Abstract
BACKGROUND
Stiffening of the proximal aorta is associated with heightened cardiovascular disease risks but can be quantified by limited methodologies (e.g., magnetic resonance imaging [MRI]). As an initial step to evaluate the emerging technique to assess proximal aortic stiffness via pulse wave velocity from the heart to the brachium (hbPWV), we determined the influences of aging on pulse wave velocity (PWV) and aortic hemodynamics.
METHOD
Using the cross-sectional and follow-up study designs, hbPWV was compared and evaluated in relation to other PWV in various arterial segments. Arterial path lengths were measured by the 3-dimensional arterial tracing of MRI.
RESULTS
In the cross-sectional study including 190 subjects (aged 19–79 years), hbPWV exhibited one of the largest age-related increases and a stronger correlation with age (r = 0.790) compared with the other measures of PWV including carotid-femoral PWV, brachial-ankle PWV, and PWV of muscular arteries (r = 0.445–0.688). In addition, hbPWV was correlated with aortic systolic blood pressure (BP) and augmentation index (r = 0.380 and 0.433, respectively) after controlling for brachial systolic BP. These results were confirmed by the 10-year follow-up study involving 84 individuals (53 years at baseline). The decadal changes in hbPWV were significantly correlated with the corresponding changes in several aortic hemodynamic variables (e.g., aortic systolic BP, augmentation pressure, and augmentation index) (r = 0.240–0.349).
CONCLUSIONS
The present findings indicate that hbPWV is a potential marker of proximal aortic stiffening that reflects age-related changes and aortic hemodynamics.
Keywords: arterial stiffness, blood pressure, hypertension, magnetic resonance imaging, pulse wave analysis, pulse wave velocity
Large elastic arteries (e.g., aorta and carotid arteries) dampen cyclic mechanical forces of cardiac pulsations by extending and recoiling against intermittent cardiac blood flow ejection. This function commonly referred to as the “Windkessel” function acts to attenuates left ventricular (LV) afterload, preserves coronary perfusion, and protects vulnerable microvasculature such as the brain and kidneys.1 The proximal aorta (e.g., the ascending aorta and aortic arch) makes the greatest contribution to Windkessel function because of the prominent elasticity and the anatomical location closest to LV. Large cohort studies indicated that deteriorated proximal aortic Windkessel function is associated with heightened cardiovascular disease (CVD) risks, cardiovascular events, and all-cause mortalities.2–5
Currently, carotid–femoral pulse wave velocity (cfPWV) is considered to be the reference standard technique as recently described in the consensus statements on arterial stiffness.6,7 In addition, there is a growing recognition that brachial-ankle pulse wave velocity (baPWV) could be a surrogate index for cfPWV as it is one of few measures of arterial stiffness that have been incorporated in the routine clinical settings.8,9 A recent meta-analysis using individual participant data from prospective cohort studies indicates baPWV could be an independent predictor of the risk of the development of CVD in adults without preexisting CVD.8 However, these measures do not involve the segment of the proximal aorta because the segments from the ascending aorta to the carotid artery and to the brachium are omitted from the effective path lengths used for cfPWV and baPWV calculations.10 Some methodologies such as phase contrast magnetic resonance imaging (MRI) can quantify proximal aortic stiffness, but the expense and technical expertise limit the use of such techniques. Therefore, very little information is available as to how aging and other relevant factors would influence proximal aortic stiffness.4,11,12
One of the promising methodologies to evaluate the stiffness of the proximal aorta is heart-to-brachium pulse wave velocity (hbPWV).13,14 Its measurement includes the segment of the proximal aorta and does not require the technical placement of transducers. Pulse transit time for hbPWV can be evaluated fairly easily by simultaneous recordings of the heart sound or electrocardiograph and brachial arterial pulse waves recorded with the high-fidelity sensor (e.g., air-plethysmography) embedded in the blood pressure (BP) cuff. We have recently proposed a validated estimation formula for arterial path length from the heart to the brachium using MRI.15 In the present study, we evaluated the influence of aging and aortic hemodynamic factors on hbPWV. To determine whether hbPWV provides unique and distinct information from other pulse wave velocity (PWV), hbPWV was compared and evaluated in relation to other PWV in various arterial segments. In addition, the analyses were conducted using the cross-sectional and longitudinal study designs.
METHODS
Subjects
This study consisted of 2 investigations of different nature: a cross-sectional (study 1) and a 10-year longitudinal follow-up (study 2) study. A total of 190 adults (100 men, 19–79 years) participated in study 1. Twenty-seven participants were taking prescribed antihypertensive (n = 15), cholesterol lowering (n = 7), diabetic (n = 4), and other (e.g., anticoagulation, thyroid hormone) (n = 14) medications. For the reason of MRI scanning, exclusion criteria included pregnancy or patients with electrically, magnetically, or mechanically active implants, intracranial aneurysm clips, prior CVD, epileptic seizures, or claustrophobic symptoms.
In study 2, we studied 84 volunteers (51 men, 63 ± 14 years) who were followed up for approximately 10 years.16 Smokers and pregnant women were excluded at the premeasurement. No one took any medication at the time of premeasurement, whereas some patients were taking prescribed antihypertensive (n = 19), cholesterol lowering (n = 6), and diabetic (n = 3) medications at the postmeasurement. Women had not taken any hormone replacement therapy during the follow-up period. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the institutional review board. All subjects provided written informed consent to participate.
Experimental protocol
Subjects abstained from alcohol intake and strenuous physical activity for at least 24 hours. Furthermore, all measurements were performed after 3 hours of fasting and an abstinence from caffeine. Upon arrival, subjects underwent body height and weight assessments, which were followed by supine rest for more than 10 minutes and cardiovascular measurements. Cardiovascular measurements were performed under supine resting conditions in a quiet, temperature-controlled room (24–26 °C).
PWV measurement
Heart rate, BP, and pulse wave velocity (PWV = arterial path length/arterial pulse transit time) were measured noninvasively with the automated cardiovascular screening device (VP-1000plus; Omron Healthcare, Kyoto, Japan) equipped with an electrocardiogram, a phonocardiogram, 4-extremity BP cuffs involving air-plethysmographic sensors, and 2 applanation tonometry sensors. Carotid and femoral artery pulse waves were obtained using arterial applanation tonometry incorporating an array of 15 micro-piezoresistive transducers placed on the carotid and femoral arteries.15,17,18 The applanation tonometry sensors were not used in study 2 as the premeasurements did not include such setups. Bilateral brachial and posterior-tibial arterial pressure waveforms were recorded by extremities cuffs connected to air-plethysmographic sensors wrapped on both arms and ankles. Heart sound was recorded by a phonographic sensor that was placed on the chest. Pulse transit times were determined from the time delay between the second heart sound and dicrotic notch on the right brachial arterial waveforms or between the proximal and distal “foot” waveforms. As shown in Supplementary Figure 1, the “foot” (i.e., the commencement of the sharp systolic upstroke) and the dicrotic notch were automatically detected by a band-path filter (5–30 Hz)19 using a LabVIEW-based software provided by Colin AT company (Komaki, Japan). The validity of determination from the time delay (Tcb) between the second heart sound and dicrotic notch was confirmed in the subanalyses. In randomly selected 100 subjects (50 men), pulse transition time between carotid and brachial arterial pulse waves was obtained by the foot-to-foot method (the carotid “foot” to the brachial “foot”) and the dicrotic notch method (the second heart sound to the dicrotic notch of brachial arterial waveform).
Arterial path lengths were obtained by 2 different ways as follows: In study 1, arterial path lengths were computed by the 3-dimensional artery tracing of transverse MRI with ImageJ (1.45s; NIH, Bethesda, MD, USA). Transverse MRI was taken at end diastole (gradient echo method, echo time/repetition time 11.0/4.2 millisecond, flip angle 258°, field of view 400 mm, slice thickness 5 mm, interslice gap 0 mm) by a 1.0 T MRI system (Magnetom Impact, Siemens Japan, Tokyo, Japan).17 The accuracy of the 3-dimensional artery tracing was verified as previously reported.20 In study 1, PWV was evaluated at the following segments from the MRI-derived arterial path lengths: between the carotid and femoral sites (cfPWV), from the heart (e.g., the aortic root) to the right brachium (hbPWV), between the right carotid and the right brachium (cbPWV), from the right femoral artery to the right ankle (faPWV), and between the right brachium and the right ankle (baPWV).17 Arterial path lengths for cfPWV were measured by the body surface distance, and those for hbPWV (Lhb)15 and baPWV (Lba)19,21,22 were derived from the following equations:
In study 2, hbPWV and baPWV were evaluated using the height-based equations above.
Aortic hemodynamics
Using the generalized transfer function-based central BP measurement system (SphygmoCor; AtCor Medical, Sydney, Australia), aortic BP was synthesized from carotid arterial BP waveform.23 Synthesized aortic BP waveform was calibrated by equating the aortic mean and diastolic BP to the brachial artery values acquired with the vascular screening system. Brachial mean BP was calculated by the following equation: mean BP = (systolic BP−diastolic BP) / 3 + diastolic BP. The pressure height of the peak above the shoulder of the wave was defined as augmentation pressure (AP). The AP by aortic pulse pressure (PP) was calculated as the augmentation index (AIx).24
Statistical analyses
Relationships between age and measurements of interests were determined by one-way analysis of variance. Regarding PWV, the interaction between age and the arterial segment was determined by the two-way repeated-measures analysis of variance. Multiple comparisons were performed with Scheffe’s post hoc test in the case of significant F value. Paired t test was applied for pre- and postmeasurements comparisons in study 2. Simple and partial correlation analyses were performed to determine relations of interests. Differences in correlation coefficients were evaluated with the Fisher r-to-z transformation. To validate the determination from the time delay between the second heart sound and dicrotic notch, the regression analysis and Brand and Altman’s plot were used. All data are reported as mean ± SD. Statistical significance was set a priori at P values less than 0.05.
RESULTS
Subjects’ characteristics and results of validation study are presented in Supplementary Table 1 and Supplementary Figure 2. In comparison with the foot-to-foot method, the dicrotic notch method exhibited a strong correlation (r = 0.902, P < 0.0001; Supplementary Figure 2) though it was significantly shorter (45.4 ± 5.3 vs. 48.7 ± 5.8 millisecond, P < 0.001). The difference between these methods significantly correlated with age (r = 0.343, P < 0.001), heart rate (r = 0.331, P < 0.001), and diastolic BP (r = 0.232, P = 0.034), but not with systolic (r = 0.023, P = 0.823) and mean BP (r = 0.133, P = 0.187).
Study 1: Table 1 presents subject characteristics in study 1. Brachial and aortic systolic BP were greater with increasing age categories. Aortic PP, AP, and AIx were significantly higher in 2 older age categories than in 2 younger age categories. Heart rate and brachial PP were not different among the age categories.
Table 1.
Subject characteristics in study 1
| Age category | ||||
|---|---|---|---|---|
| Variables | < 35 years | 35–49 years | 50–64 years | ≥ 65 years |
| N, men/women | 20/16 | 16/17 | 35/42 | 21/23 |
| Age, years | 27.0 ± 4.6 | 43.3 ± 4.8* | 59.9 ± 3.1*† | 69.2 ± 3.2*†‡ |
| Height, cm | 166.2 ± 8.3 | 165.2 ± 8.1 | 160.4 ± 8.1* | 158.0 ± 8.6*† |
| Body weight, kg | 61.7 ± 11.9 | 63.2 ± 11.5 | 59.1 ± 9.3 | 56.8 ± 8.7 |
| BMI, kg/m2 | 22.2 ± 3.1 | 23.0 ± 3.0 | 22.9 ± 2.6 | 22.7 ± 2.2 |
| Heart rate, bpm | 58.1 ± 8.8 | 60.1 ± 8.4 | 60.4 ± 7.5 | 60.1 ± 7.2 |
| Brachial SBP, mm Hg | 112 ± 15 | 119 ± 14 | 126 ± 16* | 130 ± 16*† |
| Brachial DBP, mm Hg | 65 ± 10 | 74 ± 10* | 78 ± 10* | 79 ± 10* |
| Brachial PP, mm Hg | 47 ± 7 | 45 ± 7 | 48 ± 10 | 50 ± 10 |
| Aortic SBP, mm Hg | 99 ± 14 | 110 ± 15* | 120 ± 16*† | 123 ± 15*† |
| Aortic MBP, mm Hg | 65 ± 10 | 74 ± 10* | 78 ± 10* | 79 ± 10* |
| Aortic DBP, mm Hg | 65 ± 10 | 74 ± 10* | 78 ± 10* | 79 ± 10* |
| Aortic PP, mm Hg | 33 ± 7 | 35 ± 8 | 41 ± 10*† | 43 ± 9*† |
| Aortic AP, mm Hg | 6.2 ± 3.7 | 8.8 ± 4.3 | 14.9 ± 6.0*† | 16.3 ± 6.3*† |
| Aortic AIx, % | 18.8 ± 10.4 | 24.3 ± 9.3 | 35.7 ± 8.9*† | 37.0 ± 8.8*† |
Abbreviations: AIx, augmentation index; AP, augmentation pressure; BMI, body mass index; DBP, diastolic blood pressure; MBP, mean blood pressure; PP, pulse pressure; SBP, systolic blood pressure.
Data are mean ± SD. “*”, “†”, and “‡” indicate significant differences from <35 years, 35–49 years, and 50–64 years, respectively (by Scheffe post hoc test).
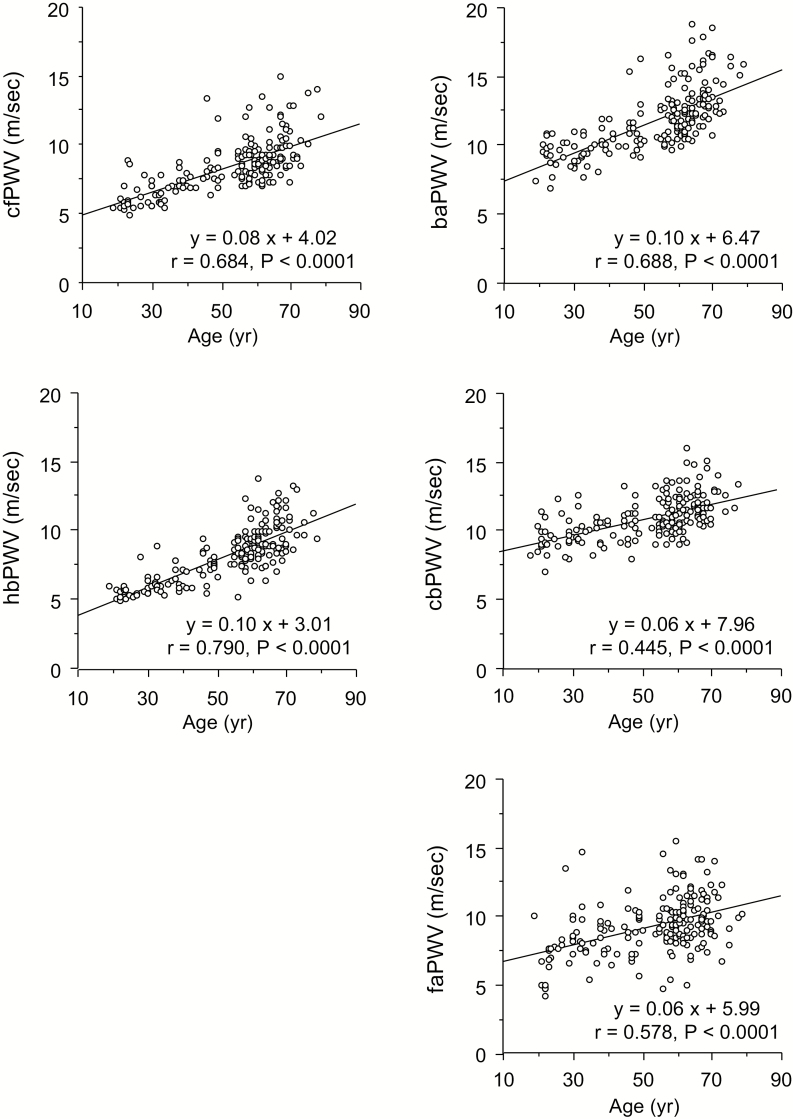
As shown in Figure 1, all the MRI-based PWV measures displayed were significantly correlated with age (r = 0.445–0.790, P < 0.0001 for all). Correlation coefficient with age was significantly greater in hbPWV compared with those of other PWVs (P < 0.05 for all). Similar results were observed in PWVs calculated from body surface and height-based arterial path lengths (Supplementary Table 2).
Figure 1.
Scatter plots between age and pulse wave velocity (PWV) of various arterial segments in study 1. Abbreviations: baPWV, brachial-ankle PWV; cbPWV, carotid-brachial PWV; cfPWV, carotid-femoral PWV; faPWV, femoral-ankle PWV; hbPWV, heart-brachial PWV.
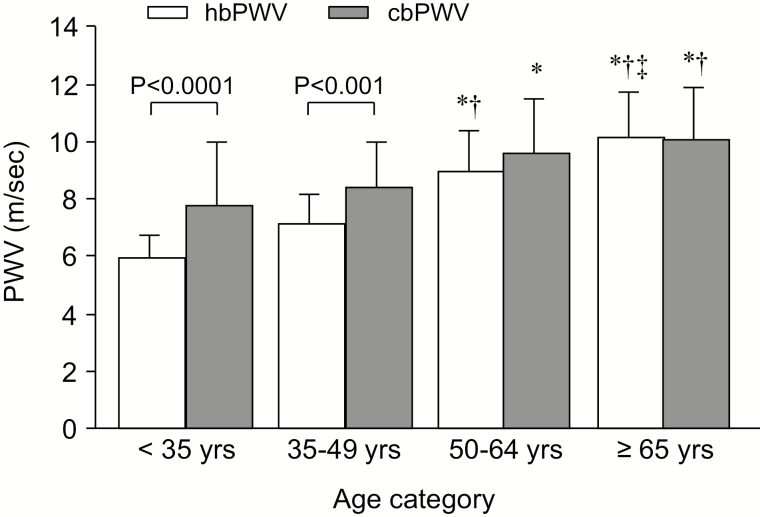
Figure 2 shows a comparison of hbPWV and cbPWV in each age category. Both PWV measures increased gradually throughout the age range. Notably, there were significant differences between hbPWV and cbPWV in the 2 younger age categories, but these measures were comparable after 50 years of age.
Figure 2.
Comparison of heart-brachial pulse wave velocity (hbPWV) and carotid-brachial PWV (cbPWV) in each age category in study 1. Data indicate mean ± SD. “*”, “†”, and “‡” indicate significant differences from <35 years, 35–49 years, and 50–64 years, respectively (by Scheffe post hoc test).
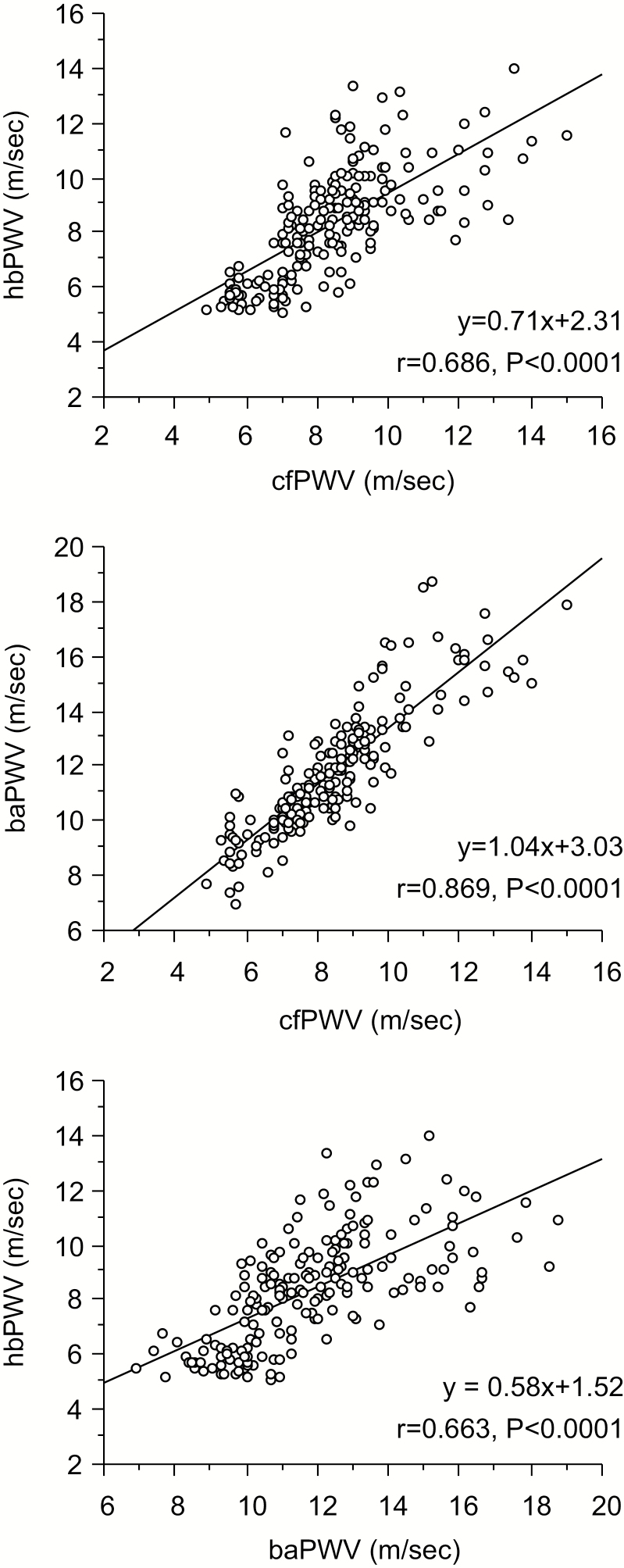
Figure 3 presents correlations between MRI-based PWVs. baPWV was strongly correlated with cfPWV (r = 0.869, P < 0.0001), whereas hbPWV exhibited modest correlations with cfPWV (r = 0.686, P < 0.0001) and baPWV (r = 0.663, P < 0.0001). Table 2 presents simple and partial correlational analyses between selected PWV and aortic hemodynamics. All the selected PWVs were correlated with all the hemodynamic variables in simple correlational analyses. hbPWV had the stronger correlations with aortic systolic BP and AIx after controlling for brachial systolic BP. Similar results were obtained in PWVs calculated from body surface and height-based arterial path lengths (Supplementary Table 2).
Figure 3.
Relationships among carotid-femoral pulse wave velocity (cfPWV), heart-brachial PWV (hbPWV), and brachial-ankle PWV (baPWV) in study 1.
Table 2.
Results of simple and partial correlation analyses between selected magnetic resonance imaging (MRI)-based pulse wave velocity (PWV) and aortic hemodynamic variables in study 1
| cfPWV | baPWV | hbPWV | |
|---|---|---|---|
| Simple correlation coefficient | |||
| Aortic SBP | 0.632 | 0.700 | 0.629 |
| Aortic MBP | 0.591 | 0.654 | 0.610 |
| Aortic DBP | 0.554 | 0.609 | 0.606 |
| Aortic PP | 0.510 | 0.570 | 0.444 |
| Aortic AP | 0.552 | 0.582 | 0.533 |
| Aortic AIx | 0.472 | 0.469 | 0.503 |
| Partial correlation coefficient (controlling for age) | |||
| Aortic SBP | 0.444 | 0.552 | 0.417 |
| Aortic MBP | 0.434 | 0.531 | 0.463 |
| Aortic DBP | 0.360 | 0.445 | 0.432 |
| Aortic PP | 0.353 | n.s. | 0.227 |
| Aortic AP | 0.246 | 0.295 | n.s. |
| Aortic AIx | n.s. | n.s. | n.s. |
| Partial correlation coefficient (controlling for brachial SBP) | |||
| Aortic SBP | 0.306 | 0.311 | 0.380 |
| Aortic MBP | n.s. | n.s. | 0.280 |
| Aortic DBP | n.s. | n.s. | 0.281 |
| Aortic PP | n.s. | n.s. | n.s. |
| Aortic AP | 0.339 | 0.350 | 0.324 |
| Aortic AIx | 0.393 | 0.390 | 0.432 |
Abbreviations: AIx, augmentation index; AP, augmentation pressure; baPWV, brachial-ankle pulse wave velocity; cfPWV, carotid-femoral pulse wave velocity; DBP, diastolic blood pressure; hbPWV, heart-brachial pulse wave velocity; MBP, mean blood pressure; n.s., non significant; PP, pulse pressure; SBP, systolic blood pressure.
Numbers are significant Pearson’s r values.
Study 2: Selected characteristics of subjects in study 2 are summarized in Table 3. Height decreased whereas body mass index, heart rate, systolic BP, PP, baPWV, and hbPWV increased after the 10-year follow-up period. Body weight, brachial diastolic BP, aortic AP, and AIx did not change significantly.
Table 3.
Subjects characteristics in study 2
| Before | After | |
|---|---|---|
| Age, years | 53 ± 14 | 63 ± 14 |
| Height, cm | 163.7 ± 8.7 | 162.7 ± 9.3* |
| Body weight, kg | 60.9 ± 11.1 | 61.0 ± 9.8 |
| BMI, kg/m2 | 22.6 ± 3.1 | 23.0 ± 2.8* |
| Heart rate, bpm | 59.2 ± 6.8 | 61.4 ± 9.4* |
| Brachial SBP, mm Hg | 121 ± 16 | 126 ± 15* |
| Brachial DBP, mm Hg | 75 ± 12 | 77 ± 11 |
| Brachial PP, mm Hg | 46 ± 8 | 49 ± 11* |
| Aortic SBP, mm Hg | 111 ± 16 | 118 ± 16* |
| Aortic MBP, mm Hg | 91 ± 13 | 94 ± 11* |
| Aortic DBP, mm Hg | 75 ± 12 | 77 ± 11 |
| Aortic PP, mm Hg | 36 ± 7 | 41 ± 12* |
| Aortic AP, mm Hg | 10.7 ± 6.9 | 10.1 ± 10.9 |
| Aortic AIx, % | 29.1 ± 16.0 | 25.8 ± 22.2 |
| baPWV, m/s | 13.4 ± 2.6 | 14.9 ± 3.2* |
| hbPWV, m/s | 9.4 ± 1.7 | 10.7 ± 1.8* |
Abbreviations: AIx, augmentation index; AP, augmentation pressure; baPWV, brachial-ankle pulse wave velocity; BMI, body mass index; DBP, diastolic blood pressure; hbPWV, heart-brachial pulse wave velocity; MBP, mean blood pressure; PP, pulse pressure; SBP, systolic blood pressure.
Data are mean ± SD. *significant difference (vs. before, by paired t test).
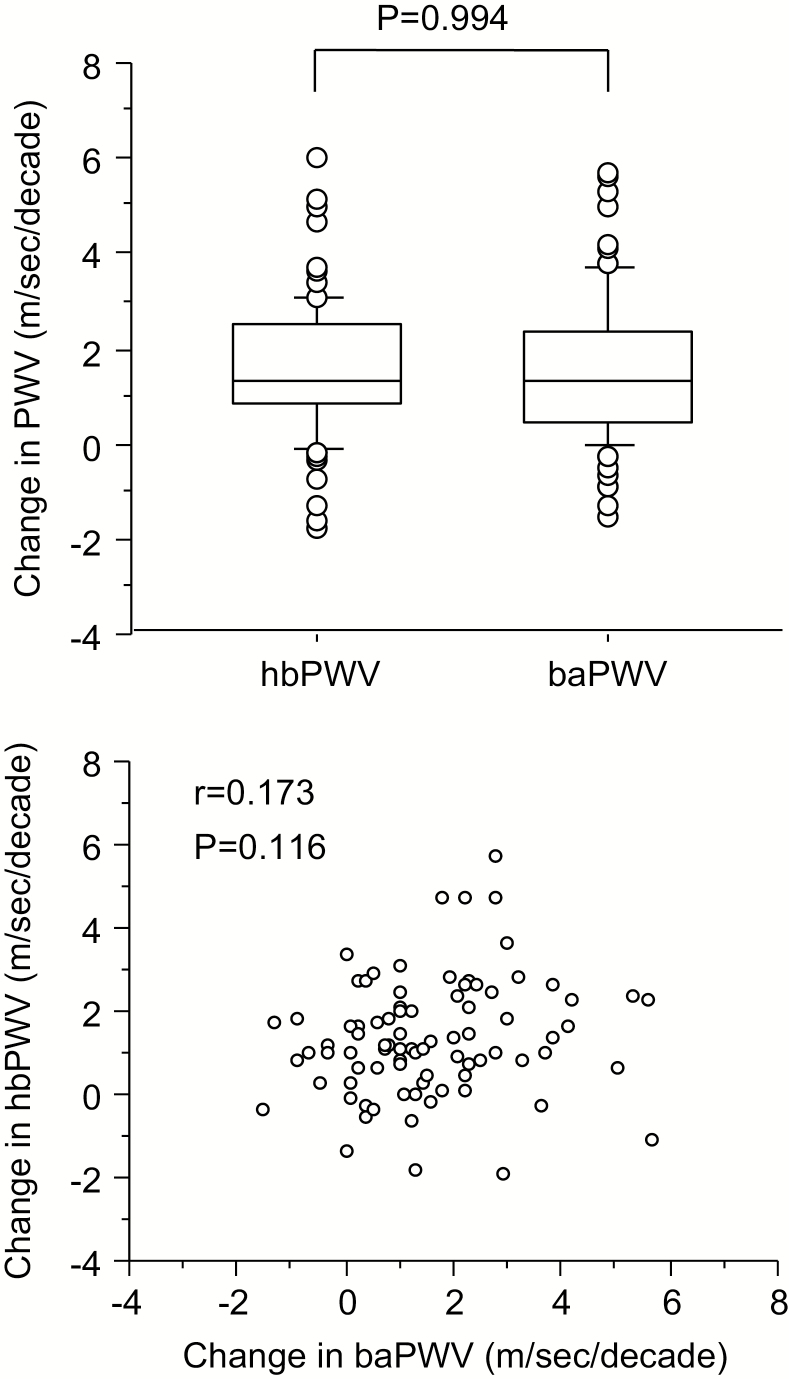
The magnitudes of decadal changes in hbPWV and baPWV were similar (P = 0.994; Figure 4, top). However, changes in hbPWV were not associated with changes in baPWV (r = 0.173, P < 0.05; Figure 4, bottom). As summarized in Table 4, decadal changes in aortic systolic BP were correlated with corresponding changes in baPWV (r = 0.228, P < 0.05) and hbPWV (r = 0.349, P < 0.05). Decadal changes in aortic AP and AIx were correlated with corresponding changes in hbPWV but not with those in baPWV.
Figure 4.
Comparison between decadal changes in heart-brachial pulse wave velocity (hbPWV) and brachial-ankle PWV (baPWV) in study 2: the box and whisker plot (top); the scatter plot (bottom).
Table 4.
Results of simple correlation analyses between changes in pulse wave velocity (PWV) and corresponding changes in aortic hemodynamic variables in study 2
| ΔhbPWV | ΔbaPWV | |
|---|---|---|
| ΔAortic SBP, mm Hg | 0.349 | 0.228 |
| ΔAortic MBP, mm Hg | 0.471 | 0.272 |
| ΔAortic DBP, mm Hg | 0.439 | n.s. |
| ΔAortic PP, mm Hg | n.s. | n.s. |
| ΔAortic AP, mm Hg | 0.289 | n.s. |
| ΔAortic AIx, % | 0.240 | n.s. |
Abbreviations: AIx, augmentation index; AP, augmentation pressure; baPWV, brachial-ankle pulse wave velocity; DBP, diastolic blood pressure; hbPWV, heart-brachial pulse wave velocity; MBP, mean blood pressure; n.s., non significant; PP, pulse pressure; SBP, systolic blood pressure.
Numbers are significant Pearson’s r values.
DISCUSSION
Salient findings of this study were as follows. Among all the PWV measures, hbPWV exhibits one of the largest age-related increases and significantly stronger correlation with age. In addition, there were significant differences between hbPWV and cbPWV in the 2 younger age categories, but these measures were comparable after 50 years of age. hbPWV demonstrated significant correlations with aortic hemodynamic variables such as aortic systolic BP and aortic AIx. These correlations remained significant even after controlling for brachial systolic BP. Even in the 10-year follow-up study, decadal change in hbPWV was significantly associated with the corresponding changes in aortic hemodynamic variables. These results suggest that hbPWV is an index of arterial stiffness that displays pronounced age-associated changes and reflects hemodynamic factors in the aorta. Our present findings provide evidence that hbPWV is a potential marker of subclinical stiffening of the proximal aorta and call for further clinical evidence.
Age-related changes
Our cross-sectional investigation revealed that hbPWV exhibits the strongest correlation with age among selected measures of PWV. Age-related stiffening is remarkable in elastic artery than muscular artery.25,26 The projected arterial tree evaluated by hbPWV includes both the proximal aorta and muscular arteries (e.g., subclavian and brachial arteries), but the proximal aorta part seems to occupy <20%.17 Nevertheless, the extent of increase in hbPWV was modestly larger than that of cfPWV (+0.10 m/s/decade vs. +0.08 m/s/decade evaluated by the slope of regression line with age). On the other hand, cbPWV, which omits the section of the proximal aorta from the arterial path of hbPWV, showed a much smaller increase (+0.06 m/s/decade). For example, Figure 1 suggests that a subject aged 50 years exhibits 12.5 % and 5.5 % of decadal increases in hbPWV and cbPWV, respectively. Here, assuming that subject’s body height does not change much for a decade as seen in study 2 (~1 cm) and that the ascending aortic length corresponds 20% of the arterial path length for hbPWV, the ascending aortic PWV would increase by approximately 16% for a decade. Taken together, these results suggest that hbPWV undergoes marked age-related increases presumably as it reflects the aging-induced stiffening of the proximal aortic region.
Currently, very little information is available regarding the age-related changes in the proximal aortic properties despite the recognition that it is the most important arterial segment for buffering and cushioning cardiac pulsations.4,11,12 A study using MRI technique12 reported similar PWV values among the proximal- (including the aortic root, the ascending aorta, and proximal descending aorta), mid- (including the proximal and mid-descending thoracic aorta), and distal- (including the mid-thoracic and abdominal aorta) sections in individuals <55 years of age, whereas the remarkable aortic stiffness gradient from the proximal to the distal region in individuals ≥55 years of age. In another study using phase-contrast MRI, aortic arch PWV increased dramatically after 50 years of age.11 The present comparisons of PWV among age categories indicate that hbPWV demonstrated significantly lower values than cbPWV in younger age categories, whereas the difference gradually diminished with increasing age categories and abolished at older age categories. These results suggest that stiffness of the proximal artery reaches to that of muscular arteries (e.g., subclavian and brachial arteries) after 50 years of age. In our 10-year follow-up study, hbPWV increased by 14% for a decade in apparently healthy individuals 53 years of age at baseline (from 9.4±1.7 to 10.7±1.8 m/s). This extent of elevation was similar to the difference in hbPWV between the 2 oldest age categories in the cross-sectional comparison (9.0 ±1.4 vs. 10.2±1.5 m/s). In this context, a large cohort study demonstrated a 18% increase in aortic arch PWV over the 10-year follow-up period.4 Thus, the age-related changes in hbPWV observed in the present study are consistent with previous studies using different modalities that assessed the stiffness of the proximal aorta.
Relationship with aortic hemodynamics
The proximal aorta, the most elastic segment of the blood vessel directly connected to LV, would strongly contribute to dampening cyclic mechanical forces of cardiac pulsations. Accordingly, it is plausible to hypothesize that the age-related aortic stiffening of the proximal region possesses stronger interaction with aortic hemodynamics compared with that of the distal aortic region evaluated by other PWV measures. hbPWV was modestly correlated with most of the selected aortic hemodynamic variables as cfPWV and baPWV did. However, the 10-year follow-up data indicated that decadal changes in hbPWV were more strongly correlated with corresponding changes in aortic hemodynamic variables. It should be emphasized here that aortic hemodynamic indices such as aortic systolic BP, PP, and AIx, are independent predictors of future CVD events and all-cause mortality.27–30 Therefore, our findings strongly suggest that hbPWV is a potential marker of subclinical arterial stiffening. Prospective data linking hbPWV to clinical endpoints are warranted.
Study limitations
There are some drawbacks of this study that should be mentioned. First, arterial path lengths were directly measured by MRI in the cross-sectional investigation but not in the follow-up study. However, the estimation equation for hbPWV has been validated by comparison with those obtained with 3-dimensional MRI.15 Second, we conducted the 10-year follow up study but could not evaluate clinical events or end point because the sample size was relatively small and the participants were apparently healthy at baseline. Third, we evaluated the longitudinal change of baPWV instead of cfPWV, the reference standard of central arterial stiffness assessment. However, baPWV has been recognized as a useful predictor of risk of total cardiovascular events and all-cause mortality as a surrogate of cfPWV.31 Finally, in study 2, as arterial path lengths were estimated by functions of height not by MRI, the age-related elongation of arterial lengths could not be accounted for in the analysis. A cross-sectional study with large sample size32 demonstrated that the path length for invasive aortic PWV was relatively constant across all age groups although body heights were shorter with advancing age of groups. These results may indicate that body surface measurement and height-based estimation of arterial path length could be a potential confounding artifact for the comparison of aortic PWV between younger and older populations. In our longitudinal observation, however, individual shrinkage of body height was very slight (~1 cm). Furthermore, its impact might be negligible because age-related change in PWV could be mainly attributed to the shorten pulse transition time.18
CONCLUSION
As an initial step to evaluate the emerging technique to assess proximal aortic stiffness via hbPWV, we determined the influence of aging on PWV and aortic hemodynamic measures. hbPWV exhibits a stronger correlation with age and aortic hemodynamic factors such as aortic systolic BP and AIx. Our present findings demonstrate that hbPWV is a potential marker of subclinical arterial stiffening.
PERSPECTIVES
In this study, using both cross-sectional and longitudinal follow-up studies, we demonstrated that hbPWV increases markedly with advancing age and is associated with aortic hemodynamic factors. Given the simplicity of the technique, hbPWV assessment has the potential to be a promising technique that is suitable for routine clinical settings. However, in order for hbPWV to be recognized as an important clinical tool, a more thorough analysis that links this PWV measure to clinical outcomes is needed.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Koichiro Hayashi, Dr Mutsuko Hieda, and Ms Mitsuho Watanabe for their technical assistance. This study was supported by special coordination funds of the Japanese Ministry of Education, Culture, Sports, Science, and Technology (KAKENHI 16700499 and 17H02186 to JS).
REFERENCES
- 1. O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005; 46:200–204. [DOI] [PubMed] [Google Scholar]
- 2. Ohyama Y, Ambale-Venkatesh B, Noda C, Kim JY, Tanami Y, Teixido-Tura G, Chugh AR, Redheuil A, Liu CY, Wu CO, Hundley WG, Bluemke DA, Guallar E, Lima JAC. Aortic arch pulse wave velocity assessed by magnetic resonance imaging as a predictor of incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis). Hypertension 2017; 70:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ohyama Y, Ambale-Venkatesh B, Noda C, Chugh AR, Teixido-Tura G, Kim JY, Donekal S, Yoneyama K, Gjesdal O, Redheuil A, Liu CY, Nakamura T, Wu CO, Hundley WG, Bluemke DA, Lima JAC. Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging the multi-ethnic study of atherosclerosis. Circ-Cardiovasc Imag 2016; 9: e004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohyama Y, Teixido-Tura G, Ambale-Venkatesh B, Noda C, Chugh AR, Liu CY, Redheuil A, Stacey RB, Dietz H, Gomes AS, Prince MR, Evangelista A, Wu CO, Hundley WG, Bluemke DA, Lima JA. Ten-year longitudinal change in aortic stiffness assessed by cardiac MRI in the second half of the human lifespan: the multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging 2016; 17:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Redheuil A, Wu CO, Kachenoura N, Ohyama Y, Yan RT, Bertoni AG, Hundley GW, Duprez DA, Jacobs DR Jr, Daniels LB, Darwin C, Sibley C, Bluemke DA, Lima JAC. Proximal aortic distensibility is an independent predictor of all-cause mortality and incident CV events: the MESA study. J Am Coll Cardiol 2014; 64:2619–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 7. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American heart association. Hypertension 2015; 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, Nakamura M, Ohkubo T, Watada H, Munakata M, Ohishi M, Ito N, Nakamura M, Shoji T, Vlachopoulos C, Yamashina A; Collaborative Group for J-BAVEL (Japan Brachial-Ankle Pulse Wave Velocity Individual Participant Data Meta-Analysis of Prospective Studies) Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension 2017; 69:1045–1052. [DOI] [PubMed] [Google Scholar]
- 9. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension 2012; 60:556–562. [DOI] [PubMed] [Google Scholar]
- 10. Tomoto T, Maeda S, Sugawara J. Relation between arterial stiffness and aerobic capacity: importance of proximal aortic stiffness. Eur J Sport Sci 2017; 17:571–575. [DOI] [PubMed] [Google Scholar]
- 11. Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, Kachenoura N, Bluemke D, Lima JA. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension 2010; 55:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rogers WJ, Hu YL, Coast D, Vido DA, Kramer CM, Pyeritz RE, Reichek N. Age-associated changes in regional aortic pulse wave velocity. J Am Coll Cardiol 2001; 38:1123–1129. [DOI] [PubMed] [Google Scholar]
- 13. Koizumi M, Shimizu H, Shimomura K, Oh-I S, Tomita Y, Kudo T, Iizuka K, Mori M. Relationship between hyperinsulinemia and pulse wave velocity in moderately hyperglycemic patients. Diabetes Res Clin Pract 2003; 62:17–21. [DOI] [PubMed] [Google Scholar]
- 14. Tsuchikura S, Shoji T, Kimoto E, Shinohara K, Hatsuda S, Koyama H, Emoto M, Nishizawa Y. Brachial-ankle pulse wave velocity as an index of central arterial stiffness. J Atheroscler Thromb 2010; 17:658–665. [DOI] [PubMed] [Google Scholar]
- 15. Sugawara J, Tomoto T, Tanaka H. Arterial path length estimation for heart-to-brachium pulse wave velocity. Hypertens Res 2018; 41:444–450. [DOI] [PubMed] [Google Scholar]
- 16. Sugawara J, Tomoto T, Noda N, Matsukura S, Tsukagoshi K, Hayashi K, Hieda M, Maeda S. Effects of endothelin-related gene polymorphisms and aerobic exercise habit on age-related arterial stiffening: a 10-year longitudinal study. J Appl Physiol (1985) 2018; 124: 312–320. [DOI] [PubMed] [Google Scholar]
- 17. Sugawara J, Hayashi K, Tanaka H. Arterial path length estimation on brachial-ankle pulse wave velocity: validity of height-based formulas. J Hypertens 2014; 32:881–889. [DOI] [PubMed] [Google Scholar]
- 18. Sugawara J, Hayashi K, Tanaka H. Arterial path length for arterial stiffness: methodological consideration. Am J Hypertens 2016; 29:1237–1244. [DOI] [PubMed] [Google Scholar]
- 19. Sugawara J, Hayashi K, Yokoi T, Cortez-Cooper MY, DeVan AE, Anton MA, Tanaka H. Brachial-ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens 2005; 19:401–406. [DOI] [PubMed] [Google Scholar]
- 20. Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging 2008; 1:739–748. [DOI] [PubMed] [Google Scholar]
- 21. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002; 25:359–364. [DOI] [PubMed] [Google Scholar]
- 22. Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement—a survey of 12517 subjects. Atherosclerosis 2003; 166:303–309. [DOI] [PubMed] [Google Scholar]
- 23. Tanaka H, Tomoto T, Kosaki K, Sugawara J. Arterial stiffness of lifelong Japanese female pearl divers. Am J Physiol Regul Integr Comp Physiol 2016; 310:R975–R978. [DOI] [PubMed] [Google Scholar]
- 24. Kelly R, Hayward C, Avolio A, O’Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 1989; 80:1652–1659. [DOI] [PubMed] [Google Scholar]
- 25. Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 1983; 68:50–58. [DOI] [PubMed] [Google Scholar]
- 26. Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol 1998; 18:127–132. [DOI] [PubMed] [Google Scholar]
- 27. Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 2007; 50:197–203. [DOI] [PubMed] [Google Scholar]
- 28. Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome: the Strong Heart Study. J Am Coll Cardiol 2009; 54:1730–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens 2010; 28:384–388. [DOI] [PubMed] [Google Scholar]
- 30. Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 2010; 31:1865–1871. [DOI] [PubMed] [Google Scholar]
- 31. Sugawara J, Tanaka H. Brachial-ankle pulse wave velocity: myths, misconceptions, and realities. Pulse (Basel) 2015; 3:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weber T, Wassertheurer S, Hametner B, Parragh S, Eber B. Noninvasive methods to assess pulse wave velocity: comparison with the invasive gold standard and relationship with organ damage. J Hypertens 2015; 33:1023–1031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.