Abstract
Purpose
Studies on returning variants of uncertain significance (VUS) have predominantly included patients with a personal or family history of cancer and cancer-associated gene VUS. This study examined health behaviors amongst participants with cardiomyopathy-associated gene VUS, but without a personal history of cardiomyopathy.
Methods
Sixty-eight eligible participants without apparent cardiomyopathy who received VUS in cardiomyopathy-associated genes completed a survey of health behaviors, disclosure, distress, uncertainty, positive experiences, decisional conflict, and perceived value. Medical records of participants who reported cardiac testing because of their VUS were reviewed for testing indication(s).
Results
Two participants had cardiac testing due to their VUS alone. Four had cardiac testing because of their VUS and other clinical indications and 12 changed health behaviors, including one participant who was subsequently diagnosed with cardiomyopathy. Distress, uncertainty, and decisional conflict were low (means= 1.2, 4.2, 24.5; scale ranges= 0–30, 0–45, 15–75, respectively), and positive experiences and perceived value were moderate (means= 12.4, 14.4; scale range= 0–20, 4–20, respectively). Greater perceived value was associated with greater likelihood to engage in health behaviors (p= 0.04).
Conclusion
VUS can be returned to apparently unaffected individuals with modest use of healthcare resources, minimal behavioral changes, and favorable psychological reactions.
Keywords: Healthcare use, health behaviors, psychological reactions, variants of uncertain significance, genome sequencing
INTRODUCTION
Variants of uncertain significance (VUS) are a common challenge in clinical genomics.1 Whether VUS should be returned is a major question as clinicians and investigators incorporate genomics into their practice and research. Although participants have positive attitudes toward the potential receipt of VUS,2 there is mixed evidence about how VUS affect health behaviors.
The predominant outcomes that have been studied are surveillance and prophylactic surgery related to the risk of breast and/or ovarian cancer following BRCA1/2 genetic testing. These studies have primarily been conducted amongst women who had a sufficient personal or family history of those cancers to recommend genetic testing. There are conflicting findings about whether women with VUS in BRCA1/2 are less likely3,4 or just as likely5 to pursue surveillance or surgery as those with pathogenic variants. These differences in levels of surveillance and surgery could be attributed to variations among study populations in the magnitude of participants’ cancer risks based on personal and family histories. A few studies have attempted to control for this by reviewing the medical records of patients who received VUS to distinguish surveillance or surgery that is clinically indicated based on personal or family history from that which is not. Some showed appropriate levels of screening and/or surgeries,6,7 while others identified a subset of women who had surgery and/or screening beyond what was indicated by their personal and family history.8 Regarding other health behaviors, two studies found that the majority of VUS recipients disclosed their result to at least one family member.9,10
The psychological reactions to VUS have also been studied, although there has been variability in the outcome measures used. The most commonly-studied outcome is test-related distress, and correlations of distress and health behavior changes after a diagnosis of cancer have been identified.11 However, there is not consistency among findings on how test-related distress is affected by receipt of VUS. Some studies showed that women who receive VUS from BRCA1/2 testing have distress levels similar to women who receive negative results,6 while others suggested that the women with VUS have significantly greater levels of test-related distress.12 A stronger personal or family history of cancer has been associated with greater levels of test-related distress.13
Most studies on outcomes of receipt of VUS were conducted with women who received BRCA1/2 VUS and had a personal and/or family history of relevant cancers. Although VUS are not generally returned to healthy participants, it is critical to research a range of result return practices so that future recommendations are evidence-based.14 In keeping with this objective, we designed a study to utilize genomic screening, as opposed to clinically-indicated genetic testing, to identify VUS in cardiomyopathy-associated genes in participants not recruited for a personal or family history of cardiomyopathy (Figure S1). The VUS results were split into two groups using a single computational algorithm15 (VUS-Low and VUS-High, which were based on the likelihood of deleteriousness assigned by the algorithm) in order to study whether participants distinguished between them. We published findings from a 2-week follow-up survey, which found that most participants did not have significantly differing responses to subcategories of VUS (e.g., VUS-low versus VUS-High) but they accurately recalled the meaning of their result and intended to share it with family members and their doctor(s), and 46% intended to change their health behaviors.16
Here, we present the findings from an 8-month follow-up survey from the same study, which measured distinct outcomes. The primary aim of the present study was to describe health behaviors, as opposed to intentions, in response to receipt of VUS amongst unaffected participants. The secondary aim was to determine if there were any differences in demographics or psychological reactions between participants who engaged in health behaviors due to their VUS versus those who did not.
MATERIALS AND METHODS
Participants were recruited from an ancillary project within the ClinSeq® study,17 which consents subjects for sequencing and return of individual results. Participants with VUS in one of 20 cardiomyopathy-associated genes were contacted about their interest in receiving a variant with unclear implications for their heart disease risk. Of the 104 contacted, 81 participants consented, had their results validated in a CLIA laboratory, and received their results in a standardized, in-person session. Participants were told that their results were categorized as VUS based on a modification of the ACMG/AMP criteria, that there were no published data on those specific variants, and that variants had been subcategorized into either a VUS-High or VUS-Low category for research purposes based on scores from a single predictive algorithm (CADD)15. Two weeks following result disclosure, participants received a letter summarizing their result and a link to a survey with questions about their health behavior intentions. Details about this study design, and results of the two-week survey have been published.16
Eighty participants from the VUS study were recruited via telephone and mail to take an eight-month follow-up survey. One participant was not recruited due to administrative oversight, and one was later excluded from analysis due to a personal history of cardiomyopathy.
Survey measures included:
Health behaviors: Participants were asked if they saw additional providers, had cardiac testing (echocardiogram, electrocardiogram, stress test), or made changes to their diet, exercise, or medication in response to their VUS.
Disclosure of VUS: Participants were asked whether they disclosed their VUS to their physicians, partner/spouse, children, siblings, or parents.
Psychological impact: Participants completed a version of the Multidimensional Impact of Cancer Risk Assessment (MICRA)18 modified to refer to VUS results. The MICRA has 23 items forming three subscales: test-related distress (six items; α= 0.90; e.g., “feeling upset about my result”), uncertainty (nine items; α= 0.75; e.g., “worrying about my risk of becoming sick or ill”), and positive experiences (four items; α= 0.80; e.g., “feeling satisfied with family communication about my genetic result”). Responses were summed to create subscale scores. A higher positive experience score indicated a less positive experience.
Decisional conflict: Participants completed the Decisional Conflict Scale,19 comprised of 15-items (α= 0.97; e.g., “I am satisfied with my decision”). Responses were summed to create a total score.
Perceived value: Perceived value was measured with a new, 4-item scale (α= 0.90; “My sequence result is… valuable for maintaining my future health/valuable for maintaining my family’s future health/useful to my physician” and “I trust my sequence result.”)
All participants who reported on the survey that they had engaged in health behaviors (receiving cardiac testing or changing diet, exercise, or medications) due to their VUS were contacted via telephone to confirm if the behaviors were undertaken in response to the VUS (Supplementary Materials and Methods). Participants who reported these behaviors on their survey and confirmed them via telephone were categorized as engaging in health behaviors due to their VUS. Participants who reported having cardiac testing since their ClinSeq® enrollment were asked to release those records. The records for participants who reported any testing due to their VUS (N= 9) were reviewed to determine indications for testing.
Means and standard deviations were calculated using Microsoft Excel (2017). Chi-squared and t-tests were performed to compare participants who engaged in health behaviors due to their VUS versus those who did not on categorical and continuous outcomes, respectively, using GraphPad QuickCalcs (2017). Cronbach alpha values were calculated using University of Connecticut Excel Spreadsheet to Calculate Instrument Reliability Estimates (2017).
This study was approved by the National Human Genome Research Institute Institutional Review Board.
RESULTS
Sixty-eight participants (85.0%) completed surveys. Most had at least a college degree (54, 79.4%), were non-Hispanic White (54, 79.4%), and had results classified as a VUS-High (41, 60.3%). Following enrollment, nine participants (13.2%) disclosed a family history of cardiomyopathy. There were no differences in health behaviors between demographic categories, VUS-High and VUS-Low groups, those with a family history of cardiomyopathy or sudden cardiac death and those without, those who previously received a genetic testing result through ClinSeq® and those who did not, and those with coronary artery disease and those without. (Table 1). Most participants told their spouse (61, 90.0%), at least one child (37, 54.4%), and their physician (36, 52.9%) about their VUS.
Table 1.
Demographic Characteristics and Psychological Reactions to Variants of Uncertain Significance (VUS)
| 3 | All Participants (N= 68) n (%) |
Participants with Healthcare Use or Health Behavior Change (n= 15)a n (%) |
Participants without Healthcare Use or Health Behavior Change (n= 51)a n (%) |
|
|---|---|---|---|---|
| Demographics | ||||
| Female | 33 (48.5%) | 7 (46.7%) | 26 (50.1%) | |
| College graduate or beyond | 54 (79.4%) | 12 (80.0%) | 42 (82.3%) | |
| Non-Hispanic/White | 54 (79.4%) | 10 (66.7%) | 43 (84.3%) | |
| Bin 4 (CVD)b | 13 (19.1%) | 2 (13.3%) | 11 (21.6%) | |
| VUS-High | 41 (60.3%) | 9 (60.0%) | 31 (60.8%) | |
| Previous genetic result from ClinSeq® | 42 (61.8%) | 11 (73.3%) | 31 (60.8%) | |
| Family history of cardiomyopathy | 9 (13.2%) | 1 (6.7) | 8 (15.7%) | |
| Family history of sudden cardiac death | 17 (25.0%) | 4 (26.7%) | 13 (25.5%) | |
| Psychological Reactions (Range) | Mean (SD) | Mean (SD) | Mean (SD) | |
| MICRA distress subscale (0–30) | 1.2 (3.1) | 2.5 (5.2) | 0.9 (2.0) | |
| MICRA uncertainty subscale (0–45) | 4.2 (4.9) | 6.1 (6.1) | 3.7 (4.5) | |
| MICRA positive experiences subscale (0–20) | 12.4 (6.3) | 11.0 (6.5) | 13.0 (6.1) | |
| Decisional Conflict Scale (15–75) | 24.5 (12.2) | 23.1 (11.1) | 25.3 (12.6) | |
| Perceived value (4–20) | 14.4 (4.6) | 16.5c (3.4) | 14.0c (4.3) | |
Two participants were not reached for telephone follow-up and are not included in either column
Based on Framingham Heart Study score. Bin 4= presence of cardiovascular disease.
Participants who reported health behavior changes had significantly higher levels of perceived value than participants who did not (t= 2.066, p= 0.043)
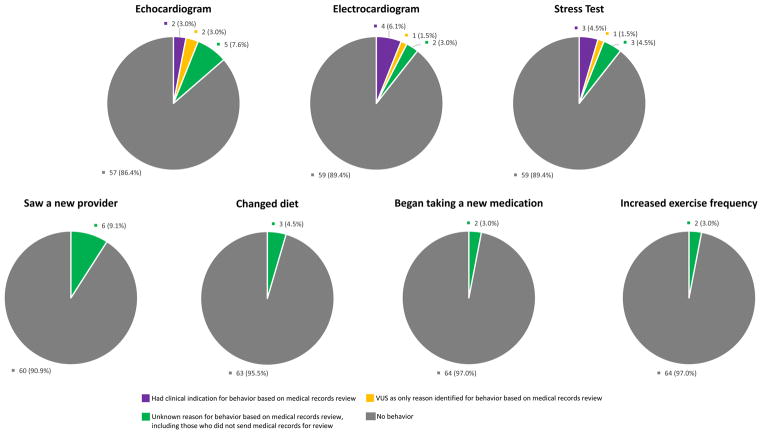
Fifteen participants confirmed via telephone that they engaged in health behaviors due to their VUS, nine of whom reported receiving cardiac testing (e.g., echocardiogram, cardiac stress test). Based on record review, only two participants had no other clinical indication for their testing. Four participants had testing due to a clinical indication, two of whom had testing due to clinical indications and receipt of their VUS. Of these two, one had a personal history of hypertension and a family history of cardiomyopathy. She received an echocardiogram about four weeks after her result disclosure, followed by an electrocardiogram six weeks later, and was found to have borderline concentric left ventricular hypertrophy. The second individual had a personal history of shortness of breath and coronary artery disease. Twelve weeks after receiving his result, he had an electrocardiogram and a stress test, both of which were normal. The remaining three participants had unknown reasons for cardiac testing according to the review of their records. Regarding other health behaviors, 12 participants made changes: six saw new providers, two changed their diets (e.g., ate less red meat), two added medication (e.g., CoQ10 and fish oil), one increased exercise frequency, and one changed her diet and exercise frequency (Figure 1).
Figure 1.
Self-Reported Health Behavior Changes Following Variants of Uncertain Significance (VUS) Disclosure
Test-related distress, uncertainty, and decisional conflict levels were low and levels of perceived value and positive experiences were moderate. Participants who reported changing their health behaviors had higher levels of perceived value (p= 0.043) than those who did not change their health behaviors (Table 1).
DISCUSSION
Given that VUS are already being returned to patients and are going to increase in frequency as panel testing becomes more widely used,3,4,6–9,16 this study provides data on healthcare outcomes following their receipt amongst healthy individuals. These results show modest healthcare utilization attributable to the receipt of cardiomyopathy VUS, suggesting that VUS can be returned to well-educated, clinically unaffected patients without excessive use of healthcare resources. Although nine participants reported their VUS as the reason for additional care, four of them had other clinical indications for that care. Only two participants received unnecessary healthcare. Amongst the participants who reported cardiac testing due to their VUS and had a clinical indication to do so according to their medical records review was one participant who was found to have borderline concentric left ventricular hypertrophy. This participant had hypertension and a family history of cardiomyopathy but did not receive appropriate cardiac testing until her result was returned. This example, taken with the data about the rarity of testing due to VUS alone, suggests that VUS may prompt conversations between patients and physicians about warranted clinical evaluations. Although this alone is not a sufficient reason to return VUS, it may be one benefit, and broader data on potential benefits of returning VUS are needed for designing future studies and informing policies.14
Negative psychological outcomes following disclosure of VUS were uncommon, which is consistent with research finding that patients generally adapt well to genetic testing results, particularly in the long-term.20 It may be that positive outcomes are reported in studies of genetic testing because participants only pursue such testing if they anticipate that they will be able to cope well with the results. Participants in our study with greater levels of perceived value were more likely to engage in health behaviors after receipt of VUS, suggesting that this factor merits investigation in future studies.16 Data on why participants engage in health behaviors may help identify who would benefit from additional education or counseling. This was a descriptive study with a small, homogeneous sample; further studies with larger, more diverse populations are needed.
Supplementary Material
Acknowledgments
The authors thank the ClinSeq® participants, and Dr. William M.P. Klein for his contribution to development of survey measures. This research was funded by grant HG200387 04 from the Intramural Research Program of the National Human Genome Research Institute.
Footnotes
CONFLICT OF INTEREST
IMM, KLL, TAL, DN, JJJ, and BBB have no conflicts to disclose. LGB receives royalties from Genentech Corp., is an unpaid advisor to Illumina Corp., and receives honoraria from Wiley-Blackwell Inc.
Supplementary information is available at the Genetics in Medicine website.
References
- 1.Bertier G, Hetu M, Joly Y. Unsolved challenges of clinical whole-exome sequencing: a systematic literature review of end-users' views. BMC Med Genomics. 2016;9(1):52. doi: 10.1186/s12920-016-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Facio FM, Eidem H, Fisher T, et al. Intentions to receive individual results from whole-genome sequencing among participants in the ClinSeq study. Eur J Hum Genet. 2013;21(3):261–265. doi: 10.1038/ejhg.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter S, Haroun I, Graham TC, Eisen A, Kiss A, Warner E. Variants of unknown significance in BRCA testing: impact on risk perception, worry, prevention and counseling. Ann Oncol. 2013;24(Suppl 8):viii69–viii74. doi: 10.1093/annonc/mdt312. [DOI] [PubMed] [Google Scholar]
- 4.Garcia C, Lyon L, Littell RD, Powell CB. Comparison of risk management strategies between women testing positive for a BRCA variant of unknown significance and women with known BRCA deleterious mutations. Genet Med. 2014;16(12):896–902. doi: 10.1038/gim.2014.48. [DOI] [PubMed] [Google Scholar]
- 5.Vos J, Gomez-Garcia E, Oosterwijk JC, et al. Opening the psychological black box in genetic counseling. The psychological impact of DNA testing is predicted by the counselees' perception, the medical impact by the pathogenic or uninformative BRCA1/2-result. Psychooncology. 2012;21(1):29–42. doi: 10.1002/pon.1864. [DOI] [PubMed] [Google Scholar]
- 6.Culver JO, Brinkerhoff CD, Clague J, et al. Variants of uncertain significance in BRCA testing: evaluation of surgical decisions, risk perception, and cancer distress. Clin Genet. 2013;84(5):464–472. doi: 10.1111/cge.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray ML, Cerrato F, Bennett RL, Jarvik GP. Follow-up of carriers of BRCA1 and BRCA2 variants of unknown significance: variant reclassification and surgical decisions. Genet Med. 2011;13(12):998–1005. doi: 10.1097/GIM.0b013e318226fc15. [DOI] [PubMed] [Google Scholar]
- 8.Ready K, Gutierrez-Barrera AM, Amos C, et al. Cancer risk management decisions of women with BRCA1 or BRCA2 variants of uncertain significance. Breast J. 2011;17(2):210–212. doi: 10.1111/j.1524-4741.2010.01055.x. [DOI] [PubMed] [Google Scholar]
- 9.Solomon I, Harrington E, Hooker G, et al. Lynch syndrome limbo: Patient understanding of variants of uncertain significance. Journal of Genetic Counseling. 2017 doi: 10.1007/s10897-017-0066-y. No Pagination Specified. [DOI] [PubMed] [Google Scholar]
- 10.Bradbury AR, Patrick-Miller L, Egleston BL, et al. When parents disclose BRCA1/2 test results: Their communication and perceptions of offspring response. Cancer. 2012;118(13):3417–3425. doi: 10.1002/cncr.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnoll RA, Malstrom M, James C, et al. Correlates of tobacco use among smokers and recent quitters diagnosed with cancer. Patient Educ Couns. 2002;46(2):137–145. doi: 10.1016/s0738-3991(01)00157-4. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill SC, Rini C, Goldsmith RE, Valdimarsdottir H, Cohen LH, Schwartz MD. Distress among women receiving uninformative BRCA1/2 results: 12-month outcomes. Psychooncology. 2009;18(10):1088–1096. doi: 10.1002/pon.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dijk S, Timmermans DR, Meijers-Heijboer H, Tibben A, van Asperen CJ, Otten W. Clinical characteristics affect the impact of an uninformative DNA test result: the course of worry and distress experienced by women who apply for genetic testing for breast cancer. J Clin Oncol. 2006;24(22):3672–3677. doi: 10.1200/JCO.2005.03.7259. [DOI] [PubMed] [Google Scholar]
- 14.Jarvik GP, Amendola LM, Berg JS, et al. Return of genomic results to research participants: the floor, the ceiling, and the choices in between. Am J Hum Genet. 2014;94(6):818–826. doi: 10.1016/j.ajhg.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawal T, Lewis KL, Johnston JJ, et al. Disclosure of Cardiac Variants of Uncertain Significance Results in an Exome Cohort. Clin Genet. 2018 doi: 10.1111/cge.13220. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biesecker LG, Mullikin JC, Facio FM, et al. The ClinSeq Project: piloting large-scale genome sequencing for research in genomic medicine. Genome Res. 2009;19(9):1665–1674. doi: 10.1101/gr.092841.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cella D, Hughes C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002;21(6):564–572. [PubMed] [Google Scholar]
- 19.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 20.Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10(1):19–32. doi: 10.1097/GIM.0b013e31815f524f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.