Abstract
Background:
Attention Deficit/Hyperactivity Disorder (ADHD) is a major sequela of traumatic brain injury (TBI) in youth. The objective of this study was to examine whether ADHD symptoms are differentially associated with genetic risk and brain structure in youth with and without a history of TBI.
Methods:
Medical history, ADHD symptoms, genetic, and neuroimaging data were obtained from a community sample of youth. ADHD symptom severity was compared between those with and without TBI (TBI N=418; noTBI N=3193). The relationship of TBI history, genetic vulnerability, brain structure, and ADHD symptoms was examined by assessing 1) ADHD polygenic score (discovery sample ADHD N=19,099, control N=34,194), 2) basal ganglia volumes and 3) fractional anisotropy in the corpus callosum and corona radiata.
Results:
Youth with TBI reported greater ADHD symptom severity compared to those without TBI. Polygenic score was positively associated with ADHD symptoms in youth without TBI but not in youth with TBI. The negative association between the caudate volume and ADHD symptoms was not moderated by history of TBI. However, the relationship between ADHD symptoms and structure of the genu of the corpus callosum was negative in youth with TBI and positive in youth without TBI.
Conclusions:
The identification of distinct ADHD etiology in youth with TBI provides neurobiological insight into the clinical heterogeneity in the disorder. Results indicate that genetic predisposition to ADHD does not increase the risk for ADHD symptoms associated with TBI. ADHD symptoms associated with TBI may be a result of a mechanical insult, rather than neurodevelopmental factors.
Keywords: attention-deficit/hyperactivity disorder, traumatic brain injury, magnetic resonance imaging, diffusion tensor imaging, polygenic score, youth
Introduction
Traumatic brain injury (TBI) in youth is associated with cognitive, intellectual, social and behavioural problems (1). Even in mild cases of TBI - which are by far the most common(2) - a significant proportion of children will experience persistent psychiatric symptoms associated with traumatic injury to the developing brain(3), making it an important risk factor to be addressed by treating physicians and mental health professionals. Attention problems and disinhibited behavior are common sequelae of TBI(4); as many as 50% of children develop symptoms of attention-deficit/hyperactivity disorder (ADHD) soon after TBI(5). In many cases these symptoms resolve over time; however, in some children they persist and children are diagnosed with ADHD. Regardless of prior psychiatric history or severity of TBI(6), 14–21% of youth admitted to hospital with TBI will present with a new ADHD diagnosis post injury(7, 8). This prevalence makes ADHD the most common disorder to develop following TBI in youth. ADHD is thought to have a complex etiology; it is highly heritable(9, 10) and has been associated with alterations to several neural circuits(11). While there are some established psychosocial premorbid risk factors for the development of ADHD post TBI(7, 8), to date, it is not clear whether genetic risk for ADHD contributes to risk of developing ADHD following TBI and whether the neural substrates associated with ADHD symptoms are the same or different when a TBI has occurred.
ADHD is characterized by a persistent pattern of age-inappropriate levels of hyperactivity, impulsivity, and inattention(12), and is associated with a range of negative health and psychosocial outcomes(13). Recently, approximately 20,000 ADHD cases and 35,000 matched controls were analyzed in the largest ADHD genome-wide association study (GWAS) to date, which identified 12 genetic risk loci that reached genome wide significance(14). Genes identified by GWAS studies are thought to act through a spectrum of biological mechanisms to contribute to ADHD pathogenesis(15) with each of these risk loci have small cumulative effects. A polygenic risk score computed from the recent GWAS data accounted for 5.5 % of variance in ADHD diagnosis and the proportion of the heritability of ADHD can be explained by all common variants was 0.22(14). However, GWAS studies have yet to fully accounted for the high heritability of ADHD estimated in twin studies (~75– 90%)(9, 10), potentially due to nonadditive genetic influences, gene-environment correlation, not examining rare variants and genetic heterogeneity. Reduced volume and altered shape of the basal ganglia (caudate, putamen, accumbens and the globus pallidus), have been consistently implicated in the pathogenesis of ADHD and are the most replicated structural finding across neuroimaging studies to date(16–18). The basal ganglia are richly interconnected components of neuroanatomical loops that connect the cortex and thalamus. These circuits regulate many cognitive processes that are impaired in ADHD such as executive function, inhibition of behaviour, and modulation of reward pathways(19).
TBI is most frequently caused by rapid acceleration-deceleration of the head that may or may not be accompanied by head impact. In addition to focal damage, impact, torsion, tension, and compression forces initiate traumatic axonal injury, which is accompanied by myelin changes in the brain(20, 21). Although traumatic axonal injury is heterogeneous and diffuse, there is a typical anatomical pattern of injuries that results from the movement of the brain relative to the skull. According to studies of post-mortem axonal damage(22, 23) and in vivo neuroimaging(24–26), traumatic axonal injury occurs typically in the areas of gray matter-white matter junctions of the corona radiata, the corpus callosum, and in more severe cases, the brainstem. Recent meta-analyses have also identified alterations of the corona radiata and corpus callosum in individuals with ADHD(27–30).
Here, we characterize and contrast the etiology of ADHD symptoms in youth who have experienced a mild TBI against youth who have no history of TBI. To the best of our knowledge we provide the first description of the contribution of genetics and brain structure to the expression of ADHD symptoms in a large community sample of youth with and without a history of mild TBI. Specifically, we test whether having a history of mild TBI modulates the association between ADHD symptoms and 1) polygenic risk for ADHD based on the most recent Psychiatric Genetics Consortium GWAS(14); 2) volumes of ADHD-related brain structures (caudate, putamen, accumbens and globus pallidus) from T1-weighted MRI; and 3) fractional anisotropy (FA) derived from diffusion tensor imaging (DTI) of white matter tracts affected by traumatic axonal injury (corpus callosum and corona radiata). Together, these analyses aim to address whether ADHD symptoms are associated with similar or different genetic and neural substrates when there is a history of TBI, with implications for both prognosis and treatment targets.
Methods
Sample Characterization and Analyses
Philadelphia Neurodevelopmental Cohort
Participants were subjects from the Philadelphia Neurodevelopmental Cohort, a population-based sample of children and adolescents ages 8 to 22(31) (see Supplemental Methods). Participants (age 11–21) and parents or guardians (age 8–17) were administered a structured interview, the GOASSESS(32) that screened for psychopathology and included a comprehensive medical history. The GOASSESS is abbreviated and modified from the epidemiologic version of the NIMH Genetic Epidemiology Research Branch Kiddie-SADS(33). Participants also provided blood samples for genetic analysis. A subset of 1,600 participants underwent multi-modal neuroimaging on the same 3T Siemens TIM Trio scanner(34).
Definition of TBI
Participants were separated into two groups, those with a history of mild TBI and those with no history of TBI. The mild TBI group was composed of individuals who reported at least one TBI that was accompanied by less than 30 minutes of unconsciousness, and less than 24 hours of amnesia with no skull fracture or neurosurgical intervention associated with the injury. There were very few participants with moderate or severe TBI who were excluded from the analyses, since most of these participants did not pass screening for participation in the neuroimaging component of the study. Within the mild TBI group participants who reported headache, loss of consciousness or amnesia associated with the injury were classified as high risk for persistent post injury deficits.
ADHD Symptom Severity Score
The structured interview assesses a subset of symptoms and criterion that corresponds to the diagnostic criteria for ADHD, as described in the DSM-5(12). Positive responses to the ADHD symptom questions contributed to the score and additional points were assigned based on the age of onset, environments where the difficulties occurred as well as impairment and distress associated with the symptoms (see Supplemental Methods).
Summary of Analyses
Separate analyses were performed in subsets of participants determined by the availability and quality of the data, and the participant inclusion criteria for each analysis as outlined below and depicted in Figure 1A. All analyses were performed in participants that had a minimum ADHD symptom severity score of 1 in order to test linear hypotheses (see Supplemental Methods) and excluded participants with moderate to severe medical conditions. Within each analysis there was a small number of participants missing necessary variables and these participants were excluded. Clinical data from 3611 participants was available for ADHD Symptom Analysis (TBI=418, noTBI=3193). Polygenic Score Analysis was performed only in participants of Caucasian ancestry in order to mitigate confounds due to ethnic differences between the present study sample and the ADHD GWAS(14) from which the polygenic score was derived. For this analysis, ethnic outliers and highly related individuals were excluded which left 1438 individuals (TBI=205, noTBI=1233). Participants of all ethnic backgrounds were included in the neuroimaging analyses. After excluding individuals who did not have imaging data, 833 participants with quality controlled T1-weighted MRI remained for Basal Ganglia Volume Analyses (TBI=110, noTBI=723) and 627 participants with quality-controlled DTI remained for White Matter Microstructure Analysis (TBI=86, noTBI=541). We additionally include sensitivity analyses that repeat each of these analyses in samples of participants where youth who meet criteria for ADHD diagnosis have been excluded as well as in samples of participants where youth taking medication for emotions and/or behaviours have been excluded.
Figure 1.
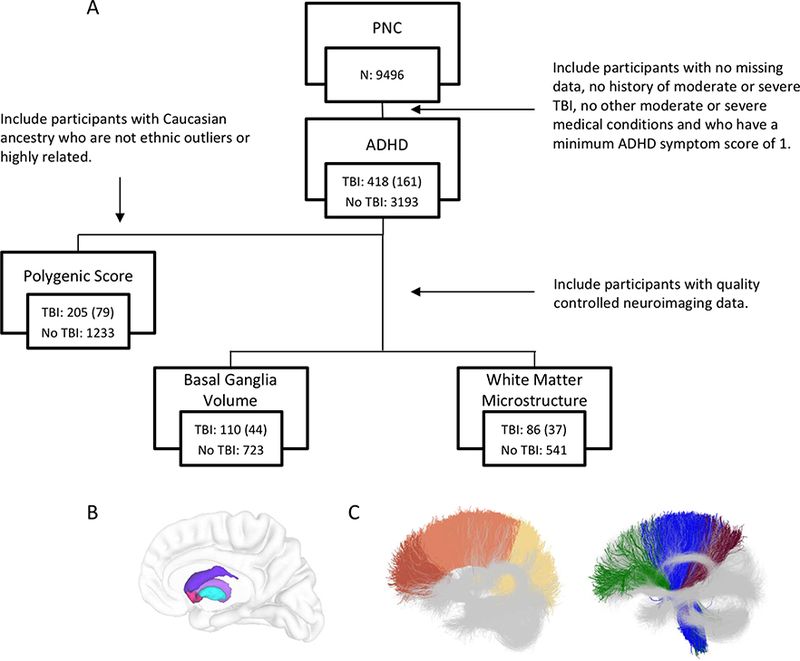
Summary of analyses. A) Eligible participants from Philadelphia Neurodevelopmental Cohort (PNC) were identified for each analysis from the total sample, first for ADHD symptom comparison between TBI groups, second for polygenic score analysis, third for basal ganglia volume analysis and forth for white matter microstructure analysis based on specific inclusion criteria summarized here and described in the Methods section. ‘TBI’ indicates number of youth with a history of mild TBI that were included in that specific analysis with the number in brackets indicating the number of these youth that were considered high risk for persistent deficits following mild TBI. ‘No TBI’ indicates the number of youth with no history of TBI included in that analysis. B) Basal ganglia regions of interest for volumetric analyses are visualized in the right hemisphere embedded in a cortical surface representation for reference: caudate (purple), putamen (violet), accumbens (pink), and globus pallidus (turquoise) C) White matter tract atlases show subdivisions examined in the corpus callosum (left): genu (red), body (orange), and splenium (yellow) and corona radiata (right): anterior (green) superior (blue) and posterior (maroon).
Data Acquisition and Processing
Genetics Data Processing
Genome-wide genetic data was assessed for quality and imputation was performed. Ethnicity was determined by multi-dimensional scaling and ethnic outliers and related individuals were identified. Details are available in the Supplemental Methods.
Polygenic Score Calculation
Polygenic ADHD risk was determined by calculating an additive genome-wide polygenic score for each participant in the sample based on summary data from the Psychiatric Genetics Consortium ADHD Subgroup GWAS in subjects of Caucasian ancestry (19,099 cases, 34,194 controls)(14, 35) (see Supplemental Methods).
Basal Ganglia Segmentation
T1-weighted image acquisition parameters have been published(34) and are described in the Supplemental Methods. The caudate, putamen, accumbens and globus pallidus volumes were automatically identified on T1-weighted images using MAGeTbrain segmentation, an extension of the multi-atlas segmentation technique(36, 37) (Figure 1B). Details are provided in the Supplemental Methods. Mean volumes of the four bilateral structures were computed and used in all analyses, as a previous large mega-analysis of the association between subcortical volumes and ADHD diagnosis did not find evidence of laterality effects(38).
White Matter Tractography
Diffusion weighted MRI acquisition parameters have been published(34) and are described in the Supplemental Methods. Diffusion weighted scans were corrected for motion and eddy current distortions using DTIPrep(39). Following quality control (see Supplemental Methods), tensors and fiber tracts were generated in 3D Slicer(40) via the SlicerDMRI project(41)(dmri.slicer.org). An automated method was used for tractography segmentation (see Supplemental Methods). Mean FA was extracted from the bilateral anterior, superior and posterior, corona radiata as well as the genu, body and splenium of the corpus callosum (Figure 1C).
Statistics
Statistical analyses were conducted using R software with multiple linear regression modeling. ADHD Symptom Analysis modeled the effect of TBI group on the ADHD symptoms score in order to compare the severity of ADHD symptoms reported in youth with and without mild TBI. This analysis included age, sex and highest level of parental education as covariates. The following 3 analyses examined whether the relationship between the variable of interest and ADHD symptom severity differed between youth with and without a history of mild TBI by modeling the interaction between the variable and TBI group on the ADHD symptoms severity score. The variable examined in the Polygenic Score Analysis was polygenic risk score for ADHD and the covariates included in this model were age, sex, highest level of parental education and the top four components from multi-dimensional scaling analysis. Variables of interest in Basal Ganglia Volume Analysis were the volume of the caudate, putamen, accumbens and globus pallidus and this model included age, sex, highest level of parental education and total brain volume as covariates. The variables examined in the White Matter Microstructure Analysis were FA of the anterior, superior and posterior corona radiata as well as the genu, body and splenium of the corpus callosum and this model included age, sex, highest level of parental education and temporal signal to noise ratio as covariates. Significant interactions of variables with TBI group were followed up with post hoc analysis of the variable’s main effects within each TBI group separately, as well as the use of the Johnson-Neyman technique to identify a region of significance that defines subgroups of participants with different ADHD symptom severity scores. When no significant interaction was detected the model was rerun without the interaction term to examine the main effect of the variable of interest on ADHD symptom severity across TBI groups. For all main effects the percent of variance explained by the key variables (polygenic score, white matter FA, basal ganglia volume) was computed by comparing the R2 of the model with the key variable to the R2 of a reduced model with covariates only (ΔR2). A linear model was also used to examine differences in these 3 types of variables between TBI groups. Interaction and group difference analyses were also performed in the subsample of youth with a history of TBI that were considered high risk for persistent deficits as described in the ‘definition of TBI’ section above. Uncorrected p values are reported and specified as significant based on bonferroni correction performed within analyses (Polygenic Score, 1 comparison, p=0.05; Basal Ganglia Volume, 4 comparisons, p=0.0125; White Matter Microstructure, 6 comparisons, p=0.0083).
Results
Sample Characteristics
Of participants that met inclusion criteria for the ADHD symptom analysis (n=3611), 11.6% (n=418) reported at least one previous mild TBI, and 38.5% (n=161) of these participants with mild TBI reported headache, loss of consciousness or amnesia associated with the injury, qualifying them as high risk for persistent deficits. Injury characteristics of the TBI participants within each analysis group are summarized in Supplemental Tables 1A, 1B, 1C and 1D. Within the ADHD symptom analysis sample, the mild TBI group was older and had a larger proportion of males compared to the group of youth with no TBI (Table 1). Age differences between TBI groups were also detected in the polygenic score, basal ganglia volume and white matter microstructure analysis samples (Supplemental Tables 2A, 2B and 2C).
Table 1.
Participant characteristics in the symptom analysis sample
| No TBI | TBI | TBI high risk | p value TBI | p value TBI high risk | |
|---|---|---|---|---|---|
| Age (mean years) | 13.6 (3.5) | 14.4 (3.4) | 15.4 (3.1) | 1.5e-6 | 4.0e-12 |
| Sex | 1592M 1595F 6U/O | 254M 163F 1U/O |
89M 71F 1U/O | 8.9e-6 | 6.2e-5 |
| Education (mean years) | 15.0 (2.5) | 15.1 (2.3) | 15.3 (2.5) | 0.15 | 0.09 |
| Medication | 378 | 63 | 27 | 0.07 | 0.07 |
| ADHD | 629 | 93 | 30 | 0.20 | 0.86 |
| Anxiety Disorder | 560 | 77 | 33 | 0.61 | 0.19 |
| Behavior Disorder |
727 | 104 | 39 | 0.36 | 0.55 |
| Mood Disorder | 424 | 82 | 33 | 5.76e-4 | 0.01 |
Mean values of continuous variables are reported with standard deviations in brackets. P values reflect differences between specified mild TBI group and no TBI group calculated with students t test for continuous variables and chi squared test for categorical variables. M: male, F: female, U/O: unknown/other. Education: highest level of parental education. Medication: number of participants who were taking medication because of emotions and/or behaviors. Diagnosed anxiety disorders include: agoraphobia, generalized anxiety disorder, panic disorder, and separation anxiety disorder. Diagnosed behavior disorders include: oppositional defiant disorder and conduct disorder. Mood disorders include: major depressive disorder and mania.
ADHD Symptom Analysis
Youth with mild TBI had a higher ADHD symptom severity score compared to those without TBI (No TBI: mean=6.8, SD=3.4; TBI: mean=7.1, SD=3.4; t(3604)=3.0; ΔR2=0.002; p=0.002). This difference did not reach our threshold for statistical significance when examining only youth with mild TBI at high risk for persistent deficits (TBI HR: mean=6.9, SD= 3.5; t(3347)=2.0; ΔR2=0.001; p=0.06).
Polygenic Score Analysis
There was a significant interaction between polygenic score and TBI group (t(1427)=−2.1; p=0.04), indicating that the relationship between polygenic score and symptom severity score differed with respect to TBI history. Polygenic score showed a strong positive association with ADHD symptom score in youth without TBI (t(1224)=3.5; ΔR2=0.009; p=0.004), and no association with ADHD symptom score in those with mild TBI (t(196)=−0.4; ΔR2=−0.004; p=0.7) (Figure 2). Assessing a region of significance indicated that low genetic risk (polygenic score<0.26) is protective against ADHD symptoms in the youth without TBI but not in those with mild TBI. This interaction did not reach significance when examining only mild TBI participants at high risk for persistent deficits (t(1301)=−1.3; p=0.2). There was no difference in mean polygenic score between the youth with and without mild TBI (TBI: mean=0.04, SD= 1.0; No TBI: mean=−0.006, SD= 1.0; t(1429)=0.7; p=0.5), as was the case when examining only high risk mild TBI participants (TBI HR: mean=0.03, SD= −0.002; t(1303)=0.5; p=0.6).
Figure 2.
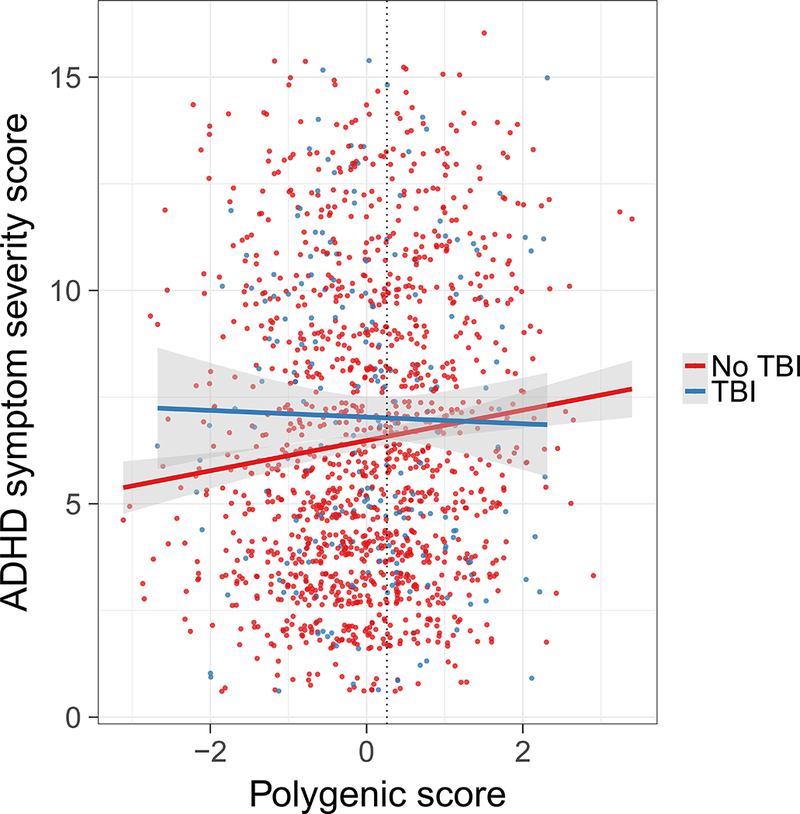
Polygenic score is differentially associated with ADHD symptoms in youth with a history of TBI. A significant interaction between polygenic score and TBI history on ADHD symptom severity is driven by a strong positive relationship in youth without a history of TBI (red) and no association in youth with a history of TBI (blue). Regression lines for those with and without a history of TBI are plotted with shaded 95% confidence intervals. Youth with mild TBI and polygenic scores to the left of the dotted vertical line have higher ADHD symptom severity scores than those with no history of TBI.
Basal Ganglia Volume Analysis
No significant interactions between structure volume and TBI group on ADHD symptom score were detected in the caudate, putamen, accumbens or globus pallidus when including either all mild TBI participants or only those considered high risk. However, a negative association between caudate volume and ADHD symptom score was found in the overall group (t(809)=−2.58; p=0.01; ΔR2=0.007) (Table 2). In addition, putamen volume was larger in high risk mild TBI youth compared to those with no TBI (t(744)=2.6; p=0.01) (Supplemental Table 3).
Table 2.
Associations between basal ganglia volumes and ADHD symptoms
| Interaction - TBI | Interaction - TBI high risk | Main Effect | |||||
|---|---|---|---|---|---|---|---|
| t value | p value | t value | p value | t value | ΔR2 | p value | |
| Caudate | 0.96 | 0.34 | 1.23 | 0.22 | −2.58 | 0.007 | 0.01 |
| Putamen | −0.12 | 0.90 | 0.71 | 0.48 | −1.09 | 0.0002 | 0.27 |
| Accumbens | 0.99 | 0.33 | 0.91 | 0.37 | −1.17 | 0.0004 | 0.24 |
| Globus Pallidus | −0.78 | 0.44 | −0.60 | 0.55 | −1.41 | 0.001 | 0.16 |
Interaction refers to the interaction between structure volume and TBI group on ADHD symptom severity score when examining all participants with mild TBI (TBI) as well as the subset at high risk for persistent deficits (TBI high risk). No significant interactions were detected and the model was rerun without the interaction term to assess main effects of structure volume on symptom severity (Main Effect). Raw p values are reported; effects significant at a Bonferroni corrected p<0.05 are indicated in bold.
White Matter Microstructure Analysis
A significant interaction between FA in the genu of the corpus callosum and TBI group on ADHD symptom score was detected when examining participants with mild TBI at high risk for persistent deficits (t(570)=−2.83; p=0.005). FA in the genu of the corpus callosum showed a positive association with ADHD symptom score in youth without TBI (t(535)=2.52; ΔR2=0.01; p=0.012), but negative association with ADHD symptom score in youth with TBI (t(31)=−3.43; ΔR2=0.25; p=0.0017) (Supplemental Figure 1). Assessing a region of significance indicated that high FA in this tract (>0.49) is associated with lower levels of ADHD symptom severity in the youth with mild TBI than in those without TBI. No significant interactions or main effects were detected in the corpus callosum body, splenium or corona radiata (Table 3). There were no significant differences in tract FA between TBI groups (Supplemental Table 4).
Table 3.
Associations between white matter tract FA and ADHD symptoms
| Interaction - TBI | Interaction - TBI high risk | Main Effect | |||||
|---|---|---|---|---|---|---|---|
| t value | p value | t value | p value | t value | ΔR2 | p value | |
| Corpus Callosum Genu | −1.38 | 0.17 | −2.83 | 0.005 | |||
| Corpus Callosum Body | −0.04 | 0.97 | −1.43 | 0.15 | 0.98 | −0.0005 | 0.33 |
| Corpus Callosum Splenium | −1.14 | 0.25 | −1.98 | 0.05 | 1.12 | 0.0004 | 0.26 |
| Anterior Corona Radiata |
0.67 | 0.50 | 0.36 | 0.72 | 0.46 | −0.001 | 0.64 |
| Superior Corona Radiata | 0.16 | 0.87 | −1.08 | 0.28 | 0.05 | −0.002 | 0.96 |
| Posterior Corona Radiata |
−0.52 | 0.61 | −0.56 | 0.58 | 1.56 | 0.002 | 0.12 |
Interaction refers to the interaction between tract FA and TBI group on ADHD symptom severity score when examining all participants with mild TBI (TBI) as well as the subset at high risk for persistent deficits (TBI high risk). Where significant interactions were observed, the relationship between tract FA and symptoms was assessed in separate groups with and without a history of TBI. When the interaction was not significant the model was rerun without the interaction term to assess effect of tract FA on ADHD symptoms (Main Effect). Raw p values are reported; effects significant at a Bonferroni corrected p<0.05 are indicated in bold.
Sensitivity Analyses
All results remain highly similar when we rerun these analyses in samples of participants where youth who meet criteria for ADHD diagnosis have been excluded (Supplemental Results and Supplemental Tables 5A and 5B) as well as in samples of participants where youth taking medication for emotions and/or behaviours have been excluded (Supplemental Results and Supplemental Tables 6A and 6B).
Discussion
Here we examined genetic and brain structural correlates of ADHD symptoms in a community sample of youth with and without a history of mild TBI. Youth with mild TBI reported increased ADHD symptoms severity, consistent with previously studies(7, 8). Assessment of the relationship between polygenic risk and ADHD symptoms revealed that, as expected, polygenic score was associated with increased ADHD symptom severity; though this relationship was driven by youth with no TBI. Caudate volume was negatively associated with number of ADHD symptoms, regardless of TBI history. In contrast, the volume of the putamen, accumbens and globus pallidus were not associated with number of ADHD symptoms. Intriguingly, an interaction between FA in the genu of the corpus callosum and TBI group on ADHD symptoms indicated that the direction of association between FA and ADHD symptoms was dependent on whether or not participant had a history of TBI. Overall the results of this study suggest that when ADHD symptoms occurs in conjunction with mild TBI, established genetic risk for ADHD may not be an important risk factor. Additionally, they point to common brain structures associated with ADHD symptoms with a potentially different underlying cellular level disruption in the genu of the corpus callosum contributing to symptoms manifestation.
Polygenic risk for ADHD was strongly associated with ADHD symptoms in youth with no TBI and showed no association with ADHD symptoms in those with mild TBI. This suggests that in individuals who experience TBI, the physical insult to the brain and resulting alterations in structure and function may be influential contributors to the presentation of post TBI ADHD symptoms, whereas additive genetic predisposition contributes to developmentally acquired ADHD. These results oppose the idea that those with higher polygenic score may be more vulnerable to developing ADHD symptoms following brain injury but are consistent with a recent study of soldiers with TBI, that found that the genetic predisposition to persistent post-concussion symptoms following TBI does not have substantial overlap with genetic predisposition to neurodegenerative and psychiatric diseases(42).
We found that TBI history did not influence the relationship between basal ganglia volumes and ADHD symptoms. A large body of research has demonstrated reduced striatum and globus pallidus volumes associated with ADHD diagnosis and symptoms(16–18). Recently a large mega-analysis confirmed the relationship between decreased volume in the caudate and putamen and accumbens and ADHD but did not find any differences in the volumes of the globus pallidus in individuals with ADHD(38). Here we detect a weak negative relationship in the caudate, demonstrating that reduced caudate volume is associated with ADHD symptom severity score, but not in the putamen, accumbens and globus pallidus. The major difference between the cited studies and this one is that here we are examining a dimensional variable, ADHD symptom severity score, in a community sample rather than ADHD diagnosis in a clinical sample. Additionally, the psychiatric assessment used in this study to create the ADHD severity score is an abbreviated version of the full K-SADS that is used for diagnosis of ADHD(32). That half of the symptoms of ADHD were sampled here may also contribute to the discrepancy in findings related to the putamen and accumbens.
There was a differential direction of association between FA and ADHD symptoms in the genu of the corpus callosum in youth with and without a history of TBI. This suggests that different means of disruption that correspond to different types of white matter pathology in this tract may contribute to ADHD symptoms in each TBI group. Decreases in FA in the genu of the corpus callosum associated with ADHD symptoms in youth with mild TBI may reflect damaged axons, reduced myelination, inflammation, edema or a combination of these factors(43, 44). However, the interpretation of the pathophysiology that is driving the opposing direction of association in youth with no history of TBI is less straightforward. Many previous studies that use a categorical approach have demonstrated decreased white matter FA in participants with ADHD diagnosis compared to healthy controls (27, 45). Conversely, using a dimensional approach, the literature in children and adolescents indicates a significant amount of support for positive associations between FA and ADHD symptom load in widespread brain regions including the genu or the corpus callosum(28, 46). One possible interpretation is that it reflects compensatory accelerated development of the corpus callosum that is associated with ADHD symptoms. In general these results are consistent with a roll for circuits that contain genu of the corpus callosum in attention processes and ADHD(27, 28, 30, 47) and a particular vulnerability of the genu of the corpus callosum to axonal injury(22–26).
A recent review concluded that “there is a paucity of evidence available to definitively guide management of attention problems after pediatric TBI”(48). As attention is a prerequisite for behavioral and neurocognitive functioning, attention deficits have been shown to relate to daily life problems after paediatric TBI(4). This study suggests that the etiology of developmental versus acquired ADHD may be distinct, providing a description of divergent genetic risk and distinct as well as non-distinct neural substrates. This indicates that fundamental differences exist between acquired and developmental ADHD despite similarities in behavioral features and is in line with previous evidence of distinct neuropsychological impairments(49, 50) and response to treatment(51, 52). Population based estimates in adolescent samples for self-report lifetime concussion are approximately 20%(53, 54), higher than the 11% of youth who reported a previous mild TBI in this cohort. Conversely, population estimates of ADHD in youth range from 5–9%(33, 55, 56), lower than the 18% of participants who met diagnostic criteria in this cohort(32). Irrespective of the exact numbers it stands that a significant number of ADHD cases in the general population have co-occurring ADHD and mild TBI with approximately half of these ADHD cases acquired post TBI(7). Consequently, TBI history should be evaluated by treating clinicians. The identification of children at highest risk of ADHD following TBI may be facilitated by in vivo neuroimaging that evaluates damage to the corpus callosum in conjunction with other known predisposing factors such as preinjury adaptive functioning, psychosocial adversity and socioeconomic status(7, 8).
There are several important limitations to this study. First, ADHD is a risk factor for and major sequela of TBI in youth. In this retrospective analysis we cannot distinguish pre and post injury ADHD and must assume that the group of participants with a history of TBI are reporting symptoms that manifest both before and after injury. Previous studies indicate that of the children who have experienced TBI, half of ADHD cases were present prior to the injury and half developed post injury(7). Nonetheless we were able to identify differences in associations with symptom severity scores between injury and non-injury groups. Future work will need to examine these associations in a prospective manner. Second, we used a polygenic score based on common risk variants to assess genetic risk for ADHD symptoms, which does not take into account all genetic risk. Third, each set of analyses examined different samples dependent on the availability of that type of data and specific exclusion criteria. Because the sample sizes differed between analyses, the analyses were powered to detect different effect sizes (see Supplemental Results). Fourth, in line with using a dimensional approach(57), we generated the ADHD severity score from the GOASESS structured interview, though we acknowledge that this continuous score has not been validated. Future work would benefit from using the Strengths and Weaknesses of ADHD-symptoms and Normal-behavior (SWAN) rating scale which unlike the scale used here is bidirectional and can capture the full spectrum of the population distribution for ADHD symptoms(58). Lastly, replication of these results in a comparable dataset will be an important next step.
Medical history that includes a mild TBI is common in this large community sample of youth and is associated with no elevated genetic risk as well as distinct and non-distinct neural substrates. The identification of characteristic ADHD etiology in youth with a history of TBI is a first step towards understanding neurobiological and clinical heterogeneity, which will ideally pave the way for the development of tailored interventions that may differ among youth with ADHD, informed by presence or absence of TBI history.
Supplementary Material
Acknowledgments
Support for the collection of the data sets was provided by grant RC2MH089983 awarded to Raquel Gur and RC2MH089924 awarded to Hakon Hakonarson. All participants were recruited through the Center for Applied Genomics at The Children’s Hospital in Philadelphia. dbGaP Study Accession: phs000607.v2.p2.
We gratefully acknowledge the funding provided by the SickKids Foundation and the following National Institutes of Health (NIH) grants: R01MH099167 (to A.V.), U01CA199459 (to L.J.O), R03NS088301(to L.J.O.), P41 EB015898 National Center for Image-Guided Therapy, and P41 EB015902 Neuroimage Analysis Center.
This data was presented in abstract form at the 2016 American College of Neuropsychopharmacology annual meeting and the 2017 Society for Biological Psychiatry annual meeting.
Footnotes
Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yeates KO (2010): Mild traumatic brain injury and postconcussive symptoms in children and adolescents. J Int Neuropsychol Soc. 16:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, et al. (2004): Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med.28–60. [DOI] [PubMed] [Google Scholar]
- 3.Max JE (2014): Neuropsychiatry of pediatric traumatic brain injury. Psychiatr Clin North Am. 37:125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konigs M, Heij HA, van der Sluijs JA, Vermeulen RJ, Goslings JC, Luitse JS, et al. (2015): Pediatric Traumatic Brain Injury and Attention Deficit. Pediatrics. 136:534–541. [DOI] [PubMed] [Google Scholar]
- 5.Max JE, Lansing AE, Koele SL, Castillo CS, Bokura H, Schachar R, et al. (2004): Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Dev Neuropsychol. 25:159–177. [DOI] [PubMed] [Google Scholar]
- 6.Bloom DR, Levin HS, Ewing-Cobbs L, Saunder AE, Song J, Fletcher JM, et al. (2001): Lifetime and novel psychiatric disorders after pediatric traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 40:572–579. [DOI] [PubMed] [Google Scholar]
- 7.Levin H, Hanten G, Max J, Li X, Swank P, Ewing-Cobbs L, et al. (2007): Symptoms of attention-deficit/hyperactivity disorder following traumatic brain injury in children. J Dev Behav Pediatr. 28:108–118. [DOI] [PubMed] [Google Scholar]
- 8.Max JE, Schachar RJ, Levin HS, Ewing-Cobbs L, Chapman SB, Dennis M, et al. (2005): Predictors of secondary attention-deficit/hyperactivity disorder in children and adolescents 6 to 24 months after traumatic brain injury. J Am Acad Child Adolesc Psychiatry. 44:1041–1049. [DOI] [PubMed] [Google Scholar]
- 9.Hawi Z, Cummins TD, Tong J, Johnson B, Lau R, Samarrai W, et al. (2015): The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry. 20:289–297. [DOI] [PubMed] [Google Scholar]
- 10.Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. (2005): Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 57:1313–1323. [DOI] [PubMed] [Google Scholar]
- 11.Purper-Ouakil D, Ramoz N, Lepagnol-Bestel AM, Gorwood P, Simonneau M (2011): Neurobiology of attention deficit/hyperactivity disorder. Pediatr Res. 69:69R–76R. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association (2013): Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- 13.Erskine HE, Norman RE, Ferrari AJ, Chan GC, Copeland WE, Whiteford HA, et al. (2016): Long-Term Outcomes of Attention-Deficit/Hyperactivity Disorder and Conduct Disorder: A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry. 55:841–850. [DOI] [PubMed] [Google Scholar]
- 14.Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. (2018): Discovery Of The First Genome-Wide Significant Risk Loci For ADHD. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke B, Neale BM, Faraone SV (2009): Genome-wide association studies in ADHD. Hum Genet. 126:13–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw P, De Rossi P, Watson B, Wharton A, Greenstein D, Raznahan A, et al. (2014): Mapping the development of the basal ganglia in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 53:780–789 e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison-Wright I, Ellison-Wright Z, Bullmore E (2008): Structural brain change in Attention Deficit Hyperactivity Disorder identified by meta-analysis. BMC Psychiatry. 8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakao T, Radua J, Rubia K, Mataix-Cols D (2011): Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 168:1154–1163. [DOI] [PubMed] [Google Scholar]
- 19.Biederman J (2005): Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry. 57:1215–1220. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong RC, Mierzwa AJ, Sullivan GM, Sanchez MA (2016): Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury. Neuropharmacology. 110:654–659. [DOI] [PubMed] [Google Scholar]
- 21.Hannawi Y, Stevens RD (2016): Mapping the Connectome Following Traumatic Brain Injury. Curr Neurol Neurosci Rep. 16:44. [DOI] [PubMed] [Google Scholar]
- 22.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR (1989): Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 15:49–59. [DOI] [PubMed] [Google Scholar]
- 23.Peerless SJ, Rewcastle NB (1967): Shear injuries of the brain. Can Med Assoc J. 96:577–582. [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JJ, Gean AD (2011): Imaging for the diagnosis and management of traumatic brain injury. Neurotherapeutics. 8:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, et al. (2014): Neuroimaging after mild traumatic brain injury: Review and meta-analysis. Neuroimage Clin. 4:283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gentry LR, Godersky JC, Thompson B (1988): MR Imaging of Head Trauma: Review of distribution and radiopathologic features of traumatic lesions. Am J Roentgenology. 150:663–672. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Hu X, Ouyang L, He N, Liao Y, Liu Q, et al. (2016): A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev. 68:838–847. [DOI] [PubMed] [Google Scholar]
- 28.van Ewijk H, Heslenfeld DJ, Zwiers MP, Faraone SV, Luman M, Hartman CA, et al. (2014): Different mechanisms of white matter abnormalities in attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 53:790–799 e793. [DOI] [PubMed] [Google Scholar]
- 29.van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J (2012): Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 36:1093–1106. [DOI] [PubMed] [Google Scholar]
- 30.Onnink AM, Zwiers MP, Hoogman M, Mostert JC, Dammers J, Kan CC, et al. (2015): Deviant white matter structure in adults with attention-deficit/hyperactivity disorder points to aberrant myelination and affects neuropsychological performance. Prog Neuropsychopharmacol Biol Psychiatry. 63:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satterthwaite TD, Connolly JJ, Ruparel K, Calkins ME, Jackson C, Elliott MA, et al. (2015): The Philadelphia Neurodevelopmental Cohort: A publicly available resource for the study of normal and abnormal brain development in youth. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, et al. (2015): The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merikangas K, Avenevoli S, Costello J, Koretz D, Kessler RC (2009): National comorbidity survey replication adolescent supplement (NCS-A): I. Background and measures. J Am Acad Child Adolesc Psychiatry. 48:367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. (2014): Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 86:544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Schizophrenia Consortium Purcell SM, Wray NR Stone JL, Visscher PM O Donovan MC, et al. (2009): Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakravarty MM, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P, et al. (2013): Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp. 34:2635–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL (2006): The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage. 30:359–376. [DOI] [PubMed] [Google Scholar]
- 38.Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, et al. (2017): Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry. 4:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, et al. (2014): DTIPrep: quality control of diffusion-weighted images. Front Neuroinform. 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. (2012): 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 30:1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norton I, Essayed WI, Zhang F, Pujol S, Yarmarkovich Y, Golby AJ, et al. (2017): SlicerDMRI: Open Source Diffusion MRI Software for Brain Cancer Research. Cancer Research. 77:e101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polimanti R, Chen CY, Ursano RJ, Heeringa SG, Jain S, Kessler RC, et al. (2016): Cross-Phenotype Polygenic Risk Score Analysis of Persistent Post-Concussive Symptoms in U.S. Army Soldiers with Deployment-Acquired Traumatic Brain Injury. Journal of neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Assaf Y, Pasternak O (2008): Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 34:51–61. [DOI] [PubMed] [Google Scholar]
- 44.Tu TW, Williams RA, Lescher JD, Jikaria N, Turtzo LC, Frank JA (2016): Radiological-pathological correlation of diffusion tensor and magnetization transfer imaging in a closed head traumatic brain injury model. Ann Neurol. 79:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ameis SH, Lerch JP, Taylor MJ, Lee W, Viviano JD, Pipitone J, et al. (2016): A Diffusion Tensor Imaging Study in Children With ADHD, Autism Spectrum Disorder, OCD, and Matched Controls: Distinct and Non-Distinct White Matter Disruption and Dimensional Brain-Behavior Relationships. Am J Psychiatry. 173:1213–1222. [DOI] [PubMed] [Google Scholar]
- 46.Wu ZM, Bralten J, Cao QJ, Hoogman M, Zwiers MP, An L, et al. (2016): White Matter Microstructural Alterations in Children with ADHD: Categorical and Dimensional Perspectives. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stave EA, De Bellis MD, Hooper SR, Woolley DP, Chang SK, Chen SD (2017): Dimensions of Attention Associated With the Microstructure of Corona Radiata White Matter. J Child Neurol.883073816685652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Backeljauw B, Kurowski BG (2014): Interventions for attention problems after pediatric traumatic brain injury: what is the evidence? PM R. 6:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinopoli KJ, Schachar R, Dennis M (2011): Traumatic brain injury and secondary attention-deficit/hyperactivity disorder in children and adolescents: the effect of reward on inhibitory control. J Clin Exp Neuropsychol. 33:805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slomine BS, Salorio CF, Grados MA, Vasa RA, Christensen JR, Gerring JP (2005): Differences in attention, executive functioning, and memory in children with and without ADHD after severe traumatic brain injury. J Int Neuropsychol Soc. 11:645–653. [DOI] [PubMed] [Google Scholar]
- 51.Ripley DL, Morey CE, Gerber D, Harrison-Felix C, Brenner LA, Pretz CR, et al. (2014): Atomoxetine for attention deficits following traumatic brain injury: results from a randomized controlled trial. Brain Inj. 28:1514–1522. [DOI] [PubMed] [Google Scholar]
- 52.Jin C, Schachar R (2004): Methylphenidate treatment of attention-deficit/hyperactivity disorder secondary to traumatic brain injury: a critical appraisal of treatment studies. CNS Spectr. 9:217–226. [DOI] [PubMed] [Google Scholar]
- 53.Ilie G, Boak A, Adlaf EM, Asbridge M, Cusimano MD (2013): Prevalence and correlates of traumatic brain injuries among adolescents. JAMA. 309:2550–2552. [DOI] [PubMed] [Google Scholar]
- 54.Veliz P, McCabe SE, Eckner JT, Schulenberg JE (2017): Prevalence of Concussion Among US Adolescents and Correlated Factors. JAMA. 318:1180–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willcutt EG (2012): The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 9:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007): The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 164:942–948. [DOI] [PubMed] [Google Scholar]
- 57.Miller G (2010): Psychiatry. Beyond DSM: seeking a brain-based classification of mental illness. Science. 327:1437. [DOI] [PubMed] [Google Scholar]
- 58.Swanson JM, Schuck S, Porter MM, Carlson C, Hartman CA, Sergeant JA, et al. (2012): Categorical and Dimensional Definitions and Evaluations of Symptoms of ADHD: History of the SNAP and the SWAN Rating Scales. Int J Educ Psychol Assess. 10:51–70. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


