Abstract
Background
There are no host biomarkers of risk for HIV-associated cryptococcal meningitis (CM) except CD4+ T-cell deficiency. At present, serum cryptococcal antigen (CrAg) screening of those with CD4 <100 cells/µL is used to identify persons at risk for HIV-associated CM. We determined if plasma antibody profiles could discriminate CrAg+ from CrAg- patients.
Methods
We performed serological analyses of 237 HIV-infected asymptomatic Zimbabwean patients with CD4 <100 cells/µL; 125 CrAg- and CrAg+ but cerebrospinal fluid CrAg- by CrAg lateral flow assay. We measured plasma immunoglobulin M (IgM), immunoglobulin G (IgG) 1, and IgG2 concentrations by Luminex, and titers of Cryptococcus neoformans (Cn) glucuronoxylomannan (GXM) polysaccharide and naturally occurring Laminarin (natural Lam, a β-(1–3)-glucan linked polysaccharide)-binding IgM and IgG by enzyme-linked immunosorbent assay.
Results
GXM-IgG, -IgM, and -IgG2 levels were significantly higher in CrAg+ patients, whereas natural Lam-IgM and Lam-IgG were higher in CrAg- patients before and after adjustment for age, sex, and CD4 T-cell count, despite overlap of values. To address this variability and better discriminate the groups, we used Akaike Information Criteria to select variables that independently predicted CrAg+ status and included them in a receiver operating characteristic curve to predict CrAg status. By inclusion of CD4, GXM-IgG, GXM-IgM, and Lam-IgG, -IgG2, and -IgM, this model had an 80.4% probability (95% confidence interval, 0.75–0.86) of predicting CrAg+ status.
Conclusions
Statistical models that include multiple serological variables may improve the identification of patients at risk for CM and inform new directions in research on the complex role that antibodies may play in resistance and susceptibility to CM.
Keywords: antibody, cryptococccal antigenenmia, glucuronoxylomannan, HIV, IgG, IgM, immunoglobulin, Laminarin, Sub-Saharan Africa, Zimbabwe
Cryptococcus neoformans (Cn) is the leading cause of meningitis among individuals living with HIV [1]. Using the 2014 Joint UN Programme on HIV and AIDS estimates, annual global deaths from cryptococcal meningitis (CM) were 181 100, with 75% of the deaths occurring in sub-Saharan Africa (SSA) [2]. Despite increased access to HIV testing and antiretroviral therapy (ART) in SSA, the incidence of CM is largely unchanged [3]. Notably, 40% of patients with CM in Southeast Asia and SSA were on ART at the time of presentation, many for several months [4].
There are no validated host biomarkers of risk for CM, except CD4 T-cell deficiency. In SSA and elsewhere, the risk of HIV-associated CM is assessed by serum cryptococcal antigen (CrAg) testing of those with ≤100 CD4 T cells/µL [5]. Although this is cost-effective [6], HIV-associated CM also occurs in patients with CD4 T-cell counts ≥100 cells/µL [7], and most HIV-uninfected patients with CM have CD4 T cells in the normal range [8]. Therefore, serological biomarkers of risk for CM could cast a wider net and enable earlier risk stratification. Biomarkers of CM may also provide new insight into host factors that promote dissemination of Cn and inform development of immune-based therapy and vaccines.
HIV-infected (HIV+) persons with advanced immunosuppression and CD4 T-cell deficiency are at the highest risk for reactivating latent Cn, but not all do so. The role of cell-mediated immunity in resistance to Cn in animals and humans is well known, but antibody is also important [9, 10]. In mice, the absence of B-1 cells and/or natural immunoglobulin M (IgM) increases Cn dissemination to the brain [11–13]. Defined cryptococcal glucuronoxylomannan (GXM) capsular polysaccharide-specific immunoglobulin G (IgG) monoclonal antibodies (Mabs) protect mice against lethal Cn challenge [9], but high doses can fail to do so, as GXM-IgG-Cn immune complexes can inhibit phagocytosis and other host defense mechanisms [14]. A similar phenomenon is plausible in humans, particularly those who express the FcγRIII allele (V158), which binds serum IgG-GXM complexes with higher affinity than a low-affinity allele (F158) and is associated with CM in HIV+ and HIV-uninfected (HIV-) persons [15, 16].
In numerous studies, HIV+ and HIV- patients with CM and HIV+ persons had higher levels of GXM-IgG than, respectively, those without CM and HIV- persons [8, 17, 18]. On the other hand, GXM-IgM was lower in patients with than without HIV-associated CM and solid organ transplant recipients who developed post-transplant CM compared with those who did not [15, 18–20]. Nonetheless, GXM IgM and IgG levels can be variable and are not sufficient to identify patients who are at risk for CM. Therefore, we sought to determine if multiple antibody variables could better identify HIV+ patients at risk for CM. To test this idea, in addition to GXM-binding antibodies, we measured levels of plasma immunoglobulins and naturally occurring Laminarin (Lam)-binding antibodies in asymptomatic cryptococcal antigen–positive (CrAg+) and –negative (CrAg-) HIV-infected patients. HIV infection has a profound effect on B-cell activation and immunoglobulin levels [21, 22]. Antibodies that bind Lam, a mainly 1–3-β glucan-linked polysaccharide, bind fungi, including Cn [23, 24] and are part of the natural human antibody repertoire [25].
METHODS
Ethical Approvals
This study was approved by the Joint Research Ethics Committee of the University of Zimbabwe College of Health Sciences and Parirenyatwa Group Hospitals (JREC/113/15), the Medical Research Council of Zimbabwe (MRCZ/B/881), and the Institutional Research Board (IRB) at the Albert Einstein College of Medicine (Bronx, NY). Sample storage and specimen shipment were approved by the Research Council of Zimbabwe (RCZ). All participants included in this substudy had provided written informed consent for sample storage, future use, and shipment in the parent CryptoART study.
Study Population
This is a substudy of the CryptoART study (ClinicalTrials.gov Identifier: NCT02434172). The cohort consisted of banked plasma samples from HIV+ persons originally recruited into the CryptoART study in Harare, Zimbabwe, between April 2015 and June 2016. The study was a 12-month prospective implementation science study in which serum CrAg testing by CrAg lateral flow assay (LFA) was conducted in HIV-1-infected individuals (age ≥18 years) with CD4 T-cell counts <100 cells/µL receiving care at outpatient treatment care facilities in Harare, Zimbabwe. Eligible participants were required to have no clinical symptoms suggestive of meningitis. A screening questionnaire excluded participants with symptoms suggestive of meningitis or focal neurological signs that included a headache, confusion, stiff neck, vision changes, seizures, altered behavior, and focal weakness. Participants were excluded if they had a recent history of CM within 2 weeks of screening, previous allergy or other reaction to amphotericin B and/or fluconazole, an estimated glomerular filtration rate (eGFR) of ≤30 mL/min, or alanine transaminase ≥5 times the upper limit of normal. Pregnant women were excluded. None of the participants included in this substudy were on antiretroviral therapy, and none had a history of CM or evidence of disseminated cryptococcosis, as defined by a positive blood or cerebrospinal fluid (CSF) fungal culture.
The samples used in this substudy included a subset of serum CrAg- and CrAg+ but CSF CrAg- patients matched by age, sex, and CD4 T-cell count who did not have clinical or laboratory evidence of cryptococcal dissemination (Supplementary Table 1).
Measurement of GXM and Natural Lam Antibody Titers
Plasma GXM-IgM, GXM-IgG, and Lam-IgM and -IgG titers were determined by enzyme-linked immunosorbent assay as previously described [26]. Lam is a branched chain polysaccharide composed mainly of 1–3-β glucan linkages [24]; β glucans are part of the Cn cell wall [27, 28]. Plasma samples were used at an initial 1:10 dilution and serially diluted in a 96-well Costar plate (Corning Inc., Kennebunk, ME) coated with 10 ug/mL of GXM (from CN 24067) or Lam (Sigma-Aldrich). Plates were incubated with plasma, washed, and incubated with isotype-specific goat antihuman alkaline phosphatase labeled Abs for 1 hour (GAH IgM-AP, GAH IgG-AP; Southern Biotech, Birmingham, AL) and then p-nitrophenyl phosphatase (Thermo Scientific, Rockford, IL), after which the absorbance at 405 nm was measured with a VERSAmax plate reader (Molecular Devices, Sunnyvale, CA). Titers were defined as the absorbance (405 nm) of the highest dilution to give a signal ≥2 times the background (wells without serum) with the dilution factor.
Measurement of Immunoglobulin Levels
Total immunoglobulin levels of IgM, IgG1, and IgG2 in plasma samples were determined by Luminex as previously described [26] using a MILLIPLEX MAP kit (human immunoglobulin isotyping magnetic beads; EMD Millipore Corp., Billerica, MA) according to the manufacturer’s instructions.
Statistical Analysis
Serological results were compared between the CrAg+ and CrAg- groups using the Wilcoxon rank test for significance. Spearman correlations were used to seek associations between GXM-IgG levels and CrAg titers. The log-transformed serological results were further compared using linear regression models with adjustment for age, sex, and log-CD4 count. P values of <.05 were considered statistically significant. To identify serological variables that can predict CrAg status, we performed a stepwise model selection using the Akaike Information Criterion (AIC) [29] in a logistic regression model with age, sex, log-CD4, and all log-transformed serological results. We assessed the discriminatory accuracy of the selected model by the receiver operating characteristic (ROC) curve (R, version 2.3, and R library pROC, R Development Core Team, http://www.R-project.org) and the corresponding area under the curve (AUC). To evaluate the contribution of each variable in the model, we compared the AUCs before and after removing each variable from the selected models. ROC curves plot the true-positive rate (sensitivity) vs the false-positive rate (1 - specificity) at a continuum of thresholds; this predicts a participant’s CrAg status if the estimated probability exceeds a particular threshold. An ROC curve of a nonpredictive model is a straight line with an AUC of 0.50.
RESULTS
We measured antibody levels in banked plasma samples from 237 HIV+ participants from the parent CryptoART study. This included 125 participants who were serum CrAg- and 112 who were serum CrAg+ but CSF CrAg-, with a median (interquartile range) serum CrAg titer of 1:20 (1:5–1:80). The baseline demographic and clinical characteristics of the CrAg+ and CrAg- groups were not statistically significantly different (Supplementary Table 1).
Plasma Antibody Levels
GXM Antibodies
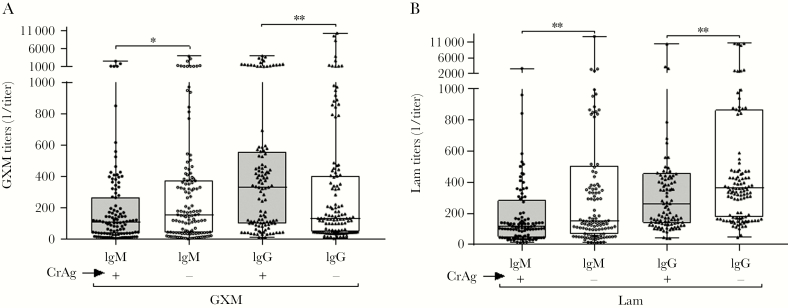
GXM-IgG titers were significantly higher in CrAg+ than CrAg- participants before and after adjustment for age, sex, and CD4 (Figure 1A, Table 1). GXM-IgM titers were significantly lower in CrAg+ than CrAg- participants, but statistical significance was attenuated (P = .08) after adjustment for age, sex, and CD4 (Figure 1A, Table 1).
Figure 1.
Glucuronoxylomannan (GXM) antibody titers determined by enzyme-linked immunosorbent assay. The inverse of (A) GXM–immunoglobulin M (IgM) and GXM–immunoglobulin G (IgG) and (B) Laminarin (Lam)-IgM and Lam-IgG titers, depicted as medians and interquartile ranges, are shown on the y-axis for each group shown on the x-axis. Cryptococcal antigen (CrAg) status is represented by (+) or (-) to indicate positive or negative CrAg status, respectively. *P < .05; **P ≤ .001, Wilcoxon rank-sum test.
Table 1.
Associations of Plasma GXM, Lam, and Immunoglobulin Levels With CrAg Status
| Univariate Analysisa | Multiple Regressionb | ||||
|---|---|---|---|---|---|
| CrAg+, Median (IQR) | CrAg-, Median (IQR) | P Value | Coefficient | P Value | |
| IgM | 951 (1066) | 785 (408) | .001 | .296 | 0 |
| IgG1 | 8165 (6275) | 7246 (5498) | .074 | .048 | .525 |
| IgG2 | 2280 (2252) | 1281 (1733) | 1.3e-5 | .598 | .001 |
| GXM-IgM | 110 (229) | 155 (332) | .044 | –.335 | .08 |
| GXM-IgG | 331 (448) | 131 (357) | .006 | .448 | .012 |
| Lam-IgM | 115 (229) | 153 (428) | .006 | –.448 | .005 |
| Lam-IgG | 265 (317) | 364 (689) | .004 | –.39 | .003 |
Abbreviations: CrAg, cryptococcal antigen; GXM, glucuronoxylomannan; IgG, immunoglobulin G; IgM, immunoglobulin M; IQR, interquartile range; Lam, Laminarin.
aWilcoxon rank test.
bRegression: log-transformed levels of antibodies compared between CrAg+ and CrAg- participants with adjustment for age, sex, and logCD4
We also determined GXM-IgG1 and GXM-IgG2 levels of a subset of 10 CrAg+ and 10 CrAg- participants with the highest GXM-IgG levels and a subset of 9 CrAg+ and 10 CrAg- participants with low GXM-IgG levels, as described previously [17, 30]. We excluded 1 CrAg+ sample from this and all other data sets because the patient was CSF CrAg+. GXM-IgG1 titers were statistically significantly higher in CrAg+ participants with high GXM-IgG titers, despite a range of values (Supplementary Figure 1A). There were no differences in the GXM-IgG2 titers of CrAg+ and CrAg- participants with low GXM-IgG titers (Supplementary Figure 1B). There was no significant correlation between CrAg titer and GXM-IgG, GXM-IgG1, or GXM-IgG2 levels (Supplementary Table 1). However, for the subset of participants with high GXM-IgG, the Spearman coefficient for the correlation between CrAg titer and GXM-IgG1 was 0.48, but at P = 0.19, it did not reach statistical significance, perhaps because of the small sample size.
Lam Antibodies
Lam-IgM and Lam-IgG titers were significantly lower in CrAg+ than CrAg- participants before and after adjustment for age, sex, and CD4 (Figure 1B, Table 2).
Table 2.
Logistic Regression to Predict Positive CrAg Identified by a Backward Variable Selection Approach Using Akaike Information Criteria
| Variable | Estimate | SE | Z Value | P Value |
|---|---|---|---|---|
| (Intercept) | –5.319792 | 3.213608 | –1.655 | .09784 |
| Age | –0.005644 | 0.020427 | –0.276 | .78233 |
| SexM | –0.011674 | 0.326033 | –0.036 | .97144 |
| Log_CD4 | –0.270405 | 0.192485 | –1.405 | .16008 |
| Log_GXM-IgM | –0.192239 | 0.153771 | –1.250 | .21124 |
| Log_GXM-gG | 0.698657 | 0.159256 | 4.387 | 1.15e-05 |
| Log_Lam-IgM | –0.192441 | 0.202436 | –0.951 | .34179 |
| Log_Lam-IgG | –0.978921 | 0.235184 | –4.162 | 3.15e-05 |
| Log_IgG1 | –0.190509 | 0.313837 | –0.607 | .54383 |
| Log_IgG2 | 0.358727 | 0.128147 | 2.799 | .00512 |
| Log_IgM | 1.387287 | 0.328529 | 4.223 | 2.41e-05 |
Abbreviations: CrAg, cryptococcal antigen; GXM, glucuronoxylomannan; IgG, immunoglobulin G; IgM, immunoglobulin M; Lam, Laminarin.
Plasma Immunoglobulins
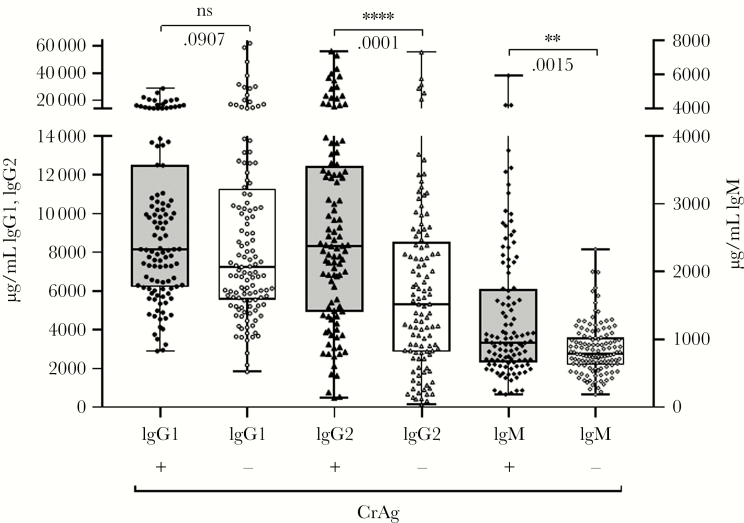
The concentrations of IgM and IgG2 were significantly higher in the plasma of CrAg+ than CrAg- participants before and after adjustment for age, sex, and CD4 (Figure 2, Table 1).
Figure 2.
Total immunglobulin levels determined by Luminex. Levels of immunoglobulin M (IgM), immunoglobulin G (IgG) 1, and IgG2, depicted as medians with interquartile ranges, are shown on the left (IgG1) and right (IgG2, IgM) y-axis for each isotype and group shown on the x-axis. Cryptococcal antigen (CrAg) status is represented by (+) or (-) to indicate positive or negative CrAg status, respectively. **P ≤ .001; ****P ≤ .0001, Wilcoxon rank-sum test. Abbreviation: ns, not significant.
Associations of Antibody Levels With CrAg Status
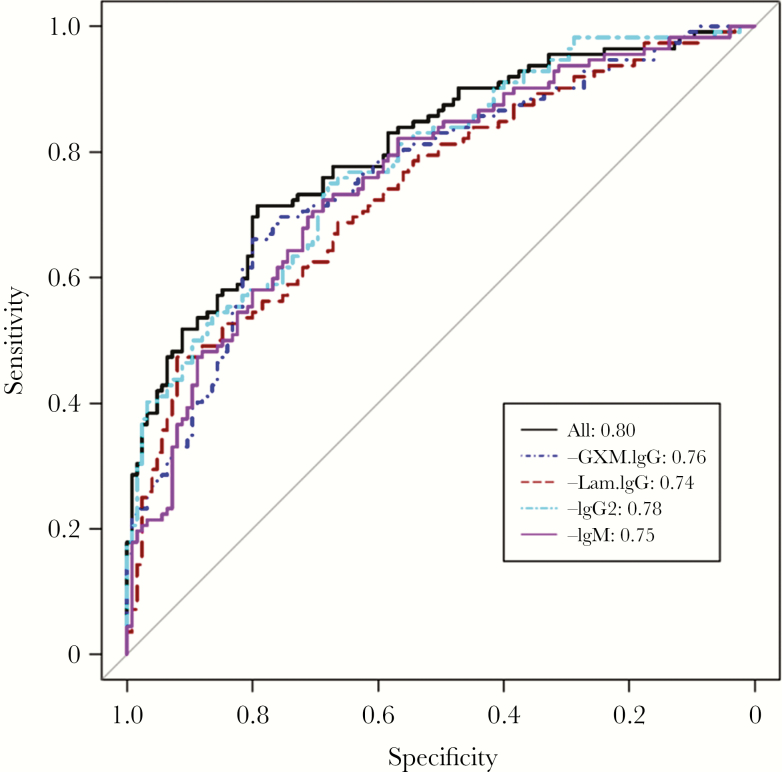
IgM, IgG2, and GXM-IgG were each independently positively associated with CrAg+ status, whereas Lam-IgG was negatively associated with CrAg+ status (Table 1). Use of a backward variable selection based on AIC [29] to identify markers that may predict CrAg+ status (Table 2) resulted in an AUC of 80.4% (95% confidence interval [CI], 0.75–0.86) when GXM-IgG, Lam-IgG, IgG2, IgM, CD4, and GXM-IgM were included in the model (Figure 3). Thus, this model had an 80% probability to predict CrAg+ status. After individually removing these variables from the model, CD4 and GXM-IgM did not affect the AUC, but the AUC was lower when we removed GXM-IgG, Lam-IgG, IgG2, and IgM (Figure 3). As a comparison, the model with age, sex, and CD4 did not have any prediction ability with an AUC of 0.53 (95% CI, 0.46–0.60).
Figure 3.
Receiver operating characteristic (ROC) curve. The ROC curve for all selected variables (black line) compared with models after removal of each of the prediction variables in the model indicated in the box (dark blue dashed line, glucuronoxylomannan [GXM]–immunoglobulin G [IgG]; brown dashed line, Laminarin [Lam]-IgG; light blue dashed line, IgG2; pink solid line, immunoglobulin M [IgM]). The area under the ROC curve with all selected variables is 0.804 (95% confidence interval, 0.748–0.859).
DISCUSSION
CD4 T-cell counts <100 cells/μL are an important risk factor for HIV-associated CM but are insufficient to predict which patients will develop HIV-associated CM. To seek serological biomarkers of risk, we measured Cn GXM capsule-binding and natural 1–3-β-glucan-binding antibody and immunoglobulin levels in plasma samples from asymptomatic HIV-infected CrAg+ and CrAg- individuals with <100 CD4 T cells. We found that CrAg+ participants had statistically significantly higher levels of GXM-IgG, IgG2, and IgM and lower levels of Lam-IgM and IgG than CrAg- participants, which remained significant after adjustment for age, sex, and CD4 T-cell count. Like many biological variables, antibody levels of CrAg+ and CrAg- participants overlapped. To improve our ability to differentiate the groups, we developed a prediction model that included multiple antibody variables. The model selected 6 variables, GXM-IgG, Lam-IgG, IgG2, IgM, GXM-IgM, and CD4, for inclusion in an ROC curve that had an 80% ability to predict CrAg+ status, however, removal of GXM-IgM and CD4 did not affect the model. As single host factors are rarely able to predict susceptibility to many if not most diseases, our data suggest that statistical modeling is a promising tool to identify host biomarkers of risk for complex diseases like CM.
GXM-IgG titers were higher in the plasma of CrAg+ than CrAg- participants. This parallels previous reports in which GXM-IgG was higher in HIV+ and HIV- patients with CM than without CM [8, 17, 18]. There is ample evidence that serum CrAg heralds symptomatic CM [31], and GXM-IgG was an independent predictor of CrAg+ status in our cohort. However, we did not find a correlation between GXM-IgG and CrAg titer, perhaps due to the small sample size or because CrAg does not faithfully reflect fungal burden. Soluble GXM can form complexes with GXM-IgG, but dissociation of such complexes did not affect measurements in a recent study [8].
Although descriptive, our data suggest that certain mechanistic effects of GXM-IgG identified in mice may be plausible in humans. For example, mouse GXM-IgG protected mice against lethal Cn, but high doses interfered with host defense in the setting of high inocula and abrogated protection [14]. GXM-IgG can promote intracellular update without killing [32], which can facilitate Cn transport to the brain in infected phagocytic cells, as described for Cn-infected human mononuclear cells in mice [33]. A similar scenario is plausible in humans given that Cn-human IgG immune complexes exhibited enhanced binding to and triggered more of a cytotoxic response upon binding with the high-affinity Fc-gamma-RIII allele V158 [15]. Expression of the V158 allele has been associated with CM in HIV+ and HIV- individuals [15, 16].
Plasma Lam-IgM and Lam-IgG were lower in CrAg+ than CrAg- participants and were inversely associated with CrAg+ status, although only the association with Lam-IgG was significant after adjustment for age, sex, and CD4. Lam-IgG was also independently predictive of CrAg- status. Lam-binding antibodies mediate protection in mice against multiple fungi, including Cn [23, 24, 27] via binding to cell wall 1–3-β glucans. Given that Lam-binding antibodies are part of the natural pre-immune human serological repertoire [25] and bind to 1–3-β glucans [25], it is reasonable to posit they may play a beneficial role in protection against human CM. In support of this idea, a Lam-IgG monoclonal antibody inhibited Cn growth in vitro and protected mice against a lethal Cn challenge [23], and human Cn cell wall 1–3-β-glycosylceramide-binding IgG inhibited Cn growth in vitro [34]. Lam-IgM and IgG were also lower in HIV+ patients with cryptococcal immune reconstitution inflammatory syndrome (C-IRIS) than without C-IRIS [26]. Neither the aforementioned nor this study included HIV- persons or mechanistic studies. Nonetheless, our findings suggest the hypothesis that human antibodies may enhance resistance to CM and worthy of investigation that bind fungal beta-glucans.
Hypergammaglobulinemia is a hallmark of advanced HIV infection [21], including in African patients [22]. Although IgG2 is often lower in HIV+ than HIV- Caucasians [35], IgG2 and IgM were each elevated in HIV+ Nigerians [36]. We found that plasma IgM and IgG2 were higher in CrAg+ than CrAg- participants and were independently associated with CrAg+ status. In CrAg+ participants, IgM and IgG2 levels may reflect B-cell activation by Cn. IgM memory B cells produce naturally occurring antibodies, and IgG2 is the main IgG subclass of human GXM antibodies [26, 30, 37]. In experimental models, IgM conferred protection against Cn dissemination in mice [11, 13], and mouse GXM-human-IgG2 chimeric Mabs protected mice against lethal Cn, but chimeric IgG1 was not protective [38]. Notably, IgG1 did not differ between CrAg+ and CrAg- participants in our cohort. In humans, serum IgG2, not IgG1, from volunteer recipients of an experimental GXM conjugate vaccine was the main mediator of human mononuclear cell phagocytosis of Cn [30]. We were not able to measure GXM-IgG1 or GXM-IgG2 in our entire cohort, but GXM-IgG1 titers of a subset of CrAg+ participants with the highest GXM-IgG titers were statistically significantly higher than those of CrAg- participants. This finding pertains to a small subset of high-titer GXM-IgG samples and requires validation in a larger cohort. Nonetheless, it suggests that production of GXM-IgG1 may be associated with risk for CM, because it lacks functional efficacy against Cn. This hypothesis warrants investigation as efficacy of mouse GXM-IgG Mabs was a function of IgG subclass [9].
In summary, our data show significant differences in levels of IgG2, IgM, and GXM and natural Lam antibodies in the plasma of asymptomatic HIV+ ART-naïve CrAg+ and CrAg- individuals in SSA. Our study has several limitations; we did not have an HIV- control group, we were not able to perform IgG subclass analyses or mechanistic experiments, and the participants had advanced immunosuppression that may have affected serological variables. Nonetheless, we were able to construct a model with an 80% probability of predicting CrAg+ status in our cohort. We hope to test this model in other cohorts of HIV+ persons. If validated, the availability of a multiple antibody detection platform could improve upon current CrAg screening for CM in SSA, where the scarcity of amphotericin B contributes to treatment failure and mortality. In addition, our data suggest that basic scientific studies of the role that GXM and beta-glucan-(natural Lam) binding antibodies and IgG subclasses play in the pathogenesis of human CM could bring a deeper understanding of resistance and susceptibility to this disease. Finally, validation of the model that emerged from this study could pave the way for improved identification of other patients at risk for CM, for example, HIV+ patients with higher CD4 counts, solid organ transplant recipients, patients with other immunodeficiency states [39], and patients on biologics [40].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the CryptoART study team for enrolling patients into the parent study. We thank Professor Alan McGregor for coordinating this work between the University of Zimbabwe College of Health Sciences in Harare, Zimbabwe, and the Albert Einstein College of Medicine in the Bronx, New York.
Trial registration. The parent study is registered at ClinicalTrials.gov (Identifier: NCT00830856).
Financial support. The parent CrytoART study was funded by National Institutes of Health (NIH) 1U1GH000737. LP was supported by NIH grant AI097096. TAM was supported by NIH Career Development Award K08AI104348.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Veltman JA, Bristow CC, Klausner JD. Meningitis in HIV-positive patients in sub-Saharan Africa: a review. J Int AIDS Soc 2014; 17:19184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scriven JE, Lalloo DG, Meintjes G. Changing epidemiology of HIV-associated cryptococcosis in sub-Saharan Africa. Lancet Infect Dis 2016; 16:891–2. [DOI] [PubMed] [Google Scholar]
- 4. Beardsley J, Wolbers M, Kibengo FM, et al. ; CryptoDex Investigators Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 2016; 374:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Longley N, Jarvis JN, Meintjes G, et al. Cryptococcal antigen screening in patients initiating ART in South Africa: a prospective cohort study. Clin Infect Dis 2016; 62:581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jarvis JN, Harrison TS, Lawn SD, et al. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS One 2013; 8:e69288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tugume L, Rhein J, Hullsiek KH, et al. HIV-associated cryptococcal meningitis occurring at relatively higher CD4 counts. J Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rohatgi S, Nakouzi A, Carreño LJ, et al. Antibody and B cell subset perturbations in human immunodeficiency virus-uninfected patients with cryptococcosis. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casadevall A, Pirofski L. Insights into mechanisms of antibody-mediated immunity from studies with Cryptococcus neoformans. Curr Mol Med 2005; 5: 421–33. [DOI] [PubMed] [Google Scholar]
- 10. Rohatgi S, Pirofski LA. Host immunity to Cryptococcus neoformans. Future Microbiol 2015; 10:565–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Subramaniam KS, Datta K, Quintero E, et al. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol 2010; 184:5755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rohatgi S, Pirofski LA. Molecular characterization of the early B cell response to pulmonary Cryptococcus neoformans infection. J Immunol 2012; 189:5820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dufaud C, Rivera J, Rohatgi S, Pirofski LA. Naïve B cells reduce fungal dissemination in Cryptococcus neoformans infected Rag1-/- mice. Virulence 2018; 9:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taborda CP, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with IgG. J Immunol 2003; 170:3621–30. [DOI] [PubMed] [Google Scholar]
- 15. Rohatgi S, Gohil S, Kuniholm MH, et al. Fc gamma receptor 3A polymorphism and risk for HIV-associated cryptococcal disease. MBio 2013; 4:e00573–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meletiadis J, Walsh TJ, Choi EH, et al. Study of common functional genetic polymorphisms of FCGR2A, 3A and 3B genes and the risk for cryptococcosis in HIV-uninfected patients. Med Mycol 2007; 45:513–8. [DOI] [PubMed] [Google Scholar]
- 17. Fleuridor R, Lyles RH, Pirofski L. Quantitative and qualitative differences in the serum antibody profiles of human immunodeficiency virus-infected persons with and without Cryptococcus neoformans meningitis. J Infect Dis 1999; 180: 1526–35. [DOI] [PubMed] [Google Scholar]
- 18. Subramaniam K, French N, Pirofski LA. Cryptococcus neoformans-reactive and total immunoglobulin profiles of human immunodeficiency virus-infected and uninfected Ugandans. Clin Diagn Lab Immunol 2005; 12:1168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jalali Z, Ng L, Singh N, Pirofski LA. Antibody response to Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan in patients after solid-organ transplantation. Clin Vaccine Immunol 2006; 13:740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Subramaniam K, Metzger B, Hanau LH, et al. IgM(+) memory B cell expression predicts HIV-associated cryptococcosis status. J Infect Dis 2009; 200:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lane CH, Masur H, Edgar LC, et al. Abnormalities of B cell activation and immunoregulation in patients with AIDS. N Engl J Med 1983; 309:453–8. [DOI] [PubMed] [Google Scholar]
- 22. Lugada ES, Mermin J, Asjo B, et al. Immunoglobulin levels amongst persons with and without human immunodeficiency virus type 1 infection in Uganda and Norway. Scand J Immunol 2004; 59:203–8. [DOI] [PubMed] [Google Scholar]
- 23. Rachini A, Pietrella D, Lupo P, et al. An anti-beta-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect Immun 2007; 75:5085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Torosantucci A, Bromuro C, Chiani P, et al. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 2005; 202:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiani P, Bromuro C, Cassone A, Torosantucci A. Anti-beta-glucan antibodies in healthy human subjects. Vaccine 2009; 27:513–9. [DOI] [PubMed] [Google Scholar]
- 26. Yoon HA, et al. Association between plasma antibody responses and risk for Cryptococcus-associated immune reconstitution inflammatory syndrome. J Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feldmesser M, Kress Y, Mednick A, Casadevall A. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J Infect Dis 2000; 182:1791–5. [DOI] [PubMed] [Google Scholar]
- 28. Kondori N, Edebo L, Mattsby-Baltzer I. A novel monoclonal antibody recognizing beta(1-3) glucans in intact cells of Candida and Cryptococcus. APMIS 2008; 116:867–76. [DOI] [PubMed] [Google Scholar]
- 29. Aho K, Derryberry D, Peterson T. Model selection for ecologists: the worldviews of AIC and BIC. Ecology 2014; 95:631–6. [DOI] [PubMed] [Google Scholar]
- 30. Zhong Z, Pirofski LA. Opsonization of Cryptococcus neoformans by human anticryptococcal glucuronoxylomannan antibodies. Infect Immun 1996; 64: 3446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jarvis JN, Lawn SD, Vogt M, et al. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis 2009; 48:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alvarez M, Burn T, Luo Y, et al. The outcome of Cryptococcus neoformans intracellular pathogenesis in human monocytes. BMC Microbiol 2009; 9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charlier C, Nielsen K, Daou S, et al. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun 2009; 77:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodrigues ML, Travassos LR, Miranda KR, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun 2000; 68:7049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lucey DR, Hendrix CW, Andrzejewski C, et al. Comparison by race of total serum IgG, IgA, and IgM with CD4+ T- cell counts in North American persons infected with the human immunodeficiency virus type 1. J Acq Immun Def Synd 1992; 5:325–32. [PubMed] [Google Scholar]
- 36. Uko GP, Griffiths M, Dawkins RL, et al. IgG2 associated hypergammaglobulinaemia in some Nigerians with HIV infection. Afr J Med Med Sci 1994; 23:385–9. [PubMed] [Google Scholar]
- 37. DeShaw M, Pirofski L. Antibodies to Cryptococcus neoformans capsular polysaccharide glucuronoxylomannan are ubiquitous in the serum of HIV+ and HIV- individuals. Clin Exp Immunol 1995; 99:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beenhouwer DO, Yoo EM, Lai CW, et al. Human immunoglobulin G2 (IgG2) and IgG4, but not IgG1 or IgG3, protect mice against Cryptococcus neoformans infection. Infect Immun 2007; 75:1424–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panackal AA, Rosen LB, Uzel G, et al. Susceptibility to cryptococcal meningoencephalitis associated with idiopathic CD4+ lymphopenia and secondary germline or acquired defects. Open Forum Infect Dis 2017; 4(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis 2018; 66:140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.