Abstract
For mechanical force to induce changes in cellular behaviors, two main processes are inevitable; perception of the force and response to it. Perception of mechanical force by cells, or mechanosensing, requires mechanical force-induced conformational changes in mechanosensors. For this, at least one end of the mechanosensors should be anchored to relatively fixed structures, such as extracellular matrices or the cytoskeletons, while the other end should be pulled along the direction of the mechanical force. Alternatively, mechanosensors may be positioned in lipid bilayers, so that conformational changes in the embedded sensors can be induced by mechanical force-driven tension in the lipid bilayer. Responses to mechanical force by cells, or mechanotransduction, require translation of such mechanical force-induced conformational changes into biochemical signaling. For this, protein-protein interactions or enzymatic activities of mechanosensors should be modulated in response to force-induced structural changes. In the last decade, several molecules that met the required criteria of mechanosensors have been identified and proven to directly sense mechanical force. The present review introduces examples of such mechanosensors and summarizes their mechanisms of action.
Keywords: Lipid bilayer model, Mechanical force, Mechanosensors, Tethered model
INTRODUCTION
Various types of cells in our body constantly experience mechanical forces in daily life. Among the five traditional human senses, hearing and touch are dependent on mechanical forces, such as vibration and pressure, which are recognized mainly by sensory neurons. Muscle cells are formed and generated as a result of exercise, suggesting that muscle precursors respond to the mechanical stretch and can be expanded/differentiated into muscles (1, 2). Endothelial cells lining the lumen of blood vessels are exposed to blood flow, causing a shear force on the cells (3). Perturbation of blood flow-altering shear forces can change the gene expression profile in endothelial cells, thus increasing the risk of atherosclerosis (4). Similarly, changes in blood flow at the injury sites can enhance platelet activation (5). Epithelial cells lining the renal tube recognize and respond to the osmotic pressure caused by ion transport across the cell membrane (6). In most of the adherent cultured cells, intracellular tension is observed in the actin cytoskeleton, connected to focal adhesions, due to actomyosin contraction to balance against the stiffness of the extracellular environment (7, 8). Thus, altered environmental stiffness can lead to changes in the degree of intracellular tension, which works as an internal mechanical force. Mesenchymal stem cells can respond to this type of force and differentiate into various cell types according to the stiffness of their extracellular environment (9).
Described above are the examples in which mechanical force induces physiological effects within the cells. How then, can cells sense and respond to mechanical force? The mechanical force acting on cells eventually results in deformations of cellular structure. To be recognized by cells as a signal, the deformation must be converted into a biochemical signal, such as a change in enzymatic activity or a protein-protein interaction. Two major hypotheses have been suggested to explain as for how cells recognize such deformations (10). In one hypothesis, proteins tethered to either cell-cell or cell-extracellular matrix (ECM) contacts are suggested to work as “mechanosensors” that can “feel” the force and translate it into a biochemical signal. When the tethered proteins are pulled by mechanical force in the opposite direction from the tethered site, the molecules undergo stretching resulting in conformational changes. These changes can expose a binding site for other proteins to interact with (Fig. 1A) or disrupt an existing protein-protein interaction (Fig. 1B), which can turn on signaling in a manner similar to protein-protein interactions involved in various cellular signaling pathways initiated by growth factors or hormones (11). Alternatively, conformational changes resulting from mechanical force-induced stretch can directly modulate the enzymatic activities of the proteins (Fig. 1C), such as ion channels, resulting in the initiation of cell signaling (12). Since this explanation relies on proteins tethered to adhesive structures, this explanation is termed as the “tethered model”.
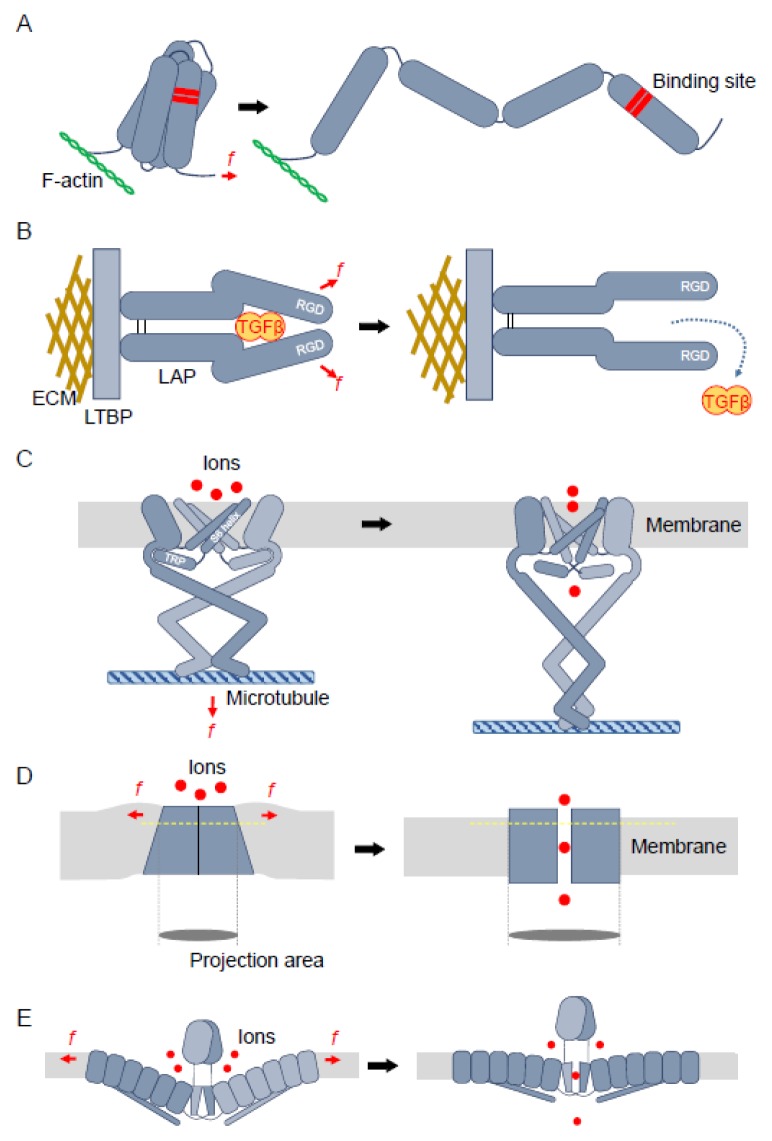
Fig. 1.
Hypothetical schematic model for mechanosensing mechanisms of various types of mechanosensors. (A) The cytoskeletal proteins linked to the actin cytoskeleton (F-actin) and adhesive structures that can undergo structural changes in response to mechanical force. The structural change can expose a binding site for other proteins to interact with, which can induce biochemical signaling. (B) Force acting on the ECM-tethered latency-associated peptide (LAP) by cells via integrin can induce a structural change in LAP. Due to the structural change, transforming growth factor (TGF) β can be released from the LAP complex. RGD; Arg-Gly-Asp (integrin binding site), ECM; extracellular matrix. (C) A stretchgated ion channel in Drosophila, NOMPC (no mechanoreceptor potential C), embedded in the membrane. Two of its four subunits are shown. S6 helices from each subunit block the passage of ions. These helices are linked to TRP domains that are captured by the cytoplasmic domains of the channel (left). The mechanical force that can stretch the cytoplasmic domain tethered to the microtubule can induce disposition of the TRP domains, which in turn induce structural changes in the S6 helices, leading to the opening of the channel (right). (D) The closed conformation of the TRAAK channel adopts a wedge shape, causing distortion of the lipid bilayer nearby (left). The open conformation of the channel adopts a cylinder shape (right). The projection areas of the cross-sections of the channel (yellow dotted lines) are shown in both the conformations. (E) Schematic illustrations of two subunits of Piezo1 are shown. Each of its three subunits has a curved conformation in the lipid bilayer, making a ‘dimple’ on the membrane (left). The central pore is suggested to be opened by tension in the lipid bilayer, which may flatten out the subunits (right).
In the other explanation, lipid bilayers are important in sensing mechanical stress. The force acting upon cells can cause deformation to entire cells, inducing stretching and/or bending of the lipid bilayer in the cellular membrane. The conformation of integral membrane proteins, especially their membrane-spanning regions or transmembrane domains (TMDs), is largely determined by interactions with nearby lipid bilayers (13). This allows the mechanical force-induced changes in the physical properties of the lipid bilayer to influence the conformation of integral membrane proteins, enabling them to adapt to the altered environment within the lipid bilayer (14). Subsequently, the resulting conformational change induces changes in protein-protein interactions or enzymatic activity (Fig. 1D, E). This explanation has been termed as the “lipid bilayer model” and is widely accepted as the opening mechanism for mechano-gated ion channels (15). In some cases, specialized cellular structures, such as stereocilia, involved in hearing by cochlea of the inner ear or cilia on the endothelial cell membrane, are involved in the sensation of flow (16) and play roles in sensing mechanical force. Although the structures by themselves do not seem to sense force or initiate signaling, they may sensitize or boost the structural changes in the actual mechanosensors, such as tethered cytoskeletal proteins or ion channels, by being sensitively deformed by mechanical force.
In the last decade, our understanding of mechanosensitivity has greatly improved, thanks to the identification of mechanosensors, demonstrations of their direct responses to mechanical force, and determination of their three-dimensional structures. In this review, we have attempted to list representative examples of mechanosensors and discuss their mechanosensing mechanisms.
MECHANOSENSING BY TETHERED PROTEINS
Theoretically, a protein that works as a mechanosensor of the tethered model should possess at least two properties: First, when stretched against the direction of its linkage to the cytoskeleton and/or ECM, the protein should undergo conformational changes. Second, the conformational changes should be linked to changes in its enzymatic activity or interactome, which would induce biochemical signaling. Listed below are the examples of such tethered proteins.
Cytoskeletal proteins
The first cytoskeletal protein to be identified as a mechanosensor of the tethered model was talin (17), a cytoskeletal protein connecting integrin-mediated focal adhesions and the actin cytoskeleton (18). In the experiment, the N-terminal and C-terminal ends of the talin rod domain were attached to a glass surface and magnetic beads, respectively. The beads were pulled using magnetic tweezers in the presence of fluorescently labeled vinculin molecules (17). The number of vinculin molecules bound to the talin head domain was measured by observing spontaneous photobleaching (drop in fluorescence intensity over several minutes) of vinculin using total internal reflection fluorescence microscopy. The pulling force actually increased the number of vinculin interactions to the talin rod domain. In addition, single-molecule force extension spectroscopy aided in detecting unfolding or structural changes in the talin rod domain in response to the pulling force (Fig. 1A) (17). A similar approach was taken to monitor force sensing at cadherin-mediated cell-cell adhesions (19). Using the above mentioned experimental settings, binding of vinculin to α-catenin, a cytoskeletal protein present between cell-cell contacts and the actin cytoskeleton, was proven to be regulated by stretching force. When extended by mechanical force, a vinculin binding site on α-catenin gets exposed. Subsequent interaction between the two molecules led to stabilization of the extended open conformation of α-catenin (19). These types of sensors also include an actin cross-linking protein, filamin A, and a giant protein stabilizing the thick filament in sarcomere, titin (11).
Adhesion receptors
An adhesion molecule found in the vascular cell-cell contact area, PECAM-1, might be another example of a direct mechanosensor tethered to the cytoskeleton, vimentin, and/or actomyosin (20). Shear stress applied to endothelial cells causes a tensional force in the cytoplasmic tail of PECAM-1 and activates Src family kinase-mediated signaling in a PECAM-1-dependent manner (21). The magnetic bead-induced force applied directly to PECAM-1 in endothelial cells also generates similar signaling events to those which result from the application of shear stress (22), although how PECAM-1 provokes signaling events upon shear stress remains unclear (21).
Extracellular ligands
Mechanosensing in the tethered model can also be observed during the activation of extracellular ligands as well. Transforming growth factor (TGF) β is released in a latent form encircled by a “straitjacket” region of latency-associated peptide (LAP) (23). The LAP is associated with latent TGF-β-binding proteins (LTBPs), which in turn bind to the ECM. In addition, LAP interacts with integrins through its integrin-binding Arg-Gly-Asp (RGD) motif. Thus, LAP holding TGFβ can be localized between the ECM and integrins (24). Single-molecule force spectroscopy and simulation studies have shown that mechanical force exerted on LAP can induce conformational changes, which result in the release of TGFβ (Fig. 1B) (25, 26). Accordingly, when the ECM-tethered LTBP-LAP-TGFβ complex experiences tensional force via integrins present on the cell membrane, structural changes in LAP are induced, disrupting the LAP-TGFβ interaction and releasing the growth factor. In this way, mechanical force can initiate conventional chemical ligand-mediated signaling events. Another example of sensors in this class would be von Willebrand factor (VWF), an extracellular ligand of GPIbα located on platelet surfaces during hemostasis. VWF is a multimeric protein present in blood plasma or released from endothelial cells and platelets (27). In the multimeric state, each monomeric VWF is assembled into a helical tubule structure in an end-to-end fashion. Once it is attached to subendothelial collagen at the site of injury, the complex is largely elongated by shear force, thus exposing many GPIbα binding sites that were buried while in the coil conformation, and forming a long, uncoiled, and rope-like structure to which platelets can be attached (28).
Ion channels
NOMPC (no mechanoreceptor potential C; also known as TRPN1) channel, a mechano-gated ion channel responsible for mechanosensing in Drosophila (29), is an example of a mechanosensor which shows changes in its activity upon the application of mechanical force. The channel consists of four identical subunits, each of which contains six transmembrane α-helices (S1–S6) (30). The pore domain of the channel is formed by S5 and S6 from each subunit, with the intersubunit interaction of four S6 helices at the middle of the pore blocking the passage of ions (30). An unusual feature of the channel is its 29 ankyrin repeats in the cytoplasmic domain, which associate with the microtubule network in the dendritic tips of campaniform sensory neurons, one of the mechanoreceptor organs in Drosophila (31), and also in cultured insect cells (32). Cryo-electron microscopic (cryo-EM) study showed that the ankyrin repeats form a helical-spring bundle which captures the C-terminal TRP domains connected to S6 helices (Fig. 1C) (30). Thus, structural changes in ankyrin repeats by mechanical force-induced tension can induce displacement of the TRP domain, which in turn induces structural changes in the S6 helix, leading to the opening of the pore. As the NOMPC channel is both tethered to the cell surface and the gigantic microtubule network, any mechanical force inducing disposition of the channel in the membrane from the cytoskeleton would induce strain in the ankyrin repeats and result in the opening of the pore (Fig. 1C).
MECHANOSENSING BY INTEGRAL MEMBRANE PROTEINS AND THE LIPID BILAYER
A mechanosensor of the lipid bilayer model should directly sense changes in the shape and/or the tension in the lipid bilayer induced by mechanical forces acting upon the cells. How could this be possible? First, force-induced topological changes of TMDs of the mechanosensor could be the basis of mechanosensation. The hydrophobic surfaces of the TMDs of membrane proteins should match with that of the lipid bilayer (14). The mechanical force that stretches the membrane would result in thinning of the membrane, thus inducing “hydrophobic mismatches” between the TMDs and the lipid bilayer. This mismatch could be relieved either by changing the topology of the TMDs (e.g. tilting) and/or TMD aggregation within the lipid bilayer or by inducing distortion of lipids near the TMD, to minimize the exposed hydrophobic region (13). As will be described below, the lipid-embedded region, a bundle of TMDs, of a possible mechanosensor of the lipid bilayer model often adopts a wedge or cone shape, affecting the nearby lipids to adopt a distorted configuration rather than making a planar lipid bilayer (Fig. 1D) (33). Consequently, the mechanical force does not induce further distortion of the lipid bilayer. Instead, it preferentially induces topological changes in the bundle of TMDs of the mechanosensor (14). When these changes are linked to the changes in enzymatic activity and/or TMD interactome, biochemical signaling is initiated. Second, mechanical force-induced increase in tension between the integral membrane proteins and lipids could also be the basis of mechanosensation (14). If the tension is large enough, it can induce expansion of the cross-section area (projection area) of integral membrane proteins at the lipid-water interface (Fig. 1D, E) (34), which causes structural changes in the mechanosensor, initiating a biochemical signaling. The following are examples of such mechanosensors that can directly respond to the stretch of the lipid bilayer.
Ion channels
One way to distinguish a bona fide mechanosensor from its indirect effectors would be to test its mechanical force-induced changes in the enzymatic activity or TMD-mediated protein-protein interactions in reconstituted liposomes (35). The electrophysiological method has enabled some ion channels to be tested in the reconstituted system, proving them to be direct mechanosensors. The activation of an E. coli ion channel, MscL, by pressure in a cell-free pure lipid system was the first demonstration of the mechanosensor in a purified system (36). Later, improvements in the membrane protein preparation methods, e.g. lipoprotein-based nanodiscs (37), and the development of cryo-EM-based structural determination of membrane proteins (38) provided clues for understanding mechanosensitivity of those mechanosensitive channels.
TREK-1, a K+ channel with four transmembrane segments and two pores (K2P channel), was first recognized as a stretch-activated channel in mammals (39, 40). Later, its related K+ channels, belonging to the same K2P channel family, TRAAK (41) and TREK-2 (42), were also suggested as mechanosensors. Recently, purified TRAAK and TREK1 embedded in an artificial lipid bilayer were proven to respond directly to mechanical force, both positive and negative pressure relative to atmospheric pressure (43). Structural studies showed that both TRAAK and TREK-2 channels have distinct ‘up’ and ‘down’ conformations (33, 34, 44). In the up conformation (open state), TM4 is shifted up, making a central cavity below the selective filter open to the cytosol. In the down conformation (closed state), TM4 is shifted downward, forming an intramembrane opening in the cavity so that lipid acyl chains can be inserted into the opening to block the central cavity, thus inhibiting the passage of ions through the channels. Importantly, the up conformation shows an overall cylinder shape in the lipid bilayer, while the down conformation shows a wedge shape, which induces deformation of the lipid bilayer (Fig. 1D). As membrane tension induced by mechanical force adds more free energy cost to a wedge-shaped conformation, it, therefore, favors the cylinder shape, thus promoting the mechanical opening of the channels (Fig. 1D) (33, 34). In addition, the cross-sectional area in the cytoplasmic leaflet is expanded in up conformation so that it occupies more space in the plane of the lipid bilayer than in the down conformation (Fig. 1D). Consequently, in the stretched lipid bilayer under mechanical tension, the open state would be favored (33, 34).
Piezo1 and Piezo2 are another types of cation channel that are known to be mechanically activated (45). Genetic ablation of Piezo1 leads to embryonic lethality due to impaired vascular development, suggesting that Piezo1 plays a role as a shear-stress sensor responsible for endothelial cell organization and survival (46, 47). Piezo2 is known to be expressed in sensory neurons of the dorsal root ganglia and the Merkel cell-neurite complex, a gentle touch receptor in the skin, and is responsible for their mechanosensitive activity (48, 49). Global and sensory neuron-specific ablation of Piezo2 causes respiratory distress and death in newborn mice (50). When purified Piezo1 was reconstituted into droplet lipid bilayers, it opened in response to osmotic pressure, as well as physical stretching force, thus demonstrating its inherent mechanosensitive characteristic (51). Recent cryo-EM studies on Piezo1 revealed a major breakthrough in the field, by showing that Piezo1 forms a trimeric structure consisting of a three-bladed propeller shape embedded in the lipid bilayer with a central ion pore that closes in response to constrictions in the cytosol (52, 53). Very interestingly, each propeller consisted of a total of six Piezo repeats (with 4 TMDs) and the inner and outer helices possessed a pronounced bend, forming a dimple on the surface of the membrane (Fig. 1E) (53). Thus, increased tension by a mechanical force acting on the membrane was suggested to expand the structure and flatten the Piezo1 dimple on the membrane (Fig. 1E), leading to an increase in the projection area and opening the channel (54–56).
Nuclear pore complex
Recent evidence suggests that gating of the nuclear pore complex (NPC) can be regulated directly by force applied to the nucleus. For example, increased tension in stress fibers spanning across the nucleus was suggested to apply force to the nucleus and regulate NPC gating (57). In addition, direct application of downward force on top of the nucleus using atomic force microscopy induced nuclear membrane flattening and nuclear pore opening (58). Intriguingly, the NPC gating by the force was independent of the linker of the nucleoskeleton-cytoskeleton complex and the actin cytoskeleton (58), suggesting that NPC gating might be regulated directly by the force-induced flattening of nuclear membrane and/or changes in its curvature. Although the exact mechanism of NPC gating needs to be investigated, the studies described above suggest that the NPC can work as a mechanosensor gated by mechanical force applied to the lipid bilayer in the nuclear membrane and that the complex can respond to the force by regulating the translocation of proteins, such as transcription factors, across the nuclear envelope.
CONCLUSION AND FUTURE PERSPECTIVES
Thanks to the intensive research on the mechanisms of mechanosensation during the last decade, we now have an idea of how cells sense mechanical forces and how this can be translated into chemical signaling events. As described above, mechanosensing requires a mechanical tension-induced conformational change in the proteins anchored to relatively stationary positions and translation of those changes into a biochemical signal. Based on these properties, the mechanosensors identified so far can be divided into two classes as the cytoskeleton/ECM-tethered and the lipid-embedded types. They can also be divided into two groups depending on their translation method, one in which their activities change and the other in which their intermolecular interactomes change. The combination of such criteria results in four different types of mechanical sensor.
The first type of sensor is anchored to the ECM or cytoskeleton, where force-induced structural changes to the sensors expose cryptic binding site(s) that are originally buried within the sensor. Examples of this type of sensor include talin, α-catenin, TGF β, and VWF (Fig. 1A, B). The second type is also anchored to stationary positions, but a force-induced structural change modulates its activity, such as ion conductivity of NOMPC (Fig. 1C). The third type of sensor includes membrane proteins in which force-induced structural changes resulting from tension in the lipid bilayer modulates their activities, as is seen in the cases of TRAAK, TREKs, and Piezo channels (Fig. 1D, E). The fourth type of sensor, if there is, could be membrane proteins in which conformational changes resulting from tension are linked to changes in their different intermolecular interactions. Considering that transmembrane proteins account for ~30% of total proteins and that more than half of these proteins contain at least two TMDs, the number of TMDs existing in the hydrophobic environment and the complexity of the TMD interactome are expected to exceed those of cytosolic proteins. Due to the diversity of TMDs and possible topological changes caused by mechanical force, the alteration in intermolecular TMD interactions might be a way to sense mechanical force and translate them into biochemical signals. However, as far as we know, this type of mechanosensor has not yet been identified. One of the difficulties in identifying such sensors may result from the difficulty in detecting TMD interactions, which should be altered by mechanical force in a pure lipid environment. The identification of this type of sensor will further expand our knowledge of mechanosensation.
Chemical signals, such as hormones and growth factors, activate specific receptors. The mechanical force may also induce specific responses in cells. However, assuming that each cell may contain more than one mechanosensor, mechanical force can activate all available mechanosensors within the cell. As a result, how cells can respond specifically to seemingly nonspecific mechanical force is one of the largest unanswered questions for future work. One possible answer is that mechanical force may not target just a single molecule, but instead might boost up or reduce down the entire mechanosensitive machinery within the cells, inducing systematic responses to the force-driven environmental change. In this regard, the manner in which mechanical force-induced responses can crosstalk with the conventional chemical ligand-induced cell signaling machinery would be an interesting topic for future research. Studies on this issue might also provide a molecular background for the use of mechanical force for therapeutic purposes to treat human diseases.
ACKNOWLEDGEMENTS
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (Grant NRF-2016R1A2B4009755) and a Korea University Grant.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/jbmr.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song G, Ju Y, Shen X, Luo Q, Shi Y, Qin J. Mechanical stretch promotes proliferation of rat bone marrow mesenchymal stem cells. Colloids Surf B Biointerfaces. 2007;58:271–277. doi: 10.1016/j.colsurfb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Baratchi S, Khoshmanesh K, Woodman OL, Potocnik S, Peter K, McIntyre P. Molecular Sensors of Blood Flow in Endothelial Cells. Trends Mol Med. 2017;23:850–868. doi: 10.1016/j.molmed.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesbitt WS, Westein E, Tovar-Lopez FJ, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15:665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
- 6.Beck FX, Burger-Kentischer A, Muller E. Cellular response to osmotic stress in the renal medulla. Pflugers Arch. 1998;436:814–827. doi: 10.1007/s004240050710. [DOI] [PubMed] [Google Scholar]
- 7.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Helvert S, Storm C, Friedl P. Mechanoreciprocity in cell migration. Nat Cell Biol. 2018;20:8–20. doi: 10.1038/s41556-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 11.Hu X, Margadant FM, Yao M, Sheetz MP. Molecular stretching modulates mechanosensing pathways. Protein Sci. 2017;26:1337–1351. doi: 10.1002/pro.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413:194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- 13.Nyholm TK, Ozdirekcan S, Killian JA. How protein transmembrane segments sense the lipid environment. Biochemistry (Mosc) 2007;46:1457–1465. doi: 10.1021/bi061941c. [DOI] [PubMed] [Google Scholar]
- 14.Anishkin A, Loukin SH, Teng J, Kung C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc Natl Acad Sci U S A. 2014;111:7898–7905. doi: 10.1073/pnas.1313364111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haswell ES, Phillips R, Rees DC. Mechanosensitive Channels: What Can They Do and How Do They Do It? Structure. 2011;19:1356–1369. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 17.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- 19.Yao M, Qiu W, Liu R, et al. Force-dependent conformational switch of alpha-catenin controls vinculin binding. Nat Commun. 2014;5:4525. doi: 10.1038/ncomms5525. [DOI] [PubMed] [Google Scholar]
- 20.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coon BG, Baeyens N, Han J, et al. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol. 2015;208:975–986. doi: 10.1083/jcb.201408103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzima E, Irani-Tehrani M, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 23.Robertson IB, Rifkin DB. Regulation of the Bioavailability of TGF-beta and TGF-beta-Related Proteins. Cold Spring Harb Perspect Biol. 2016;8:a021907. doi: 10.1101/cshperspect.a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinz B. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Buscemi L, Ramonet D, Klingberg F, et al. The single-molecule mechanics of the latent TGF-beta1 complex. Curr Biol. 2011;21:2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Dong X, Zhao B, Iacob RE, et al. Force interacts with macromolecular structure in activation of TGF-beta. Nature. 2017;542:55–59. doi: 10.1038/nature21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125:2019–2028. doi: 10.1182/blood-2014-06-528406. [DOI] [PubMed] [Google Scholar]
- 28.Springer TA. von Willebrand factor, Jedi knight of the bloodstream. Blood. 2014;124:1412–1425. doi: 10.1182/blood-2014-05-378638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- 30.Jin P, Bulkley D, Guo Y, et al. Electron cryomicroscopy structure of the mechanotransduction channel NOMPC. Nature. 2017;547:118–122. doi: 10.1038/nature22981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuthill JC, Wilson RI. Parallel Transformation of Tactile Signals in Central Circuits of Drosophila. Cell. 2016;164:1046–1059. doi: 10.1016/j.cell.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Cheng LE, Kittelmann M, et al. Ankyrin Repeats Convey Force to Gate the NOMPC Mechanotransduction Channel. Cell. 2015;162:1391–1403. doi: 10.1016/j.cell.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brohawn SG, Campbell EB, MacKinnon R. Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature. 2014;516:126–130. doi: 10.1038/nature14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brohawn SG. How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann N Y Acad Sci. 2015;1352:20–32. doi: 10.1111/nyas.12874. [DOI] [PubMed] [Google Scholar]
- 35.Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 36.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 37.Denisov IG, Grinkova YV, Lazarides AA, Sligar SG. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J Am Chem Soc. 2004;126:3477–3487. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 38.Rawson S, Davies S, Lippiat JD, Muench SP. The changing landscape of membrane protein structural biology through developments in electron microscopy. Mol Membr Biol. 2016;33:12–22. doi: 10.1080/09687688.2016.1221533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel AJ, Honore E, Maingret F, et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 41.Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- 42.Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- 43.Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A. 2014;111:3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong YY, Pike AC, Mackenzie A, et al. K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science. 2015;347:1256–1259. doi: 10.1126/science.1261512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coste B, Mathur J, Schmidt M, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranade SS, Qiu Z, Woo SH, et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci U S A. 2014;111:10347–10352. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Hou B, Tumova S, et al. Piezo1 integration of vascular architecture with physiological force. Nature. 2014;515:279–282. doi: 10.1038/nature13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ranade SS, Woo SH, Dubin AE, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516:121–125. doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo SH, Ranade S, Weyer AD, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nonomura K, Woo SH, Chang RB, et al. Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature. 2017;541:176–181. doi: 10.1038/nature20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Syeda R, Florendo MN, Cox CD, et al. Piezo1 Channels Are Inherently Mechanosensitive. Cell Rep. 2016;17:1739–1746. doi: 10.1016/j.celrep.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Q, Zhou H, Chi S, et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature. 2018;554:487–492. doi: 10.1038/nature25743. [DOI] [PubMed] [Google Scholar]
- 53.Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A, Ward AB. Structure of the mechanically activated ion channel Piezo1. Nature. 2018;554:481–486. doi: 10.1038/nature25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang X, Howard J. Structural Biology: Piezo Senses Tension through Curvature. Curr Biol. 2018;28:R357–R359. doi: 10.1016/j.cub.2018.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo YR, MacKinnon R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. Elife. 2017;6:e33660. doi: 10.7554/eLife.33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chesler AT, Szczot M. Portraits of a pressure sensor. Elife. 2018;7:e34396. doi: 10.7554/eLife.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shiu JY, Aires L, Lin Z, Vogel V. Nanopillar force measurements reveal actin-cap-mediated YAP mechanotransduction. Nat Cell Biol. 2018;20:262–271. doi: 10.1038/s41556-017-0030-y. [DOI] [PubMed] [Google Scholar]
- 58.Elosegui-Artola A, Andreu I, Beedle AEM, et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell. 2017;171:1397–1410 e1314. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]