Abstract
Traditional cancer therapies include surgery, radiation, and chemotherapy, all of which are typically non-specific approaches. Cancer immunotherapy is a type of cancer treatment that helps the immune system fight cancer. Cancer immunotherapy represents a standing example of precision medicine: immune checkpoint inhibitors precisely target the checkpoints; tumor infiltrating lymphocytes, TCR T cells, and CAR T cells precisely kill cancer cells through tumor antigen recognition; and cancer vaccines are made from patient-derived dendritic cells, tumor cell DNA, or RNA, or oncolytic viruses, thus offering a type of personalized medicine. This review will highlight up-to-date advancement in most, if not all, of the immunotherapy strategies.
Keywords: cancer, immune evasion, immunotherapy
Introduction
Recent FDA approval of immune checkpoint inhibitors and T lymphocytes expressing chimeric antigen receptors (CAR T) for cancer therapy signifies an unprecedented success in cancer immunotherapy. Cancer immunotherapy would not have reached such a milestone without advances in the area of cancer immunology. During tumor development, the immune system constantly engages with tumor cells, which undergo three phases: elimination, equilibrium, and escape.
In the elimination/immunosurveillance phase, effector immune cells, particularly effector T cells, are able to kill cancer cells on recognition of tumor antigens. In support, T cells specific for tumor antigens predicted by cutting edge tumor genome sequencing, have been detected in patients with melanoma.1 Patients with pre-existing anti-tumor immunity at diagnosis and patients with more tumor infiltrating T cells show longer survival.2 Moreover, it has been suggested that effector immune cells exist in premalignant lesions to counteract danger signals.2 In the equilibrium phase, effector immune cells are balanced by immune suppressive mechanisms (e.g. regulatory T cells (Treg)), which prevent progression of the premalignant lesion. In the escape phase, immune suppressive mechanisms outcompete effector immune cells, leading to cancer immune evasion and tumor formation.2,3
In this review, we outline mechanisms of cancer immune evasion and strategies of immunotherapy that are currently being used or explored to counteract cancer immune evasion. At the end of the review, we discuss future directions for cancer immunotherapy.
Mechanisms of cancer immune evasion
T cells need to recognize tumor antigens to kill tumor cells. Thus, one important mechanism of tumor immune evasion is that tumor cells downregulate their antigen processing/presentation machinery, such as the major histocompatibility complex (MHC) I, proteosome subunit latent membrane protein (LMP) 2 and LMP7, transporter associated with antigen processing (TAP) protein, and tapasin, preventing them from being recognized by T cells (Fig. 1).3 It is well known that IFN-induced signaling promotes antigen presentation in tumor cells,4–7 and, recently, IFN signaling has been shown to be positively regulated by Aplnr and negatively regulated by Ptpn2 and CDK4/6.4,5,7 Therefore, downregulation of IFN signaling/Aplnr or upregulation of Ptpn2/CDK4/6 may dampen antigen presentation and thus contribute to tumor immune evasion.4–7
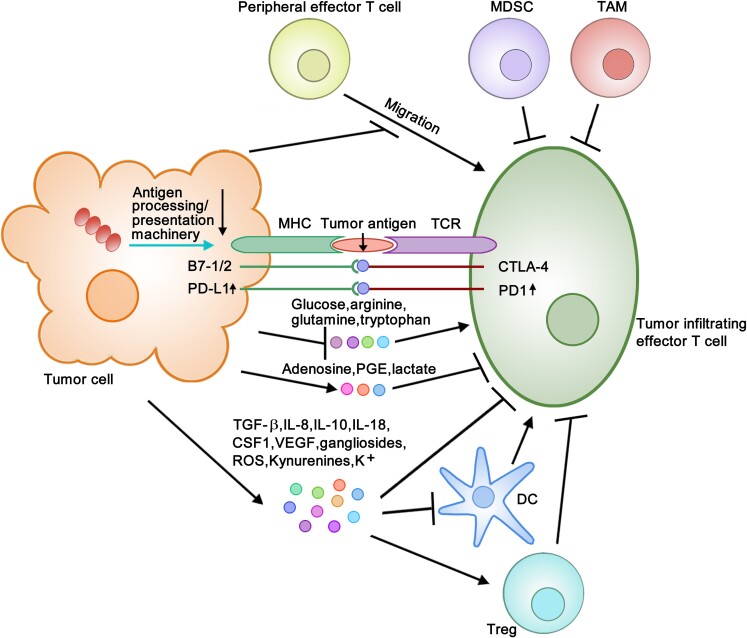
Figure 1.
Diagram of mechanisms of tumor immune evasion. Tumor cells evade immune responses through a variety of mechanisms: (i) downregulation of antigen processing and presentation machinery and thus antigen presentation (see ↓), (ii) upregulation of PD-L1 on tumor cells and PD-1 on effector T cells (see ↑) and facilitation of binding of PD-L1 and B7-1/2 to PD-1 and CTLA-4, respectively, (iii) secretion of immune suppressive modulators (TGF-β, IL-8, IL-10, IL-18, CSF1, VEGF, gangliosides, ROS, Kynurenines, K+) and metabolites (adenosine, PGE, lactate) into TME, (iv) deprivation of immune activating metabolites (glucose, arginine, glutamine, tryptophan) from TME, (v) recruitment and/or activation of Treg, MDSC and TAM, and (vi) inhibition of effector T cell infiltration.
Cancer cells can directly cause T cell exhaustion by facilitating their PD-L1 and B7-1/2 binding to immune checkpoint proteins PD-1 and CTLA-4, respectively, on T cells.3,8 Moreover, cancer cells may cause T cell exhaustion by increasing PD-L1 and PD-1 expression (Fig. 1).3,8,9 As PD-L1 expression is positively regulated by inflammatory signaling (e.g. IFN-γ, LPS, TNF-α), oncogenic signaling (e.g. Myc, Cdk5, Ras), and CMTM6- and CDK4-mediated post-translational stabilization,10 upregulation of these signals could contribute to T cell exhaustion. Because deficiency in kelch-like ECH-associated protein 1 (KEAP1) and PTEN in lung adenocarcinoma has been shown to promote PD-L1 expression,11 T cell exhaustion may be attributable to downregulation of KEAP/PTEN.
Another mechanism of cancer immune evasion is attributed to secretion of immune suppressive modulators (e.g. TGF-β, IL-8, IL-10, IL-18, CSF1, VEGF, gangliosides, ROS, Kynurenines, K+) into the tumor microenvironment (TME) by tumor cells. These immune suppressive modulators can dampen T cell function and/or dendritic cell (DC) maturation, leading to defective cross-presentation of tumor antigens to T cells.3,8,12 Tumor cells may dampen T cell function by altering metabolic composition in the TME. For example, tumor cells decrease oxygen, pH, glucose, and amino acids (e.g. glutamine, tryptophan, arginine) which promote T cell function, and increase adenosine, prostaglandin E (PGE), and lactate which inhibit T cell function.13–17 It is known that tumor cells harboring B-RAF mutations show enhanced glycolysis, leading to decreased glucose availability in TME.18 Moreover, immune suppressive cell populations in TME constitute a critical mechanism of T cell dysfunction.3 These cells include Treg that can be activated by suppressive modulators (e.g. TGF-β, IL-10, gangliosides) and metabolites (e.g. PGE), myeloid-derived suppressor cells (MDSC), and tumor-associated macrophages (TAM).3 Last but not the least, tumor cells may repress T cell recruitment to tumor sites (Fig. 1).19,20
Cancer immunotherapy strategies
Numerous cancer immunotherapy strategies are under investigation. A few have been approved for cancer treatment. For the ease of review, we classify these as molecular therapy, cellular therapy, and vaccination therapy.
Molecular therapy
Cytokine IL-2 functions to promote T cell growth. IL-2 has been approved by the FDA for treatment of metastatic renal cell carcinoma (RCC) and metastatic melanoma.21 IL-2 combined with EGFR TKI gefitinib has shown improved outcome in patients with advanced non-small-cell lung cancer (NSCLC).22 IL-2 is used to expand autologous tumor infiltrating lymphocytes (TIL), lymphocytes expressing transgenic TCRs (TCR T), and CAR T in vitro, and to support their growth and survival after adoptive transfer into cancer patients.21 Nonetheless, caution must be taken with use of IL-2, because it can also promote Treg proliferation and yield severe toxicities, including vascular leak syndrome (VLS), pulmonary edema, hypotension, and heart toxicities (Table 1).23
Table 1.
Advantages and disadvantages of cancer immunotherapy strategies.
| Strategy | Pros | Cons | ||
|---|---|---|---|---|
| Molecular therapy | IL-2 | Beneficial to RCC and melanoma patients; supports proliferation and survival of TIL, TCR T, and CAR T | Promotes Treg proliferation; yields severe toxicities | |
| Immune checkpoint inhibitors | Beneficial to patients with melanoma, lung cancer, kidney cancer, bladder cancer, head and neck cancer, Hodgkin lymphoma, bladder cancer, Merkel-cell carcinoma, and/or urothelial carcinoma | Primary or acquired resistance; severe side effects | ||
| Agonists of co-stimulatory receptors; inhibitors of immunosuppressive factors; agonists of T cell metabolism | Show efficacy in preclinical studies | Clinical benefits remain unclear | ||
| Cellular therapy | Adoptive T cell therapy |
|
|
|
| Depleting Treg (denileukin diftitox, anti-CD25) | Beneficial to cutaneous T cell lymphoma patients | Difficult to purify denileukin diftitox; clinical benefit is modest; may deplete effector T cells; adverse effect (e.g. vision loss); may cause autoimmunity | ||
| Inhibition of Treg function, trafficking, differentiation from naïve T cells; reprogramming Treg to effector T | Presumably safer than deletion of Treg; show promise in preclinical studies | Limited efficacy and poor specificity; some strategies (e.g. reprogramming Treg) are at conceptual stage | ||
| Vaccination | Vaccines (prevention) | HPV vaccine is beneficial for prevention of cervical, vaginal, vulvar, and anal cancer; HBV vaccine is beneficial for prevention of liver cancer | Side effects: bruising and itching; HPV vaccine does not prevent all HPV-related cancers | |
| Vaccines (therapy) | Tumor cell peptide, DNA and RNA | Show promise in preclinical studies; DNA vaccines are safe and stable | Efficacies of DNA vaccines are restricted by immune tolerance machinery; clinical benefits remain unclear | |
| Oncolytic viruses | HSV vaccine (T-VEC) is beneficial to melanoma patients | Side effects: fatigue, chills, pyrexia, nausea | ||
| DC | Sipuleucel-T is beneficial to prostate cancer patients | Side effects; Sipuleucel-T does not improve progression free survival | ||
TIL become exhausted because of upregulation of a number of immune checkpoint proteins such as PD-1, CTLA-4, Tim-3, and LAG-3, and because of the interaction between PD-1 and tumoral PD-L1 and between CTLA-4 and tumoral B7-1/2.3,8,24 The FDA-approved anti-CTLA-4 (ipilimumab), anti-PD-1 (nivolumab and pembrolizumab), and anti-PD-L1 (atezolizumab, avelumab, and durvalumab) block the inhibitory function and the interaction of these checkpoint proteins to reactivate T cells. Ipilimumab, nivolumab, and pembrolizumab were approved for treating melanoma. Nivolumab and pembrolizumab were also approved for treating lung cancer. In addition, nivolumab can be prescribed for kidney cancer, bladder cancer, head and neck cancer, and Hodgkin’s lymphoma. On the other hand, atezolizumab was approved for treating bladder cancer, avelumab for Merkel-cell carcinoma, and durvalumab for urothelial carcinoma.25,26 However, these clinically approved immune checkpoint inhibitors demonstrate clinical efficacy in only a small proportion of cancer patients. The majority of cancer patients either show primary resistance or develop acquired resistance (Table 1). New checkpoint blockers are thus being tested. For example, in a mouse model of breast cancer, anti-Tim3 was recently shown to stimulate CXCL9 expression in CD103+ DC, which may facilitate DC interaction with T cells to promote T cell effector function.27 Anti-LAG-3 may also boost T cell responses through its effect on DC.28
Co-stimulatory receptors 4-1BB, OX40, and GITR on T cells play a role in T cell activation. Agonistic anti-4-1BB, anti-OX40, and anti-GITR are being tested for their anti-tumor activities. It has been shown that anti-4-1BB stimulates T cells, whereas anti-OX40 and anti-GITR not only stimulate T cells but also inhibit Treg.29–31 Co-stimulatory molecule CD40 on DC is involved in DC activation. Agonistic anti-CD40 has shown anti-tumor effect through its activation of DC and T cell priming.32
Tumor cells secret a number of immune suppressive factors (e.g. TGF-β, PGE2, IDO, arginase, adenosine) into TME.3 Targeting these factors may reactivate T cells for anti-tumor immunity. Indeed, suppression of TGF-β or PGE2 production has been shown to restore T cell responses in head and neck squamous cell carcinoma treated with EGFR inhibitor.33 IDO inhibitors are being tested in clinical trials in patients with pancreatic cancer or other cancers.34,35 An arginase inhibitor, CB-1158, is being studied in a phase I clinical trial in patients with advanced solid tumors,35 and targeting of adenosine pathways or adenosine receptors shows therapeutic effects in preclinical studies.36
Defects in mitochondrial metabolism compromise TIL function.22 Thus, targeting metabolic pathways in T cells may benefit cancer patients. A recent study showed that a PPAR-α agonist promotes fatty acid catabolism in CD8+ T cells and enhances CD8+ T cell response against tumors.37 T cell metabolism has been shown to be regulated by immune checkpoint proteins. For example, CTLA-4 negatively regulates the PI3K/Akt/mTOR/c-Myc/Hif1α pathway- and co-receptor CD28-mediated glucose uptake and/or glycolysis; and PD-1 inhibits mTOR and PGC-1α metabolic pathways.22,38 Thus, blockade of CTLA-4 or PD-1 on CD8+ TIL cells increases their glucose uptake and reinvigorates their function.38 On the other hand, blockade of PD-L1 on tumor cells dampens glucose uptake and glycolysis of tumor cells, leading to increased glucose availability in TME that also promotes T cell function.39
Cellular therapy
Adoptive T cell therapy is a type of therapy involving in vitro expansion of patient-derived tumor antigen-specific T cells and their re-infusion into patients. Adoptive transfer of TIL has shown promise in patients with metastatic melanoma in a variety of clinical trials. However, because TIL are extremely limited in numbers, generating sufficient TIL cells for adoptive T cell therapy can be complicated and time-consuming.40,41 Adoptive transfer of TIL has not shown benefit in other cancers (Table 1). In this context, peripheral blood T cells may be a better choice. Indeed, blood T cells are used to generate genetically engineered T cells, namely TCR T and CAR T that express transgenic TCRs and CARs, respectively.
TCR T cells that target melanocyte differentiation antigen MART-1 show durable responses in metastatic melanoma. Nonetheless, on target, off-tumor toxicity is apparent in normal melanocytes in the skin, eye, and cochlea. TCR T cells that target cancer-testis antigen MAGE-A3 also cause fatal neurotoxicity and cardiotoxicity. On the other hand, TCR T cells that target cancer-testis antigen NY-ESO-1 display clinical efficacy without obvious toxicities in a phase I/II trial in multiple myeloma patients (Table 1).42 TCR T cells that are directed to neoantigens, a type of tumor antigens only expressed in tumor cells but not normal cells, are presumably safer than those targeting tumor antigens shared by normal tissues. However, such TCR T cells have not been tested in clinic.
CAR T cells express CARs that contain the signaling domains of TCR ζ chain, CD28, OX40, and/or 4-1BB, and a single-chain variable fragment (scFv) that is derived from the variable domains (antigen binding domains) of antibody heavy and light chains. CAR T cells target surface proteins or glycan on tumor cells. There are more than 250 clinical trials on CAR T cells. Among them, CAR T cells that target CD19 of B cell markers (CD19 CAR T) have been approved by the FDA to treat pre-B cell acute lymphoblastic leukemia and diffuse large B cell lymphoma.40,41 Other CAR T cells, such as HER2 CAR T, CAIX CAR T, CEACAM5 CAR T, mesothelin CAR T, and GD2 ganglioside CAR T, have either limited clinical efficacy or unacceptable toxicities (e.g. severe cytokine release syndrome, neurologic complications).40,41 Other drawbacks of CAR T cells include difficulties in penetrating solid tumors, outrageously high cost, and lengthened production (Table 1). Strategies to conquer these pitfalls have been extensively discussed elsewhere.43,44
Treg cells constitute a critical suppressive cell population in TME. Treg depletion may thus benefit cancer patients. Denileukin diftitox (trade name: Ontak) is a fusion protein combining IL-2 and diphtheria toxin. On binding to IL-2 receptor CD25 that is enriched on Treg, denileukin diftitox releases diphtheria toxin into Treg, leading to Treg depletion.45 Denileukin diftitox was approved by the FDA for treating cutaneous T cell lymphoma; however, it was discontinued in 2014 because of production issues.46 Other strategies to deplete Treg include anti-CD25 antibody-based therapies and chemotherapies (e.g. cyclophosphamide, fludarabine).47,48
As indiscriminate removal of Treg may cause autoimmune diseases (Table 1), it may be more advantageous to inhibit Treg function using antibodies against Treg functional markers, such as CTLA-4 and GITR, and/or to block Treg trafficking to tumor sites using methyl gallate or AMD3100.47,48 Given that Treg can be differentiated from naïve T cells or converted from effector T cells, blockade of these processes (e.g. using antibody against TGF-β) may evoke anti-tumor immune responses.47 In addition, a concept of reprogramming Treg to effector T cells is emerging (Table 1).47
Finally, recent transcriptome profiling has revealed that intratumoral Treg express a tumor-specific gene signature (e.g. MAGEH1, IL1R2, TFRC, FCRL3),49 indicating that targeting of these molecules would not affect Treg outside of tumors and thus its side effects would be limited. Complementary to Treg modulation, MDSC, macrophage, and type II NK cells in TME may be targeted to lift their suppression of anti-tumor immunity.3,50 In this context, antagonistic anti-IL-18 reduces MDSC, leading to attenuation of multiple myeloma progression,51 and antibodies against MICA on tumor cells increase NK cell-mediated tumor killing.52
Vaccination therapy
Another type of cancer immunotherapy is vaccination therapy, with a few vaccines aimed at preventing occurrence of cancer. Because cervical, vaginal, vulvar, and anal cancers are caused by human papillomavirus (HPV) infection and liver cancer is caused by hepatitis B virus (HBV) infection, the FDA has approved HPV vaccine to prevent healthy people from developing cervical, vaginal, vulvar, and/or anal cancers, and HBV vaccine for prevention of liver cancer.53
Most cancer vaccines, however, are therapeutic vaccines, including peptide vaccines derived from tumor antigens and vaccines derived from cancer cell DNA or RNA.54,55 Oncolytic virus vaccines represent standing examples of therapeutic vaccines. Oncolytic viruses (e.g. Epstein–Barr virus (EBV), Merkel cell polyomavirus (MCPyV), human T-lymphotropic virus 1 (HTLV-1), herpes virus (HSV)) selectively infect tumor cells and cause immunogenic cell death (ICD), leading to a damage-associated molecular pattern (DAMP) response that increases calreticulin, extracellular ATP, HMGB1, and ANXA1, and subsequent DC activation and cross-presentation of tumor antigens to T cells. Oncolytic viruses can also boost anti-tumor T cell immunity through pathogen-associated molecular pattern (PAMP)-mediated phagocytosis of cancer cells by DC.56 T-VEC, a HSV vaccine, has been approved by the FDA to treat melanoma patients. Autologous DC pulsed with tumoral peptides/proteins/DNA/mRNA, whole tumor cell lysate, or with intact tumor cells are well-known therapeutic vaccines.57,58 Currently, there are more than 200 DC vaccines in clinical trials.58 Sipuleucel-T, a DC-contained antigen-presenting cell (APC) vaccine derived from patient blood and pulsed with a fusion protein of prostatic acid phosphatase (PAP) and GM-CSF, has been approved by the FDA to treat prostate cancer patients.59 Finally, human and murine induced pluripotent stem cells (iPSC) expressing tumor-associated antigens may be used as therapeutic vaccines, because they can induce an antigen-specific anti-tumor T cell response.60
The most significant development in cancer vaccine studies is to include agonists of various innate immune receptors, particularly TLR, to vaccine formulations. These agonists include TLR-3 agonist poly I:C, TLR-4 agonist monophosphoryl lipid A, TLR-5 agonist flagellin, TLR-7 agonist imiquimod, and TLR-9 agonist CpG.61 A major challenge in cancer vaccine development is how to generate strong and long-lasting anti-tumor immunity, which may be achieved by optimal delivery of well-chosen tumor-associated antigens. The advantages and disadvantages of cancer vaccination are exemplified in Table 1. Readers are referred to a recent publication for further information.60
Immunotherapy drug resistance and combination therapy
Among all of the immunotherapy strategies aforementioned, immune checkpoint inhibitors have received the most attentions. As monotherapies, these inhibitors have shown long-term benefits in 40% of melanoma patients, 80% of Hodgkin’s lymphoma patients, and 50% of Merkel cell carcinoma patients.62 However, immune checkpoint inhibitors, as well as CAR T cells and other immunotherapies that are being used in clinics, do not benefit the majority of cancer patients. In addition, many patients who initially benefit from these therapies develop acquired resistance. The mechanisms that underlie primary resistance include: (i) low antigenic mutations in tumor cells, (ii) aberrant expression of genes in tumor cells that promote mesenchymal transition, cell adhesion, extracellular matrix remodeling, angiogenesis, wound healing, and immunosuppression (e.g. IL-10),63 and (iii) downregulation of IFN-α receptor 1 in CD8+ T cells.64 The mechanisms that underlie acquired resistance include: (i) loss of target antigen expression in tumor cells (in the case of adoptive T cell transfer),65 (ii) increased expression of immune checkpoint ligands on tumor cells, and (iii) accumulation of immunosuppressive cells such as Treg in TME.38 Furthermore, primary and acquired resistance may be explained by some common mechanisms, for instance, tumor cell-intrinsic defects in the antigen presentation machinery and in IFN-γ/IFN-γ receptor signaling pathway and T cell-intrinsic expression pattern of immune checkpoint proteins.6,66 For the latter, primary resistance may be result from T cell dysfunction mediated by multiple checkpoint proteins (e.g. PD-1, CTLA-4, Lag-3, Tim-3), and thus is not reversible by sole blockade of the PD-1/PD-L1 axis or CTLA-4, whereas acquired resistance may result from T cell reinvigoration-induced IFN-γ increases in tumor MHC expression/antigen presentation and thus enhanced TCR signaling, leading to upregulation of alternative immune checkpoints (e.g. Tim-3, Lag-3, TIGIT) on T cells.38
A major approach to overcome immunotherapy drug resistance is combination therapy.65 There are more than 1100 clinical trials on combination therapy that combine an immune checkpoint inhibitor with: (i) chemotherapy, radiation, molecularly targeted therapy (e.g. HDAC inhibitor, CDK4/6 inhibitor), or metabolic therapy (e.g. MCT1 inhibitor, agonistic anti-4-1BB) that may not only shrink tumor mass but also induce ICD, leading to DAMP response-mediated DC activation and subsequent tumor antigen cross-presentation, (ii) a pattern recognition receptor (PRR) agonist (e.g. STING agonist), (iii) co-stimulatory signals on APC (e.g. anti-CD40), and (iv) adoptive T cell transfer (CAR T or TCR T), oncolytic viruses, or vaccines that target neoantigens. Dual checkpoint blockade (e.g. anti-CTLA-4 + anti-PD-1, anti-CTLA-4 + anti-Tim-3) is also being tested.67–69 The FDA has approved the combination of anti-PD-1 (nivolumab) with anti-CTLA-4 (ipilimumab) for treating metastatic melanoma, and the combination of anti-PD-1 (pembrolizumab) with chemotherapy drugs pemetrexed and carboplatin to treat metastatic NSCLC.
In addition to combination therapies that involve immune checkpoint inhibitor(s), combined agonistic anti-CD40 and CSF-1R inhibitor have recently been shown to suppress melanoma growth through simultaneous targeting of DC and tumor-associated macrophages (TAM).70 As T cells in TME usually lose metabolic competitiveness (e.g. decreased glucose uptake and glycolysis) and supplementation of pyruvate, the final product of glycolysis, can increase TIL activation and function in clear cell RCC,71 an agent that increases metabolic competitiveness of T cells combined with cancer vaccines or adoptive T cell transfer may achieve satisfactory efficacy.24
Prediction of responsiveness of cancer patients to immunotherapy
The efficacies of current immunotherapy drugs vary considerably from patient to patient, highlighting the importance of identifying personalized biomarkers to predict who is likely to respond to a specific therapy. A number of biomarkers have been identified: (i) High mutational loads correlate with good drug response. For example, melanoma, NSCLC, bladder cancer, gastric cancer, and squamous cell carcinoma of the head and neck (SCCHN) that bear high mutational loads have high response rate to anti-PD1, whereas pancreatic and prostate cancer that harbor low mutational loads show poor response to anti-PD1.71 However, there is at least one exception: RCC has a modest number of mutations but is reasonably sensitive to checkpoint inhibitors.72 (ii) Tumors showing high levels of PD-1 and/or PD-L1 are generally sensitive to anti-PD-1/PD-L1 treatment.10 However, PD-1+ T cells in tumors can be so exhausted that they cannot be rescued by PD-1 blockade. These highly exhausted cells could be CD38hi CD101hi cells.73 By the same token, PD-L1+ cancer cells may make T cells too exhausted to be effectively treated with PD-L1 blockade. The irreversibility of these exhausted T cells may be related to other inhibitory mechanisms (e.g. other checkpoint proteins). Thus, improved understanding of other inhibitory mechanisms in individual patients will be helpful for better prediction of patient responses to PD-1/PD-L1 blockade. (iii) Tumors bearing high DNA mismatch repair (MMR) deficiency are likely to be amendable by PD-1/PD-L1 blockade. MMR deficiency can be identified by loss of one or more of the MMR proteins (e.g. MSH2, MSH3, MSH6, MLH1, PMS1, PMS2) or by emergence of MMR deficiency-induced microsatellite instability (MSI) that is manifested by single-nucleotide variants (SNVs) and/or insertion-deletion (indel) mutations.74–76 The FDA has recently approved that any cancers with high MSI can be treated with anti-PD-1 (pembrolizumab). (iv) Expression of IFN-γ-response genes in tumors is predictive of good drug response.10 In agreement, high density of IFN-γ-secreting CD8+ T cells or presence of IFN-γ-secreting clonally expanded T cells at the invasive edge of tumors correlates with positive response.10,72 (v) Emergence of tertiary lymphoid structures (TLS) that comprise a T cell zone harboring T cells and DC and a follicular zone containing B cells is often associated with a favorable prognosis. This is because TLS are often correlated with high overall T cell infiltration.77,78 (vi) Presence of oncolytic virus (e.g. EBV, MCPyV, HTLV-1, HSV) could serve as a positive biomarker for immunotherapy drug response.72 Another biomarker may be the gut microbiome. In this aspect, it was found that microbiome high in bifidobacter species increased tumor response to anti-PD-L1 therapy and microbiome high in bacteroides species increased response to anti-CTLA-4 therapy.72
Understanding the relationship between tumors and their immune landscape may facilitate prediction of patient response to immunotherapy. In support, it has been reported that triple negative (ER/PR/HER2-) breast tumors contain more CD8+ T cells than HER2+ breast tumors, suggesting that ER/PR/HER2- breast cancer patients, when treated with immunotherapy drugs, would show better disease-free survival.79 Loss of LATS1/2 of hippo tumor suppressor pathway in melanoma cells is associated with CD8+ T cell infiltration,80 whereas amplification of oncogenic MYC, NOTCH2, and FGFR1 in pancreatic ductal adenocarcinoma (PDAC) correlates with low CD8+ T function and immune checkpoint molecules,81 suggesting that immunotherapy could be considered for melanoma with defective expression of LATS1/2, but not for PDAC with MYC, NOTCH2, and FGFR1 amplification. Furthermore, recently published Pan-Cancer Atlas data that were collected from 10 000 tumors and 33 diverse cancer types have globally revealed the immune signatures of different tumor types and thus may serve as an excellent resource for prediction of immunotherapy response. According to the data, cancers may be classified into six immuno-subtypes: wound healing (C1), IFN-γ dominant (C2), inflammatory (C3), lymphocyte depleted (C4), immunologically quiet (C5), and TGF-β dominant (C6). C1, including colorectal cancer, lung squamous cell carcinoma, breast invasive carcinoma (BRCA) luminal A, SCCHN classical, and the chromosomally unstable gastrointestinal subtype that bear high frequency of mutation in driver gene TP53, PIK3CA, PTEN, or KRAS, shows increased expression of angiogenic genes, high proliferation rate, and elevated T helper 2 (Th2) cells. C2, including BRCA, gastric, ovarian, SCCHN, and cervical tumors that bear frequent mutation in driver genes detected in C1 and in HLA-A/B and CASP8, shows high proliferation rate, enriched M1/M2 macrophage, elevated CD8 signal, and increased TCR diversity. C3, including most RCC, prostate adenocarcinoma, PDAC, and papillary thyroid carcinomas that bear mutation in B-RAF, CDH1, or PBRM1, or low levels of aneuploidy and somatic copy number alterations, shows low to moderate proliferation and enriched Th17 and Th1 genes. C4, including adrenocortical carcinoma, pheochromocytoma, paraganglioma, liver hepatocellular carcinoma, and gliomas that exhibit CTNNB1, EGFR, or IDH1 mutation, shows dominant macrophage signature and elevated M2 macrophage response with Th1 suppressed. C5, consisting mostly of brain lower grade gliomas that exhibit IDH1, ATRX, or CIC mutation, or low levels of aneuploidy and somatic copy number alterations, shows enriched M2 macrophages and low lymphocyte. C6, consisting of mixed tumors that are enriched in KRAS G12 mutation, shows an increase in TGF-β signature, lymphocyte infiltration with comparable frequency of Th1 and Th2 cells, and TCR diversity. Overall, the data suggest that C3 has the most favorable outcome, C1 and C2 have less prognostic index than C3, whereas C4 and C6 have the poorest outcome.82
Conclusion and future directions
Cancer immunotherapy emerges as a cancer therapy with precision. Because it is a targeted and/or personalized therapy, cancer immunotherapy is presumably safer than traditional surgery, chemotherapy, and radiotherapy. Relentless efforts have led to FDA approval of a number of immunotherapy drugs, notably immune checkpoint inhibitors anti-PD-1/PD-L1/CTLA-4 and CD19 CAR T, for a variety of cancer types. However, primary or acquired resistance of the majority of cancer patients to the clinically approved immunotherapy agents highlights the need for new immunotherapy strategies.
Researchers are now exploring targeting of Treg, MDSC, TAM, and NK cells. Noting that gut microbiome affects cancer immunotherapy,83,84 manipulation of microbiota may be considered to improve immunotherapy efficacies. Some chemotherapy drugs such as anthracyclines, oxaliplatin, doxorubicin, and cyclophosphamide can induce ICD, leading to anti-cancer immune responses. Other small molecules (e.g. CDK4/6 inhibitor, B-RAF inhibitor) increase tumor antigen and/or MHC expression.5,85 These small molecules as immunotherapy drugs are supposedly more advantageous than biologics (e.g. antibodies, CAR T), because they can (i) target intracellular pathways, (ii) induce acute anti-tumor effects and avoid systemic immunogenicity, thereby improving therapeutic index, (iii) easily penetrate solid tumors, (iv) be orally administered allowing flexible dosing, and (v) be cost-effective.86,87
On the one hand, identification of biomarkers for prediction of patient response to immunotherapy drugs is essential. Moreover, it is equally important to develop appropriate experimental models to test drug responsiveness. In this aspect, a number of mouse models have been established;44 however, each of them have drawbacks.44 That being said, patient-derived xenografts (PDX) with tumor tissues orthotopically implanted or patient-derived organoids co-cultured with lymphocytes may be a better option.88
Finally, recently published Pan-Cancer Atlas data could not only advise mutational loads and neoantigen abundance of a given tumor type that are positively correlated with patient response to immune checkpoint inhibitors, but also predict driver mutations that may be used to develop personalized immunotherapies such as vaccines, tumor-specific T cells, and CAR T cells.89
Acknowledgments
This work was supported in part by National Institutes of Health (Grant No. R01GM108661) and the National Natural Science Foundation of China (Grant No. 81572850).
Conflict of interest statement
None declared.
References
- 1. Gros A, Parkhurst MR, Tran E, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 2016;22:433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finn OJ. A Believer’s Overview of Cancer Immunosurveillance and Immunotherapy. J Immunol 2018;200:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015;35:S185–98. [DOI] [PubMed] [Google Scholar]
- 4. Manguso RT, Pope HW, Zimmer MD, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 2017;547:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548:471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gao J, Shi LZ, Zhao H, et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016;167:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel SJ, Sanjana NE, Kishton RJ, et al. Identification of essential genes for cancer immunotherapy. Nature 2017;548:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med 2012;209:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Y, Liang X, Dong W, et al. Tumor-Repopulating Cells Induce PD-1 Expression in CD8+ T Cells by Transferring Kynurenine and AhR Activation. Cancer Cell 2018;33:480–94. [DOI] [PubMed] [Google Scholar]
- 10. Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 checkpoint. Immunity 2018;48:434–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Best SA, De Souza DP, Kersbergen A, et al. Synergy between the KEAP1/NRF2 and PI3K Pathways Drives Non-Small-Cell Lung Cancer with an Altered Immune Microenvironment. Cell Metab 2018;27:935–43. [DOI] [PubMed] [Google Scholar]
- 12. Eil R, Vodnala SK, Clever D, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016;537:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato Y, Ozawa S, Miyamoto C, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int 2013;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, et al. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 2017;14:11–31. [DOI] [PubMed] [Google Scholar]
- 15. Scharping NE, Delgoffe GM. Tumor Microenvironment Metabolism: A New Checkpoint for Anti-Tumor Immunity. Vaccines (Basel) 2016;4:pii:E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011;11:393–410. [DOI] [PubMed] [Google Scholar]
- 17. Speiser DE, Ho PC, Verdeil G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol 2016;16:599–611. [DOI] [PubMed] [Google Scholar]
- 18. Hall A, Meyle KD, Lange MK, et al. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E) BRAF oncogene. Oncotarget 2013;4:584–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng D, Kryczek I, Nagarsheth N, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015;527:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol 2017;17:559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenberg SA. IL-2: The First Effective Immunotherapy for Human Cancer. J Immunol 2014;192:5451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bersanelli M, Buti S, Camisa R, et al. Gefitinib plus interleukin-2 in advanced non-small cell lung cancer patients previously treated with chemotherapy. Cancers (Basel) 2014;6:2035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016;5:e1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sugiura A, Rathmell JC. Metabolic Barriers to T Cell Function in Tumors. J Immunol 2018;200:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. LaFleur MW, Muroyama Y, Drake CG, et al. Inhibitors of the PD-1 Pathway in Tumor Therapy. J Immunol 2018;200:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Mingo Pulido Á, Gardner A, Hiebler S, et al. TIM-3 Regulates CD103+ Dendritic Cell Function and Response to Chemotherapy in Breast Cancer. Cancer Cell 2018;33:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lichtenegger FS, Rothe M, Schnorfeil FM, et al. Targeting LAG-3 and PD-1 to Enhance T Cell Activation by Antigen-Presenting Cells. Front Immunol 2018;9:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin GH, Liu YAmbagala T, et al. Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PLoS One 2010;5:e11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang D, Armstrong AA, Tam SH, et al. Functional optimization of agonistic antibodies to OX40 receptor with novel Fc mutations to promote antibody multimerization. MAbs 2017;9:1129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aspeslagh S, Postel-Vinay S, Rusakiewicz S, et al. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer 2016;52:50–66. [DOI] [PubMed] [Google Scholar]
- 32. Vonderheide RH. The Immune Revolution: A Case for Priming, Not Checkpoint. Cancer Cell 2018;33:563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumai T, Oikawa K, Aoki N, et al. Tumor-derived TGF-β and prostaglandin E2 attenuate anti-tumor immune responses in head and neck squamous cell carcinoma treated with EGFR inhibitor. J Transl Med 2014;12:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bilir C, Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): Only an enzyme or a checkpoint controller? J Oncol Sci 2017;3:52–6. [Google Scholar]
- 35. Marin-Acevedo JA, Soyano AE, Dholaria B, et al. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol 2018;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Whiteside TL. Targeting adenosine in cancer immunotherapy: a review of recent progress. Expert Rev Anticancer Ther 2017;17:527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Kurupati R, Liu L, et al. Enhancing CD8+ T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell 2017;32:377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thommen DS, Schumacher TN. T Cell Dysfunction in Cancer. Cancer Cell 2018;33:547–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang CH, Qiu J, O’Sullivan D, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell 2015;162:1229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. June CH, O’Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer. Science 2018;359:1361–5. [DOI] [PubMed] [Google Scholar]
- 41. June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: a race to the finish line. Sci Transl Med 2015;7:280ps7. [DOI] [PubMed] [Google Scholar]
- 42. Rapoport AP, Stadtmauer EA, Binder-Scholl GK, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med 2015;21:914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim WA, June CH. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017;168:724–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Srivastava S, Riddell SR. Chimeric Antigen Receptor T Cell Therapy: Challenges to Bench-to-Bedside Efficacy. J Immunol 2018;200:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Litzinger MT, Fernando R, Curiel TJ, et al. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood 2007;110:3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Z, Zheng Q, Zhang H, et al. Ontak-like human IL-2 fusion toxin. J Immunol Methods 2017;448:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Byrne WL, Mills KH, Lederer JA, et al. Targeting regulatory T cells in cancer. Cancer Res 2011;71:6915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014;27:1–7. [DOI] [PubMed] [Google Scholar]
- 49. Chao JL, Savage PA. Unlocking the Complexities of Tumor-Associated Regulatory T Cells. J Immunol 2018;200:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ostrand-Rosenberg S, Fenselau C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol 2018;200:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakamura K, Kassem S, Cleynen A, et al. Dysregulated IL-18 Is a Key Driver of Immunosuppression and a Possible Therapeutic Target in the Multiple Myeloma Microenvironment. Cancer Cell 2018;33:634–48. [DOI] [PubMed] [Google Scholar]
- 52. Ferrari de Andrade L, Tay RE, Pan D, et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 2018;359:1537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Castle PE, Maza M. Prophylactic HPV vaccination: past, present, and future. Epidemiol Infect 2016;144:449–68. [DOI] [PubMed] [Google Scholar]
- 54. Yang B, Jeang J, Yang A, et al. DNA vaccine for cancer immunotherapy. Hum Vaccin Immunother 2014;10:3153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science 2018;359:1355–60. [DOI] [PubMed] [Google Scholar]
- 56. Russell SJ, Barber GN. Oncolytic Viruses as Antigen-Agnostic Cancer Vaccines. Cancer Cell 2018;33:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tanyi JL, Bobisse S, Ophir E, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med 2018;10:pii: eaao5931. [DOI] [PubMed] [Google Scholar]
- 58. Santos PM, Butterfield LH. Dendritic Cell-Based Cancer Vaccines. J Immunol 2018;200:443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anassi E, Ndefo UA. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P T 2011;36:197–202. [PMC free article] [PubMed] [Google Scholar]
- 60. Kooreman NG, Kim Y, de Almeida PE, et al. Autologous iPSC-Based Vaccines Elicit Anti-tumor Responses In Vivo. Cell Stem Cell 2018;22:501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Duthie MS, Windish HP, Fox CB, et al. Use of defined TLR ligands as adjuvants within human vaccines. Immunol Rev 2011;239:178–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schmidt C. The benefits of immunotherapy combinations. Nature 2017;552:S67–9. [DOI] [PubMed] [Google Scholar]
- 63. Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell 2016;165:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Katlinski KV, Gui J, Katlinskaya YV, et al. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer Cell 2017;31:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Patel SA, Minn AJ. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018;48:417–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zappasodi R, Merghoub T, Wolchok JD. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell 2018;33:581–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Menk AV, Scharping NE, Rivadeneira DB, et al. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J Exp Med 2018;215:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wiehagen KR, Girgis NM, Yamada DH, et al. I Combination of CD40 Agonism and CSF-1R Blockade Reconditions Tumor-Associated Macrophages and Drives Potent Antitumor Immunity. Cancer Immunol Res 2017;5:1109–21. [DOI] [PubMed] [Google Scholar]
- 71. Siska PJ, Beckermann KE, Mason FM, et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2017;2:pii: 93411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Philip M, Fairchild L, Sun L, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017;545:452–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kheirelseid EA, Miller N, Chang KH, et al. Mismatch repair protein expression in colorectal cancer. J Gastrointest Oncol 2013;4:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu Y, Sethi NS, Hinoue T, et al. Comparative Molecular Analysis of Gastrointestinal Adenocarcinomas. Cancer Cell 2018;33:721–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sautès-Fridman C, Lawand M, Giraldo NA, et al. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front Immunol 2016;7:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Engelhard VH, Rodriguez AB, Mauldin IS, et al. Immune Cell Infiltration and Tertiary Lymphoid Structures as Determinants of Antitumor Immunity. J Immunol 2018;200:432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wellenstein MD, de Visser KE. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018;48:399–416. [DOI] [PubMed] [Google Scholar]
- 80. Moroishi T, Hayashi T, Pan WW, et al. The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 2016;167:1525–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Balli D, Rech AJ, Stanger BZ, et al. Immune Cytolytic Activity Stratifies Molecular Subsets of Human Pancreatic Cancer. Clin Cancer Res 2017;23:3129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Thorsson V, Gibbs DL, Brown SD, et al. The Immune Landscape of Cancer. Immunity 2018;48:812–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gopalakrishnan V, Helmink BA, Spencer CN, et al. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018;33:570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zitvogel L, Ma Y, Raoult D, et al. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018;359:1366–70. [DOI] [PubMed] [Google Scholar]
- 85. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dhanak D, Edwards JP, Nguyen A, et al. Small-Molecule Targets in Immuno-Oncology. Cell Chem Biol 2017;24:1148–60. [DOI] [PubMed] [Google Scholar]
- 87. Adams JL, Smothers J, Srinivasan R, et al. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov 2015;14:603–22. [DOI] [PubMed] [Google Scholar]
- 88. Weeber F, Ooft SN, Dijkstra KK, et al. Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery. Cell Chem Biol 2017;24:1092–1100. [DOI] [PubMed] [Google Scholar]
- 89. Bailey MH, Tokheim C, Porta-Pardo E, et al. Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 2018;173:371–85. [DOI] [PMC free article] [PubMed] [Google Scholar]