Abstract
Background:
Patients with immune-mediated diseases on immunosuppressive therapies have more infectious episodes than healthy individuals, yet vaccination practices by physicians for this patient population remain suboptimal.
Objectives:
To evaluate the safety and efficacy of vaccines in individuals exposed to immunosuppressive therapies and provide evidence-based clinical practice recommendations.
Methods:
A literature search for vaccination safety and efficacy in patients on immunosuppressive therapies (2009-2017) was conducted. Results were assessed using the Grading of Recommendation, Assessment, Development, and Evaluation system.
Results:
Several immunosuppressive therapies attenuate vaccine response. Thus, vaccines should be administered before treatment whenever feasible. Inactivated vaccines can be administered without treatment discontinuation. Similarly, evidence suggests that the live zoster vaccine is safe and effective while on select immunosuppressive therapy, although use of the subunit vaccine is preferred. Caution regarding other live vaccines is warranted. Drug pharmacokinetics, duration of vaccine-induced viremia, and immune response kinetics should be considered to determine appropriate timing of vaccination and treatment (re)initiation. Infants exposed to immunosuppressive therapies through breastmilk can usually be immunized according to local guidelines. Intrauterine exposure to immunosuppressive agents is not a contraindication for inactivated vaccines. Live attenuated vaccines scheduled for infants and children ⩾12 months of age, including measles, mumps, rubella, and varicella, can be safely administered as sufficient time has elapsed for drug clearance.
Conclusions:
Immunosuppressive agents may attenuate vaccine responses, but protective benefit is generally maintained. While these recommendations are evidence based, they do not replace clinical judgment, and decisions regarding vaccination must carefully assess the risks, benefits, and circumstances of individual patients.
Keywords: vaccination, immunosuppression, immune-mediated disease
Immunosuppressive treatments dysregulate immunity and increase the risk of infections and associated morbidity and mortality.1-4 While immunization significantly mitigates these risks, vaccination rates in patients with immune-mediated diseases (IMDs) treated with immunosuppressants remain suboptimal, primarily due to the absence of physician recommendations.5-7 The reticence of physicians to vaccinate these patients may reflect the challenges of weighing the quality of protection achieved while on immunosuppressive treatment with the perceived risks of disease aggravation and vaccine-induced adverse events (AEs). This underscores the importance of improving physician awareness and the need for comprehensive guidelines for the management of this patient population. This publication aims to critically evaluate evidence regarding the safety and efficacy of vaccinating individuals exposed to immunosuppressive therapies and provide evidence-based clinical practice recommendations.
Current knowledge of the safety and efficacy of vaccination in this setting is mainly derived from nonrandomized trials and observational studies using postimmunization antibody titres to vaccine antigens as surrogate markers of protection. Although it may be argued that this measure imperfectly reflects the magnitude and quality of the immune response, it is the best-characterized and most routinely used correlate of protective immunity for commercially available vaccines.8-14 As most vaccines mediate protection through humoral responses that block infection, viremia, or bacteremia,15 postimmunization serological titres and functional antibody characteristics are valuable indications of vaccine efficacy.
Methods
A multidisciplinary committee comprising gastroenterologists (J.K.M., A.B., B.B., A.H.S.), dermatologists (K.A.P., M.G., R.B., V.H.), rheumatologists (B.H., J.E.P., J.W., S.J.), and infectious disease specialists with expertise in vaccinology (D.K., D.C.V.) was assembled to develop guidelines on the practical management of patients considering vaccination while on immunosuppressive therapies. Synapse Medical Communications performed literature searches in accordance to the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) system across multiple databases (Embase, MEDLINE, PubMed).16 Major search terms included vaccination, rheumatoid arthritis (RA), inflammatory bowel disease (IBD), psoriatic arthritis (PsA), psoriasis (PsO), autoimmune disease, and concepts of interest such as specific vaccines, disease-modifying antirheumatic drugs (DMARDs), glucocorticoids, and biologic agents. The literature search identified clinical trials, meta-analyses, systematic reviews, observational studies, case series, and existing guidelines published from 2009 to 2017. Reference lists were manually searched to identify relevant articles and included based on the committee’s discretion. Published studies were reviewed by the committee and assessed for content and GRADE evidence levels.16 The quality of evidence was rated as “high” (indicating that further research is unlikely to change the confidence in the estimate of effect), “moderate” (implying that further research is likely to have an impact on the confidence in the estimate of effect), “low” (suggesting that further research is likely to have a strong impact on the confidence in the estimate of effect), or “very low” (meaning that any estimate of effect is very uncertain).
The Steering Committee (K.A.P. [chair], J.K.M., D.K., B.H.) developed the initial statements, which underwent 2 rounds of revisions according to feedback received from all authors. All 14 members voted on a web-based platform to determine the level of agreement for each statement using a 5-point scale (strongly agree, agree, neutral, disagree, strongly disagree). Statements achieving ⩾75% agreement were included in the guidelines. Of the 15 statements considered, 2 statements were rejected (see Appendix 1, available online).
The strength of a recommendation was evaluated according to GRADE and rated as “strong” when desirable consequences clearly outweighed undesirable consequences, “conditional” when desirable consequences probably outweighed undesirable consequences, or “weak” when the balance between desirable and undesirable consequences was closely balanced or uncertain. Clinical practice recommendations are listed in Table 1.
Table 1.
Recommendation Statements.
|
Statement 1: In patients newly diagnosed with immune-mediated diseases, we recommend that immunization status be assessed and age- and condition-appropriate vaccines be administered prior to initiation of immunosuppressive treatment. Strong recommendation; moderate-level evidence. |
| Inactivated vaccines |
|
Statement 2a: To optimize the immunogenicity of inactivated vaccines in treatment-naive patients with immune-mediated conditions, we suggest that immunization be performed at least 2 weeks prior to initiation of immunosuppressive therapy, whenever possible. Conditional recommendation; moderate-level evidence. |
|
Statement 2b: Among patients with immune-mediated diseases currently receiving immunosuppression, we recommend that immunosuppressive treatment not be interrupted for administration of inactivated vaccine. Strong recommendation; moderate-level evidence. |
|
Statement 2c: In patients with immune-mediated diseases treated with rituximab who require optimal vaccine immunogenicity, we recommend that immunization be deferred to ⩾5 months after the last dose and at least 4 weeks prior to the subsequent dose of rituximab. Strong recommendation; low-level evidence. |
| Live attenuated vaccines: Herpes zoster |
|
Statement 3a: To optimize the immunogenicity of the live attenuated herpes zoster vaccine in treatment-naive patients with immune-mediated conditions, we suggest immunization be performed at least 2 to 4 weeks prior to initiation of immunosuppressive therapy. Conditional recommendation; moderate-level evidence. |
|
Statement 3b: In patients with immune-mediated diseases on immunosuppressive agents, the live attenuated herpes zoster vaccine can be safely administered to patients at risk, but the subunit vaccine is the preferred alternative. Individual situations should be assessed for patients treated with a combination of immunosuppressive drugs, if the live vaccine is being considered. Strong recommendation; moderate-level evidence. |
| Other live attenuated vaccines |
|
Statement 4a: In treatment-naive patients with immune-mediated diseases who are vaccinated with live attenuated vaccines, we recommend that the duration of viremia following immunization be considered when determining the optimal time to initiate immunosuppressive therapy. Strong recommendation; very low-level evidence. |
|
Statement 4b: In patients with immune-mediated diseases who interrupt immunosuppressive treatment prior to vaccination, we recommend that the duration of viremia following immunization be considered when determining the optimal time to reinitiate immunosuppressive therapy. Strong recommendation; very low-level evidence. |
|
Statement 4c: In patients with immune-mediated diseases on immunosuppressive agents, we suggest that live attenuated vaccines be administered when individual benefits outweigh the perceived risks. Conditional recommendation; low-level evidence. |
|
Statement 4d: In situations where patient safety is a paramount concern and the clinical situation allows, we suggest that immunosuppressive treatment be interrupted for a duration based on drug pharmacokinetics prior to immunization with live vaccines. Conditional recommendation; low-level evidence. |
| Vaccination of infants with early exposure to immunosuppressive agents |
|
Statement 5a: In infants exposed to immunosuppressive agents in utero during the third trimester, we recommend that inactivated vaccines be administered according to the local immunization schedule. Strong recommendation; very low-level evidence. |
|
Statement 5b: In infants exposed to immunosuppressive agents in utero during the third trimester, we recommend that the MMR and varicella vaccines be administered according to the local immunization schedule. Strong recommendation; low-level evidence. |
|
Statement 5c: In infants breastfed by mothers on immunosuppressive regimens, we recommend that inactivated and live attenuated vaccines be administered according to the local immunization schedule without delay. Strong recommendation; very low-level evidence. |
Abbreviation: MMR, measles, mumps, rubella.
Good Clinical Practice Statement
Statement 1: In patients newly diagnosed with immune-mediated diseases, we recommend that immunization status be assessed and age- and condition-appropriate vaccines be administered prior to initiation of immunosuppressive treatment.
GRADE: Strong recommendation; moderate-level evidence
Vote: 64.3% strongly agree, 28.6% agree, 7.1% neutral
Evidence Summary
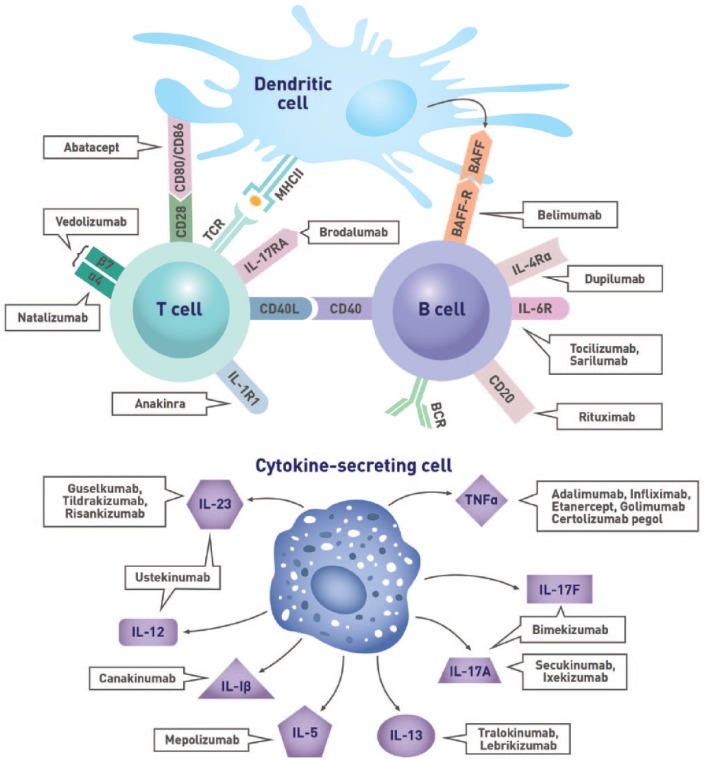
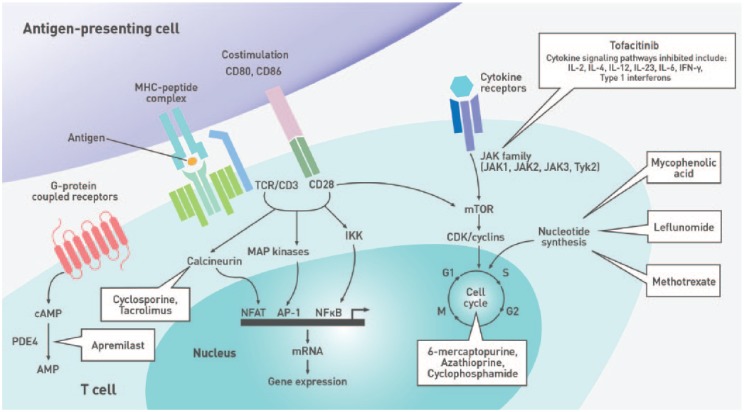
Studies in patients with type 1 diabetes, celiac disease, and IBD showed that individuals with these IMDs who are not treated with immunosuppressive agents generally have comparable serologic responses to vaccination as healthy individuals.17-20 As several DMARDs and biologic agents interfere with the immune response to vaccines (Figure 1, Figure 2, Table 2, and Table 3), it is imperative to assess the patient’s immunization history at diagnosis and administer age- and disease-appropriate vaccines according to local guidelines prior to treatment initiation to optimize efficacy. Concerns regarding the risk of disease exacerbation are unfounded as studies have shown that immunization did not generally cause clinically significant worsening of underlying IMDs (Tables 2 and 3). Indeed, a meta-analysis evaluating the impact of influenza and pneumococcal vaccination in systemic lupus erythematosus (SLE) demonstrated that immunization had no significant effect on the SLE disease activity index (SLEDAI) score.21 While some studies reported increased joint pain following the administration of pneumococcal or influenza vaccines to patients with RA, spondyloarthritis (SpA), or PsA,22,23 these symptoms were transient and self-resolving.
Figure 1.
Immunological targets of biologic agents.
Figure 2.
Immune pathways targeted by nonbiologic disease-modifying antirheumatic drugs.
Table 2.
Safety and Efficacy of Vaccination in Patients on Biologic Agents.
| Vaccine | Biologic Agent | Patient Population | Efficacy | Safety |
|---|---|---|---|---|
| Inactivated and subunit vaccines | ||||
| Cholera (oral) | Vedolizumab | Healthy individuals | No significant difference in seroconversion rates but diminished the magnitude of antibody titre increase131 | Well tolerated131 |
| Hepatitis A | TNFi (pooled) | RA | Diminished humoral response compared to healthy individuals, but 86% of patients achieved seroprotection with 2 vaccine doses132 | Well tolerated and did not result in exacerbation of disease activity132 |
| IBD | Diminished humoral response to the vaccine28 | NA | ||
| Hepatitis B | Infliximab | IBD | Reduced humoral response to the vaccine133 | NA |
| TNFi (pooled) | SpA | Diminished humoral response to the vaccine134 | NA | |
| IBD | Humoral response unaffected by TNFi treatment; however, patients with IBD generally had lower responses than healthy controls regardless of treatment135 | NA | ||
| Vedolizumab | Healthy individuals | No significant difference131 | Well tolerated131 | |
| Influenza | Abatacept | RA | Results are variable but may reduce humoral response to the vaccine136,137 | Well tolerated136,137 |
| Adalimumab | RA | No significant effect138 | Well tolerated138 | |
| Belimumab | SLE | Lower fold-increase in titres for some influenza strains compared with controls139 | NA | |
| Certolizumab pegol | RA | No significant effect45 | Well tolerated45 | |
| Infliximab | RA | No significant effect140 | Well tolerated and did not exacerbate disease activity140 | |
| IBD | Diminished humoral response141,142 | Well tolerated, without incidence of serious adverse events 141,142 | ||
| Rituximab | RA | Cellular responses maintained but diminished humoral response to the vaccine32-36 | Well tolerated32-34 | |
| Secukinumab | Healthy individuals | No significant effect among individuals who received a single secukinumab dose143 | Well tolerated143 | |
| Tocilizumab | RA | No significant effect43,144 | Well tolerated and did not result in exacerbation of disease activity43,144 | |
| TNFi (pooled) | RA, PsO, PsA, SpA, and other immune-mediated diseases | Results are variable but may result in suppression of humoral response to the vaccine44,46,58,145-147
TNFi monoclonal antibodies such as infliximab and adalimumab may have a greater suppressive effect than etanercept58 |
Generally well tolerated44,58
May trigger short-lasting PsA exacerbation following vaccination22 Higher incidence of mild, systemic reactions such as fever, arthralgia, and nasal congestion in TNFi-treated patients58 |
|
| IBD | Diminished humoral response to the vaccine24,26,29 | Well tolerated26 | ||
| Neisseria meningitidis | Secukinumab | Healthy individuals | No significant effect among individuals who received a single secukinumab dose143 | Well tolerated143 |
| Pneumococcal (polysaccharide or conjugate) | Abatacept | RA | Results are variable but may reduce humoral response to the polysaccharide and conjugate vaccines137,148,149 | Well tolerated137,148,149 |
| Adalimumab | RA | No significant effect on the immunogenicity of the polysaccharide vaccine138 | Well tolerated138 | |
| Certolizumab pegol | RA | No significant effect on the pneumococcal polysaccharide vaccine45 | Well tolerated45 | |
| Etanercept | RA | May reduce humoral response to the conjugate vaccine150 | Well tolerated150 | |
| Infliximab | RA | No significant effect on immunogenicity of the polysaccharide vaccine151 | NA | |
| IBD | Reduced humoral response to the polysaccharide vaccine27 | Well tolerated27 | ||
| Golimumab | RA | Reduced fold-increase in vaccine-specific IgG titres in response to the polysaccharide vaccine but maintained opsonophagocytic function152 | Well tolerated152 | |
| Rituximab | RA | Diminished humoral response to the polysaccharide and conjugate vaccines37,148,153 | Well tolerated148,153 | |
| Tocilizumab | RA | No significant effect on the immunogenicity of the polysaccharide or conjugate vaccines51,144,148,154 | Well tolerated51,144,148,154 | |
| TNFi (pooled) | RA, SpA | No significant effect on the immunogenicity of the polysaccharide and conjugate vaccines23,37,47 | Well tolerated, but some patients treated with methotrexate or TNFi reported a transient worsening of joint pain 1 week after vaccination23 | |
| IBD | Diminished humoral response to the polysaccharide and conjugate vaccines18,25,27,30 | Well tolerated25,27 | ||
| Ustekinumab | PsO | No significant effect on the immunogenicity of the polysaccharide vaccine155 | Higher incidence of mild injection site reactions in ustekinumab-treated patients but otherwise well tolerated155 | |
| Tetanus | Abatacept | Type 1 diabetes | Achieved protective titres but diminished the magnitude of recall humoral response compared to controls156 | NA |
| Rituximab | RA | Recall response not significantly affected153 | Well tolerated153 | |
| Tocilizumab | RA | No significant effect on recall humoral response to tetanus toxoid154 | Well tolerated154 | |
| Ustekinumab | PsO | No significant difference155 | Higher incidence of mild injection site reactions but otherwise well tolerated155 | |
| TNFi (pooled) | IBD | TNFi monotherapy had no significant effect on the immunogenicity of booster vaccination157 | Well tolerated, without incidence of disease flares157 | |
| Pertussis | TNFi (pooled) | IBD | TNFi monotherapy had no significant effect on the immunogenicity of booster vaccination157 | Well tolerated, without incidence of disease flares157 |
| Live attenuated vaccines | ||||
| Herpes zoster | TNFi (pooled) | RA, PsA, PsO, AS, IBD | Vaccination effectively protected patients from disease74 | Vaccination was not associated with short-term increase in herpes zoster risk74 |
| Measles, mumps, rubella | Etanercept | JIA | No significant effect on humoral response to vaccination; insignificant trend toward lower cellular response80 | Well tolerated and did not cause disease exacerbation80 |
| Vedolizumab | IBD | Single case report of a patient achieving a positive measles antibody index following revaccination158 | No adverse effect observed158 | |
| Yellow fever | Infliximab | RA | Similar response rates following revaccination in infliximab-treated patients with RA and controls; trend toward lower titres in patients, but analysis limited by small study numbers81 | No adverse effect observed81 |
Abbreviations: AS, ankylosing spondylitis; IBD, inflammatory bowel disease; IgG, immunoglobulin G; JIA, juvenile idiopathic arthritis; NA, not available; PsA, psoriatic arthritis; PsO, psoriasis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SpA, spondyloarthritis; TNFi, tumour necrosis factor α inhibitor.
Table 3.
Safety and Efficacy of Vaccination in Patients on Nonbiologic DMARDs and Corticosteroids.
| Vaccine | Drug | Patient Population | Efficacy | Safety |
|---|---|---|---|---|
| Inactivated and subunit vaccines | ||||
| Hepatitis A | Methotrexate | RA | Reduced humoral response to the vaccine among patients treated with a mean dose of 15 mg/wk132 | NA |
| Hepatitis B | Corticosteroids | IBD | Reduced humoral response among patients who received ⩾2 vaccine doses while on corticosteroid therapy159 | NA |
| Thiopurines | IBD | Reduced humoral response to the vaccine133 | NA | |
| Haemophilus influenzae type b | Thiopurines | IBD | No significant effect160 | Vaccination did not exacerbate disease activity160 |
| Human papillomavirus | Antimalarials | SLE | No significant effect161 | Well tolerated and did not result in exacerbation of disease activity161 |
| Calcineurin inhibitors | SLE | No significant effect, but study limited by small sample size161 | Well tolerated and did not result in exacerbation of disease activity161 | |
| Corticosteroids | SLE | No significant effect among patients receiving a mean prednisolone dose of 4.8 mg/d161 | Well tolerated and did not result in exacerbation of disease activity161 | |
| Mycophenolate | SLE | Mycophenolate mofetil dose inversely correlated with vaccine-specific antibody titres for some serotypes following vaccination161 | Well tolerated161 | |
| Thiopurines | SLE | No significant effect161 | Well tolerated and did not result in exacerbation of disease activity161 | |
| Influenza | Anti-malarials | RA, SpA, SLE | No significant effect38,46,162
May restore influenza vaccine immunogenicity in patients with SLE receiving prednisone or other immune-suppressive therapy38 |
Well tolerated and did not result in exacerbation of disease activity46,162 |
| Calcineurin inhibitors | Solid organ transplant | Variable effect on vaccine immunogenicity but may reduce humoral response163-167 | Well tolerated without impact on allograft function163,166,167 | |
| Corticosteroids | RA, SpA, IBD, Sjögren syndrome | No significant effect, 44,45,142,168,169 particularly at mean prednisone-equivalent doses ⩽10 mg/d44,45,169 | Well tolerated38,44,142,168,169
Increased incidence of disease flares and rise in autoantibody titres among patients with SLE with low response to the vaccine39 |
|
| SLE | Reduced seroconversion rates observed,38-40 particularly at prednisone-equivalent doses ⩾10 mg/d38,39 | |||
| Leflunomide | RA, PsA, AS, SLE | Reduced humoral response to vaccine compared to healthy controls46,168 | Well tolerated and did not exacerbate disease activity46,168 | |
| Methotrexate | RA, SpA | Reduced humoral response to the vaccine (mean dose in studies: 16-20 mg/wk)44-46 | Well tolerated43,44,46 | |
| Mycophenolate | Solid organ transplant | Reduced humoral response to the vaccine166,167,170-174 | Well tolerated without incidence of allograft rejection166,167,170-172,174 | |
| Sulfasalazine | RA, SpA, other inflammatory diseases | No significant effect162 | Well tolerated162 | |
| Thiopurines | Sjögren syndrome | No significant effect169 | Well tolerated and did not result in exacerbation of disease activity40,169,175,176 | |
| SLE | Reduced seroconversion and seroprotection rates40,175 | |||
| Wegener granulomatosis | No significant effect176 | |||
| IBD | Reduced humoral response to H1N1142 | NA | ||
| Tofacitinib | RA | Diminished humoral responses in patients treated with methotrexate + tofacitinib combination therapy but not in those treated with tofacitinib alone or methotrexate alone50 | NA | |
| Pneumococcal (polysaccharide or conjugate) | Calcineurin inhibitors | Solid organ transplant | May diminish humoral response to some pneumococcal serotypes in the polysaccharide vaccine163 | Well tolerated without incidence of allograft rejection163 |
| RA | No significant effect on the immunogenicity of the polysaccharide vaccine49 | NA | ||
| Corticosteroids | RA, SpA, and various inflammatory conditions | No significant effect on the immunogenicity of conjugate and polysaccharide vaccines,23,45,47,138,154 particularly at mean prednisone-equivalent doses <20 mg/d23,45,47,138
Prednisone-equivalent doses ⩾20 mg/d tended to result in poor serologic response to polysaccharide vaccine177 |
Well tolerated without incidence of disease exacerbation23,138,177 | |
| Methotrexate | RA | Reduced humoral response to the conjugate and polysaccharide vaccines23,45,47-52 | Well tolerated,23,49,51 but some patients treated with methotrexate reported a transient worsening of joint pain 1 week after vaccination23 | |
| Mycophenolate | Solid organ transplant | Reduced recall humoral response to the vaccine178 | NA | |
| Thiopurines | IBD | No significant effect27 | Well tolerated27 | |
| Tofacitinib | RA | Diminished humoral response among patients on combination therapy with tofacitinib + methotrexate50 | NA | |
| Tetanus | Calcineurin inhibitors | Chronic uveitis | No significant effect, but study limited by small sample size179 | NA |
| Mycophenolate | Solid organ transplant | Reduced recall humoral response to the vaccine178 | NA | |
| Sulfasalazine | Healthy individuals | Diminished fold-increase in antibody titres following booster vaccination180 | NA | |
| Live attenuated vaccines | ||||
| Cholera (oral) | Antimalarials | Healthy individuals | Coadministration of chloroquine and live cholera vaccine reduced seroconversion rates181 | Well tolerated181 |
| Herpes zoster | Corticosteroids | RA, PsO, PsA, AS, IBD, various inflammatory conditions | Immunogenic and effectively protected vaccinated patients from disease for up to 2 years74,182 | Vaccine was well tolerated with no incidence of vaccine-related varicella75,182
However, 1 study reported a 3-fold increased HZ risk in patients on immune-suppressive therapy75 |
| Methotrexate | RA | No significant effect among patients receiving 15-25 mg/wk72 | Generally well tolerated, but 1 patient without existing immunity developed cutaneous dissemination of the vaccine strain 16 days after vaccination (2 days after initiating tofacitinib treatment)72 | |
| Thiopurines | IBD | Low-dose thiopurine treatment blunted the cellular and humoral response to vaccine183 | Well tolerated and did not result in disease exacerbation183 | |
| Tofacitinib | RA | No significant effect among patients on background methotrexate vaccinated 2-3 weeks prior to starting tofacitinib treatment72 | Generally well tolerated, but 1 patient without existing immunity developed cutaneous dissemination of the vaccine strain 16 days after vaccination (2 days after initiating tofacitinib treatment)72 | |
| Measles, mumps, rubella | Methotrexate | JIA | Nonsignificant trend toward reduced humoral and cellular recall responses among patients on a mean dose of ⩽12 mg/m2 body surface area80 | No disease exacerbation, need for increased treatment doses, or severe adverse events resulting from vaccination76,80 |
| Typhoid + cholera (oral) | Antimalarials | Healthy Individuals | No significant effect181 | Well tolerated181 |
| Yellow fever | Calcineurin inhibitors | Solid organ transplant | NA | Well tolerated, but study limited by small sample size79 |
| Corticosteroids | RA and other inflammatory conditions | No significant effect on seroprotection rates among patients receiving a median prednisone-equivalent dose of 7 mg/d78 | Vaccination did not result in serious adverse events; however, the frequency of moderate to severe local reactions was 8-fold higher among corticosteroid-treated patients compared with healthy adults78 | |
| Methotrexate | RA, PsO, scleroderma, PsA | Methotrexate doses ranging from 10-30 mg/wk did not significantly affect humoral response as measured by plaque reduction neutralization test82 | Well tolerated82 | |
Abbreviations: AS, ankylosing spondylitis; HZ, herpes zoster; IBD, inflammatory bowel disease; JIA, juvenile idiopathic arthritis; NA, not available; PsA, psoriatic arthritis; PsO, psoriasis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SpA, spondyloarthritis.
Biologics
Biologics may impede optimal immune responses to certain vaccines (Table 2). For example, patients with IBD receiving tumour necrosis factor α inhibitors (TNFi) tended to have attenuated humoral responses to influenza, pneumococcal, and hepatitis A virus (HAV) vaccines.18,24-30 A meta-analysis of 9 studies (n = 728) revealed that patients with IBD on TNFi monotherapy had a significantly lower probability of achieving adequate immune responses to routine vaccinations, including those against hepatitis B virus (HBV), HAV, influenza, and pneumococcus, compared with untreated patients (odds ratio [OR], 0.32; 95% confidence interval [CI], 0.21-0.49; P < .001).31
Rituximab (RTX)–based B-cell depletion therapy also lowered antibody titres and seroprotection rates in response to influenza vaccines compared with DMARDs and/or prednisone.32-35 In addition, RTX treatment was often associated with failure to attain protective antibody titres against all influenza strains contained within the vaccine.32,34,36 Similarly, a meta-analysis demonstrated the negative impact of RTX on pneumococcal vaccine response rates, with RTX-treated patients with RA (n = 88) having significantly poorer responses to both the 6B (OR, 0.25; 95% CI, 0.11-0.58; P = .001) and 23F (OR, 0.21; 95% CI, 0.04-1.05; P = .06) serotypes compared with controls.37
Nonbiologic Agents
Corticosteroids and many DMARDs negatively affect vaccine immunogenicity (Table 3). For example, treatment with prednisone-equivalent doses ⩾10 mg/d diminished humoral responses to influenza vaccines in patients with SLE.38,39 A meta-analysis of 15 studies demonstrated that, compared with healthy individuals, corticosteroid treatment lowered the probability of seroconversion in patients with SLE, with relative risk ratios (RRs) of 0.66 (95% CI, 0.53-0.82), 0.49 (95% CI, 0.26-0.91), and 0.51 (95% CI, 0.24-1.09) for influenza H1N1, H3N2, and B, respectively.40 Similarly, methotrexate (MTX) suppressed humoral responses to both influenza41-46 and pneumococcal vaccines.23,45,47-52 In 1 study, patients with RA without preexisting influenza or Streptococcus pneumoniae immunity receiving either placebo (n = 36) or placebo + MTX (n = 78; mean MTX dose of 17.2 mg/wk) were immunized with the influenza and 23-valent pneumococcal polysaccharide (PPSV23) vaccines.45 Four weeks after vaccination, the placebo group achieved an influenza vaccine response rate of 84.6% (95% CI, 70.7%-98.5%) vs 50.9% (95% CI, 37.9%-63.9%) for the placebo + MTX group. Likewise, 89.3% (95% CI, 77.8%-100.0%) of placebo-treated patients achieved ⩾2-fold antibody titre increases to ⩾3 of 6 pneumococcal antigens tested compared with only 50.0% (95% CI, 37.3%-62.7%) of MTX-treated patients. The suppressive effect of MTX was further exemplified in studies by Park et al,41,42 which showed that patients with RA on MTX had a poorer vaccine response to the seasonal influenza vaccine than those in whom treatment was withheld for 2 weeks before and after vaccination and those who discontinued treatment for 2 to 4 weeks after vaccination.
Inactivated Vaccines
Statement 2a: To optimize the immunogenicity of inactivated vaccines in treatment-naive patients with immune-mediated conditions, we suggest that immunization be performed at least 2 weeks prior to initiation of immunosuppressive therapy, whenever possible.
GRADE: Conditional recommendation; moderate-level evidence
Vote: 71.4% strongly agree, 28.6% agree
Evidence Summary
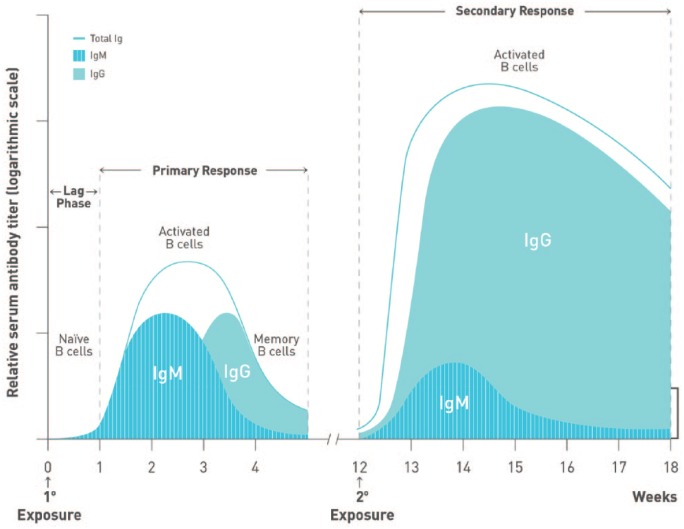
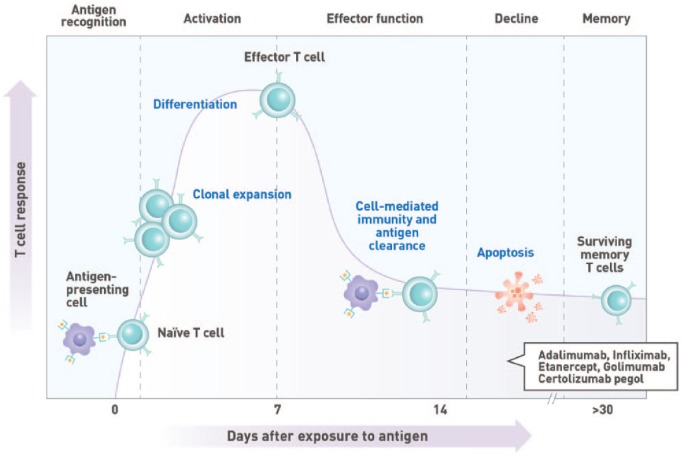
To optimize efficacy, the length of time required to develop robust immune responses to administered vaccines should be considered (Figures 3 and 4). Booster immunizations reactivate immune memory and induce high vaccine-specific IgG titres as quickly as 7 days after vaccination.53-55 In contrast, humoral responses to primary vaccination are characterized by the initial production of antigen-specific, low-affinity immunoglobulin M (IgM) antibodies after a lag phase lasting up to a week postimmunization.55,56 Higher affinity and avidity immunoglobulin G (IgG) antibodies eventually become detectable in the blood within 10 to 14 days57 and may take up to 4 to 6 weeks to achieve maximum levels.53,56 It is therefore ideal to defer immunosuppressive treatment for ⩾2 weeks after vaccination to allow sufficient time to develop a robust and protective humoral response.
Figure 3.
Kinetics of antibody response following immunization. The kinetics of B-cell activation and antibody (immunoglobulin M [IgM], immunoglobulin G [IgG]) production during primary and secondary responses to antigen are depicted. The primary response is characterized by a short lag phase lasting approximately 1 week, followed by the production of low-affinity IgM. IgG becomes detectable within 10 to 14 days after antigen exposure. Conversely, secondary responses reactivate memory B cells, resulting in quicker responses and higher IgG titres than those observed during a primary response.
Figure 4.
Kinetics of T-cell response. T-cell activation is initiated by the recognition of antigenic peptides displayed on the surface of antigen-presenting cells in the draining lymph nodes. This results in the clonal expansion of the activated T cell and the acquisition of effector cell function within approximately 1 week of antigen exposure. Cellular immunity plays a vital role in the clearance of intracellular pathogens and the development of robust T-cell–dependent humoral immune responses.
One study demonstrated the benefit of deferring treatment following vaccination. In this study, humoral response to the seasonal influenza vaccine was compared between MTX-treated patients with RA who stayed on treatment and those who withheld treatment for 2 weeks postvaccination.41 Patients who discontinued treatment for 2 weeks postvaccination had a significantly higher rate of satisfactory response to all 4 influenza antigens contained within the vaccine compared with those who stayed on MTX (46% vs 22%; P < .001). Similarly, another study compared the humoral response of patients with RA who continued MTX or withheld treatment for 2 weeks before and after vaccination or 4 weeks after vaccination.42 Compared to patients who remained on treatment, those who discontinued MTX for 2 weeks before and after vaccination (31.5% vs 51%; P = .044) or 4 weeks postvaccination (31.5% vs 46.2%; P = .121) tended to have greater response rates to all 3 influenza vaccine antigens.
Statement 2b: Among patients with immune-mediated diseases currently receiving immunosuppression, we recommend that immunosuppressive treatment not be interrupted for administration of inactivated vaccines.
GRADE: Strong recommendation; moderate-level evidence
Vote: 28.6% strongly agree, 64.3% agree, 7.1% neutral
Evidence Summary
Treatment with immunosuppressive agents should not affect the decision to administer inactivated vaccines to patients with IMDs. While vaccine antigenicity may be attenuated compared with healthy individuals, humoral response is not abolished and significant increases in antigen-specific antibody titres, often reaching protective levels, are generally achieved. In a study by Adler et al,44 DMARD-treated patients (n = 28) with RA, SpA, vasculitis, or connective tissue disease (CTD) achieved lower fold-increases in vaccine-specific antibody titres than healthy controls (n = 40) after receiving the influenza A/H1N1 vaccine (7.7 vs 13.3). Despite this, 79% of patients still achieved seroprotective titres ⩾1:40. While the seroprotection rate in DMARD-treated patients is reduced compared with healthy individuals (98%), the majority still benefitted from vaccination despite the negative effect of immunosuppressive treatment on vaccine immunogenicity.
Several studies established the safety and tolerability of inactivated vaccines in patients with IMDs on immunosuppressive therapies (Tables 2 and 3). No serious vaccine-related AEs have been reported, although differences in the incidence of mild AEs have been observed. One such study noted higher rates of mild systemic reactions such as fever (8.3% vs 0.9%; P = .01), arthralgia (12.5% vs 4.3%; P = .03), and nasal congestion (13.3% vs 4.3%; P = .014) among patients treated with TNFi compared with healthy controls after receiving the A/H1N1 vaccine.58
In patients receiving intermittent treatment in whom optimal vaccine immunogenicity is desired and the clinical situation allows, vaccination can be given at the nadir of immunosuppression. For immunosuppressive agents given as a bolus at intervals exceeding 4 weeks, such as infliximab (IFX) or cyclophosphamide, it is suggested that the vaccine be administered midcycle or 2 weeks prior to the next dose. Alternatively, immunosuppressive therapy may be temporarily discontinued prior to vaccination. The length of treatment discontinuation should take drug pharmacokinetics (Tables 4 and 5) and dosage into consideration.
Table 4.
Pharmacokinetic Half-Lives of Biologic Agents.
| Family | Biologic | Isotype | Target | Half-Life | Status |
|---|---|---|---|---|---|
| TNF inhibitors | Adalimumab | human IgG1 | TNFα | 10-20 days184 | Approved |
| Etanercept | IgG1 Fc domain + TNF receptor extracellular ligand-binding domain | TNFα, LTα (TNFβ) | 4.2 days185 | Approved | |
| Certolizumab pegol | Humanized Fab′ conjugated to polyethylene glycol | TNFα | 14 days186 | Approved | |
| Golimumab | Human IgG1қ | TNFα | 11-12 days187 | Approved | |
| Infliximab | Chimeric IgG1κ | TNFα | 7.7-14.7 days188 | Approved | |
| Interleukin inhibitors | Dupilumab | Human IgG4 | IL-4Rα | NA189,a | Approved |
| Mepolizumab | Humanized IgG1κ | IL-5 | 16-22 days190 | Approved | |
| Tocilizumab | Humanized IgG1κ | IL-6R | 11-13 days191 | Approved | |
| Sarilumab | Human IgG1 | sIL-6Rα, mIL-6Rα | Initial: 8-10 days Terminal: 2-4 days192 |
Approved | |
| Anakinra | IL-1 receptor antagonist | IL-1R1 | 4-6 hours193 | Approved | |
| Canakinumab | Human IgG1қ | IL-1β | 26 days194 | Approved | |
| Tralokinumab | Human IgG4 | IL-13 | 17.7 days195 | In development | |
| Lebrikizumab | Humanized IgG4 | IL-13 | 25 days196 | In development | |
| Secukinumab | Human IgG1қ | IL-17A | 27 days197 | Approved | |
| Ixekizumab | Humanized IgG4 | IL-17A | 13 days198 | Approved | |
| Bimekizumab | Humanized IgG1 | IL-17A, IL-17F | 17-22 days199 | In development | |
| Brodalumab | Human IgG2қ | IL-17RA | NA200,b | Approved | |
| Ustekinumab | Human IgG1 | IL-12, IL-23 | 15-32 days201 | Approved | |
| Guselkumab | Human IgG1λ | IL-23 | 15-18 days202 | Approved | |
| Risankizumab | Human IgG1 | IL-23 | 20-28 days203 | In development | |
| Tildrakizumab | Humanized IgG1қ | IL-23 | 24.5 days204 | In development | |
| Nemolizumab | Humanized IgG2 | IL-31RA | 12.6-16.5 days205 | In development | |
| B-cell inhibitor | Rituximab | Chimeric IgG1κ | CD20 | 20.8 days59 | Approved |
| Belimumab | Human IgG1λ | BAFF (BLyS) | 12.5-19.4 days206 | Approved | |
| Integrin blockers | Vedolizumab | Humanized IgG1 | α4β7 | 25 days207 | Approved |
| Natalizumab | Humanized IgG4қ | α4 | 9.6-11.1 days208 | Approved | |
| Costimulatory modulator | Abatacept | CTLA-4 extracellular domain + modified IgG1 Fc domain | CD80, CD86 | 13.1-16.7 days209 | Approved |
Abbreviations: BAFF, B-cell–activating factor; BLyS, B-lymphocyte stimulator; CD, cluster of differentiation; CTLA-4, cytotoxic T-lymphocyte–associated protein 4; Fab′, fragment antibody binding; Fc, fragment constant; IgG, immunoglobulin G; IL, interleukin; IL-1R1, interleukin 1 receptor 1; IL-17RA, interleukin 17 receptor A; LT, lymphotoxin; mIL-6Rα, membrane-bound IL-6 receptor α; NA, not available; sIL-6Rα, soluble IL-6 receptor α; TNF, tumour necrosis factor.
As dupilumab has a distinct target-mediated phase and the instantaneous half-life decreases over time to values close to zero, its terminal half-life cannot be calculated or used for any practical purposes.
Expected be similar to endogenous IgG.
Table 5.
Pharmacokinetic Half-Lives of Nonbiologic DMARDs.
| Family | Drug | Half-Life |
|---|---|---|
| JAK family kinase inhibitor | Tofacitinib | 3 hours210 |
| Folate antagonist | Methotrexate | <30 mg/m2: 3-10 hours211
⩾30 mg/m2: 8-15 hours211 |
| Calcineurin inhibitor | Tacrolimus | 35 hours212 |
| Cyclosporine | 18 hours213 | |
| Alkylating agent | Cyclophosphamide | 7 hours214 |
| Antimalarials | Chloroquine | 40 days215 |
| Hydroxychloroquine | 50 days216 | |
| Purine analog | 6-Mercaptopurine | 1.5 hours217,a |
| Azathioprine | 5 hours218,b | |
| Sulfa drug | Sulfasalazine | 6-8 hours219 |
| Inosine monophosphate dehydrogenase inhibitor | Mycophenolate mofetil | 17.9 hours for MPA220 |
| Mycophenolate sodium | 11.7 hours for MPA 15.7 hours for MPAG metabolite221 |
|
| Glucocorticoid | Prednisolone | 2-4 hours222 |
| Phosphodiesterase-4 inhibitor | Apremilast | 8.9-9.7 hours223 |
| Isoxazole | Leflunomide | 14 days224 |
Abbreviations: JAK, Janus kinase; MPA, mycophenolic acid; MPAG, mycophenolic acid glucuronide.
Active metabolites have longer half-lives.
For azathioprine’s sulfur-containing metabolites.
Studies in RA have demonstrated that discontinuing MTX treatment until 2 weeks after immunization with the seasonal influenza vaccine increases vaccine response without increasing the risk of flares compared to those who stayed on treatment.41 However, flares tended to be more common among patients who received a 4-week MTX treatment break around the time of vaccination.42 Therefore, while a washout period before vaccine administration may improve the patient’s immune response to the vaccine, clinicians should use their judgement to evaluate the risks vs benefits of treatment disruption.
Statement 2c: In patients with immune-mediated diseases treated with rituximab who require optimal vaccine immunogenicity, we recommend that immunization be deferred to ⩾5 months after the last dose and at least 4 weeks prior to the subsequent dose of rituximab.
GRADE: Strong recommendation; low-level evidence
Vote: 14.3% strongly agree, 78.6% agree, 7.1% disagree
Evidence Summary
RTX pharmacokinetics does not directly correlate with B-cell reconstitution following treatment. Despite having a half-life of approximately 21 days,59 1 study showed that B-cell reconstitution occurred after a mean of 8 months from the last RTX dose (n = 24; range of 5-13 months).60 In line with this finding, patients immunized with the trivalent influenza vaccine >5 months after RTX infusion (n = 13) exhibited greater fold-increases in antibody titres for influenza H1N1 (2.1 vs 1.1; P = .02), H3N2 (1.7 vs 1.3; P = .23), and B (3.6 vs 1.6; P = .01) than patients vaccinated ⩽5 months (n = 16) after their last RTX dose.34 Similarly, another study reported that patients with RA vaccinated 6 to 10 months after their last RTX infusion (n = 12) exhibited modestly restored IgG responses and significant increases in antibody titres for the H3N2 and H1N1 vaccine strains compared with those vaccinated 4 to 8 weeks after RTX treatment (n = 11).33,35
For most IMDs, RTX is typically administered at most every 6 months, unless clinical evaluation indicates a need for alternative dosing (in RA, RTX is administered every 6 to >12 months, depending on response duration).61 Therefore, to allow for a degree of B-cell reconstitution, as well as provide sufficient time to mount an adaptive immune response to the vaccine, it is recommended that inactivated vaccines be given at least 5 months following the last RTX dose and approximately 1 month prior to subsequent B-cell depleting therapy. When feasible, titres assessing seroconversion should be considered.
Live Attenuated Herpes Zoster Vaccine
The live attenuated herpes zoster (HZ) vaccine is indicated for adults ⩾50 years of age to prevent HZ and postherpetic neuralgia. It contains the Oka varicella-zoster virus strain (VZV), which is present at higher viral titres (19 400 plaque-forming units [pfu]/dose) than that used for the primary prevention of chickenpox (1350 pfu/dose).62,63 Among those with previous exposure and immunologic memory to VZV, the vaccine mitigates disease risk by boosting the anti-VZV adaptive immune responses.
Typically, live vaccines are more immunogenic than their inactivated counterparts due to their ability to mimic natural infection and effectively activate the humoral and cellular arms of the immune system.53 However, the HZ subunit vaccine (HZ/su) recently approved in Canada and the United States may actually provide a more effective alternative to the live attenuated vaccine. In 2 phase 3 clinical trials (ZOE-50, ZOE-70), the HZ/su vaccine efficacy over a mean follow-up of 3.7 years was 97.2% (95% CI, 93.7-99.0; P < .001) in individuals aged ⩾50 years and was 91.3% (95% CI, 86.8-94.5; P < .001) in those aged ⩾70 years.64,65 These rates were greater than the 51.3% reduction in HZ incidence observed in individuals ⩾60 years immunized with the live attenuated vaccine in the Shingles Prevention Study.66 Furthermore, the subunit vaccine had an efficacy of 88.8% (95% CI, 68.7-97.1; P < .001) against postherpetic neuralgia in individuals ⩾70 years old. In the Shingles Prevention Study, efficacy against postherpetic neuralgia was 66.8% in the same age group vaccinated with the live attenuated vaccine.64,66 To date, however, there are no studies directly comparing the 2 vaccines. There are also no published efficacy data on the use of the subunit vaccine in cohorts with IMDs.
As the adjuvant, AS01B, is a component of the HZ/su vaccine, there has been some anecdotal concern regarding the risk of autoimmune/inflammatory syndrome induced by adjuvants (ASIA). However, pooled data from the clinical trials have shown no significant differences in the incidence of IMDs up to a year postvaccination between individuals receiving HZ/su (90/14 645; 0.6%) and those receiving placebo (105/14 660; 0.7%).67 In addition, a subgroup analysis of patients with a history of IMD showed comparable incidences of possible postvaccination disease exacerbation in the HZ/su (27/983; 2.8%) and placebo (27/960; 2.8%) groups, suggesting that the vaccine is likely safe in this patient population.67
Due to its safety and efficacy, the Centers for Disease Control and Prevention (CDC) now recommends the use of the HZ/su vaccine over the live attenuated version.68 However, should the live vaccine be considered, the following statements aim to address concerns regarding its use in patients with IMDs on immunosuppressive therapies.
Statement 3a: To optimize the immunogenicity of the live attenuated herpes zoster vaccine in treatment-naive patients with immune-mediated conditions, we suggest immunization be performed at least 2 to 4 weeks prior to initiation of immunosuppressive therapy.
GRADE: Conditional recommendation; moderate-level evidence
Vote: 21.4% strongly agree, 78.6% agree
Evidence Summary
The live attenuated HZ vaccine should ideally be administered prior to initiation of immunosuppressive therapy. Some guidelines recommend HZ vaccination 3 to 4 weeks prior to starting therapy,69,70 likely due to evidence demonstrating the presence of the vaccine virus strain up to 4 weeks after vaccination.71 Further prospectively designed studies are needed; however, evidence from 1 study suggests that HZ vaccination is safe and effective when administered as early as 2 weeks before treatment initiation.72 HZ vaccine immunogenicity was assessed in patients with RA on MTX (15-25 mg/wk) who received tofacitinib (n = 55) or placebo (n = 57) 2 to 3 weeks postvaccination.72 Six weeks after immunization, the fold-increase in VZV-specific IgG titres (2.11 vs 1.74) and number of VZV-specific T cells (1.50 vs 1.29) were comparable between the tofacitinib and placebo groups, respectively, suggesting that tofacitinib did not interfere with humoral or cellular immune responses to the vaccine. Immunization was generally safe, with only a single case of cutaneous dissemination of the vaccine strain observed in a patient without preexisting VZV immunity.72
Statement 3b: In patients with immune-mediated diseases on immunosuppressive agents, the live attenuated herpes zoster vaccine can be safely administered to patients at risk, but the subunit vaccine is the preferred alternative. Individual situations should be assessed for patients treated with a combination of immunosuppressive drugs, if the live vaccine is being considered.
GRADE: Strong recommendation; moderate-level evidence
Vote: 64.3% strongly agree, 21.4% agree, 14.3% neutral
Evidence Summary
According to the CDC, treatment with low-dose MTX (⩽0.4 mg/kg/wk), azathioprine (⩽3.0 mg/kg/d), 6-mercaptopurine (⩽1.5 mg/kg/d), short-term steroid therapy lasting <2 weeks, or prednisone-equivalent doses <20 mg/d is not sufficiently immunosuppressive to preclude the use of live vaccines.73 Conversely, treatment discontinuation lasting 1 or 3 months before live vaccine administration is recommended for patients on high-dose systemic steroid therapy lasting >2 weeks or immunomodulatory biologics, respectively.73 Data, like those expected from the VERVE trial (NCT02538757) assessing the outcomes of HZ vaccination in TNFi users, are required to accurately assess the risks in specific patient populations. In the interim, retrospective analyses provided insights into the risks and benefits of HZ vaccination in patients with IMDs receiving immunosuppressive therapies.
A retrospective cohort study demonstrated the safety and efficacy of the HZ vaccine in patients ⩾60 years with RA, PsA, ankylosing spondylitis (AS), PsO, or IBD treated with biologics (TNFi, abatacept, or RTX), DMARDs, and/or oral glucocorticoids (n = 463 541).74 During the 42-day window in which infection risk with the vaccine strain is greatest, none of the patients exposed to biologics (n = 633) developed varicella or HZ. Furthermore, vaccination lowered the HZ incidence over a 2-year median follow-up in biologic-treated, vaccinated patients (incidence ratio [IR], 8.5; 95% CI, 5.1-14.4) vs unvaccinated patients (IR, 16.0; 95% CI, 15.2-16.8). Among the 551 patients on TNFi, the IR of HZ in vaccinated patients was 8.5 (95% CI, 4.8-15.0) vs 15.9 (95% CI, 15.1-16.8) for those unvaccinated. Similarly, among immunized DMARD- and glucocorticoid-treated patients, the IRs were 7.0 (95% CI, 4.7-10.3) and 10.3 (95% CI, 6.7-15.8), respectively, vs 13.6 (95% CI, 13.1-14.2) and 17.2 (95% CI, 16.5-17.9) for their respective unvaccinated counterparts.74
Notably, 1 study reported that patients with IMDs on immunosuppressive therapy at the time of HZ vaccination (n = 4826) were at greater risk of HZ 42 days postvaccination (OR, 2.99; 95% CI, 1.58-5.70) than those who discontinued immunosuppressive treatment >30 days before vaccination (n = 9728).75 However, it is important to note that no cases of disseminated VZV from the vaccine strain were observed, and latent zoster virus reactivation was speculated to be the probable cause.
Taken together, studies on the use of the live attenuated HZ vaccine on patients with IMDs on immunosuppressive therapies suggest that it is generally safe and well tolerated. It is important to note that most patients receiving the HZ vaccine would have had previous VZV exposure and immunity, which decreases the risk of disseminated disease from the vaccine strain even in individuals on immunosuppressive treatment. However, caution may be warranted in patients without preexisting immunity to the virus. Serologic testing for VZV prior to immunization with the live attenuated vaccine should be considered.
Other Live Vaccines
Statement 4a: In treatment-naive patients with immune-mediated diseases who are vaccinated with live attenuated vaccines, we recommend that the duration of viremia following immunization be considered when determining the optimal time to initiate immunosuppressive therapy.
GRADE: Strong recommendation; very low-level evidence
Vote: 21.4% strongly agree, 71.4% agree, 7.1% neutral
Statement 4b: In patients with immune-mediated diseases who interrupt immunosuppressive treatment prior to vaccination, we recommend that the duration of viremia following immunization be considered when determining the optimal time to reinitiate immunosuppressive therapy.
GRADE: Strong recommendation; very low-level evidence
Vote: 21.4% strongly agree, 57.1% agree, 21.4% neutral
Evidence Summary
While there is currently no evidence regarding the optimal time to initiate immunosuppressive therapies after immunization with live attenuated vaccines, it is intuitive that risks would be mitigated if treatment is initiated after vaccine-induced viremia clears, whenever the clinical situation allows. Table 6 lists data regarding the length of viremia resulting from commercially available live vaccines.
Table 6.
Length of Viremia Following Vaccination With Live Attenuated Vaccines.
| Vaccine | Length of Viremia |
|---|---|
| Varicella (Oka strain) | The vaccine strain could not be isolated up to 14 days postvaccination in children,225 but 1 study detected the vaccine strain by PCR up to 5 weeks after immunization in 5 of 166 (3%) asymptomatic children given the varicella vaccine.226 |
| Herpes zoster (Oka strain) | Varicella zoster virus DNA can be detected by PCR analysis in 16% (11/67) of individuals 2 weeks postvaccination227 and up to 4 weeks in 6% (2/36) of individuals >60 years old.71 |
| Yellow fever | Viremia after primary immunization wanes within 7 days postimmunization228 and is generally cleared within 2 weeks of vaccination.229 |
| Measles | The vaccine strain has not been isolated from human blood after immunization of healthy children,230 but a study on macaques has shown the persistence of the Schwarz vaccine strain 7 to 9 days postvaccination.231 |
| Mumps | There is a low risk of viremia with the mumps vaccine strains; however, the incidence of aseptic meningitis occurring 2 to 3 weeks after vaccination suggests that the potential is maintained in some vaccine strains. The frequency of vaccine-associated aseptic meningitis varies from approximately 1 in 1.8 million doses for the Jeryl Lynn strain to as high as 1 in 336 for the Urabe AM9 strain.232 |
| Rubella | Viremia was documented 7 to 21 days postvaccination in some adults receiving the primary vaccination but not in children.233 |
| Live polio (type 2 Sabin) | In adults, free virus is present in the serum between 2 and 5 days after vaccine administration, with antibody-bound virus being present up to 8 days after vaccination.234
In children aged ⩽17 months, free virus can be detected up to 8 days after vaccination.235 |
Abbreviation: PCR, polymerase chain reaction.
Statement 4c: In patients with immune-mediated diseases on immunosuppressive agents, we suggest that live attenuated vaccines be administered when individual benefits outweigh the perceived risks.
GRADE: Conditional recommendation; low-level evidence
Vote: 14.3% strongly agree, 64.3% agree, 14.3% neutral, 7.1% disagree
Evidence Summary
The use of yellow fever (YF); measles, mumps, and rubella (MMR); and oral typhoid vaccines in patients on immunosuppressive therapy has not been extensively examined. While further investigation is necessary, small observational studies provided insights into the safety and efficacy of live vaccines in this patient population.76-82 Recall humoral and cellular responses to MMR in children with juvenile idiopathic arthritis (JIA) treated with etanercept + MTX (mean dose of 12 mg/m2 body surface area [BSA] once weekly [QW]) (n = 5) or low-dose MTX alone (mean dose of 9 mg/m2 BSA QW; n = 5) were not significantly different from healthy children (n = 22).80 Moreover, the MMR vaccine was demonstrated to be safe for patients with JIA, with no reports of aggravated disease activity, increased medication use, or severe AEs following vaccination.76,80
Primary or secondary YF vaccination also induced protective serologic responses in the majority of IFX-treated patients with RA (94% [16/17]),81 and adults with chronic inflammatory conditions receiving systemic corticosteroid therapy for a median of 10 months (100% [20/20 patients examined]; prednisone-equivalent dose: 5-20 mg/d).78 In addition, patients with IMDs on MTX (n = 11), prednisolone (n = 1), leflunomide (n = 1), or etanercept (n = 2) achieved postvaccination neutralizing antibody titres comparable to healthy controls.82 In all studies, the YF vaccine was administered without adverse sequelae, although patients in 1 study experienced a higher frequency of transient local reactions (RR, 8.0; 95% CI, 1.4-45.9), including erythema, tenderness, and pain compared with healthy individuals.78
A systematic review by Croce et al83 noted that of the 253 YF vaccine doses administered to patients with IMDs or solid organ transplants, only 1 case of vaccine-related infection was reported. The fatal case of vaccine-associated viscerotropic disease occurred in a patient with SLE and RA treated with dexamethasone and possibly MTX.83 While the patient was given a vaccine from a lot associated with >20 times the risk of viscerotropic disease,83 it is possible that the underlying autoimmunity and immunosuppressive treatment may have also contributed to the fatal outcome. Therefore, prudent assessment of the risks and benefits of vaccination in each individual patient is necessary to prevent potentially deleterious consequences. Whenever possible, physicians should assess the patient’s risk of YF exposure and vaccinate accordingly prior to initiation of immunosuppressive regimens.
Statement 4d: In situations where patient safety is a paramount concern and the clinical situation allows, we suggest that immunosuppressive treatment be interrupted for a duration based on drug pharmacokinetics prior to immunization with live vaccines.
GRADE: Conditional recommendation; low-level evidence
Vote: 28.6% strongly agree, 64.3% agree, 7.1% neutral
Evidence Summary
There is evidence to suggest that live vaccines may be safely given to patients with IMDs treated with immunosuppressive agents. However, due to factors such as advanced age, disease severity, comorbidities, and potent immunosuppressive medications, some patients may be at greater risk of AEs or disseminated infection after receiving live attenuated vaccines. For instance, the risk of developing YF vaccine-associated viscerotropic disease increases with age, with elderly individuals having a 5000-fold increased incidence (0.05%) compared to children (0.00001%).84 In situations where patient safety is of paramount concern and the decision is made to interrupt immunosuppressive therapy prior to immunization, the duration of the treatment break prior to vaccine administration should ideally take drug pharmacokinetics into consideration (Tables 4 and 5) to minimize the interference of immunosuppressive agents on vaccine response and their potential effect on risk of disseminated infection.
Vaccination of Infants With Early Exposure to Immunosuppressive Agents
Statement 5a: In infants exposed to immunosuppressive agents in utero during the third trimester, we recommend that inactivated vaccines be administered according to the local immunization schedule.
GRADE: Strong recommendation; very low-level evidence
Vote: 57.1% strongly agree, 42.9% agree
Evidence Summary
Biologics
Serological responses to routine childhood vaccinations in infants exposed to TNFi up to the third trimester (final dose at 17-39 weeks’ gestation) were described in 3 observational studies and 1 case report.85-88 Adequate and protective responses to tetanus, pneumococcal, and diphtheria vaccines were generally achieved, although some variability in Haemophilus influenzae type b (Hib) vaccine immunogenicity was observed.85,88 Furthermore, routine childhood vaccinations were well tolerated, resulting in no safety concerns or severe AEs.85-91
More recently, data from the Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes (PIANO) registry comparing vaccine responses in infants born of mothers who were exposed to biologic therapies (IFX, adalimumab, vedolizumab, certolizumab pegol, golimumab, natalizumab, or ustekinumab) vs those unexposed during gestation revealed no significant differences in seroprotection rates to the Hib (21/38 [71%] vs 4/8 [50%]; P = .41) and tetanus toxoid vaccines (33/41 [80%] vs 6/8 [75%]; P = .66) between the 2 groups.92
Three case reports examined the efficacy of routine childhood immunizations such as tetanus, diphtheria, and HBV among infants exposed to RTX up to the third trimester (last RTX dose at 30-34 weeks’ gestation).93-95 Despite all infants exhibiting low94 or undetectable93,95 B-cell counts at birth, protective antibody levels were generally achieved following vaccination without serious AEs.
DMARDs and Glucocorticoids
Two case-control studies examined HBV vaccine immunogenicity among infants born to mothers treated with dexamethasone, thiopurines, or cyclosporine for autoimmunity or CTD during pregnancy.96,97 Despite exposure to immunosuppressive drugs in utero, these infants achieved protective antibody titres and exhibited comparable IgG serum levels, lymphocyte counts, and other immune parameters to unexposed infants. Importantly, vaccination with inactivated vaccines was demonstrated to be safe. In a study involving 30 infants exposed to thiopurines due to maternal IBD during gestation, diphtheria, tetanus, pertussis, inactivated polio, Hib, and pneumococcal conjugate vaccines were safely administered without serious AEs or complications.98
Exposure to higher doses of immunosuppressive agents in the solid organ transplant setting did not significantly affect the immune response to childhood vaccinations.99 Of the 17 infants born to renal transplant recipient mothers treated with thiopurines, tacrolimus, prednisone, and/or cyclosporine during pregnancy, 82% exhibited B-cell numbers lower than the 10th percentile of normal Brazilian values (280/mm3) while 29.4% exhibited abnormalities in other lymphocyte populations at birth. Despite this, primary immunization with Hib, pneumococcal, and tetanus vaccines was safely administered and resulted in protective antibody titres that were comparable to those of infants unexposed to immunosuppressive agents in utero.
Statement 5b: In infants exposed to immunosuppressive agents in utero during the third trimester, we recommend that the MMR and varicella vaccines be administered according to the local immunization schedule.
GRADE: Strong recommendation; low-level evidence
Vote: 42.9% strongly agree, 57.1% agree
Evidence Summary
Live attenuated MMR and varicella vaccines are indicated for children 12 to 15 months of age.100,101 At this time, <1% of the initial biologic agent levels should be present in the infant based on the known half-life of 30 to 52.5 days of maternally derived IgG antibodies.102-104 In the case of in utero RTX exposure, continued presence of the biologic agent in the infant was shown to delay B-cell development in some cases. However, circulating CD20+ B-cell numbers were shown to normalize 3 to 6 months after birth, possibly suggesting integrity to adequately respond to vaccines given at later time points.93,94,105 In fact, studies demonstrated that infants exposed to either TNFi or RTX in utero achieved protective antibody levels without complications upon completion of the MMR vaccine regimen.85,90,93,95
Similarly, nonbiologic immunomodulatory agents with relatively long half-lives such as leflunomide (14 days) and antimalarials (40-50 days) are expected to be cleared or present in minute concentrations that are unlikely to negatively affect the immunogenicity or safety of vaccines given ⩾12 months after birth.
Statement 5c: In infants breastfed by mothers on immunosuppressive regimens, we recommend that inactivated and live attenuated vaccines be administered according to the local immunization schedule without delay.
GRADE: Strong recommendation; very low-level evidence
Vote: 35.7% strongly agree, 57.1% agree, 7.1% neutral
Evidence Summary
Biologic Agents
Due to the inefficient transfer of IgG antibodies across the mammary epithelium, only low levels of IgG-based biologics are typically found in breastmilk.87,106-112 Any antibodies ingested by the infant are not taken up by the intact intestinal mucosa113 and are subject to degradation by proteolytic enzymes in the stomach and intestine.113,114 Consequently, breastfeeding while being treated with Ig-based biologics poses little to no risk of infant drug exposure, as evidenced by the continuous decline in serum levels of biologics acquired through gestational exposure in breastfed infants.87,110,115 For this reason, infants breastfed by mothers on biologic therapy should receive routine immunizations without delay according to local immunization guidelines.
Of note, subtherapeutic IFX levels were observed in an infant exposed to the drug solely through breastfeeding.112 Five days after maternal infusion with IFX, the biologic agent was detected in the infant (1700 ng/mL), despite being only partially breastfed. No AEs were observed in the child, and the level present in the infant’s serum is below the therapeutic range and not expected to interfere with the safety or efficacy of childhood immunizations. However, the mechanism of infant absorption of the biologic in this case is unclear, and further investigation may be warranted.
Nonbiologic Agents
Hydrocortisones, antimalarials, azathioprine metabolites, sulfasalazine, MTX, cyclosporine, and tacrolimus have been detected in breastmilk at concentrations lower than maternal serum levels.116-124 At these levels, the weight-adjusted exposure of the breastfed infant is estimated to be minimal (Table 7)125 and would likely have little effect on vaccine immunogenicity and efficacy. However, live attenuated vaccines should be administered with caution to infants breastfed by mothers on cyclophosphamide as cases of neutropenia and hematopoiesis suppression were documented in infants exposed to this agent through breastmilk.126,127
Table 7.
Exposure of Infants to Immunosuppressive Agents Through Breastmilk.
| Drug | Estimated Infant Exposure |
|---|---|
| Chloroquine | 0.55%119,a |
| Hydroxychloroquine | 2%118,b |
| Tacrolimus | 0.3%123,b |
| Cyclosporine | 0.2%-1.1%236,b |
| Prednisolone | 0.1%120,a |
| Azathioprine | 6-MP metabolite: <0.09%121,b |
| Sulfasalazine | 5.9%119,a |
| Methotrexate | NA122,c |
Abbreviations: 6-MP, 6-mercaptopurine; NA, not available.
Percent of maternal dose ingested.
Infant drug exposure corrected for maternal and infant body weight (weight-adjusted dose).
Expected to result in minimal exposure of infant to methotrexate due to the low milk/plasma ratio (0.08) observed.
Discussion
Consensus Level
These guidelines present recommendations on general immunization practices for patients with IMDs treated with immunosuppressive therapies, as well as infants exposed to such agents during the third trimester of pregnancy or through breastfeeding. Consensus was reached on 13 statements, with 2 statements being rejected (Appendix 1).
Comparison With Other Guidelines
In contrast to the recommendations in this publication, several guidelines, including those from the National Advisory Committee on Immunization (NACI), Advisory Committee on Immunization Practices, American College of Gastroenterology, and European Crohn’s and Colitis Organization, have suggested waiting at least 3 to 4 weeks after immunization with live vaccines prior to commencing immunosuppressive therapy.69,70,73,128 In addition, the 2014 NACI guidelines recommended that the live HZ vaccine not be given to individuals receiving high-dose corticosteroids or immune-suppressing medications, unless treatment has been discontinued for a period of 3 days to up to 1 year prior to vaccination, depending on the medication.128
However, in individuals with prior exposure and immunity to VZV, the live attenuated HZ vaccine acts as a booster immunization, which elicits a secondary immune response with faster kinetics than primary vaccination. Studies on tofacitinib in patients with RA demonstrated that treatment initiation within 2 weeks of vaccination did not compromise the safety or efficacy of the vaccine.72 Furthermore, data from retrospective studies suggest that vaccination even while on immunomodulatory biologics (TNFi, abatacept, or RTX), DMARDs, and glucocorticoids is safe and effective,74 potentially eliminating the need for long wait times between vaccination and treatment initiation. Presented with this evidence, the committee deemed the live attenuated HZ vaccine to be relatively safe for individuals with preexisting immunity to varicella, even in those on certain immunosuppressive treatments. In treatment-naive patients, deferring the initiation of immunosuppressive therapy 2 to 4 weeks postvaccination should be sufficient to mount an effective adaptive response to the vaccine.
While this manuscript was being prepared for publication, the NACI released the 2018 recommendations on the use of HZ vaccines, which state that the HZ/su vaccine, not the live attenuated vaccine, may be considered for immunocompromised adults aged ⩾50 years.129 This echoes the CDC recommendation that the subunit vaccine be considered over the live attenuated vaccine for immunocompetent adults ⩾50 years of age or those anticipating immunosuppression.68 Aligned with the NACI and CDC guidelines, this publication preferentially recommends considering the subunit vaccine for patients with IMDs on immunosuppressive regimens until further data are available.
With regards to vaccinating patients on RTX, the Public Health Agency of Canada recommends that treatment be discontinued 6 to 12 months prior to vaccination with live or inactivated vaccines,130 whereas a minimum washout period of 5 months preimmunization is recommended in this publication. While longer washout periods would be ideal and allow B-cell numbers to rebound following B-cell depletion therapy, extended treatment cessation is not always feasible if disease control is to be maintained. Thus, in patients requiring RTX infusion every 6 months, the committee determined that immunizing patients 5 months following the last RTX dose and at least 4 weeks prior to the subsequent dose would allow for a degree of B-cell reconstitution and sufficient time to mount an adaptive immune response to the vaccine.
Limitations
While these guidelines were developed according to the best evidence available to date, the body of evidence regarding the safety and efficacy of vaccination among individuals receiving immunosuppressive therapy for IMDs remains incomplete. Large-scale clinical trials assessing the effectiveness of vaccines in this patient population have not been performed, and data regarding the safety and efficacy of immunization for patients on recently approved drugs and biologic agents are currently lacking. Furthermore, antibody thresholds may be an imperfect surrogate for vaccine-induced protection, and the lack of more accurate measures of vaccine efficacy may hinder our complete understanding of how immunosuppressive drugs and biological agents affect the immunological response to immunization.
Conclusions
The use of immunomodulatory biologics, DMARDs, and glucocorticoids may result in attenuation but not abolishment of the immune response to vaccines. The risks and benefits of immunization should be weighed to determine patient eligibility and appropriate timing of vaccine administration relative to immunosuppressive therapy.
While specialists endorse the importance of giving age- and disease-appropriate vaccines to patients with IMDs, primary care physicians (PCPs) are often tasked with carrying out immunizations in these patients. To ensure that necessary vaccines are given and patients receive consistent care across health care providers, communication between specialists and PCPs is imperative. Should the need arise, these guidelines may serve to inform not only specialists but also family physicians and other health care providers on issues regarding the safety and efficacy of vaccines in patients with IMDs on immunosuppressive therapies.
Supplemental Material
Supplemental material, Appendix_1_Final for Vaccination Guidelines for Patients With Immune-Mediated Disorders on Immunosuppressive Therapies by Kim A. Papp, Boulos Haraoui, Deepali Kumar, John K. Marshall, Robert Bissonnette, Alain Bitton, Brian Bressler, Melinda Gooderham, Vincent Ho, Shahin Jamal, Janet E. Pope, A. Hillary Steinhart, Donald C. Vinh and John Wade in Journal of Cutaneous Medicine and Surgery
Acknowledgments
We thank Synapse Medical Communications, Oakville, Ontario, which provided medical writing assistance in the form of drafting and revising as per authors’ directions and in accordance with the standards set out by the International Committee of Medical Journal Editors. Medical writing support was funded by the Dermatology Association of Ontario.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Kim Papp is an advisory board participant, steering committee member, investigator, speaker, and/or consultant for AbbVie, Akros, Allergan, Amgen, Anacor, Astellas, AstraZeneca, Baxalta, Baxter, Boehringer Ingelheim, Bristol-Myers Squibb, CanFite, Celgene, Coherus, Dermira, Dow Pharma, Eli Lilly, Forward Pharma, Galderma, Genentech, GSK, Janssen, Kyowa Hakko Kirin, LEO Pharma, MedImmune, Meiji Seika Pharma, Merck (MSD), Merck Serono, Mitsubishi Pharma, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Takeda, UCB, and Valeant. Boulos Haraoui is an advisory board participant, speaker, consultant, and/or investigator for Amgen, AbbVie, Janssen, Lilly, Merck, Pfizer, Novartis, and UCB. Deepali Kumar is an advisory board participant, speaker, investigator, and/or consultant for Astellas, GSK, Janssen, Oxford Immunotec, Pfizer, Qiagen, and Sanofi. John K. Marshall is an advisory board participant, speaker, and/or consultant for AbbVie, Allergan, Celgene, Celltrion, Ferring, Hoffman-La Roche, Hospira, Janssen, Lilly, Merck, Pfizer, Procter & Gamble, Shire, and Takeda. Robert Bissonnette is an advisory board participant, investigator, speaker, and/or consultant for AbbVie, Amgen, Boehringer Ingelheim, BMS, Celgene, Eli Lilly, Galderma, GSK Stiefel, Immune, Incyte, Janssen, Kineta, Leo Pharma, Merck, Novartis, Pfizer, and Xenoport and is a shareholder of Innovaderm Research. Alain Bitton is an advisory board participant, speaker, and/or investigator for AbbVie, Janssen, Takeda, Shire, Ferring, Pfizer, Merck, and Pharmascience. Brian Bressler is an advisor and/or speaker for AbbVie, Actavis, Allergan, Amgen, Celgene, Ferring, Genentech, Janssen, Merck, Pendopharm, Pfizer, Shire, and Takeda and received research support from AbbVie, Alvine, Amgen, Atlantic Pharmaceuticals, Boehringer Ingelheim, BMS, Celgene, Genentech, GlaxoSmithKline, Janssen, Merck, Qu Biologic, Red Hill Pharma, and Takeda. Melinda Gooderham is an advisory board participant, investigator, consultant, and/or speaker for AbbVie, Actelion Pharmaceuticals, Akros Pharma, Amgen, Arcutis, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dermira, Eli Lilly, Galderma, GSK, Janssen, LEO Pharma, MedImmune, Novartis, Pfizer, Regeneron Pharmaceuticals, Roche, Sanofi Genzyme, Sun Pharmaceutical Industries, UCB, and Valeant Pharmaceuticals. Vincent Ho is an advisory board participant, investigator, and/or speaker for Abbvie, Lilly, Novartis, Janssen, and Sanofi. Shahin Jamal is an advisory board participant for Abbvie, Amgen, BMS, Celgene, Eli Lilly, Janssen, Merck, Novartis, Pfizer, Roche, Sandoz, Sanofi Aventis, and UCB. Janet E. Pope is a consultant for Lilly, Merck, Novartis, Pfizer, Roche, Sanofi, Sandoz, and UCB and received research grants from Merck, Pfizer, Roche, UCB, and Seattle Genetics. A. Hillary Steinhart is an advisory board participant, speaker, investigator, and/or consultant for Abbvie, Amgen, Arena Pharmaceuticals, Celgene, Ferring, Genentech, Hoffmann-La Roche, Hospira, Janssen, Merck, Pfizer, Pharmascience, Red Hill Biopharma, and Takeda. Donald C. Vinh is an advisory board participant, speaker, consultant, and/or investigator for Cidara, CSL Behring Canada, Merck Canada, and Shire Canada. John Wade is an advisory board participant and consultant for Abbvie, Amgen, BMS, Celgene, Janssen, Lilly, Novartis, Roche, Sanofi, and UCB.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Development of the vaccination guidelines was funded by the Dermatology Association of Ontario.
ORCID iD: Robert Bissonnette  https://orcid.org/0000-0001-5927-6587
https://orcid.org/0000-0001-5927-6587
References
- 1. Yun H, Yang S, Chen L, et al. Risk of herpes zoster in auto-immune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016;68:2328-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shigayeva A, Rudnick W, Green K, et al. Invasive pneumococcal disease among immunocompromised persons: implications for vaccination programs. Clin Infect Dis. 2016;62:139-147. [DOI] [PubMed] [Google Scholar]
- 3. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287-2293. [DOI] [PubMed] [Google Scholar]
- 4. McKinnon JE, Maksimowicz-McKinnon K. Autoimmune disease and vaccination: impact on infectious disease prevention and a look at future applications. Transl Res. 2016;167:46-60. [DOI] [PubMed] [Google Scholar]
- 5. Assala M, Groh M, Blanche P, et al. Pneumococcal and influenza vaccination rates in patients treated with corticosteroids and/or immunosuppressive therapies for systemic autoimmune diseases: a cross-sectional study. Joint Bone Spine. 2017;84:365-366. [DOI] [PubMed] [Google Scholar]
- 6. Lawson EF, Trupin L, Yelin EH, Yazdany J. Reasons for failure to receive pneumococcal and influenza vaccinations among immunosuppressed patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2015;44:666-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hua C, Morel J, Ardouin E, et al. Reasons for non-vaccination in French rheumatoid arthritis and spondyloarthritis patients. Rheumatology (Oxford). 2015;54:748-750. [DOI] [PubMed] [Google Scholar]
- 8. Taranger J, Trollfors B, Lagergård T, et al. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J Infect Dis. 2000;181:1010-1013. [DOI] [PubMed] [Google Scholar]
- 9. Amanna IJ, Messaoudi I, Slifka MK. Protective immunity following vaccination: how is it defined? Hum Vaccin. 2008;4:316-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond). 1972;70:767-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen RT, Markowitz LE, Albrecht P, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036-1042. [DOI] [PubMed] [Google Scholar]
- 12. Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14:839-846. [DOI] [PubMed] [Google Scholar]
- 13. Käyhty H, Peltola H, Karanko V, Mäkelä PH. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. [DOI] [PubMed] [Google Scholar]
- 14. Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179:489-492. [DOI] [PubMed] [Google Scholar]
- 15. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marseglia GL, Scaramuzza A, d’Annunzio G, Comolli G, Gatti M, Lorini R. Successful immune response to a recombinant hepatitis B vaccine in young patients with insulin-dependent diabetes mellitus. Diabet Med. 1996;13:630-633. [DOI] [PubMed] [Google Scholar]
- 18. Melmed GY, Agarwal N, Frenck RW, et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148-154. [DOI] [PubMed] [Google Scholar]
- 19. Zanoni G, Contreas G, Valletta E, Gabrielli O, Mengoli C, Veneri D. Normal or defective immune response to hepatitis B vaccine in patients with diabetes and celiac disease. Hum Vaccin Immunother. 2015;11:58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schappi MG, Meier S, Bel M, Siegrist CA, Posfay-Barbe KM. Protective antibody responses to influenza A/H1N1/09 vaccination in children with celiac disease. J Pediatr Gastroenterol Nutr. 2012;54:817-819. [DOI] [PubMed] [Google Scholar]
- 21. Puges M, Biscay P, Barnetche T, et al. Immunogenicity and impact on disease activity of influenza and pneumococcal vaccines in systemic lupus erythematosus: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2016;55:1664-1672. [DOI] [PubMed] [Google Scholar]
- 22. Caso F, Ramonda R, Del Puente A, et al. Influenza vaccine with adjuvant on disease activity in psoriatic arthritis patients under anti-TNF-alpha therapy. Clin Exp Rheumatol. 2016;34:507-512. [PubMed] [Google Scholar]
- 23. Kapetanovic MC, Roseman C, Jönsson G, Truedsson L, Saxne T, Geborek P. Antibody response is reduced following vaccination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum. 2011;63:3723-3732. [DOI] [PubMed] [Google Scholar]
- 24. Launay O, Abitbol V, Krivine A, et al. Immunogenicity and safety of influenza vaccine in inflammatory bowel disease patients treated or not with immunomodulators and/or biologics: a two-year prospective study. J Crohns Colitis. 2015;9:1096-1107. [DOI] [PubMed] [Google Scholar]
- 25. Lee CK, Kim HS, Ye BD, et al. Patients with Crohn’s disease on anti–tumor necrosis factor therapy are at significant risk of inadequate response to the 23-valent pneumococcal polysaccharide vaccine. J Crohns Colitis. 2014;8:384-391. [DOI] [PubMed] [Google Scholar]
- 26. Andrisani G, Frasca D, Romero M, et al. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-alpha agents: effects of combined therapy with immunosuppressants. J Crohns Colitis. 2013;7:301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fiorino G, Peyrin-Biroulet L, Naccarato P, et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2012;18:1042-1047. [DOI] [PubMed] [Google Scholar]
- 28. Park SH, Yang SK, Park SK, et al. Efficacy of hepatitis A vaccination and factors impacting on seroconversion in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:69-74. [DOI] [PubMed] [Google Scholar]
- 29. Frasca D, Andrisani G, Diaz A, Felice C, Guidi L, Blomberg BB. AID in aging and autoimmune diseases. Autoimmunity. 2013;46:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kantso B, Halkjaer SI, Thomsen OO, et al. Immunosuppressive drugs impairs antibody response of the polysaccharide and conjugated pneumococcal vaccines in patients with Crohn’s disease. Vaccine. 2015;33:5464-5469. [DOI] [PubMed] [Google Scholar]
- 31. Nguyen DL, Nguyen ET, Bechtold ML. Effect of immunosuppressive therapies for the treatment of inflammatory bowel disease on response to routine vaccinations: a meta-analysis. Dig Dis Sci. 2015;60:2446-2453. [DOI] [PubMed] [Google Scholar]
- 32. Oren S, Mandelboim M, Braun-Moscovici Y, et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis. 2008;67:937-941. [DOI] [PubMed] [Google Scholar]
- 33. van Assen S, Holvast A, Benne CA, et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 2010;62:75-81. [DOI] [PubMed] [Google Scholar]
- 34. Arad U, Tzadok S, Amir S, et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine. 2011;29:1643-1648. [DOI] [PubMed] [Google Scholar]
- 35. Westra J, van Assen S, Wilting KR, et al. Rituximab impairs immunoglobulin (Ig)M and IgG (subclass) responses after influenza vaccination in rheumatoid arthritis patients. Clin Exp Immunol. 2014;178:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eisenberg RA, Jawad AF, Boyer J, et al. Rituximab-treated patients have a poor response to influenza vaccination. J Clin Immunol. 2013;33:388-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti–tumor necrosis factor alpha, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2014;66:1016-1026. [DOI] [PubMed] [Google Scholar]
- 38. Borba EF, Saad CG, Pasoto SG, et al. Influenza A/H1N1 vaccination of patients with SLE: can antimalarial drugs restore diminished response under immunosuppressive therapy? Rheumatology (Oxford). 2012;51:1061-1069. [DOI] [PubMed] [Google Scholar]
- 39. Crowe SR, Merrill JT, Vista ES, et al. Influenza vaccination responses in human systemic lupus erythematosus: Impact of clinical and demographic features. Arthritis Rheum. 2011;63:2396-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang Y, Wang H, Wan L, Lu X, Tam WW. Is systemic lupus erythematosus associated with a declined immunogenicity and poor safety of influenza vaccination? A systematic review and meta-analysis. Medicine (Baltimore). 2016;95:e3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018;77:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76:1559-1565. [DOI] [PubMed] [Google Scholar]
- 43. Mori S, Ueki Y, Hirakata N, Oribe M, Hidaka T, Oishi K. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann Rheum Dis. 2012;71:2006-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adler S, Krivine A, Weix J, et al. Protective effect of A/H1N1 vaccination in immune-mediated disease—a prospectively controlled vaccination study. Rheumatology (Oxford). 2012;51:695-700. [DOI] [PubMed] [Google Scholar]
- 45. Kivitz AJ, Schechtman J, Texter M, Fichtner A, de Longueville M, Chartash EK. Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: results from a single-blind randomized phase IV trial. J Rheumatol. 2014;41:648-657. [DOI] [PubMed] [Google Scholar]
- 46. Ribeiro AC, Guedes LK, Moraes JC, et al. Reduced seroprotection after pandemic H1N1 influenza adjuvant-free vaccination in patients with rheumatoid arthritis: implications for clinical practice. Ann Rheum Dis. 2011;70:2144-2147. [DOI] [PubMed] [Google Scholar]
- 47. Kapetanovic MC, Saxne T, Sjõholm A, Truedsson L, Jönsson G, Geborek P. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology (Oxford). 2006;45:106-111. [DOI] [PubMed] [Google Scholar]
- 48. Kapetanovic MC, Roseman C, Jönsson G, Truedsson L. Heptavalent pneumococcal conjugate vaccine elicits similar antibody response as standard 23-valent polysaccharide vaccine in adult patients with RA treated with immunomodulating drugs. Clin Rheumatol. 2011;30:1555-1561. [DOI] [PubMed] [Google Scholar]
- 49. Migita K, Akeda Y, Akazawa M, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tacrolimus. Arthritis Res Ther. 2015;17:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75:687-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mori S, Ueki Y, Akeda Y, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tocilizumab therapy. Ann Rheum Dis. 2013;72:1362-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kapetanovic MC, Nagel J, Nordstrom I, Saxne T, Geborek P, Rudin A. Methotrexate reduces vaccine-specific immunoglobulin levels but not numbers of circulating antibody-producing B cells in rheumatoid arthritis after vaccination with a conjugate pneumococcal vaccine. Vaccine. 2017;35:903-908. [DOI] [PubMed] [Google Scholar]
- 53. Siegrist CA. Vaccine immunology. In: SA Plotkin, WA Orenstein, PA Offit, eds. Vaccines. 5th ed. Philadelphia, PA: Elsevier; 2008:17-36. [Google Scholar]
- 54. Halperin BA, Morris A, Mackinnon-Cameron D, et al. Kinetics of the antibody response to tetanus-diphtheria-acellular pertussis vaccine in women of childbearing age and postpartum women. Clin Infect Dis. 2011;53:885-892. [DOI] [PubMed] [Google Scholar]
- 55. Murphy KP. Janeway’s Immunobiology. 8th ed. New York, NY: Garland Science, Taylor & Francis Group, LLC; 2012. [Google Scholar]
- 56. Vauloup-Fellous C, Grangeot-Keros L. Humoral immune response after primary rubella virus infection and after vaccination. Clin Vaccine Immunol. 2007;14:644-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Flehmig B, Staedele H, Xueref C, Vidor E, Zuckerman J, Zuckerman A. Early appearance of neutralizing antibodies after vaccination with an inactivated hepatitis A vaccine. J Infect. 1997;35:37-40. [DOI] [PubMed] [Google Scholar]
- 58. França IL, Ribeiro AC, Aikawa NE, et al. TNF blockers show distinct patterns of immune response to the pandemic influenza A H1N1 vaccine in inflammatory arthritis patients. Rheumatology (Oxford). 2012;51:2091-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hoffmann-La Roche Limited. RITUXAN [product monograph]. October 31, 2014. [Google Scholar]
- 60. Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:613-620. [DOI] [PubMed] [Google Scholar]
- 61.6-month interval dosing: Rituxan dosing schedule. http://www.rituxanforra-hcp.com/dosing/6-month-interval. Accessed November 29, 2017.
- 62. Merck Canada, Inc. ZOSTAVAX II [product monograph]. July 5, 2017. [Google Scholar]
- 63. Merck Canada, Inc. VARIVAX III [product monograph]. January 25, 2016. [Google Scholar]
- 64. Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375:1019-1032. [DOI] [PubMed] [Google Scholar]
- 65. Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087-2096. [DOI] [PubMed] [Google Scholar]
- 66. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284. [DOI] [PubMed] [Google Scholar]
- 67. GlaxoSmithKline Biologicals. SHINGRIX vaccines and related biological products advisory committee briefing document. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVacBloodVaccinesandOtherB/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM575192.pdf. Accessed November 29, 2017.
- 68. Centers for Disease Control and Prevention. Shingles (herpes zoster): vaccination. https://www.cdc.gov/shingles/vaccination.html. Accessed June 26, 2018.
- 69. Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241-258. [DOI] [PubMed] [Google Scholar]
- 70. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [DOI] [PubMed] [Google Scholar]
- 71. Pierson DL, Mehta SK, Gilden D, et al. Varicella zoster virus DNA at inoculation sites and in saliva after Zostavax immunization. J Infect Dis. 2011;203:1542-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Winthrop KL, Wouters AG, Choy EH, et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II trial. Arthritis Rheumatol. 2017;69:1969-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kroger AT, Duchin J, Vazquez M. General best practice guidelines for immunization: best practices guidance of the Advisory Committee on Immunization Practices. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html. Accessed April 20, 2017.
- 74. Zhang J, Xie F, Delzell E, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cheetham TC, Marcy SM, Tseng HF, et al. Risk of herpes zoster and disseminated varicella zoster in patients taking immunosuppressant drugs at the time of zoster vaccination. Mayo Clin Proc. 2015;90:865-873. [DOI] [PubMed] [Google Scholar]
- 76. Heijstek MW, Pileggi GC, Zonneveld-Huijssoon E, et al. Safety of measles, mumps and rubella vaccination in juvenile idiopathic arthritis. Ann Rheum Dis. 2007;66:1384-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oliveira AC, Mota LM, Santos-Neto LL, Simoes M, Martins-Filho OA, Tauil PL. Seroconversion in patients with rheumatic diseases treated with immunomodulators or immunosuppressants, who were inadvertently revaccinated against yellow fever. Arthritis Rheumatol. 2015;67:582-583. [DOI] [PubMed] [Google Scholar]
- 78. Kernéis S, Launay O, Ancelle T, et al. Safety and immunogenicity of yellow fever 17D vaccine in adults receiving systemic corticosteroid therapy: an observational cohort study. Arthritis Care Res (Hoboken). 2013;65:1522-1528. [DOI] [PubMed] [Google Scholar]
- 79. Azevedo LS, Lasmar EP, Contieri FL, et al. Yellow fever vaccination in organ transplanted patients: is it safe? A multicenter study. Transpl Infect Dis. 2012;14:237-241. [DOI] [PubMed] [Google Scholar]
- 80. Borte S, Liebert UG, Borte M, Sack U. Efficacy of measles, mumps and rubella revaccination in children with juvenile idiopathic arthritis treated with methotrexate and etanercept. Rheumatology (Oxford). 2009;48:144-148. [DOI] [PubMed] [Google Scholar]
- 81. Scheinberg M, Guedes-Barbosa LS, Mangueira C, et al. Yellow fever revaccination during infliximab therapy. Arthritis Care Res (Hoboken). 2010;62:896-898. [DOI] [PubMed] [Google Scholar]
- 82. Wieten RW, Jonker EF, Pieren DK, et al. Comparison of the PRNT and an immune fluorescence assay in yellow fever vaccinees receiving immunosuppressive medication. Vaccine. 2016;34:1247-1251. [DOI] [PubMed] [Google Scholar]
- 83. Croce E, Hatz C, Jonker EF, Visser LG, Jaeger VK, Buhler S. Safety of live vaccinations on immunosuppressive therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation or after bone-marrow transplantation: a systematic review of randomized trials, observational studies and case reports. Vaccine. 2017;35:1216-1226. [DOI] [PubMed] [Google Scholar]
- 84. World Health Organization. Vaccine safety basics, module 2: types of vaccine and adverse reactions. http://vaccine-safety-training.org/overview-and-outcomes-2.html. Accessed May 19, 2017.
- 85. Bortlik M, Duricova D, Machkova N, et al. Impact of anti–tumor necrosis factor alpha antibodies administered to pregnant women with inflammatory bowel disease on long-term outcome of exposed children. Inflamm Bowel Dis. 2014;20:495-501. [DOI] [PubMed] [Google Scholar]
- 86. Zelinkova Z, de Haar C, de Ridder L, et al. High intra-uterine exposure to infliximab following maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther. 2011;33:1053-1058. [DOI] [PubMed] [Google Scholar]
- 87. Vasiliauskas EA, Church JA, Silverman N, Barry M, Targan SR, Dubinsky MC. Case report: evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol. 2006;4:1255-1258. [DOI] [PubMed] [Google Scholar]
- 88. Sheibani S, Cohen R, Kane S, Dubinsky M, Church JA, Mahadevan U. The effect of maternal peripartum anti-TNF alpha use on infant immune response. Dig Dis Sci. 2016;61:1622-1627. [DOI] [PubMed] [Google Scholar]
- 89. Steenholdt C, Al-Khalaf M, Ainsworth MA, Brynskov J. Therapeutic infliximab drug level in a child born to a woman with ulcerative colitis treated until gestation week 31. J Crohns Colitis. 2012;6:358-361. [DOI] [PubMed] [Google Scholar]
- 90. Johnsson A, Avlund S, Grosen A, Julsgaard M. Chicken pox infection in a three months old infant exposed in utero to adalimumab. J Crohns Colitis. 2013;7:e116-e117. [DOI] [PubMed] [Google Scholar]
- 91. de Lima A, Zelinkova Z, van der Ent C, Steegers EA, van der Woude CJ. Tailored anti-TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut. 2016;65:1261-1268. [DOI] [PubMed] [Google Scholar]
- 92. Beaulieu DB, Ananthakrishnan AN, Martin C, Cohen RD, Kane SV, Mahadevan U. Use of biologic therapy by pregnant women with inflammatory bowel disease does not affect infant response to vaccines. Clin Gastroenterol Hepatol. 2018;16:99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mandal PK, Dolai TK, Bagchi B, Ghosh MK, Bose S, Bhattacharyya M. B cell suppression in newborn following treatment of pregnant diffuse large B-cell lymphoma patient with rituximab containing regimen. Indian J Pediatr. 2014;81:1092-1094. [DOI] [PubMed] [Google Scholar]
- 94. Klink DT, van Elburg RM, Schreurs MW, van Well GT. Rituximab administration in third trimester of pregnancy suppresses neonatal B-cell development. Clin Dev Immunol. 2008;2008:271363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Friedrichs B, Tiemann M, Salwender H, Verpoort K, Wenger MK, Schmitz N. The effects of rituximab treatment during pregnancy on a neonate. Haematologica. 2006;91:1426-1427. [PubMed] [Google Scholar]
- 96. Cimaz R, Meregalli E, Biggioggero M, et al. Alterations in the immune system of children from mothers treated with immunosuppressive agents during pregnancy. Toxicol Lett. 2004;149:155-162. [DOI] [PubMed] [Google Scholar]
- 97. Biggioggero M, Borghi MO, Gerosa M, Trespidi L, Cimaz R, Meroni PI. Immune function in children born to mothers with autoimmune diseases and exposed in utero to immunosuppressants. Lupus. 2007;16:651-656. [DOI] [PubMed] [Google Scholar]
- 98. de Meij TG, Jharap B, Kneepkens CM, van Bodegraven AA, de Boer NK. Long-term follow-up of children exposed intrauterine to maternal thiopurine therapy during pregnancy in females with inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38:38-43. [DOI] [PubMed] [Google Scholar]
- 99. Dinelli MI, Ono E, Viana PO, et al. Response to immunization in children born to renal transplant recipients using immunosuppressive drugs during gestation. Vaccine. 2016;34:404-407. [DOI] [PubMed] [Google Scholar]
- 100. Public Health Agency of Canada. Canada’s provincial and territorial routine (and catch-up) vaccination programs for infants and children. http://healthycanadians.gc.ca/healthy-living-vie-saine/immunization-immunisation/schedule-calendrier/alt/infants-children-vaccination-enfants-nourrissons-eng.pdf. Accessed October 5, 2016.
- 101. Centers for Disease Control and Prevention. 2017. Recommended immunizations for children from birth through 6 years old. https://www.cdc.gov/vaccines/parents/downloads/parent-ver-sch-0-6yrs.pdf2017. Accessed June 6, 2017.
- 102. Shahid NS, Steinhoff MC, Hoque SS, Begum T, Thompson C, Siber GR. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet. 1995;346:1252-1257. [DOI] [PubMed] [Google Scholar]
- 103. Sarvas H, Seppälä I, Kurikka S, Siegberg R, Mäkelä O. Half-life of the maternal IgG1 allotype in infants. J Clin Immunol. 1993;13:145-151. [DOI] [PubMed] [Google Scholar]
- 104. Wiener AS. The half-life of passively acquired antibody globulin molecules in infants. J Exp Med. 1951;94:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Decker M, Rothermundt C, Hollander G, Tichelli A, Rochlitz C. Rituximab plus CHOP for treatment of diffuse large B-cell lymphoma during second trimester of pregnancy. Lancet Oncol. 2006;7:693-694. [DOI] [PubMed] [Google Scholar]
- 106. Grosen A, Julsgaard M, Kelsen J, Christensen LA. Infliximab concentrations in the milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2014;8:175-176. [DOI] [PubMed] [Google Scholar]
- 107. Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558. [DOI] [PubMed] [Google Scholar]
- 108. Ben-Horin S, Yavzori M, Katz L, et al. Adalimumab level in breast milk of a nursing mother. Clin Gastroenterol Hepatol. 2010;8:475-476. [DOI] [PubMed] [Google Scholar]
- 109. Mahadevan U, Wolf DC, Dubinsky M, et al. Placental transfer of anti–tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:286-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Murashima A, Watanabe N, Ozawa N, Saito H, Yamaguchi K. Etanercept during pregnancy and lactation in a patient with rheumatoid arthritis: drug levels in maternal serum, cord blood, breast milk and the infant’s serum. Ann Rheum Dis. 2009;68:1793-1794. [DOI] [PubMed] [Google Scholar]
- 111. Berthelsen BG, Fjeldsøe-Nielsen H, Nielsen CT, Hellmuth E. Etanercept concentrations in maternal serum, umbilical cord serum, breast milk and child serum during breastfeeding. Rheumatology (Oxford). 2010;49:2225-2227. [DOI] [PubMed] [Google Scholar]
- 112. Fritzsche J, Pilch A, Mury D, Schaefer C, Weber-Schoendorfer C. Infliximab and adalimumab use during breastfeeding. J Clin Gastroenterol. 2012;46:718-719. [DOI] [PubMed] [Google Scholar]
- 113. Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3:442-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ling J, Koren G. Challenges in vaccinating infants born to mothers taking immunoglobulin biologicals during pregnancy. Expert Rev Vaccines. 2016;15:239-256. [DOI] [PubMed] [Google Scholar]
- 115. Julsgaard M, Christensen LA, Gibson PR, et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology. 2016;151:110-119. [DOI] [PubMed] [Google Scholar]
- 116. Khan AK, Truelove SC. Placental and mammary transfer of sulphasalazine. BMJ. 1979;2:1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Akintonwa A, Gbajumo SA, Mabadeje AF. Placental and milk transfer of chloroquine in humans. Ther Drug Monit. 1988;10:147-149. [DOI] [PubMed] [Google Scholar]
- 118. Nation RL, Hackett LP, Dusci LJ, Ilett KF. Excretion of hydroxychloroquine in human milk. Br J Clin Pharmacol. 1984;17:368-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Østensen M, Khamashta M, Lockshin M, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8:209-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ost L, Wettrell G, Björkhem I, Rane A. Prednisolone excretion in human milk. J Pediatr. 1985;106:1008-1011. [DOI] [PubMed] [Google Scholar]
- 121. Moretti ME, Verjee Z, Ito S, Koren G. Breast-feeding during maternal use of azathioprine. Ann Pharmacother. 2006;40:2269-2272. [DOI] [PubMed] [Google Scholar]
- 122. Johns DG, Rutherford LD, Leighton PC, Vogel CL. Secretion of methotrexate into human milk. Am J Obstet Gynecol. 1972;112:978-980. [DOI] [PubMed] [Google Scholar]
- 123. Zheng S, Easterling TR, Hays K, et al. Tacrolimus placental transfer at delivery and neonatal exposure through breast milk. Br J Clin Pharmacol. 2013;76:988-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Østensen M, Brown ND, Chiang PK, Aarbakke J. Hydroxychloroquine in human breast milk. Eur J Clin Pharmacol. 1985;28:357. [DOI] [PubMed] [Google Scholar]
- 125. Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810. [DOI] [PubMed] [Google Scholar]
- 126. Durodola JI. Administration of cyclophosphamide during late pregnancy and early lactation: a case report. J Natl Med Assoc. 1979;71:165-166. [PMC free article] [PubMed] [Google Scholar]
- 127. Amato D, Niblett JS. Neutropenia from cyclophosphamide in breast milk. Med J Aust. 1977;1:383-384. [DOI] [PubMed] [Google Scholar]
- 128. National Advisory Committee on Immunization (NACI). Update on the use of herpes zoster vaccine. http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-92-2014-eng.pdf. Accessed June 26, 2018.
- 129. National Advisory Committee on Immunization (NACI). Updated Recommendations on the Use of Herpes Zoster Vaccines. Public Health Agency of Canada; Ottawa, 2018. [Google Scholar]
- 130. Public Health Agency of Canada. Canadian immunization guide: part 3—vaccination of specific populations. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-8-immunization-immunocompromised-persons.html#a25. Accessed June 26, 2018.
- 131. Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64:77-83. [DOI] [PubMed] [Google Scholar]
- 132. Askling HH, Rombo L, van Vollenhoven R, et al. Hepatitis A vaccine for immunosuppressed patients with rheumatoid arthritis: a prospective, open-label, multi-centre study. Travel Med Infect Dis. 2014;12:134-142. [DOI] [PubMed] [Google Scholar]
- 133. Andrade P, Santos-Antunes J, Rodrigues S, Lopes S, Macedo G. Treatment with infliximab or azathioprine negatively impact the efficacy of hepatitis B vaccine in inflammatory bowel disease patients. J Gastroenterol Hepatol. 2015;30:1591-1595. [DOI] [PubMed] [Google Scholar]
- 134. Salinas GF, De RL, Barendregt B, et al. Anti-TNF treatment blocks the induction of T cell–dependent humoral responses. Ann Rheum Dis. 2013;72:1037-1043. [DOI] [PubMed] [Google Scholar]
- 135. Belle A, Baumann C, Bigard MA, et al. Impact of immunosuppressive therapy on hepatitis B vaccination in inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 2015;27:877-881. [DOI] [PubMed] [Google Scholar]
- 136. Ribeiro AC, Laurindo IM, Guedes LK, et al. Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2013;65:476-480. [DOI] [PubMed] [Google Scholar]
- 137. Alten R, Bingham CO, III, Cohen SB, et al. Antibody response to pneumococcal and influenza vaccination in patients with rheumatoid arthritis receiving abatacept. BMC Musculoskelet Disord. 2016;17:231-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kaine JL, Kivitz AJ, Birbara C, Luo AY. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272-279. [PubMed] [Google Scholar]
- 139. Chatham WW, Wallace DJ, Stohl W, et al. Effect of belimumab on vaccine antigen antibodies to influenza, pneumococcal, and tetanus vaccines in patients with systemic lupus erythematosus in the BLISS-76 trial. J Rheumatol. 2012;39:1632-1640. [DOI] [PubMed] [Google Scholar]
- 140. Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis. 2006;65:191-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm Bowel Dis. 2016;22:638-647. [DOI] [PubMed] [Google Scholar]
- 142. Hagihara Y, Ohfuji S, Watanabe K, et al. Infliximab and/or immunomodulators inhibit immune responses to trivalent influenza vaccination in adults with inflammatory bowel disease. J Crohns Colitis. 2014;8:223-233. [DOI] [PubMed] [Google Scholar]
- 143. Chioato A, Noseda E, Stevens M, Gaitatzis N, Kleinschmidt A, Picaud H. Treatment with the interleukin-17A-blocking antibody secukinumab does not interfere with the efficacy of influenza and meningococcal vaccinations in healthy subjects: results of an open-label, parallel-group, randomized single-center study. Clin Vaccine Immunol. 2012;19:1597-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Tsuru T, Terao K, Murakami M, et al. Immune response to influenza vaccine and pneumococcal polysaccharide vaccine under IL-6 signal inhibition therapy with tocilizumab. Mod Rheumatol. 2014;24:511-516. [DOI] [PubMed] [Google Scholar]
- 145. Kapetanovic MC, Saxne T, Nilsson JÅ, Geborek P. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology (Oxford). 2007;46:608-611. [DOI] [PubMed] [Google Scholar]
- 146. Kubota T, Nii T, Nanki T, et al. Anti–tumor necrosis factor therapy does not diminish the immune response to influenza vaccine in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2007;17:531-533. [DOI] [PubMed] [Google Scholar]
- 147. Polachek A, Korobko U, Mader-Balakirski N, et al. Immunogenicity and safety of vaccination against seasonal 2012 influenza virus among patients with psoriatic arthritis and psoriasis. Clin Exp Rheumatol. 2015;33:181-186. [PubMed] [Google Scholar]
- 148. Kapetanovic MC, Saxne T, Jönsson G, Truedsson L, Geborek P. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther. 2013;15:R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Migita K, Akeda Y, Akazawa M, et al. Effect of abatacept on the immunogenicity of 23-valent pneumococcal polysaccharide vaccination (PPSV23) in rheumatoid arthritis patients. Arthritis Res Ther. 2015;17:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rákóczi É, Perge B, Végh E, et al. Evaluation of the immunogenicity of the 13-valent conjugated pneumococcal vaccine in rheumatoid arthritis patients treated with etanercept. Joint Bone Spine. 2016;83:675-679. [DOI] [PubMed] [Google Scholar]
- 151. Visvanathan S, Keenan GF, Baker DG, Levinson AI, Wagner CL. Response to pneumococcal vaccine in patients with early rheumatoid arthritis receiving infliximab plus methotrexate or methotrexate alone. J Rheumatol. 2007;34:952-957. [PubMed] [Google Scholar]
- 152. Migita K, Akeda Y, Akazawa M, et al. Opsonic and antibody responses to pneumococcal polysaccharide in rheumatoid arthritis patients receiving golimumab plus methotrexate. Medicine (Baltimore). 2015;94:e2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bingham CO, III, Looney RJ, Deodhar A, et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum. 2010;62:64-74. [DOI] [PubMed] [Google Scholar]
- 154. Bingham CO, III, Rizzo W, Kivitz A, Hassanali A, Upmanyu R, Klearman M. Humoral immune response to vaccines in patients with rheumatoid arthritis treated with tocilizumab: results of a randomised controlled trial (VISARA). Ann Rheum Dis. 2015;74:818-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Brodmerkel C, Wadman E, Langley RG, et al. Immune response to pneumococcus and tetanus toxoid in patients with moderate-to-severe psoriasis following long-term ustekinumab use. J Drugs Dermatol. 2013;12:1122-1129. [PubMed] [Google Scholar]
- 156. Weinberg A, Boulware D, Dighero B, Orban T. Effect of abatacept on immunogenicity of vaccines in individuals with type 1 diabetes. Vaccine. 2013;31:4791-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Dezfoli S, Horton HA, Thepyasuwan N, et al. Combined immunosuppression impairs immunogenicity to tetanus and pertussis vaccination among patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:1754-1760. [DOI] [PubMed] [Google Scholar]
- 158. Wichmann A, Krugliak CN, Rubin DT. Safety and efficacy of live measles vaccine administered to a Crohn’s disease patient receiving vedolizumab. Am J Gastroenterol. 2016;111:577. [DOI] [PubMed] [Google Scholar]
- 159. Sempere L, Almenta I, Barrenengoa J, et al. Factors predicting response to hepatitis B vaccination in patients with inflammatory bowel disease. Vaccine. 2013;31:3065-3071. [DOI] [PubMed] [Google Scholar]
- 160. Dotan I, Werner L, Vigodman S, et al. Normal response to vaccines in inflammatory bowel disease patients treated with thiopurines. Inflamm Bowel Dis. 2012;18:261-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mok CC, Ho LY, Fong LS, To CH. Immunogenicity and safety of a quadrivalent human papillomavirus vaccine in patients with systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 2013;72:659-664. [DOI] [PubMed] [Google Scholar]
- 162. Gabay C, Bel M, Combescure C, et al. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum. 2011;63:1486-1496. [DOI] [PubMed] [Google Scholar]
- 163. Dengler TJ, Strnad N, Bühring I, et al. Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplantation. 1998;66:1340-1347. [DOI] [PubMed] [Google Scholar]
- 164. Versluis DJ, Beyer WE, Masurel N, Wenting GJ, Weimar W. Impairment of the immune response to influenza vaccination in renal transplant recipients by cyclosporine, but not azathioprine. Transplantation. 1986;42:376-379. [DOI] [PubMed] [Google Scholar]
- 165. Willcocks LC, Chaudhry AN, Smith JC, et al. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant. 2007;7:2006-2011. [DOI] [PubMed] [Google Scholar]
- 166. Salles MJ, Sens YA, Boas LS, Machado CM. Influenza virus vaccination in kidney transplant recipients: serum antibody response to different immunosuppressive drugs. Clin Transplant. 2010;24:E17-E23. [DOI] [PubMed] [Google Scholar]
- 167. Siegrist CA, Ambrosioni J, Bel M, et al. Responses of solid organ transplant recipients to the AS03-adjuvanted pandemic influenza vaccine. Antivir Ther. 2012;17:893-903. [DOI] [PubMed] [Google Scholar]
- 168. Elkayam O, Amir S, Mendelson E, et al. Efficacy and safety of vaccination against pandemic 2009 influenza A (H1N1) virus among patients with rheumatic diseases. Arthritis Care Res (Hoboken). 2011;63:1062-1067. [DOI] [PubMed] [Google Scholar]
- 169. Pasoto SG, Ribeiro AC, Viana VS, et al. Short and long-term effects of pandemic unadjuvanted influenza A(H1N1)pdm09 vaccine on clinical manifestations and autoantibody profile in primary Sjogren’s syndrome. Vaccine. 2013;31:1793-1798. [DOI] [PubMed] [Google Scholar]
- 170. Baluch A, Humar A, Eurich D, et al. Randomized controlled trial of high-dose intradermal versus standard-dose intramuscular influenza vaccine in organ transplant recipients. Am J Transplant. 2013;13:1026-1033. [DOI] [PubMed] [Google Scholar]
- 171. Azevedo LS, Gerhard J, Miraglia JL, et al. Seroconversion of 2009 pandemic influenza A (H1N1) vaccination in kidney transplant patients and the influence of different risk factors. Transpl Infect Dis. 2013;15:612-618. [DOI] [PubMed] [Google Scholar]
- 172. Scharpé J, Evenepoel P, Maes B, et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant. 2008;8:332-337. [DOI] [PubMed] [Google Scholar]
- 173. Smith KG, Isbel NM, Catton MG, Leydon JA, Becker GJ, Walker RG. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol Dial Transplant. 1998;13:160-164. [DOI] [PubMed] [Google Scholar]
- 174. Keshtkar-Jahromi M, Argani H, Rahnavardi M, et al. Antibody response to influenza immunization in kidney transplant recipients receiving either azathioprine or mycophenolate: a controlled trial. Am J Nephrol. 2008;28:654-660. [DOI] [PubMed] [Google Scholar]
- 175. Holvast A, Huckriede A, Wilschut J, et al. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann Rheum Dis. 2006;65:913-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Holvast A, Stegeman CA, Benne CA, et al. Wegener’s granulomatosis patients show an adequate antibody response to influenza vaccination. Ann Rheum Dis. 2009;68:873-878. [DOI] [PubMed] [Google Scholar]
- 177. Fischer L, Gerstel PF, Poncet A, et al. Pneumococcal polysaccharide vaccination in adults undergoing immunosuppressive treatment for inflammatory diseases—a longitudinal study. Arthritis Res Ther. 2015;17:151-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Struijk GH, Minnee RC, Koch SD, et al. Maintenance immunosuppressive therapy with everolimus preserves humoral immune responses. Kidney Int. 2010;78:934-940. [DOI] [PubMed] [Google Scholar]
- 179. Palestine AG, Roberge F, Charous BL, Lane HC, Fauci AS, Nussenblatt RB. The effect of cyclosporine on immunization with tetanus and keyhole limpet hemocyanin (KLH) in humans. J Clin Immunol. 1985;5:115-121. [DOI] [PubMed] [Google Scholar]
- 180. Trollmo C, Gudmundsson S, Feltelius N, Rogberg S, Smedegård G, Klareskog L. Sulphasalazine inhibits human antigen-specific immune responses in vivo. Ann Rheum Dis. 2007;66:481-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kollaritsch H, Que JU, Kunz C, Wiedermann G, Herzog C, Cryz SJ., Jr. Safety and immunogenicity of live oral cholera and typhoid vaccines administered alone or in combination with antimalarial drugs, oral polio vaccine, or yellow fever vaccine. J Infect Dis. 1997;175:871-875. [DOI] [PubMed] [Google Scholar]
- 182. Russell AF, Parrino J, Fisher CL, Jr, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine. 2015;33:3129-3134. [DOI] [PubMed] [Google Scholar]
- 183. Wasan SK, Zullow S, Berg A, Cheifetz AS, Ganley-Leal L, Farraye FA. Herpes zoster vaccine response in inflammatory bowel disease patients on low-dose immunosuppression. Inflamm Bowel Dis. 2016;22:1391-1396. [DOI] [PubMed] [Google Scholar]
- 184. AbbVie Corporation. HUMIRA [product monograph]. February 26, 2016. [Google Scholar]
- 185. Amgen Canada, Inc. ENBREL [product monograph]. October 19, 2015. [Google Scholar]
- 186. UCB Canada, Inc. CIMZIA [product monograph]. January 15, 2014. [Google Scholar]
- 187. Janssen, Inc. SIMPONI [product monograph]. November 25, 2014. [Google Scholar]
- 188. Janssen, Inc. REMICADE [product monograph]. July 22, 2015. [Google Scholar]
- 189. Kovalenko P, DiCioccio AT, Davis JD, et al. Exploratory population PK analysis of dupilumab, a fully human monoclonal antibody against IL-4R alpha, in atopic dermatitis patients and normal volunteers. CPT Pharmacometrics Syst Pharmacol. 2016;5:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. GlaxoSmithKline, Inc. NUCALA [product monograph]. August 30, 2016. [Google Scholar]
- 191. Hoffmann-La Roche Limited. ACTEMRA [product monograph]. October 29, 2015. [Google Scholar]
- 192. Sanofi-Aventis Canada, Inc. KEVZARA [product monograph]. January 12, 2017. [Google Scholar]
- 193. Swedish Ophan BioVitrum AB. KINERET [product monograph]. December 23, 2010. [Google Scholar]
- 194. Novartis Pharmaceuticals Canada, Inc. ILARIS [product monograph]. July 14, 2016. [Google Scholar]
- 195. Baverel PG, Jain M, Stelmach I, et al. Pharmacokinetics of tralokinumab in adolescents with asthma: implications for future dosing. Br J Clin Pharmacol. 2015;80:1337-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Noonan M, Korenblat P, Mosesova S, et al. Dose-ranging study of lebrikizumab in asthmatic patients not receiving inhaled steroids. J Allergy Clin Immunol. 2013;132:567-574. [DOI] [PubMed] [Google Scholar]
- 197. Novartis Pharmaceuticals Canada, Inc. COSENTYX [product monograph]. February 27, 2015. [Google Scholar]
- 198. Eli Lilly Canada, Inc. TALTZ [product monograph]. May 25, 2016. [Google Scholar]
- 199. Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Valeant Pharmaceuticals North America LLC. SILIQ (brodalumab) [prescribing information]. February 2017. [Google Scholar]
- 201. Janssen, Inc. STELARA [product monograph]. March 9, 2015. [Google Scholar]
- 202. Janssen, Inc. TREMFYA [product monograph]. November 10, 2017. [Google Scholar]
- 203. Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116-124. [DOI] [PubMed] [Google Scholar]
- 204. Zandvliet A, Glasgow S, Horowitz A, et al. Tildrakizumab, a novel anti-IL-23 monoclonal antibody, is unaffected by ethnic variability in Caucasian, Chinese, and Japanese subjects. Int J Clin Pharmacol Ther. 2015;53:139-146. [DOI] [PubMed] [Google Scholar]
- 205. Nemoto O, Furue M, Nakagawa H, et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2016;174:296-304. [DOI] [PubMed] [Google Scholar]
- 206. GlaxoSmithKline. BENLYSTA [product monograph]. April 16, 2014. [Google Scholar]
- 207. Takeda Canada, Inc. ENTYVIO [product monograph]. January 25, 2015. [Google Scholar]
- 208. Biogen Canada, Inc. TYSABRI [product monograph]. August 31, 2016. [Google Scholar]
- 209. Bristol-Myers Squibb. ORENCIA [product monograph]. April 3, 2017. [Google Scholar]
- 210. Pfizer Canada, Inc. XELJANZ [product monograph]. April 16, 2014. [Google Scholar]
- 211. Apotex, Inc. APO-METHOTREXATE [product monograph]. November 27, 2014. [Google Scholar]
- 212. Astellas Pharma Canada, Inc. PROGRAF [product monograph]. April 27, 2011. [Google Scholar]
- 213. Novartis Pharmaceuticals Canada, Inc. NEORAL [product monograph]. January 9, 2015. [Google Scholar]
- 214. Baxter Corporation. PROCYTOX [product monograph]. September 7, 2012. [Google Scholar]
- 215. Browning DJ. Hydroxychloroquine and Chloroquine Retinopathy. New York, NY: Springer; 2014:35-63. [Google Scholar]
- 216. Mylan Pharmaceuticals ULC. Mylan-Hydroxychloroquine [product monograph]. February 4, 2016. [Google Scholar]
- 217. SteriMax, Inc. Mercaptopurine Tablets USP [product monograph]. November 19, 2014. [Google Scholar]
- 218. Triton Pharma, Inc. IMURAN [product monograph]. January 31, 2013. [Google Scholar]
- 219. Pfizer Canada, Inc. SALAZOPYRIN [product monograph]. December 5, 2013. [Google Scholar]
- 220. Hoffmann-La Roche Limited. CellCept/CellCept i.v. [product monograph]. March 9, 2015. [Google Scholar]
- 221. Novartis Pharmaceuticals Canada, Inc. MYFORTIC [product monograph]. December 17, 2014. [Google Scholar]
- 222. Sanofi-Aventis Canada, Inc. PEDIAPRED [product monograph]. May 1, 2006. [Google Scholar]
- 223. Celgene, Inc. OTEZLA [product monograph]. June 9, 2015. [Google Scholar]
- 224. Apotex, Inc. APO-LEFLUNOMIDE [product monograph]. February 9, 2016. [Google Scholar]
- 225. Asano Y, Itakura N, Hiroishi Y, et al. Viral replication and immunologic responses in children naturally infected with varicella-zoster virus and in varicella vaccine recipients. J Infect Dis. 1985;152:863-868. [DOI] [PubMed] [Google Scholar]
- 226. Gomi Y, Ozaki T, Nishimura N, et al. DNA sequence analysis of varicella-zoster virus gene 62 from subclinical infections in healthy children immunized with the Oka varicella vaccine. Vaccine. 2008;26:5627-5632. [DOI] [PubMed] [Google Scholar]
- 227. Levin MJ, Cai GY, Lee KS, et al. Varicella-zoster virus DNA in blood after administration of herpes zoster vaccine. J Infect Dis. 2018;217:1055-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Reinhardt B, Jaspert R, Niedrig M, Kostner C, L’age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol. 1998;56:159-167. [DOI] [PubMed] [Google Scholar]
- 229. Wheelock EF, Sibley WA. Circulating virus, interferon and antibody after vaccination with the 17-D strain of yellow-fever virus. N Engl J Med. 1965;273:194-198. [DOI] [PubMed] [Google Scholar]
- 230. Katz SL, Kempe CH, Black FL, et al. Studies on an attenuated measles-virus vaccine, VIII: general summary and evaluation of the results of vaccine. N Engl J Med. 1960;263:180-184. [DOI] [PubMed] [Google Scholar]
- 231. van Binnendijk RS, Poelen MC, van AG, de VP, Osterhaus AD. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles vaccines in the presence of passively acquired antibodies. J Infect Dis. 1997;175:524-532. [DOI] [PubMed] [Google Scholar]
- 232. Rubin SA, Plotkin RA. Mumps vaccine. In: SA Plotkin, WA Orenstein, PA Offit, eds. Vaccines. 6th ed. Philadelphia, PA: Elsevier; 2013:419-446. [Google Scholar]
- 233. Balfour HH, Jr, Groth KE, Edelman CK, Amren DP, Best JM, Banatvala JE. Rubella viraemia and antibody responses after rubella vaccination and reimmunization. Lancet. 1981;1:1078-1080. [DOI] [PubMed] [Google Scholar]
- 234. Melnick JL, Proctor RO, Ocampo AR, Diwan AR, Ben-Porath E. Free and bound virus in serum after administration of oral poliovirus vaccine. Am J Epidemiol. 1966;84:329-342. [DOI] [PubMed] [Google Scholar]
- 235. Horstmann DM, Opton EM, Klempferer R, Llado B, Vignec AJ. Viremia in infants vaccinated with oral poliovirus vaccine (Sabin). Am J Hyg. 1964;79:47-63. [DOI] [PubMed] [Google Scholar]
- 236. Moretti ME, Sgro M, Johnson DW, et al. Cyclosporine excretion into breast milk. Transplantation. 2003;75:2144-2146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_1_Final for Vaccination Guidelines for Patients With Immune-Mediated Disorders on Immunosuppressive Therapies by Kim A. Papp, Boulos Haraoui, Deepali Kumar, John K. Marshall, Robert Bissonnette, Alain Bitton, Brian Bressler, Melinda Gooderham, Vincent Ho, Shahin Jamal, Janet E. Pope, A. Hillary Steinhart, Donald C. Vinh and John Wade in Journal of Cutaneous Medicine and Surgery