Abstract
Rationale:
The infralimbic cortex (IL) and its downstream projection target the nucleus accumbens shell (NAshell) mediate the active suppression of cocaine-seeking behavior. Although an optogenetic approach would be beneficial for stimulating the IL and its efferents to study their role during reinstatement of cocaine seeking, the use of channelrhodopsin introduces significant difficulties, as optimal stimulation parameters are not known.
Objectives:
The present experiments utilized a stable step-function opsin (SSFO) to potentiate endogenous activity in the IL and in IL terminals in the NAshell during cocaine-seeking tests to determine how these manipulations affect cocaine-seeking behaviors.
Methods:
Rats first underwent 6-h access cocaine self-administration followed by 21 – 27 days in the homecage. Rats then underwent cue-induced and cocaine-primed drug-seeking tests during which the optogenetic manipulation was given. The same rats then underwent extinction training, followed by cue-induced and cocaine-primed reinstatements.
Results:
Potentiation of endogenous IL activity did not significantly alter cue-induced or cocaine-primed drug seeking following the homecage period. However, following extinction training, enhancement of endogenous IL activity attenuated cue-induced reinstatement by 35% and cocaine-primed reinstatement by 53%. Stimulation of IL terminals in the NAshell did not consistently alter cocaine-seeking behavior.
Conclusion:
These results suggest the utility of an SSFO-based approach for enhancing activity in a structure without driving specific patterns of neuronal firing. However, the utility of an SSFO-based approach for axon terminal stimulation remains unclear. Moreover, these results suggest that the ability of the IL to reduce cocaine seeking depends, at least in part, on rats first having undergone extinction training.
Keywords: optogenetics, channelrhodopsin, reinstatement, incubation of craving, cocaine seeking
Introduction
Evidence implicates the infralimbic cortex (IL), a region of the rodent medial prefrontal cortex, as part of the neurocircuitry that inhibits cocaine seeking (Augur et al. 2016; Gutman et al. 2017; LaLumiere et al. 2010; LaLumiere et al. 2012; Muller Ewald and LaLumiere 2017; Peters et al. 2009; Peters et al. 2008; Van den Oever et al. 2013). Work suggests that pharmacological IL activation, via either direct AMPA receptor activation or potentiation, reduces cocaine-primed and cue-induced reinstatement following cocaine self-administration and at least 8 days of extinction training (LaLumiere et al. 2012; Peters et al. 2008). Furthermore, IL activity regulates the extinction of cocaine-seeking behaviors, as pharmacological or optogenetic disruption of IL activity during an extinction training session or during the post-session consolidation period disrupts extinction learning (Gutman et al. 2017; LaLumiere et al. 2010).
Considering the limitations of traditional pharmacological methods, research using optogenetic-based stimulation would be a valuable approach for investigating the role of the IL during cocaine seeking sessions. Yet, the use of standard optogenetic tools such as channelrhodopsin (ChR2) to stimulate IL activity also presents difficulties, as their use depends on identification of effective stimulation parameters, potentially necessitating an unwieldy number of experiments to identify optimal stimulation frequencies (Huff et al. 2016; Huff et al. 2013). An alternative optogenetic approach that avoids this issue is the use of stable-step function opsins (SSFOs), in which brief illumination induces a long-lasting depolarized state in transduced cells (Yizhar et al. 2011a; Yizhar et al. 2011b). Consequently, activated neurons become more sensitive to depolarizing inputs, providing a method for enhancing endogenous activity in a structure. Moreover, because the open-state half-life of SSFOs is ~29 minutes following brief illumination (Yizhar et al. 2011b), animals do not need to remain connected to optical fibers during behavioral testing. Thus, the present study investigated the utility of an SSFO-based approach for enhancing IL activity during cocaine-seeking sessions.
The current work also addressed fundamental questions regarding the ability of the IL to inhibit cocaine-seeking behavior. Little work has manipulated IL activity during cocaine seeking following 6-h access cocaine self-administration, an important issue if such a model produces behavioral phenotypes that better mimic patterns observed in human drug taking (Ahmed and Koob 1998; Lenoir et al. 2012; Pickens et al. 2011). Recent work using 2-h access cocaine self-administration suggests that the ability of the IL to suppress cocaine-seeking behavior depends on animals having experienced prior extinction training (Augur et al. 2016). However, other work based on a 6-h access model suggests the development of endogenous changes in synaptic composition in projections from the IL to the nucleus accumbens shell (NAshell) without prior extinction training (Ma et al. 2014), which appear to be sufficient to decrease cue-driven cocaine seeking. Therefore, whether IL activation suppresses cocaine seeking following a drug-free period in the homecage (without extinction training) remains unclear.
To address these questions, the present study used SSFOs to enhance IL activity during cocaine seeking in rats that had previously been trained to self-administer cocaine under a 6-h access paradigm. Following self-administration, rats underwent a drug-free period in the homecage, followed by cue-induced and cocaine-primed drug-seeking tests. The same rats then underwent extinction training followed by cue-induced and cocaine-primed reinstatement. Rats received enhancement of IL activity via SSFO activation or sham stimulation prior to each drug-seeking test, in a counterbalanced within-subjects design, to determine how enhancing endogenous IL activity influences cocaine seeking under these different conditions.
Materials and Methods
The materials and methods section in the Online Resource provides greater detail of experimental procedures. For the sake of brevity, this section provides the critical information for understanding the Results and Discussion.
Rats and Surgery
Male Sprague-Dawley rats (~300 g at time of surgery, n = 41) underwent implantation of an intra-jugular catheter and stereotaxic surgery for bilateral virus injection of AAV5-CaMKIIα-hChR2(C128S/D156A)-eYFP or AAV5-CAMKIIα-eYFP (control) into the IL and bilateral fiber optic implantation in the IL (Experiments 1 and 2) or the NAshell (Experiment 3).
Optogenetic Manipulation
To enhance IL activity, the present experiments used a stable step-function opsin, a modified form of channelrhodopsin that, like channelrhodopsin, is activated by 473 nm light. However, channel mutations (C128S and D156A) of this particular opsin delay channel closing time in the dark (i.e., when illumination is not being given) (Yizhar et al. 2011b). This allows for high stability of the active state, with an open-state half-life of approximately 29 min, which is ideal for examining behavior in freely moving animals. SSFOs can also be deactivated with 561 nm light which enables high experimental control over manipulations (Yizhar et al. 2011b). Moreover, SSFOs show lower peak photocurrent (231.08 ± 31.19 pA) than traditional channelrhodopsins. As a result, SSFOs can be used to put neurons into a more depolarized state, thereby increasing sensitivity to endogenous neuronal inputs instead of directly driving neuronal activity.
To activate the SSFOs in Experiments 1 and 3, rats’ implanted fiber optics were connected to fiber optic leashes 1 min prior to the behavioral test session, and rats then received 473 nm laser stimulation (2 s duration, ~ 2 mW). Sham treatment involved following the same procedures, but no laser stimulation was provided. One min following the end of the behavioral test session, rats were connected to the 561 nm laser (10 s duration, ~ 10 mW) to close the SSFO channels. The same procedures were followed in Experiment 3, although rats did not express SSFOs.
Electrophysiological Verification
For ex vivo verification of SSFO functionality, coronal brain slices were obtained from rats expressing SSFOs in the IL. Whole-cell patch-clamp recordings were performed on eYFP-positive neurons. Light was delivered to the slice through an optical fiber from a 473 nm laser and a 561 nm laser. For in vivo electrophysiological verification, a 16-channel microwire array surrounding a fiber optic was implanted into the IL. SSFOs were activated using 2 s of 473 nm laser in awake, freely moving, drug naïve rats. Two minutes after laser onset, SSFO channels were closed with 10 s of 561 nm laser. This process was repeated for a total of 40 cycles. Local field potentials were filtered between 1 and 100 Hz using wide-band boards. Spectrograms were calculated using a 2-cycle Morlet from EEGLAB’s newtimef.m function.
Behavioral Procedures
The materials and methods section in the Online Resource and timelines in the figures (panels A and G of Figures 2-4) provide greater detail regarding the behavioral procedures for the experiments. In brief, self-administration training was carried out for 6 h/d, 6 d/week for a minimum of 10 d (mean +/− SEM = 10.70 +/− 0.23), with a minimum of 40 lever presses during the last 2 d sessions. Self-administration occurred in standard operant conditioning chambers equipped with 2 levers, a cue light, and a 4500 Hz-tone generator. During cocaine self-administration, active lever presses resulted in a 50 μl infusion containing 200 μg of cocaine (0.6 mg/kg/infusion) and the presentation of light and tone cues (cocaine-HCL, dissolved in 0.9% saline and kindly provided by the National Institute on Drug Abuse).
One d after the last self-administration session, rats underwent a single 1-h cue-induced drug-seeking session, in which active lever presses yielded the cocaine-associated cues (light and tone) but no cocaine infusions, which served as the incubation-of-craving baseline test. Following this, rats were kept in the homecage for 21–27 d (mean +/− SEM = 23.43 +/− 0.39 d), following which rats underwent another 1-h cue-induced cocaine-seeking session to test for incubation of cocaine craving. Following the incubation test, rats underwent 3 d in the homecage followed by two cue-induced drug-seeking tests (1 h each), separated by 3 d in the homecage. These tests were given in combination with optical manipulations (sham vs. illumination), which were administered in a counterbalanced manner, using a within-subjects design. After 3 more d in the homecage, rats underwent two cocaine-primed (5 mg/kg) drug-seeking tests (1 h each) in combination with counterbalanced optical manipulations, again separated by 3 d in the home cage.
Rats then underwent extinction training for a minimum of 8 d (1 h/d), in which active lever presses did not yield cocaine infusions or cocaine-associated cues. When rats reached criterion (< 15 active lever presses/h for 2 consecutive d), they underwent cue-induced and cocaine-primed reinstatement tests in the same manner as described above, with at least 3 d of re-extinction to criterion separating each reinstatement test.
In Experiment 1, all rats expressed SSFOs in IL pyramidal cells and underwent the above behavioral procedures receiving SSFO-mediated enhancement of IL activity during drug-seeking sessions. Experiment 2 was conducted to determine whether the findings of Experiment 1 were due to the effects of light alone. Rats expressing eYFP alone (no opsin) in the IL underwent the same procedures as in Experiment 1. Although the theoretical utility of SSFOs would appear to derive from placing dendrites and cell bodies into a more depolarized state, recent work suggests that activation of SSFOs in axon terminals of transduced neurons also alters behavior (Liu et al. 2016). Experiment 3 was conducted to determine the utility of an SSFO-based approach in stimulating IL axonal terminals in the NAshell during cocaine-seeking sessions. In this experiment, rats expressed SSFOs in the IL and received fiber optic implants aimed at the NAshell (the downstream target of the IL known to play a role in the inhibition of cocaine seeking; Augur et al. 2016). Rats then underwent the same procedures as in Experiment 1, but with SSFO-mediated activation of the IL axonal terminals in the NAshell.
Locomotor activity testing
Upon completion of cocaine-seeking experiments, the effects of SSFO activation on locomotor activity were examined in the same rats. Rats received optical manipulations in a counterbalanced manner (each test separated by 2 d) identical to that described above and were placed into the open field chamber for 15 min.
Histological Analysis
In brief, brains were coronally sectioned, mounted on gelatin-coated slides and stained with Cresyl violet to verify fiber optic termination points. Slices also underwent immunohistochemical staining to verify virus expression. Rats with misplaced virus expression or fiber optics were excluded from analysis (see Online Resource for more details).
Statistical Analysis
For all experiments, lever presses during cocaine-seeking sessions prior to extinction training were analyzed using a within-subjects t-test in GraphPad Prism (GraphPad Software, La Jolla, CA). For reinstatement tests, lever presses during the extinction baseline (an average of the 2 d immediately preceding reinstatement) and reinstatement tests were examined using a one-way repeated-measures ANOVA. For every cocaine-seeking test, lever pressing was also examined by dividing the 1-h test session into four 15 min bins and using a two-way repeated-measures ANOVA, with both time and manipulation (sham vs. laser) as within-subject factors. Open field data were analyzed using a within-subjects t-test.
Results
For brevity, inactive lever presses during cocaine-seeking tests are reported in the Online Resource (Figures OR2 – OR4) and are not discussed in the main text as the observed effects did not reveal any differences that were of relevance for the major questions of the study.
Figure 1A shows a schematic diagram of fiber optic termination points in the IL (first panel) and representative histological images, including immunohistochemical staining (second panel), fluorescent imaging (third panel), and Cresyl violet staining (fourth panel), indicating viral expression and/or optical fiber tracks in the IL. Figure 1B shows a schematic diagram of fiber optic termination points in the NAshell (first panel), a representative histological image including immunohistochemical staining of axon terminations from the IL in the NAshell (second panel) and Cresyl violet staining of optical fiber tracks in the NAshell (third panel). Figure 1C shows ex-vivo electrophysiological confirmation of functional SSFO expression in the IL during whole-cell recordings. Illumination with 473 nm light produced robust inward current; illumination with 561 nm light produced a return to baseline current. Figure ID shows in vivo electrophysiological confirmation of functional SSFO expression in the IL. A pulse of blue light increased 40-60 Hz activity, but did not significantly alter LFP activity in other frequency ranges. IL activity was returned to baseline levels following yellow laser illumination (p < .05).
Figure 1. Confirmation of Stable Step-function Opsin expression and function.
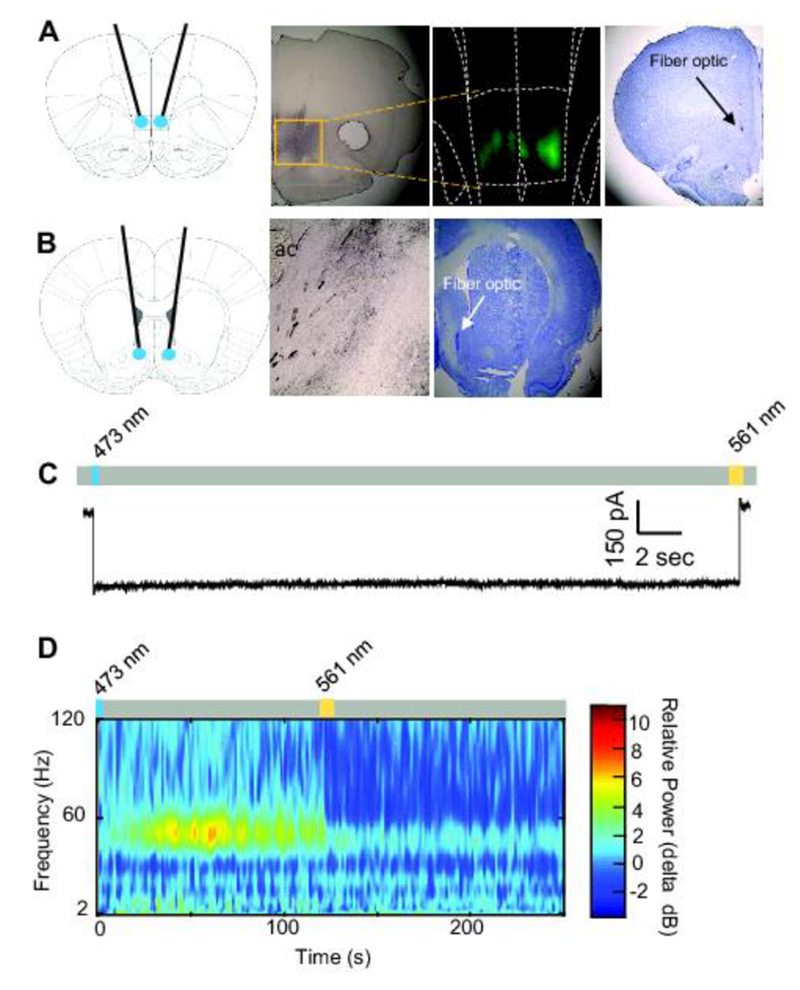
A. Schematic of fiber-optic placement targeted at the IL (first panel). Anti-eYFP immunohistochemical staining from an SSFO-transduced rat (second panel). Fluorescent image shows SSFO expression in the IL (third panel). Cresyl violet staining shows a fiber optic terminating in the IL (fourth panel). B. Schematic of fiber-optic placement targeted at the NAshell (first panel). Anti-eYFP immunohistochemical staining of axonal fibers from the IL in the NAshell from an SSFO-transduced rat (second panel). Cresyl violet staining shows a fiber optic terminating in the NAshell (third panel). ac, anterior commissure C. Whole-cell voltage-clamp recording from an IL neuron transduced with SSFO. The representative trace shows inward current recorded following blue laser illumination (473 nm, 10 ms, blue rectangle). Trace shows return to baseline following yellow laser illumination (561 nm, 10 ms, yellow rectangle). D. In vivo electrophysiological recording from an IL neuron transduced with SSFO. Blue laser illumination (473 nm, 2 s, blue rectangle) enhanced 40–60 Hz activity, which was attenuated following yellow laser illumination (561 nm, 10 s, yellow rectangle).
Experiment 1. Optical enhancement of IL activity did not alter cocaine seeking following a drug-free homecage period, but attenuated cocaine seeking following extinction training.
Figure 2A illustrates the first half of the experimental timeline for Experiment 1. Figure 2B shows active and inactive lever presses and cocaine infusions during the last 10 d of self-administration. Figure 2C (left panel) shows active lever presses during the baseline cue-induced craving test and the incubation-of-craving test. A paired t-test revealed a significant difference such that rats showed higher levels of active lever presses during the incubation-of-craving test compared to the baseline-craving test (t(9) = 2.998, p = 0.015). Figure 2C (right panel) shows the active lever presses from Figure 2C (left panel) divided into 15-min bins. A two-way repeated-measures ANOVA revealed significant effects of time within the session (F(3, 27) = 12.45, p < 0.001) and between baseline and incubation time points (F(1, 9) = 8.987, p = 0.015) and no interaction (F(3, 27) = 1.817, p = 0.16).
Figure 2. SSFO-mediated enhancement of IL activity during cocaine-seeking tests following the drug-free homecage period and extinction training.
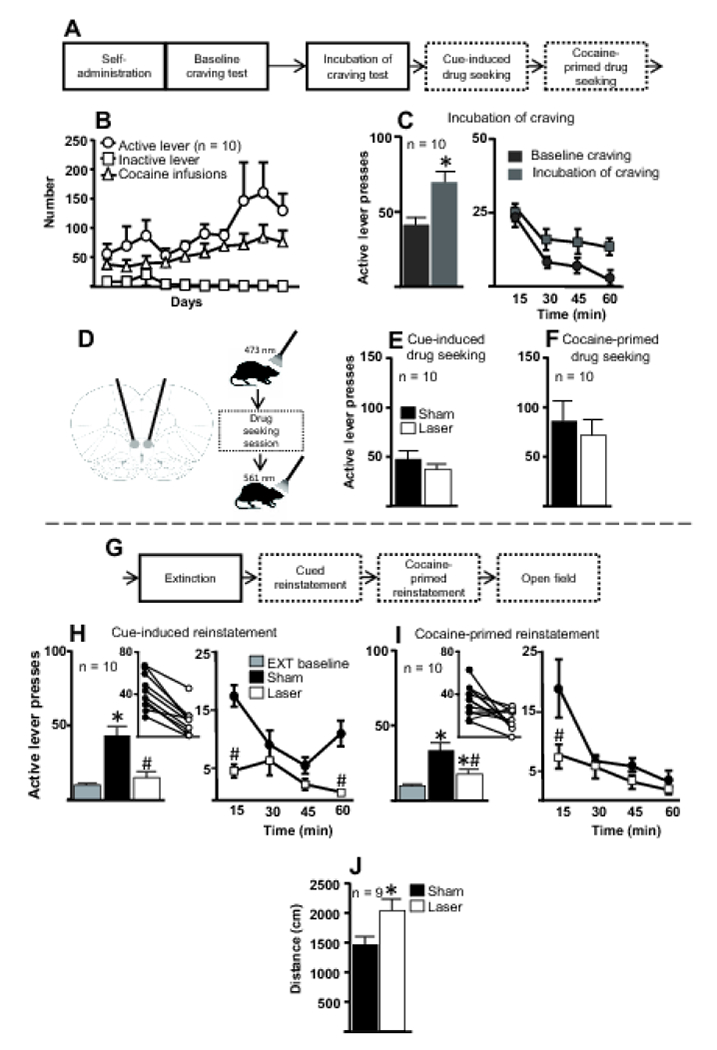
A. First part of the experimental timeline for Experiment 1 in which IL activity was enhanced with SSFOs during cocaine-seeking tests. B. Number of active and inactive lever presses and cocaine infusions for the last 10 d of cocaine self-administration for all rats included in the final analysis. C. Following the last d of self-administration (baseline craving) and the last d of the drug-free homecage period (incubation of craving), rats underwent 1-h cue-induced drug-seeking tests. Left, Rats made significantly more cocaine-seeking responses during the incubation-of-craving test than during the baseline-craving test, demonstrating incubation of cocaine craving. Right, Active lever presses from baseline- and incubation-of-craving tests, broken down into 15-min bins. *, p < 0.05 compared to baseline test. D. Schematic diagrams of fiber optic placement (left) and manipulations given during cocaine-seeking and open field tests (right). One min before the session, a 473 nm laser was used to activate the SSFOs and, following the session, a 561 nm laser was used to close SSFO channels. E. Active lever presses during the cue-induced cocaine-seeking test. Activation of SSFOs in the IL did not alter cue-induced cocaine seeking. F. Active lever presses during the cocaine-primed (5 mg/kg) drug-seeking test. Activation of SSFOs in the IL did not alter cocaine-primed drug seeking. G. Experimental timeline for subsequent behavioral procedures in Experiment 1. H. Left, Activation of SSFOs in the IL during cue-induced reinstatement significantly decreased cocaine seeking following extinction training. Inset, All rats decreased cocaine-seeking behavior following laser treatment compared to sham treatment. Right, Cue-induced reinstatement data divided into 15-min bins suggest that, following laser treatment, rats pressed the active lever less during the first and fourth bins compared to sham treatment. *, p < 0.05 compared to extinction baseline; #, p < 0.05 relative to sham treatment. EXT, extinction. I. Left, Enhancement of IL activity with an SSFO during cocaine-primed reinstatement significantly decreased cocaine seeking following extinction training. *, p < 0.05 relative to extinction baseline; #, p < 0.05 relative to sham treatment. Inset, All rats but one decreased cocaine-seeking behavior following laser treatment compared to sham treatment. Right, Cocaine-primed reinstatement data divided into 15-min bins suggest that rats pressed the active lever less during the first bin following laser treatment compared to sham treatment. J. Rats traveled a significantly greater distance in the open-field test following laser treatment compared to sham treatment. Note the decrease in subjects from 10 to 9 due to one death. *, p < 0.05 compared to sham treatment.
Figure 2D provides schematic diagrams showing fiber optic placements (left panel) and the sequence of events for each drug-seeking session and locomotor test (right panel). Figure 2E shows active lever presses during the cue-induced drug-seeking session. A paired t-test revealed no significant differences in active lever pressing between sham and laser manipulations (t(9) = 1.45, p = 0.18). Within-session analysis of the cue-induced drug-seeking session divided into 15-min bins did not reveal significant differences between sham and laser treatments throughout the session (Figure OR1A). Figure 2F shows active lever presses during the cocaine-primed drug-seeking session. As with the cued test, a paired t-test revealed no significant effect of sham vs. laser stimulation on active lever presses (t(9) = 1.069, p = 0.31). Within-session analysis divided into 15-min bins did not reveal significant differences between sham and laser treatments throughout the session (Figure OR1B).
Figure 2G shows the continuation of the timeline for Experiment 1. Figure 2H (left panel) shows active lever presses for the cue-induced reinstatement test. A one-way repeated-measures ANOVA indicated a significant effect (F(2, 18) = 33.9, p < 0.0001). Post-hoc analyses revealed that following sham stimulation rats pressed the active lever significantly more than they did during the extinction baseline and after receiving laser stimulation (p < 0.001). There was no significant difference in active lever presses between the extinction baseline and laser stimulation (p > 0.05). All rats decreased cocaine-seeking behavior following laser treatment compared to sham treatment (Figure 2H inset). To determine whether SSFO-mediated activation produced time-dependent effects across the 1-h session, the active lever presses for the cue-induced reinstatement from Figure 2H (left panel) were divided into 15-min bins and analyzed (right panel). A two-way repeated-measures ANOVA revealed a significant effect of time (F(3, 27) = 9.205, p < 0.001), a significant effect of sham vs. laser (F(1, 9) = 56.78, p < 0.001), and a significant interaction (F(3, 27) = 5.293, p = 0.0053). Post-hoc analysis indicated that laser-treated rats pressed the active lever significantly less compared to sham-treated rats during bins 1 and 4 (p < 0.001) but not during the other bins.
Figure 2I (left panel) shows active lever presses for the cocaine-primed reinstatement. A one-way repeated-measures ANOVA indicated a significant effect (F(2, 18) = 12.25, p = 0.0019). Post-hoc analyses revealed significant reinstatement of cocaine seeking following sham treatment (p < 0.05) and following laser treatment (p < 0.05), compared to extinction baseline. However, optical IL activation significantly decreased cocaine seeking compared to sham treatment (p < 0.05). All rats but one showed reduced cocaine-seeking following laser treatment compared to sham treatment (Figure 2I inset). Figure 2I (right panel) shows active lever presses for cocaine-primed reinstatement divided into 15-min bins. A two-way repeated-measures ANOVA revealed a significant effect of time (F(3, 27) = 13.87, p < 0.001), a significant effect of treatment (F(1, 9) = 7.189, p = 0.025), and a significant interaction (F(3, 27) = 3.011, p = 0.047). Post-hoc analysis indicated that following laser treatment rats pressed the active lever significantly less than following sham treatment during bin 1 (p < 0.001).
Figure 2J shows the distance traveled during an open field test. A paired t-test revealed that following laser stimulation, rats traveled greater distances compared to sham stimulation (t(8) =2.818, p = 0.022).
Experiment 2: Illumination of IL cells expressing eYFP did not affect cocaine seeking.
Figure 3A illustrates the first half of the experimental timeline for Experiment 2. Figure 3B shows active and inactive lever presses and cocaine infusions during the last 10 d of self-administration. Figure 3C (left panel) shows active lever presses during the baseline-craving test and during the incubation-of-craving test. A paired t-test revealed a trend-level difference such that rats pressed the active lever more during the incubation-of-craving test than during the baseline-craving test (t(10) = 1.854, p = 0.093). Figure 3C (right panel) shows active lever presses from 3C (left panel) divided into 15-min bins. A two-way repeated-measures ANOVA revealed a significant effect of time (F(3, 30) = 17.47, p < 0.0001), a trend-level difference between baseline and incubation (F(1, 10) = 3.483, p = 0.093), and no significant interaction (F(3, 30) = 0.495, p = 0.68). Visual inspection of the 15 min data suggests that the incubated rats pressed the active lever more than they did during the baseline test but that the overall magnitude was not as large as was observed in the other two experiments.
Figure 3. Illumination of IL cells expressing eYFP during cocaine-seeking tests following the drug-free homecage period and extinction training.
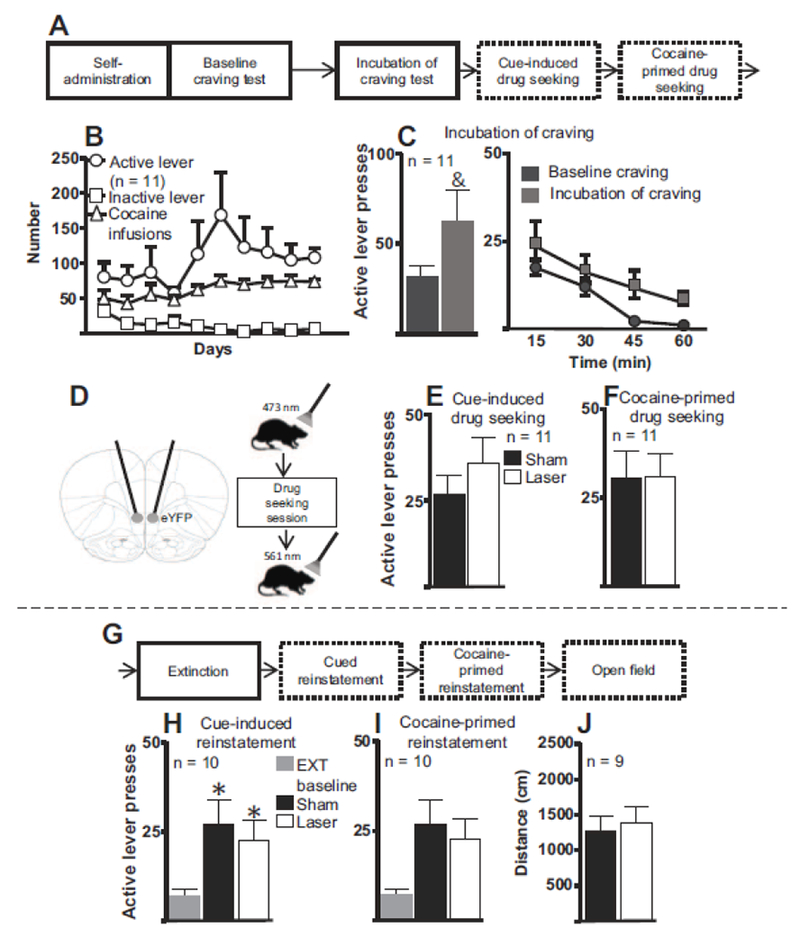
A. First part of experimental timeline for Experiment 2 in which IL cell bodies expressing eYFP only (i.e., no SSFO expression) were illuminated during cocaine-seeking tests. B. Number of active and inactive lever presses and cocaine infusions for the last 10 d of cocaine self-administration for all rats included in the final analysis. C. Following the last d of self-administration (baseline craving) and the last d of the homecage period (incubation of craving), rats underwent 1-h cue-induced drug-seeking tests. Left, There was a trend toward increased active lever pressing during the incubation-of-craving test compared to the baseline-craving test. Right, Active lever presses from baseline- and incubation-of-craving tests, broken down into 15-min bins. &, p < 0.10 compared to baseline test. D. Schematic diagrams of fiber optic placement (left) and manipulations given during cocaine-seeking and open field tests (right). One min before the session, a 473 nm laser was used and, following the session, a 561 nm was used. E. Active lever presses during the cue-induced cocaine seeking test. IL illumination did not alter cue-induced drug seeking. F. Active lever presses during the cocaine-primed (5 mg/kg) drug-seeking test. IL illumination did not alter cocaine-primed drug seeking. G. Experimental timeline for subsequent behavioral procedures in Experiment 2. H. IL illumination during cue-induced reinstatement did not significantly alter cocaine seeking following extinction training. *, p < 0.05 compared to extinction baseline. EXT, extinction. Note decrease in subjects from 11 to 10 due to one rat’s death. I. IL illumination during cocaine-primed reinstatement did not significantly alter cocaine seeking following extinction training. J. Laser treatment did not significantly alter rats’ distance traveled in the open-field test compared to sham treatment. Note the decrease in subjects from 10 to 9 due to one rat’s death.
Figure 3D provides schematic diagrams showing fiber optic placements (left panel) and the sequence of events for each drug-seeking session and locomotor test (right panel). Figure 3E shows active lever presses during the cue-induced drug-seeking test. A paired t-test revealed no significant differences in active lever pressing between sham and laser stimulation (t(10) = 1.443, p = 0.17). Figure 3F shows active lever presses during the cocaine-primed drug-seeking test. A paired t-test revealed no significant effect of sham vs. laser stimulation on active lever presses (t(10) = 0.0262, p = 0.979). Within-session analysis of lever presses divided into 15-min bins for both cue-induced and cocaine-primed drug seeking did not reveal significant differences between sham and laser treatments throughout the sessions (Figures OR1C and OR1D). Figure 3G shows the continuation of the timeline for Experiment 2. Figure 3H shows active lever presses for the cue-induced reinstatement test. A one-way repeated-measures ANOVA indicated a significant effect during the reinstatement session (F(2, 18) = 7.951, p = 0.0065). Post-hoc analyses revealed that rats significantly reinstated compared to extinction baseline regardless of whether they received sham or laser illumination (p < 0.05 for both cases). There were no significant differences between sham and laser treatments (p > 0.05). Figure 3I shows active lever presses for cocaine-primed reinstatement. A one-way repeated-measures ANOVA indicated no significant differences between extinction baseline, sham and laser treatments (F(2, 18) = 1.569, p = 0.24). Visual inspection of the data suggest that the laser treatment did not produce any obvious alterations in cocaine seeking during this test. Within-session analysis of lever presses divided into 15-min bins for cue-induced and cocaine-primed reinstatement did not reveal significant differences between sham and laser treatments throughout the sessions (Figures OR1E and OR1F).
Figure 3J shows the distance traveled during an open field test. A paired t-test revealed that laser stimulation did not significantly alter distance traveled (t(8) = 0.750, p = 0.474) compared to sham stimulation.
Experiment 3: Optical stimulation of IL axonal terminals in the NAshell attenuated cue-induced, but not cocaine-primed, drug seeking following a drug-free homecage period and had no effect on cocaine seeking following extinction training.
Figure 4A illustrates the first part of the experimental timeline for Experiment 3. Figure 4B shows active and inactive lever presses and cocaine infusions during the last 10 d of self-administration. Figure 4C (left panel) shows active lever presses during the baseline-craving test and the incubation-of-craving test. A paired t-test revealed a significant difference such that rats showed higher levels of active lever presses during the incubation-of-craving test compared to the baseline-craving test (t(15) = 4.88, p < 0.001). Figure 4C (right panel) shows active lever presses from Figure 4C (left panel) divided into 15-min bins. A two-way repeated-measures ANOVA revealed significant effects of time within the session (F(3, 45) = 17.07, p < 0.001) and between baseline and incubation timepoints (F(1, 15) = 23.86, p = 0.0002) and a trend-level interaction (F(3, 45) = 2.212, p = 0.099). Visual inspection of the data suggests that rats showed overall higher levels of lever pressing in the incubation test than they did during the baseline test.
Figure 4. Illumination of IL terminals in NAshell during cocaine-seeking tests following the drug-free homecage period and extinction training.
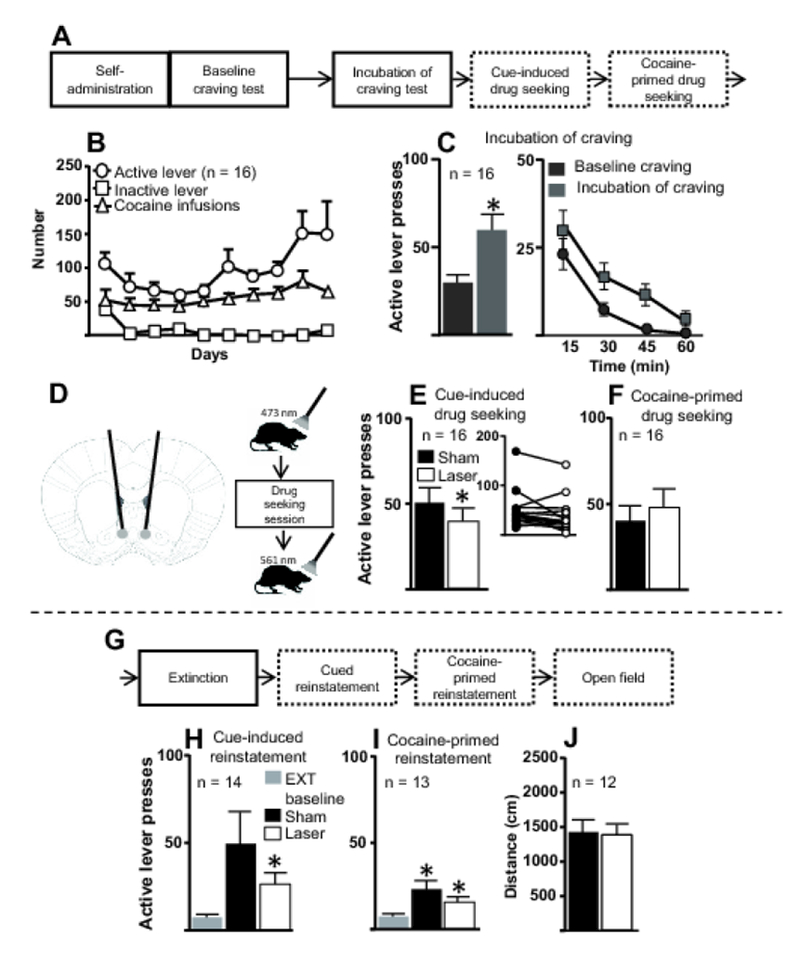
A. First part of experimental timeline for Experiment 3 in which IL terminals in the NAshell were illuminated during cocaine-seeking tests. B. Number of active and inactive lever presses and cocaine infusions for the last 10 d of cocaine self-administration for all rats included in the final analysis. C. Following the last d of self-administration (baseline craving) and the last d of the drug-free homecage period (incubation of craving), rats underwent 1-h cue-induced drug-seeking tests. Left, Rats pressed the active lever more during the incubation-of-craving test than during the baseline craving test, demonstrating incubation of cocaine craving. Right, Active lever presses from baseline- and incubation-of-craving tests, broken down into 15-min bins. *, p < 0.05 compared to baseline test. D. Schematic diagrams of fiber optic placement (left) and manipulations given during cocaine-seeking and open field tests (right). One min before the session, a 473 nm laser was used and, following the session, a 561 nm laser was used. E. Left, Active lever presses during the cue-induced cocaine seeking test. Laser treatment significantly attenuated cocaine seeking compared to sham treatment. Inset, Active lever presses for individual rats following either sham or laser treatment. *, p < 0.05 compared to sham treatment. F. Active lever presses during the cocaine-primed (5 mg/kg) drug-seeking test. Illumination of IL terminals in the NAshell did not alter cocaine-primed drug seeking. G. Experimental timeline for subsequent behavioral procedures in Experiment 3. H. Illumination of IL terminals in the NAshell during cue-induced reinstatement did not significantly alter cocaine seeking following extinction training. *, p < 0.05 compared to extinction baseline. EXT, extinction. Note the decrease in subjects from 16 to 14 due to 2 deaths. I. Illumination of IL terminals in the NAshell during cocaine-primed reinstatement did not significantly alter cocaine seeking following extinction training. *, p < 0.05 relative to extinction baseline. Note the decrease in subjects from 14 to 13 due to failure to re-extinguish following the cue-induced reinstatement. J. Laser treatment did not significantly alter rats’ distance traveled in the open field test compared to sham treatment. Note the decrease in subjects 13 to 12 due to one rat’s death prior to open field testing.
Figure 4D provides schematic diagrams showing fiber optic placements (left panel) and the sequence of events for each drug-seeking session and locomotor test (right panel). Figure 4E shows active lever presses during the cue-induced drug-seeking session. A paired t-test revealed significant differences in active lever pressing between sham and laser treatments (t(15) = 2.21, p = 0.042). Laser treatment attenuated active lever pressing in most, though not all, subjects (Figure 4E inset). Within-session analysis divided into 15-min bins revealed significant differences between sham and laser treatments but no interaction (Figure OR1G). Figure 4F shows active lever presses during the cocaine-primed drug-seeking sessions. A paired t-test revealed no significant effect of sham vs. laser stimulation (t(15) = 1.222, p = 0.24). Within-session analysis of lever presses divided into 15-min bins revealed no significant differences between sham and laser treatments throughout the session (Figure OR1H).
Figure 4G shows the continuation of the timeline for Experiment 3. Figure 4H shows active lever presses for the cue-induced reinstatement test. A one-way repeated-measures ANOVA indicated a significant effect (F(2, 26) = 4.523, p = 0.044). Post-hoc analyses revealed a trend-level difference between sham stimulation and extinction baseline (p < 0.1) and a significant difference between laser treatment and extinction baseline (p < 0.05). There was no significant difference between sham and laser treatment (p > 0.05). Within-session analysis divided into 15-min bins did not reveal significant differences between sham and laser treatments throughout the session (Figure OR1I).
Figure 4I shows active lever presses for the cocaine-primed reinstatement. A one-way repeated-measures ANOVA indicated a significant effect (F(2, 24) = 10.23, p = 0.0016). Post-hoc analyses revealed that rats showed significant cocaine-primed reinstatement following both sham and laser treatments, as compared to the extinction baseline (p < 0.05 in both cases). Following laser treatment, rats showed a trend toward decreased reinstatement compared to sham treatment (p < 0.10). Within-session analysis divided into 15-min bins did not reveal significant differences between sham and laser treatments throughout the session (Figure OR1J).
Figure 4J shows the distance traveled during an open field test. A paired t-test revealed that laser stimulation did not significantly alter distance traveled (t(11) = 0.319, p = 0.755) compared to sham stimulation.
Discussion
The present work found that, after a drug-free homecage period, following 6-h cocaine self-administration, enhancement of IL pyramidal cell activity via SSFOs did not alter cocaine-seeking behavior in rats. In contrast, after the same rats underwent extinction training, enhancement of IL activity attenuated cocaine seeking, as reflected in decreased active lever pressing during reinstatement. Together, the present findings indicate that extinction training is necessary to recruit IL neuronal activity in directing cocaine-seeking behavior. Moreover, these results suggest the utility of an SSFO-based approach for activating a brain region without driving specific activity patterns.
Enhancement of IL activity via SSFOs attenuated cocaine seeking following extinction training but not following a drug-free homecage period
Prior work suggests that the ability of the IL to inhibit cocaine seeking depends on extinction training itself (LaLumiere et al. 2010; Peters et al. 2008). Indeed, recent evidence using Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) found that IL activation attenuates cocaine seeking only following extinction training and not following a period in the homecage (Augur et al. 2016). Although such evidence suggests an interaction between extinction training and the capacity of the IL to inhibit cocaine seeking, at least one study suggests that the passage of time following cocaine self-administration (i.e., 45 days in the homecage) is sufficient to induce changes at IL-NAshell synapses, which, when reversed, increase cocaine seeking during a cue-induced drug-seeking test (Ma et al. 2014). The present study found that enhancement of IL activity attenuated cocaine seeking only after rats had undergone extinction training, suggesting that extinction-induced plasticity is required for IL activation to influence cocaine-seeking behaviors.
One observation from the present work is that the initial baseline- and incubation-of-craving tests may be considered extinction training, as during these tests active lever presses are not rewarded with cocaine infusions. This raises the question of why no manipulation effects were observed in the first drug-seeking tests. One possibility is that this brief exposure to the extinction contingencies did not induce sufficient plasticity in the IL, or relevant circuits, for later enhancement of IL activity to modulate cocaine-seeking behavior. Indeed, in Experiment 1, during both the initial cue-induced and cocaine-primed drug-seeking tests, IL activation decreased drug seeking, but this reduction did not rise to the level of statistical significance. This observation is in line with the findings of Peters et al. (2008), which suggest that one session of extinction training does not sufficiently “recruit” IL activity to modulate cocaine-seeking behavior.
SSFO enhancement of cell body activity
In accordance with past work, our results suggest that SSFO-mediated activation of cell bodies reliably produced a small depolarizing current. This SSFO-mediated current has been suggested to enhance activity in a structure without directly driving action potential patterns (Liu et al. 2016; Yizhar et al. 2011a; Yizhar et al. 2011b). This represents a significant advantage over the use of traditional ChR2 constructs, which require the identification of stimulation parameters to drive neuronal activity in behaviorally relevant ways, a process that can necessitate a large number of experiments and varies depending on brain region or behavior under study (Huff et al. 2016; Huff et al. 2013). SSFOs also represent an advantage over DREADD approaches, as SSFOs can be activated with one wavelength of light, and deactivated with another, increasing temporal control over the manipulation.
Our in vivo electrophysiological recordings suggest that SSFOs enhance IL activity particularly in the gamma range. As the purpose of the in vivo electrophysiological recordings was to demonstrate the effectiveness of SSFO activation in an in vivo preparation in addition to the ex vivo data shown by us and others (Yizhar et al. 2011b), the present findings should not be interpreted as indicating that the behavioral effects were due to enhanced gamma rhythm activity. Nonetheless, work by other laboratories suggests that increases in gamma power are linked to a variety of processes including attention, working memory, and action selection, in both primates and rodents (Beshel et al. 2007; Donnelly et al. 2014; Fries et al. 2007; Fries et al. 2001; Pesaran et al. 2002; Tallon-Baudry et al. 1999).
SSFO enhancement of terminal activity
As the theoretical utility of SSFOs derives from their ability to bring neuronal cell bodies closer to threshold for firing, and thus increase sensitivity to excitatory inputs, the usefulness of light application to SSFO-transduced terminals would seem unclear on a theoretical level. However, prior work suggests that such an approach may be effective (Liu et al. 2016). As evidence suggests that the NAshell is involved in the regulation of reward seeking and is a critical projection target for the IL during the inhibition of cocaine-seeking behaviors (Augur et al. 2016; Di Chiara 2002; Gorelova and Yang 1997; Kelley 2004; LaLumiere et al. 2012; Ma et al. 2014; Peters et al. 2008), we investigated the use of SSFOs to stimulate IL terminals in the NAshell using the present behavioral paradigm.
Our results revealed that illumination of SSFO-transduced IL terminals in the NAshell significantly decreased cocaine seeking during cue-induced, but not cocaine-primed, drug-seeking tests prior to extinction training. Following extinction training, such terminal activation had no effect on cue-induced reinstatement and only a trend-level effect on cocaine-primed reinstatement. Although generally consistent with a role for the IL-NAshell pathway in reducing cocaine seeking, the specific and limited nature of the present findings was unexpected and potentially spurious. Furthermore, the effects observed with terminal SSFO activation on cue-induced drug seeking were not particularly large, compared to those observed during reinstatement in Experiment 1. Indeed, the statistical significance from the cue-induced drug-seeking test largely resulted from the use of a paired analysis in which most rats showed a relatively small decrease in cocaine seeking following terminal SSFO manipulation. Thus, even if the result is not spurious, the small size of the effect warrants further consideration. Although mechanisms of action regarding SSFO illumination at axonal terminals are not yet clear, studies suggest that the effects of SSFOs on terminals likely involve secondary mechanisms of neurotransmitter release, such as Ca 2+ influx (Liu et al. 2016). As a consequence, the effects of activation of SSFOs in terminals may be quite different than effects on cell bodies and may not be as physiologically relevant as the SSFO-mediated activation of cell bodies, though this requires further study. Alternatively, the size of the effect may indicate the importance of multiple IL efferents in modulating cocaine-seeking behavior, and thus terminal activation in a single pathway may not be capable of replicating the effects observed with cell body activation.
Consideration of open field test
Results from our open field test indicate that enhancement of IL activity (Experiment 1) leads to an increase in motor output. However, because there is an increase in motor output, this likely does not account for the decrease in lever pressing seen in Experiment 1. It is possible that the increase in general motor behavior seen following laser treatment could cause animals to exhibit specific decreases in lever-directed behavior. However, this is unlikely as the decreases in lever pressing behavior only occurred in 2 out of the 4 cocaine seeking tests, namely the cue-induced and cocaine-primed reinstatement tests. If the differences in lever pressing were due to motoric effects, we would expect to see differences between sham and laser stimulation in all cocaine-seeking tests.
Conclusion
The present findings suggest that the ability of the IL to reduce cocaine seeking depends, at least in part, on a rat having experienced extinction training. Furthermore, our findings suggest the utility of an SSFO-based approach for activating a structure without driving specific patterns of activity, though whether SSFO-mediated terminal manipulation represents a valuable approach to targeting pathways remains unclear.
Supplementary Material
Acknowledgments
Funding and Disclosure: R.T.L was supported by National Institute of Health R01 DA034684. J.A.W. was supported by the Department of Veterans Affairs (Merit Award), National Institute on Drug Abuse R01 DA037216, National Heart, Lung and Blood Institute R01 HL113863 and the Carver Foundation. N.S.N. was supported by National Institute of Health R01 NS089470. The authors declare no conflicts of interest.
Footnotes
Conflict of interest statement:
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science (New York, NY) 282: 298–300. [DOI] [PubMed] [Google Scholar]
- Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, Peters J (2016) Chemogenetic Activation of an Extinction Neural Circuit Reduces Cue-Induced Reinstatement of Cocaine Seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience 36: 10174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshel J, Kopell N, Kay LM (2007) Olfactory Bulb Gamma Oscillations Are Enhanced with Task Demands. The Journal of Neuroscience 27: 8358–8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G (2002) Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behavioural brain research 137: 75–114. [DOI] [PubMed] [Google Scholar]
- Donnelly NA, Holtzman T, Rich PD, Nevado-Holgado AJ, Fernando AB, Van Dijck G, Holzhammer T, Paul O, Ruther P, Paulsen O, Robbins TW, Dalley JW (2014) Oscillatory activity in the medial prefrontal cortex and nucleus accumbens correlates with impulsivity and reward outcome. PLoS One 9: e111300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W (2007) The gamma cycle. Trends in neurosciences 30: 309–16. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R (2001) Modulation of Oscillatory Neuronal Synchronization by Selective Visual Attention. Science (New York, NY) 291: 1560–1563. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Yang CR (1997) The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience 76: 689–706. [DOI] [PubMed] [Google Scholar]
- Gutman AL, Nett KE, Cosme CV, Worth WR, Gupta SC, Wemmie JA, LaLumiere RT (2017) Extinction of Cocaine Seeking Requires a Window of Infralimbic Pyramidal Neuron Activity after Unreinforced Lever Presses. The Journal of neuroscience : the official journal of the Society for Neuroscience 37: 6075–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, Emmons EB, Narayanan NS, LaLumiere RT (2016) Basolateral amygdala projections to ventral hippocampus modulate the consolidation of footshock, but not contextual, learning in rats. Learning & memory (Cold Spring Harbor, NY) 23: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff ML, Miller RL, Deisseroth K, Moorman DE, LaLumiere RT (2013) Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proceedings of the National Academy of Sciences of the United States of America 110: 3597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE (2004) Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neuroscience and biobehavioral reviews 27: 765–76. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW (2010) The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learning & memory (Cold Spring Harbor, NY) 17: 168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW (2012) Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. The European journal of neuroscience 35: 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Guillem K, Koob GF, Ahmed SH (2012) Drug specificity in extended access cocaine and heroin self-administration. Addiction biology 17: 964–76. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Cai L, Li Y, Chen B, Dong Y, Huang YH (2016) Prefrontal Cortex to Accumbens Projections in Sleep Regulation of Reward. The Journal of neuroscience : the official journal of the Society for Neuroscience 36: 7897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schluter OM, Huang YH, Dong Y (2014) Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83: 1453–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller Ewald VA, LaLumiere RT (2017) Neural systems mediating the inhibition of cocaine-seeking behaviors. Pharmacology, biochemistry, and behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA (2002) Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nature neuroscience 5: 805–11. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ (2009) Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & memory (Cold Spring Harbor, NY) 16: 279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW (2008) Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of neuroscience : the official journal of the Society for Neuroscience 28: 6046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011) Neurobiology of the incubation of drug craving. Trends in neurosciences 34: 411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Kreiter A, Bertrand O (1999) Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Visual neuroscience 16: 449–59. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Rotaru DC, Heinsbroek JA, Gouwenberg Y, Deisseroth K, Stuber GD, Mansvelder HD, Smit AB (2013) Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 33: 18225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K (2011a) Optogenetics in neural systems. Neuron 71: 9–34. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K (2011b) Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477: 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


