental study to evaluate whether a protocol for oral hygiene care was effective in reducing rates of pneumonia in both intubated and non-intubated patients admitted with acute ischemic stroke or intracerebral hemorrhage [69]. After instituting the protocol which consisted of campus-wide nursing education to perform systematic assessment of oral health and use of Sage Oral Care kits (0.05% cetylpyridinium chloride and 1.5% hydrogen peroxide and suction toothbrush, rates of VAP decreased from 14% to 10% (p=0.022). Although there was a higher proportion of intracerebral hemorrhage patients in the pre-protocol cohort (47% vs 31%), a post-hoc analysis showed no effect modification by stroke subtype on the efficacy of intervention.
Prevention of Central Line Associated Blood Stream Infections in the Neurosciences Intensive Care Unit
In two reports, Fukunaga et al. reported on the efficacy of povidone-iodine ointment and gauze dressings in reducing rates of central line associated blood stream infections (CLABSI) in a mixed neurological ICU population [70, 71]. They showed that application of a 10% povidone-iodine ointment covered with gauze and a Tegaderm resulted in less CLABSI and central line colonization than use of Tegaderm alone (0/1000 days CLABSI, 0.6/1000 days colonization vs. 5.22/1000 days CLABSI, 9/1000 days colonization, both p<0.05).
Elsayed et al. reported on the efficacy of a multi-intervention approach in reducing CLABSI rates in a mixed Neuro ICU population through use of pre-packed central line kits including all necessary sterile equipment, campus-wide education, a central line bundle, antiseptic coated catheters, and Biopatches[72]. The CLABSI rate decreased from 6.24 to 3.7 per 1000/catheter days in the first year (no p-value reported). Continued enforcement of the intervention led to 0 CLABSIs by year 4.
Prevention of Urinary Tract Infections in the Neurosciences Intensive Care Unit
Wisniewski et al. reported on the efficacy of a nursing driven protocol in a neurosurgical ICU to perform meatal and foley care using chlorhexidine wipes to reduce rates of catheter associated urinary tract infections (CAUTIs) [73]. The CAUTI rate decreased from 5.8/1000 device days to 0/1000 device days (p<0.001).
Samuel et al. described a protocol that included daily assessment of catheter need according to preset criteria, urine culture only with a positive urinalysis if other causes of fever were excluded, nursing foley maintenance strategies, and compliance audits [74]. After implementing the protocol with a 6-month education phase, the CAUTI rate in the neurosciences ICU decreased from 3% to 1% (p=0.001) and length-of-stay decreased from 3 to 2 days (p=0.02) though mortality and post-ICU disposition did not change.
Patel et al. reported on a physician-directed daily checklist to assess whether a urinary catheter was still needed and a nursing-directed protocol to utilize bladder scanning and clean intermittent catheterization prior to insertion of a urinary catheter [75]. They found a non-significant decrease in CAUTI rate from 3.8 to 2.9 infections/1000 catheter days (p=0.8) and a decrease in the percentage of catheter days in the ICU (69% to 50%, p=0.02). Lusby et al. reported on a similar protocol which also utilized chlorhexidine baths and reflex cultures only after positive urinalysis. CAUTI rates decreased from 8.6 to 3.7/1000 patient days (p=0.001)[76].
CONCLUSION
Preventing hospital-acquired infections is an important focus in neurosciences ICUs. There are numerous infection prevention strategies available. The reviewed literature highlights the need for collaborative, multi-disciplinary, and multi-pronged approaches. Rates of VRI, SSI, VAP, CAUTI, and CLABSI can approach zero with persistence and a team-based approach. Given the impact preventing these infections may have on patient-centered outcomes as well as their use as important benchmarks for regulatory agencies and payers on measuring the quality of care, developing protocols and auditing compliance for prevention infection should be standard practice in modern neurosciences ICUs.
Supplementary Material
References
- 1.Gould CV, Umscheid CA, Agarwal RK, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–26. doi: 10.1086/651091. [DOI] [PubMed] [Google Scholar]
- 2.Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96–105. doi: 10.1093/cid/ciu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muscedere J, Dodek P, Keenan S, et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: prevention. J Crit Care. 2008;23(1):126–37. doi: 10.1016/j.jcrc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le C, Guppy KH, Axelrod YV, et al. Lower complication rates for cranioplasty with peri-operative bundle. Clin Neurol Neurosurg. 2014;120:41–4. doi: 10.1016/j.clineuro.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Hale M, Coppa N, Dogan A, Townes J. A multi-disciplinary performance improvement project to reduce craniotomy surgical site infections. American journal of infection control. 2012;40(5):e47. [Google Scholar]
- 9.Adogwa O, Fatemi P, Perez E, et al. Negative pressure wound therapy reduces incidence of postoperative wound infection and dehiscence after long-segment thoracolumbar spinal fusion: A single institutional experience. Spine Journal. 2014;14(12):2911–7. doi: 10.1016/j.spinee.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Bader MK, Littlejohns L, Palmer S. Ventriculostomy and intracranial pressure monitoring: In search of a 0% infection rate. Heart and Lung: Journal of Critical Care. 1995;24(2):166–72. doi: 10.1016/s0147-9563(05)80012-3. [DOI] [PubMed] [Google Scholar]
- 11.Korinek AM, Reina M, Boch AL, et al. Prevention of external ventricular drain--related ventriculitis. Acta Neurochir (Wien) 2005;147(1):39–45. doi: 10.1007/s00701-004-0416-z. discussion -6. [DOI] [PubMed] [Google Scholar]
- 12.Dasic D, Hanna SJ, Bojanic S, Kerr RSC. External ventricular drain infection: The effect of a strict protocol on infection rates and a review of the literature. British journal of neurosurgery. 2006;20(5):296–300. doi: 10.1080/02688690600999901. [DOI] [PubMed] [Google Scholar]
- 13.Harrop JS, Sharan AD, Ratliff J, et al. Impact of a standardized protocol and antibiotic-impregnated catheters on ventriculostomy infection rates in cerebrovascular patients. Neurosurgery. 2010;67(1):187–91. doi: 10.1227/01.NEU.0000370247.11479.B6. discussion 91. [DOI] [PubMed] [Google Scholar]
- 14.Honda H, Jones JC, Craighead MC, et al. Reducing the incidence of intraventricular catheter-related ventriculitis in the neurology-neurosurgical intensive care unit at a tertiary care center in St Louis, Missouri: An 8-year follow-up study. Infection control and hospital epidemiology. 2010;31(10):1078–81. doi: 10.1086/656377. [DOI] [PubMed] [Google Scholar]
- 15.Leverstein-van Hall MA, Hopmans TE, van der Sprenkel JW, et al. A bundle approach to reduce the incidence of external ventricular and lumbar drain-related infections. J Neurosurg. 2010;112(2):345–53. doi: 10.3171/2009.6.JNS09223. [DOI] [PubMed] [Google Scholar]
- 16.Amini S, Fauerbach L, Archibald L, Friedman W, Layon A. Ventriculostomy-placement bundle and decreased ventricular infections: A single institution’s experience. Crit Care Med. 2011;39:139. doi: 10.3171/2012.11.JNS121336. [DOI] [PubMed] [Google Scholar]
- 17.Rahman M, Whiting JH, Fauerbach LL, Archibald L, Friedman WA. Reducing ventriculostomy-related infections to near zero: the eliminating ventriculostomy infection study. Joint Commission journal on quality and patient safety / Joint Commission Resources. 2012;38(10):459–64. doi: 10.1016/s1553-7250(12)38061-6. [DOI] [PubMed] [Google Scholar]
- 18.Kubilay Z, Amini S, Fauerbach LL, et al. Decreasing ventricular infections through the use of a ventriculostomy placement bundle: Experience at a single institution. Journal of neurosurgery. 2013;118(3):514–20. doi: 10.3171/2012.11.JNS121336. [DOI] [PubMed] [Google Scholar]
- 19.Hill M, Baker G, Carter D, et al. A multidisciplinary approach to end external ventricular drain infections in the neurocritical care unit. Journal of Neuroscience Nursing. 2012;44(4):188–93. doi: 10.1097/JNN.0b013e3182527672. [DOI] [PubMed] [Google Scholar]
- 20.Lwin S, Low SW, Choy DK, Yeo TT, Chou N. External ventricular drain infections: successful implementation of strategies to reduce infection rate. Singapore Med J. 2012;53(4):255–9. [PubMed] [Google Scholar]
- 21.Flint AC, Rao VA, Renda NC, et al. A simple protocol to prevent external ventricular drain infections. Neurosurgery. 2013;72(6):993–9. doi: 10.1227/NEU.0b013e31828e8dfd. [DOI] [PubMed] [Google Scholar]
- 22.Camacho EF, Boszczowski I, Freire MP, et al. Impact of an Educational Intervention Implanted in a Neurological Intensive Care Unit on Rates of Infection Related to External Ventricular Drains. PLoS ONE. 2013;8(2):e50708. doi: 10.1371/journal.pone.0050708. no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zakaria R, Tripathy S, Srikandarajah N, Rothburn MM, Lawson DDA. Reduction of drain-associated cerebrospinal fluid infections in neurosurgical inpatients: A prospective study. Journal of Hospital Infection. 2013;84(3):215–21. doi: 10.1016/j.jhin.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Chatzi M, Karvouniaris M, Makris D, et al. Bundle of measures for external cerebral ventricular drainage-associated ventriculitis. Crit Care Med. 2014;42(1):66–73. doi: 10.1097/CCM.0b013e31829a70a5. [DOI] [PubMed] [Google Scholar]
- 25.Angulo MN, Fagaragan L, Tabbilos SJ, et al. Improving the practice on external ventricular drains: Risk and cost reduction through a multi-collaborative approach. Neurocrit Care. 2015;(1):S138. [Google Scholar]
- 26.Phan K, Schultz K, Huang C, et al. External ventricular drain infections at the Canberra Hospital: A retrospective study. J Clin Neurosci. 2016 doi: 10.1016/j.jocn.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Wong GK, Poon WS, Ng SC, Ip M. The impact of ventricular catheter impregnated with antimicrobial agents on infections in patients with ventricular catheter: interim report. Acta neurochirurgica Supplement. 2008;102:53–5. doi: 10.1007/978-3-211-85578-2_11. [DOI] [PubMed] [Google Scholar]
- 28.Zabramski JM, Whiting D, Darouiche RO, et al. Efficacy of antimicrobial-impregnated external ventricular drain catheters: A prospective, randomized, controlled trial. Journal of neurosurgery. 2003;98(4):725–30. doi: 10.3171/jns.2003.98.4.0725. [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez-Gonzalez R, Boto GR, Fernandez-Perez C, del Prado N. Protective effect of rifampicin and clindamycin impregnated devices against Staphylococcus spp. infection after cerebrospinal fluid diversion procedures. BMC Neurology. 2010;10(93) doi: 10.1186/1471-2377-10-93. no pagination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright K, Young P, Brickman C, et al. Rates and determinants of ventriculostomy-related infections during a hospital transition to use of antibiotic-coated external ventricular drains. 2013;34(5):E12. doi: 10.3171/2013.2.FOCUS12271. [DOI] [PubMed] [Google Scholar]
- 31.Mikhaylov Y, Wilson TJ, Rajajee V, et al. Efficacy of antibiotic-impregnated external ventricular drains in reducing ventriculostomy-associated infections. Journal of Clinical Neuroscience. 2014;21(5):765–8. doi: 10.1016/j.jocn.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Verberk JDM, Berkelbach van der Sprenkel JW, Arts MP, et al. Preventing ventriculostomy-related infections with antibiotic-impregnated drains in hospitals: a two-centre Dutch study. Journal of Hospital Infection. 2016;92(4):401–4. doi: 10.1016/j.jhin.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Shekhar H, Kalsi P, Dambatta S, Strachan R. Do antibiotic-impregnated external ventriculostomy catheters have a low infection rate in clinical practice? A retrospective cohort study. British journal of neurosurgery. 2016;30(1):64–9. doi: 10.3109/02688697.2015.1096903. [DOI] [PubMed] [Google Scholar]
- 34.Abla AA, Zabramski JM, Jahnke HK, Fusco D, Nakaji P. Comparison of two antibiotic-impregnated ventricular catheters: a prospective sequential series trial. Neurosurgery. 2011;68(2):437–42. doi: 10.1227/NEU.0b013e3182039a14. discussion 42. [DOI] [PubMed] [Google Scholar]
- 35.Fichtner J, Guresir E, Seifert V, Raabe A. Efficacy of silver-bearing external ventricular drainage catheters: A retrospective analysis. Journal of neurosurgery. 2010;112(4):840–6. doi: 10.3171/2009.8.JNS091297. [DOI] [PubMed] [Google Scholar]
- 36.Wong GKC, Poon WS, Lyon D, Wai S. Cefepime vs. Ampicillin/Sulbactam and Aztreonam as antibiotic prophylaxis in neurosurgical patients with external ventricular drain: Result of a prospective randomized controlled clinical trial. Journal of clinical pharmacy and therapeutics. 2006;31(3):231–5. doi: 10.1111/j.1365-2710.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 37.Poon WS, Ng S, Wai S. CSF antibiotic prophylaxis for neurosurgical patients with ventriculostomy: a randomised study. Acta neurochirurgica Supplement. 1998;71:146–8. doi: 10.1007/978-3-7091-6475-4_43. [DOI] [PubMed] [Google Scholar]
- 38.Alleyne CH, Jr, Hassan M, Zabramski JM, et al. The efficacy and cost of prophylactic and periprocedural antibiotics in patients with external ventricular drains. Neurosurgery. 2000;47(5):1124–9. doi: 10.1097/00006123-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 39.Murphy RKJ, Liu B, Srinath A, et al. No additional protection against ventriculitis with prolonged systemic antibiotic prophylaxis for patients treated with antibiotic-coated external ventricular drains. Journal of neurosurgery. 2015;122(5):1120–6. doi: 10.3171/2014.9.JNS132882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dellit TH, Chan JD, Fulton C, et al. Reduction in Clostridium difficile infections among neurosurgical patients associated with discontinuation of antimicrobial prophylaxis for the duration of external ventricular drain placement. Infection control and hospital epidemiology. 2014;35(5):589–90. doi: 10.1086/675828. [DOI] [PubMed] [Google Scholar]
- 41.Williams TA, Leslie GD, Dobb GJ, Roberts B, van Heerden PV. Decrease in proven ventriculitis by reducing the frequency of cerebrospinal fluid sampling from extraventricular drains. J Neurosurg. 2011;115(5):1040–6. doi: 10.3171/2011.6.JNS11167. [DOI] [PubMed] [Google Scholar]
- 42.Wong GKC, Poon WS, Wai S, et al. Failure of regular external ventricular drain exchange to reduce cerebrospinal fluid infection: Result of a randomised controlled trial. Journal of Neurology Neurosurgery and Psychiatry. 2002;73(6):759–61. doi: 10.1136/jnnp.73.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duncan C, Laurie K, Lynch M. Reducing the frequency of external ventricular drainage set changes may reduce the incidence of clinically defined ventriculitis. Australian Critical Care. 2011;24(1):69. [Google Scholar]
- 44.Schodel P, Proescholdt M, Ullrich OW, Brawanski A, Schebesch KM. An outcome analysis of two different procedures of burr-hole trephine and external ventricular drainage in acute hydrocephalus. J Clin Neurosci. 2012;19(2):267–70. doi: 10.1016/j.jocn.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Scheithauer S, Schulze-Steinen H, Hollig A, et al. Significant reduction of external ventricular drainage-associated meningoventriculitis by chlorhexidine-containing dressings: A before-after trial. Clinical Infectious Diseases. 2016;62(3):404–5. doi: 10.1093/cid/civ887. [DOI] [PubMed] [Google Scholar]
- 46.Bookland MJ, Sukul V, Connolly PJ. Use of a cyanoacrylate skin adhesive to reduce external ventricular drain infection rates. J Neurosurg. 2014;121(1):189–94. doi: 10.3171/2013.12.JNS13700. [DOI] [PubMed] [Google Scholar]
- 47.May AK, Fleming SB, Carpenter RO, et al. Influence of broad-spectrum antibiotic prophylaxis on intracranial pressure monitor infections and subsequent infectious complications in head-injured patients. Surgical Infections. 2006;7(5):409–17. doi: 10.1089/sur.2006.7.409. [DOI] [PubMed] [Google Scholar]
- 48.Lewis A, Sen R, Hill TC, et al. Antibiotic prophylaxis for subdural and subgaleal drains. 2016:1–5. doi: 10.3171/2016.4.JNS16275. [DOI] [PubMed] [Google Scholar]
- 49.Kramer AH, Roberts DJ, Zygun DA. Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit Care. 2012;16(5):R203. doi: 10.1186/cc11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ignelzi RJ, VanderArk GD. Analysis of the treatment of basilar skull fractures with and without antibiotics. J Neurosurg. 1975;43(6):721–6. doi: 10.3171/jns.1975.43.6.0721. [DOI] [PubMed] [Google Scholar]
- 51.Hoff JT, Brewin AU, HS Letter: Antibiotics for basilar skull fracture. J Neurosurg. 1976;44(5):649. doi: 10.3171/jns.1976.44.5.0649. [DOI] [PubMed] [Google Scholar]
- 52.Klastersky J, Sadeghi M, Brihaye J. Antimicrobial prophylaxis in patients with rhinorrhea or otorrhea: a double-blind study. Surg Neurol. 1976;6(2):111–4. [PubMed] [Google Scholar]
- 53.Demetriades D, Charalambides D, Lakhoo M, Pantanowitz D. Role of prophylactic antibiotics in open and basilar fractures of the skull: a randomized study. Injury. 1992;23(6):377–80. doi: 10.1016/0020-1383(92)90011-g. [DOI] [PubMed] [Google Scholar]
- 54.Eftekhar B, Ghodsi M, Nejat F, Ketabchi E, Esmaeeli B. Prophylactic administration of ceftriaxone for the prevention of meningitis after traumatic pneumocephalus: results of a clinical trial. Journal of neurosurgery. 2004;101(5):757–61. doi: 10.3171/jns.2004.101.5.0757. [DOI] [PubMed] [Google Scholar]
- 55.Ratilal BO, Costa J, Pappamikail L, Sampaio C. Antibiotic prophylaxis for preventing meningitis in patients with basilar skull fractures. Cochrane Database Syst Rev. 2015;(4):CD004884. doi: 10.1002/14651858.CD004884.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sirvent JM, Torres A, El-Ebiary M, et al. Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. American journal of respiratory and critical care medicine. 1997;155(5):1729–34. doi: 10.1164/ajrccm.155.5.9154884. [DOI] [PubMed] [Google Scholar]
- 57.Acquarolo A, Urli T, Perone G, et al. Antibiotic prophylaxis of early onset pneumonia in critically ill comatose patients. A randomized study. Intensive care medicine. 2005;31(4):510–6. doi: 10.1007/s00134-005-2585-5. [DOI] [PubMed] [Google Scholar]
- 58.Valles J, Peredo R, Burgueno MJ, et al. Efficacy of single-dose antibiotic against early-onset pneumonia in comatose patients who are ventilated. Chest. 2013;143(5):1219–25. doi: 10.1378/chest.12-1361. [DOI] [PubMed] [Google Scholar]
- 59.Schwarz S, Al-Shajlawi F, Sick C, Meairs S, Hennerici MG. Effects of prophylactic antibiotic therapy with mezlocillin plus sulbactam on the incidence and height of fever after severe acute ischemic stroke: the Mannheim infection in stroke study (MISS) Stroke. 2008;39(4):1220–7. doi: 10.1161/STROKEAHA.107.499533. [DOI] [PubMed] [Google Scholar]
- 60.Harms H, Prass K, Meisel C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS One. 2008;3(5):e2158. doi: 10.1371/journal.pone.0002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weireter LJ, Jr, Collins JN, Britt RC, et al. Impact of a Monitored Program of Care on Incidence of Ventilator-Associated Pneumonia: Results of a Longterm Performance-Improvement Project. Journal of the American College of Surgeons. 2009;208(5):700–4. doi: 10.1016/j.jamcollsurg.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 62.Schmitz M. Preventing ventilator-associated pneumonia in the neuroscience intensive care unit: A multidisciplinary approach. American journal of infection control. 2013;(1):S89–S90. [Google Scholar]
- 63.Frattalone A, Ziai W, Fellerman D. Effect of independent monitoring and quality improvement interventions on the rate of ventilator associated pneumonia in a neurocritical care unit. Neurocrit Care. 2011;(1):S264. [Google Scholar]
- 64.Korinek AM, Laisne MJ, Nicolas MH, et al. Selective decontamination of the digestive tract in neurosurgical intensive care unit patients: a double-blind, randomized, placebo-controlled study. Crit Care Med. 1993;21(10):1466–73. doi: 10.1097/00003246-199310000-00013. [DOI] [PubMed] [Google Scholar]
- 65.Hammond JMJ, Potgieter PD. Neurologic disease requiring long-term ventilation: The role of selective decontamination of the digestive tract in preventing nosocomial infection. Chest. 1993;104(2):547–51. doi: 10.1378/chest.104.2.547. [DOI] [PubMed] [Google Scholar]
- 66.Cabrera Santana M, Pena Morant V, Sanchez Ramirez C, et al. Selective decontamination of the digestive tract (SDD) effects on nosocomial infections in a neurotraumatic intensive care unit (ICU) in a tertiary-care hospital. Intensive care medicine. 2013;39:S271–S2. [Google Scholar]
- 67.Seguin P, Tanguy M, Laviolle B, Tirel O, Malledant Y. Effect of oropharyngeal decontamination by povidone-iodine on ventilator-associated pneumonia in patients with head trauma. Crit Care Med. 2006;34(5):1514–9. doi: 10.1097/01.CCM.0000214516.73076.82. [DOI] [PubMed] [Google Scholar]
- 68.Seguin P, Laviolle B, Dahyot-Fizelier C, et al. Effect of oropharyngeal povidone-iodine preventive oral care on ventilator-associated pneumonia in severely brain-injured or cerebral hemorrhage patients: a multicenter, randomized controlled trial. Crit Care Med. 2014;42(1):1–8. doi: 10.1097/CCM.0b013e3182a2770f. [DOI] [PubMed] [Google Scholar]
- 69.Wagner C, Marchina S, Deveau JA, et al. Risk of stroke-associated pneumonia and oral hygiene. Cerebrovascular Diseases. 2016;41(1–2):35–9. doi: 10.1159/000440733. [DOI] [PubMed] [Google Scholar]
- 70.Fukunaga A, Naritaka H, Fukaya R, Tabuse M, Nakamura T. Our method of povidone-iodine ointment and gauze dressings reduced catheter-related infection in serious cases. Dermatology. 2006;212(Suppl 1):47–52. doi: 10.1159/000089199. [DOI] [PubMed] [Google Scholar]
- 71.Fukunaga A, Naritaka H, Fukaya R, Tabuse M, Nakamura T. Povidone-iodine ointment and gauze dressings associated with reduced catheter-related infection in seriously ill neurosurgical patients. Infection control and hospital epidemiology. 2004;25(8):696–8. doi: 10.1086/502464. [DOI] [PubMed] [Google Scholar]
- 72.Elsayed A, Mahanes D, Nathan B, Gress D. Prevention of catheter-related blood stream infection in the neurointensive care unit. Neurocrit Care. 2010;13:S140. [Google Scholar]
- 73.Wisniewski P, Mulatre M, Ibrahim J, Ashworth S, Aguirre L. Decrease in CAUTI rate following adoption of new protocols in the ICU. Crit Care Med. 2013;(1):A277. [Google Scholar]
- 74.Samuel S, Bertin M, Rasmussen P, Manno E, Frontera J. Implementation of cauti prevention protocol in the neuro ICU lowers CAUTI rates and length of stay. Crit Care Med. 2014;(1):A1550. [Google Scholar]
- 75.Patel S, Ibrahim J, Smith C, Safcsak K, Ashworth S. Strategies to reduce urinary catheter usage and rate of catheter-associated urinary tract infections. Crit Care Med. 2014;(1):A1552. [Google Scholar]
- 76.Lusby M, Williams MH, Blaber B, et al. A multi-faceted program to reduce the rates of catheter-associated urinary tract infections. Neurocrit Care. 2014;(1):S41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.