Summary
Our previous study revealed that Yin Yang 1(YY1) played an important part in promoting interleukin (IL)‐6 production in rheumatoid arthritis (RA). However, whether YY1 has any role in regulation of IL‐8 in RA remains unclear. YY1 and IL‐8 expression in RA patients were analyzed by real‐time polymerase chain reaction (PCR). Ingenuity pathway analysis (IPA) was used to analyze the signaling pathway involved in YY1‐induced IL‐8 production. The expression of YY1 and proteins involved in the pathway were detected by Western blot and enzyme‐linked immunosorbent assay (ELISA). Migration of neutrophils was performed by chemotaxis assay. In this study, we found that high expression of IL‐8 was positively associated with YY1 expression in RA. Blocking YY1 expression by YY1‐short hairpin (sh)RNA lentivirus reduced IL‐8 production. Mechanistically, we showed YY1 activated IL‐8 production via the phosphatidylinositol‐3‐kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway. Further, using a co‐culture system consisting of peripheral blood mononuclear cells (PBMC) and neutrophils, we found that migration of neutrophils would be inhibited by YY1 RNA interference. Finally, using the collagen‐induced arthritis animal model, we showed that treatment with the YY1‐shRNA lentivirus led to reduction of IL‐8 levels and attenuation of inflammation and neutrophil infiltration in vivo. Our results reveal a role of YY1 involved in neutrophil infiltration in RA via the PI3K/Akt/mTOR/IL‐8 signaling pathway. YY1 may be a new therapeutic target for treatment of RA.
Keywords: interleukin‐8, neutrophils, rheumatoid arthritis, YY1
Introduction
Rheumatoid arthritis (RA), a chronic inflammatory autoimmune disease that affects approximately 1% of the world’s population, is characterized by neutrophil infiltration, joint destruction and function disability 1. It is established that gene–environmental interaction contributes to the development of RA. Our previous study showed that transcription factor YY1 (Yin Yang 1) plays an important role in the pathogenesis of RA by regulating the production of interleukin (IL)‐6, contributing to the process of RA 2. However, due to the complex mechanism of RA, whether or not YY1 has any other role in the pathogenesis of RA is still unclear.
It is well known that neutrophils are the major leukocytes in RA joints and play a crucial role in the development of RA 3, 4. In RA, the mechanisms of neutrophil activation and migration are changed 5. In the early stages of the disease, neutrophils first migrate to the synovial fluid (SF), where they phagocytose immune complexes, release protease‐rich granules and produce large quantities of reactive oxygen species (ROS), leading eventually to the erosion and destruction of cartilage 6. Migration and recruitment of neutrophils to the RA joints is a key pathogenesis of RA which is often mediated by chemokines 7. IL‐8 is a member of the CXC chemokine family, which acts as neutrophil chemotactic factor. It can be secreted by monocytes, macrophages, epithelial cells, endothelial cells and airway smooth muscle cells 8. Studies have shown that elevated IL‐8 can promote the recruitment of neutrophils which contribute to the occurrence of chronic inflammation diseases, such as RA 4, psoriasis 9, inflammatory bowel disease 10 and palmoplantar pustulosis 11.
However, whether YY1 plays any role in the neutrophil infiltration in RA joints, and whether IL‐8 contributes to this process, is unclear. In this study, we aimed to investigate the role of YY1 in IL‐8 production and neutrophil infiltration in RA.
Materials and methods
Patients
One hundred RA patients [22 men and 78 women, aged 26–78 years, mean ± standard deviation (s.d.) 51 ± 12 years], 100 osteoarthritis (OA) and 100 healthy controls (HD) were enrolled into the study. Diagnosis of RA patients fulfilled the 2010 American College of Rheumatology/European League Against Rheumatism criteria for RA 12. Diagnosis of OA based on the OARSI guidelines 13 and the guidelines of the Chinese Rheumatology Association. The clinical characteristics of RA, OA and HD are shown in Table 1. The study was approved by the Institutional Medical Ethics Review Board of the First Affiliated Hospital of Fujian Medical University (IEC‐FOM‐013‐1.0). Written or verbal consent were provided for the patients in the study.
Table 1.
Demographic characteristics of rheumatoid arthritis (RA) patients, osteoarthritis (OA) patients and healthy donors
| Clinical data | RA | OA | HD |
|---|---|---|---|
| Age, mean (range), years | 51·05 (26–78) | 53·25 (19–78) | 51·79 (49–55) |
| Sex, no. female | 78 | 66 | 64 |
| Sex, no. male | 22 | 34 | 36 |
| WBC, mean ± s.d., 109/l | 8·00 ± 2·43 | 7·36 ± 2·54 | 6·14 ± 1·56 |
| RBC, mean ± s.d., 1012/l | 4·39 ± 0·41 | 4·43 ± 0·54 | 4·70 ± 0·44 |
| PLT, mean ± s.d., 109/l | 262·66 ± 63·18 | 254·15 ± 88·36 | 267·10 ± 63·67 |
| HGB, mean ± s.d., g/l | 128·70 ± 15·57 | 130·32 ± 16·31 | 141·70 ± 14·69 |
| RDW, mean ± s.d., % | 14·43 ± 1·70 | 13·76 ± 1·36 | 13·00 ± 0·80 |
| ACPA, median (IQR), RU/ml | 125·8 (151·8) | 1 (0·7) | – |
| CRP, median (IQR), mg/l | 10·25 (12·4) | 12·6 (8·22) | – |
| RF, median (IQR), IU/ml | 107 (172·95) | < 10·2 (0) | – |
| ESR, median (IQR), mm/h | 25 (26·75) | 20 (24·75) | – |
RA = rheumatoid arthritis; OA = osteoarthritis; HD = healthy donors; ACPA = anti‐cyclic citrullinated peptide antibodies; RF = rheumatoid factor; ESR = erythrocyte sedimentation rate; CRP = C‐reactive protein; IQR = interquartile range; s.d. = standard deviation.
Animals
Male DBA/1J mice, aged 6–8 weeks, were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Science. Mice were maintained under pathogen‐free conditions. All experiments were performed according to the committee guidelines and approved by the Animal Experiment Center of the Fujian Medical University (SYXK (Fujian) 2012‐0001).
Peripheral blood mononuclear cell (PBMC) isolation and culture
PBMC were isolated from the peripheral blood of RA and OA patients and HD controls according to the manufacturer’s instructions. In brief, PBMC were isolated immediately by Ficoll (TBD Science, Tianjin, China) and resuspended in RPMI‐1640 medium with 10% fetal bovine serum at a cell concentration of 105/ml. These PBMC were stimulated by Dynabeads human T‐activator CD3/CD28 (gibco, Carlsbad, CA, USA) and human IL‐2 recombinant protein (eBioscience, San Diego, CA, USA). After 24 h, these activated PBMC were infected with YY1 over‐expressing lentivirus (LV‐YY1), YY1 short hairpin (sh)RNA lentivirus (LV‐YY1‐shRNA) or control lentivirus (LV‐NC) [multiplicity of infection (MOI) = 10] and 5 μg/ml of polybrene. All the lentivirus was obtained from GenePharma (Shanghai, China). The primers used for lentivirus construction are shown in Supporting information, Table 1; 48 h later, cell cultures were collected for further use.
Establishment and treatment of collagen‐induced arthritis (CIA)
CIA mice were induced as previously described 2. On day 1, male DBA/1J mice were injected intradermally with 150 mg bovine type II collagen (Chondrex, Redmond, WA, USA) dissolved in 0·05 M acetic acid and emulsified in Freund’s complete adjuvant. On day 21, 75 mg collagen II in Freund’s incomplete adjuvant was administered to the mice by intradermal injection. Joint inflammation was evaluated from 28 days after the first immunization, with a scale of 1–4. The total score for each mouse was 16, based on four paws. When the CIA mice had an arthritic score of 2–3, the mice were divided into two groups and treated with LV‐YY1‐shRNA or LV‐NC, respectively, by tail intravenous injection, 4 × 107 transducing units (TU)/mouse.
Histopathology
CIA mice were killed 70 days post‐immunization,. The knee joints were dissected and fixed in 10% phosphate‐buffered formalin for 2 days, then decalcified in 10% ethylenediamine tetraacetic acid (EDTA) for 15 days. The specimens were embedded in paraffin, and serial paraffin sections were cut throughout the joint by a microtome. The sections were stained with hematoxylin and eosin (H&E) and observed by light microscopy.
Real‐time PCR analysis
Total RNA was extracted from cells, and real‐time PCR was performed as previously described 14. Briefly, total RNA was extracted from cells using a TransZol Up Plus RNA Kit (Transgen, Beijing, China). Messenger RNA (mRNA) was converted to cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA). Two‐step real‐time PCR was performed using SYBR Premix Ex Taq (Takara, Dalian, China), according to the manufacturer’s instructions. The primers used in this study are shown in Supporting information, Table 1. Thermocycler conditions were as follows: initial holding at 95°C for 30 s, followed by a two‐step PCR process that consisted of 95°C for 3 s and 65°C for 30 s for 40 cycles. Data were collected, and quantitative analysis was performed using an ABI Prism 7500 sequence detection systemwThe glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) gene was used as the endogenous control. Gene expression was then calculated as the difference in cycle threshold (ΔCt) between the target gene and GAPDH; relative expression of target genes was calculated as 2–ΔCt.
Western blot analysis
Protein immune blotting was performed as previously described 15. Briefly, cell lysates were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS‐PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore Corporation, Bedford, MA, USA) at 90 V for 90 min. The expression of YY1 and the phosphorylation of phosphatidylinositol‐3‐kinase (PI3K) and Akt were analyzed using specific antibodies (Abcam, Cambridge, MA, USA). After washing with phosphate‐buffered saline (PBS), the membranes were incubated with horseradish peroxidase (HRP)‐conjugated goat anti‐rabbit immunoglobulin (Ig)G at room temperature for 1 h followed by washing with PBS. The target proteins were examined with an enhanced chemiluminescence (ECL) system (Beyotime, Shanghai, China) and visualized with ChemiDoc XRS+ System (Bio‐Rad, Hercules, CA, USA). The grey value of the band was analyzed by Image J software.
ELISA detection
PBMC from RA patients were isolated and stimulated with IL‐2 (10 IU/ml; R&D Systems, Minneapolis, MN, USA) and Dynabeads human T‐activator CD3/CD28 (gibco). After 24 h, the cells were infected with LV‐YY1‐shRNA, LV‐YY1 or LV‐NC (MOI = 10) lentivirus and 5 μg/ml of polybrene. Signaling pathway inhibitors, including 50 μM LY294002 (inhibitor of PI3K), 100 μM BAY 11‐7082 [inhibitor of nuclear factor kappa B (NF‐κB)], 50 μM SB203580 [inhibitor of mitogen‐activated protein kinase (MAPK)], 10 μM SP600125 [inhibitor of Janus kinase (JNK)] and 50 μM rapamycin (inhibitor of mammalian target of rapamycin (mTOR)], were added to LV‐YY1‐treated PBMC. Cell supernatant was collected 48 h later and diluted (1 : 1000) for the measurement of IL‐8 by ELISA, according to the manufacturer’s recommendations (R&D Systems).
Neutrophil isolation
Neutrophils were isolated from peripheral blood of RA patients. Briefly, neutrophils were isolated under sterile conditions immediately by Ficoll (TBD Science). Residual erythrocytes were removed by hypotonic lysis, and the cells were resuspended in RPMI‐1640 medium supplemented with 10% fetal bovine serum at a cell concentration of 105/ml. The neutrophil viability was confirmed using trypan blue staining.
Transwell migration assay
PBMC from RA patients, in the presence of Dynabeads human T‐activator CD3/CD28 (gibco) and human IL‐2 recombinant protein (eBioscience, San Diego, CA, USA), resuspended at a concentration of 5 × 105 cells/ml in RPMI‐1640 and 10% fetal bovine serum and treated with L V‐YY1‐shRNA or LV‐NC lentivirus, were added to the lower chamber. Neutrophils isolated from RA patients were added to the top chamber; 8 h later the filters were fixed with 4% paraformaldehyde for 15 min. The cells remained on fixed filters and were observed under optical microscope and counted with Wright–Giemsa staining.
Microarray and ingenuity pathway analysis (IPA)
The IPA analysis was performed as in our previous study 2. In brief, in the presence of Dynabeads human T‐activator CD3/CD28 (gibco) and IL‐2 (10 IU/ml, R&D Systems), PBMC from RA patients were treated with LV‐YY1‐shRNA or LV‐NC lentivirus for 4 h. Then, the PBMC was collected and total RNA from was extracted by the standard TRIzol method. Affymetrix array was performed by Genechme Company Limited (Shanghai, China) using human primeview GeneChip. The signaling pathways involved were performed by IPA.
Statistical analysis
All the experiments were performed in triplicate or more. Data were presented as mean ± standard deviation (s.d.) or n (%). Analysis of variance was performed to determine differences among groups, and Student’s t‐test was used to analyze the differences between two groups. Comparisons of categorical variables were conducted using χ2 testing. Correlation analyses were performed using Spearman’s correlation test. Arthritic score of CIA mice was analyzed by repeated measured analysis of variance (anova). A P‐value less than 0·05 was considered as statistical difference for the statistical analyses. Statistical analyses were performed by the Statistical Package for the Social Sciences, version 22.0 (SPSS Inc, Chicago, IL, USA), Microsoft Excel 2016 (Microsoft, Redmond, WA, USA) or GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA).
Results
YY1 and IL‐8 levels are increased in RA patients
Our previous studies have shown that YY1 plays a critical role in RA by inducing IL‐6 production. Many studies have found that IL‐8 is also involved in the pathophysiology of RA 16, 17. In order to explore the role of YY1 in the production of IL‐8, the expression of YY1 and IL‐8 in peripheral blood derived from RA, OA patients and healthy donors was examined. The results showed that the mRNA expression and protein levels of YY1 (Fig. 1) and IL‐8 (Fig. 1c) were increased in RA patients compared with OA patients and HD, and there was a significant positive association between YY1 and IL‐8 expression (Fig. 1d).
Figure 1.
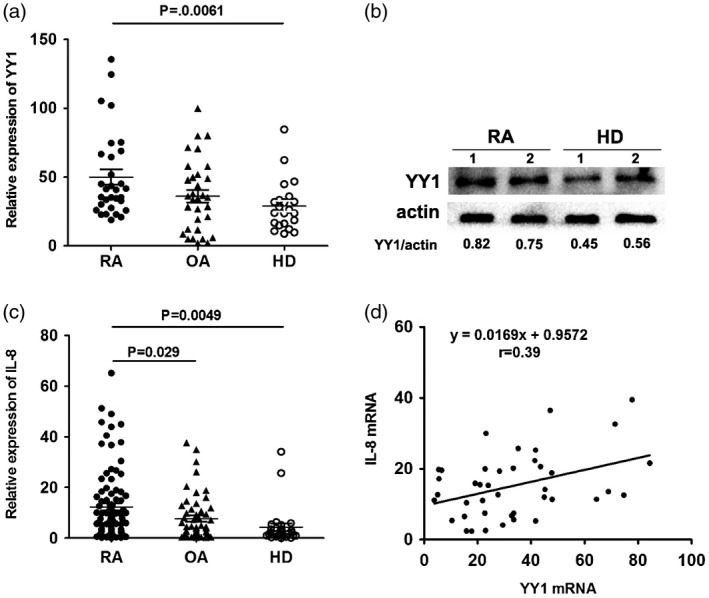
Yin Yang 1 (YY1) and IL‐8 were over‐expressed in rheumatoid arthritis (RA) patients. (a) Relative expression of YY1 in RA patients (n = 32), osteoarthritis (OA) patients (n = 32) and healthy donors (n = 22) was detected by real‐time polymerase chain reaction (PCR). (b) Protein level of YY1 in RA patients and health donors determined by Western blot. (c) Relative expression of interleukin (IL)‐8 in RA patients (n = 32), OA patients (n = 32) and health donors (n = 22) detected by real‐time PCR. (d) Correlation analysis between YY1 and IL‐8 expression in RA patients.
Inhibited YY1 expression affected PI3K/AKT and mTOR signaling
IL‐8 plays an important role in the development of RA, and many signaling pathways are involved in the regulation of IL‐8. In the previous study, we observed that YY1 expression was positively correlated with IL‐8 level; we then questioned whether there were signaling pathways involved in this procession. To clarify this, we treated PBMC from RA patients with lentivirus of LV‐YY1‐shRNA or LV‐NC for microarray assay. The inhibitory effect of LV‐YY1‐shRNA was confirmed in our previous study 2. Volcano plot analysis showed that expression of 757 genes showed a significant difference between LV‐NC‐ and LV‐YY1‐shRNA‐treated PBMC, including IL‐6, YY1 and IL‐8. The x‐axis is the logarithmic transformation with 2 as base of the fold change, and the y‐axis is the logarithmic transformation with 10 as base of the statistical significance (Fig. 2a). Moreover, decreased YY1 mRNA and protein expression was confirmed by real‐time PCR and Western blot assay (Fig. 2). We further analyzed the changes in the signaling pathways by IPA, and the analysis result was shown by Z‐score, showing that the PI3K/AKT and mTOR signal pathways were significantly inhibited in LV‐YY1‐shRNA‐treated PBMC in RA patients (Fig. 2).
Figure 2.
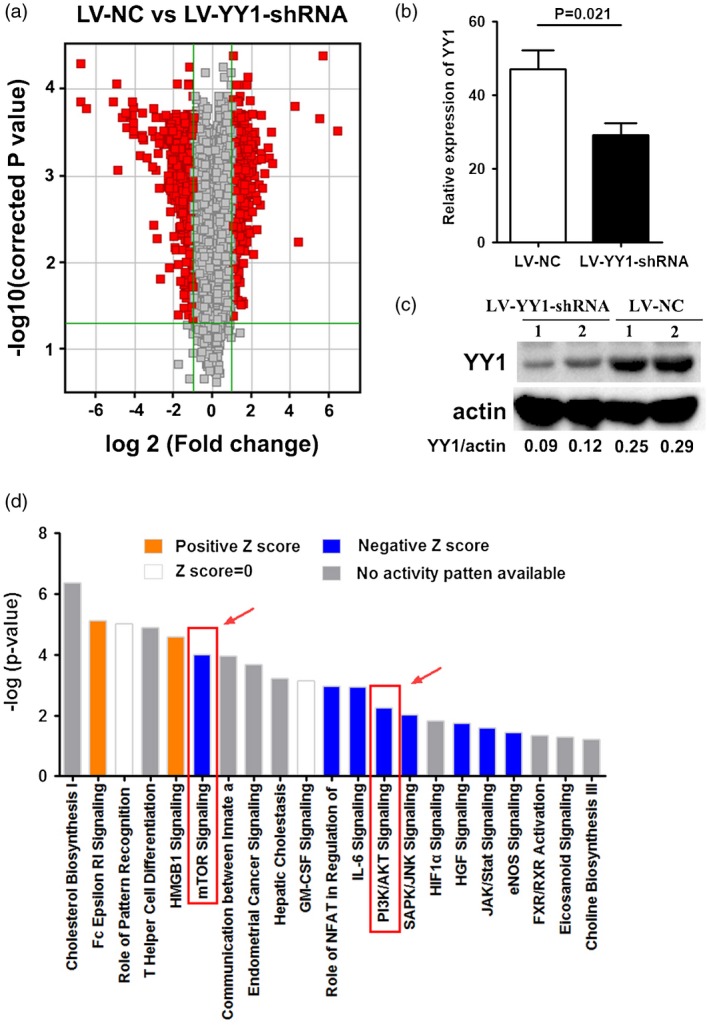
Mammalian target of rapamycin (mTOR) signaling was restrained by Yin Yang 1 (YY1) blockade. (a) Volcano plot analysis of differences in gene distribution between lentivirus‐control (LV‐NC) or LV‐YY1‐short hairpin (shRNA)‐treated peripheral blood mononuclear cells (PBMC). The x‐axis is the logarithmic transformation with 2 as base of the fold change. The y‐axis is the logarithmic transformation with 10 as base of the statistical significance. (b) mRNA expression of YY1 in LV‐NC‐ or LV‐YY1‐shRNA‐treated PBMC detected by real‐time polymerase chain reaction (PCR). (c) Protein level of YY1 in LV‐NC‐ or LV‐YY1‐shRNA‐treated PBMC detected by Western blot. (d) The histogram of the signaling pathway shows the enrichment of the differentially expressed genes in the classical signaling pathway, and the analysis was shown by Z‐score. Positive Z‐score (yellow) indicated that the pathway was significantly activated. Negative Z‐score (blue) indicated that the pathway was significantly suppressed.
YY1 induced IL‐8 production via the PI3K/Akt signaling pathway
It is well known that mTOR is a key kinase downstream of PI3K/AKT, which regulates tumor cell proliferation, growth 18, 19 and inflammation 20, 21, etc. As we found that the mTOR signaling pathway might take part in the production of IL‐8 induced by YY1 in PBMC from RA patients, we next detected several downstream signaling pathways of YY1 using known inhibitors of several pathways. The results showed that PI3K, NF‐κB and mTOR inhibitors significantly inhibit the production of IL‐8 in LV‐YY1‐treated PBMC from RA patients (Fig. 3a). To further validate the signaling pathway in LV‐YY1‐shRNA‐treated cells, a Western blot assay was performed for detection of PI3K/Akt phosphorylation protein levels. The results showed that the phosphorylation of PI3K and Akt protein was significantly decreased in YY1 blocking PBMC (Fig. 3). Therefore, we believe that interfering with the expression of YY1 in lymphocytes will inhibit the release of IL‐8 through the PI3K‐Akt‐mTOR signaling pathway.
Figure 3.
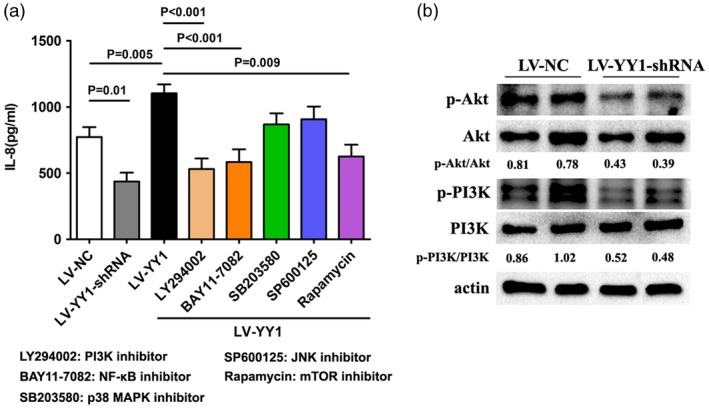
Signaling pathways involved in Yin Yang 1 (YY1)‐induced interleukin (IL)‐8 production in lymphocytes of rheumatoid arthritis (RA). (a) Peripheral blood mononuclear cells (PBMC) isolated from RA patients were treated with lentivirus‐control (LV‐NC), LV‐YY1‐short hairpin (sh)RNA or LV‐YY1. Then, 50 μM LY294002 (inhibitor of PI3K), 100 μM BAY 11‐7082 [inhibitor of nuclear factor kappa B (NF‐κB)], 50 μM SB203580 [inhibitor of mitogen‐activated protein kinase (MAPK)], 10 μM SP600125 [inhibitor of Janus kinase (JNK)] and 50 μM rapamycin [inhibitor of mammalian target of rapamycin (mTOR)] was added to LV‐YY1‐treated PBMC. After 48 h, cell supernatant was collected for detection of IL‐8 by enzyme‐linked immunosorbent assay (ELISA). (b) Western blot analysis of the PI3K, Akt and phosphorylated PI3K, Akt expression in PBMC treated with LV‐NC or LV‐YY1‐shRNA. The grey value of p‐Akt/Akt and phosphatidylinositol‐3‐kinase (p‐PI3K)/PI3K was calculated by Image J.
Blocking of YY1 reduced inflammation in CIA mice
As we found that YY1 induced IL‐8 production via the PI3K‐Akt‐mTOR signaling pathway in vitro, we determined whether YY1 plays a role in joint inflammation in vivo. We injected LV‐YY1‐shRNA and LV‐NC, respectively, into CIA mice through the tail vein. The results showed that the inflammation score in the joints were significantly decreased in LV‐YY1‐shRNA‐treated CIA mice (Fig. 4a). Compared to the LV‐NC‐treated mice, the mRNA and protein expression of YY1 in LV‐YY1‐shRNA‐treated CIA mice was also decreased (Fig. 4). Consistently, we can see that the joints from LV‐NC‐treated mice were infiltrated with leukocytes. Moreover, the cartilage destruction and pannus formation in LV‐NC‐treated mice was more serious than that in LV‐YY1‐shRNA‐treated mice (Fig. 4d). Furthermore, cytokine expression in the lymph node of LV‐NC‐ or LV‐YY1‐shRNA‐treated CIA mice was also examined. The results showed that the relative expression of IL‐8, IL‐6, IL‐17A, IL‐21 and IFN‐γ were significantly decreased in LV‐YY1‐shRNA‐treated CIA mice (Fig. 5). Taken together, these results suggested that blocking of YY1 ameliorated the inflammatory reaction of the CIA mice.
Figure 4.

Blocking of Yin Yang 1 (YY1) attenuated inflammation in collagen‐induced arthritis (CIA) mice. (a) Clinical inflammatory score of CIA mice treated with lentivirus‐control (LV‐NC) (n = 5) or lentivirus (LV‐)YY1‐short hairpin (sh)RNA (n = 5). The inflammation score in the joints were significantly decreased in LV‐YY1‐shRNA‐treated CIA mice (P = 0·029). *P < 0·05. (b,c) mRNA and protein expression of YY1 in LV‐NC or LV‐YY1‐shRNA‐treated CIA mice. (d) Histopathology of joint tissue sections of CIA mice treated with LV‐NC (n = 5) or LV‐YY1‐shRNA (n = 5) by hematoxylin and eosin (H&E) staining. Original magnification ×200. Pannus formation, cartilage destruction and neutrophils infiltration is indicated by the green arrows.
Figure 5.

Cytokine mRNA expression in lymph node of lentivirus‐control (LV‐NC) or LV‐Yin Yang 1 (YY1)‐short hairpin (sh)RNA‐treated collagen‐induced arthritis (CIA) mice. (a–f) Relative expression of tumor necrosis factor (TNF)‐α, interferon (IFN)‐γ, interleukin (IL)‐21, IL‐17A, IL‐8 or IL‐6 in lymph node of LV‐NC (n = 5) or LV‐YY1‐shRNA (n = 5)‐treated CIA mice.
Blocking of YY1 suppressed neutrophil infiltration
IL‐8 plays a major role in neutrophil migration through vascular endothelium and focal recruitment at inflammation sites. Our studies showed that blocking of YY1 could significantly decrease the expression of IL‐8. To further identify whether YY1‐blockage IL‐8 secretion in PBMC has functional activity for reducing neutrophil migration, we established a mouse model of CIA and treated mice with LV‐YY1‐shRNA or LV‐NC. The joints of the mice were obtained and cut into pathological sections for Wright–Giemsa staining. The microscopic results showed that the number of neutrophil infiltrations decreased significantly in the LV‐YY1‐shRNA‐treated CIA mice (Fig. a,b). In addition, chemotaxis assay results showed that LV‐YY1‐shRNA‐treated PBMC significantly reduced migration of neutrophils isolated from peripheral blood of RA patients (Fig. 6). Based on these results, our data demonstrated that blocking of YY1 activity with LV‐YY1‐shRNA could reduce neutrophil infiltration and migration by down‐regulated IL‐8 production.
Figure 6.
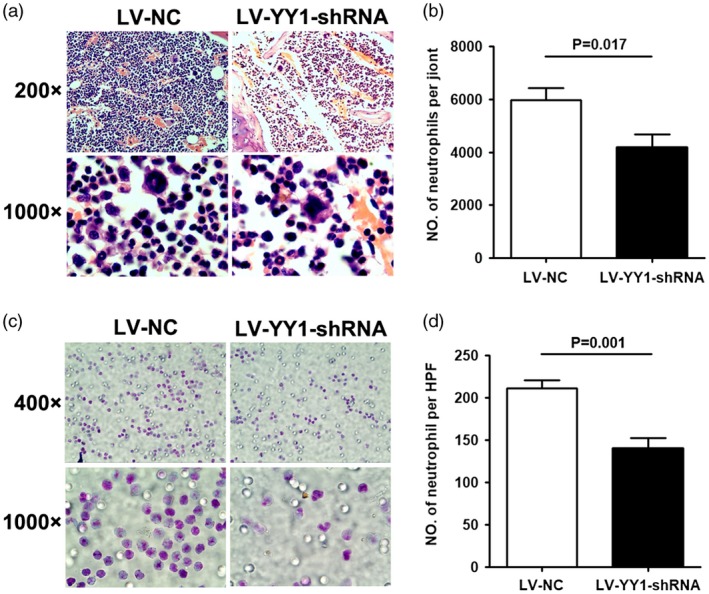
Blocking of Yin Yang 1 (YY1) reduced neutrophil migration. (a) Neutrophils of joint tissue sections of collagen‐induced arthritis (CIA) mice treated with lentivirus‐control (LV‐NC) (n = 5) or LV‐YY1‐short hairpin (sh)RNA (n = 5) by Wright–Giemsa staining. Original magnification ×200 and ×1000. Neutrophils are indicated by the green arrows. (b) Number of neutrophils per joint tissue sections of LV‐NC‐ or LV‐YY1‐shRNA‐treated CIA mice. (c) Migration of neutrophils in response to the culture supernatant from peripheral blood mononuclear cells (PBMC) treated with LV‐NC (n = 5) or LV‐YY1‐shRNA (n = 5) by Wright–Giemsa staining. Original magnification ×400 and ×1000. (d) Number of migrated neutrophils in condition of LV‐NC‐ or LV‐YY1‐shRNA‐treated PBMC.
Discussion
Although the pathogenesis of RA is unknown, accumulated evidence has shown that a large number of neutrophils were present in synovial fluid and deposited on the articular surface of RA patients, which can be activated by immune complexes (such as RF), and perform a completely different function than those isolated from healthy individuals 7. On one hand, neutrophils possess the cytotoxic potential to promote the destruction of articular cartilage which, owing to their ability to release protease‐rich granules, produce high amounts of ROS 5. On the other hand, it can interact with fibroblast‐like synoviocytes (FLS) and lymphocytes to promote intra‐articular and peripheral inflammation and other immune responses characteristic of RA 22. Many interventions used to treat RA have inhibitory effects on neutrophils. Non‐steroidal anti‐inflammatory drugs (NSAIDs) and corticosteroids reduce neutrophil adherence, degranulation and ROS production. Disease‐modifying anti‐rheumatic drugs (DMARDs), such as methotrexate, induce neutrophil apoptosis and reduce neutrophil migration 7. Tocilizumab reduces the number of neutrophils 23. Animal experiments show that neutrophil‐depleting antibody‐treated mice have a slowly progressive disease, and neutrophil depletion can prevent joint inflammation if the antibodies are given before the induction of arthritis 6. Moreover, some studies have shown that neutrophils and T helper type 17 (Th17) cells are key to the onset and perpetuation of RA. In early RA, increased levels of cytokines related with neutrophil activation and recruitment were found 6.
IL‐8, a small protein family of 8–10 kDa, is an important cytokine that promotes the chemotaxis of neutrophils to the inflammation site of the joints. IL‐8 exhibits pro‐angiogenic activity and the ability to induce blood vessel formation and angiogenesis 24. It is also a proinflammatory CXC chemokine associated with neutrophil chemotaxis and the pathogenesis of some chronic inflammatory diseases of neutrophil infiltration 11.
YY1 is a transcription factor with the ability to activate and inhibit gene transcription. It is a widely distributed transcription factor belonging to the GLI‐Kruppel family of zinc finger protein 25. Studies have shown that YY1 is over‐expressed in many types of tumors, such as breast cancer 26, osteosarcoma 27, cervical cancer, hepatoblastoma, brain tumor 28, ovarian cancer, colon cancer, multiple myeloma 29, esophageal squamous cell carcinoma 30 and non‐Hodgkin lymphoma 31, etc. Our previous studies have shown that YY1 can promote the pathogenesis of RA by promoting the production of Th17 cells 2. However, whether YY1 is involved in IL‐8 production and neutrophil infiltration remains unknown.
In this study, we first examined the expression of YY1 and IL‐8 in RA patients. The results showed that they were significantly elevated in the peripheral blood of RA patients, and there is a positive correlation between YY1 and IL‐8 levels. Similarly, in animal models of arthritis, we found that the level of joint inflammation was significantly decreased and the expression of YY1 and IL‐8 in lymph nodes were also significantly decreased in LV‐YY1‐shRNA‐treated CIA mice. These results indicated that YY1 has some relation to the expression of IL‐8. However, the activity of YY1 has not been measured. Whether the activity of YY1 is related to IL‐8 needs further study.
How does YY1 induce IL‐8 production in RA? To clarify the signaling pathway through which YY1 promotes IL‐8 production, a microarray assay was performed to identify the genes changed in LV‐YY1‐shRNA‐treated cells. The signaling pathways involved were performed by IPA, and we found that the mTOR signaling pathway was inhibited in LV‐YY1‐shRNA‐treated cells. As mTOR is a key kinase downstream of PI3K/AKT, which regulates tumor cell growth and proliferation 18, 19 and is implicated in an increasing number of pathological conditions, including cancer 32, diabetes 33, neurodegeneration 34 and inflammation 20, 21, etc., we evaluated five well‐known pathways using known inhibitors to treat YY1‐over‐expressed lymphocytes and found that PI3K, NF‐κB and mTOR signaling pathways have an impact on IL‐8 production. Moreover, the phosphorylation of PI3K and Akt protein was significantly decreased in YY1 blocking lymphocytes. All the results revealed that YY1 can induce IL‐8 production via the PI3K‐AKT‐mTOR signal pathway.
Further, in order to investigate whether the YY1‐induced IL‐8 production has some effect on neutrophil infiltration in RA, the neutrophil infiltration in CIA mice and in vitro were observed. We found that compared with LV‐NC‐treated CIA mice, the inflammation score and the neutrophil infiltration in the joints of LV‐YY1‐shRNA‐treated CIA mice was significantly decreased. Next, we treated PBMC isolated from RA patients with LV‐YY1‐shRNA in vitro; Transwell migration experiments suggested that blocking of YY1 in PBMC could significantly reduce the migration of neutrophils. Taken together, these results suggest that inhibition of YY1 attenuates neutrophil infiltration by decreasing IL‐8 production in RA.
Recently, a study showed that a novel NF‐κB/YY1/miR‐10a/NF‐κB regulatory circuit promoted the secretion of NF‐κB‐mediated inflammatory cytokines, including IL‐6 and IL‐8 35. Moreover, another study found that YY1, a long non‐coding RNA ANRIL binding transcription factor, was required for IL‐6 and IL‐8 expression under TNF‐α treatment in coronary artery disease 36. These findings were consistent with our results. However, it is not known if any other signaling pathway is involved in YY1‐regulated IL‐8 production. Our present study revealed that YY1 could promote neutrophil infiltration by regulating IL‐8 production via the PI3K‐AKT‐mTOR signal pathway in RA, which may perfect the mechanism of YY1‐regulated inflammation in RA. Combined with our previous studies 2, we believe that YY1 can promote Th17 cell production and neutrophil infiltration. Therefore, targeting YY1 may be the key strategy for future RA treatment.
Disclosures
The authors declare that they have no conflicts of interest.
Author contributions
Y. H. and Z. Z. participated in the design of the study and drafted the manuscript. Z. X. and S. C. participated in analysis and interpretation of data. J. L. participated in the design of the study, performed the statistical analyses and revised the manuscript. Q. O. participated in the design of the study and revised the manuscript.
Supporting information
Fig. S1. The viability of PBMC cells treated with lentivirus. PBMC from RA patients were treated with LV‐YY1‐shRNA or LV‐NC lentivirus, the viability of PBMC was detected by MTT assay. There is no significant difference between LV‐NC, LV‐YY1‐shRNA and control groups.
Fig. S2. The viability of PBMC cells treated with lentivirus. PBMC from RA patients were treated with LV‐NC, LV‐YY1‐shRNA or LV‐YY1 lentivirus, the viability of PBMC was detected by MTT assay. There is no significant difference between LV‐NC, LV‐YY1‐shRNA, LV‐YY1 and control groups.
Fig. S3. Signaling pathway involved endogenous IL‐8 production. PBMC from RA patients were stimulated with IL‐2 (10IU/ml) and Dynabeads Human T‐activator CD3/CD28. After 24hr, signaling pathway inhibitors 50μM LY294002, 100μM BAY 11‐7082, 50μM SB203580, 10μM SP600125 (inhibitor of JNK) and 50μM rapamycin were added. 48hr later, cell supernatant was collected for the measurement of IL‐8 by ELISA.
Table S1. The sequences of primers used in this study
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (81871710, 81401340), a grant from Fujian Provincial Department of Science and Technology (2017J01190) and a grant from Fujian Science Fund for Distinguished Young Scholars (2018J06022).
References
- 1. McInnes IB, Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017; 389:2328–37. [DOI] [PubMed] [Google Scholar]
- 2. Lin J, He Y, Chen J, Zeng Z, Yang B, Ou Q. A critical role of transcription factor YY1 in rheumatoid arthritis by regulation of interleukin‐6. J Autoimmun 2017; 77:67–75. [DOI] [PubMed] [Google Scholar]
- 3. Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol 2001; 167:1601–8. [DOI] [PubMed] [Google Scholar]
- 4. Ahn JK, Huang B, Bae EK et al The role of alpha‐defensin‐1 and related signal transduction mechanisms in the production of IL‐6, IL‐8 and MMPs in rheumatoid fibroblast‐like synoviocytes. Rheumatology (Oxf) 2013; 52:1368–76. [DOI] [PubMed] [Google Scholar]
- 5. Cascao R, Rosario HS, Souto‐Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: more than simple final effectors. Autoimmun Rev 2010; 9:531–5. [DOI] [PubMed] [Google Scholar]
- 6. Cascao R, Moura RA, Perpetuo I et al Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res Ther 2010; 12:R196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol 2014; 10:593–601. [DOI] [PubMed] [Google Scholar]
- 8. Hedges JC, Singer CA, Gerthoffer WT. Mitogen‐activated protein kinases regulate cytokine gene expression in human airway myocytes. Am J Respir Cell Mol Biol 2000; 23:86–94. [DOI] [PubMed] [Google Scholar]
- 9. Wu P, Ma G, Zhu X et al Cyr61/CCN1 is involved in the pathogenesis of psoriasis vulgaris via promoting IL‐8 production by keratinocytes in a JNK/NF‐kappaB pathway. Clin Immunol 2017; 174:53–62. [DOI] [PubMed] [Google Scholar]
- 10. Walczak A, Przybylowska K, Dziki L et al The lL‐8 and IL‐13 gene polymorphisms in inflammatory bowel disease and colorectal cancer. DNA Cell Biol 2012; 31:1431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sung HJ, Choi S, Lee JW, et al Inhibition of human neutrophil activity by an RNA aptamer bound to interleukin‐8. Biomaterials 2014; 35:578–89. [DOI] [PubMed] [Google Scholar]
- 12. Aletaha D, Neogi T, Silman AJ et al 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–81. [DOI] [PubMed] [Google Scholar]
- 13. McAlindon TE, Bannuru RR, Sullivan MC et al OARSI guidelines for the non‐surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014; 22:363–88. [DOI] [PubMed] [Google Scholar]
- 14. Lin J, Li N, Chen H, Liu C, Yang B, Ou Q. Serum Cyr61 is associated with clinical disease activity and inflammation in patients with systemic lupus erythematosus. Medicine 2015; 94:e834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin J, Huo R, Xiao L et al A novel p53/microRNA‐22/Cyr61 axis in synovial cells regulates inflammation in rheumatoid arthritis. Arthritis Rheum 2014; 66:49–59. [DOI] [PubMed] [Google Scholar]
- 16. Kontny E, Szczepanska K, Kowalczewski J et al The mechanism of taurine chloramine inhibition of cytokine (interleukin‐6, interleukin‐8) production by rheumatoid arthritis fibroblast‐like synoviocytes. Arthritis Rheum 2000; 43:2169–77. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell TS, Moots RJ, Wright HL. Janus kinase inhibitors prevent migration of rheumatoid arthritis neutrophils towards interleukin‐8, but do not inhibit priming of the respiratory burst or reactive oxygen species production. Clin Exp Immunol 2017; 189:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007; 12:9–22. [DOI] [PubMed] [Google Scholar]
- 19. Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006; 441:424–30. [DOI] [PubMed] [Google Scholar]
- 20. Lee D‐F, Kuo H‐P, Chen C‐T et al IKKβ suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell 2007; 130:440–55. [DOI] [PubMed] [Google Scholar]
- 21. Weichhart T, Costantino G, Poglitsch M et al The TSC‐mTOR signaling pathway regulates the innate inflammatory response. Immunity 2008; 29:565–77. [DOI] [PubMed] [Google Scholar]
- 22. Carmona‐Rivera C, Carlucci PM, Moore E et al Synovial fibroblast‐neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol 2017; 2. doi: 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaber T, Hahne M, Strehl C et al Disentangling the effects of tocilizumab on neutrophil survival and function. Immunol Res 2016; 64:665–76. [DOI] [PubMed] [Google Scholar]
- 24. Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N. Chemokines regulate IL‐6 and IL‐8 production by fibroblast‐like synoviocytes from patients with rheumatoid arthritis. J Immunol 2001; 167:5381–5. [DOI] [PubMed] [Google Scholar]
- 25. Shi Y, Seto E, Chang LS, Shenk T. Transcriptional repression by YY1, a human GLI‐Krüppel‐related protein, and relief of repression by adenovirus E1A protein. Cell 1991; 67:377–88. [DOI] [PubMed] [Google Scholar]
- 26. Wan M, Huang W, Kute TE et al Yin Yang 1 plays an essential role in breast cancer and negatively regulates p27. Am J Pathol 2012; 180:2120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Nigris F, Zanella L, Cacciatore F et al YY1 overexpression is associated with poor prognosis and metastasis‐free survival in patients suffering osteosarcoma. BMC Cancer 2011; 11:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Crea F, Hurt EM, Farrar WL. Clinical significance of polycomb gene expression in brain tumors. Mol Cancer 2010; 9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Potluri V, Noothi SK, Vallabhapurapu SD et al Transcriptional repression of Bim by a novel YY1‐RelA complex is essential for the survival and growth of multiple myeloma. PLOS ONE 2013; 8:e66121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo J, Zhou X, Ge X et al Upregulation of Ying Yang 1 (YY1) suppresses esophageal squamous cell carcinoma development through heme oxygenase‐1. Cancer Sci 2013; 104:1544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Falzone L, Libra M, Bonavida B. Overexpression of the transcription factor Yin Yang 1 in non‐Hodgkin lymphoma is associated with chemo‐immune resistance. Clin Lymph Myeloma Leuk 2016; 16:S119–20. [Google Scholar]
- 32. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2010; 12:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol 2005; 17:596–603. [DOI] [PubMed] [Google Scholar]
- 34. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mu N, Gu J, Huang T et al A novel NF‐kappaB/YY1/microRNA‐10a regulatory circuit in fibroblast‐like synoviocytes regulates inflammation in rheumatoid arthritis. Sci Rep 2016; 6:20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou X, Han X, Wittfeldt A et al Long non‐coding RNA ANRIL regulates inflammatory responses as a novel component of NF‐kappaB pathway. RNA Biol 2016; 13:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The viability of PBMC cells treated with lentivirus. PBMC from RA patients were treated with LV‐YY1‐shRNA or LV‐NC lentivirus, the viability of PBMC was detected by MTT assay. There is no significant difference between LV‐NC, LV‐YY1‐shRNA and control groups.
Fig. S2. The viability of PBMC cells treated with lentivirus. PBMC from RA patients were treated with LV‐NC, LV‐YY1‐shRNA or LV‐YY1 lentivirus, the viability of PBMC was detected by MTT assay. There is no significant difference between LV‐NC, LV‐YY1‐shRNA, LV‐YY1 and control groups.
Fig. S3. Signaling pathway involved endogenous IL‐8 production. PBMC from RA patients were stimulated with IL‐2 (10IU/ml) and Dynabeads Human T‐activator CD3/CD28. After 24hr, signaling pathway inhibitors 50μM LY294002, 100μM BAY 11‐7082, 50μM SB203580, 10μM SP600125 (inhibitor of JNK) and 50μM rapamycin were added. 48hr later, cell supernatant was collected for the measurement of IL‐8 by ELISA.
Table S1. The sequences of primers used in this study


