Abstract
Staphylococcus aureus capsular polysaccharides (CP) are important virulence factors under evaluation as vaccine antigens. Clinical S. aureus isolates have the biosynthetic capability to express either CP5 or CP8 and an understanding of the relationship between CP genotype/phenotype and S. aureus epidemiology is valuable. Using whole genome sequencing, the clonal relatedness and CP genotype were evaluated for disease-associated S. aureus isolates selected from the Tigecycline Evaluation and Surveillance Trial (T.E.S.T) to represent different geographic regions in the United States (US) during 2004 and 2009–10. Thirteen prominent clonal complexes (CC) were identified, with CC5, 8, 30 and 45 representing >80% of disease isolates. CC5 and CC8 isolates were CP type 5 and, CC30 and CC45 isolates were CP type 8. Representative isolates from prevalent CC were susceptible to in vitro opsonophagocytic killing elicited by anti-CP antibodies, demonstrating that susceptibility to opsonic killing is not linked to the genetic lineage. However, as not all S. aureus isolates may express CP, isolates representing the diversity of disease isolates were assessed for CP production. While approximately 35% of isolates (primarily CC8) did not express CP in vitro, CP expression could be clearly demonstrated in vivo for 77% of a subset of these isolates (n = 20) despite the presence of mutations within the capsule operon. CP expression in vivo was also confirmed indirectly by measuring an increase in CP specific antibodies in mice infected with CP5 or CP8 isolates. Detection of antigen expression in vivo in relevant disease states is important to support the inclusion of these antigens in vaccines. Our findings confirm the validity of CP as vaccine targets and the potential of CP-based vaccines to contribute to S. aureus disease prevention.
Introduction
S. aureus is a highly successful pathogen with the potential to cause a range of severe life threatening diseases (e.g. pneumonia, bacteremia, endocarditis and sepsis) and represents a public health burden [1–4]. Vulnerability to this pathogen is further highlighted by the prevalence of antibiotic resistance among S. aureus isolates, exemplified by the emergence of methicillin-resistant S. aureus (MRSA), which often necessitates the use of more complex treatment options. Initially, MRSA was primarily confined to healthcare settings, hence referred to as healthcare-associated MRSA (HA-MRSA) [2, 4, 5]; however, the epidemiology of MRSA changed at the turn of the century with the emergence and spread of MRSA isolates among individuals not previously exposed to healthcare settings. These community-associated MRSA (CA-MRSA) isolates are associated predominantly with skin and soft tissue infections (SSTIs), but have also been linked to clinical syndromes such as necrotizing pneumonia and severe sepsis [6]. In the US, clinical isolates of CA-MRSA have been dominated by a single genetic background, USA300, a pulsotype within sequence type 8 (ST8) and clonal complex 8 (CC8), as defined by multilocus sequence typing (MLST) [7, 8]. More recently, USA300 clones have emerged among healthcare-associated infections, superseding USA100 clone (associated with ST5) as a cause of bloodstream and nosocomial infections in some geographic locations [9, 10]. Furthermore, the contribution of methicillin-susceptible S. aureus (MSSA) to the burden of S. aureus disease has arguably been under-appreciated [11, 12]. Whereas MRSA infections are often associated with the spread of a limited number of clones, MSSA infections are caused by a wide range of genetically diverse isolates [13, 14]. The continuous evolution of S. aureus disease epidemiology, high mortality rates, and longer hospital stays associated with S. aureus invasive infections have necessitated the development of a vaccine to reduce morbidity and mortality attributed to this pathogen.
S. aureus capsular polysaccharides (CP) are virulence factors that can protect the pathogen from complement binding and subsequent phagocytic killing by neutrophils [15–17]. Although 13 putative capsular polysaccharides have been reported, only isolates that express capsular polysaccharide type 5 (CP5) or 8 (CP8) have been associated with disease [18]. Both polysaccharides consist of similar trisaccharide repeat units of N-acetyl mannosaminuronic acid (ManNAc), N-acetyl-l-fucosamine (l-FucNAc), and N-acetyl-d-fucosamine (d-FucNAc) [19] but differ in the linkages between the sugars and the sites of O-acetylation on the monosaccharide units [16]. Generally, S. aureus isolates contain the genetic machinery to express either CP5 or CP8, and the close association between CP type and clonal complex (CC) strongly suggests that these pathways are not subject to genetic switching to escape anti-capsular immunity [20, 21]. This is in contrast to other bacterial pathogens, which do utilize capsule switching, most likely to escape anti-capsular immunity (e.g. Streptococcus pneumoniae and Neisseria meningitidis) [22–24].
While the capsule can shield the pathogen from host immune defense mechanisms, it also presents a vulnerability to the pathogen since anti-capsular antibodies elicited by the host can opsonize the pathogen, facilitating uptake and killing by neutrophils [16]. Several highly successful vaccines to other bacterial pathogens target CP, including those against Haemophilus influenzae type b, S. pneumoniae and N. meningitidis, serogroups A, C, W, and Y [25–27]. Given these successes, S. aureus vaccine candidates based on CP have been tested clinically. To increase the immunogenicity of the CP and to induce T-cell memory, S. aureus CP5 and CP8 were conjugated to carrier proteins for use in preclinical and early clinical evaluations [28–30]. Preclinical studies with CP5 and CP8 antibodies or vaccination with CP conjugates showed some evidence of protection in animal models of S. aureus infection and that polyclonal antibodies to staphylococcal CP are opsonic for encapsulated S. aureus [28, 31–34]. Staphvax (by Nabi Biopharmaceutical), a first generation vaccine containing CP5 and CP8 coupled to detoxified recombinant Pseudomonas aeruginosa exotoxin A was the first to enter clinical efficacy phase 3 trials but failed to demonstrate efficacy in end-stage renal disease patients [35, 36]. The lack of efficacy of Staphvax raised concerns that S. aureus isolates may be deficient in CP expression, or that the patient population targeted was not competent in eliciting the appropriate protective immune responses. In addition, the vaccine didn’t consistently generate functional, bacterial killing responses in that patient population [35]. Furthermore, Scully et al. (2018) demonstrated that O-acetylation of the S. aureus capsular polysaccharide has to be maintained in the CP5 and 8 conjugates for them to induce bacterial killing antibodies [37]. Passive immunization has also been evaluated to prevent S. aureus infections. A Phase II trial of a polyclonal immune globulin (Ig) of anti-CP5 and anti-CP8 antibodies (Altastaph; by Nabi Biopharmaceutical) failed to show a significant difference in clinical outcomes among patients with complicated S. aureus bacteremia [38]. However, in another Phase II trial enrolled patients with documented S. aureus bacteremia who received Altastaph in addition to standard therapy or placebo, the vaccine induced CP antibodies were insufficient to significantly reduce S. aureus bacteremia [39]. Liu et al. (2017) attributed the inconsistency of protective efficacy of passive CP8 immunization to the release of solube CP8 by some S. aureus isolates, which can possibly interfere with antibody binding to the encapsulated bacterial surface [40–42]. Using lessons learned from previous clinical trials, the inclusion of multiple staphylococcal antigens would likely result in a more effective vaccine given the complexity of S. aureus and its myriad of virulence factors [43–45]. Thus, several next-generation multi-antigen vaccines containing CP conjugates are currently in development. The most advanced vaccine in clinical development is a 4 antigen S. aureus vaccine (SA4Ag) candidate that is being evaluated in a phase 2b/3 efficacy study [31, 46]. SA4Ag is composed of CP5 and CP8 individually conjugated to to the nontoxic mutant form of diphtheria toxin (cross-reactive material 197 [CRM197]), recombinant form of clumping factor A (rmClfA) and recombinant non-lipidated form of manganese transport protein C (MntC) [31, 47, 48].
Confirmation of antigen expression is important to support the inclusion of these antigens in a prophylactic vaccine. Due to the difficulty of studying bacterial gene expression in vivo, many studies have been performed under in vitro conditions to mimic the host environment, including nutrient limitation and low oxygen conditions [49, 50]. These studies have provided valuable information; however, the full complexity of the in vivo environment cannot be adequately mimicked. It has been reported that a significant proportion of clinical isolates do not express CP when grown in vitro [51–53]. This observation was first noted for isolates belonging to the USA300 lineage, and remained to be evaluated in other lineages [54, 55]. CP expression is highly regulated by complex genetic mechanisms and subject to in vitro and in vivo physiological signals, including those encountered in the host [56–63]. Recent data have emerged showing that a subset of S. aureus isolates that do not produce capsule in vitro, express the capsular polysaccharide when harvested from mice infected with these isolates [21, 31, 64]. To expand upon this observation, a prospective analysis of disease causing isolates collected in the US either in 2004 or 2009–10 as part of the T.E.S.T surveillance trial was performed to investigate CP genotype distribution and expression in the context of S. aureus epidemiology in the US.
Materials and methods
Ethics statement
For the experiments with blood from subjects vaccinated with Sa3Ag (3-antigen S. aureus vaccine, ClinicalTrials.gov NCT01018641), the protocol, informed consent, and all associated documents were approved by the Human Research Ethics Committees for each of the participating study sites: Site 001, Alfred Research and Ethics Unit; Site 002, Bellberry Human Research Ethics Committee; Site 003, Children's, Youth and Women's Health Services Human Research Ethics Committee; Site 004, Princess Margaret Hospital for Children Ethics Committee; Site 005, Royal Children's Hospital and Health Services District Ethics Committee. Written informed consent was obtained from all subjects prior to study entry. The clinical study was conducted in compliance with Good Clinical Practice (GCP) guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, the International Ethical Guidelines for Biomedical Research Involving Human Subjects, and the principles of the Declaration of Helsinki.
All S. aureus Isolates including those obtained from the CDC and the global collection of Tigecycline Evaluation and Surveillance Trial were not linked to their associated patient data.
All animal studies were conducted according to Pfizer local and approved global Institutional Animal Care and Use Committee (IACUC) guidelines. The animal protocol is PRL-2011-00102 and it was approved by the Pfizer, Inc., Pearl River IACUC. Pfizer, Inc., Pearl River utilizes the Guide for the Care and Use of Laboratory Animals (Guide, NRC, 2011) as the primary standard for all animals under its institutional animal care and use program and also complies with the United States Department of Agriculture’s Animal Welfare Act (AWA) and Animal Welfare Regulations “Blue Book” (USDA/APHIS, November 2013) for regulated species (this does not apply to this protocol). In all instances where Guide recommendations are different from AWA regulations for covered species, the mandated governing standard shall apply. Female, CD1 mice 8 to 12 weeks old (Charles River Laboratories) were used and were humanely euthanized 6 h post infection by slow fill inhalation of CO2 followed by cervical dislocation or thoracotomy as a confirmatory method. No adverse events occurred and animals did not experience more than momentary pain or distress.
Isolate selection
We obtained 516 S. aureus isolates that were collected as part of the global Tigecycline Evaluation and Surveillance Trial (T.E.S.T), now ATLAS (Antimicrobial Testing Leadership And Surveillance). For the purpose of conducting a longitudinal assessment of S. aureus isolates associated with disease in the USA, we prospectively selected healthcare centers across 6 US census regions (East North Central, East South Central, Middle Atlantic, South Atlantic, West North Central, West South Central) that were participating between 2004 and 2010 (five sites). Additional seven sites active at the time of our study were included. A total of 516 S. aureus clinical isolates derived from 2004 (n = 117), 2009 (n = 162) and 2010 (n = 237) were collected and analyzed. In line with T.E.S.T requirements, the participating US hospital sites collected consecutive isolates from patients with a documented infection of nosocomial or community origin. Only one isolate per patient was permitted and all body sites were considered as acceptable clinical sources. S. aureus isolates were cultured from specimens of patients aged 0 to 95 in various clinical settings, with samples broadly defined as derived from either inpatient (medicine general, medicine ICU, pediatric general, pediatric ICU, surgery general, and surgery ICU) or outpatient wards (clinic, office, emergency room and nursing home-rehab) [65]. Culture identification and data management were coordinated by a single reference laboratory (Laboratories International for Microbiology Studies, International Health Management Associates [IHMA], Schaumburg, IL). For the purpose of our study, all US isolates were grouped into HA-SA (obtained from inpatient wards) and community-associated (CA-SA, obtained from outpatient wards) infections and categorized into four main types defined as wound, invasive (organism is cultured from synovial and pleural fluids, blood or bone), respiratory or other infections.
For the in vivo expression experiments, additional isolates from a contemporary US collection were also tested. These isolates were obtained from the Centers for Disease Control and Prevention (CDC) from a reference collection of 1,984 MRSA isolates collected in 2005–2006 by the Active Bacterial Core surveillance system [66]. The clinical and epidemiologic characteristics of the these isolates are described in Murphy et al. [67].
Construction of a PFESA0119 Δcap5HIJK ermC strain
All PCRs were performed with iProof High-Fidelity DNA polymerase (Bio-Rad, Hercules, CA), and all restriction endonucleases were purchased from New England Biolabs (Ipswich, MA). A Δcap5HIJK ermC knockout cassette, consisting of two ~1.1 kb fragments (cap-1 and cap-2) flanking the erythromycin resistance gene, ermC, was constructed. The primer sets (with relevant restriction sites in bold font) and DNA templates used to amplify each of the fragments were: (i) cap-1: oLH291 (GCT GCT CCC GGG GAC AAT AGT TGG TAC AAG GCC)/oLH277 (GTA GCA TGT CTC ATT CAA TTG CTA TCC TCA TCG TCA TTT CC), S. aureus strain Newman genomic DNA; (ii) ermC: oLH278 (GGA AAT GAC GAT GAG GAT AGC AAT TGA ATG AGA CAT GCT AC)/oLH279 (CGA CGT CCT TTT TAT TAA TGA AAA CTG GTT TAA GCC GAC), pE194; (iii) cap-2: oLH280 (GTC GGC TTA AAC CAG TTT TCA TTA ATA AAA AGG ACG TCG)/oLH281 (GCT GCT GTC GAC TCT CGT GCA ATT CCT TAC TCG), S. aureus strain Newman genomic DNA. These three products were spliced together by amplification with oLH291 and oLH281, and the knockout cassette was cloned into pSPT181 [68] at the XmaI and SalI sites. After electroporation and chromosomal integration into S. aureus strain RN4220, the final Δcap5HIJK ermC mutant strain was obtained after plasmid excision by secondary recombination. This mutation was transduced into S. aureus isolate PFESA0119 essentially as described elsewhere [69]. In the resulting erythromycin resistant transductants, deletion of cap5HIJK was confirmed by PCR. Mutants were shown to be of the expected strain lineage with a Qualicon RiboPrinter (DuPont, Wilmington, DE).
Whole genome sequencing of S. aureus isolates
Isolated colonies of pure cultures of S. aureus were cultured in TSB in 2 mL 96-well blocks. Genomic DNA was isolated using the Qiagen QIAcube system according to manufacturer’s protocol. Tagged DNA libraries were created using a method adapted from a standard Illumina Indexing protocol [70]. Whole genome sequencing was performed on the Illumina HiSeq 2000 platform with 100 bp paired-end reads. The Illumina sequence data were submitted to the European Nucleotide Archive (ENA) under accession numbers ERS234133- ERS432212 (S1 Table).
Whole genome sequence data analysis
Annotated assemblies were produced as previously described [71]. Briefly, de novo assembly of whole genome sequences was performed using Velvet v1.2 [72] with Velvet Optimiser v2.2.5 (http://www.vicbioinformatics.com/software.velvetoptimiser.shtml)). Contigs were scaffolded with SSPACE [73] and sequence gaps closed using GapFiller [74]. The draft assemblies have been submitted to the ENA with accession numbers (ERS234133- ERS432212) provided in S1 Table. The assembled contigs were annotated using Prokka v1.11 [75] and S. aureus specific database from RefSeq [76]. In silico PCR was used to determine the SCCmec type amongst mecA positive MRSA isolates [77, 78].
Isolate typing
S. aureus isolates were characterized by MLST [79] and spa typing (based on DNA sequence analysis of the S. aureus-specific staphylococcal protein A (spa) gene) [80]. For the MLST, the allelic profiles were extracted from the assembled genomes using an in-house developed pipeline, MLST check (https://github.com/sanger-pathogens/mlst_check). Novel allelic profiles and novel allele sequences were submitted to MSLT database curator (http://saureus.beta.mlst.net) and new STs were assigned. For the spa typing, the spa gene was extracted by an in silico PCR using previously described primers (http://www.ridom.de/doc/Ridom_spa_sequencing.pdf) and the spa types were assigned using the spaTyper application (http://spatyper.fortinbras.us). Presence of genes of interest (mecA, pvl, cap operon) was analyzed using the Short Read Sequence Typer 2 [81]. The capsular polysaccharide type was determined based on the presence of the type-specific capHIJK genes. In silico PCR was used to determine the SCCmec type amongst mecA positive MRSA isolates [77, 78].
Opsonophagocytic assays (OPA)
OPA were performed as previously described [16]. OPA titers of sera from ten subjects vaccinated with Sa3Ag (pre-vaccination bleed and 29 days post-vaccination bleed) [82] were determined against CP5 and CP8 isolates representing the four most prevalent clonal complexes. OPA titers are defined as the reciprocal of the highest serum dilution that kills 50% of the bacteria in the test. Each sample was tested in replicate and geometric mean titers with 95% confidence interval were calculated using data from the ten subjects and from two independent experiments. Negative samples were arbitrarily assigned a titer of 50, which is half of the lowest serum dilution tested in the OPA. The specificity of the OPA was determined by preabsorbing immune serum with homologous capsular polysaccharides CP5 (140 μg/mL) or CP8 (320 μg/mL). After overnight incubation at 4°C, anti-CP antibody depleted sera were used in the OPA described above. Due to limited availability of serum volume, the PFESA2069 isolate was tested with depleted sera from 7 out of 10 vaccinated subjects.
Detection of capsule production in vitro by Luminex-based assays
As not all 516 isolates were tested in this assay, the proportion that may not express capsule in vitro was estimated by calculating the % CP-negative isolates within each spa/CC group tested and then extrapolating to the % of the total population (n = 516) based on the distribution of each given CC. For example, 47 isolates of CC8 (37% of the total S. aureus population) were tested for in vitro capsule expression and since 41 CC8 isolates (87% of CC8) were CP-negative, the % CP-negative CC8 isolates was estimated to be 32% of the total population. The Luminex assays used to detect capsule expression in vitro are based on several steps including polysaccharide sample preparation, generation of monoclonal antibodies, coupling of CP5 and CP8 antigens to Luminex MagPlex microspheres, and analysis of samples by singleplex liquid array system, Luminex 200.
Polysaccharide sample preparation
S. aureus isolates were grown overnight on Columbia salt agar (CSA) plates and isolated colonies were then resuspended in Dulbecco’s PBS (DPBS) buffer without Ca2+/Mg2+ to one OD600. The cell suspension was then autoclaved at 121°C for 30 min as previously described [83]. Cells were pelleted by centrifugation (3,000 x g, 15 min) and supernatants were used in the Luminex assays.
Monoclonal antibodies (mAbs)
CP5 and CP8 specific mAbs were generated by immunizing mice (Swiss Webster, BALB/c, or SJL) with polysaccharides conjugated to the CRM197 carrier protein. These polysaccharides were produced according to the methods as described in Fattom et al. [84]. The CP were conjugated to cross-reacting CRM197 (cross-reacting material 197), a nontoxic recombinant mutant of diphtheria toxin, in a conjugation process using 1,1′-carbonyldiimidazole/1,1′-carbonyl-di-(1,2,4-triazole) (described in US patent US 8568735 B2). Splenocytes were fused with the non-secreting myeloma cell line X63Ag8.653 to generate hybridomas that were screened for the appropriate serotype specificity. The CP5-specific mAb CP5-5-1 is of the IgG1 subclass and the CP8-specific mAb CP8-119-11 is of the IgG2b subclass. Both mAbs were purified on Protein A column. The mAbs were conjugated with phycoerythrin (PE) and purified on Protein A column by Chromaprobe, Inc. (Maryland Hts., MO).
Coupling of CP5 and CP8 to Luminex MagPlex microspheres
CP5 and CP8 conjugated to poly-lysine (CP5-pLL and CP8-pLL) were passively coated individually onto unique Luminex MagPlex carboxylated microspheres. CP5-pLL (50 ng/mL final) or CP8-pLL (12.5 ng/mL final) were added to a 1 mL bead suspension (1.25 x 107 microspheres) in DPBS and incubated for 2.5 h at room temperature with mixing. After unbound antigen was washed, the microspheres were blocked and stored in DPBS containing 1% BSA and 0.05% NaN3.
Analysis of samples by singleplex liquid array system, Luminex 200
CP5 and CP8 detection assays were performed individually using an antigen competitive assay format. CP5 or CP8 coated microspheres in DPBS containing 0.05% Tween-20 and 0.02% NaN3 were added to wells (2,500 beads/well) of the assay plate (Costar 3912). CP5 or CP8 reference standards serially diluted 2.5-fold in DPBS containing 0.05% Tween-20 and 0.02% NaN3 were added in triplicate to wells to create a 12-point titration standard curve. The concentration range of the reference standards was 0.06–1500 ng/mL and 0.01–250 ng/mL for CP5 and CP8 respectively. A volume of 50 μL of unknown samples was tested in duplicate. Finally, 50 μL of mAb CP5-5-1-PE (600 ng/mL) or mAb CP8-119-11-PE (105 ng/mL) in DPBS containing 0.05% Tween-20 and 0.02% NaN3 were added to each well of the assay plate. The plate was covered and incubated with shaking at 300 rpm at room temperature for 60 min. The plates were then washed three times with DPBS containing 0.05% Tween-20 and 0.02% NaN3. After the final wash, 100 μL of DPBS containing 0.05% Tween-20 and 0.02% NaN3 was added to each well and the plates were read at high reporter setting using a Bio-Plex reader (Bio-Plex 200 systems; Bio-Rad Laboratories, Hercules, CA). The quantity of CP5 or CP8 polysaccharide in test samples was derived from the CP5 or CP8 reference standard curves fitted using 4-Parameter Logistic (4PL) nonlinear regression analysis.
Bioinformatics analysis of the cap operon and SNP calling
Single nucleotide polymorphisms (SNPs) in the cap operon were detected by mapping the paired-end reads against cap operons of selected reference isolates of S. aureus, using SMALT version 0.7.4. (https://www.sanger.ac.uk/resources/software/smalt/). Different reference sequences were used for CP5 and CP8 isolates. Furthermore, for each CP type two different reference genomes were used to verify identified polymorphisms and only the consensus SNPs were further analyzed (those found by mapping to both reference genomes of the same CP type). The CP5 isolates were mapped against the cap operon from the ST22 HO 5096 0412 isolate (GenBank accession HE681097) [85] and the SNPs were verified by mapping against the ST5 N315 isolate (GenBank accession BA000018). The CP8 isolates were mapped against the cap operon of the ST1 MSSA476 isolate (GenBank accession BX571857) and the SNPs were verified by mapping against the M013 isolate (ST59, accession number NC_016928). In silico PCR extraction of individual genes for all analyzed isolates was used to confirm the mapping results and analyses listed above.
To determine the distribution of all identified cap polymorphisms in the context of S. aureus phylogeny, evolutionary relationships between all isolates were reconstructed. Whole genome SNPs were detected by mapping the paired-end reads against the S. aureus N315 reference genome using SMALT. The generated whole genome sequence alignment was curated to exclude accessory regions (mobile genetic elements identified from the N315 reference genome). Based on the core genome SNP alignment an approximately-maximum-likelihood phylogenetic tree was constructed with FastTree software using generalized time reversible (GTR) model with GAMMA method of correction for among site rate variation. The phylogenetic tree was annotated with the distribution of identified SNPs and indels (S3 Fig).
Detection of capsule production in vivo
Immunofluorescence microscopy assay (IFA) for the detection of surface polysaccharides
IFA was used to detect in vivo expression in S. aureus isolates. S. aureus isolation from infected animals and immunofluorescence staining were performed as previously described [64]. The accuracy of IFA in confirming capsule expression was first demonstrated using three different S. aureus strains; wild type Reynolds (CP5), its isogenic CP5-negative mutant, and the CP8-expressing isogenic Reynolds derivative in which cap5H-cap5K was replaced with cap8H-cap8K of the cap8 gene cluster [86]. The strains were tested in a blinded fashion with two independent experiments per strain. Bacteremia (intraperitoneal challenge with 7×107 to 2×108 CFU S. aureus per animal) and wound infection (a 1-cm incision is made in the right thigh muscle of mice then closed with a suture, and 5 μL of an S. aureus suspension (5 × 107 CFU) was introduced into the muscle incision under the suture) models were used. Female, CD1 mice 8 to 12 weeks old (Charles River Laboratories) were used in the in vivo experiments Duplicate samples were tested, one with CP5 specific antibody and the other with CP8 specific antibody. After specificity of the CP5 and CP8 antibodies had been confirmed with the S. aureus isogenic derivatives of strain Reynolds, strains from the T.E.S.T collection were tested in duplicate using the same IFA procedure.
Genetic comparison of challenge inocula with the bacteria recovered from infected animals
Mice (2 mice per bacterial strain) were challenged with one of three USA300 strains (PFESA0119, PFESA0029 and PFESASA0021) or Reynolds. Blood samples were collected from animals at two time points; T0 (pre-challenge) and 6 h after infection. Challenge stocks used to inoculate mice as well as blood samples were plated on Tryptic Soy Agar plates with 5% sheep blood. No colonies were recovered from the pre-challenge blood samples. Five colonies from the respective bacterial inocula and 10 colonies recovered from mice challenged with Reynolds strain (5 colonies per animal) and 20 colonies from mice challenged with each of the USA300 strains (10 colonies per strain per animal) were subjected to whole genome sequencing for comparative analysis of genotypic features and cap operon analysis.
Detection of capsule transcripts in vivo by qRT-PCR
RNA expression of two cap operon genes (cap5D, cap5E) and regulators (mgrA, sarA, agrA, RNA III and ccpA) was evaluated in animals infected with S. aureus isolates. Determination of 16S rRNA expression was included to normalize RNA expression levels of target genes. The isolates tested represented lineages that had a mixture of CP-expression positive and negative in vitro phenotypes; Reynolds, CC8-USA300 (PFESA0029, PFESA0119 and PFESA0021) and USA500 (PFESA0065) strains. Approximately, 4x108 CFU of mid-log phase bacterial cultures grown in TSB were used to infect 10–12 week old CD1 mice intraperitoneally (IP). T0 represents the bacterial challenge sample. Bacteria were harvested from ~ 1.5–2 mL of blood at 1 and 4 hr post challenge (2 mice per time point per isolate) and mixed with 2 volumes of RNAProtect Bacteria Reagent (Qiagen, Valencia, CA); the samples were processed immediately for RNA extraction where samples were mixed thoroughly by vortexing and incubated at room temperature for 10–30 min. Mixtures were centrifuged at 5,000 x g for 10 min and supernatants removed. Pellets were suspended in 500 uL of RNA Protect and homogenized with an Omni tissue homogenizer (TH) (Omni International, GA) assembled with disposable Soft Tissue Omni Tip plastic homogenizing probes. Homogenates were centrifuged at 5,000 x g for 10 min and supernatants removed. Pellets were suspended in 1 mL of RLT buffer/β-mercaptoethanol buffer (QIAgen RNeasy kit), homogenized and centrifuged as above. Pellets were suspended in 110 μL of 2X TE buffer and transferred to 2 mL tubes harboring 50mg of glass beads (Sigma). A volume of 385 μL of RLT/β-mercaptoethanol buffer were used to rinse the 15 mL tubes and combined with the 110 uL 2x TE. Bacterial lysates were generated by beating the tubes at 1/30 s for 10 min in a Tissue Lyser (Qiagen) and cleared by centrifugation at 14,000 rpm for 2 min. A volume of 450 μL of lysate were mixed with 250 μL100% ethanol and applied to an RNeasy column (QIAgen). RNA was purified according to the QIAgen RNeasy Kit instructions with on-column DNase treatment to remove genomic DNA. RNA was eluted twice in 40 μL RNase-free water, quantified on a Nanodrop and stored -80°C. RNA integrity was evaluated by Bioanalyzer 2100 (Agilent, Santa Clara, CA).
For in vivo transcript profiling, 10 μL of RNA was reverse transcribed using SuperScript Vilo Master Mix (Invitrogen, Carlsbad, CA) and the resulting cDNA was diluted 5 fold with RNase-free water. Real-Time PCR reactions were assembled by adding 4 μL of diluted cDNA, 400 nM forward and reverse primers, 200 nM fluorescent-labeled probe (Applied Biosystems, Foster City, CA) and 5 μL of Taqman Fast Advanced Master Mix (Applied Biosystems) in a total volume of 10 μL. Reactions were performed using the 7900HT Fast Real-Time PCR System (Applied Biosystems) under the following conditions: 30 s at 95°C and 40 cycles of 3 s at 95°C and 30 s at 60°C. In some instances, transcripts were measured in one-step real-time PCR reactions assembled with 4 μL of RNA, 400 nM forward and Reverse primer, 200 nM Fluorescently labeled probe, 9.5 μL of EXPRESS One-Step qRT-PCR SuperMix (Applied Biosystems) in a total volume of 15 μL. The AB 7900HT cycling conditions consisted of 15 min at 55°C for cDNA synthesis, 2 min at 95°C and 40 cycles of 15 s at 95°C and then followed by 1 min at 60°C. For each transcript, the number of copies was determined from standard curves generated using 101 to 107 copies of plasmid harboring the corresponding gene. Normalized expression of transcripts was then expressed as copies per 107 copies of 16S rRNA.
Competitive Luminex immunoassay (cLIA)
cLIA was used to measure the level of serum antibody titers against CP5 and CP8 antigens in animals challenged with USA300 isolates (PFESA0029 [CDC3], PFESA0119 and PFESA0119 Δcap5HIJK ermC). CD1 mice (7–9 weeks of age, 10 per group) were challenged with three intraperitoneal injections of ~ 2x106 CFU/animal in 0.5 mL at 0, 6 and 14 weeks and bled before vaccination and two weeks after the final inoculation. CP5 antibody responses were measured using a CP5 specific cLIA assay. CP5 and CP8 antigens were coupled to a distinct fluorescent Luminex microsphere. Antigen-coated microspheres were incubated overnight at 4°C with appropriately diluted serum samples, controls, or reference standard serum. Antigen-specific functional mouse mAbs were then added to the microsphere/serum mixture and bound mAbs were detected with R-Phycoerythrin (PE) labeled rat anti-mouse IgG1 secondary antibody (Southern Biotech) using a BioPlex reader (BioRad) to measure fluorescence. In this competitive assay, the magnitude of the fluorescent PE signal is inversely proportional to the amount of antigen-specific antibody in the sample.
CP8 immune responses were also measured in animals challenged with CP8 S. aureus isolates including five CC12 isolates (PFESA1194, PFESA1305, PFESA1405, PFESA1502, PFESA2455) and two CC188 isolates (PFESA2058 and PFESA2089). CD1 mice (7–9 weeks of age, 10 per group) were administered with three intraperitoneal injections of ~ 2x106 CFU/animal in 0.5 mL at 0, 4 and 12 weeks and bled at week 27. CP8 antibody responses were measured using a CP8 specific cLIA assay as described above for CP5 cLIA assay.
Statistical analyses
The categorical variables in the molecular epidemiology analyses were compared using either the χ2 or likehood ratio test. A P value of <0.05 was considered significant. The cLIA serology data were described with geometric mean titer (GMT) and corresponding 95% confidence intervals (CIs). The CIs were constructed by back transformation of the confidence limits computed for the mean of the logarithmically transformed assay data based on Student t distribution. P-values based on 2-sample t-test or Wilcoxon test were presented to identify potential differences in immunoglobulin concentration.
Results
Patient demographics
The demographics of the patients with S. aureus infections in 2004 and 2009–10 are shown in Table 1. The differences in % males (P = 0.462) and median age (P = 0.717) were not significant among patients from the two time periods.
Table 1. Patient demographics and characteristics of S. aureus isolates.
| 2004 | 2009–10b | P-Valuec | |||
|---|---|---|---|---|---|
| Ward source | Inpatient (n = 80) |
Outpatient (n = 37) |
Inpatient (n = 294) |
Outpatient (n = 95) |
|
|
Patient characteristics | |||||
| Male, % | 59 | 59 | 56 | 53 | 0.4622 |
| Median age, yrs | 50 | 50 | 53 | 53 | 0.7168 |
|
Infection Characteristics | |||||
| MRSA, n (%) | 56 (70) | 30 (81.1) | 126 (42.9) | 48 (50.5) | <0.001 |
| Infection type, n | 0.1137 | ||||
| Wound | 25 | 18 | 65 | 39 | |
| Respiratory | 20 | 1 | 103 | 1 | |
| Invasivea | 21 | 2 | 66 | 14 | |
| Other | 14 | 16 | 60 | 41 | |
| CP genotypes | 0.2987 | ||||
| CP5, % | 71 | 86 | 70 | 73 | |
| CP8, % | 29 | 14 | 30 | 27 | |
a Invasive infections include blood, pleural and synovial body fluids and bone infections.
b Ten isolates collected in 2009–10 had an unknown ward source.
c P values compare variables between the two time periods.
Clinical characteristics and sources of isolates
In total, 516 S. aureus isolates, collected through the T.E.S.T program from 12 hospitals in 6 US census regions in 2004 (n = 117) and 2009–10 (n = 399), were analyzed. A list of the 516 isolates is shown in S1 Table. No ward information was available for 10 isolates collected in 2009–10. The epidemiological characteristics of the isolates are described in Table 1. The proportion of MRSA infections among all S. aureus infections in inpatient and outpatient wards combined was significantly higher in 2004 (74%, 86/117) compared to 2009–10 (45%, 179/399) (P < 0.001). The majority of S. aureus isolates evaluated were obtained from healthcare-associated S. aureus (HA-SA) infections (72%, 374/516) and approximately 25% of these isolates were derived from invasive infections.
Population structure of S. aureus isolates
Clonal complexes
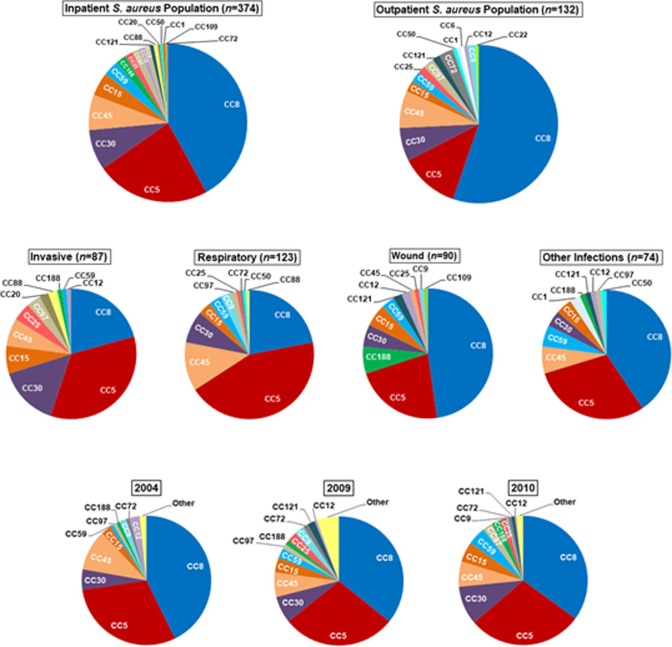
The S. aureus population in the US, over the time period surveyed, included 20 clonal complexes (CC) comprised of 69 sequence types (ST, S2 Table). Overall, 13 CC represented 98% of disease causing isolates (Fig 1). Four of these CC represented >80% of isolates (Fig 1): CC8 (37%), CC5 (29%), CC30 (8%) and CC45 (7%). No clear distinction was observed between disease-causing CC in the healthcare setting compared to the community; four major lineages caused disease in both settings including CC5, 8, 30 and 45 (Fig 1A). There was a significant association between the isolate genotype (CC) and the type of clinical infection in inpatient wards (P = 0.036). For example, CC5 isolates were largely implicated in HA-SA invasive and respiratory infections, whereas CC8 isolates accounted for the majority of HA-SA wound infections (Fig 1B). The distribution of S. aureus isolates collected from inpatient wards at different census regions was reflective of the total S. aureus population with high prevalence of CC5 and CC8 isolates (S1 Fig).
Fig 1. Clonal complex (CC) distribution among disease-causing S. aureus isolates in the US.
(A) All isolates associated with S. aureus infections in healthcare and community settings. (B) S. aureus isolates associated with the main types of clinical infections in healthcare settings. (C). Temporal variation in the distribution of CC amongst S. aureus isolates.
The distribution of clonal complexes among disease isolates collected during 2004 (n = 117), 2009 (n = 162) and 2010 (n = 237) is illustrated in Fig 1C. In 2004, 13 CC were associated with disease, whereas 20 CC were associated with disease in 2009–10. For all time periods, CC8 and CC5 constituted the two most prevalent lineages followed by CC30 and CC45. While there was a trend in emergence of CC59 over time, the variation in the distribution of CC among 2004, 2009 and 2010 isolates was not statistically significant (P = 0.138).
spa type distribution
A total of 124 spa types were identified in 97% (n = 501 of 516) of isolates where a spa type was defined (S2 Table). The distribution of prevalent spa types across the population in association with different infections reflected the CC distribution (S2 Fig). The most common spa types t008 (28%, 146/516) and t002 (20%, 105/516) were associated with the two dominant CC in the population, CC8 and CC5, respectively. CC30 was mostly associated with spa type t012, while CC45 had greater spa type diversity compared to other prominent CC where a single spa type dominated each lineage.
CC8-USA300 lineage
USA300 isolates belong to CC8, contain the SCCmec type IV element, and carry signature toxin genes such as Panton-Valentine Leukocidin (PVL) [87, 88]. The molecular epidemiology of CC8-USA300 isolates analyzed here was described previously [89]. Briefly, of the 191 CC8 isolates in the S. aureus population, 154 isolates (81%, 154/191) were typed as the USA300 clone. The majority of USA300 isolates (86%, 132/154) were genotyped as spa t008. The majority of these isolates (84%, 129/154) were MRSA. Most infections caused by USA300 isolates were associated with skin and wound infections (41%, 63/154). A relatively small number of USA300 isolates were obtained from invasive infections (7.8%, 12/154). The CC8 lineage also contained four representatives of the USA500 clone and six additional isolates closely related to USA500 but descending from a distinct node (USA500-like) [89]. All USA500 and USA500-like isolates belonged to spa type t064.
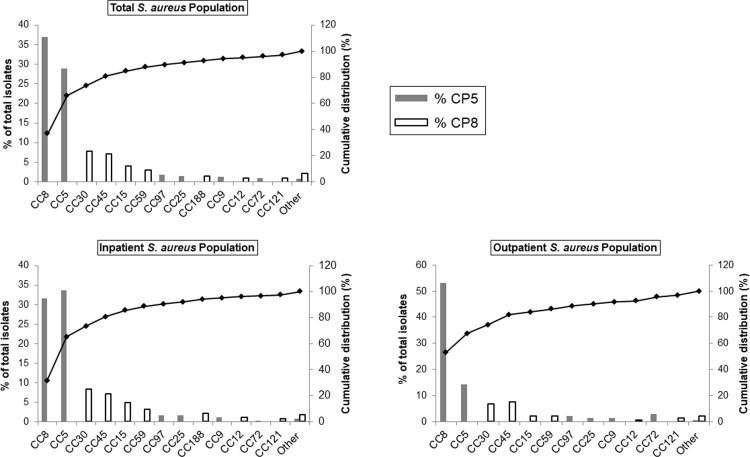
Capsular polysaccharide genotype distribution
Genotyping revealed that all study isolates possessed either cap5 or cap8 specific genes to direct the biosynthesis of CP5 or CP8, respectively (72% CP5 and 28% CP8). As previously reported [20], capsule genotypes were highly correlated with isolate lineage (Fig 2). Most MRSA isolates were CP5 (96%, 254/265), whereas MSSA isolates had a nearly equal distribution between CP5 and CP8 (S3 Fig). A CP5 genotype was significantly more prevalent among isolates collected in either time period from both inpatient and outpatient wards (P < 0.001) (Table 1 and Fig 2). The prevalence of CP5 isolates was higher among invasive isolates (67%, 70/105). The same trend was observed for other infection types where CP5 was the dominant genotype amongst clinical isolates. No association was observed between CP genotype distribution and either the type of infection (P = 0.724) or the ward source (P = 0.208).
Fig 2. Distribution of clonal complexes (CC) and capsular polysaccharide (CP) genotypes by ward source.
Opsonophagocytic killing of diverse clinical S. aureus isolates with immune sera
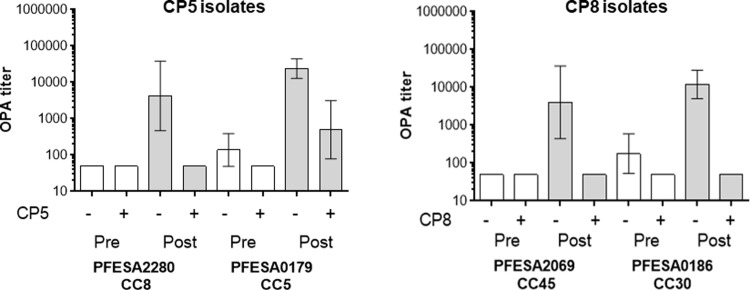
Clinical sera from human subjects immunized with an investigational vaccine (SA3Ag) containing CP5- and CP8-CRM197 conjugates [82] were evaluated for the ability to kill encapsulated S. aureus isolates in opsonophagocytic assays (OPA). OPA analyses of representative isolates from the four prominent CC (5, 8, 30 and 45), were conducted to evaluate the breadth of coverage of this investigational vaccine. An OPA titer was defined as the reciprocal of the sample serum dilution required to kill 50% of the bacteria in the test. Geometric mean OPA titers and 95% confidence intervals (CI) were calculated for samples (n = 10) tested in two independent experiments (Fig 3). OPA titers in sera from SA3Ag immunized subjects increased substantially compared to preimmune sera and for all isolates tested (Fig 3). Preabsorption of test sera with homologous capsule blocked the majority of vaccine elicited opsonic killing, illustrating the functional activity of antibody directed to the CP antigens.
Fig 3. Killing of diverse S. aureus clinical isolates by human immune sera in CP5/CP8 OPA assays.
Opsonophagocytic activity of clinical sera from ten vaccinated subjects (Pre: prevaccination bleed and Post: 29 days post vaccination bleed) were determined against CP5 and CP8 isolates representing the four most prevalent clonal complexes. Bars reflect geometric mean OPA titers from sera of ten immunized subjects tested in two separate experiments with 95% confidence intervals (error bars). CP specificity of the OPAs was demonstrated with anti-CP antibody depleted sera (CP5/8 +) generated as described in the Material and Methods section.
Detection of capsule expression in vitro by Luminex based assays
OPA demonstrated that antibodies elicited to the CP conjugate antigens contained in SA3Ag were able to kill diverse S. aureus clinical isolates. Since OPA are complex functional assays that are time consuming to develop for a large number of strains, CP5/8 detection assays were developed using a competitive antigen binding assay format to evaluate capsule expression in vitro for a larger panel of S. aureus clinical isolates. Initially, five randomly selected representatives from each of the 13 prominent CC among US disease causing isolates were tested. To account for the spa diversity within a given CC, additional isolates within each lineage were also evaluated (Table 2). All CP5 isolates tested were negative in the CP8 assay and vice versa. Among the representative CC 9, 15, 25, 30, 45, 59, 72 and 121 isolates tested, all expressed capsule in vitro (Table 2). However, CC5, 8, 12, 97 and 188 contained some isolates for which capsule production in vitro was below detectable levels (approximately 35% of the total isolates tested) (Table 2).
Table 2. In vitro and in vivo capsule expression by S. aureus isolates from major disease-causing clonal complexes (CC) and associated prevalent spa types.
| CC (n) |
CP genotype | spa types testeda (n) | CP in vitro phenotype (n) | cap operon mutation typeb (n) | CP in vivo phenotypec (n) |
|---|---|---|---|---|---|
| CC8 (191) | CP5 | t008d (146) | POS (2) | No (1) | NT |
| 1 (1) | POS (1) | ||||
| NEG (21) | 1, 2, 4 (2) | POS (1) | |||
| 1, 2, 3, 4 (19) | POS (13) | ||||
| NEG (5) | |||||
| t064d (12) | POS (3) | No (1) | NT | ||
| 1 (2) | POS (2) | ||||
| NEG (14) | 1, 2 (14) | POS (2) | |||
| NEG (1) | |||||
| t024d (9) | NEG (3) | 1, 2, 4 (1) | NT | ||
| 1,2,3,4 (2) | POS (1) | ||||
| t121 (3) | NEG (2) | 1, 2, 4 (1) | NT | ||
| 1,2,3,4 (1) | |||||
| t681 (3) | NEG (1) | 1,2,3,4 (1) | NT | ||
| t334 (2) | POS (1) | No | NT | ||
| CC5 (149) | CP5 | t002 (105) | POS (4) | No | NT |
| t045 (8) | POS (1) | No | NT | ||
| t062 (8) | POS (1) | No | NT | ||
| NEG (1) | 5 (1) | ||||
| t179 (3) | POS (2) | No | NT | ||
| t306 (3) | POS (2) | No | NT | ||
| t668 (1) | POS (1) | No | NT | ||
| CC30 (40) | CP8 | t012 (13) | POS (1) | No | NT |
| t018 (4) | POS (1) | ||||
| t021 (4) | POS (1) | ||||
| t338 (4) | POS (2) | ||||
| t2387 (1) | POS (1) | ||||
| CC45 (37) | CP8 | t553 (4) | POS (2) | No | NT |
| t644 (4) | POS (2) | ||||
| t004 (3) | POS (2) | ||||
| t050 (2) | POS (1) | ||||
| t671 (2) | POS (1) | ||||
| t026 (1) | POS (1) | ||||
| t1964 (1) | POS (1) | ||||
| t2444 (1) | POS (1) | ||||
| CC15 (21) | CP8 | t084 (6) | POS (2) | No | NT |
| t346 (3) | POS (2) | ||||
| t605 (1) | POS (1) | ||||
| t385 (1) | POS (1) | ||||
| t094 (1) | POS (1) | ||||
|
CC59 (16) |
CP8 |
t216 (13) | POS (2) | No | NT |
| t437 (1) | POS (1) | ||||
| t3736 (1) | POS (1) | ||||
| t8419 (1) | POS (1) | ||||
|
CC97 (9) |
CP5 | t267 (4) | POS (1) | No | POS (1) |
| t2802 (1) | POS (1) | No | NT | ||
| t2297 (1) | POS (1) | No | NT | ||
| t3380 (1) | POS (1) | No | NT | ||
| t359 (1) | NEG (1) | Yese | NEG (1) | ||
|
CC25 (8) |
CP5 | t078 (4) | POS (1) | No | NT |
| t7084 (1) | POS (1) | ||||
| t258(1) | POS (1) | ||||
| t081 (1) | POS (1) | ||||
| t1315 (1) | POS (1) | ||||
| CC188 (8) | CP8 | t189 (7) | POS (4) | No | NT |
| NEG (1) | 8 (1) | POS (1) | |||
|
CC9 (7) |
CP5 | t209 (5) | POS (4) | No | NT |
| t193 (1) | POS (1) | ||||
|
CC12 (5) |
CP8 | t160 (2) | NEG (2) | 6,7 (2) | POS (1) |
| NEG (1) | |||||
| t156 (1) | POS (1) | No | POS (1) | ||
| t5318 (1) | NEG (1) | No | POS (1) | ||
| t771 (1) | NEG (1) | 6,7 (1) | POS (1) | ||
| CC121 (5) | CP8 | NA (5) | POS (5) | No | NT |
|
CC72 (5) |
CP5 | t148 (2) | POS (2) | No | NT |
| t1346 (1) | POS (1) | ||||
| t3169 (1) | POS (1) | ||||
| t1991 (1) | POS (1) |
a Representative isolates spanning the spa diversity within each lineage were tested for CP expression.
b The type of mutations in cap5/8 operons are described in Table 3.
c Results are only shown for isolates randomly selected and tested for in vivo capsule expression and NT refers to the isolates that were not tested for in vivo capsule expression.
d Additional isolates associated with these spa genotypes from contemporary collections were tested for capsule expression.
e The single CC97 isolate belonging to spa type t359 had a 1448 nucleotide deletion in cap5D-E.
Genetic analysis of the cap5/8 biosynthetic operon
To investigate the potential genetic basis for the lack of in vitro capsule expression in some isolates, whole genome sequencing data corresponding to the cap biosynthetic operon was analyzed for each of the 516 S. aureus isolates. Nucleotide polymorphisms were identified relative to the S. aureus reference isolates HO 5096 0412 (ST22) for CP5 isolates and MSSA476 (ST1) for CP8 isolates. Substantial single nucleotide polymorphism (SNP) diversity was detected within both CP5 and CP8 operons (S4 Fig). A small number of insertion/deletion (indel) mutations were also observed (S3 Fig). Most of the SNPs were detected in isolates that expressed capsule in vitro, suggesting that they do not negatively impact capsule production in vitro. For the SNP analysis of CC8 isolates, we focused on previously identified mutations that were linked to the CP-negative phenotype; whereas for the other CC, we screened the entire cap operon to correlate the occurrence of SNPs and the observed CP-negative phenotype. When compared with the respective reference isolates, a total of 8 different mutation types were identified among the isolates that lacked detectable capsule expression in vitro (Table 3). For CC8 isolates, four different mutations in the cap5 operon were identified and in vitro negative CP phenotypes were associated with isolates that carried combinations of two or more of these mutations. These mutations have been previously described [55]. The cap5 promoter had a transition mutation (T→C) positioned 73 nucleotides upstream of the ATG translation start codon of cap5A and was identified in 186 of 191 (97%) CC8 isolates. CC8 isolates with just this single mutation in the cap5 promoter did express detectable CP5 in vitro as shown in Table 2. The majority of CC8 isolates (97%, 185/191) carried a single nucleotide insertion in cap5D (poly A heptamer occurring after nucleotide position 992) resulting in a translation frameshift and the introduction of a premature stop codon. This mutation was absent in the CC8 isolates where capsule was detected when cultured in vitro. The G→T transversion mutation at nucleotide position 223 of cap5E resulted in the non-synonymous substitution, Asp75Tyr, and was detected in all USA300 isolates (81% of the total CC8 isolates, 154/191), but not in other CC8 isolates. A T→C transition mutation at nucleotide position 478 in cap5G was detected in 89% (170/191) of CC8 isolates (including spa types t008, t024, t121 and t681) and resulted in the non-synonymous substitution, Phe160Leu. USA500 and USA500-like isolates (spa type t064) harbored the cap5 promoter and the cap5D frameshift mutations, but the cap5E and cap5G mutations were not detected in these sub-lineages of S. aureus CC8 isolates.
Table 3. Summary of cap operon SNPs and indels detected in the study isolates typed as belonging to CC5, 8, 12 and 188.
| Mutation type | CC | Gene | Nucleotide position in reference gene | Nucleotide in referencea | Nucleotide in study isolates (n/total) | aa in ref.a | aa in study isolates | Codon position | Percent of isolates |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CC8 | cap5Ab | -73 | T | C (186/191) |
not applicable | not applicable | not applicable | 97% |
| 2 | CC8 | cap5Db | 992 | A | AA (185/191) | Lys | STOPd | 338 | 97% |
| 3 | CC8 | cap5Eb | 223 | G | T (154/191) |
Asp | Tyr | 75 | 81% |
| 4 | CC8 | cap5Gb | 478 | T | C (170/191) |
Phe | Leu | 160 | 89% |
| 5 | CC5 | cap5Ec | 340 | C | T (1/149) |
Gln | STOPd | 114 | 1% |
| 6 | CC12 | cap8Dc | 671 | G | del (3/5) |
Leu | STOPd | 236 | 60% |
| 7 | CC12 | cap8Ec | 683 | G | T (3/5) |
Ser | Ile | 156 | 60% |
| 8 | CC188 | cap8Oc | 690 | G | T (1/8) |
Gly | Val | 251 | 13% |
a The reference isolates used for the genetic analysis of the cap loci were HO 5096 0412 for CP5 isolates and MSSA476 for CP8 isolates.
b Previously described mutations in the cap5 operon [55].
c Novel mutations in cap5/8 operons identified this study in association with in vitro CP-negative phenotypes.
d The mutation results in truncated open reading frame.
The presence of capsule operon mutations was not restricted to the CC8 lineage of S. aureus isolates. In a single CC5-t062 isolate with a CP-negative in vitro phenotype, a premature stop codon was detected in cap5E (C→T resulting in a nonsense mutation). A different CC5 isolate of the same spa type that lacked this mutation was CP-positive in vitro. A similar genotype to phenotype correlation was observed for the CC188-t189 isolates. One isolate that did not express capsule in vitro had a unique SNP in cap8O resulting in a Gly251Val substitution. This SNP was not detected in four CC188-t189 isolates with a positive CP in vitro phenotype. Four of the five CC12 isolates did not express capsule in vitro and three of these shared a single nucleotide deletion in cap8D (position 671), resulting in a frameshift and premature stop codon. The same three isolates (two with spa type t160 and one with spa type t771) carried a G→T transversion mutation at nucleotide position 683 of cap8E resulting in a non-synonymous substitution, Ser156Ile. Interestingly, no specific mutations were detected in the fourth CC12 isolate (spa type t5318) with the CP-negative in vitro phenotype. A single CC97 isolate that did not express capsule in vitro harbored a 1448 nucleotide deletion involving the cap5D and cap5E genes.
Detection of capsule expression in vivo
Detection of surface polysaccharides in the murine model by immunofluorescence assay (IFA)
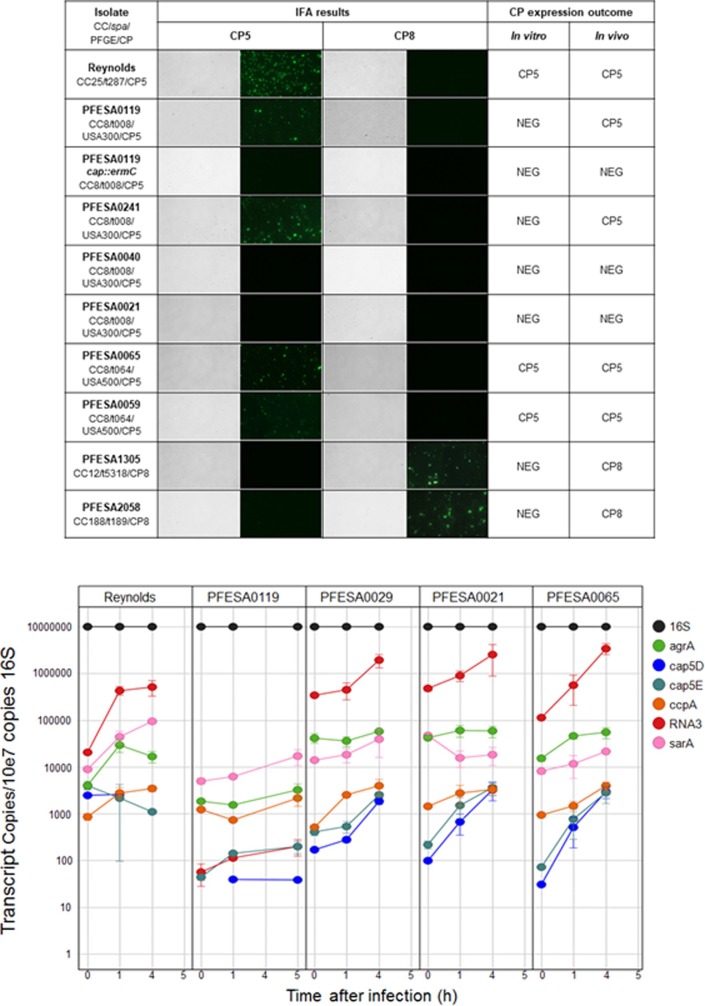
To assess whether isolates with undetectable capsule expression in vitro may express capsule in vivo, IFA was used to test the majority of S. aureus isolates that were CP-negative in vitro [21, 64]. Blood was harvested from mice 6 h following infection with S. aureus strains, and evaluated by immunohistochemistry using anti-CP5 or anti-CP8 specific antibodies. The specificity of the anti-capsular polysaccharide antibodies used to detect capsule production by IFA was confirmed using three isogenic S. aureus Reynolds strains that expressed CP5, CP8, or no capsule (S5 Fig). The CP5 mAb only detected wild type S. aureus Reynolds, the prototype serotype 5 strain. Likewise the CP8 mAb only recognized the isogenic CP8 expressing Reynolds strain; neither antibody recognized the isogenic acapsular mutant strain. Of the five CC12 isolates tested, four (spa types t160, t5318 and t771) were CP-negative in vitro while one isolate (CC12-t156) was CP-positive. As discussed previously, the in vitro CP-negative CC12 isolates either had two SNPs in their capsule operon (spa types t160 and t771), or none (spa type t5318). CP8 expression was detected in blood following infection with three of the four in vitro CP-negative CC12 isolates (IFA data is shown for a representative isolate in Fig 4A). The CC12-t160 isolate that was CP-negative in vivo had mutations in cap8D and cap8E (mutation types 6 and 7, Table 3). The second CC12-t160 isolate with the same two mutations was positive for CP8 expression in vivo, despite having no detectable capsule expression in vitro. The single CC188-t189 isolate that was phenotypically CP-negative in vitro and harbored the non-synonymous Gly251Val substitution in cap8O (mutation type 8, Table 3) did express capsule in vivo (Fig 4A). The CC5-t062 isolate that was phenotypically CP-negative in vitro and contained the CP5 mutation (cap5E) resulting in a premature termination codon was not tested in the bacteremia model.
Fig 4. Detection of CP5 or CP8 polysaccharide expression in S. aureus isolates recovered from a murine bacteremia infection model.
(A) Detection of surface polysaccharides in the murine IFA model. S. aureus was collected from the blood of infected mice 6h after infection, and stained with CP5 or CP8 antibodies. CP8 staining served as a specificity control for CP5-expressing isolates, and vice versa. As an additional control, the PFESA0119 ΔcapHIJK isogenic deletion strain (PFESA0119 cap::ermC) was included. Isolate name, CC, spa type, pulsotype and capsule genotype are indicated next to each set of images. Both differential interference contrast (DIC) and IFA images are shown for each isolate. (B) In vivo transcript analyses of gene targets in four representative S. aureus CC8 isolates; three USA300 isolates (PFESA0119, PFESA0021 and PFESA0029) and one USA500 (PFESA0065) isolate. RNA levels corresponding to regulatory and biosynthetic CP genes (agrA, cap5D, cap5E, ccpA, RNAIII and sarA) were measured by RT-PCR in the bacterial challenge (T0) inoculum, as well as in mouse blood harvested at 1 and 4h after infection. Transcript copy numbers normalized to 16S rRNA are shown for triplicate samples at each time point.
To more thoroughly investigate in vivo expression of CP5 in S. aureus CC8 isolates in light of contradictory conclusions in recent reports [21, 55, 64], additional CC8 S. aureus isolates from a contemporary US collection obtained from the CDC were included to supplement the CC8 isolates from the T.E.S.T collection. A total of 26 CC8 isolates were tested for capsule expression in mice, including 20 t008 isolates (17 of which are USA300 isolates), five t064 isolates (four of which are USA500 isolates), and one t024 USA300 isolate (Table 2). Three of the 26 CC8 S. aureus isolates in this subset expressed capsule in vitro (12%). As was seen with isolates from other lineages (e.g. CC12), no consistent association was found between the presence of mutations in the cap operon and the ability to express capsule in vivo (Table 2). Twenty of the 26 CC8 isolates tested expressed capsule in vivo (77%). Detection of capsule in vivo expression is shown for representative CC8-t008 (USA300) and CC8-t064 (USA500) isolates in Fig 4A.
To assess whether the observed in vivo expression was due to either mutations reverting in vivo or contamination by other S. aureus that are capsule producers, mice (two mice per bacterial strain tested) were challenged individually with three USA300 strains (PFESA0119, PFESA0029 and PFESASA0021) and Reynolds strain. For each of these strains, ST, spa type and cap operon sequences were identical in the challenge bacteria and the bacteria that were recovered from the animals after infection (S3 Table). There was no evidence of genetic changes or strain contamination.
Capsule polysaccharide expression in vivo was further explored by evaluating whether RNA transcripts from the cap operon could be detected in mouse blood following infection with a representative panel of USA300 (two in vivo CP-positive isolates, PFESA0119 and PFESA0029 [CDC3] and an in vivo CP-negative isolate, PFESA0021) and USA500 (in vivo CP-positive isolate PFESA0065) isolates. RNA transcripts corresponding to cap5D and cap5E were detected in vivo for each of the four isolates tested (Fig 4B).
Detection of CP5 immune responses to USA300 isolates. To further evaluate whether USA300 isolates express capsule in vivo, mice were infected with two in vivo CP-positive USA300 MRSA isolates PFESA0029 [CDC3] and PFESA0119 as well as the isogenic capsule knockout mutant (PFESA0119 Δcap5HIJK ermC). S. aureus (MSSA) strain Reynolds was used as a CP5 positive control. Sera were collected from mice before infection and 2-weeks after the final inoculation and were evaluated for the presence of CP specific antibodies. Antibody responses to CP5 were measured using a CP5-specific competitive luminex immunoassay (cLIA). Anti-CP5 antibodies were not detected in cohort mice prior to infection. However, a CP5 immune response was detected in mice infected with either of the USA300 isolates indirectly indicating CP5 capsule expression in vivo. No anti-CP5 antibodies were observed for the PFESA0119 capsule knockout mutant (Table 4).
Table 4. Demonstration of specificity of in vivo CP immune responses in mice challenged with S. aureus isolates.
CP5 antibody titers in mice challenged with CP5 S. aureus isolates (MSSA strain Reynolds, two USA300 MRSA isolates PFESA0029 [CDC3] and PFESA0119, PFESA0119 Δcap5HIJK ermC capsule knock-out strains). CD1 mice (7–9 weeks of age, 10 per group) were administered three IP injections of ~ 2x106 CFU/animal in 0.5 mL at 0, 6 and 14 weeks, and bled two weeks after the final inoculation. CP5 antibody responses were measured using a CP5 specific cLIA assay. CP5 antibody responses were detected in mice infected with strain Reynolds, and PFESA0029 and PFESA0119 isolates, while the capsule knockout mutant was not able to induce a CP5 antibody response. GMT fold change over the antibody titer of the capsule knockout mutant was calculated for all tested isolates.
| Challenge CP5 isolate | |||||
|---|---|---|---|---|---|
| CP5 cLIA titer | PFESA0119 Δcap5HIJK ermC | PFESA0119 | PFESA0029 | Reynolds | |
| Geometric mean titer (95% CI) | 5 (5–5) |
14 (5.9–33.3) |
24 (8.8–66.8) |
54 (10.7–274.8) |
|
| GMT fold change from baseline | 1 | 2.8 | 4.8 | 10.8 | |
CP8-specific cLIA was also used to evaluate immune responses to CP8 isolates that lacked capsule expression in vitro. Anti-CP8 antibodies were detected in mice infected with three CC12 isolates (PFESA1305, PFESA1405 and PFESA2455) and the CC188 isolate (PFESA2058) that harbored mutations in the cap8 operon. No immune responses were detected in mice infected with the single CC12-t160 isolate (PFESA1502) that carried the same mutations in cap8D and cap8E, but was CP-negative in vivo (Table 5). These results are consistent with the IFA data and further support the observed in vivo capsule expression for CP8 isolates.
Table 5. Detection of CP5 and CP8 immune responses in mice challenged with S. aureus isolates.
CP8 antibody titers in mice challenged with CP8 S. aureus isolates. A panel of seven different CP8 isolates representing different combinatins of in vitro and in vivo CP8 expression were used to challenge mice; five CC12 isolates (PFESA1194 (in vitro +, in vivo +), PFESA1305 (–, +), PFESA1405 (–, +), PFESA2455 (–, +), PFESA1502 (–,–)) and two CC188 isolates (PFESA2089 (+, not tested in vivo), PFESA2058 (–, +)). CD1 mice (7–9 weeks of age, 10 per group) were administered three IP injections of ~ 2x106 CFU/animal in 0.5 mL at 0, 4 and 12 weeks and bled at week 27. CP8 antibody responses were measured using a CP8 specific cLIA assay. CP8 antibody responses were detected in mice infected with all isolates except the CC12-t160 isolate (PFESA1502) that didn’t express capsule in the IFA murine model.
| Challenge CP8 Isolate | CP8 cLIA titer GMT (95% CI)a,b |
|---|---|
| PFESA1194 | 68.2 (6.3–735.7) |
| PFESA1305 | 17.3 (2.3–130) |
| PFESA1405 | 12.9 (1.3–123.2) |
| PFESA2455 | 10.4 (1.6–69.7) |
| PFESA1502 | 3 (3–3) |
| PFESA2089 | 5.7 (1.3–23.8) |
| PFESA2058 | 32.2 (2.1–499) |
a LOD values (units/mL) were 6.5 for CP5 and 3.0 for CP8. CP5 cLIA titer of all samples was 6.5.
b A CP8 positive control was used in this experiment and it had CP8 cLIA GMT of 173.3 (95% CI: 14–2155).
Discussion
Owing to the extended length of vaccine research and development, it is important to monitor the epidemiology of the target pathogen throughout the development program to ensure that the vaccine candidate under development remains appropriate. Over recent years, the US has seen a change in the epidemiology of S. aureus as exemplified by the rise of CA-MRSA USA300 isolates and their establishment in healthcare settings [9]. Analysis of these contemporary disease isolates revealed that they carried a mobile methicillin resistance cassette and specific virulence factors such as PVL, and appeared to lack capsular polysaccharides expression in vitro [55, 88, 90]. The most advanced vaccine candidate in clinical studies is SA4Ag, a four-antigen vaccine which includes CP5 and CP8 conjugated to the CRM197 carrier protein in addition to two protein antigens. The observation that emerging isolates may not express two of the vaccine’s target antigens, (CP5 or CP8) highlighted the importance to further investigate the distribution of capsule genotypes in prevalence-based studies and to assess the proportion of isolates that express capsular polysaccharides. This study is also the first that carefully and comprehensively examined the clonal relatedness (CC and spa types), CP genotype, and CP phenotype (in vitro and in vivo) amongst a panel of relevant S. aureus clinical isolates. A prevalence-based collection of such S. aureus isolates in the US was utilized to permit an epidemiological survey in a variety of clinical settings.
The S. aureus population circulating in the US during the time periods studied was represented by 13 prominent clonal complexes. In concordance with previous reports of S. aureus infection and colonization in the US and Europe, the S. aureus isolates in healthcare and community settings were primarily distributed between four major CC5, 8, 30, and 45 [91–96], with CC8 and CC5 comprising the largest number of isolates. In agreement with recent studies showing an association between invasive disease and clonality [97], we also observed a significant association between CC and type of infection (invasive vs non-invasive) (P = 0.036).
The inpatient data shows that although most S. aureus genotypes (CC) have the capacity to cause invasive disease, isolates within CC5, 8, 30 and 45 caused the majority of invasive disease. In the current study, the most prevalent spa types in the US S. aureus population were t008 and t002, consistent with results from earlier reports [14]. There was a strong association between spa type and type of infection (HA-wound vs respiratory or invasive) (P < 0.001), t008 isolates caused a higher proportion of wound infections whereas t002 isolates predominated in HA-invasive and HA-respiratory infections.
We determined CP prevalence, genotype distribution and expression in S. aureus isolates circulating in the US. Most available literature reports described capsule prevalence as determined by serotyping [53, 54, 56]. The present study is the first systematic molecular analysis of CP distribution and expression both in vitro and in vivo of S. aureus isolates causing disease in the US during 2004 and 2009–10. All S. aureus isolates harbored the genetic pathway to express either CP5 (72%) or CP8 (28%). A link was found between capsule genotype and CC-spa type, supporting the previous notion of the high clonality of the capsule antigens [67]. Among the MRSA isolates, 96% of the isolates were CP5 while 50% of the MSSA strains were CP5 (P < 0.001). Overall (MRSA and MSSA isolates combined), CP5 genotypes were the most prevalent in both inpatient and outpatient wards (P < 0.001).
For a vaccine strategy to be effective, the vaccine must contain antigens that are expressed in host microenvironments and elicit immune responses in humans. A mechanism of protection for multicomponent vaccines containing CP conjugates is to induce antibodies that facilitate the killing of the bacteria by complement mediated opsonophagocytosis which can be measured in vitro by OPA. We demonstrated in this study that humans immunized with a vaccine (SA3Ag) containing CP conjugates generated CP-specific antibodies with bactericidal activity against encapsulated isolates in OPA and that genetic lineage is not linked to the potential for a strain to be killed by anti-CP antibodies. CP-specific antibodies were detected in mice infected with two S. aureus USA300 isolates and not in mice infected with a USA300 capsule knockout mutant, indicating in vivo expression of capsule in these isolates (Table 5).
Potential vaccine candidate antigens must be present in the genome of circulating isolates, highly conserved, and expressed by S. aureus during the course of natural infection. The danger in relying on in vitro assays to preselect antigens is the potential lack of expression using standard culture conditions. Several studies comparing S. aureus gene expression in vivo to that under in vitro growth conditions indicated that the behavior of S. aureus in vivo could be significantly different from that observed in vitro [98–101]. Some candidate antigens are expressed both in vivo and in vitro (e.g. clumping factor A [21, 102, 103]), while others are poorly expressed or not detectable in in vitro grown bacteria [59, 104, 105]. Manganese transporter protein C (MntC), a protein that is not readily detected using standard culture conditions, was identified as a potential vaccine target based on in vivo expression analysis showing that MntC is rapidly upregulated by S. aureus in a murine model of infection [106]. In this study, assessing S. aureus isolates for capsule production revealed that a proportion of isolates tested within CC8 (37%) did not express capsule in vitro. Likewise, a lack of in vitro capsule expression was observed for other isolates belonging to CC5, 12, 97 and 188, albeit to a lesser extent. In vivo capsule expression analysis of representative isolates that showed no capsule expression in vitro revealed that many of these isolates were perfectly capable of expressing capsule in vivo. We therefore examined the cap operons of all isolates in the collection to identify whether any mutations within the cap operons could be linked to an in vitro CP-negative phenotype.
Both Cocchiaro et al. and Boyle-Vavra et al. [51, 55] identified specific genetic mutations in the capsular polysaccharide biosynthetic operon, which they showed by complementation experiments may contribute to the lack of observed capsule expression in vitro. In an effort to assess the relevance of these mutations with regard to capsule expression during infection, Boyle-Vavra et al. [55] conducted an in vivo analysis using a single isolate and confirmed that no capsule expression could be detected in that instance. The methodology used was different from that utilized in our previous reports where expression was detected [21, 64] so it is difficult to interpret these findings in relation to previous observations. The mutations in the cap5A promoter and the cap5D, cap5E and cap5G coding regions [55, 107] were also identified in CC8 isolates tested in this study (Table 3). We propose that only one of these mutations (type 2, cap5D) is likely to contribute to the lack of in vitro expression phenotype. The cap5D mutation is a frameshift mutation predicted to result in a truncated non-functional enzyme due to premature termination of protein synthesis. As the cap5A promoter mutation (mutation type 1) was identified in some strains that expressed capsule in vitro, this is unlikely to be the root cause of the acapsular phenotype. The cap5E (mutation type 3) and cap5G (mutation type 4) SNPs result in non-conservative amino acid substitutions (Cap5E D75Y and Cap5G F160L) with the potential to influence the activity of the corresponding enzymes. However, Cap5E D75Y maps to a location peripheral to cofactor or substrate binding sites [108], and Cap5G F160L falls beyond the boundary of gene fragments capable of genetically complementing the USA300 acapsular phenotype [55]. Additional mutations associated with other CC including CC5 (cap5E); CC12 (cap8D and cap8E) and CC188 (cap8O) were also identified in this study. In each of the cases tested, there was no correlation between these mutations and the ability of an isolate to express capsule in vivo. This was the case, even in the instance of the cap8D frame shift mutation in the two CC12 isolates (mutation type 6, Table 3), a mutation that might be expected to ablate expression of capsule functions. The third gene where a missense mutation was identified was cap8O in a CC188 isolate. This gene has been demonstrated to be essential for the production of D-ManNAcA when it is completely knocked out, however the effects of specific non-synonymous substitutions have not been characterized. These observations suggest that point/frameshift mutations within the capsular genetic pathway are generally not predictive of the ability of this pathogen to produce capsule during infection. The only clear capsule null mutation was identified in a single CC97 isolate within the collection of 516 S. aureus isolates, for which no detectable capsule expression was observed in vitro, suggesting that the acquisition of such mutations is potentially detrimental to S. aureus and thus clonal expansion of such isolates in the clinical setting may be unlikely. CC97 is an interesting lineage that is a leading cause of bovine mastitis globally [109, 110] and has several major genomic differences to the common S. aureus CC that cause disease in humans. The loss of capsule function in the CC97 isolate is consistent with the prevalence of acapsular bovine S. aureus that has evolved through mutations in capsular polysaccharide genes [51, 111].
Given our data, it seems possible that particular capsular biosynthetic enzymes that are not expressed or are inactive in vitro may be expressed or active in the in vivo environment; therefore in vitro analyses may not necessarily predict capsule expression in vivo. Rozemeijer et al. [112] reported the detection of RNA transcripts of genes encoding capsular polysaccharide in swab samples from infected patients by qRT-PCR despite the experimental challenges. The in vivo mouse experiments presented here have the disadvantage that the number of bacterial isolates that are recoverable is limited, making it difficult to assess the absolute structure of the polysaccharide being detected. Despite these limitations, sufficient bacterial RNA was isolated from mouse blood to confirm that individual gene transcripts of the cap operon were being expressed (Fig 4B).
We acknowledge that we have generated a body of data that can be considered controversial. Using specific capsular polysaccharide detection reagents and assays we show that clear differences exist between the expression of capsular polysaccharides by S. aureus disease-causing strains in vivo, compared with expression in the same strains grown in laboratory medium. At this point we can only speculate on possible mechanisms. Ouyang et al. [107] were the first to postulate that in vitro expression may be compromised due to a mutation within the cap5A promoter region. In this study we have found that this promoter mutation is not limited only to CP5 S. aureus strains that do not express capsule in vitro, it is also present in other strains where capsular polysaccharide expression is detected in vitro. A potential explanation for this was outlined by Sau et al. [113] who demonstrated that while the cap operon was transcribed as a single transcript using the cap5A associated promoter, weaker promoters were also associated with the individual genes. Likewise Ouyang et al. [107] and Gupta et al. [114] have demonstrated that the principal promoter (Pcap) of the cap operon has several different activators and repressors that bind to different promotor sites. Genetic and biochemical data may explain how cap5D frameshift mutations associated with USA300 strains can be bypassed in vivo. Our transcriptional analyses demonstrates the presence of cap5D transcript during in vivo growth, suggesting that although the translation of the cap5D coding sequence may be disrupted by a frameshift, it is possible that a post-translational bypass mechanism may be responsible for compensating for the cap5D nucleotide insertion under in vivo growth conditions. The gene products of capD and capE are similar, with the exception of a membrane bound motif associated with capD, which is thought to anchor the polysaccharide to the cell surface during synthesis [115]. Both proteins encode UDP-GlcNAc C6 dehydratases that are associated with the first steps in D-FucNAc and L-FucNAc biosynthesis, respectively. However, Miyafusa et al. [108] demonstrated that the Cap5E enzyme is capable of producing the stereoisomeric precursors of both D-FucNAc and L-FucNAc in vitro. Not only can Cap5E generate the UDP-xylo-sugar (L-FucNAc precursor), but it can also produce the UDP-arabino-sugar (D-FucNAc precursor) as byproduct. The activity of the enzyme is also likely to be regulated by exogenous metabolites due to the presence of an allosteric site identified in the crystal structure [108]. By generating both D- and L- precursors of fucose, Cap5E has the potential to bypass Cap5D, thus providing a potential mechanism for our observations which could be explored further (S6 Fig).
In conclusion, our comprehensive study of relevant S. aureus disease isolates demonstrates that although the expression of capsule may not be fully understood in vivo, a much higher proportion of the disease-causing isolates in the US have the potential to express capsule in vivo than previously predicted through analysis of the pathogen cultured in in vitro growth media. Studies have identified many genes and pathways involved in capsule production by S. aureus, but the role and mechanisms by which they regulate capsule production during infection remain to be elucidated. Our work shows that mutations in the cap operon are identified in a subset of the 16 cap genes and they are associated with specific CC. Although all USA300 and some USA500 isolates within the CC8 lineage had the cap5D frameshift mutation, many were able to express capsule in vivo. Phylogenetic analysis of USA300 and USA500 isolates in the context of a wider CC8 population revealed that these two epidemic clones are not directly related [89], in contrast to a previous suggestion that USA500 represents a progenitor of the USA300 clone [116]. In this study, less than 10% of invasive disease cases were caused by USA300 isolates, where reduced expression of CP in vitro was observed. Therefore, surveys of CP prevalence that only take into account the in vitro phenotype or use isolate collections that may not represent the target population for the vaccine, will underestimate the distribution of CP-expressing isolates and thus the potential coverage of CP conjugate containing vaccines. The expression of capsule by S. aureus is only one of several virulence mechanisms deployed by this pathogen. It is expected that for a vaccine to be effective it will need to also address additional virulence mechanisms such as binding to host structures and an ability to scavenge nutrients [33, 106, 112, 117–119]. The appropriate clinical efficacy trials are therefore crucial to demonstrate the effectiveness of a multi-antigen vaccine containing capsular polysaccharide conjugates in preventing S. aureus infections.
Supporting information
(PDF)
(PDF)
Presence of the four conserved cap5 operon mutations is indicated in green while the absence of mutations is indicated in red.
(PDF)
(TIF)
(A) All isolates associated with S. aureus infections in healthcare and community settings. (B) S. aureus isolates associated with the main types of clinical infections in healthcare settings.
(DOCX)
(DOCX)
Approximately-maximum-likelihood phylogenetic trees of analyzed S. aureus CP5 (A and C) and CP8 (B and D) isolates annotated with the distribution of cap operon SNPs (A and B) and indels in US S. aureus isolates (C and D). (A) cap operon SNPs in CP5 isolates. SNPs were identified across the cap operon based on mapping against S. aureus HO 5096 0412 reference. Isolate tracks color-coded by CC (legend below figure). Color-coding of SNPs; Green A, Blue G, Black T and Red C. (B) cap operon SNPs in CP8 isolates. SNPs were identified across the cap operon and plotted against tree of all CP8 USA isolates based on mapping against S. aureus MSSA476 reference. Isolate tracks color-coded by CC legend below figure). (C) cap operon indels in CP5 isolates. Indels were identified across the cap operon and plotted against tree of all CP5 USA isolates based on mapping against S. aureus HO 5096 0412 reference. Isolate tracks color-coded by CC (legend below figure). Color-coding of indels; Vertical magenta dots indicates insertion while dark gray indicates deletion. For each isolate, the mapping coverage is indicated by the light grey horizontal field, with white regions demonstrating deletion of the corresponding cap genomic region. As such, this figure shows the partial loss of cap5D-E in a single CC97 isolate. (D) cap operon indels in CP8 isolates. Indels were identified across the cap operon and plotted against tree of all CP8 USA isolates based on mapping against S. aureus MSSA476 reference.
(DOCX)
Three different S. aureus strains: Reynolds (CP5), its isogenic CP-negative mutant, and the CP- negative Reynolds complemented with CP8-coding sequences were used to challenge mice. The strains were tested in a blinded fashion with two independent experiments per strain. S. aureus was collected at the time of challenge (T0) and from the blood of infected mice 6 hr post infection (T6), and stained with rabbit anti-CP5, or rabbit anti-CP8 antibodies, or normal rabbit IgGs. Bright-field (A) and fluorescence photographs (B) of IFA staining are shown for each strain at both time points.
(TIF)
(A) Capsular polysaccharide type 5 is composed of repeat units of D-N-acetylmannosamine (D-ManNAc); L-N-acetylfucosamine (L-FucNAc) and D-L-acetylfucosamine (D-FucNAc). Cap5D is associated with synthesis of the D-FucNAc precursor (Li et al. Internatl. J. Med. Micro. 2014) and Cap5E is primarily associated with synthesizing L-FucNAc precursor (Miyafusa et al. FEBS Lett. 2013). (B) Cap5D is a 4, 6-dehydratase that converts UDP-D-GlcNAc to a D-FucNAc precursor, while Cap5E has 4, 6-dehydratase and 5-epimerase activity that converts UDP-D-GlcNAc to an L-FucNAc precursor but can also generate the analogous D-FucNAc precursor in a reverse epimerization reaction (Miyafusa et al. FEBS Lett. 2013). We propose that for S. aureus USA300 strains where the cap5D gene has a premature stop codon, the UDP-D-FucNAc precursor UDP-2-acetoamino-2, 6-dideoxy-a-D-xylo-4-hexulose (highlighted in orange) is derived as byproduct of the CapE epimerase reaction.
(DOCX)
Acknowledgments
We thank Pfizer colleagues; Ed Buurman for thoughtful comments and review of the manuscript, Luke Handke for constructing isogenic capH-K deletion mutants, Charles Tan for supporting the statistical analyses, Arthur Illenberger for help with the in vivo studies, Danka Pavliakova and Hong Chen for assistance with the cLIA analyses, Srinivas Kodali for biochemistry work and his help with testing isolates for capsule expression in vitro, and the following colleagues for help with the genetic analyses of the S. aureus isolates tested in the in vivo IFA experiments; Luba Andrew for sequencing and data analysis, Lorna Nunez for bioinformatics analysis and Eneida Pollozi for microbiology support, We also acknowledge Anatoly Severin for developing the proof of concept of the antigen competition Luminex-based assay.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was sponsored by Pfizer Vaccine Research who provided support in the form of salaries for authors NM, YT, ER, LH, NSM, JH, GS, BC, PL, SS, RGKD, ILS, CHJ, KUJ and ASA, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature reviews Microbiology. 2009;7(9):629–41. Epub 2009/08/15. 10.1038/nrmicro2200 ; PubMed Central PMCID: PMCPmc2871281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–71. 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 3.Goetghebeur M, Landry PA, Han D, Vicente C. Methicillin-resistant Staphylococcus aureus: A public health issue with economic consequences. Can J Infect Dis Med Microbiol. 2007;18(1):27–34. Epub 2008/10/17. ; PubMed Central PMCID: PMCPmc2542887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallen AJ, Mu Y, Bulens S, Reingold A, Petit S, Gershman K, et al. Health care-associated invasive MRSA infections, 2005–2008. JAMA. 2010;304(6):641–8. Epub 2010/08/12. 10.1001/jama.2010.1115 . [DOI] [PubMed] [Google Scholar]
- 5.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. Epub 2008/10/25. 10.1086/591861 . [DOI] [PubMed] [Google Scholar]
- 6.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23(3):616–87. 10.1128/CMR.00081-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, et al. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis. 2011;53(2):144–9. Epub 2011/06/22. 10.1093/cid/cir308 . [DOI] [PubMed] [Google Scholar]
- 8.Tenover FC, McDougal LK, Goering RV, Killgore G, Projan SJ, Patel JB, et al. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44(1):108–18. Epub 2006/01/05. 10.1128/JCM.44.1.108-118.2006 ; PubMed Central PMCID: PMCPmc1351972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diekema DJ, Richter SS, Heilmann KP, Dohrn CL, Riahi F, Tendolkar S, et al. Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: results from a nationwide surveillance study. Infect Control Hosp Epidemiol. 2014;35(3):285–92. Epub 2014/02/14. 10.1086/675283 . [DOI] [PubMed] [Google Scholar]
- 10.Pasquale TR, Jabrocki B, Salstrom SJ, Wiemken TL, Peyrani P, Haque NZ, et al. Emergence of methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of late-onset nosocomial pneumonia in intensive care patients in the USA. Int J Infect Dis. 2013;17(6):e398–403. Epub 2013/02/05. 10.1016/j.ijid.2012.12.013 . [DOI] [PubMed] [Google Scholar]
- 11.David MZ, Boyle-Vavra S, Zychowski DL, Daum RS. Methicillin-susceptible Staphylococcus aureus as a predominantly healthcare-associated pathogen: a possible reversal of roles? PloS one. 2011;6(4):e18217 Epub 2011/05/03. 10.1371/journal.pone.0018217 ; PubMed Central PMCID: PMCPmc3076382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.David MZ, Medvedev S, Hohmann SF, Ewigman B, Daum RS. Increasing burden of methicillin-resistant Staphylococcus aureus hospitalizations at US academic medical centers, 2003–2008. Infect Control Hosp Epidemiol. 2012;33(8):782–9. Epub 2012/07/05. 10.1086/666640 ; PubMed Central PMCID: PMCPmc3682488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallin M, Denis O, Deplano A, De Mendonca R, De Ryck R, Rottiers S, et al. Genetic relatedness between methicillin-susceptible and methicillin-resistant Staphylococcus aureus: results of a national survey. J Antimicrob Chemother. 2007;59(3):465–72. Epub 2007/02/10. 10.1093/jac/dkl535 . [DOI] [PubMed] [Google Scholar]
- 14.Miko BA, Hafer CA, Lee CJ, Sullivan SB, Hackel MA, Johnson BM, et al. Molecular characterization of methicillin-susceptible Staphylococcus aureus clinical isolates in the United States, 2004 to 2010. J Clin Microbiol. 2013;51(3):874–9. Epub 2013/01/04. 10.1128/JCM.00923-12 ; PubMed Central PMCID: PMCPmc3592060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidry AJ, Oliver SP, Squiggins KE, Erbe EF, Dowlen HH, Hambleton CN, et al. Effect of anticapsular antibodies on neutrophil phagocytosis of Staphylococcus aureus. J Dairy Sci. 1991;74(10):3360–9. 10.3168/jds.S0022-0302(91)78525-1 [DOI] [PubMed] [Google Scholar]
- 16.Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, et al. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother. 2013;9(3):480–7. Epub 2012/12/20. 10.4161/hv.23223 ; PubMed Central PMCID: PMCPmc3891703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thakker M, Park J-S, Carey V, Lee JC. Staphylococcus aureus Serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun. 1998;66(11):5183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sompolinsky D, Samra Z, Karakawa WW, Vann WF, Schneerson R, Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985;22(5):828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones C. Revised structures for the capsular polysaccharides from Staphylococcus aureus Types 5 and 8, components of novel glycoconjugate vaccines. Carbohydr Res. 2005;340(6):1097–106. Epub 2005/03/31. 10.1016/j.carres.2005.02.001 . [DOI] [PubMed] [Google Scholar]
- 20.Murphy E, Lin SL, Nunez L, Andrew L, Fink PS, Dilts DA, et al. Challenges for the evaluation of Staphylococcus aureus protein based vaccines: monitoring antigenic diversity. Hum Vaccin. 2011;7 Suppl:51–9. Epub 2011/01/20. . [DOI] [PubMed] [Google Scholar]
- 21.Nanra JS, Timofeyeva Y, Buitrago SM, Sellman BR, Dilts DA, Fink P, et al. Heterogeneous in vivo expression of clumping factor A and capsular polysaccharide by Staphylococcus aureus: implications for vaccine design. Vaccine. 2009;27(25–26):3276–80. Epub 2009/02/10. 10.1016/j.vaccine.2009.01.062 . [DOI] [PubMed] [Google Scholar]
- 22.Wyres KL, Lambertsen LM, Croucher NJ, McGee L, von Gottberg A, Linares J, et al. Pneumococcal capsular switching: a historical perspective. J Infect Dis. 2013;207(3):439–49. Epub 2012/11/24. 10.1093/infdis/jis703 ; PubMed Central PMCID: PMCPmc3537446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swartley JS, Marfin AA, Edupuganti S, Liu LJ, Cieslak P, Perkins B, et al. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci U S A. 1997;94(1):271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison LH, Shutt KA, Schmink SE, Marsh JW, Harcourt BH, Wang X, et al. Population structure and capsular switching of invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era—United States, 2000–2005. J Infect Dis. 2010;201(8):1208–24. Epub 2010/03/05. 10.1086/651505 ; PubMed Central PMCID: PMCPmc2838939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trotter CL, McVernon J, Ramsay ME, Whitney CG, Mulholland EK, Goldblatt D, et al. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine. 2008;26(35):4434–45. Epub 2008/07/12. 10.1016/j.vaccine.2008.05.073 . [DOI] [PubMed] [Google Scholar]
- 26.De Montalembert M, Abboud MR, Fiquet A, Inati A, Lebensburger JD, Kaddah N, et al. 13-valent pneumococcal conjugate vaccine (PCV13) is immunogenic and safe in children 6–17 years of age with sickle cell disease previously vaccinated with 23-valent pneumococcal polysaccharide vaccine (PPSV23): Results of a phase 3 study. Pediatr Blood Cancer. 2015;62(8):1427–36. Epub 2015/03/27. 10.1002/pbc.25502 . [DOI] [PubMed] [Google Scholar]
- 27.Block SL, Shepard J, Garfield H, Xie F, Han L, Dull PM, et al. Immunogenicity and Safety of a 3- and 4-Dose Vaccination Series of a Meningococcal ACWY Conjugate Vaccine in Infants: Results of a Phase 3b Randomized, Open Label Trial. Pediatr Infect Dis J. 2015. 10.1097/inf.0000000000000965 . [DOI] [PubMed] [Google Scholar]
- 28.Fattom AI, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64(5):1659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fattom AI, Sarwar J, Basham L, Ennifar S, Naso R. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect Immun. 1998;66(10):4588–92. Epub 1998/09/24. ; PubMed Central PMCID: PMCPmc108565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CY. Capsule and vaccine development In: Honeyman AL, Friedman H, Endinelli M, editor. Staphylococcus aureus infection and disease. New York, NY: Kluwer Academic/Plenum Publishers; 2001. p. 49–63. [Google Scholar]
- 31.Anderson AS, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, et al. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother. 2012;8(11):1585–94. 10.4161/hv.21872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JC, Park JS, Shepherd SE, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65(10):4146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lattar SM, Noto Llana M, Denoel P, Germain S, Buzzola FR, Lee JC, et al. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect Immun. 2014;82(1):83–91. Epub 2013/10/16. 10.1128/IAI.01050-13 ; PubMed Central PMCID: PMCPmc3911846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Gerber S, Lee JC. Antibodies to Staphylococcus aureus serotype 8 capsular polysaccharide react with and protect against serotype 5 and 8 isolates. Infect Immun. 2014;82(12):5049–55. 10.1128/IAI.02373-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vaccin Immunother. 2015;11(3):632–41. Epub 2014/12/09. 10.4161/hv.34414 ; PubMed Central PMCID: PMCPmc4514248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. New England Journal of Medicine. 2002;346(7):491–6. 10.1056/NEJMoa011297 [DOI] [PubMed] [Google Scholar]
- 37.Scully IL, Pavliak V, Timofeyeva Y, Liu Y, Singer C, Anderson AS. O-Acetylation is essential for functional antibody generation against Staphylococcus aureus capsular polysaccharide. Hum Vaccin Immunother. 2018;14(1):81–4. 10.1080/21645515.2017.1386360 PubMed PMID: PMC5791590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamin DK, Schelonka R, White R, Holley HP, Bifano E, Cummings J, et al. A blinded, randomized, multicenter study of an intravenous Staphylococcus aureus immune globulin. Journal of perinatology: official journal of the California Perinatal Association. 2006;26(5):290–5. Epub 2006/04/07. 10.1038/sj.jp.7211496 . [DOI] [PubMed] [Google Scholar]
- 39.Rupp ME, Holley HP Jr., Lutz J, Dicpinigaitis PV, Woods CW, Levine DP, et al. Phase II, randomized, multicenter, double-blind, placebo-controlled trial of a polyclonal anti-Staphylococcus aureus capsular polysaccharide immune globulin in treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51(12):4249–54. Epub 2007/09/26. 10.1128/AAC.00570-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu B, Park S, Thompson CD, Li X, Lee JC. Antibodies to Staphylococcus aureus capsular polysaccharides 5 and 8 perform similarly in vitro but are functionally distinct in vivo. Virulence. 2017;8(6):859–74. 10.1080/21505594.2016.1270494 PubMed PMID: PMC5626342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook J, Hepler R, Pancari G, Kuklin N, Fan H, Wang X-m, et al. Staphylococcus aureus capsule type 8 antibodies provide inconsistent efficacy in murine models of staphylococcal infection. Hum Vaccin. 2009;5(4):254–63. [DOI] [PubMed] [Google Scholar]
- 42.Tuchscherr LPN, Buzzola FR, Alvarez LP, Lee JC, Sordelli DO. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect Immun. 2008;76(12):5738–44. 10.1128/IAI.00874-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bagnoli F, Bertholet S, Grandi G. Inferring reasons for the failure of Staphylococcus aureus vaccines in clinical trials. Front Cell Infect Microbiol. 2012;2:16 Epub 2012/08/25. 10.3389/fcimb.2012.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: "Staphyloccocus aureus vaccines: Problems and prospects". Vaccine. 2013;31(25):2723–30. 10.1016/j.vaccine.2013.04.002 [DOI] [PubMed] [Google Scholar]
- 45.Giersing BK, Dastgheyb SS, Modjarrad K, Moorthy V. Status of vaccine research and development of vaccines for Staphylococcus aureus. Vaccine. 2016;34(26):2962–6. 10.1016/j.vaccine.2016.03.110 [DOI] [PubMed] [Google Scholar]
- 46.Mohamed N, Wang MY, Le Huec JC, Liljenqvist U, Scully IL, Baber J, et al. Vaccine development to prevent Staphylococcus aureus surgical-site infections. The British journal of surgery. 2017;104(2):e41–e54. Epub 2017/01/26. 10.1002/bjs.10454 . [DOI] [PubMed] [Google Scholar]
- 47.Scully IL, Liberator PA, Jansen KU, Anderson AS. Covering all the bases: Preclinical development of an effective Staphylococcus aureus vaccine. Front Immunol. 2014;5:109 Epub 2014/04/10. 10.3389/fimmu.2014.00109 ; PubMed Central PMCID: PMCPmc3970019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawkins J, Kodali S, Matsuka Y, McNeil L, Mininni T, Dodge I, et al. A recombinant Clumping factor A containing vaccine induces a functional response to Staphylococcus aureus that is not observed with natural exposure. submitted. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malachowa N, Whitney AR, Kobayashi SD, Sturdevant DE, Kennedy AD, Braughton KR, et al. Global changes in Staphylococcus aureus gene expression in human blood. PloS one. 2011;6(4):e18617 10.1371/journal.pone.0018617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oogai Y, Matsuo M, Hashimoto M, Kato F, Sugai M, Komatsuzawa H. Expression of virulence factors by Staphylococcus aureus grown in serum. Appl Environ Microbiol. 2011;77(22):8097–105. 10.1128/AEM.05316-11 PubMed PMID: PMC3208999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cocchiaro JL, Gomez MI, Risley A, Solinga R, Sordelli DO, Lee JC. Molecular characterization of the capsule locus from non-typeable Staphylococcus aureus. Mol Microbiol. 2006;59(3):948–60. 10.1111/j.1365-2958.2005.04978.x [DOI] [PubMed] [Google Scholar]
- 52.Lattar SM, Tuchscherr LP, Caccuri RL, Centrón D, Becker K, Alonso CA, et al. Capsule expression and genotypic differences among Staphylococcus aureus isolates from patients with chronic or acute osteomyelitis. Infect Immun. 2009;77(5):1968–75. 10.1128/IAI.01214-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roghmann M, Taylor KL, Gupte A, Zhan M, Johnson JA, Cross A, et al. Epidemiology of capsular and surface polysaccharide in Staphylococcus aureus infections complicated by bacteraemia. J Hosp Infect. 2005;59(1):27–32. Epub 2004/12/02. 10.1016/j.jhin.2004.07.014 . [DOI] [PubMed] [Google Scholar]
- 54.Sutter DE, Summers AM, Keys CE, Taylor KL, Frasch CE, Braun LE, et al. Capsular serotype of Staphylococcus aureus in the era of community-acquired MRSA. FEMS Immunol Med Microbiol. 2011;63(1):16–24. Epub 2011/06/03. 10.1111/j.1574-695X.2011.00822.x . [DOI] [PubMed] [Google Scholar]
- 55.Boyle-Vavra S, Li X, Alam MT, Read TD, Sieth J, Cywes-Bentley C, et al. USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. MBio. 2015;6(2). Epub 2015/04/09. 10.1128/mBio.02585-14 ; PubMed Central PMCID: PMCPmc4453534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17(1):218–34. 10.1128/CMR.17.1.218-234.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouyang S, Lee CY. Transcriptional analysis of type 1 capsule genes in Staphylococcus aureus. Mol Microbiol. 1997;23(3):473–82. Epub 1997/02/01. . [DOI] [PubMed] [Google Scholar]
- 58.Herbert S, Worlitzsch D, Dassy B, Boutonnier A, Fournier JM, Bellon G, et al. Regulation of Staphylococcus aureus capsular polysaccharide type 5: in vitro and in vivo inhibition by CO2. Pneumologie. 1997;51(11):1043–50. [PubMed] [Google Scholar]
- 59.McKenney D, Pouliot KL, Wang Y, Murthy V, Ulrich M, Doring G, et al. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284(5419):1523–7. Epub 1999/05/29. . [DOI] [PubMed] [Google Scholar]
- 60.Luong TT, Sau K, Roux C, Sau S, Dunman PM, Lee CY. Staphylococcus aureus ClpC divergently regulates capsule via sae and codY in strain newman but activates capsule via codY in strain UAMS-1 and in strain Newman with repaired saeS. J Bacteriol. 2011;193(3):686–94. Epub 2010/12/07. 10.1128/JB.00987-10 ; PubMed Central PMCID: PMCPmc3021213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luong TT, Lee CY. The arl locus positively regulates Staphylococcus aureus type 5 capsule via an mgrA-dependent pathway. Microbiology. 2006;152(10):3123–31. Epub 2006/09/29. . [DOI] [PubMed] [Google Scholar]
- 62.Sun F, Ji Q, Jones MB, Deng X, Liang H, Frank B, et al. AirSR, a [2Fe-2S] cluster-containing two-component system, mediates global oxygen sensing and redox signaling in Staphylococcus aureus. J Am Chem Soc. 2012;134(1):305–14. Epub 2011/11/30. 10.1021/ja2071835 ; PubMed Central PMCID: PMCPmc3257388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meier S, Goerke C, Wolz C, Seidl K, Homerova D, Schulthess B, et al. sigmaB and the sigmaB-dependent arlRS and yabJ-spoVG loci affect capsule formation in Staphylococcus aureus. Infect Immun. 2007;75(9):4562–71. Epub 2007/07/20. 10.1128/IAI.00392-07 ; PubMed Central PMCID: PMCPmc1951174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Timofeyeva Y, Scully IL, Anderson AS. Immunofluorescence microscopy for the detection of surface antigens in methicillin-resistant Staphylococcus aureus (MRSA). Methods Mol Biol. 2014;1085:85–95. Epub 2013/10/03. 10.1007/978-1-62703-664-1_4 . [DOI] [PubMed] [Google Scholar]
- 65.Bouchillon SK, Hoban DJ, Johnson BM, Johnson JL, Hsiung A, Dowzicky MJ. In vitro activity of tigecycline against 3989 Gram-negative and Gram-positive clinical isolates from the United States Tigecycline Evaluation and Surveillance Trial (TEST Program; 2004). Diagn Microbiol Infect Dis. 2005;52(3):173–9. Epub 2005/08/18. 10.1016/j.diagmicrobio.2005.06.004 . [DOI] [PubMed] [Google Scholar]
- 66.Limbago B, Fosheim GE, Schoonover V, Crane CE, Nadle J, Petit S, et al. Characterization of methicillin-resistant Staphylococcus aureus isolates collected in 2005 and 2006 from patients with invasive disease: a population-based analysis. J Clin Microbiol. 2009;47(5):1344–51. Epub 2009/03/27. 10.1128/JCM.02264-08 ; PubMed Central PMCID: PMCPmc2681881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy E, Lin SL, Nunez L, Andrew L, Fink PS, Dilts DA, et al. Challenges for the evaluation of Staphylococcus aureus protein based vaccines: monitoring antigenic diversity. Hum Vaccin. 2011;7(0):51–9. [DOI] [PubMed] [Google Scholar]
- 68.Janzon L, Arvidson S. The role of the delta-lysin gene (hld) in the regulation of virulence genes by the accessory gene regulator (agr) in Staphylococcus aureus. Embo j. 1990;9(5):1391–9. Epub 1990/05/01. ; PubMed Central PMCID: PMCPmc551825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McNamara PJ. Genetic Manipulation of Staphylococcus aureus In: Lindsay JA, editor. Staphylococcus: Molecular Genetics. 1 Norfolk, UK: Caister Academic Press; 2008. p. 89–130. [Google Scholar]
- 70.Hsu L-Y, Harris SR, Chlebowicz MA, Lindsay JA, Koh T-H, Krishnan P, et al. Evolutionary dynamics of methicillin-resistant Staphylococcus aureus within a healthcare system. Genome Biol. 2015;16(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Page AJ, De Silva N, Hunt M, Quail MA, Parkhill J, Harris SR, et al. Robust high-throughput prokaryote de novo assembly and improvement pipeline for Illumina data. Microb Genom. 2016;2(8):e000083 Epub 2017/03/30. 10.1099/mgen.0.000083 ; PubMed Central PMCID: PMCPmc5320598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–9. 10.1101/gr.074492.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27(4):578–9. Epub 2010/12/15. 10.1093/bioinformatics/btq683 . [DOI] [PubMed] [Google Scholar]
- 74.Boetzer M, Pirovano W. Toward almost closed genomes with GapFiller. Genome Biol. 2012;13(6):R56 Epub 2012/06/27. 10.1186/gb-2012-13-6-r56 ; PubMed Central PMCID: PMCPmc3446322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014:btu153. [DOI] [PubMed] [Google Scholar]
- 76.Pruitt KD, Tatusova T, Brown GR, Maglott DR. NCBI Reference Sequences (RefSeq): current status, new features and genome annotation policy. Nucleic acids research. 2012;40(Database issue):D130–5. Epub 2011/11/29. 10.1093/nar/gkr1079 ; PubMed Central PMCID: PMCPmc3245008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Milheiriço C, Oliveira DC, de Lencastre H. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(9):3374–7. 10.1128/AAC.00275-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milheiriço C, Oliveira DC, de Lencastre H. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus:‘SCCmec IV multiplex’. J Antimicrob Chemother. 2007;60(1):42–8. 10.1093/jac/dkm112 [DOI] [PubMed] [Google Scholar]
- 79.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38(3):1008–15. Epub 2000/03/04. ; PubMed Central PMCID: PMCPmc86325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harmsen D, Claus H, Witte W, Rothganger J, Claus H, Turnwald D, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol. 2003;41(12):5442–8. Epub 2003/12/10. 10.1128/JCM.41.12.5442-5448.2003 ; PubMed Central PMCID: PMCPmc309029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inouye M, Dashnow H, Raven L-A, Schultz MB, Pope BJ, Tomita T, et al. SRST2: Rapid genomic surveillance for public health and hospital microbiology labs. Genome Medicine. 2014;6(11):90 10.1186/s13073-014-0090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nissen M, Marshall H, Richmond P, Shakib S, Jiang Q, Cooper D, et al. A randomized phase I study of the safety and immunogenicity of three ascending dose levels of a 3-antigen Staphylococcus aureus vaccine (SA3Ag) in healthy adults. Vaccine. 2015;33(15):1846–54. Epub 2015/02/25. 10.1016/j.vaccine.2015.02.024 . [DOI] [PubMed] [Google Scholar]
- 83.Soell M, Diab M, Haan-Archipoff G, Beretz A, Herbelin C, Poutrel B, et al. Capsular polysaccharide types 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells, and monocytes and induce release of cytokines. Infect Immun. 1995;63(4):1380–6. Epub 1995/04/01. ; PubMed Central PMCID: PMCPmc173162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fattom A, Schneerson R, Szu SC, Vann WF, Shiloach J, Karakawa WW, et al. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect Immun. 1990;58(7):2367–74. PubMed PMID: PMC258821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koser CU, Holden MT, Ellington MJ, Cartwright EJ, Brown NM, Ogilvy-Stuart AL, et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med. 2012;366(24):2267–75. Epub 2012/06/15. 10.1056/NEJMoa1109910 ; PubMed Central PMCID: PMCPmc3715836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watts A, Ke D, Wang Q, Pillay A, Nicholson-Weller A, Lee JC. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect Immun. 2005;73(6):3502–11. 10.1128/IAI.73.6.3502-3511.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine leukocidin. Lab Invest. 2007;87(1):3–9. Epub 2006/12/06. 10.1038/labinvest.3700501 . [DOI] [PubMed] [Google Scholar]
- 88.Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46(4):1147–52. Epub 2002/03/19. 10.1128/AAC.46.4.1147-1152.2002 ; PubMed Central PMCID: PMCPmc127097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jamrozy D, Harris SR, Mohamed N, Peacock SJ, Tan CY, Parkhill J, et al. Pan-genomic perspective on the evolution of the Staphylococcus aureus USA300 epidemic. Microb Genom. 2016. Epub 05 April, 2016 10.1099/mgen.0.000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, et al. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis. 2008;198(4):561–70. Epub 2008/07/05. 10.1086/590157 . [DOI] [PubMed] [Google Scholar]
- 91.Rasmussen G, Monecke S, Ehricht R, Soderquist B. Prevalence of clonal complexes and virulence genes among commensal and invasive Staphylococcus aureus isolates in Sweden. PloS one. 2013;8(10):e77477 Epub 2013/10/17. 10.1371/journal.pone.0077477 ; PubMed Central PMCID: PMCPmc3793971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matson MA GD, Zito E, Hubler R, Jones CH, Laudet F, Severs J, Anderson AS. Longitudinal analysis of bacterial carriage rates and antibody titers to 4 conserved Staphylococcus aureus antigens comprising a novel multi-component vaccine. ICAAC Abstract 1–306; Sept 5–9; Washington DC 2014.
- 93.Feil EJ, Cooper JE, Grundmann H, Robinson DA, Enright MC, Berendt T, et al. How clonal is Staphylococcus aureus? J Bacteriol. 2003;185(11):3307–16. Epub 2003/05/20. 10.1128/JB.185.11.3307-3316.2003 ; PubMed Central PMCID: PMCPmc155367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fowler VG Jr., Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, et al. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis. 2007;196(5):738–47. Epub 2007/08/04. 10.1086/520088 . [DOI] [PubMed] [Google Scholar]
- 95.Stefani S, Chung DR, Lindsay JA, Friedrich AW, Kearns AM, Westh H, et al. Meticillin-resistant Staphylococcus aureus (MRSA): global epidemiology and harmonisation of typing methods. Int J Antimicrob Agents. 2012;39(4):273–82. 10.1016/j.ijantimicag.2011.09.030 [DOI] [PubMed] [Google Scholar]
- 96.Sharma-Kuinkel BK, Mongodin EF, Myers JR, Vore KL, Canfield GS, Fraser CM, et al. Potential influence of Staphylococcus aureus clonal complex 30 genotype and transcriptome on hematogenous infections. Open Forum Infect Dis. 2015;2(3):ofv093. Epub 2015/07/28. 10.1093/ofid/ofv093 ; PubMed Central PMCID: PMCPmc4512144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lindsay JA, Moore CE, Day NP, Peacock SJ, Witney AA, Stabler RA, et al. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J Bacteriol. 2006;188(2):669–76. Epub 2005/12/31. 10.1128/JB.188.2.669-676.2006 ; PubMed Central PMCID: PMCPmc1347281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goerke C, Fluckiger U, Steinhuber A, Zimmerli W, Wolz C. Impact of the regulatory loci agr, sarA and sae of Staphylococcus aureus on the induction of alpha-toxin during device-related infection resolved by direct quantitative transcript analysis. Mol Microbiol. 2001;40(6):1439–47. Epub 2001/07/10. . [DOI] [PubMed] [Google Scholar]
- 99.Cheung AL, Yang SJ, Bayer AS, Xiong YQ. Disparity in the in vitro versus in vivo regulation of fibronectin-binding proteins by 2 global regulators, saeRS and sigB, in Staphylococcus aureus. J Infect Dis. 2009;200(9):1371–4. Epub 2009/10/08. 10.1086/606011 ; PubMed Central PMCID: PMCPmc2814518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Wamel W, Xiong YQ, Bayer AS, Yeaman MR, Nast CC, Cheung AL. Regulation of Staphylococcus aureus type 5 capsular polysaccharides by agr and sarA in vitro and in an experimental endocarditis model. Microbial pathogenesis. 2002;33(2):73–9. Epub 2002/08/31. . [DOI] [PubMed] [Google Scholar]
- 101.Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong YQ. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004;40(1):1–9. Epub 2004/01/22. . [DOI] [PubMed] [Google Scholar]
- 102.Jenkins A, Diep BA, Mai TT, Vo NH, Warrener P, Suzich J, et al. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. MBio. 2015;6(1):e02272–14. Epub 2015/02/19. 10.1128/mBio.02272-14 ; PubMed Central PMCID: PMCPmc4337569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chaves-Moreno D, Wos-Oxley ML, Jauregui R, Medina E, Oxley AP, Pieper DH. Exploring the transcriptome of Staphylococcus aureus in its natural niche. Scientific reports. 2016;6:33174 Epub 2016/09/20. 10.1038/srep33174 ; PubMed Central PMCID: PMCPmc5027550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Etz H, Minh DB, Henics T, Dryla A, Winkler B, Triska C, et al. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proceedings of the National Academy of Sciences. 2002;99(10):6573–8. 10.1073/pnas.092569199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, et al. A novel Staphylococcus aureus Vaccine: Iron surface determinant B induces rapid antibody responses in Rhesus Macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74(4):2215–23. 10.1128/IAI.74.4.2215-2223.2006 PubMed PMID: PMC1418914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, Mininni T, et al. Staphylococcus aureus manganese transport protein C is a highly conserved cell surface protein that elicits protective immunity against S. aureus and Staphylococcus epidermidis. J Infect Dis. 2012;205(11):1688–96. Epub 2012/04/05. 10.1093/infdis/jis272 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ouyang S, Sau S, Lee CY. Promoter analysis of the cap8 operon, involved in type 8 capsular polysaccharide production in Staphylococcus aureus. J Bacteriol. 1999;181(8):2492–500. Epub 1999/04/10. ; PubMed Central PMCID: PMCPmc93676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miyafusa T, Caaveiro JM, Tanaka Y, Tsumoto K. Dynamic elements govern the catalytic activity of CapE, a capsular polysaccharide-synthesizing enzyme from Staphylococcus aureus. FEBS Lett. 2013;587(23):3824–30. Epub 2013/10/26. 10.1016/j.febslet.2013.10.009 . [DOI] [PubMed] [Google Scholar]
- 109.Smith EM, Green LE, Medley GF, Bird HE, Fox LK, Schukken YH, et al. Multilocus sequence typing of intercontinental bovine Staphylococcus aureus isolates. J Clin Microbiol. 2005;43(9):4737–43. Epub 2005/09/08. 10.1128/JCM.43.9.4737-4743.2005 ; PubMed Central PMCID: PMCPmc1234155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smyth DS, Feil EJ, Meaney WJ, Hartigan PJ, Tollersrud T, Fitzgerald JR, et al. Molecular genetic typing reveals further insights into the diversity of animal-associated Staphylococcus aureus. Journal of medical microbiology. 2009;58(Pt 10):1343–53. Epub 2009/06/17. 10.1099/jmm.0.009837-0 . [DOI] [PubMed] [Google Scholar]
- 111.Spoor LE, Richardson E, Richards AC, Wilson GJ, Mendonca C, Gupta RK, et al. Recombination-mediated remodelling of host–pathogen interactions during Staphylococcus aureus niche adaptation. Microb Genom. 2015;1(4). 10.1099/mgen.0.000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rozemeijer W, Fink P, Rojas E, Jones CH, Pavliakova D, Giardina P, et al. Evaluation of approaches to monitor Staphylococcus aureus virulence factor expression during human disease. PloS one. 2015;10(2):e0116945 Epub 2015/02/27. 10.1371/journal.pone.0116945 ; PubMed Central PMCID: PMCPmc4342157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sau S, Bhasin N, Wann ER, Lee JC, Foster TJ, Lee CY. The Staphylococcus aureus allelic genetic loci for serotype 5 and 8 capsule expression contain the type-specific genes flanked by common genes. Microbiology. 1997;143 (Pt 7):2395–405. Epub 1997/07/01. 10.1099/00221287-143-7-2395 . [DOI] [PubMed] [Google Scholar]
- 114.Gupta RK, Alba J, Xiong YQ, Bayer AS, Lee CY. MgrA activates expression of capsule genes, but not the alpha-toxin gene in experimental Staphylococcus aureus endocarditis. J Infect Dis. 2013;208(11):1841–8. Epub 2013/08/01. 10.1093/infdis/jit367 ; PubMed Central PMCID: PMCPmc3814835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li W, Ulm H, Rausch M, Li X, O'Riordan K, Lee JC, et al. Analysis of the Staphylococcus aureus capsule biosynthesis pathway in vitro: characterization of the UDP-GlcNAc C6 dehydratases CapD and CapE and identification of enzyme inhibitors. Int J Med Microbiol. 2014;304(8):958–69. Epub 2014/07/16. 10.1016/j.ijmm.2014.06.002 . [DOI] [PubMed] [Google Scholar]
- 116.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106(14):5883–8. Epub 2009/03/19. 10.1073/pnas.0900743106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moreillon P, Que YA. Infective endocarditis. Lancet (London, England). 2004;363(9403):139–49. Epub 2004/01/17. 10.1016/s0140-6736(03)15266-x . [DOI] [PubMed] [Google Scholar]
- 118.McAdow M, Kim HK, DeDent AC, Hendrickx APA, Schneewind O, Missiakas DM. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS pathogens. 2011;7(10):e1002307 10.1371/journal.ppat.1002307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Handke LD, Hawkins JC, Miller AA, Jansen KU, Anderson AS. Regulation of Staphylococcus aureus MntC expression and its role in response to oxidative stress. PloS one. 2013;8(10):e77874 Epub 2013/11/10. 10.1371/journal.pone.0077874 ; PubMed Central PMCID: PMCPmc3810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Presence of the four conserved cap5 operon mutations is indicated in green while the absence of mutations is indicated in red.
(PDF)
(TIF)
(A) All isolates associated with S. aureus infections in healthcare and community settings. (B) S. aureus isolates associated with the main types of clinical infections in healthcare settings.
(DOCX)
(DOCX)
Approximately-maximum-likelihood phylogenetic trees of analyzed S. aureus CP5 (A and C) and CP8 (B and D) isolates annotated with the distribution of cap operon SNPs (A and B) and indels in US S. aureus isolates (C and D). (A) cap operon SNPs in CP5 isolates. SNPs were identified across the cap operon based on mapping against S. aureus HO 5096 0412 reference. Isolate tracks color-coded by CC (legend below figure). Color-coding of SNPs; Green A, Blue G, Black T and Red C. (B) cap operon SNPs in CP8 isolates. SNPs were identified across the cap operon and plotted against tree of all CP8 USA isolates based on mapping against S. aureus MSSA476 reference. Isolate tracks color-coded by CC legend below figure). (C) cap operon indels in CP5 isolates. Indels were identified across the cap operon and plotted against tree of all CP5 USA isolates based on mapping against S. aureus HO 5096 0412 reference. Isolate tracks color-coded by CC (legend below figure). Color-coding of indels; Vertical magenta dots indicates insertion while dark gray indicates deletion. For each isolate, the mapping coverage is indicated by the light grey horizontal field, with white regions demonstrating deletion of the corresponding cap genomic region. As such, this figure shows the partial loss of cap5D-E in a single CC97 isolate. (D) cap operon indels in CP8 isolates. Indels were identified across the cap operon and plotted against tree of all CP8 USA isolates based on mapping against S. aureus MSSA476 reference.
(DOCX)
Three different S. aureus strains: Reynolds (CP5), its isogenic CP-negative mutant, and the CP- negative Reynolds complemented with CP8-coding sequences were used to challenge mice. The strains were tested in a blinded fashion with two independent experiments per strain. S. aureus was collected at the time of challenge (T0) and from the blood of infected mice 6 hr post infection (T6), and stained with rabbit anti-CP5, or rabbit anti-CP8 antibodies, or normal rabbit IgGs. Bright-field (A) and fluorescence photographs (B) of IFA staining are shown for each strain at both time points.
(TIF)
(A) Capsular polysaccharide type 5 is composed of repeat units of D-N-acetylmannosamine (D-ManNAc); L-N-acetylfucosamine (L-FucNAc) and D-L-acetylfucosamine (D-FucNAc). Cap5D is associated with synthesis of the D-FucNAc precursor (Li et al. Internatl. J. Med. Micro. 2014) and Cap5E is primarily associated with synthesizing L-FucNAc precursor (Miyafusa et al. FEBS Lett. 2013). (B) Cap5D is a 4, 6-dehydratase that converts UDP-D-GlcNAc to a D-FucNAc precursor, while Cap5E has 4, 6-dehydratase and 5-epimerase activity that converts UDP-D-GlcNAc to an L-FucNAc precursor but can also generate the analogous D-FucNAc precursor in a reverse epimerization reaction (Miyafusa et al. FEBS Lett. 2013). We propose that for S. aureus USA300 strains where the cap5D gene has a premature stop codon, the UDP-D-FucNAc precursor UDP-2-acetoamino-2, 6-dideoxy-a-D-xylo-4-hexulose (highlighted in orange) is derived as byproduct of the CapE epimerase reaction.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.