Abstract
Intraportal allogeneic islet transplantation has been demonstrated as a potential therapy for type 1 diabetes (T1D). The placement of islets into the liver and chronic immunosuppression to control rejection are two major limitations of islet transplantation. We hypothesize that localized immunomodulation with a novel form of FasL chimeric with streptavidin, SA-FasL, can provide protection and long-term function of islets at an extrahepatic site in the absence of chronic immunosuppression. Allogeneic islets modified with biotin and engineered to transiently display SA-FasL on their surface showed sustained survival following transplantation on microporous scaffolds into the peritoneal fat in combination with a short course (15 days) of rapamycin treatment. The challenges with modifying islets for clinical translation motivated the modification of scaffolds with SA-FasL as an off-the-shelf product. Poly(lactide-co-glycolide) (PLG) was conjugated with biotin and fabricated into particles and subsequently formed into microporous scaffolds to allow for rapid and efficient conjugation with SA-FasL. Biotinylated particles and scaffolds efficiently bound SA-FasL and induced apoptosis in cells expressing Fas receptor (FasR). Scaffolds functionalized with SA-FasL were subsequently seeded with allogeneic islets and transplanted into the peritoneal fat under the short-course of rapamycin treatment. Scaffolds modified with SA-FasL had robust engraftment of the transplanted islets that restored normoglycemia for 200 days. Transplantation without rapamycin or without SA-FasL did not support long-term survival and function. This work demonstrates that scaffolds functionalized with SA-FasL support allogeneic islet engraftment and long-term survival and function in an extrahepatic site in the absence of chronic immunosuppression with significant potential for clinical translation.
Introduction
Exogenous insulin treatment is the standard of care for type 1 diabetic (T1D) patients. However, daily insulin treatment negatively affects the quality of life and is often ineffective in preventing recurrent hyperglycemic episodes with consequent development of micro- and macro-angiopathic lesions and the development and progression of chronic complications [1]. Allogeneic islet transplantation has proven effective in improving metabolic control/quality of life and in preventing severe hypoglycemia in patients with T1D [2-6]. However, broad application of clinical allogeneic islet transplantation is limited by the chronic use of immunosuppression to control immune rejection and its sequelae [5], and transplantation into the liver, which compromises immediate post-graft islet engraftment as well as long-term survival [7].
T cells are the major culprit of both T1D and allogeneic islet graft rejection by recognizing and responding to beta cell autoantigens and allogeneic major and minor histocompatibility antigens [8-12]. T cells upregulate Fas receptor and become sensitive to FasL-mediated apoptosis [13]. Apoptosis induced by FasL interaction with Fas on immune cells plays an important role in immune homeostasis and tolerance to self-antigens [14-17], and can be applied to address allogeneic rejection [18]. FasL may also play an active role in the generation of immunoregulatory cells [19] and their function [20-23]. Several subsets of immunoregulatory cells were shown to be more resistant to Fas/FasL-mediated apoptosis, and some of which use FasL as a death molecule to eliminate antigen-reactive T cells [20-25]. We have recently demonstrated localized tolerance achieved by the direct display of SA-FasL on allogeneic islets transplanted under the kidney capsule was mediated by the elimination of alloreactive T effector cells and induction/expansion of CD4+CD25+FoxP3+ Treg cells that maintained tolerance [26]. The critical role apoptosis plays in immune tolerance (central and peripheral) combined with the potential of SA-FasL in apoptosis and generation/function of immunoregulatory cells make this an attractive molecule to modulate alloreactive immune responses for tolerance induction to allogeneic islet grafts.
The development of an extrahepatic site lacking the short and long-term complications of intraportal transplantation will significantly contribute to wide-spread application of clinical islet transplantation. For intraportal islet transplantation, islets are infused into the portal vein allowing immediate exposure of the graft to the blood, which initiates instant blood-mediated inflammatory reactions (IBMIR [27]). IBMIR is responsible for 50-80% of early loss of transplanted islets and involves the binding of tissue factor expressed on islets with platelets, resulting in their activation [7, 27]. Activated platelets trigger coagulation and complement cascades, leading to granulocyte and monocyte infiltration into the islet graft and their ultimate destruction [7, 27, 28]. Intraportal transplantation also suffers from long-term complications, including hepatic steatosis and β-cell apoptosis/non-function due to the inherent hyperglycemic environment and relatively higher concentrations of drug metabolites in the liver [29]. Porous scaffolds have demonstrated the capacity to modulate the local environment in order to promote engraftment and long-term function [30-34]. Islets can readily be seeded into the pores, while the porosity supports rapid cell infiltration for integration with the host tissue. Microporous scaffolds composed of the biocompatible, biodegradable, FDA approved copolymer of lactide and glycolide (poly(lactide-co-glycolide) (PLG), creates and maintains a space for transplanted islets while enabling control of their distribution and density within the scaffold [35, 36]. The high porosity enables nutrient diffusion, rapid host tissue infiltration and islet revascularization. These scaffolds have been decorated with extracellular matrix proteins from the basement membrane (e.g., collagen IV), which results in a significant increase in islet function post transplantation [35, 37].
In this report, we describe the attachment of SA-FasL to microporous PLG scaffolds to achieve chronic immunosuppression-free, long-term survival of allogeneic pancreatic islets transplanted into an extrahepatic site, i.e. the epididymal fat pad. The omentum has emerged as a leading candidate in human clinical trials due to its thin, highly vascularized membrane and portal draining that recreates the physiological effects of insulin in the liver [38, 39]. In mice, the epididymal fat pad has many similar features to the omentum, and we have developed scaffolds to support engraftment of transplanted islets at this site. Initial studies employed an established model in which islets are modified with SA-FasL, with subsequent transplantation onto a microporous scaffold. Subsequently, we developed procedures for modifying microporous PLG scaffolds with biotin for subsequent immobilization of SA-FasL, and characterized the binding and functionality of the immobilized protein. These studies employed islets isolated from BALB/c mice and transplanted into the epididymal fat pad of C57BL/6 mice, a fully mismatched transplantation model. The function of the transplanted islets was monitored by blood glucose levels as well as an intraperitoneal glucose tolerance test. Collectively, these studies address two major issues with clinical islet transplantation; development of an extrahepatic site for islet engraftment and overcoming immune rejection without of the use of chronic immunosuppression.
Materials and methods
Materials
Poly(lactide-co-glycolide) (75:25) (PLG) (approx. 80,000 g/mol) with a single carboxylic acid end-group and an inherent viscosity of 0.76 dL/g was purchased from Lakeshore Biomaterials (Birmingham, AL). Poly(ethylene-alt-maleic anhydride) (PEMA) was purchased from Polyscience, Inc. (Warrington, PA). EZ link Amine-PEG2-Biotin was purchased from Fisher Scientific. 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), dichloromethane (DCM), dimethylsulfoxide (DMSO), and all other reagents were purchased from Sigma Aldrich (St. Louis, MO) unless noted otherwise.
Biotinylation of PLG and characterization
PLG (890 mg, 0.011 mmol) was added to a 20 mL glass scintillation vial and dissolved in 10 mL DMSO. The carboxyl end group of PLG was activated by first adding EDC (10.6 mg, 0.056 mmol) dissolved in 1 mL DMSO followed by NHS (6.4 mg, 0.056 mmol) dissolved in 1 mL DMSO, and the reaction was allowed to stir for 15 min. Amine-PEG2-Biotin (5 mg, 0.056 mmol) was dissolved in 1 mL DMSO and added dropwise to the stirring solution of PLG-NHS and the reaction was allowed to stir overnight. Excess biotinylation reagent was removed by extraction. The reaction mixture was diluted into 200 mL of DCM and washed 4 times with 150 mL of a saturated salt solution (brine). The organic layer was dried over anhydrous sodium sulfate, filtered, concentrated by rotary evaporation, precipitated into ice cold methanol, and stored in vacuo overnight to remove residual solvents. Functionalization was confirmed with 1H-NMR (DMSO-d6).
Particle and scaffold fabrication
PLG microparticles were formed for scaffold fabrication as previously described [40]. Briefly, PLG was dissolved in 2.04 mL of DCM (6 wt%) and sonicated in 10 mL of a 1% solution of PEMA at 100% amplitude (Cole-Parmer, 130 W, 3 mm stepped tip). The emulsion was poured into 200 mL of 0.5% PEMA and the organic solvent was evaporated by stirring the emulsion overnight. The particles were recovered by washing four times with deionized water by centrifugation at 7000 x g for 15 min at 4 deg C. Particles were lyophilized for 48 hours and stored under vacuum. Biotin-PLG microparticles were similarly fabricated, however biotin-PLG conjugates were combined with unmodified PLG at a mass ratio of 3:1 (biotin-PLG:PLG) for a final concentration of 6 wt% in DCM.
Porous scaffolds were formed by mixing PLG particles with NaCl (250 μm< d < 425 μm) at a 1:30 ratio (PLG:NaCl). The mixture was pressed in a 5 mm KBr die using a Carver press at 1500 psi and foamed in CO2 at 750 psi for 16 hours. Scaffolds were leached in water for 1 hour followed by a second wash for 30 minutes. Scaffolds were disinfected by soaking them in 70% ethanol and washed with deionized water.
Particle characterization
The size and zeta potential of the particles was determined by dynamic light scattering (DLS) by mixing 10 μL of a 25 mg/mL particle solution into 990 μL of MilliQ water using a Malvern Zetasizer ZSP (Westborough, MA).
Protein loading and quantification
Particles were incubated with various concentrations of fluorophore-labeled streptavidin at 1 mg particles/mL for 20 minutes at various concentrations. Unbound streptavidin was removed by washing the particles with PBS by centrifugation (7000 x g, 5 min, 4 deg. C). To quantify the amount of fluorophore binding to the particles, particles were dissolved in DMSO and fluorescence was quantified using a plate reader (Synergy 2 (BioTek)) at 578 nm excitation and 605 nm emission.
Scaffolds were incubated with fluorescent streptavidin by applying 10 μL of the SA solution (0-40 ng/μL) to both sides of the disc (a total of 20 μL) for 20 minutes. Unbound streptavidin was removed by washing the scaffold three times with 1.5 mL of PBS. Scaffolds were dissolved in DMSO and fluorescence was quantified as described above.
Apoptosis assay
Particles (1 mg) and scaffolds were incubated with SA-FasL (Particles: 1 mL, 400 ng/μL, scaffold: 20 μL, 0-50 ng/μL) and washed as described above. To assess the ability for FasL particles or scaffolds to induce apoptosis in vitro, 1 mg/mL particles or a single scaffold was added to a 96 well plate containing A20 cells (mouse B lymphoma) at a concentration of 1.5×106 cells/mL and incubated for 18 hours. Cells were removed from the plate, stained with annexin V and propidium iodide (Life Technologies), and analyzed via flow cytometry.
Mice and recombinant proteins
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from Jackson Laboratory and bred in our specific pathogen-free animal facility at the University of Louisville using protocols approved by the Institutional Animal Care and Use Committee. Recombinant SA and SA-FasL proteins were made with the Drosophila DES expression system (Invitrogen) following standard protocols [18].
Islet isolation and engineering with SA-FasL protein
BALB/c islets were harvested from 8 to 12-week-old donors under anesthesia. Donor pancreata were perfused with 3 mL of cold Liberase TL (Roche Diagnostics) then removed and incubated for 20 minutes at 37°C. Islets were isolated using a Ficoll gradient (Sigma-Aldrich). Islets were kept overnight in RPMI-1640 medium supplemented with penicillin/streptomycin (100 U/mL and 100 μg/mL) and 10% fetal bovine serum in an incubator at 37°C with 5% CO2. Islets were transferred to a 14-mL round bottom tube and washed in PBS. Islets were then incubated in 5 μM EZ-Link™ Sulfo-NHS-LC-Biotin solution (Thermo Scientific) at 20°C for 30 minutes. After incubation, islets were washed twice in PBS to remove any unbound biotin. Then, islets were incubated in PBS containing SA-FasL protein (~200 ng SA-FasL/500-550 islets/200 μl PBS) at 20°C for 30 minutes. Islets were washed twice in PBS to remove any unbound protein before transplantation. Select biotin-PLG scaffolds were engineered by placing scaffolds to a round bottom tube and adding SA-FasL (0.5 or 2.5 μg /scaffold) diluted in PBS and incubating at 20°C for 30 minutes while rotating and shaking the tube every 10 minutes. Scaffolds were washed twice before being loaded with islets.
Islet transplantation
C57BL/6 mice were chemically induced with diabetes by intravenous (i.v.) injection of streptozotocin (200 mg/kg). Mice were monitored by reading blood glucose where ≥ 250 mg/dL for two consecutive days was considered diabetic. Islets were loaded onto PLG scaffolds (2/mouse). Diabetic mice were given anesthesia and a small incision was made on the abdomen to allow scaffolds to be placed on epididymal fat pads. Adipose tissue was wrapped around scaffolds before being returned to the abdomen. Mice were then sutured. Select mice were administered rapamycin through i.p. injection of 0.2 mg/kg daily for 15 days starting the day of transplant. Mice were monitored for diabetes and those with ≥ 250 mg/dL blood glucose level for two consecutive days considered rejecting the islet graft.
Intraperitoneal Glucose Tolerance Test
Mice were put in clean cages without food and allowed to fast for 6 hours. Mice were then injected with 25% glucose solution (2 g/kg body weight) and monitored for blood glucose levels before injection and at 10, 20, 30, 60, 90, and 120 minutes post glucose injection.
Mixed Lymphocyte Reaction
Spleen and draining lymph nodes were harvested from mice after rejection of graft or at experimental end point (> 200 days) if mice remained euglycemic up to that point. Organs were processed into single cell suspensions using frosted slides. ACK lysis buffer (ThermoFisher Scientific) was added to spleen to lyse red blood cells. Splenocytes were panned, labelled with (5(6)-Carboxyfluorescein N-hydroxysuccinimidyl ester) (CFSE), and used as responders in a standard mixed lymphocyte reaction assay [41]. Stimulator cells were prepared from either naïve BALB/c (donor) or C3H (3rd party) mice, irradiated with 200 cGy, and cocultured with equal numbers of responder cells in 96-well plates (0.1×106 cells/well). Cells were cultured in 200 μL DMEM supplemented with HEPES buffer, sodium pyruvate, penicillin/streptomycin, L-Glutamine (ThermoFisher Scientific), FBS, L-Arginine HCL, folic acid, L-Asparagine, 2-Mercaptoethanol (Sigma), and responder serum. Cells were harvested after four days of culture at 37°C and stained with Alexa 700-CD4 Ab, APC-Cy7- CD8 Ab, and 7AAD to separate dead cells (BD Pharmingen). Cells were collected using BD LSR II and analyzed using Diva software.
Statistical analysis
Flow data was tested for significance using a two tailed Welch’s t-test. Graft survival was tested for significance using the log-rank test. P values less than 0.05 were considered significant. Survival curves, IPGTT, and flow graphs were created and analyzed using GraphPad Prism software.
Results
Transplantation of SA-FasL-engineered islets on microporous scaffolds
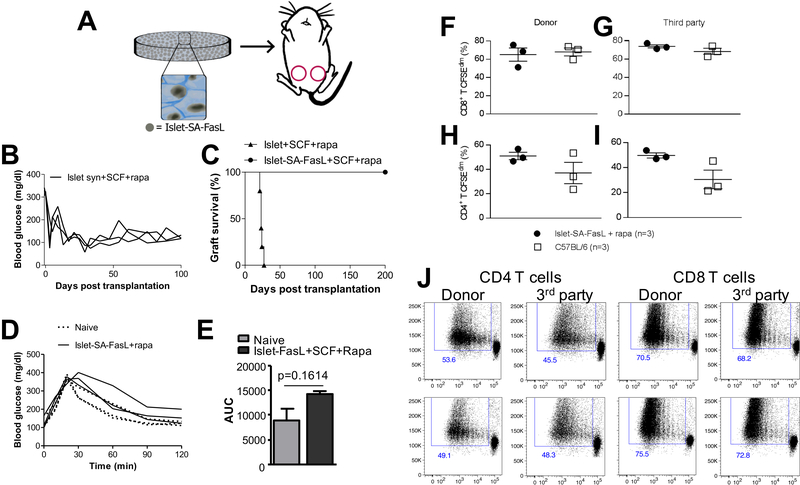
We investigated the transplantation of SA-FasL-engineered islets on microporous scaffolds implanted into the epididymal fat pad (Fig 1A). Initial studies employed syngeneic islets transplanted into streptozotocin-induced diabetic mice to determine the impact of the scaffolds and short-term rapamycin on the engraftment and function of the islets. Transplantation of the syngeneic islets led to the establishment of euglycemia within 10 days for all animals, and blood glucose levels fell below 200 mg/dL, which was used to define graft survival (Fig 1B). All animals with syngeneic islets engineered with SA-FasL had graft survival for the duration of the study (100 days). Subsequently, allogeneic islets engineered with SA-FasL were transplanted on microporous scaffolds. Unmodified islets transplanted on scaffolds with transient rapamycin underwent rejection, as indicated by increased blood glucose levels, by day 40 (Supplementary Fig. 1). Mice transplanted with allogeneic islets modified with SA-FasL and receiving transient rapamycin had graft survival that was sustained for 200 days (Fig 1C), similar to the results with syngeneic islets. Rapamycin has been previously reported to synergize with SA-FasL presentation to prolong allograft survival [26], as either factor alone results in only short-term graft function. An intraperitoneal glucose tolerance test (IPGTT) study demonstrated that the normalization of blood glucose levels by the transplanted islets was similar to that observed with naïve mice (i.e., non-diabetic) (Fig 1D, E), which is consistent with previous reports of islets transplanted on scaffolds [36, 42].
Figure 1:
SA-FasL engineered islets establish allogeneic tolerance when transplanted on PLG scaffolds. (A) Schematic showing biotinylated islets functionalized with SA-FasL are loaded on microporous PLG scaffolds and implanted in the epididymal fat pad of mice. (B) Individual tracing of blood glucose levels for syngeneic islets modified with SA-FasL and transplanted on scaffolds (SCF), with mice receiving a short course of rapamycin (n=3, mean survival time > 100 days). (C) Kaplan Meier analysis of allogeneic BALB/c islets transplanted under the short cover of rapamycin on unmodified PLG scaffolds in C57BL/6 recipients. Conditions include SA-FasL-engineered islets on scaffolds with rapamycin (n = 5, mean survival time >200 days), naïve islets on scaffolds with rapamycin (n = 5, mean survival time = 23 ± 2.19 days). (D) Intraperitoneal glucose tolerance test of long-term islet grafts compared to naïve C57BL/6 mice after fasting for 6 hours, followed by i.p. glucose injection. Blood glucose of mice was taken starting just before injection and at the indicated time points. (E) Area under curve for the intraperitoneal glucose tolerance test. The area for naïve mice compared to the transplanted mice is not significantly different (p=0.16). (F-I) T cell proliferative response from recipients of long-term (> 200 days) BALB/c SA-FasL-engineered islets mounted on unmodified PLG scaffolds plus rapamycin (n = 3) and naïve C57BL/6 as controls (n = 3). Responders were labeled with CFSE and used against BALB/c donor (F,H) and third party C3H (G,I) stimulators in a standard ex vivo mixed lymphocyte reaction. After 4 days of culture, cells were stained with antibodies against CD8 (F,G) and CD4 (H,I) molecules and incubated with 7AAD to gate out dead cells before flow cytometry analysis. (J) Representative dot plots of data presented in F, G, H and I. Bars represent mean and SEM. Asterisks represent level of significance (*p<0.05, **p<0.01) found by using a two-tailed Welch’s t-test.
We next assessed the immune competency of long-term islet graft recipients and if the observed graft survival is associated with systemic immune non-responsiveness against the donor alloantigens. T cell proliferative responses were analyzed from the spleens and draining lymph nodes of the long-term allogeneic islet graft survivors. The collected cells were labeled with CFSE and used against BALB/c donor and third party C3H stimulators in a standard ex vivo mixed lymphocyte reaction [41]. After 4 days of culture, the responses from CD8+ T cells indicated similar proliferative responses for the SA-FasL-engineered islets and an age-matched C57BL/6 control, with responses similar to both the donor and third-party stimulators (Fig 1F, G). Interestingly, CD4+ T cell responses were greater for the SA-FasL-engineered islets relative to age-matched control (Fig H, I). This response was similar for both the donor and third-party stimulators. Representative dot plots for the donor and third-party stimulators are depicted in Fig 1J. These results demonstrate that recipients of long-surviving islet allografts are immunocompetent and show lack of systemic unresponsiveness to donor antigens, demonstrating localized nature of graft protection. Collectively, these studies demonstrate that the microporous scaffolds for transplantation of SA-FasL-engineered islets to an extrahepatic, extra-renal site provides for engraftment of the islets and protection from the immune response similar to previous reports performed with transplantation under the kidney capsule [26].
Synthesis and characterization of biotin-PLG conjugates and particle formation
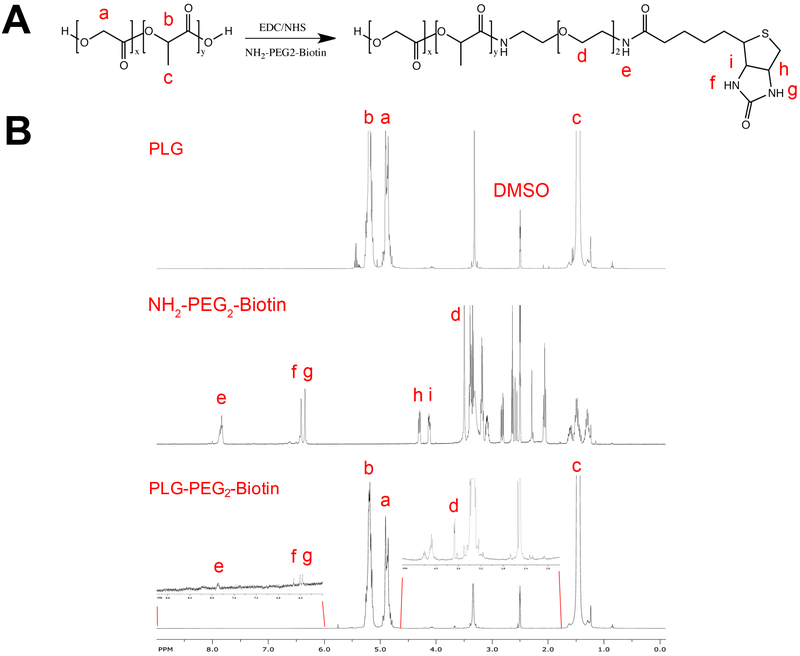
We subsequently investigated the modification of scaffolds with biotin to allow for immobilization of SA-FasL as a potential off-the-self product for immunomodulation. PLG was conjugated with a biotin linker and the biotin-PLG conjugates were subsequently formed into microspheres that were assembled into scaffolds. The carboxyl-terminal group of PLG was conjugated to the heterobifunctional linker NH2-PEG2-Biotin using carbodiimide chemistry and confirmed using 1H-NMR (Figs 2A, B). Biotin-PLG particles were prepared with a single emulsion-solvent evaporation procedure using the biotin-PLG conjugates. The particles displayed an average size of 860 ± 40 nm and a zeta potential of −16 ± 5.0 mV (Supplementary Fig 2).
Figure 2:
Characterization of biotin-PLG polymer. (A) Conjugation of NH2-PEG2-biotin to PLG via carbodiimide chemistry, resulting in biotin-PLG. (B) 1H-NMR in (D3C)2SO of PLG (top), biotin linker (middle), and biotin-PLG (bottom).
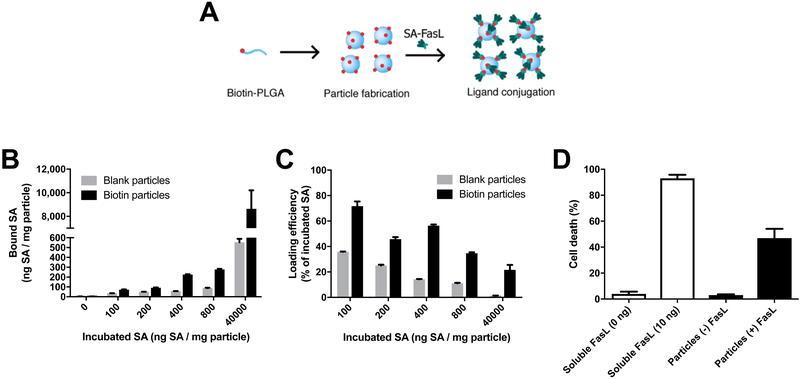
The ability of biotin-PLG particles to bind streptavidin was quantified through the maximum loading and efficiency of fluorescently-tagged streptavidin (AF568-SA) (Fig 3A). The unmodified particles non-specifically bound a small amount of AF568-SA, however the presence of biotin significantly increased the amount of bound protein. The concentration of AF568-SA incubated with particles was varied from 100 to 800 ng AF568-SA per mg of particle, which resulted in loadings of 75 to 280 ng AF568-SA per mg of particle (Fig 3B). A dose of 40,000 ng AF568-SA per mg of particle was used as a saturating dose, which produced a loading in excess of 8,000 ng AF568-SA per mg of particle. Although the amount loaded on the particle increased with greater concentration of AF568-SA, the loading did not increase linearly. The loading efficiency (defined as the amount of protein bound divided by the amount incubated) decreased from 75% to 35% as the concentration was increased from 100 to 800 ng AF568-SA per mg of particle (Fig 3C). At the maximal dose of 40,000 ng AF568-SA per mg of particle, the loading efficiency was 20% for the biotinylated particles and approached 0% for the non-biotinylated particles.
Figure 3:
Biotin-PLG particles can be efficiently conjugated with SA-FasL and induce apoptosis in mouse B lymphoma cell line. (A) Schematic of biotin-PLG particles being functionalized with SA-FasL. (B) The amount of fluorescently tagged SA bound to the particles was investigated by varying the protein concentration (0, 100, 200, 400, 800, and 40,000 ng SA/mL in 1 mL) incubated with 1 mg of biotin-PLG particles (“biotin particles”). As a control, 1 mg of unmodified PLG particles was incubated with the same SA concentrations (“blank particles”). After two spins and washes, particles were dissolved in DMSO and fluorescence was measured, n=3. (C) The loading efficiency was calculated by dividing the bound SA by the incubated SA, n=3. (D) Biotin-PLG particles functionalized with SA-FasL induce cell death in A20 cells. Particles were incubated with 400 ng / mL of SA-FasL in 1 mL with 1 mg of particles. After washing to remove unbound SA-FasL, particles were incubated with 1.5 × 105 A20 cells for 18 hours and cell death was analyzed via propidium iodide stain and flow cytometry (n=3). For comparison, soluble FasL was added to cells (0, 10 ng) and demonstrated the ability to induce cell death at low concentrations (n=3).
The bioactivity of immobilized SA-FasL was investigated next through their ability to induce apoptosis in the mouse B lymphoma cell line A20. Particles were incubated with 400 ng SA-FasL, which was selected based on the substantial difference in the binding to biotinylated and non-biotinylated particles (Fig 3B). The addition of particles modified with SA-FasL induced apoptosis in approximately 50% of the cells (Fig 3D). Biotinylated particles without SA-FasL did induce apoptosis at levels consistent with the negative control, which was approximately 1% of the cell population. The level of apoptosis induced by the particles was less than that obtained with soluble SA-FasL, which induced apoptosis in more than 90% of the cells for a dose of 10 ng.
SA-FasL loading on biotin-PLG scaffolds
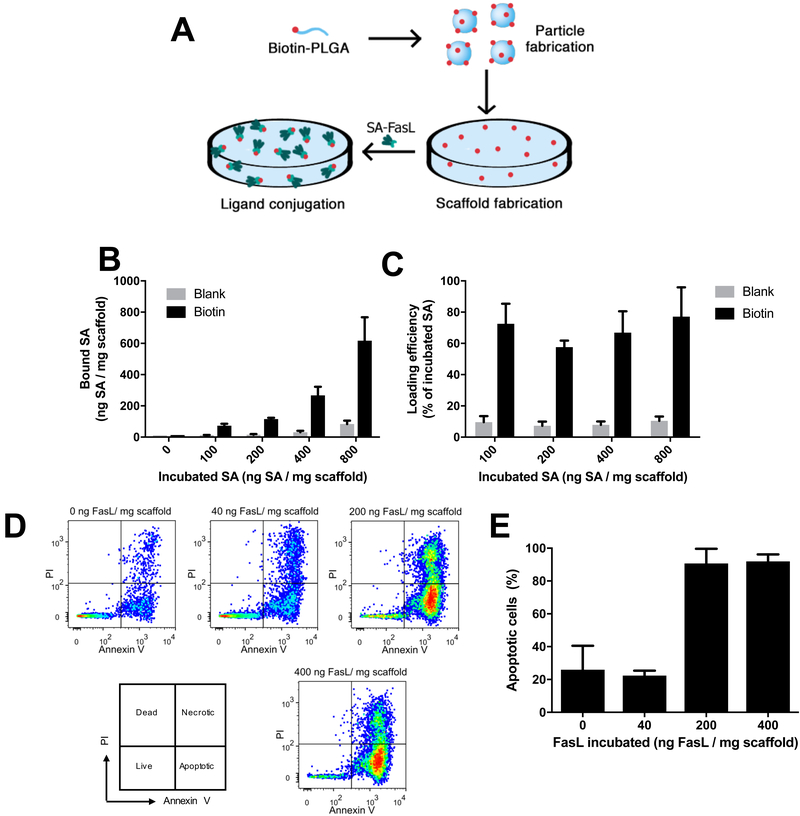
SA-FasL modified scaffolds were subsequently prepared by fabricating scaffolds from the biotinylated polymer with subsequent incubation with a solution containing SA-FasL (Fig 4A). Incubation of SA-AF568 at concentrations ranging from 100 to 800 ng SA-AF568 per mg of scaffold resulted in loadings that ranged from 70 to 620 ng SA per mg of scaffold, which was similar to particles at low concentrations (Fig 4B). The efficiency of loading was relatively constant across the range of concentrations (≈70%), which differed from what was observed with the particles that had a declining efficiency with increased concentration of SA-AF568 (Fig 4C). The increased efficiency may result from an increased surface area of the porous scaffold compared to the spherical particles. Unmodified scaffolds were used as a control and showed similar concentrations of non-specific binding as unmodified particles at higher protein concentrations.
Figure 4:
Biotin-PLG scaffolds can be efficiently conjugated with SA-FasL and induce apoptosis in mouse B lymphoma cell line. (A) Schematic of biotin-PLG particles functionalization with SA-FasL. (B) The amount of fluorescently tagged SA bound to scaffolds was investigated by varying the protein concentration (0, 100, 200, 400, and 800 ng SA/20μL) incubated with 2.5 mg biotin-PLG scaffolds (“biotin”). As a control, unmodified PLG scaffolds were incubated with the same SA concentrations (“blank”). After two washes, scaffolds were dissolved in DMSO and fluorescence was measured, n=3. (C) The loading efficiency was calculated by dividing the bound SA by the incubated SA, n=3. (D) Scaffolds were incubated with SA-FasL (0, 40, 200, and 400 ng / 20 μL). After washing to remove unbound SA-FasL, scaffolds were incubated with 1.5 × 105 A20 cells for 18 hours. Apoptotic and dead cells were analyzed via propidium iodide and Annexin V staining and flow cytometry (n=3). For comparison, 2.5 mg of soluble FasL was added to cells and induced apoptosis in the 98% of the cells (n=3). (E) Scaffolds required a minimum loading of 200 ng FasL / mg scaffold to induce apoptosis in the majority of A20 cells.
SA-FasL-loaded scaffolds had a greater efficiency for inducing apoptosis of A20 cells relative to the particles (Fig 4D, E). The induction of apoptosis was concentration-dependent as 40 ng SA-FasL/mg scaffold was not significantly different than the control but 200 ng/mg and 400 ng/mg increased apoptosis (Annexin V+) in more than 90% of the cells. These results demonstrate that SA-FasL could be functionalized to the surface of PLG scaffolds and effectively induce apoptosis in A20 cells.
SA-FasL scaffolds support allogeneic graft function without chronic immunosuppression
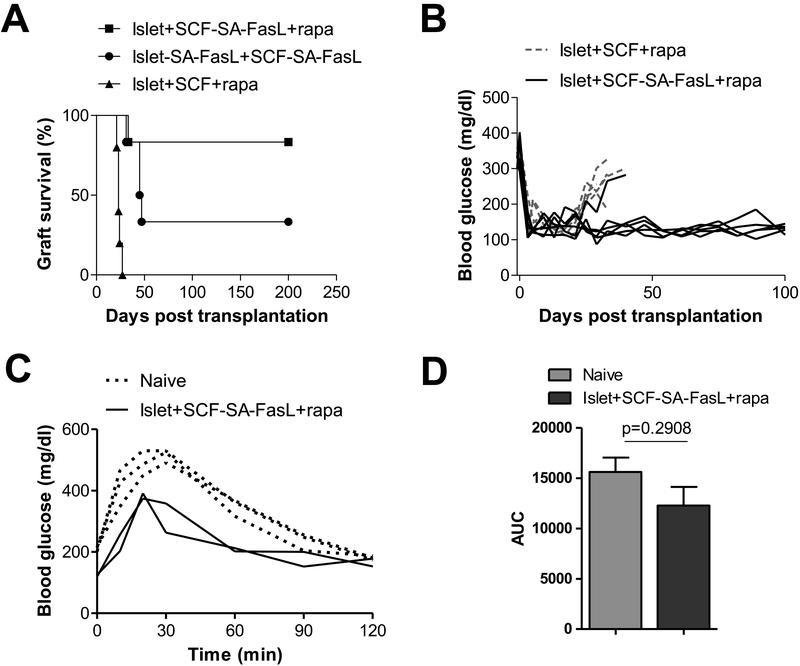
We subsequently investigated whether SA-FasL modified scaffolds could prevent allogeneic islet rejection similar to the SA-FasL-engineered islets, while also supporting engraftment and long-term function to maintain normoglycemia. Scaffolds engineered with SA-FasL were loaded with islets from BALB/c donors and transplanted into the peritoneal fat of diabetic C57BL/6 mice. Naïve islets mounted on SA-FasL-engineered PLG scaffolds along with transient rapamycin demonstrated graft survival for more than 200 days in more than 80% of the animals (Fig 5A), with one animal rejecting at day 30. Rapamycin without SA-FasL had a mean graft survival time of 23 ± 2 days (Fig 5A, B). Interestingly, when both islets and scaffolds were engineered with SA-FasL but did not receive the short course rapamycin treatment, one third of the mice established long-term tolerance while the rest rejected by day 50. For the transplantation of islets on SA-FasL modified scaffolds, normoglycemia was established within days of transplantation (Fig 5B). The blood glucose dynamics were similar between the SA-FasL-modified scaffolds and the unmodified scaffolds (with rapamycin) through day 20, at which point, the blood glucose levels began to rise for the unmodified scaffolds likely due to rejection of the islets. An IPGTT performed at day 200 demonstrated restoration of normoglycemia at a rate that was similar to naïve animals (Fig 5C, D).
Figure 5:
Islets on scaffolds conjugated with SA-FasL demonstrate robust long-term tolerance. (A) Survival of allogeneic BALB/c islets mounted on PLG scaffolds and transplanted in the epididymal fat pad of chemically diabetic C57BL/6 recipients. Groups included naïve islets mounted on SA-FasL-engineered PLG scaffolds plus rapamycin (n = 6, MST > 200 days, P=0.0007 vs rapamycin alone), SA-FasL-engineered islets mounted on SA-FasL-engineered PLG scaffolds (n = 6, MST = 46 days, P = 0.0007 vs rapamycin alone), and naïve islets transplanted under a short cover of rapamycin (n = 5, MST = 23 ± 2.19 days). All mice received islets loaded on 2 PLG scaffolds. PLG scaffolds were engineered with 2.5 μg/scaffold, except 3 mice in the islet-FasL + PLG-SA-FasL that were transplanted with PLG scaffolds engineered with 0.5μg/scaffold and all 3 animals rejected their grafts. Rapamycin was given to the indicated groups through i.p. injection at 0.2 mg/kg daily for 15 days starting on the day of transplantation. Mice were monitored twice weekly for blood glucose levels. Those with > 250 mg/dL for two consecutive readings 24 hours apart were considered diabetic and rejecting the graft. Statistical analysis was performed using log-rank test, **P<0.01, ***P<0.001. (B) Blood glucose readings of two groups of mice from (A). (C) Intraperitoneal glucose tolerance test (IPGTT) of long-term islet grafts compared to naïve C57BL/6 mice after fasting for 6 hours, followed by i.p. glucose injection. Blood glucose of mice was taken starting just before injection and at the indicated time points. (D) Area under the curve for (C).
Discussion
This report investigated the combination of SA-FasL and biomaterial scaffolds as a means to create a translatable site that supported the engraftment and long-term function of allogeneic islets at an extrahepatic and extrarenal site. Immunoprivileged sites, such as the testes, anterior chamber of the eye, brain, and tumors, have the ability to suppress destructive immune responses by various mechanisms [43-46]. Importantly, FasL was initially discovered as one of the molecules that plays a critical role in immunoprivileged sites [14, 15]. Indeed, we have recently reported that SA-FasL-engineered allogeneic islets induced localized immune privilege when transplanted under the kidney capsule [26]. Therefore, FasL not only contributes to the regulatory mechanisms in naturally occurring immunoprivileged sites in the body, yet also can be used to create “induced” immunoprivileged sites [26]. Herein, we demonstrated that allogeneic SA-FasL-engineered islets engrafted and normalized blood glucose levels for more than 200 days under a short course of rapamycin treatment (0.2 mg/kg daily starting the day of transplantation for a total of 15 doses) with transplantation on PLG scaffold into an extrahepatic site, one that has translational potential [47].
Given that localized presentation of SA-FasL on islets supported long-term function on the scaffold, we subsequently investigated the immobilization of SA-FasL to the scaffold as a means to minimize the cellular manipulation prior to transplantation. Biotin decorated PLG particles have been reported for targeted drug delivery to cancer cells [48]. Manipulating the scaffold to present SA-FasL would eliminate the steps needed for direct islet engineering, and as such save time and overcome potential undesired effects associated with engineering process, such as cell fragmentation. Moreover, scaffolds engineered with SA-FasL can be manufactured as a potential off-the-shelf immunomodulatory product for islet transplantation, expediting clinical translation. Importantly, the presentation of SA-FasL from surfaces has previously been reported not to interfere with its apoptotic function [26, 49]. To create scaffolds modified with SA-FasL, we chemically modified the polymer in solution [48, 50-52], which was subsequently formed in to particles and then into scaffolds. Initial attempts to functionalize scaffolds involved conjugation of biotin to the surface of a pre-formed scaffold, which produced inconsistent modification with SA-FasL and thus the direct modification of the polymer was pursued based on prior reports indicating enhanced target binding [53]. Stable spherical particles could only be formed from mixtures of the biotinylated and non-biotinylated polymer, as particles formed from only biotinylated polymer were unstable due to aggregation. The microspheres were employed to construct the scaffold using a gas foaming and particulate leaching process [54-56]. The scaffolds can readily be stored prior to islet seeding for transplantation; however, as a potential off-the-shelf product, further studies will be needed to test long-term storage of SA-FasL pre-engineered scaffolds for downstream applications.
The microporous structure of the scaffold enhanced protein loading and bioactivity relative to the particles. This observation likely results from the higher surface area of the scaffolds. Protein loading and efficiency were similar to other techniques like carbodiimide coupling to PLG particles [40, 57]. The presentation of SA-FasL from particles or scaffolds maintained the ability to induce apoptosis, although it was not as efficient as soluble SA-FasL, which may reflect the nature of ex vivo culture setting favoring better access of soluble SA-FasL to the Fas receptor on A20 cells. Further optimization of the PEG-biotin linker length may enable enhanced binding of SA-FasL to Fas by increasing the mobility of SA-FasL [58]. Interestingly, for concentrations between 40 and 400 ng/mg, the extent of binding was highly consistent within experiments, yet considerable variation in apoptosis was observed between experiments, suggesting a sensitivity to the protein loading or presentation within this range. Previous reports of surface modified apoptosis systems utilized surface anchored polymer chains with covalently linked anti-Fas antibodies but were only able to achieve up to 34% apoptosis in cells expressing Fas, whereas the method presented here achieved 92% apoptosis and in vivo protection of allogeneic islets [59]. This result may be due to the far greater surface density of protein (up to 150 ng/cm2 vs 1.6 ng/cm2), the reported robust apoptotic function of SA-FasL [60], and the positional display of this molecule on biotinylated scaffolds, preserving its function. These findings are consistent with our recent publication with polyethylene glycol (PEG) microparticles where we demonstrated a dose-dependent binding of SA-FasL to biotin presenting microparticles [61].
Importantly, microporous scaffolds functionalized with SA-FasL supported engraftment and function of the transplanted allogeneic islets that maintained normoglycemia for more than 200 days in the absence of chronic immunosuppression. Islets transplanted on unmodified PLG scaffolds were promptly rejected within 6-12 days after the rapamycin treatment ended, consistent with our previous results [26, 61]. The observed sustained survival and function of allogeneic islets without immunosuppression is consistent with our previously published data with islet directly engineered to transiently display SA-FasL on their surface, but not streptavidin or other apoptosis unrelated proteins used as controls [41]. Importantly, this data also consistent with our most recent study reporting sustained survival of allogeneic islets co-transplanted with biotinylated PEG microparticles presenting SA-FasL on their surface [61]. SA-FasL alone without rapamycin was able to delay rejection of all grafts with ~40% surviving for the 200-day observation period. This observation demonstrated that SA-FasL has the potential as monotherapy to achieve permanent graft survival, but complete efficacy may require further refinement of the protocol by increasing the dose of SA-FasL or duration of persistence. Our approach of functionalizing SA-FasL onto the surface of biotin-PLG scaffolds is an effective method to induce long-term function without chronic immunosuppression.
Long-term graft recipients generated T cell responses to both donor and third-party antigens at a similar magnitude to those of naïve mice. These findings demonstrate that immunomodulation with SA-FasL in context of PLG scaffolds does not result in immune incompetence of the graft recipients nor systemic tolerance to donor antigens. Lack of systemic unresponsiveness is expected given the localized nature of immunomodulation by SA-FasL and the demonstrated role of this molecule in physiological immune privilege [62]. These findings are further consistent with our published studies demonstrating that SA-FasL-engineered islets or PEG microgels presenting SA-FasL transplanted under the kidney capsule establish localized tolerance, which is maintained by CD4+CD25+FoxP3+ T regulatory cells [41, 61]. PEG microgels modified with SA-FasL that were co-transplanted with islets had approximately 90% engraftment and return to normoglycemia with transplantation into the kidney capsule, which is widely used in mouse models yet is generally considered a non-translational site. Herein, we report that modification of the microporous scaffolds with FasL results in approximately 80% engraftment and long-term function of the transplanted islets in the epididymal fat pad, which models the omentum as a translational site for islet transplantation.
The long term function in the absence of immunosuppression may result from localized Treg function. The sensitivity of Treg cells to FasL has previously been shown to be context dependent and regulated by various factors, including antigenic stimulation, proliferation, and cytokine milieu [63, 64]. Two lines of evidences from our own studies support this notion. First, we have demonstrated that Treg cells in NOD are preferentially more resistant to SA-FasL as compared with Teff cells [25]. Second, in collaboration with Askenasy and group, we have shown that sorted Treg cells modified to display SA-FasL on their surface have improved regulatory function and following adoptive trasnfer into prediabetic NOD prevented the development of diabetes [65, 66]. Importantly, SA-FasL-engineered Treg cells homed to the pancreas and regional LNs where they proliferated and induced apoptosis in Teff cells in situ via SA-FasL [66]. Our observations are also supported by a study demonstrating that cytokine-induced selective expression of FasL in the vasculature of human and mouse solid tumors results in preferential apoptosis in CD8+ T effector cells, but not Treg cells because of their expression of higher levels of c-FLIP [67]. Treg cells were shown to use FasL as an effector molecule to eliminate Teff cells [68]. Also, Treg cells expressing FasL were shown to regulate hapten-specific CD8+ T effector cell expansion through the elimination of Fas-expressing hapten-presenting DCs, resulting in the prevention of T-cell-mediated allergic immune responses in the skin [69].
A short course of rapamyicn was required for SA-FasL-induced tolerance. We have not extensively investigated the mechanistic synergy between SA-FasL and rapamycin, which we believe is complex and may operate on multiple levels. Rapamycin induces apoptosis via the mitochondria-mediated intrinsic pathway, while FasL apoptotic effect operates through extrinsic Fas-mediated pathway. As such, apoptosis mediated by these two pathways may ensure the physical elimination of pathogenic T cells. Furthermore, rapamycin has various other tolerogenic characteristics that involves generation of regulatory dendritic cells [70], T cell anergy [71], and most importantly generation and expansion of CD4+CD25+FoxP3+ T regulatory cells [72]. We [67, 73, 74] and others [67, 69] have shown that Treg cells are resistant to FasL-mediated apoptosis under inflammatory conditions. Given that Treg cells play a dominant role for the induced tolerance in our model as published previously [75, 76], we speculate that induction of apoptosis in Teff cells and generation and maintenance of Treg cells are the two potential regulatory steps targeted by SA-FasL and rapamycin for the observed synergy.
The positional display of SA-FasL on islets grafts or PLG scaffold did not negatively impact the long-term survival of islet grafts or their funciton. These observations are not in line with published studies implicating the expression of Fas by β cells in their destruction in autoimmunity setting. Unlike normal NOD female mice, which develop spontaneous diabetes within 30 weeks, NOD-lpr/lpr mice lacking a functional Fas do not develop diabetes [77]. Diabetes cannot be induced in NOD-lpr/lpr mice by adoptive transfer of diabetogenic T cells [77]. Also, transgenic expression of FasL in β cells resulted in accelerated diabetes caused by β cell suicide via Fas/FasL-induced apoptosis [78, 79]. It was shown that the expression of Fas on β cells is largely dependent on local levels of inflammatory cytokines. β cells in response to proinflammatory cytokines, such as IL-1β, TNF-α, and INF-γ, upregulate Fas on their surface and bocome senstive to FasL expressed on infiltrating lymphocytes [80, 81]. While an association with inflammatory cytokines has been proposed, the effector mechanisms that lead to β cell destruction are not well defined.
However, several lines of evidences have provided contradictory evidences against the role of Fas/FasL-mediated apoptosis in the destruction of β cells. Fas expression was not detectable on β cells from young NOD mice, but only on 1-5% of cells from older animals [82]. Treatment of NOD with an anti-FasL antibody did not prevent accelerated diabetes mediated by adoptive transfer of diabetogenic T cells [83]. Fas deficient neonatal pancreata of NOD-lpr/lpr mice, whihc do not develop spoantenous diabetes, underwent destruction when transplanted into diabetic NOD mice [83]. The resistance of NOD-lpr/lpr mice to diabetes was shown to be due to the presence of high numbers of double negative T cells expressing FasL and eliminating autoreactive T cells expressing Fas [84]. Furthermore, islets from young NOD mice are not sensitive to FasL-mediated killing unless precultured with IL-1β and IFN-γ to induce the expression of Fas [82]. Importantly, islet cells harvested from young NOD mice did not undergo apoptosis when cultured in the presence of anti-Fas Ab or FasL expressing transfectants [85]. Consistent with this observation are studies that islets of various species, including porcine, nonhuman primates, humans (Shirwan et al, unpublished observations), and rodents [76, 86], engineered to display SA-FasL on their surface did not negatively impact their viability or function both in vitro and in vivo. Extensive data in our recent publicaton [75] also demonstrate PEG microgels engineered with SA-FasL do not negatively impact islet viability and function when co-transplanted with naïve islets in an allogenic host. Taken together, these studies indicate that the precise role of Fas/FasL-mediated apoptosis in the destruction of β cells is ambiguous and may be model and condition dependent.
In conclusion, our studies demonstrate that PLG scaffolds support the engraftment and sustained survival of allogeneic islets directly engineered to transiently display SA-FasL on their surface in an extrahepatic and translational site in the absence of chronic immunosuppression. Importantly, scaffolds directly engineered with SA-FasL achieves a similar outcome as SA-FasL-engineered islets, demonstrating that immunoregulation induced in this setting does not require T cells establishing simultaneous physical interaction with both the alloantigen and SA-FasL on the target tissue, consistent with our recent study with PEG microgels presenting SA-FasL [61]. This observation suggests that T cells activated in response to alloantigens are eliminated within graft microenvironment upon encounter with SA-FasL attached to the scaffold. Importantly, the modification of biomaterial with SA-FasL avoids the complications associated with direct engineering of islet grafts and provides a potential off-the-shelf immunomodulatory product that may have applicability to a multitude of applications involving cell transplantation.
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations. However, they can be provided upon request.
Supplementary Material
Supplementary Figure 1: Blood glucose levels of islet transplanted mice in Fig. 1C
Supplementary Figure 2: Characterization of biotin-PLG particles. Dynamic light scattering of particles determined a size of 861 ± 39.2 nm (n=3) and a charge of −15.8 ± 4.98 mV (n=3).
Footnotes
Conflict of Interest: H.S., E.S.Y., A.J.G., and H.Z. are inventors on a pending patent on PEG engineered with SA-FasL and H.S. and E.S.Y. hold equity in FasCure Therapeutics, which has an option to license the SA-FasL technology from the University of Louisville.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Atkinson MA, Eisenbarth GS, Michels AW, Type 1 diabetes, Lancet 383(9911) (2014) 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bellin MD, Barton FB, Heitman A, Harmon JV, Kandaswamy R, Balamurugan AN, Sutherland DE, Alejandro R, Hering BJ, Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes, Am J Transplant 12(6) (2012) 1576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rickels MR, Schutta MH, Mueller R, Kapoor S, Markmann JF, Naji A, Teff KL, Glycemic thresholds for activation of counterregulatory hormone and symptom responses in islet transplant recipients, J Clin Endocrinol Metab 92(3) (2007) 873–9. [DOI] [PubMed] [Google Scholar]
- [4].Thompson DM, Meloche M, Ao Z, Paty B, Keown P, Shapiro RJ, Ho S, Worsley D, Fung M, Meneilly G, Begg I, Al Mehthel M, Kondi J, Harris C, Fensom B, Kozak SE, Tong SO, Trinh M, Warnock GL, Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy, Transplantation 91(3) (2011) 373–8. [DOI] [PubMed] [Google Scholar]
- [5].Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR, International trial of the Edmonton protocol for islet transplantation, N Engl J Med 355(13) (2006) 1318–30. [DOI] [PubMed] [Google Scholar]
- [6].Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA, Clinical Islet Transplantation C, Phase 3 Trial of Transplantation of Human Islets in Type 1 Diabetes Complicated by Severe Hypoglycemia, Diabetes Care 39(7) (2016) 1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Korsgren O, Nilsson B, Berne C, Felldin M, Foss A, Kallen R, Lundgren T, Salmela K, Tibell A, Tufveson G, Current Status of Clinical Islet Transplantation, Transplantation 79(10) (2005) 1289–1293. [DOI] [PubMed] [Google Scholar]
- [8].Makhlouf L, Yamada A, Ito T, Abdi R, Ansari MJ, Khuong CQ, Winn HJ, Auchincloss H Jr., Sayegh MH, Allorecognition and effector pathways of islet allograft rejection in normal versus nonobese diabetic mice, J Am Soc. Nephrol 14(8) (2003) 2168–2175. [DOI] [PubMed] [Google Scholar]
- [9].Makhlouf L, Kishimoto K, Smith RN, Abdi R, Koulmanda M, Winn HJ, Auchincloss H Jr., Sayegh MH, The role of autoimmunity in islet allograft destruction: major histocompatibility complex class II matching is necessary for autoimmune destruction of allogeneic islet transplants after T-cell costimulatory blockade, Diabetes 51(11) (2002) 3202–3210. [DOI] [PubMed] [Google Scholar]
- [10].Sleater M, Diamond AS, Gill RG, Islet Allograft Rejection by Contact-Dependent CD8(+) T cells: Perforin and FasL Play Alternate but Obligatory Roles, Am J Transplant 7(8) (2007) 1927–1933. [DOI] [PubMed] [Google Scholar]
- [11].Diamond AS, Gill RG, An essential contribution by IFN-gamma to CD8+ T cell-mediated rejection of pancreatic islet allografts, J. Immunol 165(1) (2000) 247–255. [DOI] [PubMed] [Google Scholar]
- [12].Gill RG, Rosenberg AS, Lafferty KJ, Singer A, Characterization of primary T cell subsets mediating rejection of pancreatic islet grafts., J. Immunol 143 (1989) 2176–2178. [PubMed] [Google Scholar]
- [13].Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH, Fas ligand mediates activation-induced cell death in human T lymphocytes, J Exp Med 181(1) (1995) 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA, FasL ligand-induced apoptosis as a mechanism of immune privilege, Science 270(5239) (1995) 1189–92. [DOI] [PubMed] [Google Scholar]
- [15].Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC, A role for CD95 ligand in preventing graft rejection, Nature 377 (1995) 630–2. [DOI] [PubMed] [Google Scholar]
- [16].Wu J, Zhou T, Zhang J, He J, Gause WC, Mountz JD, Correction of accelerated autoimmune disease by early replacement of the mutated lpr gene with the normal Fas apoptosis gene in the T cells of transgenic MRL-lpr/lpr mice, Proc Natl Acad Sci U S A 91 (1994) 2344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ju S-T, Panka DJ, Cul H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A, Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation, Nature 373 (1996) 444–8. [DOI] [PubMed] [Google Scholar]
- [18].Yolcu ES, Askenasy N, Singh NP, Cherradi SL, Shirwan H, Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection, Immunity 17 (2002) 795–808. [DOI] [PubMed] [Google Scholar]
- [19].Torgler R, Jakob S, Ontsouka E, Nachbur U, Mueller C, Green DR, Brunner T, Regulation of activation-induced Fas (CD95/Apo-1) ligand expression in T cells by the cyclin B1/Cdk1 complex, J Biol Chem 279(36) (2004) 37334–42. [DOI] [PubMed] [Google Scholar]
- [20].Lundin KU, Screpanti V, Omholt H, Hofgaard PO, Yagita H, Grandien A, Bogen B, CD4+ T cells kill Id+ B-lymphoma cells: FasLigand-Fas interaction is dominant in vitro but is redundant in vivo, Cancer Immunology Immunotherapy 53(12) (2004) 1135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alderson MR, Armitage RJ, Maraskovsky E, Tough TW, Roux E, Schooley K, Ramsdell F, Lynch DH, Fas transduces activation signals in normal human T lymphocytes, J Exp Med 178 (1993) 2231–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Suzuki I, Martin S, Boursalian TE, Beers C, Fink PJ, Fas Ligand Costimulates the In Vivo Proliferation of CD8+ T Cells, The Journal of Immunology 165(10) (2000) 5537–5543. [DOI] [PubMed] [Google Scholar]
- [23].Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD, Ziegler SF, Bradley LM, Adaptive Islet-Specific Regulatory CD4 T Cells Control Autoimmune Diabetes and Mediate the Disappearance of Pathogenic Th1 Cells In Vivo, The Journal of Immunology 176(8) (2006) 4730–4739. [DOI] [PubMed] [Google Scholar]
- [24].Gorbachev AV, Fairchild RL, CD4+CD25+ regulatory T cells utilize FasL as a mechanism to restrict DC priming functions in cutaneous immune responses, Eur J Immunol 40(7) (2010) 2006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Franke DD, Yolcu ES, Alard P, Kosiewicz MM, Shirwan H, A novel multimeric form of FasL modulates the ability of diabetogenic T cells to mediate type 1 diabetes in an adoptive transfer model, Mol Immunol 44(11) (2007) 2884–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yolcu ES, Zhao H, Bandura-Morgan L, Lacelle C, Woodward KB, Askenasy N, Shirwan H, Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice, Journal of immunology 187(11) (2011) 5901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bennet W, Sundberg B, Lundgren T, Tibell A, Groth CG, Richards A, White DJ, Elgue G, Larsson R, Nilsson B, Korsgren O, Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin, Transplantation 69 (2000) 711–9. [DOI] [PubMed] [Google Scholar]
- [28].Korsgren O, Review article: Acute cellular xenograft rejection, Xenotransplantation 4 (1997) 11–19. [Google Scholar]
- [29].Shapiro AM, Gallant HL, Hao EG, Lakey JR, McCready T, Rajotte RV, Yatscoff RW, Kneteman NM, The portal immunosuppressive storm: relevance to islet transplantation?, Ther Drug Monit 27(1) (2005) 35–7. [DOI] [PubMed] [Google Scholar]
- [30].R.P. D., Michael S, Jeffrey L, Richard Y, Ekaterina K, G.A. J., S.L. D., Evaluation of encapsulating and microporous nondegradable hydrogel scaffold designs on islet engraftment in rodent models of diabetes, Biotechnology and Bioengineering 0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu JMH, Zhang X, Joe S, Luo X, Shea LD, Evaluation of biomaterial scaffold delivery of IL-33 as a localized immunomodulatory agent to support cell transplantation in adipose tissue, Journal of Immunology and Regenerative Medicine 1 (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu JMH, Zhang J, Zhang X, Hlavaty KA, Ricci CF, Leonard JN, Shea LD, Gower RM, Transforming growth factor-beta 1 delivery from microporous scaffolds decreases inflammation post-implant and enhances function of transplanted islets, Biomaterials 80 (2016) 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gibly RF, Zhang X, Lowe WL, Shea LD, Porous Scaffolds Support Extrahepatic Human Islet Transplantation, Engraftment, and Function in Mice, Cell Transplantation 22(5) (2013) 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gibly RF, Zhang X, Graham ML, Hering BJ, Kaufman DB, Lowe WL, Shea LD, Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models, Biomaterials 32(36) (2011) 9677–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Salvay DM, Rives CB, Zhang X, Chen F, Kaufman DB, Lowe WL Jr., Shea LD, Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation, Transplantation 85(10) (2008) 1456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL Jr., Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation, Transplantation 82(4) (2006) 452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gibly RF, Zhang X, Lowe WL Jr., Shea LD, Porous scaffolds support extrahepatic human islet transplantation, engraftment, and function in mice, Cell Transplant 22(5) (2013) 811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cantarelli E, Piemonti L, Alternative transplantation sites for pancreatic islet grafts, Curr Diab Rep 11(5) (2011) 364–74. [DOI] [PubMed] [Google Scholar]
- [39].van der Windt DJ, Echeverri GJ, I.J.N. M., C.D.K. C., The choice of anatomical site for islet transplantation, Cell Transplantation 17 (2008) 1005–14. [PubMed] [Google Scholar]
- [40].Hunter ZN, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, Miller SD, A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease, ACS Nano 8(3) (2014) 2148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yolcu ES, Gu X, Lacelle C, Zhao H, Bandura-Morgan L, Askenasy N, Shirwan H, Induction of tolerance to cardiac allografts using donor splenocytes engineered to display on their surface an exogenous fas ligand protein, J. Immunol 181(2) (2008) 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hlavaty KA, Gibly RF, Zhang X, Rives CB, Graham JG, Lowe WL Jr., Luo X, Shea LD, Enhancing human islet transplantation by localized release of trophic factors from PLG scaffolds, Am J Transplant 14(7) (2014) 1523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhao S, Zhu W, Xue S, Han D, Testicular defense systems: immune privilege and innate immunity, Cell Mol Immunol 11(5) (2014) 428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Benhar I, London A, Schwartz M, The privileged immunity of immune privileged organs: the case of the eye, Front Immunol 3 (2012) 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC, CNS immune privilege: hiding in plain sight, Immunol Rev 213 (2006) 48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pearson RM, Casey LM, Hughes KR, Miller SD, Shea LD, In vivo reprogramming of immune cells: Technologies for induction of antigen-specific tolerance, Adv Drug Deliv Rev 114 (2017) 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chen X, Zhang X, Larson C, Chen F, Kissler H, Kaufman DB, The epididymal fat pad as a transplant site for minimal islet mass, Transplantation 84(1) (2007) 122–5. [DOI] [PubMed] [Google Scholar]
- [48].Mehdizadeh M, Rouhani H, Sepehri N, Varshochian R, Ghahremani MH, Amini M, Gharghabi M, Ostad SN, Atyabi F, Baharian A, Dinarvand R, Biotin decorated PLGA nanoparticles containing SN-38 designed for cancer therapy, Artif Cells Nanomed Biotechnol 45(3) (2017) 495–504. [DOI] [PubMed] [Google Scholar]
- [49].Yolcu ES, Zhao H, Shirwan H, Immunomodulation with SA-FasL protein as an effective means of preventing islet allograft rejection in chemically diabetic NOD mice, Transplant Proc 45(5) (2013) 1889–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Donaldson O, Huang ZJ, Comolli N, An integrated experimental and modeling approach to propose biotinylated PLGA microparticles as versatile targeting vehicles for drug delivery, Biomaterials 2(3) (2013) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pearson RM, Casey LM, Hughes KR, Wang LZ, North MG, Getts DR, Miller SD, Shea LD, Controlled Delivery of Single or Multiple Antigens in Tolerogenic Nanoparticles Using Peptide-Polymer Bioconjugates, Mol Ther 25(7) (2017) 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Casey LM, Pearson RM, Hughes KR, Liu JMH, Rose JA, North MG, Wang LZ, Lei M, Miller SD, Shea LD, Conjugation of Transforming Growth Factor Beta to Antigen-Loaded Poly(lactide- co-glycolide) Nanoparticles Enhances Efficiency of Antigen-Specific Tolerance, Bioconjug Chem 29(3) (2018) 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Valetti S, Mura S, Noiray M, Arpicco S, Dosio F, Vergnaud J, Desmaele D, Stella B, Couvreur P, Peptide conjugation: before or after nanoparticle formation?, Bioconjug Chem 25(11) (2014) 1971–83. [DOI] [PubMed] [Google Scholar]
- [54].Mooney DJ, Baldwin DF, Suh NP, Vacanti JP, Langer R, Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents, Biomaterials 17 (1996) 1417–1422. [DOI] [PubMed] [Google Scholar]
- [55].Shea LD, Smiley E, Bonadio J, Mooney DJ, DNA delivery from polymer matrices for tissue engineering, Nature Biotechnology 17 (1999) 551–4. [DOI] [PubMed] [Google Scholar]
- [56].Gibly RF, Zhang X, Graham ML, Hering BJ, Kaufman DB, Lowe WL Jr., Shea LD, Extrahepatic islet transplantation with microporous polymer scaffolds in syngeneic mouse and allogeneic porcine models, Biomaterials 32(36) (2011) 9677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yap WT, Song WK, Chauhan N, Scalise PN, Agarwal R, Miller SD, Shea LD, Quantification of particle-conjugated or particle-encapsulated peptides on interfering reagent backgrounds, BioTechniques 57(1) (2014) 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pearson RM, Sen S, Hsu HJ, Pasko M, Gaske M, Kral P, Hong S, Tuning the Selectivity of Dendron Micelles Through Variations of the Poly(ethylene glycol) Corona, ACS Nano 10(7) (2016) 6905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hume PS, Anseth KS, Inducing local T cell apoptosis with anti-Fas-functionalized polymeric coatings fabricated via surface-initiated photopolymerizations, Biomaterials 31(12) (2010) 3166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yolcu ES, Askenasy N, Singh NP, Cherradi SE, Shirwan H, Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection, Immunity 17(6) (2002) 795–808. [DOI] [PubMed] [Google Scholar]
- [61].Headen DM, Woodward KB, Coronel MM, Shrestha P, Weaver JD, Zhao H, Tan M, Hunckler MD, Bowen WS, Johnson CT, Shea L, Yolcu ES, Garcia AJ, Shirwan H, Local immunomodulation with Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance, Nat Mater 17 (2018) 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA, Fas ligand-induced apoptosis as a mechanism of immune privilege, Science 270(5239) (1995) 1189–1192. [DOI] [PubMed] [Google Scholar]
- [63].Yolcu ES, Ash S, Kaminitz A, Sagiv Y, Askenasy N, Yarkoni S, Apoptosis as a mechanism of T-regulatory cell homeostasis and suppression, Immunol. Cell Biol 86(8) (2008) 650–658. [DOI] [PubMed] [Google Scholar]
- [64].Banz A, Pontoux C, Papiernik M, Modulation of Fas-dependent apoptosis: a dynamic process controlling both the persistence and death of CD4 regulatory T cells and effector T cells, J. Immunol 169(2) (2002) 750–757. [DOI] [PubMed] [Google Scholar]
- [65].Kaminitz A, Yolcu ES, Mizrahi K, Shirwan H, Askenasy N, Killer Treg cells ameliorate inflammatory insulitis in non-obese diabetic mice through local and systemic immunomodulation, Int. Immunol 25(8) (2013) 485–494. [DOI] [PubMed] [Google Scholar]
- [66].Kaminitz A, Yolcu ES, Stein J, Yaniv I, Shirwan H, Askenasy N, Killer Treg restore immune homeostasis and suppress autoimmune diabetes in prediabetic NOD mice, J. Autoimmun 37(1) (2011) 39–47. [DOI] [PubMed] [Google Scholar]
- [67].Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, Lal P, Feldman MD, Benencia F, Coukos G, Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors, Nat. Med 20(6) (2014) 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Strauss L, Bergmann C, Whiteside TL, Human circulating CD4+CD25highFoxp3+ regulatory T cells kill autologous CD8+ but not CD4+ responder cells by Fas-mediated apoptosis, J. Immunol 182(3) (2009) 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gorbachev AV, Fairchild RL, CD4+CD25+ regulatory T cells utilize FasL as a mechanism to restrict DC priming functions in cutaneous immune responses, Eur. J. Immunol 40(7) (2010) 2006–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Horibe EK, Sacks J, Unadkat J, Raimondi G, Wang Z, Ikeguchi R, Marsteller D, Ferreira LM, Thomson AW, Lee WP, Feili-Hariri M, Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion, Transpl. Immunol 18(4) (2008) 307–318. [DOI] [PubMed] [Google Scholar]
- [71].Powell JD, Lerner CG, Schwartz RH, Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation, J Immunol 162(5) (1999) 2775–84. [PubMed] [Google Scholar]
- [72].Battaglia M, Stabilini A, Roncarolo MG, Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells, Blood 105(12) (2005) 4743–4748. [DOI] [PubMed] [Google Scholar]
- [73].Kaminitz A, Askenasy N, Yolcu ES, Immunomodulation with regulatory T cells and Fas-ligand ameliorate established inflammatory colitis, Gut 62(8) (2013) 1228–1230. [DOI] [PubMed] [Google Scholar]
- [74].Yolcu ES, Kaminitz A, Mizrahi K, Ash S, Yaniv I, Stein J, Shirwan H, Askenasy N, Immunomodulation with donor regulatory T cells armed with Fas-ligand alleviates graft-versus-host disease, Exp. Hematol 41(10) (2013) 903–911. [DOI] [PubMed] [Google Scholar]
- [75].Headen DM, Woodward KB, Coronel MM, Shrestha P, Weaver JD, Zhao H, Tan M, Hunckler MD, Bowen WS, Johnson CT, Shea L, Yolcu ES, Garcia AJ, Shirwan H, Local immunomodulation with Fas ligand-engineered biomaterials achieves allogeneic islet graft acceptance, Nat Mater (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yolcu ES, Zhao H, Bandura-Morgan L, Lacelle C, Woodward KB, Askenasy N, Shirwan H, Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing regulatory T cells in mice, J. Immunol 187(11) (2011) 5901–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Itoh N, Imagawa A, Hanafusa T, Waguri M, Yamamoto K, Iwahashi H, Moriwaki M, Nakajima H, Miyagawa J, Namba M, Makino S, Nagata S, Kono N, Matsuzawa Y, Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice, J Exp Med 186(4) (1997) 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Allison J, Georgiou HM, Strasser A, Vaux DL, Transgenic expression of CD95 ligand on islet beta cells induces a granulocytic infiltration but does not confer immune privilege upon islet allografts, Proc Natl Acad Sci U. S A 94 (1997) 3943–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, Stock PG, Baekkeskov S, Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction, Nat Med 3 (1997) 738–743. [DOI] [PubMed] [Google Scholar]
- [80].Stassi G, De Maria R, Trucco G, Rudert W, Testi R, Galluzzo A, Giordano C, Trucco M, Nitric oxide primes pancreatic beta cells for Fas-mediated destruction in insulin-dependent diabetes mellitus, J Exp Med 186 (1997) 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Suarez-Pinzon W, Sorensen O, Bleackley RC, Elliott JF, Rajotte RV, Rabinovitch A, Beta-cell destruction in NOD mice correlates with Fas (CD95) expression on beta-cells and proinflammatory cytokine expression in islets, Diabetes 48(1) (1999) 21–28. [DOI] [PubMed] [Google Scholar]
- [82].Thomas HE, Darwiche R, Corbett JA, Kay TW, Evidence that beta cell death in the nonobese diabetic mouse is Fas independent, J Immunol 163(3) (1999) 1562–1569. [PubMed] [Google Scholar]
- [83].Kim YH, Kim S, Kim KA, Yagita H, Kayagaki N, Kim KW, Lee MS, Apoptosis of pancreatic beta-cells detected in accelerated diabetes of NOD mice: no role of Fas-Fas ligand interaction in autoimmune diabetes, Eur. J Immunol 29(2) (1999) 455–465. [DOI] [PubMed] [Google Scholar]
- [84].Kim S, Kim KA, Hwang DY, Lee TH, Kayagaki N, Yagita H, Lee MS, Inhibition of autoimmune diabetes by Fas ligand: the paradox is solved, J Immunol 164(6) (2000) 2931–2936. [DOI] [PubMed] [Google Scholar]
- [85].Lee MS, Kim S, Chung JH, Lee MK, Kim KW, Fas is expressed in murine pancreatic islet cells and an insulinoma cell line but does not mediate their apoptosis in vitro, Autoimmunity 29(3) (1999) 189–199. [DOI] [PubMed] [Google Scholar]
- [86].Yolcu ES, Zhao H, Shirwan H, Immunomodulation with SA-FasL protein as an effective means of preventing islet allograft rejection in chemically diabetic NOD mice, Transplant. Proc 45(5) (2013) 1889–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Blood glucose levels of islet transplanted mice in Fig. 1C
Supplementary Figure 2: Characterization of biotin-PLG particles. Dynamic light scattering of particles determined a size of 861 ± 39.2 nm (n=3) and a charge of −15.8 ± 4.98 mV (n=3).
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations. However, they can be provided upon request.