Abstract
Key points
Restless legs patients complain about sensory and motor symptoms leading to sleep disturbances. Symptoms include painful sensations, an urge to move and involuntary leg movements.
The responsible mechanisms of restless legs syndrome are still not known, although current studies indicate an increased neuronal network excitability. Reflex studies indicate the involvement of spinal structures. Peripheral mechanisms have not been investigated so far.
In the present study, we provide evidence of increased hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channel‐mediated inward rectification in motor axons. The excitability of sensory axons was not changed.
We conclude that, in restless legs syndrome, an increased HCN current in motoneurons may play a pathophysiological role, such that these channels could represent a valuable target for pharmaceutical intervention.
Abstract
Restless legs syndrome is a sensorimotor network disorder. So far, the responsible pathophysiological mechanisms are poorly understood. In the present study, we provide evidence that the excitability of peripheral motoneurons contributes to the pathophysiology of restless legs syndrome. In vivo excitability studies on motor and sensory axons of the median nerve were performed on patients with idiopathic restless legs syndrome (iRLS) who were not currently on treatment. The iRLS patients had greater accommodation in motor but not sensory axons to long‐lasting hyperpolarization compared to age‐matched healthy subjects, indicating greater inward rectification in iRLS. The most reasonable explanation is that hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channels open at less hyperpolarized membrane potentials, a view supported by mathematical modelling. The half‐activation potential for HCN channels (Bq) was the single best parameter that accounted for the difference between normal controls and iRLS data. A 6 mV depolarization of Bq reduced the discrepancy between the normal control model and the iRLS data by 92.1%. Taken together, our results suggest an increase in the excitability of motor units in iRLS that could enhance the likelihood of leg movements. The abnormal axonal properties are consistent with other findings indicating that the peripheral system is part of the network involved in iRLS.
Keywords: threshold tracking, HCN channels, motoneuron, Restless Legs Syndrome, axonal, peripheral nervous system
Key points
Restless legs patients complain about sensory and motor symptoms leading to sleep disturbances. Symptoms include painful sensations, an urge to move and involuntary leg movements.
The responsible mechanisms of restless legs syndrome are still not known, although current studies indicate an increased neuronal network excitability. Reflex studies indicate the involvement of spinal structures. Peripheral mechanisms have not been investigated so far.
In the present study, we provide evidence of increased hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channel‐mediated inward rectification in motor axons. The excitability of sensory axons was not changed.
We conclude that, in restless legs syndrome, an increased HCN current in motoneurons may play a pathophysiological role, such that these channels could represent a valuable target for pharmaceutical intervention.
Introduction
Restless legs syndrome (RLS) is a sensorimotor disorder with multiple aetiologies. The idiopathic end of the spectrum may be influenced by common allelic variants (Winkelmann et al. 2007; Rye, 2015) and is associated with a number of co‐morbidities including peripheral neuronal diseases such as polyneuropathy, central disturbances such as stroke and multiple sclerosis, or metabolic disorders such as iron deficiency, anaemia and diabetes (Allen et al. 2014; Trenkwalder et al. 2016).
Neurophysiological studies suggest that cortical, subcortical and spinal mechanisms intensify sensory input and motor output leading to common positive symptoms, such as the urge to move, uncomfortable leg sensations and periodic leg movements (Trenkwalder & Paulus, 2010; Allen et al. 2014; Lanza et al. 2017). Transcranial magnetic stimulation (TMS) studies have reported increased excitability within the central nervous system (Lanza et al. 2017) and there is considerable evidence that the excitability of spinal circuits is increased in RLS (Bara‐Jimenez et al. 2000; Aksu & Bara‐Jimenez, 2002; Rijsman et al. 2005; Scaglione et al. 2008; Marconi et al. 2012). Clinical observations strengthen the idea of an essential role of the spinal cord in the pathophysiology of RLS: involuntary leg movements in patients with spinal cord injury are identical to sleep‐related periodic leg movements seen in RLS (Yokota et al. 1991).
The excitability of α‐motoneurons depends on intrinsic factors, such as the activity of ion channels and ion pumps. However, these factors are labile: the biophysical properties of α‐motoneurons and/or their axons undergo plastic changes after changes in activity (Gardiner et al. 2006; Hultborn, 2006), following spinal lesions (Button et al. 2008; Boland et al. 2009) and in multiple sclerosis (Ng et al. 2008). Changes in the excitability of motor axons and presumably the parent neurons occur in pathologies such as diabetes, stroke and porphyria (Horn et al. 1996; Jankelowitz et al. 2007; Lin et al. 2008), all of which are risk factors for developing RLS (Hellmann & Tschudy, 1962; Zobeiri & Shokoohi, 2014; Schlesinger et al. 2015). In these excitability studies, the symptoms of RLS were not mentioned, although secondary changes in axonal excitability in diabetes, stroke and porphyria do not necessarily cause RLS symptoms. Thus, the role of motoneuron excitability in restless legs syndrome remains unclear.
To address this unresolved question, we investigated the excitability of motoneurons in patients who had been diagnosed with idiopathic RLS (iRLS) by studying the excitability of motor axons (Bostock et al. 1998). Patients with RLS associated with other diseases of the nervous systems were excluded.
Our results suggest greater inward rectification in motor axons in iRLS, and our mathematical modelling is consistent with a hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channel‐driven increase in excitability of motor units in iRLS. This is the first evidence for abnormality in the peripheral system in the sensorimotor network involved in iRLS.
Methods
Ethical approval
Written informed consent was obtained from all patients and healthy subjects. The study conformed to the standards set by the Declaration of Helsinki, except for registration in a database. The procedures were approved by the ethics committee of the University of Göttingen (ethics approval reference number is 20/2/12) and registered by the clinical study management Göttingen.
Patients were included in the study only if they fulfilled the iRLS criteria (Allen et al. 2014). Patients suffering from ‘secondary’ RLS, such as those with clinically relevant polyneuropathy, currently existing anaemia and other neurological or sleep disorders, were not included. No patient or healthy subject was on medication for RLS at the time of the study, and none experienced twitching, cramps or sensory symptoms in the upper and lower limbs during the experimental measurements. Excitability studies were performed on motor axons in the median nerve in 34 patients (11 men and 23 women) with iRLS and 38 healthy subjects (19 men and 19 women) as normal controls. Similar studies were performed on sensory axons in the median nerve in 14 patients (five men and nine women) and 14 healthy controls (seven men and seven women).
Axonal excitability assessment
Nerve excitability studies of motor axons in the median nerves were carried out via the threshold tracking technique using Qtrac software and the extended TrondNF protocol (UCL Institute of Neurology, London, UK) (Bostock et al. 1998). Axonal excitability measurements using the threshold tracking technique are described in detail in several previous studies (Bostock et al. 1998; Burke et al. 2001; Krarup & Moldovan, 2009). As shown in Fig. 1 A, stimulation of the median nerve was performed using non‐polarizable Ag/AgCl adhesive electrodes (ECG‐electrodes; MPC International SA, Luxembourg, Luxembourg). The cathode was placed over the nerve at the wrist and the anode on the radial edge of the forearm (10 cm proximal and away from the median nerve). The compound muscle action potential (CMAP) of abductor pollicis brevis was recorded using surface electrodes connected to an isolated preamplifier (D440; Digitimer Ltd, Welwyn Garden City, UK; gain 200×). The reference electrode was placed on the proximal phalanx of the thumb. A ground electrode was placed on the palm.
Figure 1. Methodology.
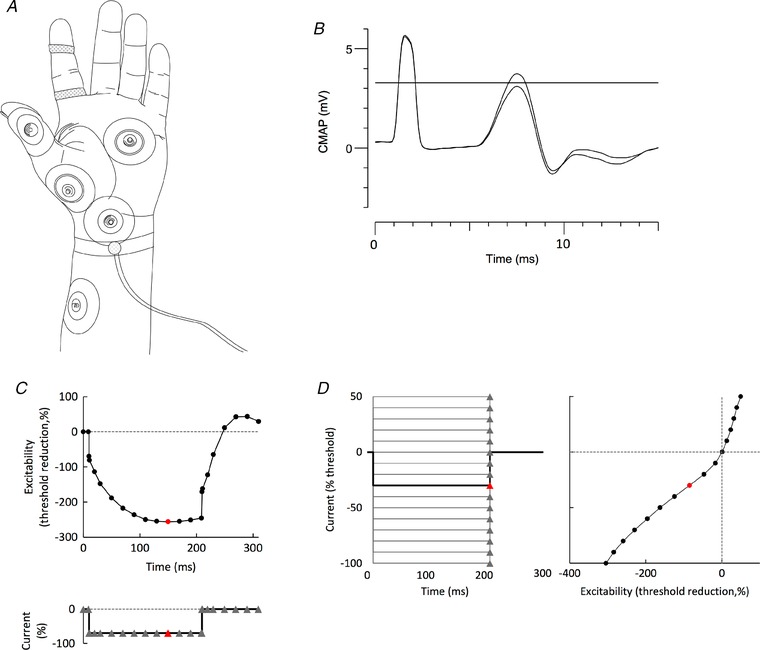
A, recording arrangement. Stimuli were delivered at the wrist (cathode) using Ag/AgCl electrodes with the anode 10 cm distal on the radial edge of the forearm. CMAPs were recorded over the thenar eminence, with the reference electrode on the proximal phalanx of the thumb. CSAPs were recorded using disposable Ag/AgCl ring electrodes on digit 2. The same ground (in the palm) was used for both motor and sensory recordings. A thermistor was strapped around the wrist to record skin temperature close to the site of stimulation (Howells et al. 2013). B, principle of threshold tracking. A target threshold was set, as indicated by the horizontal line, here ∼3 mV, which is ∼40% of the size of the maximal CMAP (or CSAP). If the target response exceeded that threshold (e.g. as demonstrated by the larger CMAP), the intensity of the next test stimulus was reduced. If the target response was less than the threshold value (as in the smaller CMAP), the intensity of the next test stimulus was increased. C and D, application of the technique to the recording of TE to a –70% hyperpolarizing current (C) and to the I–V relationship (D). C, threshold was measured at the intervals indicated by triangular markers, before, during and after a square wave hyperpolarizing current lasting 200 ms, set to –70% of the control threshold. D, threshold was measured 200 ms after the onset of 15 square‐wave polarizing currents from +50% (depolarizing, top left) to –100% (hyperpolarizing, bottom). The change in excitability in the right panel is equivalent to a conventional I–V relationship. In (C) and (D), the measurements on the threshold plots in red are indicated on the current plots in red.
Data were digitized at 10 kHz using a data acquisition system (NI USB‐6251; National Instruments, Austin, TX, USA). The QtracS software provided stimulus command pulses through the data acquisition system to an isolated linear bipolar constant current stimulator (DS5; Digitimer Ltd). Mains frequency noise was removed with an in‐line noise eliminator (Hum Bug 50/60 Hz Noise Eliminator; Quest Scientific, North Vancouver, BC, Canada). Skin temperature was monitored close to the site of stimulation with a thermistor thermometer (EcoScan Temp4 Meter; Eutech Instruments, Singapore) and was kept constant above 32°C throughout the experiment.
The excitability of sensory axons in the median nerve was assessed by stimulation at the wrist as for motor studies. The compound sensory action potential (CSAP) was recorded with ring electrodes around the index finger. The CSAP was measured peak to peak. The test pulses were of 0.5 ms in duration.
Excitability protocols
To investigate the excitability of motor axons, the extended nerve excitability protocol (TrondNF) was used (Tomlinson et al. 2010). This includes five stimulation protocols: stimulus–response (SR) curve, strength–duration (SD) properties, threshold electrotonus (TE), current–threshold (I–V) relationship and recovery cycle (RC). Excitability studies measure the excitability of a population of axons recruited around the target CMAP/CSAP, which is a fixed fraction of the maximal CMAP/CSAP (40% in the present study). The threshold was defined as the stimulus intensity required to elicit the target CMAP/CSAP (Fig. 1 B).
SR curve
A SR curve was recorded first using unconditioned stimuli of duration 1.0 ms for motor axons and 0.5 ms for sensory. Stimulus intensity was graded, starting with the maximal CMAP/CSAP and gradually reducing until no CMAP/CSAP could be recorded.
SD properties
To assess the SD properties, motor axons were stimulated with rectangular test pulses with durations of 0.2, 0.4, 0.6, 0.8 and 1.0 ms. For sensory axons, the stimulus durations were 0.1, 0.2, 0.3, 0.4 and 0.5 ms. Strength–duration time constant (SDTC) and rheobase were calculated using Weiss’ law (Bostock, 1983; Mogyoros et al. 1996).
SDTC was calculated using the formula:
where t a and t b are the test stimulation durations for the threshold currents I a and I b, respectively.
Rheobase (I rh) is the threshold current if the test stimulus could be infinitely long. It was calculated from the same data using the formula:
TE
TE measures accommodation of the threshold stimulus to long subthreshold depolarizing and hyperpolarizing conditioning currents (Burke et al. 2001). The standard currents were set as: depolarizing (+20; +40% of the control threshold current, 100 ms long) and hyperpolarizing (–20% and –40% of the control threshold, 100 ms long). To explore inward rectification in greater detail, hyperpolarizing currents, 200 and 300 ms long, were set to –70% and –100% of the control threshold, respectively (Tomlinson et al. 2010). Test pulses were applied before, during and after the long‐lasting polarizing currents (Fig. 1 C). TE parameters are termed according to the polarization (‘d’ for depolarizing and ‘h’ for hyperpolarizing) and the interval over which the parameter was measured; for example, TEd 90–100 describes a threshold change produced 90–100 ms after the onset of a depolarizing conditioning current.
I–V relationship
The I–V is a measure of rectification and is the threshold analogue of the traditional I–V relationship (Burke et al. 2001). Motor (or sensory) axons were stimulated with test pulses of duration 1 ms (or 0.5 ms) at the end of 200 ms long polarizing currents of graded strength [16 strengths, 10% steps from +50% (depolarizing) to –100% (hyperpolarizing) of the control threshold]; increase in excitability was plotted against the strength of the current pulse to produce a threshold ‘I–V’ curve (Fig. 1 D). The steepness of the I–V relationship in response to the conditioning hyperpolarizing currents is a measure of inward rectification, whereas the steepness of the curve in the depolarizing direction reflects outward rectification as a result of fast and slow K+ currents.
Recovery cycle
The recovery cycle measures the excitability following supramaximal stimulation at 18 conditioning test intervals, in a roughly logarithmic sequence from 2 to 200 ms following this supramaximal stimulus. To ensure accurate measurements at short conditioning test intervals, the CMAP (CSAP) produced by the conditioning stimulus alone was subtracted online from the combined response to the conditioning and test stimuli.
Statistical analysis
Group data are reported as the mean ± SD. Individual data were tested for normality using the Kolmogorov–Smirnov test. The mean values for the two groups (normal and iRLS) were compared using Student's t tests for unpaired data. Measures that follow a log‐normal distribution were calculated using the geometric mean and geometric SD. Multiple comparisons were corrected using the Holm–Bonferroni method (Holm, 1979).
Mathematical modelling
To explore the mechanisms responsible for increased inward rectification seen in the iRLS recordings, the group data were modelled using the ‘Bostock’ mathematical model of a human motor axon (Bostock et al. 1991), as updated by Howells et al. (2012). This contains both nodal and internodal compartments with voltage‐gated ion channels, ion pumps and leak conductances that characterize the excitability of human myelinated axons. The discrepancy between modelled and recorded data was minimized using an iterative method. The overall discrepancy was defined as the weighted sum of the Euclidean distances (or square root of the sum of the squares) between the model and group data. The weights for each of the four measures were: SD time constant = 0.5; TE = 3; RC = 1; I–V relationship = 2.
Results
Excitability of motor axons in the median nerve
In total, 72 individuals were studied, 34 patients (11 men and 23 women) with iRLS and 38 healthy volunteers (19 men and 19 women) as a normal control group. None of the iRLS patients had upper limb motor or upper limb sensory symptoms. The ages of the iRLS and control groups were similar: 51 ± 16.3 years and 47.6 ± 12.8 years, respectively (P = 0.27).
All participants underwent excitability studies on the median nerve at the wrist, and no significant discomfort was reported by the subjects. The data conformed to a normal distribution (or did so after log transformation) and the results are shown in Figs 2, 3, 4.
Figure 2. Evidence against a change in resting membrane potential.
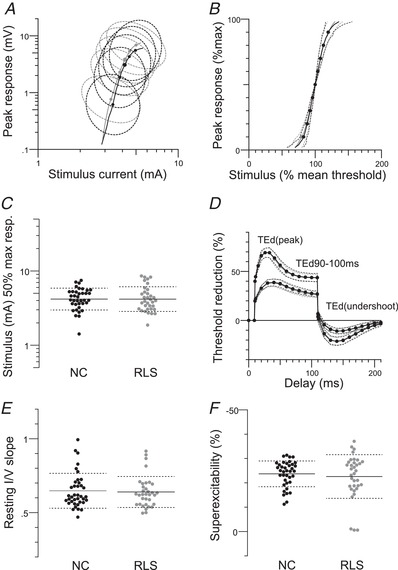
Excitability data for motor nerves in normal controls (NC) (n = 38) and RLS patients (n = 34). A and B, maximal CMAPs (P = 0.1), black: normal controls, grey: iRLS; dotted circles (A) and lines (B) indicate 1 SD). C, stimulus for a half‐maximal response (P = 0.9), black: normal controls, grey: iRLS; dotted lines indicate SD. D, mean data for depolarizing TE (note that the patient data and the control data superimpose perfectly such that it is difficult to identify separate data points). TEd (peak) (P = 0.8); TEd 90–100 ms (P = 0.86); TEd (undershoot) (P = 0.87). E, resting I–V slope (P = 0.76). F, superexcitability (P = 0.6).
Figure 3. SD properties and recovery cycles.
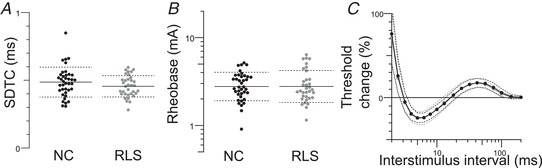
Excitability data for motor nerves in normal controls (n = 38; black lines) and RLS patients (n = 34; grey lines). Dotted lines indicate the SD. SD time constant (P = 0.09) (A) and rheobase (P = 0.82) (B) were not different. C, no significant differences in measures of the recovery cycle (relative refractory period, P = 0.87; extent of refractoriness, P = 0.6; superexcitability, P = 0.6; late subexcitability, P = 0.53).
Figure 4. Evidence for greater I h in median nerve with the best‐fit from the mathematical model.
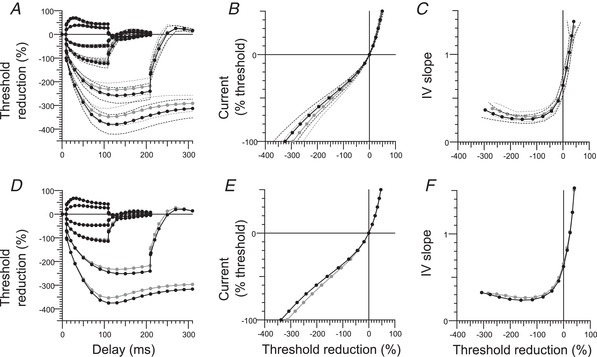
Extended excitability data for motor axons in the median nerve. A, B and C, upper row: group data (mean and SD); normal controls (n = 38; black filled circles) and RLS patients (n = 34; grey filled circles) and best‐fit from the Bostock motor axon model (D, E and F; lower row: black circles indicate the model in control subjects, and the modelled changes in RLS patients are shown as grey circles). A and D, TE for conditioning levels of +20%, –20%, +40%, –40%, –70% and –100% of control threshold. B and E, I–V for 200‐ms conditioning stimuli. C and F, I–V slope (threshold conductance): resting I–V slope (r): calculated from currents between –10% and +10%, shown at x = 0; minimal I–V slope.
There were no significant differences between the iRLS patients and controls in the maximal CMAP amplitudes (P = 0.1) (Fig. 2 A) and in threshold‐related measures: stimulus for a half‐maximal response (P = 0.9) (Fig. 2 C) and SR curves (SR slope, P = 0.35) (Fig. 2 B), SDTC (P = 0.17) (Fig. 3 A) and rheobase (P = 0.93) (Fig. 3 B).
There were no significant differences between the iRLS and control groups in measures of the recovery of excitability following a supramaximal conditioning stimulus (relative refractory period, P = 0.89, extent of refractoriness, P = 0.6; superexcitability, P = 0.73; late subexcitability, P = 0.68) (Fig. 2 F and 3 C). The measures shown in Figs 2 A–F and 3 A–C are those most sensitive to small changes in resting membrane potential (Kiernan & Bostock, 2000) and these findings suggest that there was little difference in resting membrane potential in the patients and control subjects.
In TE, the accommodation to hyperpolarization was greater in the patient group, and this difference was greater the stronger the hyperpolarization (Fig. 4). This is as would be expected with greater I h. Accordingly, the increase in threshold to hyperpolarizing currents was smaller in iRLS with conditioning currents of –70% and –100%, as shown in Fig. 4 A (for –70%: iRLS, –237.4 ± 29.2%; controls, –260.8 ± 34%; P = 0.0027, Holm–Bonferroni adjusted P = 0.011; for –100% iRLS, –354.5 ± 35%; controls, –385.3 ± 42.2%; P = 0.0014, adjusted P = 0.0072). The patients with iRLS had greater minimum I–V slope (iRLS, 0.27 ± 0.05; controls, 0.25 ± 0.04; P = 0.011, adjusted P = 0.034) (Fig. 4 C). TEh (90–100 ms) and TEh (slope 101–140 ms) were also significantly different (iRLS, –114.3 ± 15.2%; controls, –122.5 ± 18.7%; P = 0.0416, adjusted P = 0.0416; iRLS, 2.04 ± 0.34; controls, 2.28 ± 0.49; P = 0.018, adjusted P = 0.036) (Fig. 4 A and C). The resting I–V slope was not significantly different between iRLS and the control group (iRLS, 0.63 ± 0.1; controls, 0.65 ± 0.1; P = 0.75) (Figs 2 E and 4 C).
Mathematical modelling
The differences in the axonal excitability of the median nerve were modelled using the modified ‘Bostock’ model of a human motor axon. The only significant changes in axonal excitability appeared when the nerve was significantly hyperpolarized (Fig. 4 A–C: measured data, Fig 4 D–F: modelled data), indicative of a change in the hyperpolarization‐activated current, I h. All of the parameters associated with I h were examined (Fig 4 D–F) The most reasonable explanation is that HCN channels open with a lesser degree of hyperpolarization. Accordingly, the half‐activation potential for HCN channels (B q) was the single best parameter that accounted for the difference between normal controls and iRLS data. A 6 mV depolarization of B q reduced the discrepancy between the normal control model and the iRLS data by 92.1%.
Excitability of sensory axons in the median nerve
Fourteen patients with iRLS and 14 healthy controls were recruited. Based on the motor findings, these samples should have been adequately powered to detect identical changes in I h: indeed, samples of only five subjects would have a power of 81.7% to detect the change seen in motor axons at the 5% level in a one‐tailed t test (one‐tailed because the direction of change was known). However, because the properties of sensory and motor axons differ, larger samples were studied. There were no significant changes in the excitability of sensory axons in the median nerve (Fig. 5, round symbols).
Figure 5. No changes in axonal excitability in sensory axons of the median nerve in RLS.
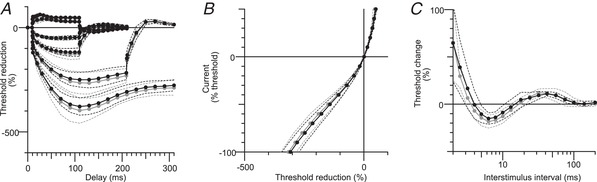
Extended excitability data. A, B and C, normal controls (n = 14; black filled circles) and RLS patients (n = 14; grey filled circles) for sensory axons (median nerve) (mean ± SD). A, TE for conditioning levels of +20%, –20%, +40%, –40%, –70% and –100% of control threshold. B, I–V relationship for 200 ms conditioning stimuli. C, recovery cycle.
Discussion
In the present study, we report data showing for the first time that the intrinsic biophysical properties of peripheral motor axons are altered in patients suffering from iRLS. Excitability studies in patients with a clinical diagnosis of iRLS not on medication have increased inward rectification in motor axons of the median nerve. Mathematical modelling supports this interpretation and suggests that the responsible mechanism is a depolarizing shift in the voltage dependence of HCN channels.
HCN activity: the ‘chicken and egg’ issue
HCN channels are known to be ‘plastic’. One major factor that regulates channel function is neuronal activity. The simplest explanation for the greater inward rectification is that it is driven by both ‘restless’ involuntary (in the context of periodic leg movements) and voluntary (in the context of the urge to move) motor activity. In many respects, this explanation complements that for patients who had suffered a stroke and in whom evidence for less HCN activity was reported (Jankelowitz et al. 2007). In addition, peripheral hypoxia, which is known to increase HCN current, has been demonstrated in RLS (Salminen et al. 2014), although this factor is probably not important for median motor axons remote from the ‘restless’ activity.
However, the evidence for greater inward rectification was found for median nerve axons, and none of the patients had relevant upper limb symptoms. This raises the possibility that greater HCN current may be a primary and generalized phenomenon, rather than secondary to focal heightened ‘restless’ contractions or peripheral hypoxia. In this regard, the threshold tracking technique has proven to be sufficiently sensitive to identify biophysical abnormalities in motor axons, even in asymptomatic motor nerves in various neurological diseases (Jankelowitz et al. 2007; Ng et al. 2008; Tomlinson et al. 2012). Moreover, a generalized disorder could also explain why the upper limbs are often symptomatic (in 21–57% of cases) (Allen et al. 2014) or become so when iRLS patients are started on high dosages of dopaminergic drugs (Allen & Earley, 1996), comprising a previously unexplained phenomenon. Further studies on iRLS patients during such treatment could clarify this issue.
In earlier studies on healthy subjects, we have demonstrated that, although the between‐subject variability of I h is high, within‐subject variability is quite low, even in test–retest situations (Tomlinson et al. 2010; Howells et al. 2013), and also that the between‐subject variability can be explained by differences in the voltage for half‐activation of HCN channels (Howells et al. 2012). Different subjects have different I h activity levels, presumably related to life‐style factors, to which I h is exquisitely sensitive (Pape, 1996; Robinson & Siegelbaum, 2003; Wahl‐Schott & Biel, 2009), and possibly genetic factors, which are prominent in idiopathic RLS (Rye, 2015; Winkelmann et al. 2007; Trenkwalder et al. 2016). In this respect, RLS patients may merely be at one end of a ‘normal’ spectrum of I h activity. It is relevant that plastic changes in the activity of I h occur largely through changes in channel gating (Biel et al. 2009; Wahl‐Schott & Biel, 2009), much as suggested by modelling in the present study and also prevously by Howells et al. (2012).
Functional consequences of greater HCN current
We presume that the greater I h on motor axons reflects greater I h in the parent motoneurons. HCN current is depolarizing and non‐inactivating and, accordingly, would increase the probability of motoneuron discharge. There was no significant change in resting membrane potential in motor axons, although the axonal change probably does not quantitatively replicates the change in the parent motoneurons. In addition, a depolarizing shift in membrane potential could have been readily offset by small changes in multiple properties, which are changes that cannot be identified in the modelling. The changes in the experimental data were not large, although this does not mean that the biological effect of these changes was small, particularly at the motoneuron level.
The kinetics of HCN channels are in the range of hundreds of milliseconds to seconds (Wahl‐Schott & Biel, 2009) and this is too slow for the channels to play a pacemaker role in the discharge of individual motoneurons. However, HCN channels might well be suited to modulate the bursting nature of the motoneurons in increased motor activity as a result of the urge to move. It is reasonable to postulate that, in the presence of increased I h, any input to the motoneuron, peripheral or descending (Fig. 6) would have a greater probability of facilitating motoneuron discharge, such that natural drives reaching the axon hillock might then trigger restless activity.
Figure 6. Restless legs syndrome is a network disorder.
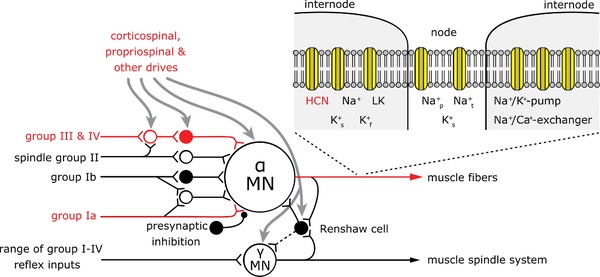
Motoneurons receive various central and peripheral inputs. It has already been shown that in RLS input to the motoneuron is increased via group IV, group I afferents and central efferents (red coloured input; Bara‐Jimenez et al. 2000; Rijsman et al. 2005; Lanza et al. 2017). Our data show for the first time that hyperexcitability in iRLS can also be found in peripheral motor axons. Na+ p: persistent sodium channel; Na+ t: transient sodium channel; K+ f: fast potassium channel; K+ s: slow potassium channel (Adapted from Gandevia, 2001).
RLS is a complex sensorimotor network disorder
Axonal excitability studies document excitability at the site of stimulation: the peripheral axon. Thus, our data show for the first time that hyperexcitability in iRLS can also be found in peripheral motor axons. It is therefore important that the findings using other neurophysiological techniques be (re)‐interpreted considering the whole network proposed for RLS, including the peripheral nervous system (Fig. 6).
Neurophysiological, neuroimaging and neuromodulatory studies indicate the increased excitability of a neuronal network encompassing cortical, subcortical and spinal areas (Lanza et al. 2017) in both idiopathic and secondary RLS (Trenkwalder et al. 2016). From a neurophysiological point of view, most techniques identify alterations in targeted structures; for example, cortical excitability in TMS studies (Magalhaes et al. 2015; Lanza et al. 2017) and spinal excitability in reflex studies (e.g. diminished inhibitory mechanisms: Rijsman et al. 2005; Scaglione et al. 2008; Marconi et al. 2012; as well as enhanced and state‐dependent flexor reflex: Bara‐Jiminez et al. 2000; Aksu & Bara‐Jiminez, 2002). QST (Bachmann et al. 2010) and cutaneous silent period studies (Isak et al. 2011) involve both peripheral nerve and spinal excitability. On their own, these studies do not control for a contribution from non‐investigated areas. For example, TMS studies need peripheral reflex studies to exclude additional spinal influences.
The excitability of spinal motoneurons depends on intrinsic factors and on the descending and peripheral influences acting upon them. The enhanced excitability seen in TMS and reflex studies could therefore have resulted from a primary change elsewhere in the neuraxis. However, the greater inward rectification in motor axons in the present study presumably reflects increased I h in motoneurons, implying a difference in the intrinsic properties of those motoneurons. We cannot exclude the fact that this change in motoneuron properties was an adaptation to a change in descending or peripheral inputs but, if this was the case, an ‘indelible mark’ would have been left on the motoneurons, altering their responsiveness.
Measurements on sensory axons did not show changes in excitability. This suggests that increased inward rectification in RLS is specific to motor axons and is not a generalized (patient‐specific) phenomenon. It should be noted that the threshold‐tracking techniques used in the present study document the biophysical properties only of large myelinated fibres, and not those of small myelinated and unmyelinated fibres, which are probably involved in RLS (Bachmann et al. 2010). Thus, future studies could be focussed on the biophysical properties of small fibres in RLS.
Clinical implications
The findings of the present study are similar to those of our previous study of benign cramp fasciculation syndrome (Czesnik et al. 2015). This is also a condition with increased muscle activity and it is tempting to suggest that similar mechanisms are responsible for the increased I h.
I h has also been implicated in neuropathic pain (Chaplan et al. 2003; Momin et al. 2008; Biel et al. 2009). Again there is an analogy to the situation with RLS in which patients complain of an urge to move the legs, usually associated with unpleasant leg sensations, often reported as pain or discomfort (Trenkwalder & Paulus, 2010). A common feature of all these conditions is hyperexcitability of the relevant neuronal networks. I h is a depolarizing current and greater I h in this network would contribute to or accentuate any hyperexcitability.
Currently, dopaminergic agents are recommended for RLS (Trenkwalder et al. 2015). The present findings suggest that modulators of I h might be beneficial when safe medications become freely available.
Taken together, we conclude that an increase in motoneuronal I h as suggested by the present axonal excitability studies plays an important role in fine‐tuning the final common path not only under physiological circumstances, but also in a variety of different clinical conditions.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
The experiments were performed in the Department of Clinical Neurophysiology, Medical School Göttingen.
DC, AW, CT, DB and WP were responsible for the conception and design of work. DC, MB, EV and OK were responsible for the acquisition of data. DC and JH were responsible for the analysis of data. DC, JH, DB and WP were responsible for the interpretation of data. DC, MB, EV, OK, DB and WP were responsible for drafting the paper. DC, JH, AW, CT, DB and WP were responsible for critically revising the paper for important intellectual content. All authors read and approved the final version of the manuscript submitted for publication.
Funding
The study was partly funded be the Germany Restless Legs Society.
Biography
Dirk Czesnik is a neurologist and currently works in the Department of Clinical Neurophysiology at the Medical School Göttingen, Germany. His current research focuses on in vivo studies in neuromuscular diseases using and developing advanced and high resolution neurophysiological in vivo techniques. Before he started his career in clinical neurophysiology, his research focused on molecular–neurophysiological studies in animals using both molecular biological and electrophysiological techniques. His experiences during that time helped to address the challenges in his future career. His aspirations are to build up a mechanism‐based neurophysiological understanding of neuromuscular symptoms in patients and to establish neurophysiological in vivo biomarkers for use in clinical studies.

Edited by: Janet Taylor & Gregory Funk
References
- Allen RP, Picchietti DL, Garcia‐Borreguero D, Ondo WG, Walters AS, Winkelmann JW, Zucconi M, Ferri R, Trenkwalder C & Lee HB (2014). Restless legs syndrome/Willis‐Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria‐history, rationale, description, and significance. Sleep Med 15, 860–873. [DOI] [PubMed] [Google Scholar]
- Allen RP & Earley CJ (1996). Augmentation of the restless legs syndrome with carbidopa/levodopa. Sleep 19, 205–213. [DOI] [PubMed] [Google Scholar]
- Aksu M & Bara‐Jimenez W (2002). State dependent excitability changes of spinal flexor reflex in patients with restless legs syndrome secondary to chronic renal failure. Sleep Med 3, 427–430. [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Rolke R, Scheidt U, Stadelmann C, Sommer M & Pavlakovic G (2010). Thermal hypoaesthesia differentiates secondary restless legs syndrome associated with small fibre neuropathy from primary restless legs syndrome. Brain 133, 762–770. [DOI] [PubMed] [Google Scholar]
- Bara‐Jimenez WM, Aksu B, Graham B, Sato S & Hallett M (2000). Periodic limb movements in sleep: state‐dependent excitability of the spinal flexor reflex. Neurology 54, 1609–1616. [DOI] [PubMed] [Google Scholar]
- Biel M, Wahl‐Schott C, Michalakis S & Zong X (2009). Hyperpolarization‐activated cation channels: from genes to function. Physiol Rev 89, 847–885. [DOI] [PubMed] [Google Scholar]
- Button DC, Kalmar JM, Gardiner K, Marqueste T, Zhong H, Roy RR, Edgerton VR & Gardiner PF (2008). Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J Physiol 586, 529–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland RA, Bostock H & Kiernan MC (2009). Plasticity of lower limb motor axons after cervical cord injury. Clin Neurophysiol 120, 204–209. [DOI] [PubMed] [Google Scholar]
- Bostock H (1983). The strength‐duration relationship for excitation of myelinated nerve: computed dependence on membrane parameters. J Physiol 341, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Baker M & Reid G (1991). Changes in excitability of human motor axons underlying post‐ischaemic fasciculations: evidence for two stable states. J Physiol 441, 537–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Cikurel K & Burke D (1998). Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve 21, 137–158. [DOI] [PubMed] [Google Scholar]
- Burke D, Kiernan MC & Bostock H (2001). Excitability of human axons. Clin Neurophysiol 112, 1575–1585. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Guo H‐Q, Lee DH, Luo L, Liu C, Kuei C, Velumian AA, Butler MP, Brown SM & Dubin AE (2003). Neuronal hyperpolarization‐activated pacemaker channels drive neuropathic pain. J Neurosci 23, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesnik D, Howells J, Negro F, Wagenknecht M, Hanner S, Farina D, Burke D & Paulus W (2015). Increased HCN channel driven inward rectification in benign cramp fasciculation syndrome. Brain 138, 3168–3179. [DOI] [PubMed] [Google Scholar]
- Gandevia SC (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 81, 1725–1789. [DOI] [PubMed] [Google Scholar]
- Gardiner P, Dai Y & Heckman CJ (2006). Effects of exercise training on alpha‐motoneurons. J Appl Physiol 101, 1228–1236. [DOI] [PubMed] [Google Scholar]
- Hellmann ES & Tschudy DP (1962). Restless legs syndrome in acute intermittent porphyria. Ann Intern Med 56, 487–489. [DOI] [PubMed] [Google Scholar]
- Holm S (1979). A simple sequentially rejective multiple test procedure. Scand J Stat 6, 65–70. [Google Scholar]
- Horn S, Quasthoff S, Grafe P, Bostock H, Renner R & Schrank B (1996). Abnormal axonal inward rectification in diabetic neuropathy. Muscle Nerve 19, 1268–1275. [DOI] [PubMed] [Google Scholar]
- Howells J, Trevillion L, Bostock H & Burke D (2012). The voltage dependence of I h in human myelinated axons. J Physiol 590, 1625–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells J, Czesnik D, Trevillion L & Burke D (2013). Excitability and the safety margin in human axons during hyperthermia. J Physiol 591, 3063–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H (2006). Spinal reflexes, mechanisms and concepts: from Eccles to Lundberg and beyond. Prog Neurobiol 78, 215–232. [DOI] [PubMed] [Google Scholar]
- Isak B, Uluc K, Salcini C, Agan K, Tanridag T & Us O (2011). A neurophysiological approach to the complex organisation of the spine: F‐wave duration and the cutaneous silent period in restless legs syndrome. Clin Neurophysiol 122, 383–390. [DOI] [PubMed] [Google Scholar]
- Jankelowitz SK, Howells J & Burke D (2007). Plasticity of inwardly rectifying conductances following a corticospinal lesion in human subjects. J Physiol 581, 927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC & Bostock H (2000). Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain 123, 2542–2551. [DOI] [PubMed] [Google Scholar]
- Krarup C & Moldovan M (2009). Nerve conduction and excitability studies in peripheral nerve disorders. Curr Opin Neurol 22, 460–466. [DOI] [PubMed] [Google Scholar]
- Lanza G, Bachmann C, Ghorayeb I, Yuping W, Ferri R & Paulus W (2017). Central and peripheral nervous system excitability in restless legs syndrome. sleep med 31, 49–60. [DOI] [PubMed] [Google Scholar]
- Lin CS‐Y, Krishnan AV, Lee MJ, Zagami AS, You HL, Yang CC, Bostock H & Kiernan MC (2008). Nerve function and dysfunction in acute intermittent porphyria. Brain 131, 2510–2519. [DOI] [PubMed] [Google Scholar]
- Magalhães SC, Kaelin‐Lang A, Sterr A, do Prado GF, Eckeli AL & Conforto AB (2015). Transcranial magnetic stimulation for evaluation of motor cortical excitability in restless legs syndrome/Willis‐Ekbom disease. Sleep Med 16, 1265–1273. [DOI] [PubMed] [Google Scholar]
- Marconi S, Scaglione C, Pizza F, Rizzo G, Plazzi G & Vetrugno R (2012). Group I nonreciprocal inhibition in restless legs syndrome secondary to chronic renal failure. Parkinsonism Relat Disord 18, 362–366. [DOI] [PubMed] [Google Scholar]
- Mogyoros I, Kiernan MC & Burke D (1996). Strength‐duration properties of human peripheral nerve. Brain 119, 439–447. [DOI] [PubMed] [Google Scholar]
- Momin A, Cadiou H, Mason A & McNaughton PA (2008). Role of the hyperpolarization‐activated current Ih in somatosensory neurons. J Physiol 586, 5911–5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K, Howells J, Pollard JD & Burke D (2008). Up‐regulation of slow K+ in peripheral motor axons: a transcriptional channelopathy in multiple sclerosis. Brain 131, 3062–3071. [DOI] [PubMed] [Google Scholar]
- Pape HC (1996). Queer current and pacemaker: the hyperpolarization‐activated cation current in neurons. Annu Rev Physiol 58, 299–327. [DOI] [PubMed] [Google Scholar]
- Rijsman RM, Stam CJ & de Weerd AW (2005). Abnormal H‐reflexes in periodic limb movement disorder; impact on understanding the pathophysiology of the disorder. Clin Neurophysiol 116, 204–210. [DOI] [PubMed] [Google Scholar]
- Robinson RB & Siegelbaum SA (2003). Hyperpolarization‐activated cation currents: from molecules to physiological function. Annu Rev Physiol 65, 453–480. [DOI] [PubMed] [Google Scholar]
- Rye DB (2015). The molecular genetics of restless legs syndrome. Sleep Med Clin 10, 227–233. [DOI] [PubMed] [Google Scholar]
- Salminen AV, Rimpilä V & Polo O (2014). Peripheral hypoxia in restless legs syndrome (Willis‐Ekbom disease). Neurology 82, 1856–1861. [DOI] [PubMed] [Google Scholar]
- Scaglione C, Vetrugno R, Plazzi G, Rizzo G, Provini F, Montagna P & Martinell P (2008). Group I nonreciprocal inhibition in primary restless legs syndrome. Mov Disord 23, 96–100. [DOI] [PubMed] [Google Scholar]
- Schlesinger I, Erikh I, Nassar M & Sprecher E (2015). Restless legs syndrome in stroke patients. Sleep Med 16, 1006–1010. [DOI] [PubMed] [Google Scholar]
- Tomlinson S, Burke D, Hanna M, Koltzenburg M & Bostock H (2010). In vivo assessment of HCN channel current (Ih) in human motor axons. Muscle Nerve 41, 247–256. [DOI] [PubMed] [Google Scholar]
- Tomlinson SE, Bostock H, Grinton B et al. (2012) In vivo loss of slow potassium channel activity in individuals with benign familial neonatal epilepsy in remission. Brain. 135, 3144–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenkwalder C, Allen R, Högl B, Paulus W & Winkelmann J (2016). Restless legs syndrome associated with major diseases: a systematic review and new concept. Neurology 86, 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenkwalder C & Paulus W (2010). Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol 6, 337–346. [DOI] [PubMed] [Google Scholar]
- Trenkwalder C, Winkelmann J, Inoue Y & Paulus W (2015). Restless legs syndrome – current therapies and management of augmentation. Nat Rev Neurol. 11, 434–445. [DOI] [PubMed] [Google Scholar]
- Wahl‐Schott C & Biel M (2009). HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 66, 470–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, Fulda S, Pütz B, Eckstein G, Hauk S, Trenkwalder C, Zimprich A, Stiasny‐Kolster K, Oertel W, Bachmann CG, Paulus W, Peglau I, Eisensehr I, Montplaisir J, Turecki G, Rouleau G, Gieger C, Illig T, Wichmann HE, Holsboer F, Müller‐Myhsok B & Meitinger T (2007). Genome‐wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet 39, 1000–1006. [DOI] [PubMed] [Google Scholar]
- Yokota T, Hirose K, Tanabe H & Tsukagoshi H (1991). Sleep‐related periodic leg movements (nocturnal myoclonus) due to spinal cord lesion. J Neurol Sci 104, 13–18. [DOI] [PubMed] [Google Scholar]
- Zobeiri M & Shokoohi A (2014). Restless leg syndrome in diabetics compared with normal controls. Sleep Disord 2014, 871751. [DOI] [PMC free article] [PubMed] [Google Scholar]


