Abstract
In the present study, a new series of 2,5-disubstituted-1,3,4-thiadiazole tethered 1,2,4-triazole, 1,3,4-thiadiazole, 1,3,4-oxadiazole and Schiff base derivatives were synthesized and characterized by IR, 1H-NMR, 13C-NMR, MS and elemental analyses. All compounds were screened for their antibacterial, antifungal and antiproliferative activity. Some of the synthesized derivatives have displayed promising biological activity.
Keywords: 1,3,4-thiadiazole; 1,2,4-triazole; 1,3,4-oxadiazole; Schiff base; antimicrobial activity; antitumor activity
1. Introduction
Aromatic five-membered nitrogen heterocycles have been potential targets of investigations by several research groups owing to their interesting biological activities and medicinal properties [1,2]. Among these, the 1,2,4-triazole scaffold constitutes the core moiety of several therapeutically active compounds as antimicrobial [3], analgesic [4], antiviral [5], antioxidant [6], anti-inflammatory [7], and anticancer [8] agents. Furthermore, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles are also important classes of azoles endowed with significant biological properties as there are several examples in the literature including antifungal [9,10], anti-inflammatory [11,12], antimicrobial [13,14], antiviral [15,16] and anticancer [17,18] activities. Additionally, many investigations showed that the clubbing of two or three heterocyclic units may significantly potentiate the antimicrobial activities [19,20,21]. In addition, Schiff bases have been the focus of numerous studies due to their wide spectrum of biological activities [22]. Moreover, azomethine Schiff bases linkages, as attractive connecting units that could bind two pharmacophores to generate an innovative bifunctional drugs, have rapidly emerge as one of the most challenging and attractive topics in drug design for the constructing of novel bioactive molecules. Based on all above considerations and as an extension of our studies on the developments of novel azoles antimicrobial agents [23,24], we disclosed the synthesis of new polyheterocyclic ring systems with anticipated antimicrobial and antiproliferative activities, by clubbing 1,3,4-thiadiazole with 1,2,4-triazole, 1,3,4-thiadiazole, 1,3,4-oxadiazole and/or Schiff base moiety in one frame work.
2. Results and Discussion
2.1. Chemistry
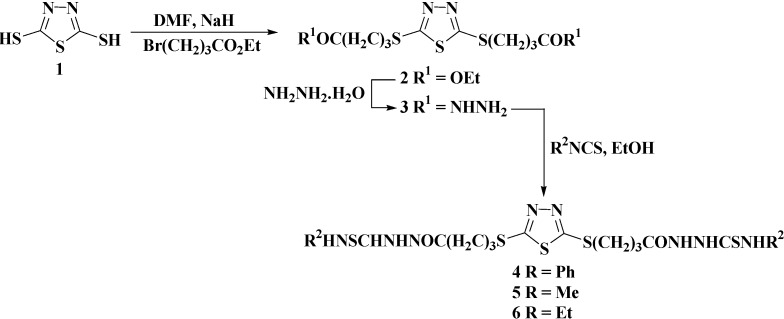
The target azoles were prepared started from 2,5-dimercapto-1,3,4-thiadiazole (1) [25] via multi-step synthesis as illustrated and outlined in Scheme 1, Scheme 2, Scheme 3 and Scheme 4.
Scheme 1.
Synthesis of bis-acid thiosemicarbazides 4–6.
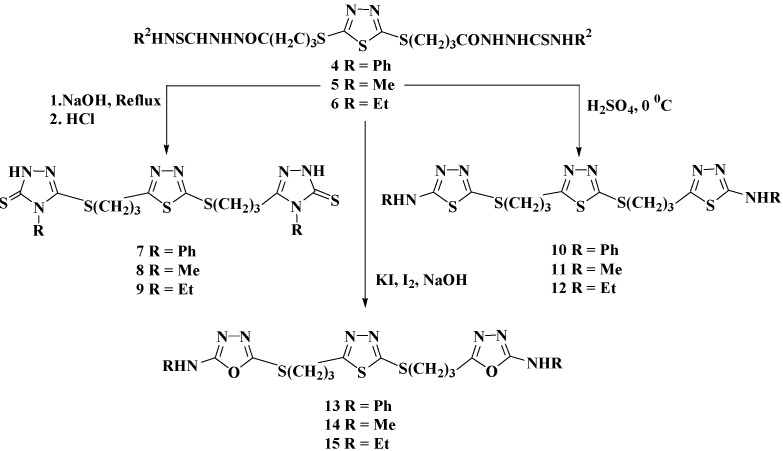
Scheme 2.
Synthesis of 1,2,4-triazoles 7–9, 1,3,4-thiadiazoles 10–12 and 1,3,4-oxadiazoles 13–15.
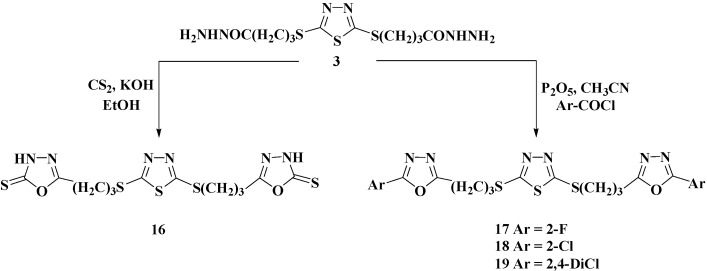
Scheme 3.
Synthesis of 1,3,4-oxadiazole derivatives 16–19.
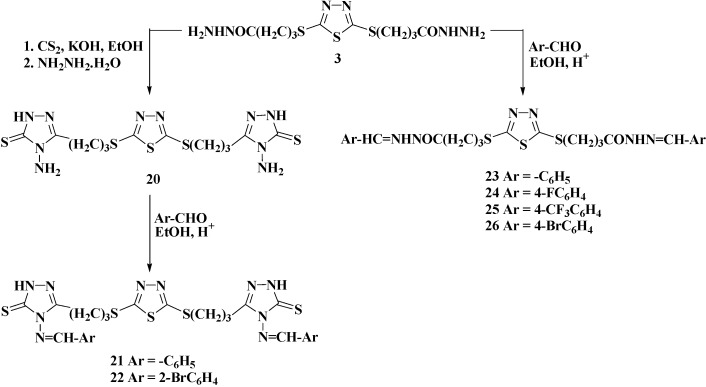
Scheme 4.
Synthesis of 4-amino-1,2,4-triazole 20 and Schiff bases 21–26.
The alkylation of 2,5-dimercapto-1,3,4-thiadiazole (1) with 4-ethylbromobutyrate in dimethylformamide in the presence of potassium carbonate as base gave diethyl 4,4′-[(1,3,4-thiadiazol-2,5-diyl)bis(sulfanediyl)]dibutanoate (2) in 92% yield (Scheme 1). The structures of the newly synthesized compounds were characterized by IR, 1H-NMR, 13C-NMR, Mass spectra and elemental analysis. Compound 2 exhibited NMR and IR spectra congruent with its assigned structure. Its IR spectrum showed strong absorption bands at 1740 and 1270 cm−1 due to (C=O) and (C-O) groups of ester 2, respectively. In addition, the appearance of two absorption bands at 2879 and 2936 cm−1 corresponding to the aliphatic (C-H) streching vibrations confirm the success of the alkylation reaction. In the 1H-NMR spectrum, the characteristic ester protons (CH2CH3) resonated as triplet at δH 2.06 ppm and quartet at δH 4.08–4.12 ppm. The butyric methylene protons CH2CH2CH2, CH2CO and SCH2 were observed as two multiplets at δH 1.78–1.82 and 1.87–1.93 ppm and triplet at δH 3.32 ppm, respectively. Moreover, the 13C-NMR spectrum confirmed the alkylation reaction through the chemical shifts at δC 20.94 ppm attributed to the methyl groups and the carbon signals of the methylene groups were recorded at δC 25.88, 27.61, 33.66 and 63.63 ppm. The signals corresponding to the C=N and C=O carbons appeared at δC 164.86 and 170.99 ppm, respectively.
Hydrazinolysis of the bis-ester 2 with hydrazine hydrate, in refluxing ethanol for 6 h, furnished the corresponding bis-acid hydrazide 3 which upon condensation with alkyl/aryl isothiocyanates, in ethanol under reflux for 6–8 h, led to the precursor’s bis-acid thiosemicarbazides 4–6 in good yields (86%–89%).
The success of the hydrazinolysis reaction was confirmed by IR, 1H-NMR and 13C-NMR analysis of compound 3. In the IR spectrum, the characteristic NH and NH2 groups of the hydrazide moiety were observed at 3250–3400 cm−1. Its 1H-NMR spectrum showed a quintet at δH 1.89–1.96 ppm characteristic for the methylene group CH2CH2CH2. The remaining methylene protons of CH2CO and SCH2 groups resonated as two triplets at δH 2.17 and 3.27 ppm, respectively. In addition, the NH2 and NH group were assigned to two singlets appearing at δH 4.22 and 9.02 ppm, respectively. In the 13C-NMR spectrum, the signals belonging to the three carbons of the methylene groups appeared at δC 18.65, 24.38 and 29.16 ppm, while the C=O amide group showed a signal at δC 166.82 ppm which provided definitive proof for the formation of the bis-acid hydrazide 3. The structure of the bis-acid thiosemicarbazides 4–6 was elucidated from their spectral analyses. Their IR spectra showed the presence of thiocarbonyl group (C=S) by the appearance of new absorption band at 1280–1310 cm−1. In their 1H-NMR spectra, the disappearance of the hydrazide (NH2, NH) protons and appearance of characteristic thiosemicarbazide NH′s in the range of δH 7.88–9.90 ppm confirmed the formation of the bis-acid thiosemicarbazides 4–6 (see experimental part). The 1H-NMR spectrum of the thiosemicarbazide 6 derived from the ethylisothiocyanate revealed the presence of two triplets related to the NH that is bonded to the ethyl group at δH 7.91 and 8.27 ppm, respectively, in a ratio 4 to 1. Additional CH2 and CH3 signals belonging to the ethyl group appeared in the aliphatic region. The 13C-NMR spectrum supported the formation of compound 6 via the appearance of new characteristic CH2 and CH3 groups related to the ethyl substituent in the aliphatic region at δC 14.46, 18.52 and 38.37, 39.04 ppm, respectively. Moreover, C=S group resonated at δC 175.60 ppm in the 13C-NMR spectrum of 6 confirming its presence in the thione form. The remaining carbons were recorded at their corresponding regions.
Thermal intramolecular cyclodehydration of compounds 4–6 has been performed in alkaline medium (2N NaOH) to give the corresponding 2,5-bis[(4-aryl/alkyl-2,4-dihydro-1,2,4-triazol-3-thione-5-yl)propylthio]-1,3,4-thiadiazole 7–9 in 84%–87% yields (Scheme 2). In contrast, the action of H2SO4 on the precursors 4–6 at 0 °C furnished new 2-aminoalkyl/aryl-1,3,4-thiadiazoles 10–12. On the other hand, compounds 4–6 were oxidatively cyclized to the corresponding alkyl/arylamino-1,3,4-oxadiazoles 13–15, in the presence of I2/KI in refluxing (4%) NaOH (Scheme 2).
The IR spectra of triazoles 7–9 showed common characteristic absorption peaks at 3200–3325 cm−1 assigned to (NH), 1267–1295 cm−1 (C=S) and 1629–1690 cm−1 (C=N) which confirmed the formation of triazole rings. From the 1H-NMR spectrum of the N-phenyl derivative 7, the disappearance of signals related to the NH group of the corresponding phenylthiosemicarbazide 4 and appearance of diagnostic triazole-NH singlet at δH 13.72 ppm was a clear evidence for the formation of triazole 7 in its thione form. Moreover, the phenyl protons resonated in the aromatic region as multiplet at δH 7.41–7.60 ppm. The peaks belonging to the same phenyl group were observed at δC 128.76–134.18 ppm in the 13C-NMR spectrum. The C=S signal appeared at δC 168.10 ppm confirming the predominance of the thione isomer. These results confirmed that compound 4 underwent ring closure to give the corresponding 1,2,4-triazole 7. The formation of the thiadiazoles 10–12 were deduced on the basis of their spectral data, which revealed the disappearance of the carbonyl (C=O) and thiocarbonyl groups in their IR spectra and the appearance of characteristic absorption bands near 1624–1657 cm−1 attributed to the C=N group. In addition, the exhibited chemical shifts obtained from their 1H-NMR and 13C-NMR spectra were all supported by the proposed structures of 10–12. Their 1H-NMR spectra confirmed the disappearance of the signals related to the -CONH and -NHCSNH- of their corresponding thiosemicarbazides 4–6 around δH 7.88–9.90 ppm. In the 1H-NMR spectrum of the N-ethyl derivative 12, the characteristic exocyclic NH proton at position 2 of the 1,3,4-thiadiazole ring resonated at δH 7.89 ppm. The aliphatic region showed two additional signals at δH 1.15 and 3.24–3.28 ppm attributed to the ethyl protons. The peaks belonging to the same group were observed at δC 14.12 and 40.04 ppm in the 13C-NMR spectrum. The spectrum also indicated the disappearance of the signals related to the C=O and C=S groups at δC 171.09 and 175.60, respectively.
Compound 14 was taken as a model compound to discuss the obtained spectroscopic data used to confirm the formation of the oxadiazoles ring. The structure was confirmed by IR, 1H-NMR, 13C-NMR and mass spectra. In the IR spectrum, a new characteristic peak appeared at 1639 cm−1 for C=N group and the disappearance of C=S at 1310 cm−1 of its corresponding starting 5. In addition, the 1H-NMR analysis revealed the disappearance of the signals related to the HNCSNH and NHCO protons at δH 7.88, 9.12 and 9.67 ppm of the corresponding methyl thiosemicarbazide 5 and only one singlet appeared at δH 9.90 ppm related to the amino group. All the other aliphatic protons appeared on their expected chemical shifts. The 13C-NMR spectrum of 14 indicated the appearance of the 1,3,4-oxadiazole carbons (C=N) at 157.85 and 168.78 ppm.
Intramolecular ring closure of the bis-acid hydrazide 3 using carbon disulfide in the presence of KOH, in refluxing ethanol for 8 h, afforded the corresponding 2-mercapto-1,3,4-oxadiazole-2-thione 16 in 82% yield (Scheme 3). Moreover, cyclocondensation of 3 with aromatic acid chlorides in the presence of P2O5 furnished the corresponding unfunctionalized 2,5-disubstituted-1,3,4-oxadiazoles 17–19 in 89%–91% yields (Scheme 3).
The IR spectrum of the oxadiazole 16 clearly showed the presence of two characteristic bands around 3210–3280 and 1267 cm−1 assigned to NH and C=S groups, respectively. In addition, the disappearance of C=O of the hydrazide group in the starting material 3 confirmed its involvement in the cyclocondensation reaction. The formation of the oxadiazole ring in the proposed structure of compound 16 was also established on the basis of its 1H-NMR spectrum in which the existence of a characteristic singlet at δH 13.74 ppm assigned to the NH proton confirming the thione form of such compound. Moreover, the disappearance of two singlets assigned to CONH and NH2 also confirmed the success of the oxidative cyclization of the bis-acid hydrazide 3. The three CH2 protons were also recorded in their respected area. In addition, the 13C-NMR analysis clearly confirmed the formation of the oxadiazole ring in its thione form through the appearance of C=S peak at δc 167.65 ppm. On the other hand, the structures of the oxadiazoles 17–19 were elucidated based on their IR spectra that showed the disappearance of the NH, NH2, and C=O groups of the starting material 3, and the appearance of characteristic absorption bands near 3069–3090 cm−1 assigned to aromatic protons. The 1H-NMR spectra displayed the presence of the aromatic protons in the region of 7.30–7.62 ppm confirming the structure of the oxadiazoles 17–19.
The treatment of the bis-acid hydrazide 3 with carbon disulphide in ethanolic potassium hydroxide solution led to the formation of potassium dithiocarbazinate salt. Its treatment with hydrazine hydrate under reflux for 8 h furnished the desired 2,5-bis[(4-amino-2,4-dihydro-1,2,4-triazol-3-thione-5-yl)propylthio]-1,3,4-thiadiazole (20) at 83% yield (Scheme 4). The significant peaks corresponding to the major functional groups were obvious in the IR spectrum of the aminotriazole 20. The band at 1310 cm−1 clearly indicated the presence of the thione group C=S, while the NH and NH2 groups appeared as a sharp band at 3220–3360 cm−1. In the 1H-NMR spectrum of compound 20 carried out in DMSO-d6, the presence of two diagnostic singlets at δH 5.32 and 13.34 ppm of the NH2 and NH protons respectively, depicting the presence of the triazole ring in its thione form. The structure of compound 20 was also supported by 13C-NMR. The signal at δC 163.38 ppm is assigned to the carbon of (C=N) linkage of triazole ring, while the signal that appeared at 177.64 ppm is assigned to the (C=S) group, which is another evidence of the existence of compound 20 in the thione form.
New Schiff bases 21–26 were successfully prepared in good yields (90%–94%), through the condensation of the 5-amino-1,2,4-triazole-3-thione 20 and/or bis-acid hydrazide 3 with several benzaldehyde derivatives in refluxing ethanol, in the presence of catalytic amount of hydrochloric acid (Scheme 4).
In the IR spectra of Schiff bases 21–26, the disappearance of the NH2 absorption bands and the appearance of sharp absorption bands at 3218–3367 cm−1 related to the NH group confirmed the success of the condensation reaction. The spectra also showed the presence of sharp absorption bands around 3050–3094 cm−1 characteristic of Sp2 hydrogen of the aromatic ring which were absent in the starting material. In the 1H-NMR spectra of compounds 21 and 22, the disappearance of NH2 signal and appearance of two singlets at δH 9.18–9.24 and 13.47–13.52 ppm attributed to H-C=N and NH protons, respectively, supported the proposed structures. In addition, the presence of C=S carbon at δC 173.41–173.81 ppm confirmed the formation of Schiff bases 21 and 22 in their thione form.
As expected, the 1H-NMR and 13C-NMR spectra of the synthesized hydrazones 23–26 analyzed in DMSO-d6 confirmed the existence of a mixture of diastereomers, i.e., E/cis and E/trans for each imino-amide moiety. These results were in agreement with that previously reported for similar hydrazones which were proved to exhibit E/Z geometrical isomers about the imine bond (HC=N) and cis/trans conformers to the carbonyl amide group [26,27]. This could presumably due to the restricted rotation around the C=N bond which could generates different steric rearrangements of hydrazone functionality in the geometric syn and anti-isomers.
Furthermore, the E-geometrical isomer and the cis/trans conformers were the more predominant forms in highly polar solvent such as DMSO-d6, while the Z-isomer appeared only in less polar solvents [28,29]. The 1H-NMR spectrum of the unsubstituted phenyl derivative 23 showed two different characteristic singlet peaks at δH 7.97 and 8.15 ppm with a ratio (2:1) and integrated totally for one proton, which is related to the imine proton (HC=N). The spectrum also showed two broad singlets at δH 11.31and 11.43 ppm integrated for the NH group with the same ratio. In addition, the absence of such pairing of signals in the 1H-NMR spectrum of the bis-hydrazide 3 compared to the bis-hydrazones 23–26 confirmed the formation these compounds as a mixture of E/cis and E/trans diastereomers. Regarding to the protons of the propyl chain of compound 23, the 1H-NMR spectrum was not so informative, as these peaks normally appear as multiple peaks. In addition to the evidence about the diastereomers formation for compound 23 by 1H-NMR, formation was also confirmed by 13C-NMR experiments. In the spectrum; each peak of compound 23 appeared as double peaks due to the presence of diasteriomeric mixture. Focusing on the aliphatic carbons this time, the propyl chain has three different carbon atoms that should be seen in the 13C-NMR spectrum. Instead of the three expected signals, a total of six signals were shown in the 13C-NMR spectrum reflecting the existence of diastereomers. The 13C-NMR spectrum also revealed the presence of two sets of signals in the downfield belonging to C=N and C=O groups of E/cis and E/trans diastereomers at δC 164.98, 167.63 and 167.63, 173.40 ppm, respectively.
2.2. Biology
2.2.1. Antimicrobial Activity
All of the newly synthesized compounds were evaluated for their in vitro growth inhibitory activities against a panel of standard strains of pathogenic microorganisms including three Gram-positive bacteria, three Gram-negative bacteria, and three strains of fungi. The antimicrobial studies were assessed by minimum inhibitory concentration (MIC) using the broth dilution method [30,31]. MIC is the highest dilution of a compound which shows clear fluid with no development of turbidity.
Antibacterial and antifungal screening revealed that some of the tested compounds exhibited good to excellent activities at concentrations ranging between 4–62.5 μg/mL.
Diethyl 4,4′-[(1,3,4-thiadiazol-2,5-diyl)bis(sulfanediyl)]dibutanoate (2) and its hydrazide derivative 3 displayed good activity against Gram-positive and Gram-negative bacterial strains at MIC 16–31.25 μg/mL and moderate activity towards fungal strains at MIC 31.25–62.5 μg/mL. Evaluating the antimicrobial activity of the synthesized 2,2′-(2,2′-(3,3′-(1,3,4-thiadiazol-2,5-diyl)bis-(sulfanediyl)bis-(propane-3,1-diyl)bis-(hydrazine-2,1-diyl)bis-(N-aryl/alkyl-2-oxoethanethioamide) 4–6, revealed that compounds exhibited good to moderate antibacterial activity at 16–31.25 μg/mL while their antifungal activity was significantly diminished. Nonetheless, 1,2,4-triazole carrying phenyl and/or alkyl substitution at N-4 7–9 showed excellent antibacterial and antifungal activities against all examined bacterial and fungal strains at 8–16 μg/mL (Table 1).
Table 1.
Antimicrobial activity expressed as MIC (μg/mL).
| Compound No. | Gram-Positive Organism a | Gram-Negative Organism b | Fungi c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sp | Bs | Sa | Pa | Ec | Kp | Af | Ca | GC | |
| 2 | 31.25 | 31.25 | 31.35 | 16 | 31.25 | 16 | 31.25 | 62.5 | 31.25 |
| 3 | 16 | 8 | 31.25 | 31.25 | 16 | 16 | 31.25 | 31.25 | 62.5 |
| 4 | 16 | 31.25 | 31.25 | 31.25 | 31.25 | 16 | 62.5 | 31.25 | 62.5 |
| 5 | 31.25 | 31.25 | 16 | 31.25 | 16 | 31.25 | 125 | 250 | 62.5 |
| 6 | 31.25 | 16 | 16 | 31.25 | 31.25 | 16 | 125 | 250 | 125 |
| 7 | 8 | 8 | 16 | 16 | 16 | 8 | 8 | 8 | 16 |
| 8 | 16 | 31.25 | 16 | 31.25 | 31.25 | 16 | 16 | 16 | 31.25 |
| 9 | 16 | 16 | 8 | 31.25 | 16 | 31.25 | 16 | 31.25 | 16 |
| 10 | 8 | 16 | 16 | 31.25 | 16 | 16 | 16 | 16 | 8 |
| 11 | 16 | 31.25 | 31.25 | 31.25 | 31.25 | 16 | 31.25 | 16 | 16 |
| 12 | 16 | 16 | 31.25 | 16 | 16 | 31.25 | 16 | 16 | 8 |
| 13 | 8 | 8 | 16 | 62.5 | 62.5 | 31.25 | 31.25 | 31.25 | 62.5 |
| 14 | 16 | 16 | 16 | 31.25 | 125 | 62.5 | 31.25 | 62.5 | 62.5 |
| 15 | 8 | 16 | 8 | 31.25 | 62.5 | 31.25 | 62.5 | 31.25 | 31.25 |
| 16 | 4 | 8 | 4 | 4 | 8 | 8 | 31.25 | 16 | 16 |
| 17 | 16 | 8 | 16 | 16 | 8 | 16 | 31.25 | 16 | 16 |
| 18 | 16 | 16 | 16 | 16 | 16 | 8 | 31.25 | 16 | 16 |
| 19 | 16 | 16 | 8 | 16 | 16 | 16 | 31.25 | 16 | 16 |
| 20 | 4 | 4 | 8 | 8 | 4 | 4 | 16 | 16 | 8 |
| 21 | 16 | 8 | 16 | 16 | 8 | 16 | 31.25 | 16 | 16 |
| 22 | 8 | 8 | 8 | 16 | 8 | 16 | 31.25 | 16 | 16 |
| 23 | 8 | 8 | 4 | 8 | 4 | 8 | 31.25 | 16 | 16 |
| 24 | 8 | 4 | 4 | 8 | 4 | 8 | 31.25 | 31.25 | 16 |
| 25 | 8 | 8 | 16 | 8 | 16 | 8 | 16 | 16 | 31.25 |
| 26 | 8 | 16 | 8 | 8 | 8 | 16 | 31.25 | 16 | 16 |
| Ciprofloxacin | ≤5 | ≤1 | ≤5 | ≤5 | ≤1 | ≤1 | - | - | - |
| Fluconazole | - | - | - | - | - | - | ≤1 | ≤1 | ≤1 |
a Gram-positive bacteria: Streptococcus pneumonia, Bacillus subtilis, Staphylococcus aureus; b Gram-negative bacteria: Pseudomonas aeuroginosa, Escherichia coli, Klebsiella pneumonia; c yeasts: Aspergillus fumigatus, Candida albicans, Geotrichum candidum.
On other hand, the antimicrobial activity of the thiadiazoles 10–12 revealed that all the tested compounds showed comparatively good activity against all bacterial and fungal strains at 8–31.25 μg/mL.
Moreover, 2-amino-1,3,4-oxadiazole derivatives 13–15 showed good and greater antibacterial activity against Gram-positive at MIC 8–16 μg/mL. Furthermore, compounds 13–15 exhibited moderate antifungal activity at MIC 31.25–62.5 μg/mL.
Among the oxadiazoles 16–19, oxadiazole 16 functionalized with thiol group at position 2 exhibited excellent antibacterial activities against all bacterial strains at MIC 4–8 μg/mL and good activity towards fungal strains at MIC 16–31.28 µg/mL (Table 1).
The incorporation of amino and thiol group into 1,2,4-triazole ring as 2,5-bis[(4-amino-2,4-dihydro-1,2,4-triazol-3-thione-5-yl)propylthio]-1,3,4-thiadiazole 20 resulted in enhancing the antimicrobial activities against all examined bacterial and fungal strains at MIC 4–16 μg/mL.
Evaluating the antibacterial activity of the Schiff bases 21–26, divulged compounds more effective against all bacteria strains at MIC 4–16 μg/mL. Particularly, Schiff bases 23, 24 comprising fluorine atom exhibited excellent inhibition at MIC 4–8 μg/mL against Gram positive bacteria.
Antifungal screening of Schiff bases 23–26 revealed that all compounds showed good antifungal activity against all examined fungal strains at MIC 16–31.5 μg/mL.
Therefore, it was concluded that 1,2,4-triazole moiety exhibits better antibacterial and antifungal activities than 1,3,4-thiadiazole or 1,3,4-oxadiazole moiety. Interestingly, the presence of azomethine linkage in hydrazones was found essential for the high antibacterial and antifungal activities of these compounds.
2.2.2. Antiproliferative Activity
In an effort to expand our investigation into potential biological activities associated with the newly synthesized compounds, an in vitro evaluation of their antiproliferative activities was carried out on four different human cancerous cell lines according to the protocol described in the ISO 10993-5 guide [32]. Only compounds shown in Table 2 exhibited a relatively high cytotoxic activity against the examined cancer cell lines and thus can be used as model compounds for the design and synthesis of new anticancer drugs.
Table 2.
The LD50 values (ng/μL) of the examined compounds on four human cancer cell lines. Values are expressed as mean ± SD of three experiments.
| Compound No. | MCF-7 | T47D | Caco II | HeLa |
|---|---|---|---|---|
| 12 | 376± 16 | 389 ± 12 | 387 ± 8 | 391 ± 10 |
| 15 | 409± 9 | 418± 10 | 418 ± 9 | 438 ± 9 |
| 18 | 673 ± 15 | 648 ± 11 | 668 ± 19 | 690 ± 17 |
| 19 | 398 ± 10 | 397 ± 9 | 373 ± 10 | 356 ± 12 |
| 22 | 519 ± 11 | 527 ± 19 | 502 ± 17 | 498 ± 23 |
| 25 | 863 ± 12 | 798 ± 18 | 678 ± 11 | 562 ± 8 |
Human breast adenocarcinoma MCF7, Human ductal breast epithelial tumor T47D, Human epithelial colorectal adenocarcinoma Caco II and Human epithelial carcinoma HeLa.
Interestingly, changing phenyl and methyl substitution in compounds 10, 11 and 13, 14 into ethyl group to yield compounds 12 and 15 appears to significantly potentiate the cytotoxic activities associated with these analogues suggesting a steric factor mediating either transport or molecular interaction of these compounds with cellular targets. The addition of one more Cl atom into the structure of compound 18 to yield compound 19 was successful in almost doubling its activity. More investigation of possible pathways by which these compounds exert their antiproliferative activity should shed light onto potential molecular targets with which the compounds may interact.
3. Experimental Section
3.1. General
Commercially available solvents and reagents were purified according to the stander procedures. All melting points were measured on a Melt-Temp apparatus and are uncorrected. Thin layer chromatography (TLC) was performed on aluminum plates Silica Gel 60 F254 (E-Merk, layer thickness 0.2 mm) with detection by UV light absorption. The IR spectra were recorded for the compounds in a KBr matrix with a FTIR-8400s-Fourier transform infrared spectrophotometer-Shimadzu. The NMR spectra were measured with Bruker spectrophotometer (400 and 600 MHz) using TMS as internal standard. ESI mass spectral data were obtained by a Finnigan LCQ spectrometer and EI mass spectra were recorded on a Finnigan MAT 95XL spectrometer. Elemental analyses were performed using an elementar Analysen-systeme GmbH-Vario EL III Element Analyzer.
3.2. Synthesis and Characterization of 2,5-Dimercapto-1,3,4-thiadiazole (1)
To a mixture of hydrazine hydrate (20 mmol) in aqueous solution of sodium hydroxide (12 N) (100 mL) was added carbon disulfide (CS2) (40 mmol) in ethanol (100 mL) at 0 °C with stirring during 30 min. Then, the mixture was refluxed for 17 h. After cooling, the mixture was acidified with diluted hydrochloric acid (HCl) and the solid formed was washed, dried and recrystallized from ethanol afforded yellow needles, Yield 98%; m.p. 160–162 °C (Lit. m.p. 162–164 °C) [25].
3.3. Synthesis and Characterization of Diethyl 4,4′-[(1,3,4-Thiadiazol-2,5diyl)bis(sulfanediyl)]dibutanoate (2)
A mixture of compound 1 (1 mmol), potassium carbonate (2.2 mmol) in DMF (20 mL) was stirred for 2 h at room temperature, then ethyl bromobutyrate (2.2 mmol) was added and the mixture was heated under reflux for 8 h. The reaction mixture was cooled and poured onto crushed ice. The product was collected by filtration, washed with water, dried and recrystallized from ethanol yielded compound 2 as colorless crystals, Yield 92%; m.p. 95–96 °C. IR (υ, cm−1): 1270 (C-O), 1634 (C=N), 1740 (C=O), 2879, 2936 (C-H al). 1H-NMR: δ 1.78–1.82 (m, 2H, CH2CH2CH2), 1.87–1.93 (m, 2H, CH2CO), 2.06 (bs, 3H, CH2CH3), 3.32 (t, 2H, J = 4 Hz, SCH2), 4.08–4.12 (q, 2H, J = 4 Hz, J = 8 Hz, OCH2) ppm. 13C-NMR: δ 20.94 (CH2CH3), 25.88 (CH2CH2CH2), 27.61 (CH2CO), 33.66 (SCH2), 63.63 (OCH2), 164.86 (C=N), 170.99 (C=O) ppm. EI MS (m/z): 378.49 (M+). Anal. Calcd for C14H22N2O4S3: C 44.42; H 5.86; N 7.40. Found: C 44.28; H 5.99; N 7.23.
3.4. Synthesis and Characterization of 4,4′-[(1,3,4-Thiadiazol-2,5-diyl)bis(sulfanediyl)]dibutanehydrazide (3)
A mixture of ester 2 (10 mmol) in ethanol (50 mL) and hydrazine hydrate (50 mmol) was refluxed for 6 h. After cooling, ethanol was removed under reduced pressure and the product formed was recrystallized from ethanol yielded compound 3 as white solid, Yield 90%; m.p. 189–191 °C. IR (υ, cm−1): 1627 (C=N), 1689 (C=O), 3250–3400 (NH, NH2). 1H-NMR: δ 1.89–1.96 (quin, 2H, CH2CH2CH2), 2.17 (t, 2H, J = 8 Hz, CH2CO), 3.27 (t, 2H, J = 8 Hz, SCH2), 4.22 (bs, 2H, NH2), 9.02 (s, 1H, NH) ppm. 13C-NMR δ 18.65 (CH2CH2CH2), 24.38 (CH2CO), 29.16 (SCH2), 147.80 (C=N), 166.82 (C=O) ppm. EI MS (m/z): 350.44 (M+). Anal. Calcd for C10H18N6O2S3: C 34.27; H 5.18; N 23.98. Found: C 34.59; H 5.07; N 24.22.
3.5. General Procedure for the Synthesis of Thiosemicarbazide Derivatives 4–6
A mixture of acid hydrazide 3 (10 mmol) in ethanol (50 mL) and the appropriate alkyl/aryl isothiocyanate (22 mmol) was refluxed for 6–8 h. After cooling, the precipitate formed was collected by filtration and recrystallized from ethanol to afford the desired product.
2,2′-(2,2′-(3,3′-(1,3,4-Thiadiazol-2,5-diyl)bis-(sulfanediyl)bis-(propane-3,1-diyl)bis-(hydrazine-2,1-diyl)bis-(N-phenyl-2-oxoethanethioamide) (4). This compound was obtained as colorless crystals, Yield 89%; m.p. 247–248 °C. IR (υ, cm−1): 1292 (C=S), 1648 (C=N), 1675 (C=O), 3209–3350 (NH). 1H-NMR: δ 1.96–2.03 (quin, 2H, CH2CH2CH2), 2.32 (t, 2H, J = 8 Hz, CH2CO), 3.21 (t, 2H, J = 8 Hz, SCH2), 7.12–7.47 (m, 5H, Ar-H), 9.57 (d, 1H, J = 4 Hz, NH), 9.62 (s, 1H, J = 4 Hz, NH), 9.90 (s, 1H, NH) ppm. 13C-NMR: δ 24.64 (CH2CH2CH2), 31.95 (CH2CO), 32.12 (SCH2), 126.20, 126.45, 128.13, 128.24, 128.30, 128.49, 128.89, 129.12, 130.06, 130.68, 130.95, 134.13, 135.31, 137.31, 142.49 (Ar-C, C=N), 173.66, 174.90 (C=O, C=S) ppm. EI MS (m/z): 620.32 (M+). Anal. Calcd for C24H28N8O2S5: C 46.43; H 4.55; N 18.05. Found: C 46.71; H 4.74; N 17.98.
2,2′-(2,2′-(3,3′-(1,3,4-Thiadiazol-2,5-diyl)bis-(sulfanediyl)bis-(propane-3,1-diyl)bis-(hydrazine-2,1-diyl)bis-(N-methyl-2-oxoethanethioamide) (5). This compound was obtained as colorless crystals, Yield 86%; m.p. 192–193 °C. IR (υ, cm−1): = 1310 (C=S), 1645 (C=N), 1660 (C=O), 3200–3310 (NH). 1H-NMR: δ 1.92–1.99 (quin, 2H, CH2CH2CH2), 2.27 (t, 2H, J = 8 Hz, CH2CO), 2.84 (d, 3H, J = 4 Hz, NCH3), 3.18 (t, 2H, J = 8 Hz, SCH2), 7.88 (d, 1H, J = 4 Hz, NH), 9.12 (d, 1H, J = 4 Hz, NH), 9.67 (bs, 1H, NH) ppm. 13C-NMR: δ 24.55 (CH2CH2CH2), 30.81 (NCH3), 31.91 (CH2CO), 32.01 (SCH2), 137.30, 142.49 (C=N), 171.37, 173.44 (C=O, C=S) ppm. EI MS (m/z): 496.45 (M+). Anal. Calcd for C14H24N8O2S5: C 33.85; H 4.87; N 22.56. Found: C 33.89; H 4.75; N 22.38
2,2′-(2,2′-(3,3′-(1,3,4-Thiadiazol-2,5-diyl)bis-(sulfanediyl)bis-(propane-3,1-diyl)bis-(hydrazine-2,1-diyl)bis-(N-ethyl-2-oxoethanethioamide) (6). This compound was obtained as colorless crystals, Yield 87%; m.p. 259–260 °C. IR (υ, cm−1): 1280 (C=S), 1635 (C=N), 1675 (C=O), 3220–3350 (NH). 1H-NMR: δ 1.05 (t, 3H, J = 8 Hz, CH2CH3), 1.93–2.00 (quin, 2H, CH2CH2CH2), 2.30 (t, 2H, J = 8 Hz, CH2CO), 3.30 (t, 2H, J = 8 Hz, SCH2), 3.42–3.48 (m, 2H, NCH2), 7.91 (t, 0.8H, J = 4 Hz, NH), 8.27 (bt, 0.2H, NH), 9.04 (s, 0.8H, NH), 9.10 (s, 0.2H, NH), 9.38 (s, 0.1H, NH), 9.68 (s, 1H, NH) ppm. 13C-NMR: δ 14.46, 18.52 (CH2CH3), 24.39, 24.40 (CH2CH2CH2), 29.37, 31.83 (CH2CO), 33.48, 33.55 (SCH2), 38.37, 39.04 (NCH2), 164.89, 164.96 (C=N), 171.09, 175.60 (C=O, C=S) ppm. EI MS (m/z): 524.30 (M+). Anal. Calcd for C16H28N8O2S5: C 36.62; H 5.38; N 21.35. Found: C 36.88; H 5.48; N 21.08.
3.6. General Procedure for the Synthesis of Triazole Derivatives 7–9
A solution of the appropriate thiosemicarbazide derivative 4–6 (1 mmol) in aqueous sodium hydroxide 2 N (10 mL) was refluxed for 6 h. The resulting solution was cooled to room temperature and acidified with diluted hydrochloride acid. The precipitate formed was filtered, washed with water and recrystallized from ethanol to give the desired product.
2,5-Bis[(4-phenyl-2,4-dihydro-1,2,4-triazol-3-thione-5-yl)propylthio]-1,2,4-triazole (7). This compound was obtained as colorless crystals, Yield 87%; m.p. 290–291 °C. IR (υ, cm−1): 1267 (C=S), 1629 (C=N), 3200–3310 (NH). 1H-NMR δ 1.74–1.79 (quin, 2H, CH2CH2CH2), 2.27 (t, 0.8H, J = 6 Hz, CH2C=N), 2.45–2.54 (m, 3.2H, CH2C=N, SCH2), 7.41–7.60 (m, 5H, Ar-H), 13.72 (s, 1H, NH) ppm. 13C-NMR: δ 23.34 (CH2CH2CH2), 24.32 (CH2C=N), 29.72 (SCH2), 128.76, 129.92, 129.95, 134.18 (Ar-C), 152.09, 168.10 (C=N, C=S) ppm. ESI MS (m/z): 585.40 (M + H)+. Anal. Calcd for C24H24N8S5: C 49.29; H 4.14; N 19.16. Found: C 49.02; H 4.25; N 19.37
2,5-Bis[(4-methyl-2,4-dihydro-1,2,4-triazol-3-thione-5-yl)propylthio]-1,2,4-triazole (8). This compound was obtained as colorless crystals, Yield 84%; m.p. 201–203 °C. IR (υ, cm−1): 1289 (C=S), 1640 (C=N), 3225–3265 (NH). 1H-NMR: δ 2.07–2.14 (quin, 2H, CH2CH2CH2), 2.50 (s, 3H, NCH3), 2.78 (t, 2H, J = 4 Hz, CH2C=N), 3.21 (t, 2H, J = 4 Hz, SCH2), 13.52 (s, 1H, NH) ppm. 13C-NMR: δ 23.60 (CH2CH2CH2), 25.16 (NCH3), 29.62 (CH2C=N), 31.53 (SCH2), 152.48, 166.58 (C=N, C=S) ppm. EI MS (m/z): 460.88 (M+). Anal. Calcd for C14H20N8S5: C 36.50; H 4.38; N 24.32. Found: C 36.73; H 4.44; N 24.16.
2,5-Bis[(4-ethyl-2,4-dihydro-1,2,4-triazol-3-thione-5-yl)propylthio]-1,2,4-triazole (9). This compound was obtained as colorless crystals, Yield 85%; m.p. 213–214 °C. IR (υ, cm−1): 1295 (C=S), 1690 (C=N), 3260–3325 (NH). 1H-NMR: δH 1.21(t, 3H, J = 6 Hz, CH2CH3), 1.91–1.96 (quin, 2H, CH2CH2CH2), 2.46 (t, 1H, J = 6 Hz, CH2C=N), 2.60 (t, 1H, J = 6 Hz, CH2C=N), 2.79 (t, 2H, J = 6 Hz, SCH2), 3.94–3.98 (q, 2H, J = 6 Hz, J = 12 Hz, NCH2), 13.49 (s, 1H, NH) ppm. 13C-NMR: δ 13.84 (CH2CH3), 23.55 (CH2CH2CH2), 25.58 (CH2C=N), 30.13 (SCH2), 39.56 (NCH2), 151.96, 166.44 (C=N, C=S) ppm. ESI MS (m/z): 489.50 (M + H)+. Anal. Calcd for C16H24N8S5: C 39.32; H 4.95; N 22.93. Found: C 39.15; H 4.83; N 23.09.
3.7. General Procedure for the Synthesis of Thiadiazole Derivatives 10–12
A mixture of the appropriate thiosemicarbazide derivative 4–6 (1 mmol) in cold concentrated sulfuric acid (15 mL) was stirred for 30 min. Then the mixture was allowed to reach room temperature. After stirring for an additional 16 h; the resulting solution was poured into ice-cold water and made alkaline to pH 8 using ammonium hydroxide. The precipitate formed was filtered; washed with water and recrystallized from ethanol to give the desired product.
2,5-Bis[(2-phenylamino-1,3,4-thiadiazol-5-yl)propylthio]-1,3,4-thiadiazole (10). This compound was obtained as white solid, Yield 82%; m.p. 235–236 °C. IR (υ, cm−1): 1624 (C=N), 3224–3292 (NH). 1H-NMR: δH 2.15–2.19 (quin, 2H, CH2CH2CH2), 3.02 (t, 2H, J = 6 Hz, CH2C=N), 3.30 (t, 2H, J = 6 Hz, SCH2), 7.49–7.84 (m, 5H, ArH), 10.13 (s, 1H, NH) ppm. 13C-NMR: δ 28.00 (CH2CH2CH2), 28.15 (CH2C=N), 32.78 (SCH2), 116.06, 126.44, 140.29, 141.04, 158.09, 163.97, 164.44 (Ar-C, C=N) ppm. EI MS (m/z): 584.83 (M+). Anal. Calcd for C24H24N8S5: C 49.29; H 4.14; N 19.16. Found: C 49.13; H 4.06; N 19.28.
2,5-Bis[(2-methylamino-1,3,4-thiadiazol-5-yl)propylthio]-1,3,4-thiadiazole (11). This compound was obtained as white solid, Yield 80%; m.p. 222–224 °C. IR (υ, cm−1): 1636 (C=N), 3217–3290 (NH). 1H-NMR: δ 2.03–2.09 (quin, 2H, CH2CH2CH2), 2.89–2.94 (m, 5H, CH2C=N, NCH3), 3.21 (t, 2H, J = 4 Hz, SCH2), 9.87 (s, 1H, NH) ppm. 13C-NMR: δC 26.89 (CH2CH2CH2), 28.34 (CH2C=N), 31.98 (SCH2), 38.69 (NCH3), 155.67, 163.72, 168.31 (C=N) ppm. ESI MS (m/z): 461.49 (M + H)+. Anal. Calcd for C14H20N8S5: C 36.50; H 4.38; N 24.32. Found: C 36.31; H 4.50; N 24.49.
2,5-Bis[(2-ethylamino-1,3,4-thiadiazol-5-yl)propylthio]-1,3,4-thiadiazole (12). This compound was obtained as white solid, Yield 81%; m.p. 280–281 °C. IR (υ, cm−1): 1657 (C=N), 3229–3309 (NH). 1H-NMR δ 1.15 (t, 3H, J = 6 Hz, CH2CH3), 2.09–2.14 (quin, 2H, CH2CH2CH2), 2.49 (t, 2H, J = 6 Hz, CH2C=N), 2.92 (t, 2H, J = 6 Hz, SCH2), 3.24–3.28 (q, 2H, J = 6 Hz, J = 12 Hz, NCH2), 7.89 (d, 1H, J = 6 Hz, NH) ppm. 13C-NMR: δ 14.12 (CH2CH3), 28.19 (CH2CH2CH2), 28.21 (CH2C=N), 32.80 (SCH2), 40.04 (NCH2), 155.88, 164.39, 168.66 (C=N) ppm. EI MS (m/z): 488.30 (M+). Anal. Calcd for C16H24N8S5: C 39.32; H 4.95; N 22.93. Found: C 39.19; H 4.83; N 22.85.
3.8. General Procedure for the Synthesis of Oxadiazole Derivatives 13–15
To a solution of the appropriate thiosemicarbazide derivative 4–6 (1 mmol) in ethanol (20 mL) was added (5 N) NaOH solution until the thiosemicarbazide completely dissolved. Then, a solution of I2/KI (5%) was added dropwise until the iodine color persists. The reaction mixture was heated under reflux for 3 h and then filtered. After cooling, the precipitate formed was filtered, washed with water and recrystallized from ethanol to afford the desired product.
2,5-Bis[(2-phenylamino-1,3,4-oxadiazol-5-yl)propylthio]-1,3,4-thiadiazole (13). This compound was obtained as colorless crystals, Yield 81%; m.p. 285–286 °C. IR (υ, cm−1): 1627 (C=N), 3223–3310 (NH). 1H-NMR: δ 1.92–2.03 (m, 2H, CH2CH2CH2), 2.55–2.62 (m, 2H, CH2C=N), 3.27 (t, 2H, J = 6Hz, SCH2), 7.31–7.57 (m, 5H, Ar-H), 8.68 (s, 1H, NH) ppm. 13C-NMR: δ 28.19 (CH2CH2CH2), 28.21 (CH2C=N), 32.80 (SCH2), 124.26, 140.33, 142.89, 148.98, 155.88 (Ar-C), 164.39, 168.66 (C=N) ppm. EI MS (m/z): 552.89 (M+). Anal. Calcd for C24H24N8O2S3: C 52.15; H 4.38; N 20.27. Found: C 52.32; H 4.49; N 20.01
2,5-Bis[(2-methylamino-1,3,4-oxadiazol-5-yl)propylthio]-1,3,4-thiadiazole (14). This compound was obtained as colorless crystals, Yield 84%; m.p. 268–269 °C. IR (υ, cm−1): 1639 (C=N), 3225–3121 (NH). 1H-NMR δ 2.05–2.12 (quin, 2H, CH2CH2CH2), 2.96–2.99 (m, 5H, CH2C=N, NCH3), 3.24 (t, 2H, J = 4 Hz, SCH2), 9.90 (s, 1H, NH) ppm. 13C-NMR: δ 27.79 (CH2CH2CH2), 28.17 (CH2C=N), 32.07 (SCH2), 38.83 (NCH3), 157.85, 168.78 (C=N) ppm. ESI MS (m/z): 429.78 (M + H)+. Anal. Calcd for C14H20N8O2S3: C 39.24; H 4.70; N 26.15. Found: C 39.41; H 4.53; N 26.27.
2,5-Bis[(2-ethylamino-1,3,4-oxadiazol-5-yl)propylthio]-1,3,4-thiadiazole (15). This compound was obtained as colorless crystals, Yield 85%; m.p. 247–248 °C. IR (υ, cm−1): 1637 (C=N), 3229–3299 (NH). 1H-NMR: δ 1.20 (t, 3H, J = 4 Hz, CH2CH3), 2.04–2.11 (quin, 2H, CH2CH2CH2), 2.97 (t, 2H, J = 4 Hz, CH2C=N), 3.24 (t, 2H, J = 4 Hz, SCH2), 3.33–3.42 (q, 2H, J = 4 Hz, J = 8 Hz, NCH2), 9.96 (s, 1H, NH) ppm. 13C-NMR: δ 13.34 (CH2CH3), 27.78 (CH2CH2CH2), 28.16 (CH2C=N), 32.17 (SCH2), 40.83 (NCH2), 157.74, 167.74 (C=N) ppm. EI MS (m/z): 456.80 (M+). Anal. Calcd for C16H24N8O2S3: C 42.09; H 5.30; N 24.54. Found: C 42.33; H 5.17; N 24.36.
3.9. Synthesis and Characterization of 2,5-Bis[(3H-1,3,4-oxadiazole-2-thione-5-yl)propylthio]-1,3,4-thiadiazole (16)
To a solution of acid hydrazide 3 (1 mmol) in ethanol (20 mL), potassium hydroxide (2.2 mmol) in water (5 mL) and carbon disulfide (10 mmol) were added. The reaction mixture was heated under reflux for 8 h. The mixture was cooled, diluted with cold water (20 mL) and acidified with diluted HCl. The precipitate formed was collected by filtration, washed with water and recrystallized from ethanol afforded the desired compound 16 as colorless needles, Yield 82%; m.p. 238–239 °C. IR (υ, cm−1): 1267 (C=S), 1692 (C=N), 3210–3280 (NH). 1H-NMR: δ 1.92–1.99 (quin, 2H, CH2CH2CH2), 2.53 (t, 2H, J = 8 Hz, CH2C=N), 3.12 (t, 2H, J = 8 Hz, SCH2), 13.74 (s, 1H, NH) ppm. 13C-NMR: δC 24.03 (CH2CH2CH2), 25.19 (CH2C=N), 31.34 (SCH2), 151.54, 167.65 (C=N, C=S) ppm. EI MS (m/z): 433.76 (M+). Anal. Calcd for C12H14N6O2S5: C 33.16; H 3.25; N 19.34. Found: C 32.97; H 3.34; N 19.36.
3.10. General Procedure for the Synthesis of Oxadiazole Derivatives 17–18
To a solution of acid hydrazide 3 (1 mmol) in acetonitrile (10 mL) was added the appropriate benzoyl chloride (1.3 mmol), and phosphorous pentoxide (5 mmol). The reaction mixture was stirred at room temperature for 20–30 min. Then, the solvent was removed under pressure and the mixture was dissolved in dichloromethane and washed with water (3 × 50 mL). The organic layer was then dried over sodium sulfate and evaporated under reduce pressure. The obtained solid was recrystallized from ethanol to afford the desired product.
2,5-Bis[(2-(2-fluoropenyl)-1,3,4-oxadiazol-5-yl)propylthio]-1,3,4-thiadiazole (17). This compound was obtained as white solid, Yield 89%, m.p. 292–294 °C. IR (υ, cm−1): 1635 (C=N), 3069–3072 (Ar-H). 1H-NMR: δ 1.90–2.04 (m, 2H, CH2CH2CH2), 2.53–2.60 (m, 2H, CH2C=N), 3.24 (t, 2H, J = 6 Hz, SCH2), 7.30–7.49 (m, 3H, Ar-H), 7.52 (dd, 1H, J = 6 Hz, J = 12 Hz, Ar-H) ppm. 13C-NMR: δ 28.09 (CH2CH2CH2), 28.24 (CH2C=N), 32.88 (SCH2), 124.28, 140.36, 142.99, 149.28, 155.48 (Ar-C), 164.29, 168.66 (C=N) ppm. EI MS (m/z): 558.35 (M+). Anal. Calcd for C24H20F2N6O2S3: C 51.60; H 3.61; N 15.04. Found: C 51.79; H 3.48; N 15.17.
2,5-Bis[(2-(2-chlorophenyl)-1,3,4-oxadiazol-5-yl)propylthio]-1,3,4-thiadiazole (18). This compound was as white solid, Yield 91%; m.p. 267–268 °C. IR (υ, cm−1): 1648 (C=N), 3073–3089 (Ar-H). 1H-NMR: δ 1.94–2.10 (m, 2H, CH2CH2CH2), 2.56–2.61 (m, 2H, CH2C=N), 3.29 (t, 2H, J = 6 Hz, SCH2), 7.41–7.57 (m, 4H, Ar-H) ppm. 13C-NMR: δ 28.19 (CH2CH2CH2), 28.32 (CH2C=N), 32.67 (SCH2), 124.37, 140.41, 142.86, 149.98, 156.12 (Ar-C), 164.26, 167.96 (C=N) ppm. EI MS (m/z): 590.37 (M+). Anal. Calcd for C24H20Cl2N6O2S3: C 48.73; H 3.41; N 14.21. Found: C 48.50; H 3.29; N 14.34.
2,5-Bis[(2-(2,5-dichlorophenyl)-1,3,4-oxadiazol-5-yl)propylthio]-1,3,4-thiadiazole (19). This compound was obtained as white solid, Yield 90%; m.p. 252–254 °C. IR (υ, cm−1): 1664 (C=N), 3075–3090 (Ar-H). 1H-NMR: δ 1.96–2.12 (m, 2H, CH2CH2CH2), 2.54–2.60 (m, 2H, CH2C=N), 3.32 (t, 2H, J = 6 Hz, SCH2), 7.48–7.55 (m, 2H, Ar-H), 7.62 (s, 1H, Ar-H) ppm. 13C-NMR: δ 27.99 (CH2CH2CH2), 28.83 (CH2C=N), 33.17 (SCH2), 125.47, 141.31, 142.66, 150.77, 157.02 (Ar-C), 165.12, 166.99 (C=N) ppm. EI MS (m/z): 658.15 (M+). Anal. Calcd for C24H18Cl4N6O2S3: C 43.65; H 2.75; N 12.72. Found: C 43.47; H 2.88; N 12.90.
3.11. Synthesis and Characterization of 2,5-Bis[(4-amino-2,4-dihydro-1,2,4-triazol-3-thione-5-yl)propylthio]-1,3,4-thiadiazole (20)
Step 1: Carbon disulfide (3 mL) was added dropwise to a solution of acid hydrazide 3 (1 mmol) in absolute ethanol (20 mL) containing potassium hydroxide (2.2 mmol) at 0 °C. The reaction was stirred at room temperature for 16 h, and then cooled and diluted with diethyl ether. The precipitate was filtered, washed with diethyl ether and dried. The potassium dithiocarbazinate salt was obtained in nearly quantitative yield and used without further purification as it was moisture sensitive.
Step 2: Hydrazine hydrate (2.5 mmol) was added to a suspension of the potassium salt (1 mmol) in water (15 mL) and the mixture was refluxed with stirring for 8 h. After cooling, it was diluted with water then acidified with aqueous hydrochloric acid. The precipitate was filtered, washed with water and recrystallized from ethanol to give the desired product 16 as colorless crystals, Yield 83%; m.p. 203–205 °C. IR (υ, cm−1): 1310 (C=S), 1654 (C=N), 3220–3360 (NH, NH2). 1H-NMR: δ 2.02–2.13 (m, 2H, CH2CH2CH2), 2.73–2.77 (m, 2H, CH2C=N), 2.91–2.98 (m, 2H, SCH2), 5.32 (s, 2H, NH2), 13.34 (s, 1H, NH) ppm. 13C-NMR: δ 23.57 (CH2CH2CH2), 25.10 (CH2C=N), 32.10 (SCH2), 163.38, 164.79 (C=N), 177.64 (C=S) ppm. ESI MS (m/z): 463.44 (M + H)+. Anal. Calcd for C12H18N10S5: C 31.15; H 3.92; N 30.27. Found: C 31.38; H 3.78; N 30.16.
3.12. General Procedure for the Synthesis of Schiff Bases 21–26
A mixture of compound 20 and/or 3 (1 mmol) in ethanol (20 mL) and the appropriate aromatic aldehyde (2.2 mmol) with drops of hydrochoric acid was refluxed for 4–6 h. After cooling, the product formed was collected by filtration and recrystallized from ethanol to furnish the desired product.
2,5-Bis[(4-benzylideneamino)-2,4-dihydro-1,2,4-triazol-3-thione-5-yl)propylthio]-1,3,4-thiadiazole (21). This compound was obtained as white solid, Yield 91%; m.p. 186–188 °C. IR (υ, cm−1): 1624 (C=N), 1268 (C=S), 3084 (Ar-H), 3288–3329 (NH). 1H-NMR: δ 2.06–2.15 (m, 2H, CH2CH2CH2), 2.67–2.73 (m, 2H, CH2C=N), 2.80–2.88 (m, 2H, SCH2), 7.40–7.53 (m, 3H, Ar-H), 7.69–7.79 (m, 2H, Ar-H), 9.18 (s, 1H, H-C=N), 13.52 (s, 1H, NH triazole) ppm. 13C-NMR: δ 23.36 (CH2CH2CH2), 25.60 (CH2C=N), 32.83 (SCH2), 126.45, 128.79, 129.40, 134.80 (Ar-C), 162.08, 163.77, 165.94 (C=N), 173.41 (C=S) ppm. ESI MS (m/z): 638.35 (M + H)+. Anal. Calcd for C26H26N10S5: C 48.88; H 4.10; N 21.92. Found: C 48.98; H 4.26; N 21.77.
2,5-Bis[(4-(2-bromobenzylideneamino)-2,4-dihydro-1,2,4-triazol-3-thione-5-yl)-propylthio]-1,3,4-thiadiazole (22). This compound was obtained as white solid, Yield 92% yield; m.p. 206–208 °C. IR (υ, cm−1): 1639 (C=N), 1247 (C=S), 3069 (Ar-H), 3214–3344 (NH). 1H-NMR: δ 2.02–2.11 (m, 2H, CH2CH2CH2), 2.63–2.71 (m, 2H, CH2C=N), 2.77–2.85 (m, 2H, SCH2), 7.45–7.58 (m, 2H, Ar-H), 7.73–7.82 (m, 2H, Ar-H), 9.24 (s, 1H, H-C=N), 13.47 (s, 1H, NH triazole) ppm. 13C-NMR: δ 23.68 (CH2CH2CH2), 25.05 (CH2C=N), 32.64 (SCH2), 124.61, 127.80, 128.34, 131.87, 133.13, 141.80 (Ar-C), 162.34, 164.18, 165.86 (C=N), 173.89 (C=S) ppm. ESI MS (m/z): 793.69 (M + H)+. Anal. Calcd for C26H24Br2N10S5: C 39.20; H 3.04; N 17.58. Found: C 39.41; H 2.86; N 17.45.
4,4′-(1,3,4-Thiadiazol-2,5-diyl)bis-(sulfanediyl)bis-(N′-(4-benzylidene)butanehyd-razide) (23). This compound was obtained as colorless crystals, Yield 93% yield; m.p. 136–138 °C. IR (υ, cm−1): 1669 (C=N), 1714 (C=O), 3064 (Ar-H), 3218–3357 (NH). 1H-NMR: δ 2.00–2.05 (quin, 2H, CH2CH2CH2), 2.34–2.38 (m, 0.7H, CH2CO), 2.80 (ddd, 1.3H, J = 4 Hz, J = 8 Hz, CH2CO), 3.29–3.36 (m, 2H, SCH2, overlaped with DMSO), 7.39–7.69 (m, 5H, Ar-H), 7.97 (s, 0.65H, H-C=N), 8.15 (s, 0.35H, H-C=N), 11.31 (s, 0.62H, CONH), 11.43 (s, 0.32H, CONH) ppm. 13C-NMR: δ 24.06, 24.64 (CH2CH2CH2), 30.76, 32.64 (CH2CO), 33.51, 33.69 (SCH2), 126.61, 126.93, 128.75, 129.64, 129.87, 134.20, 134.28, 142.67, 145.93 (Ar-C), 164.86, 164.98, 167.63 (C=N), 167.63, 173.40 (C=O) ppm. EI MS (m/z): 526.50 (M+). Anal. Calcd for C24H26N6O2S3: C 54.73; H 4.98; N 15.96. Found: C 54.95; H 5.09; N 15.78.
4,4′-(1,3,4-Thiadiazol-2,5-diyl)bis-(sulfanediyl)bis-(N′-(4-fluorobenzylidene)butane-hydrazide (24). This compound was obtained as colorless crystals, Yield 91%; m.p. 154–155 °C. IR (υ, cm−1): 1672 (C=N), 1704 (C=O), 3080 (Ar-H), 3254–3358 (NH). 1H-NMR: δ 1.91–2.00 (m, 1.5H, CH2CH2CH2), 2.26–2.30 (m, 0.5H, CH2CH2CH2), 2.71 (ddd, 1H, J = 4 Hz, J = 8 Hz, CH2CO), 3.19–3.37 (m, 3H, CH2CO, SCH2, overlaped with DMSO), 7.21 (ddd, 1.5H, J = 8 Hz, 12 Hz, Ar-H), 7.30 (dd, 0.5H, J = 8 Hz, J = 12 Hz, Ar-H), 7.59–7.67 (m, 1.5H, Ar-H), 7.85–7.88 (m, 1H, Ar-H, H-C=N), 8.07 (s, 0.25H, H-C=N), 8.64 (s, 0.25H, H-C=N), 11.23 (s, 0.65H, CONH), 11.36 (s, 0.35H, CONH) ppm. 13C-NMR: δ 24.06, 24.64 (CH2CH2CH2), 30.65, 30.75 (CH2CO), 33.50, 33.66 (SCH2), 130.38, 130.61, 130.70, 130.81, 130.84, 141.52, 144.82 (Ar-C), 160.42, 161.57, 164.97, 165.06 (C=N), 167.65, 173.38 (C=O) ppm. EI MS (m/z): 562.83 (M+). Anal. Calcd for C24H24F2N6O2S3: C 51.23; H 4.30; N 14.94. Found: C 51.42; H 4.39; N 14.98.
4,4′-(1,3,4-Thiadiazol-2,5-diyl)bis-(sulfanediyl)bis-(N′-(4-trifluoromethylbenzyli-dene)butanehydrazide (25). This compound was obtained as colorless crystals, Yield 94% yield; m.p. 185–186 °C. IR (υ, cm−1): 1648 (C=N), 1704 (C=O), 3094 (Ar-H), 3229–3342 (NH). 1H-NMR: δ 1.99–2.09 (m, 2H, CH2CH2CH2), 2.38–2.41 (m, 0.7H, CH2CO), 2.84 (ddd, 1.3H, J = 8 Hz, 12 Hz, CH2CO), 3.29–3.37 (m, 2H, SCH2, overlaped with DMSO), 7.75–7.80 (m, 2H, Ar-H), 7.84–7.91 (m, 2H, Ar-H), 8.04 (s, 0.65H, H-C=N), 8.23 (s, 0.35H, H-C=N), 11.51 (s, 0.65H, CONH), 11.65 (s, 0.35 H, CONH) ppm. 13C-NMR: δ 24.02, 24.54 (CH2CH2CH2), 30.64, 30.76 (CH2CO), 32.62, 33.46 (SCH2), 120.01, 122.71, 125.42, 125.58, 125.62, 127.16, 127.49, 128.12, 129.05, 129.13, 129.44, 129.64, 138.16, 138.30, 140.97, 144.17 (Ar-C), 164.85, 164.96, 165.05 (C=N), 167.94, 173.64 (C=O) ppm. EI MS (m/z): 662.37 (M+). Anal. Calcd for C26H24F6N6O2S3: C 47.12; H 3.65; N 12.68. Found: C 47.31; H 3.79; N 12.49.
4,4′-(1,3,4-Thiadiazol-2,5-diyl)bis-(sulfanediyl)bis-(N′-(4-bromobenzylidene)but-anehydrazide (26). This compound was obtained as colorless crystals, Yield 90%; m.p. 201–204 °C. IR (υ, cm−1): 1692 (C=N), 1709 (C=O), 3050 (Ar-H), 3249–3367 (NH). 1H-NMR: δ 1.97–2.09 (quin, 2H, CH2CH2CH2), 2.41 (ddd, 0.7H, J = 4 Hz, J = 8 Hz, CH2CO), 2.81 (ddd, 1.2H, J = 4 Hz, J = 8 Hz, CH2CO), 3.28–3.36 (m, 2H, SCH2, overlaped with DMSO), 7.41 (ddd, 1H, J = 4 Hz, J = 8 Hz, Ar-H), 7.51 (t, 0.25H, J = 4 Hz, Ar-H), 7.57–7.69 (m, 1.75H, Ar-H), 7.84–7.91 (m, 1H, Ar-H), 7.94 (s, 0.5H, H-C=N), 8.12 (s, 0.25H, H-C=N), 8.71 (s, 0.25H, Ar-H), 11.42 (s, 0.65H, CONH), 11.56 (s, 0.3H, CONH) ppm. 13C-NMR: δ 24.01, 24.58 (CH2CH2CH2), 30.61, 30.66 (CH2CO), 32.61, 32.64 (SCH2), 122.07, 122.15, 125.75, 125.99, 127.25, 128.76, 129.07, 130.75, 130.90, 131.11, 132.18, 132.36, 134.03, 135.98, 136.68, 136.79, 140.99, 144.12 (Ar-C), 160.55, 164.93, 165.03 (C=N), 167.87, 173.56 (C=O) ppm. EI MS (m/z): 682.21 (M+). Anal. Calcd for C24H24Br2N6O2S3: C 42.11; H 3.53; N 12.28. Found: C 42.34; H 3.40; N 12.45.
3.13. Biological Activity
3.13.1. Antimicrobial Susceptibility Testing
Cell Lines
All synthesized thiadiazoles derivatives 2–26 were screened for their in vitro antimicrobial activity against the standard pathogenic strains of the Regional Center for Mycology and Biotechnology (RCMB) namely; Streptococcus pneumonia RCMB 010010, Bacillus subtilis RCMB 010067, Staphylococcus aureus RCMB 010025 (Gram-positive bacteria), Pseudomonas aeuroginosa RCMB 010043, Escherichia coli RCMB 010052, Klebsiella pneumonia RCMB 010058 (Gram-negative bacteria), and the yeast-like pathogenic fungus Aspergillus fumigates RCMB 02568, Geotrichum candidum RCMB 05097 and Candida albicans RCMB 05036.
Antimicrobial Evaluation Using the MIC Assay
Antimicrobial activity of compounds 2–26 was evaluated by broth microdilution method [30,31]. The MIC determination of the synthesized compounds was carried out in side-by-side comparison with ciprofloxacin against Gram-positive and Gram-negative bacteria. The antifungal activity was assayed against yeasts in comparison with fluconazole. The minimum inhibitory concentrations of the compounds were recorded as the lowest concentration of each chemical compounds in the tubes with no turbidity (i.e., no growth) of inoculated bacteria/fungi. Test compounds (10 mg) were dissolved in dimethylsulfoxide (DMSO, 1 mL) then diluted in culture medium (Mueller-Hinton Broth for bacteria and Sabouraud Liquid Medium for fungi), further progressive dilutions to obtain final concentrations of 1, 2, 4, 8, 16, 31.25, 62.5, 125, 250 and 500 mg·mL−1. DMSO never exceeded 1% v/v. The tubes were inoculated with 105 cfu·mL−1 (colony forming unit/mL) and incubated at 37 °C for 24 h. The growth control consisting of media and media with DMSO at the same dilutions as used in the experiments was employed.
3.13.2. Antiproliferative Susceptibility Testing
Cell Lines
Human breast adenocarcinoma MCF7, Human ductal breast epithelial tumor T47D, Human epithelial colorectal adenocarcinoma Caco II and Human epithelial carcinoma HeLa cell lines were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in Dulbecco’s modified eagle medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing 10% heat inactivated fetal bovine serum (HI-FBS) (Invitrogen), 2 mmol·L−1 of L-glutamine, 50 U·mL−1 of penicillin and 50 μg·mL−1 of streptomycin. Cell lines were maintained in an atmosphere of 5% CO2 and 95 relative humidity at 37 °C. All cells used in this study were between passages 20 and 40.
Cytotoxicity Evaluation Using the MTT Assay
The cytotoxic effects associated with the examined compounds were evaluated according to the protocol described in the ISO 10993-5 guide [32]. In brief, cells were seeded at seeding density of 1 × 104 cells per well in 96-well plates and incubated to allow adhesion for 24 h. The tested compounds were dissolved in dimethylsulfoxide (DMSO and subsequently diluted in culture media (test sample). Final concentration of DMSO was maintained constant in all treatment groups within a given experiment and never exceeded 1%. Three triplicates of each concentration for all tested compounds were evaluated in three independent assays for a total of 9 triplicates.
Initially, culture medium in each well was replaced with 200 μL of either test or control solutions. DMEM samples were employed as negative controls, and 50% DMSO (v/v) in DMEM as a positive control. Both test and control samples were allowed for 48 h incubation at 37 °C in a 5% CO2 incubator. At the end of the exposure period, MTT assay was carried out as previously described [32]. Briefly, viable cell count was determined using the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay. The yellow tetrazolium dye [MTT, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] was reduced by metabolically active cells into an intracellular purple formazan product. The quantity of formazan product, as determined by the absorbance at 490 nm, is directly proportional to the number of living cells in the culture. Cell viability was calculated based on the measured absorbance relative to the absorbance of the cells exposed to the negative controls, which represented 100% cell viability.
4. Conclusions
In conclusion, we have synthesized new biologically significant heterocycles and/or Schiff bases tagged 1,3,4-thiadiazole moiety and evaluated their antimicrobial and antiproliferative activities. Antimicrobial assay results indicated that most of the synthesized thiadiazole derivatives exhibited good to excellent antimicrobial activity. From the experimental results, it could be concluded that the clubbing of 1,3,4-thiadiazole with 1,2,4-triazole nucleus and/or Schiff base azomethine linkage in one scaffold resulted in the formation of new potent antimicrobial agents. From this work, compounds 12, 15, 18, 19, 22 and 25 proved to be the most promising active molecules which are capable of inhibiting the growth of human cancer cell lines in vitro.
Acknowledgments
We gratefully acknowledge the financial support from Taibah University through the deanship of the scientific research (Grant 4060).
Author Contributions
N.R., A.M.A.-Y., and F.F.A.-B. carried out of the experimental work and cooperated in the preparation of the manuscript. N.R. and M.R.A. gave the concepts of work, interpreted the results and prepared the manuscript. S.K.B. performed the biological assays. N.R., M.R.A. and S.K.B. wrote the paper and edited English language. All authors discussed the results and commented on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Katritzky A.R., Ramsden C.A., Joule J.A., Zhdankin V.V. Handbook of Heterocyclic Chemistry. 3rd ed. Elsevier; Oxford, UK: 2010. [Google Scholar]
- 2.Gupta R.R., Kumar M., Gupta V. Heterocyclic Chemistry II. 3rd ed. Springer; New Delhi, India: 2005. Five membered heterocycles with more than two heteroatoms; p. 491. [Google Scholar]
- 3.Kaplancikli Z.A., Zitouni G.T., Ozdemir A., Revial G. New triazole and triazolothiadiazine derivatives as possible antimicrobial agents. Eur. J. Med. Chem. 2008;43:155–159. doi: 10.1016/j.ejmech.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Zitouni G., Kaplancikli Z.A., Erol K., Kilic F.S. Synthesis and analgesic activity of some triazoles and triazolothiadiazines. Farmaco. 1999;54:218–223. doi: 10.1016/S0014-827X(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 5.Farghaly A.R., El-Kashef H. Synthesis of some new azoles with antiviral potential. Arkivoc. 2006;11:76–90. [Google Scholar]
- 6.Bekircan O., Kucuk M., Kahveci B., Kolayli S. Convenient synthesis of fused heterocyclic 1,3,5-triazines from some N-acyl imidates and heterocyclic amines as anticancer and antioxidant agents. Arch. Pharm. 2005;338:365–372. doi: 10.1002/ardp.200400964. [DOI] [PubMed] [Google Scholar]
- 7.Zitouni G.T., Kaplancikli Z.A., Ozdemir A., Chevallet P., Kandilci H.B., Gumus B. Studies on 1,2,4-triazole derivatives as potential anti-inflammatory agents. Arch. Pharm. 2007;340:586–590. doi: 10.1002/ardp.200700134. [DOI] [PubMed] [Google Scholar]
- 8.Lesyka R., Vladzimirska O., Holota S., Zaprutko L., Gzella A. New 5-substituted thiazolo [3,2-b][1,2,4]triazol-6-ones: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2007;42:641–648. doi: 10.1016/j.ejmech.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Li Q., Ren J., Dong F., Feng Y., Gu G., Guo Z. Synthesis and antifungal activity of thiadiazole-functionalized chitosan derivatives. Carbohydr. Res. 2013;373:103–107. doi: 10.1016/j.carres.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Zou Y., Zhao Q., Liao J., Hu H., Shichong Y., Chai X., Xu M., Qiuye W. New triazole derivatives as antifungal agents: Synthesis via click reaction, in vitro evaluation and molecular docking studies. Bioorg. Med. Chem. 2012;22:2959–2962. doi: 10.1016/j.bmcl.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 11.Mathew V., Keshavayya J., Vaidya V.P., Giles D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. Eur. J. Med. Chem. 2007;42:823–840. doi: 10.1016/j.ejmech.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Rajak H., Kharya M., Mishra P. Synthesis of some novel oxadiazole and oxadiazoline analogues for their anti-inflammatory activity. J. Pharm. Soc. Jpn. 2007;127:1757–1764. doi: 10.1248/yakushi.127.1757. [DOI] [PubMed] [Google Scholar]
- 13.Karabasanagouda T., Adhikari A.V., Shetty N.S. Synthesis and antimicrobial activities of some novel 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines carrying thioalkyl and sulphonyl phenoxy moieties. Eur. J. Med. Chem. 2007;42:521–529. doi: 10.1016/j.ejmech.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Sadek B., Fahelelbom K.M.S. Synthesis, characterization, and antimicrobial evaluation of oxadiazole congeners. Molecules. 2011;16:4339–4347. doi: 10.3390/molecules16064339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong W., Liu Z., Liu X., Li Z., Zhao W. Synthesis and antiviral activity of new acrylamide derivatives containing 1,2,3-thiadiazole as inhibitors of hepatitis B virus replication. Eur. J. Med. Chem. 2010;45:1919–1926. doi: 10.1016/j.ejmech.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Li Z., Zhan P., Liu X. 1,3,4-Oxadiazole: A privileged structure in antiviral agents. Mini Rev. Med. Chem. 2011;13:1130–1142. doi: 10.2174/138955711797655407. [DOI] [PubMed] [Google Scholar]
- 17.Kumar D., Kumar N.M., Chang K.-H., Shah K. Synthesis and anticancer activity of 5-(3-indolyl)-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010;45:4664–4668. doi: 10.1016/j.ejmech.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Ahsan M.J., Rathod V.P.S., Singh M., Sharma R., Jadav S.S., Yasmin S., Salahuddin S., Pradeep K. Synthesis, anticancer and molecular docking studies of 2-(4-chlorophenyl)-5-aryl-1,3,4-oxadiazole analogues. Med. Chem. 2013;3:294–297. [Google Scholar]
- 19.Bayrak H., Demirbas A., Karaoglu S.A., Demirbas N. Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009;44:1057–1066. doi: 10.1016/j.ejmech.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Güzeldemirci N.U., Küçükbasmaci Ӧ. Synthesis and antimicrobial activity evaluation of new 1,2,4-triazoles and 1,3,4-thiadiazoles bearing imidazo[2,1-b]thiazole. Eur. J. Med. Chem. 2010;45:63–68. doi: 10.1016/j.ejmech.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Suresh Kumar G.V., Rajendraprasad Y., Mallikarjuna B.P., Chandrashekar S.M., Kistayya C. Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1,2,4-triazole and 1,3,4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2010;45:2063–2074. doi: 10.1016/j.ejmech.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 22.Rudrapal M., De B. Chemistry and biological importance of heterocyclic Schiff’s bases. Int. Res. J. Pure Appl. Chem. 2013;3:232–249. doi: 10.9734/IRJPAC/2013/3996. [DOI] [Google Scholar]
- 23.Aouad M.R., Rezki N., Kasmi M., Aouad L., Rezki M.A. Synthesis, characterization and evaluation of antimicrobial activity of some novel 1,2,4-triazoles and 1,3,4-thiadiazoles bearing imidazole nuclues. Heterocycles. 2012;85:1141–1154. doi: 10.3987/COM-12-12456. [DOI] [Google Scholar]
- 24.Aouad M.R., Messali M., Rezki N., Ali A.A., Lesimple A. Synthesis and characterization of some novel 1,2,4-triazoles, 1,3,4-thiadiazoles and Schiff bases incorporating imidazole moiety as potential antimicrobial agents. Acta Pharm. 2015;65:117–132. doi: 10.1515/acph-2015-0011. [DOI] [PubMed] [Google Scholar]
- 25.Kuo J.-Z., Chen S.-Ê. Preparation of 2,5-dimercapto-1,3,4-thiadiazole and its derivatives. Acta Chim. Sin. 1963;29:62–63. [Google Scholar]
- 26.Karthikeyan M.S., Prasad D.J., Poojary B., Bhat K.S., Holla B.S., Kumari N.S. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg. Med. Chem. 2006;14:7482–7489. doi: 10.1016/j.bmc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Varvaresou A., Siatra-Papastaikoudi T., Tsotinis A., Tsantili-Kakoulidou A., Vamvakides A. Synthesis, lipophilicity and biological evaluation of indole-containing derivatives of 1,3,4-thiadiazole and 1,2,4-triazole. Farmaco. 1998;53:320–326. doi: 10.1016/S0014-827X(98)00024-X. [DOI] [PubMed] [Google Scholar]
- 28.Wyrzykiewicz E., Prukala D. New isomeric N-substituted hydrazones of 2-, 3- and 4-pyridinecarboxaldehydes. J. Heterocycl. Chem. 1998;35:381–387. doi: 10.1002/jhet.5570350221. [DOI] [Google Scholar]
- 29.Palla G., Predieri G., Domiano P. Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron. 1986;42:3649–3654. doi: 10.1016/S0040-4020(01)87332-4. [DOI] [Google Scholar]
- 30.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000;6:509–515. doi: 10.1046/j.1469-0691.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically Approved Standard M7-A5. 5th ed. NCCLS; Wayne, PA, USA: 2000. [Google Scholar]
- 32.ISO-International Organization for Standardization . ISO 10993-5-Biological Evaluation of Medical Devices. Part 5: Testes for in Vitro Cytotoxicity. 3rd ed. ISO; Geneva, Switzerland: 2009. [Google Scholar]