Abstract
Background
The uncoupling protein 1 (UCP1) gene has a role in mitochondrial energy expenditure in brown adipose tissue. This study aimed to investigate the effects of berberine, a benzylisoquinoline alkaloid used in traditional Chinese medicine, on energy expenditure, expression of the UCP1 gene, the cell stress protein inositol-requiring enzyme 1α (IRE1α), apoptosis genes, and macrophage phenotype (M1 and M2) in white and brown adipose tissue in an obese mouse model fed a high-fat diet.
Material/Methods
Four-week-old C57BL/6J male mice (n=20) were divided into a high-fat diet group, a normal diet group, a group treated with berberine at 100 mg/kg/d in 0.9% normal saline, and a non-treated group. Whole-body fat mass, blood glucose, insulin resistance, and oxygen expenditure during physical activity were measured. After 16 weeks, the mice were euthanized for examination of liver and adipose tissue. The expression of pro-inflammatory cytokines, apoptosis genes, thermogenic genes (including UCP1), and IRE1α, were investigated using immunohistochemistry, Western blot, and quantitative reverse transcription polymerase chain reaction (qRT-PCR), in white and brown adipose tissue. Magnetic cell sorting harvested M1 and M2 macrophages in adipose tissue. Clodronate liposomes were used to inhibit macrophage recruitment.
Results
Berberine treatment in mice fed a high-fat diet increased energy metabolism, glucose tolerance, and expression of UCP1, and reduced expression of pro-inflammatory cytokines, macrophage recruitment, and resulted in M2 macrophage polarization in white adipose tissue. Polarized M2 macrophages showed reduced expression of apoptotic genes and IRE1α.
Conclusions
Berberine improved metabolic function in a mouse model fed a high-fat diet.
MeSH Keywords: Berberine, Inflammation, Macrophages
Background
Berberine is a benzylisoquinoline alkaloid widely used in traditional Chinese medicine and has shown pharmacological activity that includes the reduction of inflammation and oxidation [1,2]. Previously published studies have shown that berberine treatment can reduce hyperglycemia and hyperlipidemia, and has protective effects on the cardiovascular and neurological systems [3–6]. The endoplasmic reticulum is an intracellular organelle required for the folding and secretion of proteins, calcium homeostasis, and the biosynthesis of lipids. Activation of endoplasmic reticulum stress signaling pathways may lead to increased cell apoptosis and alterations in lipid metabolism.
Berberine has also been shown to have a beneficial effect in reducing non-alcoholic fatty liver disease (NAFLD) and related metabolic dysregulation [7]. In a high-fat diet mouse model of obesity, berberine treatment has been shown to inhibit methylation of the microsomal triglyceride transfer protein (MTTP) gene promoter, which normally upregulates gene expression in NAFLD, and to reduce the changes of fatty liver [8]. In this previously published study, berberine was shown to influence hepatic gene expression related to the regulation of metabolism and energy expenditure [8]. In a mouse model of NAFLD, berberine treatment has been shown to reduce hyperglycemia and dyslipidemia, reduce body weight and hepatic fat content in metabolic syndrome [9,10]. However, the mechanism of the pharmacological effects of berberine remains to be explored. Currently, most studies on berberine have been directed to metabolism-related gene expression in the liver, but gene expression in other organs and tissues associated with glycometabolism and energy consumption remain to be studied, including it effects on metabolic disorders.
Berberine has anti-inflammatory and immunomodulatory effects in animal models of autoimmune disease, including autoimmune type 1 diabetes, and associated diabetic retinopathy, by reducing leukocyte infiltration and Th1/Th17 differentiation in the inflammatory response [11,12]. Reduced serum levels of IL-6 after berberine treatment have been reported in patients with diabetic nephropathy [13]. These studies support the possible role of berberine in the treatment of inflammatory and metabolic disorders.
Inflammation can be induced by metabolic disorders that result in the dysfunction of energy expenditure, which can be associated with insulin resistance, hyperglycemia, and chronic inflammation involving microvascular endothelial cells [14,15]. Inflammation represents an important risk factor for cardiovascular disease, including coronary artery disease, heart failure, and metabolic syndrome [16–18]. Adipose tissue has a role in the regulation of metabolism and increased inflammatory responses in adipose tissue have been identified in metabolic disorders and can lead to restricted energy consumption [19]. Dedifferentiation of the M2 macrophage and polarization of the M1 macrophage is an important event that accompanies inflammation in adipose tissue [20]. The uncoupling protein 1 (UCP1) gene has a role in mitochondrial energy expenditure in brown adipose tissue, and M1 macrophage activation has been shown to promote insulin resistance and type 2 diabetes [21,22].
Therefore, this study aimed to investigate the effects of berberine, a benzylisoquinoline alkaloid used in traditional Chinese medicine, on energy expenditure, expression of the UCP1 gene, the cell stress protein inositol-requiring enzyme 1α (IRE1α), apoptosis genes, and macrophage phenotype (M1 and M2) in white and brown adipose tissue in an obese mouse model fed a high-fat diet.
Material and Methods
Mouse study groups
The Animal ethic committee of our institution approved all animal experiment procedures. The animals are kept in the standard feeding condition. C57BL/6J mouse were purchased from Experimental Animals Center of the Second Affiliated Hospital of Fujian Medical University (China, Fujian). Mice were divided into a high-fat diet group (with a diet consisting of 60% fat) and a normal control group (fed standard chow), and a group treated with berberine at 100 mg/kg/day in 0.9% normal saline by gavage, and a non-treated group. The baseline weight of mice fed a high-fat diet was 16.3±2.1 gm and 15.8±2.5 gm in the control group and treatment group, respectively. The baseline weight of mice at four weeks of age fed with a normal diet was 15.9±3.1 gm and 15.5±2.6 gm in the control group and treatment group, respectively. Clodronate liposomes (50 μL) were administered twice daily per mouse for seven days, to deplete macrophages. After 16 weeks, the mice were euthanized and the livers, white fat and brown fat was dissected from each.
RNA extraction and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the white adipose tissue and brown adipose tissue for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) according to standard protocols. Between 200–400 ng of eluted RNA was obtained from 1000 μL of serum. Total RNA was reverse transcribed using hexamer primer and Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) for analysis of transcript levels. After reverse transcriptase reactions amplified cDNAs from mRNA, qRT-PCR was performed to analyze gene expression using SYBR® Green PCR Master Mix (Applied Biosystems, Carlsbad, CA). The transcriptional levels were normalized against GAPDH for mRNAs. The qRT-PCR data were analyzed using the 2−ΔΔ Ct method. The results were expressed as the fold-change compared with the control samples.
Western blot
Protein from brown adipose tissue and mouse adipose tissue-derived macrophages were prepared with lysis buffer. Equal amounts of protein were separated and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Burlington, MA, USA). Membranes were blocked with 5% dried skimmed milk powder in Tris-buffered saline (TBS) and Tween 20 (TTBS) for 2 h at 37°C and incubated overnight at 4°C with the following primary antibodies: anti-UCP1 (1: 500) (Cat No. ab2433) (Abcam, Cambridge MA, USA); anti-STAT3 (1: 1000) (Cat No. ab32360) (Abcam, Cambridge MA, USA); anti-p-STAT3 (1: 1000) (Cat No. 76315) (Abcam, Cambridge MA, USA); anti-tyrosine hydroxylase (TH) (1: 1,000) Cat No. 137869) (Abcam, Cambridge MA, USA); anti-caspase-3 (1: 1000) (Cat No. ab46794) (Abcam, Cambridge MA, USA); anti-caspase-8 (1: 1000) (Cat No. ab14106) (Abcam, Cambridge MA, USA); anti-Bax (1: 1500) (Cat No. ab203614) (Abcam, Cambridge MA, USA); anti-IRE1α (1: 1500) (Cat No. ab520321) (Abcam, Cambridge MA, USA); anti-GAPDH (1: 1000) (Cat No. sc-47778) (Santa Cruz Biotechnology, Dallas TX, USA); anti-Bip (1: 2000) (Cat No. ab78421) (Abcam, Cambridge MA, USA); anti-Chop (1: 2000) (Cat No. ab12536) (Abcam, Cambridge MA, USA), anti-eIF-2α (1: 1000) (Cat No. ab52636) (Abcam, Cambridge MA, USA), and anti-ATF-4 (1: 2000) (Cat No. ab23760) (Abcam, Cambridge MA, USA). Membranes were washed and incubated with secondary antibody for 1 h at 25°C. The intensity of the protein bands was detected by enhanced chemiluminescence (ECL) using Fusion FX software (Vilber Lourmat, Marne-la-Vallée, France).
Histology of liver and white and brown adipose tissue
Histological analysis of the liver and white and brown adipose tissue obtained from the mice post-mortem was performed according to standard laboratory protocols. Mouse liver and white and brown adipose tissue were fixed in neutral buffered formalin solution at 25°C, followed by tissue processing and embedding in paraffin wax. Tissue sections were cut at 8 μm thickness using a microtome and were placed onto glass slides. Before histochemical and immunohistochemical staining, the tissue sections were de-waxed using serial incubation in 70% ethanol, 95% ethanol, 100% ethanol, and xylene, and washed twice in TBS (50 mM Tris-Cl, 150 mM NaCl, pH 7.6) for 5 min.
Routine histochemical staining was performed with hematoxylin and eosin (H&E) (Muto Pure Chemicals, Tokyo, Japan) and oil red O (ORO) (Sigma-Aldrich, St. Louis, MO, USA), which stained lipid. For H&E staining, sections were incubated in hematoxylin for 3 min and washed in distilled water, followed by washing with 1% HCl and 70% ethanol and incubation for 5 min in eosin solution (Muto Pure Chemicals, Tokyo, Japan), washing three times in distilled water, and then dried. For ORO staining, a stock solution of ORO (Sigma-Aldrich, St. Louis, MO, USA) was diluted in 3: 2 with distilled water to make a working solution. The tissue sections were incubated in the ORO working solution for 15 min, washed in distilled water, and counterstained in hematoxylin solution for 3 min, washed and dried. The tissue sections on the glass slides were mounted in Permount Mounting Medium (Fisher Scientific, Pittsburgh, PA, USA), and a glass coverslip was added. The stained slides were visualized using an Olympus light microscope (Olympus Optical Co. Ltd., Japan).
Assessment of metabolic phenotype, glucose tolerance test, and insulin tolerance test
The assessment of metabolic phenotype was performed according to a standard procedure. For each mouse, body fat and lean mass were measured by nuclear magnetic resonance (NMR) spectroscopy. Oxygen consumption was calculated for 24 hours, based on lean mass during physical activity. For the glucose tolerance test, mice received intraperitoneal injections of glucose (1 gm/kg body weight). For the insulin tolerance test, mice were preconditioned for 4 h followed by intravenous insulin injection (0.75 U/kg body weight). Blood glucose, triglyceride (TG), cholesterol, and insulin were measured according to standard automated laboratory methods.
Stromal, vascular, and macrophages isolation from white adipose tissues
White adipose tissue and brown adipose tissue were digested with 0.5% collagenase type II (Roche) and DNase (1 mg/mL) (Sigma-Aldrich, St Louis, MO, USA) at 37 °C with mixing at 100 rpm. Digested tissues were filtered through nylon mesh (70 μM), and centrifuged at 500×g for 5 min. Pellets of the stromal vascular fraction (SVF), without fat, were collected from the bottom of the centrifuge tube, washed with PBS, and incubated with CD11b MicroBeads (Miltenyi Biotec, Cologne, Germany) for 0.5 h to isolate adipose tissue macrophages (ATMs), then separated by an LS magnetic cell sorting column (Miltenyi Biotec, Cologne, Germany).
Flow cytometry of isolated adipose tissue macrophages (ATMs)
Isolated adipose tissue macrophages were washed with PBS and incubated with the following primary antibodies: CD11b (eBioscience, ThermoFisher Scientific, Waltham, MA, USA); F4/80 (eBioscience, ThermoFisher Scientific, Waltham, MA, USA); CD11c (eBioscience, ThermoFisher Scientific, Waltham, MA, USA); CD206 (AbDSerotec, Bio-Rad, Kidlington, Oxfordshire, UK); CD45 (eBioscience, ThermoFisher Scientific, Waltham, MA, USA); Ly6G (eBioscience, ThermoFisher Scientific, Waltham, MA, USA). Fluorescence labeling, using a fluorescein-conjugated secondary antibody, was performed according to the manufacturer’s instructions, and analysis was performed using a FACSAria II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Cells with a high and low expression of CD206 were isolated and stored in the sorting tube.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 software. Values were expressed as the mean ± standard deviation (SD) of three independent experiments. The differences between groups were analyzed using an unpaired t-test. Comparisons of more than two groups, or repeat data, were performed with analysis of variance (ANOVA). P-values <0.05 were considered to be statistically significant.
Results
Berberine treatment improved metabolic function and phenotype in mice fed a high-fat diet
In the high-fat diet mouse group, a significant decrease in the ratio of fat content to body mass was found in mice treated with berberine, six weeks after feeding with a high-fat diet (Figure 1A), but this difference was not found in mice fed a normal diet and treated with berberine, and mice on a high-fat diet who were not treated with berberine (Figure 1B). These findings support that berberine treatment reduced obesity in mice on a high-fat diet. Because a decrease in body fat was not found in mice fed a normal diet when treated with berberine, this finding indicated that berberine inhibited lipid accumulation under conditions of excess fat intake and obesity, but had no effect on normal lipid metabolism. Also, berberine reduced serum leptin levels and reduced hyperinsulinemia in mice fed a high-fat diet (Figure 1C, 1D), and insulin resistance was affected, as assessed by the glucose tolerance test and the insulin tolerance test (Figure 1E, 1F). Fatty change in the liver, evaluated by hematoxylin and eosin (H&E) and oil red O (ORO) histochemical staining, was reduced after treatment with berberine in the high-fat diet obese mice (Figure 1G, 1H). Treatment with berberine significantly reduced the size of the liver and reduced the degree of fat deposition in the liver in the mice fed a high-fat diet (Figure 1I). These results showed that berberine treatment improved the metabolic function and prevented the development of hyperglycemia, hyperlipidemia, and insulin resistance in an obese mouse model fed a high-fat diet.
Figure 1.
Berberine treatment improved the metabolic phenotype in mice fed a high-fat diet (HFD). (A, B) The percentage of fat content of body weight in age-matched C57BL/6J mice on a high-fat diet (HFD) (A) and normal chow diet (B), respectively. Mice fed a high-fat diet (n=10). Mice fed a normal diet (n=10). (C, D) Serum levels of leptin (C) and insulin (D) in the treatment group (light blue) and control group (deep blue). Significantly reduced levels of metabolic hormones were measured after treatment with berberine. (E, F) Glucose tolerance test (GTT) (E) and insulin tolerance test (ITT) (F) were used for the assessment of insulin sensitivity in the control group (deep blue) and treatment group (light blue). (G–I) Representative photomicrographs of the histology of the liver show the varying extent of fatty deposits in the mice fed a high-fat diet and treated with berberine, and the control group treated with berberine. Hematoxylin and eosin (H&E). Data are presented as the mean ±SD; * P<0.05 and ** P<0.01 by Student’s t-test. The treatment group, n=10; The control group, n=10.
Berberine treatment upregulated thermogenesis genes and suppressed inflammation in white adipose tissue in mice fed a high-fat diet
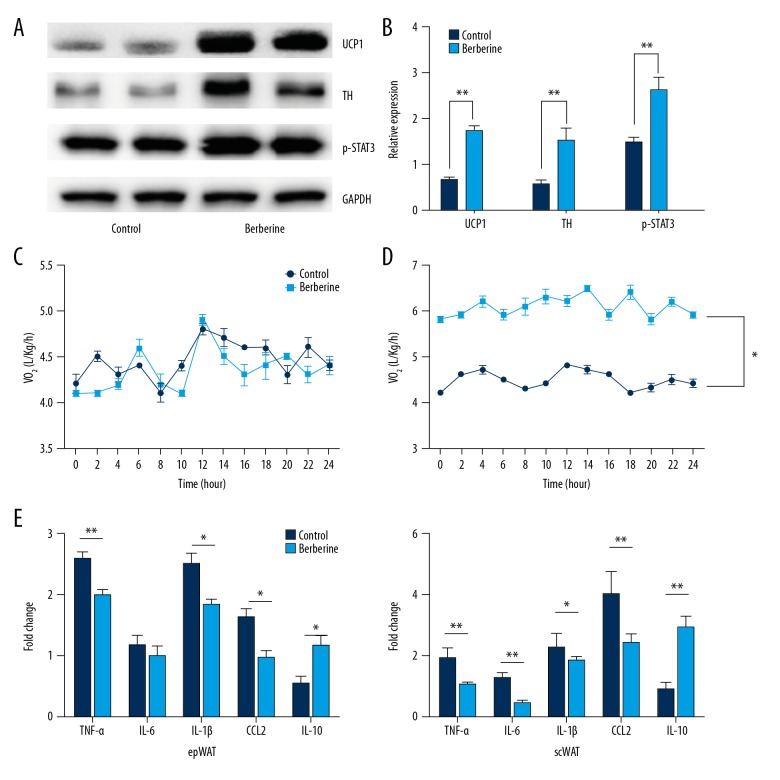
The expression of genes related to energy expenditure and inflammatory response in brown adipose and subcutaneous white adipose tissue, respectively were studied. Immunoblotting analysis showed that berberine treatment promoted the expression of the uncoupling protein 1 (UCP1) gene, a marker of thermogenesis, tyrosine hydroxylase (TH), an important rate-limiting enzyme in the heat production process (Figure 2A, 2B), and increased phosphorylation of p-STAT3 (Figure 2A, 2B). Oxygen consumption was found to be increased in mice fed a high-fat diet treated with berberine when compared with the controls, after normalization with a lean mass under conditions of similar physical activity, and no changes were noted in mice fed a normal diet (Figure 2C, 2D).
Figure 2.
Berberine treatment inhibited inflammation in white adipose tissue and promoted the expression of thermogenesis genes. (A) Representative immunoblot for the expression of uncoupling protein 1 (UCP1), tyrosine hydroxylase (TH), and p-STAT3. GAPDH was used as the control. (B) Quantitative analysis of protein expression of thermogenesis. (C, D) Oxygen expenditure in mice fed a normal diet (C) and a high-fat diet (D) after 24h. (E) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was used to show the expression of genes associated with inflammation, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-10, and chemokine (C-C motif) ligand 2 (CCL2), also known as monocyte chemoattractant protein 1 (MCP1), in subcutaneous white adipose tissue and extraperitoneal white adipose tissue, respectively. Data are presented as the mean ±SD; * P<0.05 and ** P<0.01 by Student’s t-test. (n=10).
The expression levels of pro-inflammatory cytokines interleukin (IL)-6, IL-1β, tumor necrosis factor (TNF)-α, and chemokine (C-C motif) ligand 2 (CCL2), also known as monocyte chemoattractant protein 1 (MCP1), were analyzed in extraperitoneal white adipose tissue and subcutaneous white adipose tissue. Following berberine treatment, the expression of pro-inflammatory cytokines was significantly decreased, but the expression of IL-10 increased in extraperitoneal white adipose tissue and subcutaneous white adipose tissue (Figure 2D, 2E). These results showed that berberine treatment reduced the inflammatory response in white adipose tissue, and promoted heat production in brown adipose tissue in an obese mouse model fed a high-fat diet.
Berberine treatment inhibited inflammation by recruitment of M2 macrophages and blocking M2 macrophage polarization in white adipose tissue in mice fed a high-fat diet
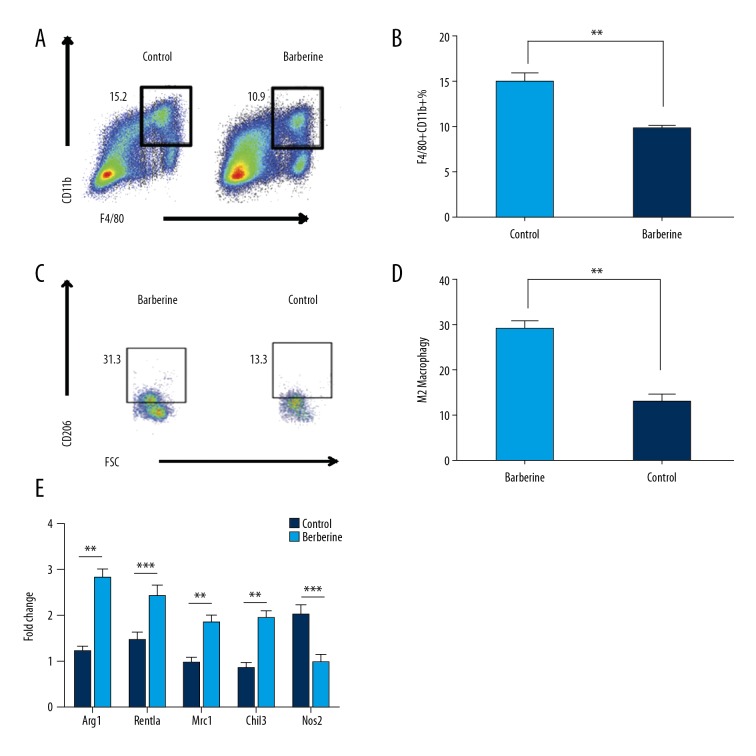
To determine the cause of reduced inflammation in white adipose tissue, flow cytometry was performed to analyze the macrophage phenotype, as key roles for tissue macrophages in metabolic dysfunction have been previously reported [23,24]. Increased recruitment of CD11b-positive and F4/80-positive cells in mice fed a high-fat diet was found. Following berberine treatment, the recruitment of CD11b-positive and F4/80-positive cells was reduced (Figure 3A, 3B). Following berberine treatment, extraperitoneal white adipose tissue M2 macrophages numbers were increased, and M1 macrophages were decreased (Figure 3C, 3D), demonstrating M2 polarization and reduced inflammation. M2 macrophage marker genes, including the arginase 1 gene (ARG1), the mannose receptor gene (MRC1), and the chitinase-like protein 3 (CHIL3) gene were also upregulated after administration of berberine. Marker genes specific for M1 macrophages, including nitric oxide synthase 2 (NOS2), were downregulated after treatment with berberine (Figure 3E).
Figure 3.
Berberine treatment was associated with M2 macrophage infiltration in white adipose tissue. (A, B) Flow cytometry plots of macrophage infiltration in adipose tissue. Figures represent the proportion of tissue macrophages in white adipose tissue on the left (A), and quantitative analysis is presented on the right (B). (C, D) Analysis of flow cytometry of M2 macrophage infiltration in adipose tissue. Representative flow cytometry plot on the left demonstrates the proportion of M2 tissue macrophages in white adipose tissue (C), and quantitative analysis is presented on the right (D). (E) M1 and M2 marker gene expression, measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR), in white adipose tissue. Data are presented as the mean ±SD; * P<0.05 and ** P<0.01 by Student’s t-test. (n=5).
To determine whether M2 polarization is the mechanism responsible for reduced inflammation, clodronate liposomes were used to remove all resident macrophages in adipose tissue. Increased expression of pro-inflammatory cytokines and reduced levels of IL-10 occurred after application of clodronate liposomes and remained below those observed for mice that were not treated with berberine (Figure 4A). Reduced expression of UCP1 in subcutaneous white adipose tissue was found at the clodronate injection site, as well as in mice treated with berberine after they were exposed to a 4°C cold environment to induce the expression of thermogenesis genes (Figure 4B, 4C). These data showed that M2 macrophage recruitment was the predominant mechanism for establishing an anti-inflammatory microenvironment in white adipose tissue, and regulating thermogenic processes. Because M2 macrophages have been previously shown to be an anti-inflammatory cell type with additional paracrine effects including upregulation immunomodulatory cells, including regulatory T-cells (Tregs), reduced M2 recruitment in white adipose tissue could result in excessive inflammatory responses, regardless of M1 macrophages. These results showed that M2 macrophage were the predominant cells in determining inflammatory homeostasis in white adipose tissue in an obese mouse model fed a high-fat diet.
Figure 4.
Clodronate liposomes, used to inhibit macrophage recruitment, promoted inflammatory gene expression, and reduced UCP1 expression. (A) Increased expression of pro-inflammatory genes and reduced expression of IL-10 in white adipose tissue (WAT). (B) Western blot of UCP1 expression in subcutaneous white adipose tissue after cold exposure. GAPDH is the loading control. (C) Quantitative analysis of UCP1 expression. Data are presented as the mean ±SD; * P<0.05 and ** P<0.01 by Student’s t-test. (n=5).
Berberine treatment suppressed M2 apoptosis and decreased endoplasmic reticulum stress of macrophages in white adipose tissue in mice fed a high-fat diet
To determine the role of M2 macrophages in white adipose tissue after berberine treatment, the expression of apoptosis-related genes was measured by immunoblotting. The results showed decreased expression of caspase-3, caspase-8, and Bax in M2 macrophages isolated from white adipose tissue, while apoptosis gene expression in M1 macrophages was unaltered (Figure 5A, 5B). This finding might indicate that berberine selectively inhibits apoptosis of M2 macrophages that are resident in white adipose tissue, which could partly explain the observed M2 polarization following berberine treatment. Also, measurement of the expression of IRE1α and other endoplasmic reticulum stress-related genes, in mice fed a high-fat diet showed decreased expression of IER1α in macrophages isolated from mice treated with berberine when compared with the control group (Figure 5C). However, the expression of other endoplasmic reticulum stress-related genes such as eIF2α, BIP, CHOP, and ATF4 was not detected in this study (Figure 5C). These results showed that that endoplasmic reticulum stress was relevant to M2 macrophage polarization and M1/M2 balance in an obese mouse model fed a high-fat diet.
Figure 5.
Berberine treatment suppressed apoptosis in M2 macrophages. (A) Representative immunoblot assay of apoptosis-related gene expression in M1 and M2 macrophages from subcutaneous white adipose tissue. GADPH is the loading control. (B) Quantitative analysis of apoptosis gene expression. GADPH is the loading control. (C) Representative immunoblot of genes related to endoplasmic reticulum stress. (D) Quantitative analysis of relative expression of genes related to endoplasmic reticulum stress. GADPH is the loading control. Data are presented as the mean ±SD; * P<0.05 and ** P<0.01 by Student’s t-test. (n=5).
Discussion
Recently, the immunomodulatory effects of berberine, a benzylisoquinoline alkaloid used in traditional Chinese medicine, have been reported in various autoimmune and inflammatory diseases, including multiple sclerosis and myocarditis [25,26]. The cardioprotective effects of berberine in myocarditis were found to reduce cardiac specific antibody production and differentiation of Th1/TH17 pro-inflammatory cells via inhibition of the JAK-STAT signaling pathway [27]. In animal models of multiple sclerosis, the administration of berberine reduced demyelination and neurological symptoms of multiple sclerosis, attributed to the effects on the sphingosine kinase 1 and sphingosine-1-phosphate (Sphk1/S1P) signaling pathway by blocking inflammatory cell infiltration into diseased sites [25]. Berberine has also been shown to reduce hyperglycemia in non-obese diabetic mouse, via activation of ERK1/2, p38, and mitogen-activated protein kinase (MAPK) signaling, which suggests that the berberine might be a candidate treatment for hyperglycemia [11]. Berberine has also been reported to prevent the destruction of islet cells resulting from autoimmune pancreatic islet cell destruction [25,27]. In cases of diabetic retinopathy, it has been reported that berberine inhibits leukocyte accumulation within the retina and prevents inflammatory damage to retinal endothelial cells [28].
The aims of the present study were to investigate the effects of berberine on energy expenditure, expression of the uncoupling protein 1 (UCP1) gene, the cell stress protein inositol-requiring enzyme 1α (IRE1α), apoptosis genes, and macrophage phenotype (M1 and M2) in white and brown adipose tissue in an obese mouse model fed a high-fat diet. The findings were that berberine treatment in mice fed a high-fat diet increased energy metabolism, glucose tolerance, and expression of UCP1, and reduced expression of pro-inflammatory cytokines, macrophage recruitment, and resulted in M2 macrophage polarization in brown adipose tissue. Reduced expression of apoptotic genes and IRE1α occurred in polarized M2 macrophages. These findings support those of previous studies on the anti-inflammatory effects of berberine. Additional immunomodulatory mechanisms for the action of berberine, including its effects on the regulation of leukocyte subsets and pro-inflammatory cells, remain to be determined. Excessive or autoimmune inflammatory responses have been previously reported in conditions of over-nutrition, resulting in pathological metabolic conditions, inflammatory disease, cardiovascular disease, and nonalcoholic fatty liver disease (NAFLD) [14–18]. There is an increasing requirement for the therapeutic control of low-grade inflammation associated with metabolic disease, including metabolic syndrome, which is characterized by hypertension, diabetes, obesity, and hyperlipidemia.
Mice fed a high-fat or nutrient-rich diet have previously been shown to have activated endoplasmic reticulum stress signaling and metabolic inflammation, with increased pro-inflammatory cytokine levels, although the mechanisms underlying the relationship between endoplasmic reticulum stress and inflammatory responses remain unknown [29,30]. Aberrant metabolic inflammation in white adipose tissue is considered to be a contributing factor to dysregulation of lipid, insulin resistance, reduced energy expenditure, and increased rates of obesity [14–16,18]. Inflammation is central to the pathophysiology of metabolic disease, as in type 2 diabetes and metabolic syndrome, over-nutrition, and obesity-induced inflammation are primary risk factors [21,22,31]. This finding is in contrast to type 1 diabetes, which is an autoimmune disease characterized by infiltrating T-lymphocytes and islet-specific auto-antibody production [32]. Innate immune cells are considered the primary mediators of inflammation in other organs and tissues, such as liver and white adipose tissue in type 2 diabetes mellitus [31]. Therefore, it is possible to hypothesize that resident macrophage populations in white adipose tissue might regulate adipose inflammation in the context of over-nutrition and metabolic disturbance.
The findings of the present study showed that berberine not only reduced hyperglycemia and insulin resistance but also enhanced energy consumption under conditions of physical exercise. Previous studies have demonstrated that M2 polarization induced by IL-4 and IL-13 resulted in an increased basal metabolic rate and increased energy consumption [33–35]. The tissue remodeling that results from the conversion of brown to white adipose tissue has been shown to be associated with M2 polarization, which also reduces metabolic disturbance and enhances thermogenesis [33]. Therefore, M2 polarization might represent a downstream biological effect that occurs after administration of immunomodulatory drugs and results in an anti-inflammatory microenvironment. In the present study, berberine, an alkaloid extracted from Chinese herbs and used in traditional Chinese medicine, has diverse pharmacologic activities in a mouse model, including the ability to induce M2 polarization in white adipose tissue in conditions of over-nutrition and metabolic disturbance due to a high-fat diet.
In this study, an attempt was made to investigate the mechanism of M2 polarization, and the results indicated that this might occur due to changes in apoptotic signaling. Apoptotic gene expression was increased in mice fed a high-fat diet and treated with berberine, and cell survival of M2 macrophages in white adipose tissue was increased, but berberine had no effect on M1 macrophages, indicating that M2 macrophages are the predominant macrophage subtype within white adipose tissue. An unexpected finding of this study was that complete depletion of macrophages impaired the metabolic improvements observed following administration of berberine, which suggests that M2 macrophages might amplify immunomodulatory effects by regulating downstream anti-inflammatory cells. The cell stress protein, inositol-requiring enzyme 1α (IRE1α), is a key molecule involved in endoplasmic reticulum stress and has previously been reported to be involved in metabolic disease and to promote M1 differentiation in mice fed a high-fat diet [33,36]. Therefore, depletion of IRE1α in white adipose tissue macrophages might improve metabolic status in chronic inflammatory diseases and in metabolic syndrome. In the present study, IRE1α expression was found to be reduced after treatment of the mice with berberine, although other marker genes related to endoplasmic reticulum stress showed no difference in expression levels. This finding might be due to the complex signal transduction pathways associated with the endoplasmic reticulum stress response.
Conclusions
The findings of this study, in a high-fat diet mouse model, showed that berberine, a traditional Chinese medicine, increased energy metabolism, glucose tolerance, and expression of the uncoupling protein 1 (UCP1) gene, and reduced expression of pro-inflammatory cytokines, macrophage recruitment, and resulted in M2 macrophage polarization in adipose tissue. Polarized M2 macrophages showed reduced expression of apoptotic genes and the cell stress protein, inositol-requiring enzyme 1α (IRE1α). Further studies are recommended to examine the molecular and the clinical effects of berberine treatment in patients with metabolic and inflammatory disease, including metabolic syndrome.
Footnotes
Source of support: Departmental sources
References
- 1.Kumar A, Ekavali, Chopra K, et al. Current knowledge and pharmacological profile of berberine: An update. Eur J Pharmacol. 2015;761:288–97. doi: 10.1016/j.ejphar.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 2.Ortiz LM, Lombardi P, Tillhon M, Scovassi AI. Berberine, an epiphany against cancer. Molecules. 2014;19:12349–67. doi: 10.3390/molecules190812349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed T, Gilani AU, Abdollahi M, et al. Berberine and neurodegeneration: A review of literature. Pharmacol Rep. 2015;67:970–79. doi: 10.1016/j.pharep.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Dong H, Zhao Y, Zhao L, Lu F. The effects of berberine on blood lipids: A systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013;79:437–46. doi: 10.1055/s-0032-1328321. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Yi X, Ma L, Zhou Y. Hepatocyte growth factor and basic fibroblast growth factor regulate atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease via the mitogen-activated protein kinase signaling pathway. Exp Ther Med. 2013;6:1121–26. doi: 10.3892/etm.2013.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan J, Zhao Y, Dong F, et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia, and hypertension. J Ethnopharmacol. 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 7.Yan HM, Xia MF, Wang Y, et al. Efficacy of berberine in patients with non-alcoholic fatty liver disease. PLoS One. 2015;10:e0134172. doi: 10.1371/journal.pone.0134172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang X, Yan H, Fei J, et al. Berberine reduces methylation of the MTTP promoter and alleviates fatty liver induced by a high-fat diet in rats. J Lipid Res. 2010;51:2504–15. doi: 10.1194/jlr.M001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing LJ, Zhang L, Liu T, et al. Berberine reducing insulin resistance by up-regulating IRS-2 mRNA expression in nonalcoholic fatty liver disease (NAFLD) rat liver. Eur J Pharmacol. 2011;668:467–71. doi: 10.1016/j.ejphar.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 10.Qiang X, Xu L, Zhang M, et al. Demethyleneberberine attenuates non-alcoholic fatty liver disease with activation of AMPK and inhibition of oxidative stress. Biochem Biophys Res Commun. 2016;472:603–9. doi: 10.1016/j.bbrc.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Cui G, Qin X, Zhang Y, et al. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420–29. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni WJ, Ding HH, Tang LQ. Berberine as a promising anti-diabetic nephropathy drug: An analysis of its effects and mechanisms. Eur J Pharmacol. 2015;760:103–12. doi: 10.1016/j.ejphar.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Luo Y, Xiong J. [Progress for treating diabetic renopathy by berberine hydrochloride in clinical and experimental researches]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32:1714–17. [in Chinese] [PubMed] [Google Scholar]
- 14.Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013;39:99–110. doi: 10.1016/j.diabet.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golia E, Limongelli G, Natale F, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. 2014;16:435. doi: 10.1007/s11883-014-0435-z. [DOI] [PubMed] [Google Scholar]
- 17.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 18.Guarner V, Rubio-Ruiz ME. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol. 2015;40:99–106. doi: 10.1159/000364934. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Ye J. Regulation of energy balance by inflammation: Common theme in physiology and pathology. Rev Endocr Metab Disord. 2015;16:47–54. doi: 10.1007/s11154-014-9306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill AA, Reid Bolus W, Hasty AH. A decade of progress in adipose tissue macrophage biology. Immunol Rev. 2014;262:134–52. doi: 10.1111/imr.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jourdan T, Godlewski G, Cinar R, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–40. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y, Yu X, Chen H, et al. Leptin deficiency shifts mast cells toward anti-inflammatory actions and protects mice from obesity and diabetes by polarizing M2 macrophages. Cell Metab. 2015;22:1045–58. doi: 10.1016/j.cmet.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choe SS, Shin KC, Ka S, et al. Macrophage HIF-2alpha ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes. 2014;63:3359–71. doi: 10.2337/db13-1965. [DOI] [PubMed] [Google Scholar]
- 24.Orr JS, Kennedy A, Anderson-Baucum EK, et al. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes. 2014;63:421–32. doi: 10.2337/db13-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo J, Chen R, Zeng S, et al. The effects of berberine on a murine model of multiple sclerosis and the SPHK1/S1P signaling pathway. Biochem Biophys Res Commun. 2017;490:927–32. doi: 10.1016/j.bbrc.2017.06.142. [DOI] [PubMed] [Google Scholar]
- 26.Lv X, Yu X, Wang Y, et al. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS One. 2012;7:e47351. doi: 10.1371/journal.pone.0047351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Zhang X, Ye L, Yuan H. Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. Biomed Pharmacother. 2016;79:222–30. doi: 10.1016/j.biopha.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 28.Tian P, Ge H, Liu H, et al. Leukocytes from diabetic patients kill retinal endothelial cells: Effects of berberine. Mol Vis. 2013;19:2092–105. [PMC free article] [PubMed] [Google Scholar]
- 29.Keestra-Gounder AM, Byndloss MX, Seyffert N, et al. NOD1 and NOD2 signaling links ER stress with inflammation. Nature. 2016;532:394–97. doi: 10.1038/nature17631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–41. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- 31.Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38:183–91. doi: 10.1016/j.diabet.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Xie Z, Chang C, Zhou Z. Molecular mechanisms in autoimmune type 1 diabetes: A critical review. Clin Rev Allergy Immunol. 2014;47:174–92. doi: 10.1007/s12016-014-8422-2. [DOI] [PubMed] [Google Scholar]
- 33.Shan B, Wang X, Wu Y, et al. The metabolic ER stress sensor IRE1alpha suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol. 2017;18:519–29. doi: 10.1038/ni.3709. [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, Tang Y, Zhou H, Xie K. A meta-analysis on correlation between interleukin-6-174G/C polymorphism and end-stage renal disease. Renal Failure. 2017;39:350–56. doi: 10.1080/0886022X.2017.1281146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galvan-Pena S, O’Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu R, Zhang QH, Lu YJ, et al. Involvement of the IRE1alpha-XBP1 pathway and XBP1s-dependent transcriptional reprogramming in metabolic diseases. DNA Cell Biol. 2015;34:6–18. doi: 10.1089/dna.2014.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]