Abstract
As a result of the wide distribution of herbal teas the data on nutritional characterisation, chemical profile and biological activity of these products are required. The decoctions of Gentiana algida, G. decumbens, G. macrophylla and G. triflora herb teas were nutritionally characterized with respect to their macronutrients, demonstrating the predominance of polysaccharides and low lipid content. Gentian decoctions were also submitted to a microcolumn RP-HPLC-UV analysis of phytochemicals demonstrating a high content of iridoids (177.18–641.04 μg/mL) and flavonoids (89.15–405.71 μg/mL). Additionally, mangiferin was detected in samples of G. triflora tea (19.89 μg/mL). Five free sugars (fructose, glucose, sucrose, gentiobiose, gentianose) were identified in all gentian teas studied, as well as six organic acids (malic, citric, tartaric, oxalic, succinic, quinic). Pectic polysaccharides with a high content of rhamnogalacturonans and arabinogalactans were also identified and characterized in gentian decoctions for the first time. Gentian tea decoctions and their specific compounds (gentiopicroside, loganic acid-6′-O-β-d-glucoside, isoorientin, isoorientin-4′-O-β-d-glucoside, mangiferin, water-soluble polysaccharides) showed a promising antimicrobial, anti-inflammatory and antioxidant potentials. Evidences obtained indicate the prospective use of gentian herb teas as food products and medicines.
Keywords: Gentiana algida, Gentiana decumbens, Gentiana macrophylla, Gentiana triflora, iridoids, flavonoids, polysaccharides, anti-inflammatory, antioxidant, antimicrobial activity
1. Introduction
The tradition of tea beverage consumption is distributed worldwide. Nowadays, the tea drinking custom is considered to be associated with the region, environmental factors, ethnic group, etc. Herbal teas constitute a separate group of tea beverages. This refers to any beverage made from the infusion or decoction of herbs, spices, or other plant materials in hot water, and usually it does not contain caffeine. The consumption of herbal teas is particularly popular among the nomadic people of Siberia (Buryats, Yakutians, Evenkies, Soyots and others). Inhabitants from different tribes apply diverse tea beverages made with an eclectic assortment of ingredients, including local raw materials. Some herbal teas are used for curative purposes. Siberian nomads have a special category of such decoctions known as bitter teas which are made chiefly from the gentianaceous plants. Such teas are widely used because of the predominance of proteins in the diet and used as a remedy in various digestive disorders ranging from simple types such as vomiting to more complex problems like dyspepsia [1,2,3,4].
Despite the archaic manner of application of bitter decoctions, it is still the customary to consume bitter herbal teas before meals as specific aperitifs. Information on the gastric stimulatory effects of four frequently used Siberian gentian herbs (Gentiana algida, G. decumbens, G. macrophylla, G. triflora) has been presented earlier, and their effectiveness has been demonstrated [5]. In several traditional medical systems (Traditional Chinese Medicine, Traditional Tibetan Medicine) decoctions of these gentians are also used as anti-inflammatory, antimicrobial, wound-healing agents, etc. [1,3,6,7]. The known literature data on chemical composition and biological activities relates primarily to the roots of the gentians but little information is available about the herbs [8,9], confirming the poor general knowledge level of the nutritional, chemical, and pharmacological data of decoctions from the herbs G. algida, G. decumbens, G. macrophylla and G. triflora, and suggesting a need for further work in this direction.
In addition to the known low-molecular weight compounds, an investigation of the chemical composition and biological activity of the polymeric plant compounds is of particular interest. As water is a universal extractant that allows dissolution of most plant polymers, polysaccharides are therefore usual components of any plant-derived tea product. According to known data, in the Gentiana genus only G. rigescens [10] and G. scabra root polysaccharides [11] have been previously characterised. To the best of our knowledge, no information about the chemical composition and bioactivity of polysaccharides of the Gentiana herbs has been previously reported.
In the present work, the nutritional characterisation, and free sugars and organic acids profiling of G. algida, G. decumbens, G. macrophylla and G. triflora herbal teas were performed, and gentian decoctions were submitted to a detailed analysis of phytochemicals (iridoids, phenolic compounds, polysaccharides). Antimicrobial, anti-inflammatory, and antioxidant activities of gentian tea preparations and their representative phytochemicals were investigated.
2. Results and Discussion
2.1. Nutritional Profiles of Bitter Gentian Teas Decoctions
The results of the nutritional characterisation of the decoctions of four bitter gentian teas including organoleptic parameters (colour, odour, taste) and nutritional compounds (macronutrients, free sugars, organic acids) are presented in Table 1.
Table 1.
Organoleptic and nutritional characteristics of four gentian tea decoctions a.
| Parameter | G. algida | G. decumbens | G. macrophylla | G. triflora |
|---|---|---|---|---|
| Extractives b | 272 ± 8 | 224 ± 8 | 263 ± 9 | 345 ± 12 |
| Organoleptic characteristics | ||||
| Color | Light-brown | Yellow | Yellow | Dark-yellow |
| Odor | Herbal | Herbal | Herbal | Herbal |
| Taste | Bitter, intensive | Bitter, light | Bitter, light | Bitter, medium |
| Bitter index c | 9300 (4200–12,000) | 500 (170–600) | 1000 (520–1200) | 3050 (1300–4800) |
| Nutritional characteristics | ||||
| Carbohydrates b | 98.39 ± 3.05 | 95.23 ± 2.76 | 107.11 ± 3.42 | 151.99 ± 4.71 |
| Protein b | 22.65 ± 0.77 | 22.75 ± 0.68 | 40.01 ± 1.12 | 30.91 ± 0.95 |
| Lipids b | <0.1 | <0.1 | <0.1 | <0.1 |
| Ash b | 8.63 ± 0.43 | 9.39 ± 0.49 | 5.18 ± 0.23 | 6.47 ± 0.32 |
| Energy d | 0.48 | 0.47 | 0.59 | 0.73 |
| Total free amino acids b | 2.39 ± 0.06 | 1.37 ± 0.03 | 3.84 ± 0.11 | 2.19 ± 0.06 |
| Free sugars | ||||
| Fructose b | 2.00 ± 0.05 | 8.18 ± 0.20 | 1.42 ± 0.04 | 7.42 ± 0.22 |
| Glucose b | 16.37 ± 0.39 | 32.64 ± 0.75 | 2.03 ± 0.05 | 51.57 ± 1.34 |
| Sucrose b | 26.35 ± 0.63 | 20.26 ±0.45 | 24.32 ± 0.58 | 25.28 ± 0.61 |
| Gentiobiose b | 4.16 ± 0.09 | 6.51 ± 0.15 | 9.20 ± 0.22 | 13.24 ± 0.30 |
| Gentianose b | 5.72 ± 0.15 | 21.80 ± 0.59 | 31.93 ± 0.83 | 37.76 ± 1.06 |
| Total free sugars b | 54.60 | 89.39 | 68.90 | 135.27 |
| Organic acids | ||||
| Malic acid b | 9.64 ± 0.29 | 15.63 ± 0.43 | 5.39 ± 0.15 | 4.87 ± 0.11 |
| Citric acid b | 4.16 ± 0.11 | 6.11 ± 0.15 | 4.83 ± 0.12 | 9.31 ± 0.22 |
| Tartaric acid b | 0.72 ± 0.02 | 1.24 ± 0.02 | 0.94 ± 0.02 | 0.52 ± 0.01 |
| Oxalic acid b | tr. | tr. | 0.11 ± 0.00 | 0.09 ± 0.00 |
| Succinic acid b | tr. | tr. | tr. | 0.12 ± 0.00 |
| Quinic acid b | tr. | tr. | tr. | tr. |
| Total organic acids b | 14.52 | 22.98 | 11.27 | 14.91 |
a standard brewing—1 g plant material/100 mL water; b mg/100 mL decoction; c median value (min–max values); d kcal/100 mL decoction; tr.—traces amounts (<limit of quantification).
Extractives ranged from 224 mg/g (G. decumbens) to 345 mg/g (G. triflora), demonstrating the good extractability of the gentian tea compounds by boiling water. Colour and odour of the four gentian teas were typical for herbal teas, though the tastes were characterised as having bitterness of different intensity. Bitter indices varied from 500 units in G. decumbens to 9300 units in G. algida. Generally, organoleptic characteristics allow us to describe these gentian teas as specific products with atypical but pleasant tastes and odours.
Among macronutrients, carbohydrates were the most abundant compounds, reaching 95.23–151.99 mg/100 mL decoction, followed by proteins (22.65–40.01 mg/100 mL decoction). Low lipid content (<0.1 mg/100 mL decoction), ash (5.18–9.39 mg/100 mL decoction) and energy content (0.47–0.73 kcal/100 mL decoction) were detected in the gentian teas.
Five free sugars were identified in the gentian teas, including glucose, fructose and sucrose, which were usual for plants, as well as two oligosaccharides, gentiobiose [β-d-glucopyranosyl-(1→6)-d-glucopyranose] and gentianose [β-d-glucopyranosyl-(1→6)-α-d-glucopyranosyl-(1→2)-β-d-fructofuranose], specific for the Gentianaceae family [12]. Sucrose was a dominant free sugar in G. algida, glucose in G. decumbens and G. triflora, and gentianose in G. macrophylla. The presence of the five mentioned sugars had been shown previously in G. macrophylla and G. decumbens flowers [12]. The determination of the free sugar composition of G. algida and G. triflora and quantification of free sugar in the four species were realised in this work for the first time.
Organic acids in the gentian teas included six compounds—malic, citric, tartaric, oxalic, succinic and quinic acids. The total content of organic acids in the teas was 11.27–22.98 mg/100 mL decoction. Malic acid was the prevalent compound in G. algida, G. decumbens, and G. macrophylla. In contrast, citric acid dominated in G. triflora tea. Malic, citric, tartaric, and succinic acids have potent biological properties like antimicrobial [13], cardioprotective [14], and antiplatelet properties [15]. The low content of oxalic acid (tr.—0.11 mg/100 mL decoction) should be noted, as it is characterised as an antinutrient compound due to its inhibitory effect on mineral bioavailability [16].
As far as we know, this is the first report on nutritional characterisation of four gentian products that proved to be equilibrated valuable herbs rich in carbohydrates and proteins, and poor in fat and calories.
2.2. Phytochemical Profiles of Bitter Gentian Tea Decoctions
Quantification of low-molecular weight phytochemicals in the decoctions of bitter gentian tea was realised by an microcolumn (MC)-RP-HPLC-UV procedure [17]. Previously, the presence of the 12 compounds including five iridoids (loganic acid, loganic acid-6′-O-β-d-glucoside, swertiamarin, sweroside, gentiopicroside), six flavone-C/O-glycosides (isoorientin, isoorientin-4′-O-β-d-glucoside, isovitexin, saponarin, isosaponarin, isoscoparin) and mangiferin were found in the hydro-ethanolic extracts of gentian herbs of Siberian origin [5]. The present work aimed the quantification of mentioned compounds in the gentian herb decoctions. All the contents are summarised in Table 2.
Table 2.
Content of iridoids, flavonoids and mangiferin in bitter gentian tea decoctions, μg/mL (±SD).
| Compound | G. algida | G. decumbens | G. macrophylla | G. triflora |
|---|---|---|---|---|
| Iridoids | ||||
| Loganic acid | 106.42 ± 1.91 | 32.69 ± 0.59 | 40.09 ± 0.72 | 33.04 ± 0.66 |
| Loganic acid-6′-O-β-d-glucoside | n.d. | 523.10 ± 10.99 | 123.08 ± 2.34 | 592.41 ± 13.03 |
| Swertiamarin | 26.38 ± 0.47 | tr. | 4.02 ± 0.06 | tr. |
| Sweroside | 23.03 ± 0.46 | n.d. | 2.91 ± 0.05 | n.d. |
| Gentiopicroside | 259.57 ± 4.67 | n.d. | 7.08 ± 0.13 | 15.59 ± 0.34 |
| Subtotal | 415.40 | 555.79 | 177.18 | 641.04 |
| Flavonoids | ||||
| Isoorientin | 173.60 ± 2.77 | 14.83 ± 0.27 | 27.83 ± 0.53 | 120.22 ± 1.92 |
| Isoorientin-4′-O-β-d-glucoside | tr. | 40.71 ± 0.89 | 111.10 ± 2.22 | 204.43 ± 3.88 |
| Isovitexin | 37.96 ± 0.75 | 18.11 ± 0.34 | 40.04 ± 0.92 | 9.70 ± 0.19 |
| Saponarin | 25.62 ± 0.46 | 15.50 ± 0.25 | 6.12 ± 0.13 | 29.31 ± 0.59 |
| Isosaponarin | n.d. | tr. | tr. | 21.22 ± 0.45 |
| Isoscoparin | n.d. | tr. | 12.04 ± 0.26 | 20.83 ± 0.48 |
| Subtotal | 237.18 | 89.15 | 197.13 | 405.71 |
| Xanthones | ||||
| Mangiferin | n.d. | n.d. | n.d. | 19.89 ± 0.46 |
| Subtotal | n.d. | n.d. | n.d. | 19.89 |
| Total | 652.58 | 644.94 | 374.31 | 1066.64 |
n.d.—not detected (<limit of detection); tr.—traces amounts (<limit of quantification).
The amounts of the specific components found varied among the different gentian species. The results showed that the total content of quantifiable compounds in the gentian tea samples displayed a 2.9-fold variation. G. triflora tea had the highest total compound content (1066.64 μg/mL), followed in order by G. algida (652.58 μg/mL), G. decumbens (644.94 μg/mL) and G. macrophylla (374.31 μg/mL). Gentiopicroside was found in the highest concentration in G. algida tea (259.57 μg/mL), while the lowest content of the compound was detected in G. triflora (15.59 μg/mL) and G. macrophylla teas (7.08 μg/mL). Detectable quantities of sweroside and swertiamarin were found in samples of G. algida (23.03 and 26.38 μg/mL, respectively) and G. macrophylla teas (2.91 and 4.02 μg/mL, respectively). G. decumbens and G. triflora teas had the highest concentration of loganic acid-6′-O-β-d-glucoside, 523.10 and 592.41 μg/mL, respectively. Quantifiable prevalence of isoorientin was noted only in G. algida tea (173.60 μg/mL). Isoorientin-4′-O-β-d-glucoside was a dominant flavonoid in the other gentian teas, with the highest content in G. triflora (204.43 μg/mL). In the case of G. triflora teas, mangiferin concentrations were 19.89 μg/mL.
The data obtained allow us to calculate the total uptake of the specific compounds after application of the standard dosage of gentian teas (100 mL). It was 37.43 mg for G. macrophylla tea, 64.49 mg for G. decumbens tea, 65.26 mg for G. algida tea, and 106.66 mg for G. triflora tea. The HPLC quantification results are clear evidence that gentian tea decoctions are a good source of iridoids and phenolic compounds.
It should be noted that the iridoid content in G. algida and G. decumbens decoctions are close (415.40 and 555.79 μg/mL, respectively) but the bitter index of G. algida decoction is 18.6 times more that of G. decumbens decoction. We decided to find out the reasons for this contradiction. First we determined the bitter indices of all phytochemicals (iridoids, flavonoids and mangiferin) identified in the gentian teas. As expected, gentiopicroside was the most bitter compound, with a bitterness index of 14,500 (min–max 11,000–16,500). This parameter is slightly different from the commonly reported value (12,000) but this difference should be attributed to some subjectivity of the sensory analysis. Sweroside has a lower bitterness of 9500 (min–max 6000–12,500), followed by swertiamarin with a bitter index of 7200 (min–max 4100–8600). Structural features such as a saturated C5–C6 bond (sweroside) and the presence of a hydroxyl function at the C5-atom (swertiamarin) are the reasons for the decreased bitter taste. Loganic acid may be characterised as a non-bitter compound with a dominant salty taste, and loganic acid-6′-O-β-d-glucoside is tasteless. Neither flavonoids nor mangiferin have any taste. Therefore, only three compounds can cause influence on taste receptors—gentiopicroside, sweroside and swertiamarin.
2.3. Polysaccharide Characterisation of Bitter Gentian Teas
The yields of water soluble polysaccharide fractions (WSPF) were 19.34 mg/g, 6.27 mg/g, 22.18 mg/g, and 16.31 mg/g for G. algida, G. decumbens, G. macrophylla and G. triflora, respectively (Table 3).
Table 3.
General parameters and monosaccharide compositions of water soluble polysaccharide fractions of bitter gentian teas.
| Parameter | G. algida | G. decumbens | G. macrophylla | G. triflora |
|---|---|---|---|---|
| Yield, mg/g | 19.34 | 6.27 | 22.18 | 15.31 |
| Total carbohydrate content, mg/g | 946.14 ± 24.59 | 931.63 ± 24.15 | 932.07 ± 22.37 | 954.91 ± 24.83 |
| Uronic acid content, mg/g | 802.37 ± 20.86 | 732.64 ± 18.31 | 824.63 ± 22.26 | 797.68 ± 20.74 |
| Protein content, mg/g | 16.27 ± 0.41 | 22.86 ± 0.48 | 21.15 ± 0.51 | 18.37 ± 0.38 |
| , ° | +116 | +101 | +124 | +110 |
| Reaction with I2 | negative | negative | negative | negative |
| Reaction with resorcinol | negative | negative | negative | negative |
| Reaction with Yariv reagent | positive | positive | positive | positive |
| Monosaccharide composition, mol % | ||||
| Ara | 9.4 | 8.0 | 4.2 | 10.6 |
| Gal | 8.4 | 12.9 | 7.3 | 7.7 |
| Glc | 2.7 | 7.4 | 1.8 | 1.6 |
| Man | 0.9 | 2.1 | 1.2 | 1.1 |
| Rha | 5.6 | 4.8 | 6.3 | 2.8 |
| Xyl | 1.4 | 0.9 | 0.8 | 1.1 |
| GalA | 71.6 | 63.9 | 78.4 | 75.1 |
| GlcA | tr. | tr. | tr. | tr. |
tr.—traces amounts (<limit of quantification).
All isolated WSPF were characterised by a high total carbohydrate content (931.63–954.91 mg/g) and low protein content (16.27–22.86 mg/g). High uronic acid content (732.64–824.63 mg/g) was reflected in the specific rotation values (+101–+124°) and indicated the presence of polygalacturonan compounds in the WSPF. Negative reactions with iodine and resorcinol declared the absence of starch and inulin-derived polymers. Interestingly, all WSPF gave a positive reaction with Yariv reagent, which is specific for arabinogalactan-protein complexes (AGP).
The monosaccharide compositions of the WSPF of the four bitter gentian teas demonstrated a dominance of galacturonic acid, with the smallest amount in G. decumbens (63.9 mol %) and largest in G. macrophylla (78.4 mol %) (Table 3). Content of arabinose and galactose were 4.2 mol %–10.6 mol % and 7.3 mol %–12.9 mol %, respectively. Rhamnose, glucose, xylose and mannose were detected in all samples, as well as glucuronic acid in trace levels. Generally, the monosaccharide compositions of the WSPF were typical for pectic polysaccharides containing polygalacturonans, rhamnogalacturonans and arabinogalactans [18].
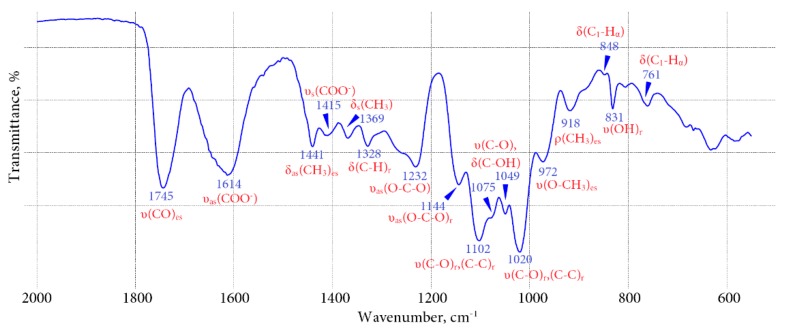
FT-IR spectra of the WSPF of the four bitter gentian teas are very close to each other, similar to those for pectin polysaccharides [19]. The FT-IR spectrum of the WSPF of G. algida bitter tea is shown in Figure 1. It contains specific bands assigned to stretching C=O vibrations of esterified groups (1745 cm−1) and asymmetric stretching vibrations of free carboxylic groups (1624 cm−1) [20]. Additional bands at 1441 cm−1, 1415 cm−1, 1369 cm−1, and 972 cm−1 may be ascribed to the ester and carboxylate groups. The band at 1328 cm−1 was assigned to C–H bending vibrations of the pyranoid ring. The characteristic “anomeric region” bands for α-linkages at 848 cm−1 and 761 cm−1 were observed [21]. The presence of two intense bands at 1102 cm−1 and 1020 cm−1 which coincided with vibrations of glycosidic bonds and pyranoid rings was due to the high homogalacturonan content. In contrast, the low intensity of bands at 1075 cm−1 and 1049 cm−1, specific for rhamnogalacturonans, indicates their low content [22].
Figure 1.
FT-IR spectra of water-soluble polysaccharide fraction of G. algida bitter tea.
Based on the data obtained, we can conclude that the water soluble polysaccharides identified in the gentian bitter teas are pectins with a high content of homogalacturonan compounds as well as rhamnogalacturonans and arabinogalactans. Despite the fact that further investigations are needed to understand the structural features of gentian herb polysaccharides, as part of this work we carried out chemical examination of the four Gentiana herb polysaccharides for the first time. It is known that the plant pectins possess a wide spectrum of biological activity—immune-stimulating, anticancer, hypoglycaemic, anti-inflammatory and anticoagulant properties [18]. Taking into account the high therapeutic efficiency of plant pectic polysaccharides, the Gentiana genus might be deduced as a prospective source of biologically active polymeric nutrients.
2.4. Biological Activity of Bitter Gentian Teas
2.4.1. Anti-Inflammatory Activity
Carrageenan-induced rat paw edema was used to study the anti-inflammatory activity of the four gentian teas. The model of inflammation caused by carrageenan is an acute and reproducible model which results in a quantifiable increase of paw size (edema) and is also modulated by inhibitors within the inflammatory cascade [23].
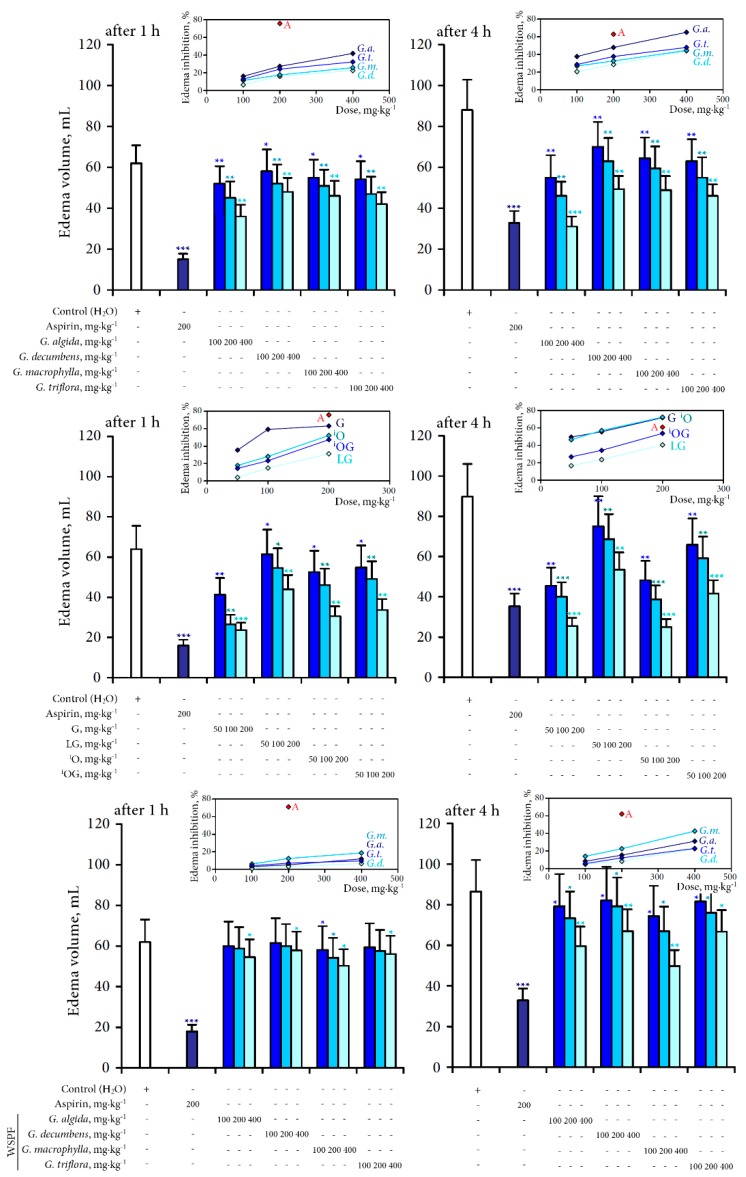
Aspirin, a common anti-inflammatory drug, was used as a reference substance. In our results, all investigated teas administered orally in doses of 100–400 mg/kg displayed a marked and similar intensity of action (Figure 2).
Figure 2.
Anti-inflammatory activity of four gentian teas and individual compounds on carrageenan-induced paw edema in rats after 1 and 4 h after injection. Bars show the values of edema volume (mL). Values of edema shown are mean ± SD (n = 12). Asterisks indicate statistically significant values from the control (*—p < 0.05; **—p < 0.01; ***—p < 0.001). On cuts—percentage of edema inhibition (A—aspirin, G.a.—G. algida, G.d—G. decumbens, G.m.—G. macrophylla, G.t.—G. triflora, G—gentiopicroside, LG—loganic acid-6′-O-β-d-glucoside, iO—isoorientin, iOG—isoorientin-4′-O-β-d-glucoside, WSPF—water-soluble polysaccharide fractions).
All the gentian tea extracts demonstrated a dose-dependent mode of suppressive action and were able to reduce edema size after 1–4 h after injection of the inflammatory agent. The percentage of edema inhibition was more expressed for G. algida tea (16.1%–41.9% after 1 h, 37.5%–64.8% after 4 h) and less expressed for G. decumbens tea (6.5%–22.6% after 1 h, 20.3%–43.8% after 4 h). G. algida tea in a dose of 400 mg/kg after 4 h showed activity similar to aspirin (200 mg/kg).
Among the main phytochemicals identified in the gentian teas gentiopicroside was the most active compound reduced edema size after 1 h after injection of the inflammatory agent in doses 50–200 mg/kg at 35.47%–63.12% comparing with control. However, after 4 h activities of gentiopicroside and isoorientin were close (49.44%–71.60% and 46.33%–72.27%, respectively). The possible reason of this feature is various bioaccessibility/bioavailability values for both compounds. Gentiopicroside is a compound with high bioavailability value [24] in contrast to isoorientin characterised as low-bioavailable compound [25]. Finally, the mentioned facts may play an important role in the process of bioactivity implementation. Loganic acid-6′-O-β-d-glucoside and isoorientin-4′-O-β-d-glucoside were the compounds with a less pronounced anti-inflammatory effect.
Anti-inflammatory activity of water-soluble polysaccharide fractions of four gentians characterised as week after 1 h after injection of phlogogenic agent comparing with activity of crude decoctions and phytochemicals. Only G. macrophylla water-soluble polysaccharide fraction in dose 100–400 mg/kg inhibited the reduction of edema at 6.14%–18.74%. After 4 h the effectiveness of polysaccharide fractions was more expressed reached maximal values in dose 400 mg/kg—42.48% for G. macrophylla polysaccharides, 31.02% for G. algida polysaccharides, and 22.80% and 22.45% for G. triflora and G. decumbens polysaccharides, respectively.
Speculating about the possible reasons of the anti-inflammatory activity of bitter gentian teas, it can be concluded that a combination of chemical agents may be the main reason for the high activity. Particularly, the suppressive effects of gentiopicroside and swertiamarin have been demonstrated previously in an in vitro model system of inhibition of COX-1, COX-2 and TNF-α [26]. Loganic acid has been found to be active in a carrageenan-induced inflammation test [27]. Besides iridoids, flavonoids are known inhibitors of inflammation. The basic phenolic compound of the investigated gentian teas, an isoorientin, was previously denoted as a strong anti-inflammatory agent in carrageenan-induced inflammation in mice [28]. Also, a remarkable inhibitory effect of isoorientin on the synthesis of thromboxane B2 and leukotriene B4 has been demonstrated [29]. Isoorientin has been identified as the main active anti-inflammatory compound of Phyllostachys edulis (Carr.) J. Houz leaves due to its inhibitory activity on tumour necrosis factor (TNF), α-induced release of interleukins 6 and 8 and vascular endothelial growth factor [30]. Pectic substances may possess anti-inflammatory effectiveness. It is known that celery pectin in a model of lipopolysaccharide (LPS) induced inflammation can decrease interleukin-1β, increase interleukin-10 production, and diminish the amount of neutrophils migrating to the peritoneal cavity after LPS injection [31]. Pectic polysaccharide from alfalfa has been shown to have a significant anti-inflammatory effect against mRNA expression of the pro-inflammatory cytokine genes [32]. Comaruman, a pectin from Comarum palustre L., inhibits spontaneous and phorbol-12-myristate-13-acetate-activated adhesion of peritoneal leukocytes in vitro [33]. Thus, it is likely that the effectiveness of bitter gentian teas is due to their high content of phytochemicals known to have anti-inflammatory properties.
2.4.2. Antimicrobial Activity
The results of the determination of antimicrobial activity of the gentian tea decoctions demonstrated that all extracts inhibited the growth of six microorganisms tested including Gram-positive/negative cultures and one fungal species (Table 4). The minimum inhibitory concentration (MIC) of G. algida tea was the highest with values of 100–200 μg/mL. The most sensitive to this extract were B. subtilis, E. faecalis, E. coli and P. aeruginosa. Decoctions of G. triflora, G. macrophylla and G. decumbens tea had slightly lower antimicrobial activity (200–800 μg/mL) with better activity against E. faecalis (200 μg/mL). Among the individual compounds gentiopicroside was most active with MIC values of 100–400 μg/mL. The species sensitive to gentiopicroside were E. faecalis and E. coli. The antibacterial action of loganic acid-6′-O-β-d-glucoside was characterised as poorly effective. No inhibition of bacterial growth was shown by the flavones studied, isoorientin and isoorientin-4′-O-β-d-glucoside (MIC > 800 μg/mL), as well as water-soluble polysaccharide fractions (MIC > 1600 μg/mL).
Table 4.
Antimicrobial activity of four bitter gentian tea decoctions, gentiopicroside (G) and loganic acid-6′-O-β-d-glucoside (LG), MIC, μg/mL a.
| Microorganism | G. algida | G. decumbens | G. macrophylla | G. triflora | G | LG | PC b |
|---|---|---|---|---|---|---|---|
| B. subtillis | 100 | 400 | 400 | 200 | 200 | 400 | 4.0 |
| S. aureus | 200 | 800 | 400 | 400 | 200 | >800 | 4.0 |
| E. faecalis | 100 | 200 | 200 | 200 | 100 | 400 | 32.0 |
| E. coli | 100 | 400 | 400 | 200 | 100 | 400 | 2.0 |
| P. aeruginosa | 100 | 800 | 800 | 400 | 400 | >800 | 16.0 |
| C. albicans | 200 | >800 | >800 | 800 | 400 | >800 | 8.0 |
a Lowest concentration preventing visible grow; b Positive control—streptomycin, except C. albicans—nystatin.
Previous data concerning antimicrobial activity of Gentiana herb extractions has indicated close character of activity. Extracts of G. lutea leaves and flowers with a high gentiopicroside content (38.85–48.38 mg/g) inhibited growth of 15 microorganisms with MIC values of 120–310 μg/mL [34]. G. asclepiadea extract was shown to be active against seven bacteria tested at concentrations ranging from 50–1600 μg/mL [35]. Among the gentian phytochemicals, the compound with the widest spectrum of activity was found to be gentiopicroside [34]. Loganic acid derivatives have also been shown to be effective against pathogenic bacteria and fungi [36]. Concerning information about the antibacterial activity of flavone-C/O-glycosides, known data differ. No activity of isoorientin against common bacteria and fungi has been reported previously [37]. Lately some activities of flavone-C/O-glycosides have been demonstrated [38]. Additional information is needed to establish the role of gentian flavonoids in the manifestation of the antibacterial effect of extractions. It deserves special attention because of information about the presence of synergistic interaction of gentian phytochemicals discussed previously [34]. Nonetheless, application of gentian tea decoctions showed an antimicrobial effect against various microorganisms and could be beneficial for treatment of bacterial infections.
2.4.3. Antioxidant Activity
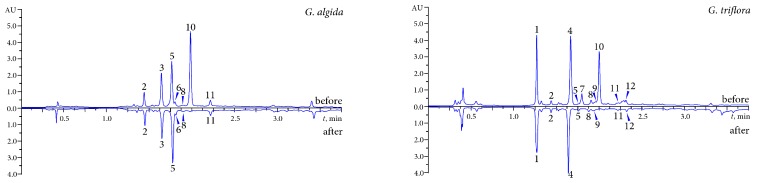
Preliminary characterisation of antioxidant potential was carried out using a DPPH-HPLC procedure, that is HPLC separation of the samples pre-treated with an excess of DPPH• followed by comparison with an HPLC chromatogram of an untreated sample. The reaction between an antioxidant and radical results in the oxidation of the antioxidant, which leads to a decrease of the corresponding peak areas in the chromatograms. The comparison of the HPLC chromatograms of untreated and radical-treated samples allows determination of the most active compounds. Chromatograms of G. algida and G. triflora tea decoctions spiked with DPPH• radicals are shown in Figure 3, which presents obviously reduced peak areas for some compounds in comparison with untreated samples.
Figure 3.
HPLC chromatograms of G. algida and G. triflora teas decoctions before and after prechromatographic reaction with DPPH-radicals. Compounds: 1—loganic acid-6′-O-β-d-glucoside; 2—loganic acid; 3—swertiamarin; 4—isoorientin-4′-O-β-d-glucoside; 5—gentiopicroside; 6—sweroside; 7—mangiferin; 8—saponarin; 9—isosaponarin; 10—isoorientin; 11—isovitexin; 12—isoscoparin.
Therefore, only one compound (isoorientin, peak 10) in G. algida, G. decumbens and G. macrophylla and two compounds (mangiferin, peak 7; isoorientin, peak 10) in G. triflora demonstrated a visible reduction of the peak area after spiking with DPPH• radicals. Both substances possessed antioxidant activity and they may be concluded as the major active compounds. All detectable iridoids and most flavone-C/O-glycosides were inactive. The results obtained showed the leading role of isoorientin and mangiferin in free radical scavenging of crude extracts of gentian teas.
The antioxidant properties of the four gentian tea decoctions were evaluated by various tests: 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) scavenging assay; 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) radical (ABTS•+) scavenging assay, superoxide radical (O2•−) scavenging assay; carotene bleaching assay (CBA). All experiments included the determination and comparative estimation of the same antioxidant factors for isoorientin and mangiferin, the main antioxidant compounds of the gentian teas. As can be observed in Table 5, G. algida is the most active antioxidant in all assays tested followed by G. triflora, G. macrophylla and G. decumbens. The high antioxidant potential of G. algida can be related to it having the highest isoorientin content. Comparative analysis of the two compounds isoorientin and mangiferin confirmed their effectiveness as antioxidants.
Table 5.
Antioxidant activity of four bitter gentian tea decoctions, isoorientin (iO) and mangiferin (Man), IC50, μg/mL a.
| Method b | G. algida | G. decumbens | G. macrophylla | G. triflora | iO | Man |
|---|---|---|---|---|---|---|
| DPPH• | 89.22 ± 3.83 ii | 287.91 ± 10.36 iii | 278.82 ± 9.87 iii | 104.86 ± 3.56 ii | 18.67 ± 0.63 i | 16.94 ± 0.52 i |
| ABTS•+ | 101.67 ± 3.15 v | 217.03 ± 7.41 vi | 187.93 ± 6.76 vi | 103.76 ± 3.32 v | 14.20 ± 0.42 iv | 12.80 ± 0.34 iv |
| O2•− | 58.63 ± 2.11 viii | > 300 | > 300 | 189.39 ± 6.81 viii | 27.69 ± 1.14 vii | 21.09 ± 0.97 vii |
| CBA | 32.04 ± 0.89 x | 84.15 ± 2.52 | 55.61 ± 1.56 x | 49.37 ± 1.43 x | 12.32 ± 0.40 ix | 9.62 ± 0.37 ix |
a Average of three analyses (±SD); b DPPH•—DPPH• radical scavenging activity; ABTS•+—ABTS•+ radical scavenging activity; O2•−—superoxide anion radical scavenging activity; CBA—carotene bleaching assay. All values correspond to mean values ± standard deviation of three replicates. Values with different letters (i–x) indicate statistically significant differences among groups at p < 0.05 by one-way ANOVA.
Previously, antioxidant properties of several gentian extractions have been analysed, demonstrating their good ability to scavenge free radicals and inhibit lipid peroxidation. Methanolic extract of the aerial part G. cruciata has been shown to be effective in DPPH•, ABTS•+ and O2•− assays with IC50 values of 1263.13, 601.15 and 135.73 μg/mL, respectively [39]. The percentage of DPPH• radical scavenging of a MeOH-extract from leaves of G. asclepiadea, G. olivierii, S. septemfida and G. verna has been measured as 35.67%–91.70% [40]. Results obtained in the present study indicated a more expressed antioxidant effect of G. algida, G. decumbens, G. macrophylla and G. triflora tea decoctions indicating the effectiveness of bitter gentian tea for regulation of the antioxidant status of humans.
3. Experimental Section
3.1. General Information
3.1.1. Chemicals, Sorbents
Biosupplies Australia Ply Ltd. (Victoria, Australia)—Yariv reagent kit (Cat. No. 100-4); Extrasynthese (Lyon, France)—gentianose (Cat. No. 4319, ≥98%), gentiobiose (Cat. No. 4125, ≥98%), gentiopicroside (Cat. No. 0216, ≥95%), isoorientin (Cat. No. 1055 S, ≥99%), isovitexin (Cat. No. 1235 S, ≥99%), loganic acid (Cat. No. 0230 S, ≥99%), saponarin (Cat. No. 1238 S, ≥98%); Sigma-Aldrich (St. Louis, MO, USA)—aspirin (Cat. No. A5376, ≥99%), anthrone (Cat. No. 319899, ≥97%), arabinose (Cat. No. A9524, ≥98%), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS; Cat. No. Al888, ≥98%), Bradford reagent (Cat. No. B6916), β-carotene (Cat. No. 22040, ≥97%), citric acid (Cat. No. 251275, ≥99%), 3,5-dimethylphenol (Cat. No. 144134, ≥99%), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•, Cat. No. D9132), DMSO (Cat. No. D4540, ≥99.5%), fructose (Cat. No. F2543, ≥98%), galactose (Cat. No. G0750, ≥99%), galacturonic acid (Cat. No. 48280, ≥97%), glucose (Cat. No. G8270, ≥99.5%), glucuronic acid (Cat. No. 851450, ≥99%), hydrogen peroxide (Cat. No. 216763, ca. 30%), lithium perchlorate (Cat. No. 431567, ≥99.99%), malic acid (Cat. No. M1210, ≥99%), mangiferin (Cat. No. 06279, ≥98%), mannose (Cat. No. M2069, ≥99%), Mueller Hinton broth (Cat. No. 70192), nystatin (Cat. No. N3503, ≥4400 USP units/mg), oleic acid (Cat. No. 01008, ≥99%), oxalic acid (Cat. No. 75688, ≥99%), perchloric acid (Cat. No. 311421, ≥70%, 99.999% trace metals basis), polyamide for CC (Cat. No. 02395), quinic acid (Cat. No. 138622, ≥98%), resorcinol (Cat. No. 398047, ≥99%), rhamnose (Cat. No. R3875, ≥99%), Sabouraud dextrose agar (Cat. No. S3181), sodium persulphate (Cat. No. 216232, ≥98%), streptomycin sulfate salt (Cat. No. S6501, ≥720 I.U./mg), succinic acid (Cat. No. S3674), sucrose (Cat. No. S7903, ≥99.5%), sweroside (Cat. No. SMB00083, ≥95%), swertiamarin (Cat. No. SMB0008O, ≥95%), tartaric acid (Cat. No. T400, ≥99%), Tween® 80 (Cat. No. P8074), xylose (Cat. No. X1500, ≥99%); Wuhan ChemFaces Biochemical Co., Ltd. (Hubei, China)—isosaponarin (Cat. No. CFN9O 133, ≥95%). Loganic acid-6′-O-β-d-glucoside and isoorientin-4′-O-β-d-glucoside were isolated previously from G. decumbens [5].
3.1.2. Equipment
UV-Vis spectrophotometry—SF-2000 UV-Vis-spectrophotometer (OKB Specter, St. Peterburg, Russia); analyt. MC-HPLC—microcolumn chromatograph Econova MiLiChrom A-02 (Novosibirsk, Russia).
3.2. Plant Material
The samples of four gentian species were collected in the flowering period in the Buryatia Republic: G. algida—Bagdarin (Bauntovskii region, 12.VII.2014, 54°28′26″ N, 113°29′16″ E, voucher specimen No. Gn/h-62/04-11/0714); G. decumbens—Mukhorshibir’ (Mukhorshibirskii region, 10.VII.2014, 51°2′14″ N, 107°55′31″ E, voucher specimen No. Gn/h-31/09-24/0714); G. macrophylla—Turka (Pribaikal’skii region, 16.VII.2014, 52°55′46″ N, 108°16′31″ E, voucher specimen No. Gn/h-71/06-12/0714); G. triflora—Posol’skoye (Kabanskii region, 25.VII.2014, 52°0′20″ N, 106°11′43″ E, voucher specimen No. Gn/h-63/04-09/0714). The species was determined by Prof. T.A. Aseeva (IGEB SB RAS, Ulan-Ude).
3.3. Oranoleptic and Nutritional Analysis
3.3.1. Decoction Preparation
The sample of dried milled herb (1 g) was added to distilled water (100 mL), heated on heater plate and boiled 10 min. The mixture was left to stand at room temperature for 15 min, and then filtered under reduced pressure.
3.3.2. Crude Composition
Organoleptic parameters (color, odor, taste) of bitter gentian teas were determined accordingly AHPA guidance on Organoleptic Analysis [41]. Extractives, bitter index and ash were determined accordingly WHO recommendations [42]. The protein content was estimated by Bradford method using BSA as a reference substance [43]. The lipid content was determined by extracting a known weight of dried gentian tea decoction with chloroform–methanol mixture (4:1) using Soxhlet apparatus. Carbohydrate content was determined with spectrophotometric phenol–sulphuric acid method [44]. Energy was calculated according to the following equation: Energy (kcal) = 4 × (g protein + g carbohydrate) + 9 × (g lipids). Free amino acids content was determined with ninhydrin method [42].
3.3.3. Free Sugars Composition
Free sugars were determined by a Milichrom A-02 microcolumn HPLC system, using Separon 5-NH2 column (1 × 60 mm, Ø 1 μm; Tessek Ltd.; Prague, Czechia), column temperature was 20 °C. Mobile phase was acetonitrile–water 75:25. The injection volume was 1 μL, and elution was at 100 μL/min. Detector wavelength was 190 nm. Reference sugars retention times (t, min): fructose (11.73), glucose (13.24), sucrose (17.45), gentiobiose (18.46), gentianose (19.94). The results were expressed in mg per 100 mL of decoction. HPLC chromatograms of the reference carbohydrate mixture and gentian tea samples are presented as the Supplementary Material (Supplementary Material Figure S1).
3.3.4. Organic Acids Composition
Organic acids were determined by a Milichrom A-02 microcolumn HPLC system, using a ProntoSIL-120-5-C18 AQ column (1 × 70 mm, Ø 5 μm; Metrohm AG; Herisau, Switzerland), column temperature was 35 °C. Eluent A was 0.2 М LiClO4 in 0.01 M HClO4 and eluent B was acetonitrile. The injection volume was 1 μL, and elution was at 50 μL/min. Gradient programme: 0–20 min, 1% B; 20–25 min, 1%–10% B. Detector wavelength was 210 nm. Reference organic acids retention times (t, min): oxalic (2.17), tartaric (4.03), malic (5.93), citric (9.74), succinic (11.02), quinic (12.22). The results were expressed in mg per 100 mL of decoction.
3.4. MC-RP-HPLC-UV Quantification of Phytochemicals in Bitter Gentian Teas
MC-RP-HPLC-UV experiments were performed on an Econova MiLiChrom A-02 microcolumn chromatograph (Novosibirsk, Russia) coupled with a UV-detector, using a ProntoSIL-120-5-C18 AQ column (1 × 50 mm, Ø 1 μm; Metrohm AG; Herisau, Switzerland); the column temperature was 35 °C. Eluent A was 0.2 М LiClO4 in 0.006 M HClO4 and eluent B was acetonitrile. The injection volume was 1 μL, and elution was at 600 μL/min. Gradient program: 0–2.5 min, 5%–35% B; 2.5–4 min, 35%–70% B. UV-detector wavelengths were 254 nm (iridoids) and 334 nm (flavonoids, mangiferin). Decoctions of gentian herb teas prepared accordingly a protocol described in Section 3.3.1 were filtered through a 0.22 μm PTFE syringe filter before injection into the HPLC system for analysis. Stock solutions of standards were made by accurately weighing 1 mg of loganic acid, loganic acid-6′-O-β-d-glucoside, swertiamarin, sweroside, gentiopicroside, isoorientin, isoorientin-4′-O-glucoside, isovitexin, saponarin, isosaponarin, isoscoparin, and mangiferin and dissolving it in 20 mL of methanol/DMSO in a volumetric flask. The appropriate amounts of stock solutions were diluted with methanol in order to obtain standard solutions containing 0.25–1.00 mg/mL. As all the compounds used for quantification were well-separated in experiment conditions mixtures of standards were analyzed. Prepared solutions were stored at 4 °C for no more than 72 h. The results are presented as mean values ± SD (standard deviations) of the three replicates.
3.5. Polysaccharide Analysis
The sample of dried milled herb (100 g) was added to distilled water (10 L), heated on a boiled water bath (1 h) and after cooling to room temperature water extract was filtered under reduced pressure and concentrated down in vacuo to 200 mL. The concentrated residue was mixed with 95% ethanol (1:5) and after 2 h the precipitate was centrifuged at 3000 g. The crude polysaccharide fraction was redissolved in 200 mL of water. The Sevag method [45] was used for deproteinisation, and was followed by dialysis for 48 h against distilled water using dialysis tubes with an MW-cut off of 2 kDa (Sigma-Aldrich, St. Louis, MO, USA). The non-dialysed part was loaded on to a KU-2-8 cation-exchange resin column (H+-form, 200 g; Closed Joint-Stock Company Tokem, Kemerovo, Russia) which was eluted with 2 L of distilled water. Eluate was concentrated in vacuo up to 200 mL and then liophylized. WSPF obtained were off-white powders.
Total carbohydrate content (TCC) was determined with the spectrophotometric phenol-sulphuric acid method [44]; content of uronic acids (UA) was estimated by the 3,5-dimethylphenol method calculated as galacturonic acid [46]; and the proteins were determined by the Bradford method using Coomassie G250 [43]. The optical rotation was measured at 40 °С for 1% sample solutions in 0.5% KOH using a SM-3 polarimeter (Zagorskii Optiko-Mekhanicheskii Zavod, Zagorsk, Russia) equipped with a 1 dm cell. Reactions of WSPF solutions with iodine, resorcinol and Yariv reagent were performed accordingly [47,48,49]. IR spectra were registered in a spectral range of 4000–600 cm−1 using a FT-801 infrared Fourier spectrometer (Simex, Novosibirsk, Russia) coupled with single reflection ATR device. The hydrolysis procedure and HPLC analysis conditions of the released products were as described by us previously [50].
3.6. Biological Activity Assays
The experimental procedures relating to the animals were authorised by the Institute of General and Experimental Biology’s Ethical Committee (protocol No. LM-0324, 27.01.2012) before starting the study and were conducted under the internationally accepted principles for laboratory animal use and care.
3.6.1. Carrageenan-Induced Paw Edema in Male Rats
Sprague Dawley rats weighing ca. 200–250 g were purchased from the “Pushchino” Laboratory Animal Breeding House (Moscow, Russia), and were kept in polyethylene boxes under a controlled temperature of 25 °C, humidity of 55% and a 12 h light/dark cycle, with free access to standard food and water. Animals were weighed and randomised into 14 groups of 12 animals each. Control group: water treatment; positive drug group: aspirin treatment (200 mg/kg); four bitter gentian tea groups: intragastrically administered three doses (100, 200, 400 mg/kg), once a day for seven days. For the determination of effects on acute inflammation, the carrageenan-induced paw oedema model described by Yesilada et al. [51] was employed with modifications. One hour after the last delivery, 100 μL of a 1% solution of carrageenan in 0.9% physiological saline was injected subcutaneously into the subplantar region of the right hind paw. The paw volume was measured with a water displacement plethysmometer 37140 (Ugo Basile, Varese, Italy) at 1–4 h after induction of inflammation. The percentage inhibition of paw volume in the drug-treated group was compared with the control group. Inhibitory activity was calculated according to the following formula described in the literature [52]:
| (1) |
where I is the inhibition of oedema, VS is the displacement volume after carrageenan administration and V0 is the displacement volume before carrageenan administration.
3.6.2. Antimicrobial Activity
The bitter gentian tea decoctions and individual compounds were individually tested against six microorganisms. The following microbial strains were used in this research: Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 6538P), Enterococcus faecalis (ATCC 12952), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27835); the test fungus was Candida albicans (ATCC 10231). All test microbial strains were obtained from the Institute of Biochemistry and Physiology of Microogranisms (Pushchino, Russia). Bacterial strains were cultured overnight at 37 °C in nutrient agar and fungi were cultured on Sabouraud dextrose agar at 28 °C for 5 days. The material obtained was suspended in sterile water with a concentration of 5 × 105 CFU/mL for bacterial strains and 3 × 104 CFU/mL for C. albicans. The MICs of the bitter gentian tea decoctions and individual compounds against tested microorganisms were determined based on a microdilution method in 96 multi-well microtiter plates [53] with slight modification as described by Mihailović et al. [35]. All tests with bacterial strains were performed in Mueller-Hinton broth (MHB), and for C. albicans Sabouraud dextrose broth (SDB) was used. A volume of 50 µL stock solutions of bitter gentian tea decoctions or individual compounds (in 50% DMSO, 1.6 mg/mL) were added into the first row of the plate. To all other wells 50 µL of MHB or SDB was added. A volume of 50 µL from the first test well was pipetted into the second well of each microtiter line, and then 50 µL of scalar dilution was transferred from the second to the ninth well. To each well, 10 µL of resazurin indicator solution (6.5 mg/mL in sterile distilled water) and 30 µL of nutrient broth were added. Finally, 10 µL of bacterial suspension (5 × 105 CFU/mL) and C. albicans spore suspension (3 × 104 CFU /mL) was added to each well. In tests with fungi instead of resazurin indicator solution, 10 µL of SDB was added. For each strain, the growth conditions and the sterility of the medium were checked. Standard antibiotic streptomycin was used to control the sensitivity of the tested bacteria, whereas nystatin was used as control against C. albicans. Plates were placed in an incubator at 37 °C for 24 h for the bacteria and at 28 °C for 48 h for C. albicans. The visual growth was then assessed. Any colour change from purple to pink or colourless was recorded as positive. The lowest concentration at which there was no observed colour change was taken as the MIC value for bacterial strains, and the lowest concentrations without visible growth were defined as concentrations that completely inhibited fungal growth. All tests were repeated in triplicate.
3.6.3. Antioxidant Activity
The DPPH-HPLC-UV procedure was realized as described previously [54]. The DPPH• radical scavenging activity (DPPH•) was assessed as described by Asker and Shawky [55]; the ABTS•+ radical scavenging activity (ABTS•+-SA) was measured using the method of Ding et al. [56]; the determination of superoxide anion scavenging activity (O2•−-SA) was measured in phenazine methosulphatenicotinamide adenine dinucleotide-nitroblue tetrazolium systems using the method of Ozen et al. [57]; β-carotene bleaching assay (CBA) was performed in β-carotene-oleic acid-DMSO-H2O2-system [58].
3.7. Statistical Analysis
Statistical analyses were performed using a one-way analysis of variance (ANOVA), and the significance of the mean difference was determined by Duncan’s multiple range test. Differences at p < 0.05 were considered statistically significant. The results are presented as mean values ± SD (standard deviations) of the three replicates.
4. Conclusions
In conclusion, the present study demonstrated that four gentian teas (G. algida, G. decumbens, G. macrophylla, G. triflora) used in Siberia could be distinguished plant products in regard to their nutritional and chemical profiles, as well as their potential medical uses. The results showed the equilibrated composition of the nutraceutical compounds and specific phytochemicals in the gentian herb decoctions. The gentian teas preparations demonstrated expressed anti-inflammatory, antimicrobial and antioxidant activities the most significant for G. algida tea. Chemical features as a high gentiopicroside and isoorientin content may be responsible for the pharmacological effectiveness. The gentian teas bioactivity can be used in the food industries and medicine.
Acknowledgments
The authors acknowledge the financial support provided by The Russian Foundation for Basic Research, Project No. 16-03-00039, and the Presidium of SD RAS, Project No. VI.62.1.8.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/11/19674/s1.
Author Contributions
D.N.O. and N.I.K. designed research; N.I.K., N.K.C., L.P.K. and L.N.V. performed research and analyzed the data; D.N.O. and N.I.K. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of G. algida, G. decumbens, G. macrophylla, G. triflora plants and extracts are available from the authors.
References
- 1.Anonymous. In: Buryats. Abaeva L.L., Zhukovskaya N.L., editors. Nauka; Moscow, Russia: 2004. p. 633. [Google Scholar]
- 2.Anonymous. In: Traditions of the Buryat Cuisine. Mikhailova V.T., editor. Printhouse L.T.; Ulan-Ude, Bria: 2010. p. 93. [Google Scholar]
- 3.Makarov A.A. Plant Remedies of the Traditional Yakutian Medicine. Yakutian Scientific Center; Yakutsk, Russia: 1974. p. 64. [Google Scholar]
- 4.Anonymous. In: Traditions and Innovations in Life and Culture of the Siberian Peoples. Rusakova L.M., editor. SD RAS; Novosibirsk, Russia: 1983. p. 138. [Google Scholar]
- 5.Olennikov D.N., Kashchenko N.I., Chirikova N.K., Tankhaeva L.M. Iridoids and flavonoids of four Siberian gentians: Chemical profile and gastric stimulatory effect. Molecules. 2015;20:19172–19188. doi: 10.3390/molecules201019172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hostettmann-Kaldas M., Hostettmann K., Sticher O. Xanthones, flavones and secoiridoids of American Gentiana species. Phytochemistry. 1981;20:443–446. doi: 10.1016/S0031-9422(00)84162-X. [DOI] [Google Scholar]
- 7.Tan R.X., Wolfender J.-L., Ma W.G., Zhang L.X., Hostettmann K. Secoiridoids and antifungal aromatic acids from Gentiana algida. Phytochemistry. 1996;41:111–116. doi: 10.1016/0031-9422(95)00599-4. [DOI] [PubMed] [Google Scholar]
- 8.Tan R.X., Wolfender J.-L., Zhang L.X., Ma W.G., Fuzzati N., Marston A., Hostettmann K. Acyl secoiridoids and antifungal constituents from Gentiana macrophylla. Phytochemistry. 1996;42:1305–1313. doi: 10.1016/0031-9422(96)00149-5. [DOI] [PubMed] [Google Scholar]
- 9.Wang S., Xu Y., Jiang W., Zhang Y. Isolation and identification of constituents with activity of inhibiting nitric oxide production in RAW 264.7 macrophages from Gentiana triflora. Planta Med. 2013;79:680–686. doi: 10.1055/s-0032-1328460. [DOI] [PubMed] [Google Scholar]
- 10.Li X., Zhou J.Y., Yang Q.W., Chen Y., Piao Y.A., Li H.Y. Inhibition activities of polysaccharide (RG4–1) from Gentiana rigescens against RSV. J. Asian Nat. Prod. Res. 2011;13:512–522. doi: 10.1080/10286020.2011.573628. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z., Wang C., Su T., Zhang J. Antioxidant and immunological activities of polysaccharides from Gentiana scabra Bunge roots. Carbohydr. Polym. 2014;112:114–118. doi: 10.1016/j.carbpol.2014.05.077. [DOI] [PubMed] [Google Scholar]
- 12.Massias M., Carbonnier J., Mohlo D. Implications chimiotaxonomiques de la répartition des substances osidiques dans le genre Gentiana L. Bull. Mus. Nat. Hist. Natur. Sci. Phys. Chim. N. 1977;13:41–54. [Google Scholar]
- 13.Eswaranandam S., Hettiarachchy N.S., Johnson M.G. Antimicrobial activity of citric, lactic, malic, or tartaric acids and nisin-incorporated soy protein film against Listeria monocytogenes, Escherichia coli O157:H7, and Salmonella gaminara. J. Food Sci. 2004;69:FMS79–FMS84. doi: 10.1111/j.1365-2621.2004.tb13375.x. [DOI] [Google Scholar]
- 14.Tang X., Liu J., Dong W. The cardioprotective effects of citric Acid and l-malic acid on myocardial ischemia/reperfusion injury. Evid.-Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/820695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q.C., Zhao Y., Bian H.M. Antiplatelet activity of a novel formula composed of malic acid, succinic acid and citric acid from Cornus officinalis fruit. Phytother. Res. 2013;27:1894–1896. doi: 10.1002/ptr.4934. [DOI] [PubMed] [Google Scholar]
- 16.Guimarães R., Barros L., Dueñas M., Calhelha R.C., Carvalho A.M., Santos-Buelga C., Queiroz M.J., Ferreira I.C. Nutrients, phytochemicals and bioactivity of wild Roman chamomile: A comparison between the herb and its preparations. Food Chem. 2013;15:718–725. doi: 10.1016/j.foodchem.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Olennikov D.N. Chemical investigation of Anagallidium dichotomum and anticholinesterase activity of its constituents. Chem. Nat. Comp. 2013;49:1137–1139. doi: 10.1007/s10600-014-0842-y. [DOI] [Google Scholar]
- 18.Paulsen B.S., Barsett H. Bioactive pectic polysaccharides. Adv. Polym. Sci. 2005;186:69–101. [Google Scholar]
- 19.Kačuráková M., Wilson R.H. Development in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydr. Polym. 2001;44:291–303. doi: 10.1016/S0144-8617(00)00245-9. [DOI] [Google Scholar]
- 20.Čopíková J., Synytsyá A., Černá M., Kaasová J., Novotná M. Application of FT-IR spectroscopy in detection of food hydrocolloids in confectionery jellies and food supplements. Czech J. Food Sci. 2001;19:51–56. [Google Scholar]
- 21.Synytsyá A., Čopíková J., Matějka P., Machovič V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003;54:97–106. doi: 10.1016/S0144-8617(03)00158-9. [DOI] [Google Scholar]
- 22.Kačuráková M., Capek P., Sasinková V., Wellner N., Ebringerová A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000;43:195–203. doi: 10.1016/S0144-8617(00)00151-X. [DOI] [Google Scholar]
- 23.Winiyard P.G., Willoughby D.A. Inflammation Protocols. Humana Press; New York City, NY, USA: 2003. p. 365. [Google Scholar]
- 24.Wang C.-H., Cheng X.-M., Bligh S.W.A., White K.N., Branford-White C.-J., Wang Z.-T. Pharmacokinetics and bioavailability of gentiopicroside from decoctions of Gentianae and Longdan Xiegan Tang after oral administration in rats—Comparison with gentiopicroside alone. J. Pharm. Biomed. Anal. 2007;44:1113–1117. doi: 10.1016/j.jpba.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Shi P., Lin X., Yao H. Metabolism and plasma pharmacokinetics of isoorientin, a natural active ingredient, in Sprague-Dawley male rats after oral and intravenous administration. Xenobiotica. 2015;45:999–1008. doi: 10.3109/00498254.2015.1028513. [DOI] [PubMed] [Google Scholar]
- 26.Huang W.-J., Niu H.-S., Lin M.-H., Cheng J.-T., Hsu F.-L. Antihyperglycemic effect of catalpol in streptozotocin-induced diabetic rats. J. Nat. Prod. 2010;73:1170–1172. doi: 10.1021/np9008317. [DOI] [PubMed] [Google Scholar]
- 27.Recio D.L.M., Giner R.M., Manez S., Rios J.H. Structural consideration on the iridoids as anti-inflammatory agents. Planta Med. 1994;60:232–234. doi: 10.1055/s-2006-959465. [DOI] [PubMed] [Google Scholar]
- 28.Küpeli E., Aslan M., Gürbüz I., Yesilada E. Evaluation of in vivo biological activity profile of isoorientin. Z. Naturforsch. 2004;59c:787–790. doi: 10.1515/znc-2004-11-1204. [DOI] [PubMed] [Google Scholar]
- 29.Odontuya G., Hoult J.R.S., Houghton P.J. Structure-activity relationship for anti-inflammatory effect of luteolin and its derived glucosides. Phytother. Res. 2005;19:782–786. doi: 10.1002/ptr.1723. [DOI] [PubMed] [Google Scholar]
- 30.Wedler J., Daubitz T., Schlotterbeck G., Butterweck V. In vivo anti-inflammatory and wound-healing potential of a Phyllostachys edulis leaf extract—Identification of isoorientin as an active compound. Planta Med. 2014;80:1678–1684. doi: 10.1055/s-0034-1383195. [DOI] [PubMed] [Google Scholar]
- 31.Ovodova R.G., Golovchenko V.V., Popov S.V., Popova G.Y., Paderin N.M., Shashkov A.S., Ovodov Y.S. Chemical composition and anti-inflammatory activity of pectic polysaccharide isolated from celery stalks. Food Chem. 2009;114:610–615. doi: 10.1016/j.foodchem.2008.09.094. [DOI] [Google Scholar]
- 32.Chen L., Liu J., Zhang Y., Dai B., An Y., Yu L. Structural, thermal, and anti-inflammatory properties of a novel pectic polysaccharide from Alfalfa (Medicago sativa L.) stem. J. Agric. Food Chem. 2015;63:3219–3228. doi: 10.1021/acs.jafc.5b00494. [DOI] [PubMed] [Google Scholar]
- 33.Popov S.V., Popova G.Y., Ovodova R.G., Ovodov Y.S. Antiinflammatory activity of the pectic polysaccharide from Comarum palustre. Fitoterapia. 2005;76:281–287. doi: 10.1016/j.fitote.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 34.Savikin K., Menković N., Zdunić G., Stević T., Radanović D., Janković T. Antimicrobial activity of Gentiana lutea L. extracts. Z. Naturforsch. 2009;64c:339–342. doi: 10.1515/znc-2009-5-606. [DOI] [PubMed] [Google Scholar]
- 35.Mihailović V., Vuković N., Nićiforović N., Solujić S., Mladenović M., Mašković P., Stanković M.S. Studies on the antimicrobial activity and chemical composition of the essential oils and alcoholic extracts of Gentiana asclepiadea L. J. Med. Plants Res. 2011;5:1164–1174. [Google Scholar]
- 36.Graikou K., Aligianis N., Chinou I.B., Harvala C. Cantleyoside-dimethyl-acetal and other iridoid glucosides from Pterocephalus perrenis—Antimicrobial activities. Z. Naturforsch. C. 2002;57:95–99. doi: 10.1515/znc-2002-1-217. [DOI] [PubMed] [Google Scholar]
- 37.Cottiglia F., Loy G., Garau D., Floris C., Casu M., Pompei R., Bonsignore L. Antimicrobial evaluation of coumarins and flavonoids from the stems of Daphne gnidium L. Phytomedicine. 2001;8:302–305. doi: 10.1078/0944-7113-00036. [DOI] [PubMed] [Google Scholar]
- 38.Xue Y.Q., Song J., Ye S.P., Yuan K. Separation, identification and its antibacterial activity of glycosylflavones in Lophantherum gracile Brong. West China J. Pharm. Sci. 2009;24:218–220. [Google Scholar]
- 39.Mihailović V., Mišić D., Matić S., Mihailović M., Stanić S., Vrvić M.M., Katanić J., Mladenović M., Stanković N., Boroja T., et al. Comparative phytochemical analysis of Gentiana cruciata L. roots and aerial parts, and their biological activities. Ind. Crops Prod. 2015;73:49–62. doi: 10.1016/j.indcrop.2015.04.013. [DOI] [Google Scholar]
- 40.Senol F.S., Tuzun C.Y., Toker G., Orhan I.E. An in vitro perspective to cholinesterase inhibitory and antioxidant activity of five Gentiana species and Gentianella caucasea. Int. J. Food Sci. Nutr. 2012;63:802–812. doi: 10.3109/09637486.2012.676031. [DOI] [PubMed] [Google Scholar]
- 41.Anonymous. Organoleptic Analysis of Herbal Ingredients. American Herbal Products Association; Silver Spring, MD, USA: 2013. p. 37. [Google Scholar]
- 42.Anonymous. Quality Control Methods for Herbal Materials. World Health Organization; Geneva, Switzerland: 2011. p. 173. [Google Scholar]
- 43.Bradford M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;76:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 44.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 45.Sevag M.G., Lackman D.B., Smolens J. The isolation of the components of Streptococcal nucleoproteins in serologically active form. J. Biol. Chem. 1938;124:425–436. [Google Scholar]
- 46.Usov A.T., Bilan M.I., Klochkova N.G. Polysaccharides of algae. 48. Polysaccharide composition of several calcareous red algae: Isolation of alginate from Corallina pilulitara P. et R. (Rhodophyta, Corallinaceae) Bot. Mar. 1995;35:43–51. [Google Scholar]
- 47.Olennikov D.N., Stolbikova A.V., Rokhin A.V., Khobrakova V.B., Tankhaeva L.M. Polysaccharides from Fabaceae. V. α-Glucan from Sophora flavescens roots. Chem. Nat. Comp. 2011;47:1–6. doi: 10.1007/s10600-011-9817-4. [DOI] [Google Scholar]
- 48.Olennikov D.N., Tankhaeva L.M. A quantitative assay for total fructans in burdock (Arctium spp.) roots. Russ. J. Bioorg. Chem. 2011;37:893–898. doi: 10.1134/S1068162011070181. [DOI] [Google Scholar]
- 49.Togola A., Inngjerdingen M., Diallo D., Barsett H., Rolstad B., Michaelsen T.E., Paulsen B.S. Polysaccharides with complement fixing and macrophage stimulation activity from Opilia celtidifolia, isolation and partial characterization. J. Ethnopharmacol. 2007;115:423–431. doi: 10.1016/j.jep.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Olennikov D.N., Rokhin A.V. Water-soluble glucans from true cardamom (Elettaria cardamomum White at Maton) seeds. Appl. Biochem. Microbiol. 2013;49:182–187. doi: 10.1134/S0003683813010134. [DOI] [PubMed] [Google Scholar]
- 51.Yesilada E., Kupeli E. Berberis crataegina root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats. J. Ethnopharmacol. 2002;79:237–248. doi: 10.1016/S0378-8741(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 52.Olajide A.A., Olubusayo A.S., Modupe M.J. Studies on the anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. J. Ethnopharmacol. 2000;71:179–186. doi: 10.1016/S0378-8741(99)00200-7. [DOI] [PubMed] [Google Scholar]
- 53.Sarker S.D., Nahar L., Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olennikov D.N., Kashchenko N.I., Chirikova N.K. A novel HPLC-assisted method for investigation of the Fe2+-chelating activity of flavonoids and plant extracts. Molecules. 2014;19:18296–18316. doi: 10.3390/molecules191118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asker M.M.S., Shawky B.T. Structural characterization and antioxidant activity of an extracellular polysaccharide isolated from Brevibacterium otitidis BTS 44. Food Chem. 2010;123:315–320. doi: 10.1016/j.foodchem.2010.04.037. [DOI] [Google Scholar]
- 56.Ding H., Chou T., Liang C. Antioxidant and antimelanogenic properties of rosmarinic acid methyl ester from Origanum vulgare. Food Chem. 2010;123:254–262. doi: 10.1016/j.foodchem.2010.04.025. [DOI] [Google Scholar]
- 57.Ozen T., Demirtas I., Aksit H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011;124:58–64. doi: 10.1016/j.foodchem.2010.05.103. [DOI] [Google Scholar]
- 58.Olennikov D.N., Tankhaeva L.M., Agafonova S.V. Antioxidant components of Laetiporus sulphureus (Bull.: Fr.) Murr. fruit bodies. Appl. Biochem. Microbiol. 2011;47:419–425. doi: 10.1134/S0003683811040107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.