Abstract
Many animals can change the size, shape, texture and color of their regenerated coats in response to different ages, sexes, or seasonal environmental changes. Here we propose that the feather core branching morphogenesis module can be regulated by sex hormones or other environmental factors to change feather forms, textures or colors, thus generating a large spectrum of complexity for adaptation. We use sexual dimorphisms of the chicken to explore the role of hormones. A long-standing question is whether the sex-dependent feather morphologies are autonomously controlled by the male or female cell types, or extrinsically controlled and reversible. We have recently identified core feather branching molecular modules which control the anterior-posterior (BMP, Wnt gradient), medio-lateral (Retinoic signaling, Gremlin), and proximo-distal (Sprouty, BMP) patterning of feathers. We hypothesize that morpho-regulation, through quantitative modulation of existing parameters, can act on core branching modules to topologically tune the dimension of each parameter during morphogenesis and regeneration. Here we explore the involvement of hormones in generating sexual dimorphisms using exogenously delivered hormones. Our strategy is to mimic male androgen levels by applying exogenous dihydrotestosterone and aromatase inhibitors to adult females and to mimic female estradiol levels by injecting exogenous estradiol to adult males. We also examine differentially expressed genes in the feathers of wildtype male and female chickens to identify potential downstream modifiers of feather morphogenesis. The data show male and female feather morphology and their color patterns can be modified extrinsically through molting and resetting the stem cell niche during regeneration.
Keywords: Feathers, Sex hormones, Hormone therapy, Sexual dimorphism, Morpho-regulation
Graphical Abstract
We propose that feather morphogenesis modules can be regulated by sex hormones or other environmental factors to change feather forms, textures or colors, thus generating a large spectrum of complexity for adaptation. We examine the roles of sex hormones in avian feather sexual dimorphisms by mimicking male androgen levels in female chickens and female androgen levels in males. We also examine differentially expressed genes in wildtype male and female chickens to identify potential downstream modifiers of feather morphogenesis.
Introduction
An animal’s coat is strongly influenced by environmental changes which can induce the skin to shed its appendages (hairs, feathers) and grow a new coat with characteristics more suited to the altered environment (C.-C. Chen & Chuong, 2012; C.-M. Chuong, Randall, Widelitz, Wu, & Jiang, 2012; Prum, 2017; Schneider, Schmidt-Ullrich, & Paus, 2009). We consider that the environment at least acts at three levels to regulate skin appendage morphogenesis and regeneration; the micro-environment, macro-environment and mega-environment. 1) Factors within the skin appendage stem cell niche (signaling molecules, growth factors, adhesion molecules, etc.) that can influence stem cell activation and cycling are said to arise from the micro-environment (C.-F. Chen et al., 2015; Kandyba et al., 2013). 2) Factors within the body but outside of the skin appendages (hormones, neurons, etc) arise from the macro-environment and may influence skin appendage cycling and growth (Valerie Anne Randall, 2007; Schneider et al., 2009). Hormones, the subject of this paper, fall within this category. 3) Finally, mega-environmental factors from outside of the body such as the temperature or photoperiod can also have a large impact on skin appendage characteristics. The local circadian clock can affect hair follicle TA cell proliferation (Plikus et al., 2013). Light stimulation of photosensors in the eyes has been shown to induce sympathetic nerves to provoke the release of norepinephrine in the skin which activates hair growth (S. M.-Y. Fan et al., 2018).
The response to these environmental changes can be mediated by altered hormone levels which trigger the end of one hair / feather cycle and the beginning of another. In males, androgens can induce androgenetic alopecia (male pattern baldness) on the scalp (Heilmann-Heimbach, Hochfeld, Paus, & Nöthen, 2016). Castration of early aged males was found to prevent male pattern baldness later in life (Hamilton, 1942). We still do not know the mechanism of the preferential effect on scalp and head hairs, even though we learned some time ago that the androgen receptor is localized within the dermal papilla (V A Randall et al., 1993). Alterations of hormone levels due to endocrinopathies have been found to cause dramatic changes in human skin appendages. For example, polycystic ovarian syndrome (PCOS) can cause elevated levels of androgens leading to region-specific hirsutism and male pattern baldness in women (Jabbour, 2013). Hirsutism increases the formation of terminal hairs in women with a typical male-pattern distribution on the chest, lower abdomen, back and face (Blank, Helm, McCartney, & Marshall, 2008). As a result of these and other studies, transgender women are commonly treated with anti-androgens to decrease hair growth on the body and face (Tangpricha & den Heijer, 2017). Seasonal influence on region-specific hair growth is apparent in animals and molecular studies have been carried out in red deer and horses (Osthaus et al., 2018; Thornton et al., 2001). Even human hair growth shows some seasonal influence (V A Randall & Ebling, 1991). This may be regulated, in part, by Thyoxine which has been shown to influence the circadian clock in human hair follicles (Hardman, Haslam, Farjo, Farjo, & Paus, 2015). Furthermore, seasonal changes also can alter hair color to attract a mate in spring and then provide camouflage in the winter, as seen in hamsters (Paul, George, Zucker, & Butler, 2007) and many other animals. Non-sex hormone morphogens from local dermis can also affect hair growth. For instance, BMPs in the adipose tissue beneath the skin can synchronize hair cycling, producing hair waves which traverse the mouse body (Plikus et al., 2008) and PDGF from adipose tissue can also affect hair stem cell activity (Festa et al., 2011). These reports all suggest a role for hormones in establishing sexually dimorphic effects on skin appendages.
Among vertebrate species, birds have the most dramatic changes of their coats in response to different seasons. Male and female birds can have drab coats in the winter and males of many avian species tend to have bright coats during the mating season. Interestingly, sexual dimorphisms begin to appear at puberty, suggesting that they are regulated, at least in part, by hormonal changes. Therefore, birds are excellent models in which to study how hormones might regulate sexually dimorphic feather morphogenesis and regeneration (Widelitz et al., 2003).
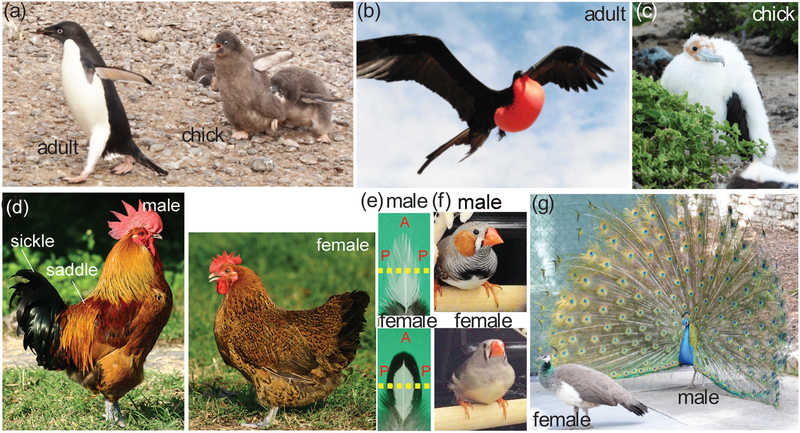
Feathers go through a few replacement cycles prior to sexual maturity. Upon hatching, precocial chicks are covered with downy feathers that provide warmth but do not display sexual dimorphisms. Cyclic renewal of the feathers enables the replacement of downy feathers with juvenal and then sexually dimorphic mature adult feather phenotypes from the same feather follicles (Mayer, Chuong, & Widelitz, 2004; Prum, 2017). Adult feathers show regional and sexually dimorphic differences in size (length and width, regulated by the duration of the feather cycle) in texture (fluffy; plumulaceous and stiff; pennaceous), regulated by the absence or presence of a barbule that links neighboring barbs and in color patterning. Feather forms can be radially symmetric (downy feathers), bilaterally symmetric (contour feathers) or bilaterally asymmetric (flight feathers). The feather types are distributed with regional-specificity in specific tracts. Feathers within a tract tend to have similar characteristics but frequently show graded size differences across the tract. The diversity of feather shapes, sizes, textures and colors at different life stages, in response to hormones and seasons is enormous (Fig. 1) and enables the birds to adapt to ecological niches at different life stages and within different varied environments.
FIGURE 1.
Macro-environment (age, sex) and Mega-environment (seasonal and ecological alterations) can affect feather morphology in terms of shapes, colors, and textures. Feather morphology can be dramatically altered due to macro-enviromental changes brought about by life stages and advancing age. Precocial chicks are covered with downy feathers. After molting, adults are cover with contour feathers. Examples shown in (a) Penguin, (b) adult Frigate bird, . (c) young Frigate bird, (d) adult Taiwan country chicken (male, left panel; female, right panel), (e) Wing covert feathers of adult Silver Laced Wyandotte chickens (male, top panel; female bottom panel). Adopted from Lin et al., (2013), (f) Zebra finches, Taeniopygia guttata, (male, top panel; female bottom panel), (g) Peacocks and peafowls are reknown for their sexually dimorphic feathers displayed during mating season. a,b,c,g, photos by CM Chuong. A, anterior; P, posterior.
We and others previously found that feather morphology is formed by the activation of a number of molecular pathways. The feather collar houses the feather bulge stem cells (Yue, Jiang, Widelitz, & Chuong, 2005). Transient amplifying cell proliferation occurs in the collar region (Fig. 2, red zone). At a higher position, in ramogenic zones, barb branching starts to form (Fig. 2, bottom of green zone). Barb branching is regulated by Bone Morphogenetic Proteins (BMPs) and their antagonists (Yu, Wu, Widelitz, & Chuong, 2002). The next step is the expression of NCAM (C. M. Chuong & Edelman, 1985a, 1985b) and Sonic hedgehog (Shh) (Harris, Fallon, & Prum, 2002; Ting-Berreth & Chuong, 1996; Yu et al., 2002). This periodic branching process is mediated by filapodial interactions among basal cells and involves FGF and Notch signaling (Cheng et al., 2018). The rachis is positioned along the anterior-posterior axis by a Wnt3a gradient (Yue, Jiang, Widelitz, & Chuong, 2006), followed by cell arrangement changes involving PCP (J. Lin & Yue, 2018). The barb generative zone, where barb formation initiates, is positioned by Grem1 (Li et al., 2017). The size of the rachis, the backbone of each feather, is controlled by BMP and GDF10 (Li et al., 2017; Yu et al., 2002). Along the proximal-distal axis, branching is regulated by the relative activities of Sprouty and FGFs (Yue, Jiang, Wu, Widelitz, & Chuong, 2012). Asymmetric feather vane morphology is controlled by a retinoic acid gradient (Li et al., 2017).
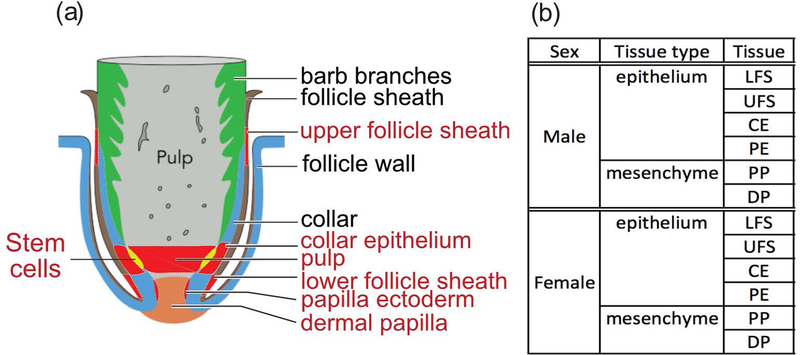
FIGURE 2.
Tissue collections from sickle feathers used for RNA-seq. (a) Structures of a growing feather follicle. Six regions of tissue collected for RNA-seq are marked and labeled in red. Adopted and modified from (C. M. Chuong, Yeh, Jiang, & Widelitz, 2012). (b) Source of duplicate samples used for RNA-seq from the epithelial components including the upper follicle sheath (UFS), lower follicle sheath (LFS), collar epithelium (CE) and papillary ectoderm (PE). Source of duplicate samples from mesenchymal components including the pulp (PP) and dermal papilla (DP).
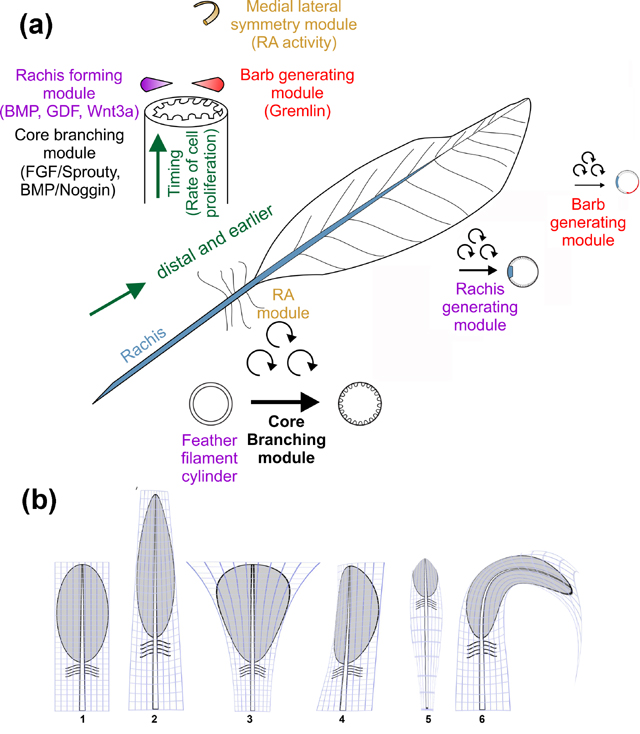
These findings have led us to postulate diverse feather forms are generated via the combination of a core branching modules and many morpho-modulatory modules (Fig. 3A). There is a core branching module that specifies how a basic feather template forms. Each step here represents an evolutionary novelty (qualitative changes), such as the generation of barbs, rachis and barbules, which set up a hierarchical feather template. On top of it, there are different “morpho-modulatory modules” that adjust the length of the feather, width of the feather vane, ratio of pennaceous versus plumulaceous regions, etc. (quantitative changes, Fig. 3B). The switch regulating these modules are optional in feathers from different body regions or at different moltings in the life of a bird (Fig. 3). Feather molting gives feathers a chance to renew their phenotypes (C.-M. Chuong, Yeh, Jiang, & Widelitz, 2013).
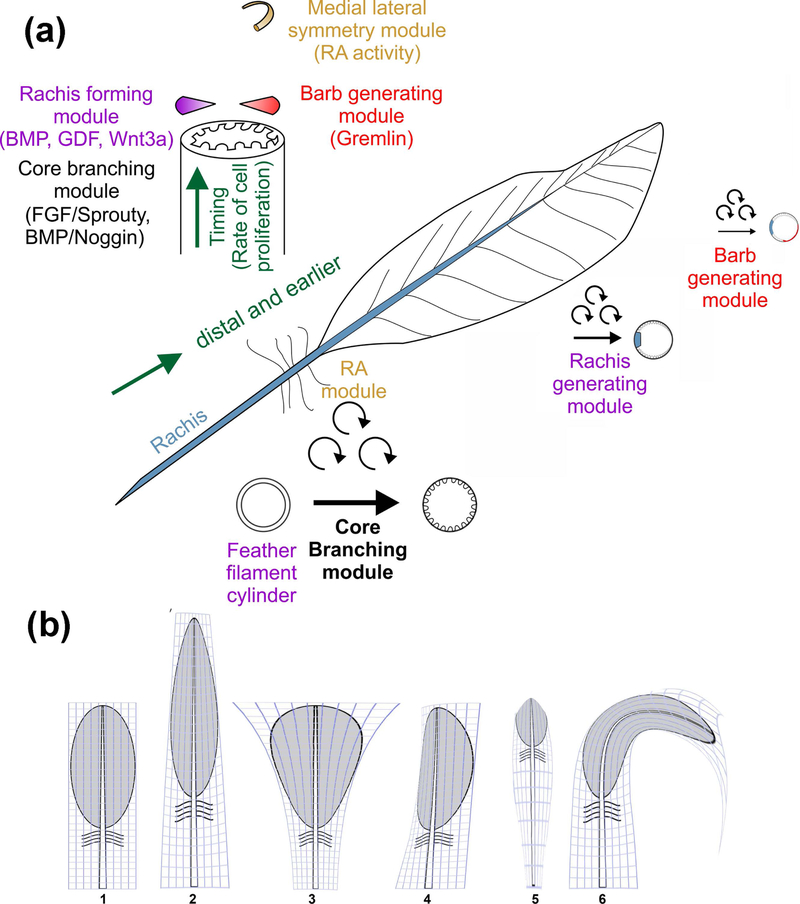
FIGURE 3.
Schematic diagram introducing the concept of feather morphogenesis. (a) An evolutionary novel event set up new branch types with qualitative advancement. The core branching module converts cylindrical feather filaments into barb branches. The rachis forming module sets up the rachis, converting radial to bilateral symmetric feather forms. The position of the barb generating module helps set up medial – lateral feather vane asymmetry. Distal feathers are formed first, with stem cells continuously generated from the proximal portion. This can be appreciated by viewing both the cross-sectional view (upper left) and whole feather view. The calamus in the base of the rachis has a cylindrical filament. (b) The concepts of morpho-regulation. Morpho-regulation modulates each dimension of feather forms quantitatively, and can generate diverse feather forms based on a prototypic feather. Molecules involved in each module are listed in the figure. By modulating the relative strengh of the molecular signal, each component can be amplified or shrunken or bent, topologically and disproportionately (i.e., by anisotropic positioned signaling modules) to generate different feather forms. (1) contour feather to (2) an elongate symmetric feather, (3) a fan shaped feather, (4) an asymmetric feather, (5) a filoplume or (6) a curved feather. The figure is drawn in the style of “On the Growth and Form” (Thompson, 1917).
Following molting and in each feather regeneration stage, these modules can be regulated by the surrouding stem cell niche, whose properties can be modulated by environmental factors such as sex hormones, circadian rhythm changes, seasonal changes etc. to generate diverse feather forms for best adaptation.
To further explore the role of hormones in sexual dimorphisms of skin appendages, we used the chicken feather model to examine the default feather phenotype in the absence of sex hormone stimulation (Mayer et al., 2004). We wondered whether manipulating hormone levels to mimic those in the opposite sex might alter feather phenotypes; perhaps all female feathers would take on male characteristics with androgen stimulation, or all feathers on male birds would resemble the female phenotype in the presence of estrogen, or alternatively hormones might have no effect on feather morphology, suggesting that feather shape occurs through a hormone-independent mechanism (Fig. 4a, c).
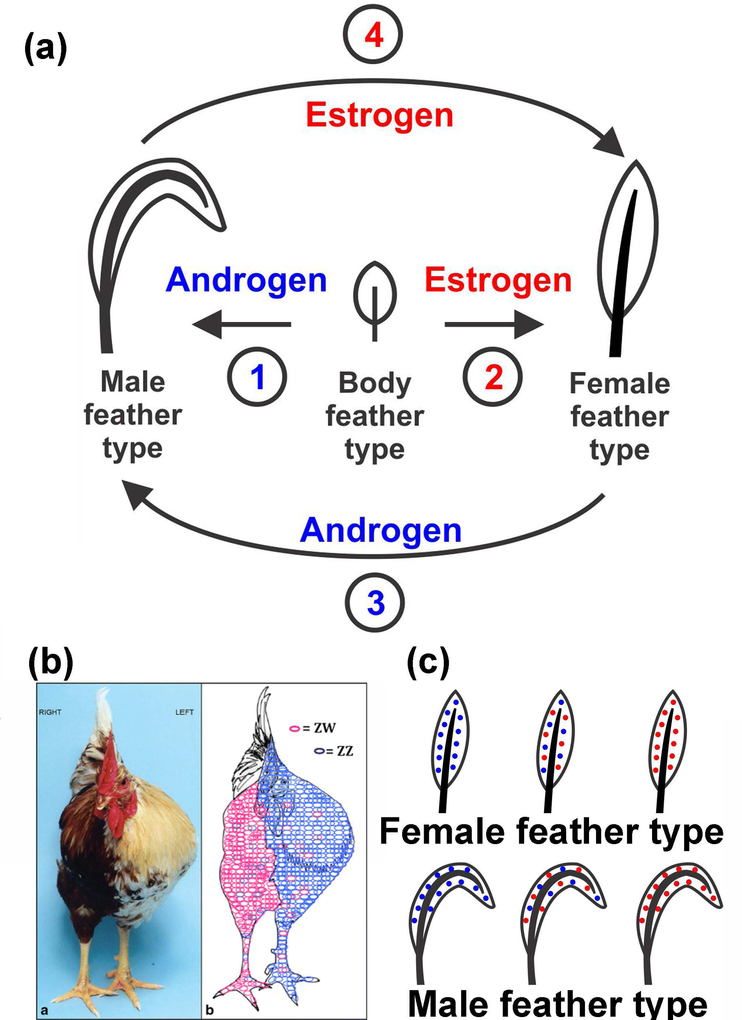
FIGURE 4.
Hypothetical model for the formation of sexually dimorphic feather forms. (a) Do hormones mediate a response to environmental change which led to feather diversity or is the response mediating by another means? If hormones play the leading role, is there a default feather phenotype in the absence of hormone stimulation? Do hormones stimulate the conversion of a sexless feather into a male or female feather type? Can hormones also induce male or female type feathers to undergo sex reversal in the next feather generation? Our data suggest that hormones can influence feather phenotype morphogenesis. Either body feathers are the default feather phenotype and are converted to the male feather forms by stimulation with Androgens (1) or to the female forms by stimulation with Estrogens (2). An alternate model has the default feather forms as female which are converted to male by the action of Androgens (3). Another alternate model has the default feather forms as male which are converted to female by the action of Estrogens (4). Revised from (Mayer et al., 2004). (b) A chicken gynandromorph (displaying characteristics of both sexes), male on the left and female on the right. Fluorescence in situ hybridization demonstrated the possible role of cell autonomous sex identity in the morphogenesis of sexual dimorphisms. From (Clinton et al., 2012). (c) Schematic showing that male, chimeric or female feathers can assume female or male morphology under proper environmental conditions. Sexual dimorphic organ shapes (feather shapes) is at a different scale than the sex of cells (chromosome ZW genotyping). Therefore, male and female shaped feathers can be composed of genetically male (blue dots) or female (red dots) cells, or a mixture of both.
As opposed to mammals, in birds, females are heterogametic (ZW) while males are the homogametic (ZZ) sex. The ratio of androgens to estrogens was proposed to regulate gonad determination (Bogart, 1987). More recently, doublesex and mab-3-related transcription factor 1 (DMRT1) was proposed to act in a dose-dependent manner (Hirst, Major, & Smith, 2018). DMRT1 is encoded on the Z chromosome. While females have a single copy of the Z chromosome, males have 2 copies. By suppressing DMRT1, genetic males showed a partial conversion toward becoming females. This was discerned by a loss of Sox2 expression and a gain of aromatase, the female enzyme that converts androgens to estrogens. The left gonad also became more ovary-like after DMRT1 suppression. During this conversion, the right gonad showed variable effects on DMRT1 expression but still expressed aromatase (Hirst et al., 2018).
Here we explore the involvement of hormone pathways in guiding feather morphogenesis. We examine whether the decision occurs at the molecular, cell, cell collective (a feather) or body region level (Fig. 4a–c). To begin to answer these questions, we took a hormone therapy approach to see how manipulating hormone levels might influence regenerating feather phenotype morphogenesis by injecting estradiol or testosterone to the leg or by implanting Femara (Letrazole, Novartis) pellets that slowly release an aromatase activity inhibitor in adult chickens. We surmise that a hypothetical enhancer regulates the expression of a key molecule within a core morphogenesis module that subsequently controls basic feather morphology. This can occur in response to changes in the endogenous or exogenous environment. For example, hormones are known to bind to enhancers and alter gene expression. Increased hormone levels at puberty or during mating season may bind to enhancers and modulate downstream molecular signals that subsequently may alter the feather cycle time (regulating feather and branch lengths), feather shape, texture and coloring to enhance extant feather diversity.
On the other hand, some investigators have identified a genetic component that bestows maleness or femaleness to individual cells. This cell autonomous sex identity was studied in three chickens that were morphologically male on one side and female on the other (gynandromorphs). We discuss this as well.
Materials and Method
Animals and Ethics Statement
Black-feathered Taiwan country chickens used for hormone treatments were from the National Chung Hsing University, Taiwan, ROC poultry farm. Animal experiments in this treatment were conducted according to the protocol approved by the Institutional Animal Care and Use Committees of National Chung Hsing University (Taichung, Taiwan). White Leghorn Chickens used for RNA-seq were from Charles River SPAFAS, Animal experiments in this collection were conducted according to the protocol approved by the Institutional Animal Care and Use Committee of the University of Southern California.
Hormone disturbance by injection of hormone precursors or by implanting pellets
To explore the response of feather sexual dimorphisms to hormone replacement therapy, we injected estradiol (Estradiol Benzoate (0.05 mg/ml; Tafoong, Taiwan) and testosterone (200 mg/ml diluted to 2.8 mg/ml in 99.5 % ethanol; Yai-Yu, Taiwan) to the leg muscles of adult wildtype male and female Taiwan country chickens, once per week to achieve 200 mg/70kg as suggested by the manufacturer (Vet Medicine) to mimic hormone levels present in the opposite sex. We then characterized the effects of manipulating androgen and estrogen levels on sexually dimorphic feather lengths, textures and shapes.
To test the role of hormones in feather morphogenesis, we implanted Femara (Letrazole, Novartis, 2.5 mg pellets), an aromatase inhibitor, subcutaneously every 2 weeks in the loose skin behind the neck of female birds through a minor incision that is closed with a single suture. This location was chosen to block the host birds’ ability to remove the pellets. Upon completion of the experiment, birds were euthanized and tissues harvested for further characterization. Experimental design is shown in Fig. S1.
RNA-sequencing
Libraries were prepared from epithelium and mesenchyme using the standard protocol (Ping Wu et al., 2015). RNA was extracted using Trizol reagent (Invitrogen). 1–2 ug of total RNA from each sample was used to construct an RNA-seq library using the TruSeq RNA sample preparation v2 kit (Illumina). Sequencing (75 bp single-end read) was performed using NextSeq 500 at the USC Molecular Genomics Core. The chicken galGal4 assembly including un-placed and un-localized scaffolds and Ensembl Release81 annotation were downloaded from the UCSC Genome Browser on 2016.2.6. (Speir et al., 2016). The alignment, quantification, normalization, and differential expression analysis will be performed by STAR 2.4.1d (Ramírez, Dündar, Diehl, Grüning, & Manke, 2014), HTSeq-count 0.6.0 (Kel et al., 2003), TMM (Robinson & Oshlack, 2010), and edgeR (Robinson, McCarthy, & Smyth, 2010), respectively. RNA-Seq raw data will be accessible at NCBI GEO (accession number: GSE120823).
Results
Effects of hormones on adult chicken feathers
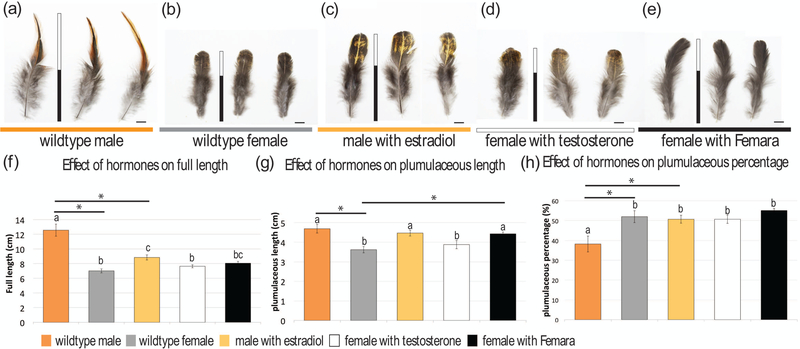
After injection of estradiol or testosterone to the leg muscles of adults once per week, we first measured the length of regenerating control (wildtype) vs treated saddle feathers on adult birds at 21 days (3 weeks) when both female and male feathers should be at their maximal size (Fig. S1). At 11.37 cm ± 0.35 cm long, the wildtype male saddle feathers (n = 14) were significantly different from 6.73 ± 0.12 cm for female saddle feathers (n = 12) (Fig. 5a, b, f). Treating males with exogenous estradiol (n = 12) significantly reduced saddle feather length to 9.20 ± 0.10 cm which is 2.17 cm shorter than that of control males (Fig. 5c, f). Treating females with exogenous testosterone (n = 15) increased feather length slightly to 7.28 ± 0.10 cm which is 0.55 cm longer than those seen in control females (Fig. 5d, f). Blocking the conversion of androgens to estradiol with Femara (n = 21) produced the longest female feathers at a length of 8.47 ± 0.21 cm which is 1.74 cm longer than control female feathers (Fig. 5e, f).
FIGURE 5.
Representative phenotypes and size variation of regenerated saddle feathers three weeks after hormone treatment. (a) Wildtype male feathers from three individual Taiwan country chickens. (b) Wildtype female feathers. (c) Male feathers treated with estradiol. (d) Female feathers treated with testosterone. (e) Female feathers treated with Femara. The vertical bars in each panel show the average total length of feathers and the black portion indicates the average plumulaceous portion of feathers. Scale bar 1 cm. (f) Chart showing the effect of hormones on the full length of feathers. (g) Chart showing the effect of hormones on the length of the plumulaceous portion of feathers. (h) Chart showing the effect of hormones on the percentage of the total feather length that is plumulaceous. The significant differences of LSMES at P< 0.05 are marked as asterisks.
In addition to the feather length, we looked at the effects of hormones on feather texture. The proximal end of saddle feathers are plumulaceous (barbs are fluffy and not interconnected) to provide warmth to the birds while the distal branches are pennaceous (barbs are interconnected and form a vane; Fig. 5a–e, marked with black and white bars, respectively). We next measured the proportion of each of these two regions to determine whether hormones might influence the ratios of their distributions. Both lengths or proportions of the proximal plumulaceous component are significantly different between male and female saddle feathers (Fig. 5g, h). The proximal, plumulaceous component of male saddle feathers occupies 49% of the total feather length, whereas this region accounts for 61% in control female feathers. Treating males with estradiol increased the plumulaceous segment to 55% of feather length. Treating females with testosterone reduced the plumulaceous region slightly to 59% while inhibiting aromatase slightly increased the segment to 63% of the total length (Fig. 5h).
Then we examined the effects of manipulating hormone levels on the shape of regenerated feathers. Feathers were plucked and allowed to regenerate after exposure to exogenous testosterone, estradiol, or aromatase for 9 weeks. Control female saddle feathers are rounded while male saddle feathers are tapered to a point at the distal tip (Fig. 5a, b). Injecting estradiol to male birds induced the conversion of the male saddle feather morphology to the rounded form found on females (Fig. 5c). Injecting testosterone to female birds had no significant effect on feather morphology; the feathers retained their female phenotype (Fig. 4d). Similarly, the saddle feathers retained their rounded distal end after the implantation of Femara (aromatase inhibitor) pellets (Fig. 5e). Based on the literature, injections of estradiol to male brown leghorns produced female feathering while ovarectomy of females induced a male feathering (Juhn & Gustavson, 1930). Our data and those of Juhn and Gustavson suggest that estradiol may regulate the appearance of the female feathering phenotype.
The pigmentation patterns were also perturbed by hormone treatments. The brown color (distribution of pheomelanin) was usually located in the margin of plumulaceous male saddle feathers (Fig. 5a). However, the color pattern of feathers from estradiol injected males looks more like control female feathers with yellow patches from the center to the margin (Fig. 5b, c). Treating females with exogenous testosterone had no obvious effects on color patterning (Fig. 5d). In contrast, blocking the conversion of androgens to estradiol with Femara on females regenerated whole black feathers (Fig. 5e).
RNA-seq analysis of female versus male feathers
We previously found that feather shape and size can be regulated by molecular signals. The positioning of dermal GDF10 and GREM1 establish the rachidial zone and barb generative zone, respectively which sets the size of the two vanes on the feather. Feather asymmetry is determined by an anterior-posterior Wnt gradient. Graded changes in feather size are established by a retinoic acid gradient which further regulates feather asymmetry by controlling epithelial cell shapes (Li et al., 2017). Our findings suggest that hormones may contribute to regional specificity, but other factors must respond locally to produce region-specific skin appendage phenotypes (feathers - flight, saddle, rectrice, contour, etc or scales – scutate, reticulate, etc) within each tract.
To gain further insights into the genes that might mediate hormonal effects leading to sexual dimorphisms, we compared expression profiles between adult white leghorn female and male chicken feathers collected 6–17 days after plucking. For these studies feathers were collected from the hormone responsive sickle tract of 2 chickens per sample and duplicate samples were used per feather component (n = 2 chickens per replicate × 2 replicates). From each sample, we then collected epithelial parts from the upper follicle sheath (UFS), lower follicle sheath (LFS), collar epithelium (CE) and papillar ectoderm (PE). We also collected mesenchymal parts from the pulp (PP) and dermal papilla (DP) (Fig. 2). These regions were chosen based on our assumption of epidermal progenitor and potential dermal niche regions.
Transcriptome data are shown (Table 1). We first examined genes that were up-regulated in all components of female feathers. Only ZP1 (Zona pellucida 1) (Okumura et al., 2015) belongs in this category. Other genes that were upregulated in all female feather components except for the dermal papilla include ATP5A1W (ATP Synthase, H+ Transporting, Mitochondrial F1 Complex, Alpha Subunit 1, Cardiac Muscle) and SpinW (Spindlin 1-W) (Dawson, Dos Remedios, & Horsburgh, 2016). We also identified genes that were always up-regulated in all components of male feathers. These include KLF4, involved in cellular reprogramming (Tsai et al., 2010) and the orphan nuclear receptors NR4A2 and NR4A3. We next found genes that were always up-regulated in female epithelial cells including BKJ, an avian beta-keratin-related gene (Hartl & Bister, 1995); C1QTNF5, involved in obesity-related inflammation (Lee et al., 2005) and FGF7. Additionally, we identified the upregulated gene encoding Sox10 in male collar epithelium, which has been implicated in feather pigmentation in male epithelial cells (Gunnarsson et al., 2011). Some of these genes have been implicated in feather morphogenesis and color patterning but a full understanding and functional verification of their involvement will require more studies.
TABLE 1.
RNA-seq results showing the list of up-regulated genes in specific components of female and male sickle feathers. DEGs: Differentially expressed genes.
| Tissue | Number of DEGs | Up-regulated female | Up-regulated male | ||
|---|---|---|---|---|---|
| UFS | 566 | SMAD7 | SPRY1, SPRY3 | ||
| HSD17B2 | ATF3, CSRNP1, MMP13, SOCS3, KRT20, KLF4, NR4A2, NR4A3 | ||||
| ZP1, ATPS5A1W, SpinW | |||||
| LFS | 187 | SMAD7, WNT4, WNT16 | SPRY1, SPRY3 | ||
| PTCH2 | CILP2, KLF4, NR4A2, NR4A3 | ||||
| Myosin | |||||
| KRT6A, KRT8, ZP1, ATPS5A1W, SpinW | |||||
| CE | 246 | DIO2 | WNT2B, WNT7A | ||
| SMAD7, FGF7, GREM1, DKK2 | PMEL, MLANA, TYR, TYRP1, SOX10 | ||||
| ASIP | KLF4, NR4A2, NR4A3 | ||||
| FGF7, BJK, C1QTNF5, COL6A1, ZP1, ATPS5A1W, SpinW | |||||
| PE | 63 | GDF10 | ATF3, SERPINB2, KLF4, NR4A2, NR4A3 | ||
| SMAD7 | |||||
| FLT4, HGF/SF, MMRN1, TFPI2, TIE1, COL8A1, ZP1, ATPS5A1W, SpinW | |||||
| PP | 1367 | SMAD7, Pgo2, RSPO1, RSPO3, Wnt5B, BMP2 | SERP4, BMP3 | ||
| GDF10 | ESR1, SRD5A2 | ||||
| COL8A1, ZP1, ATPS5A1W, SpinW | PMEL, MLANA, TYR, TYRP1 | ||||
| SERPINB2, ZNF536 | |||||
| DP | 142 | Myosin | SPRY3 | ||
| AR | ATF3, CYR61, KLF4, NR4A2, NR4A3 | ||||
| ZP1 | |||||
We next explored molecules that have been implicated in how seasons and hormones might influence feather cycling and morphology. Since feathers must are shed and then regrown in response to diurnal cycles, we looked for molecules involved in light sensing. DIO2 was found to be upregulated in the female collar epithelium. This places the sensors in close proximity to the feather stem cell compartment (Yue et al., 2005). Since sexual dimorphisms involve dramatic changes in feather morphology we examined where molecules involved in feather shaping are expressed. Myosin, which is involved in feather asymmetry (Li et al., 2013) is expressed in the female lower follicle sheath and dermal papilla. GDF10 (Growth differentiation factor 10) which positions the rachis is expressed in the female papillar ectoderm and in the pulp, while GREM1 which positions the barb generative zone is up-regulated in the female collar epithelium (Li et al., 2017). Next, since hormones are known to function through their receptors, we explored where genes encoding hormone receptors and hormone modifiers were expressed. Our results were surprising. The androgen receptor (AR) is upregulated in the female dermal papilla while the estrogen receptor alpha (ESR1) is upregulated in the male pulp. However, these data only represent a single time point. It is possible that hormone receptor levels fluctuate, and these data need to be acquired for additional timepoints to better interpret this finding. SRD5A2 (5 alpha-reductase) which converts testosterone to dihydrotestosterone (Scaglione et al., 2017) is upregulated in the male pulp. HSD17B2 which converts active steroid hormones to less active forms (L. Wu et al., 1993) is present in the female upper follicle sheath. Lastly, we studied molecules known to be involved in pigmentation, since in birds, males often are more colorful than females. Pigmentation is controlled by ASIP expressed in the female collar epithelium which may act to suppress the formation of bright colors; whereas, PMEL, MLANA, TYR, TYRP and SOX10 which are involved in pigment synthesis (Mort, Jackson, & Patton, 2015) are expressed in the male collar epithelium and pulp.
Other genes known to be involved in feather periodic patterning and feather morphology are also differentially expressed. In female feathers, SMAD7 is expressed in all female tissues except the dermal papilla. SMAD7 acts to inhibit TGF-beta signaling and has been shown to block hair development (Klopcic et al., 2007; Owens, Han, Li, & Wang, 2008). PTCH2, WNT4, and WNT16 are upregulated in the lower follicle sheath. FGF7 and DKK2 are up-regulated in the collar epithelium. Pgo2, RSPO1, RSPO3, Wnt5B, BMP2 are expressed in the pulp. BMP2, suppresses cell migration and establishes the borders of the feather dermal condensation (Michon, Forest, Collomb, Demongeot, & Dhouailly, 2008). In male feathers, SPRY1, SPRY3 which suppress FGF activity are expressed in the lower follicle sheath; WNT2B, promotes the formation of human basal cell carcinomas (Katoh, 2005) and WNT7A, whose gradient produces a restricted nuclear beta-catenin zone that establishes feather polarity (Li et al., 2013) are expressed in the collar epithelium; and SFRP4 and BMP3 are expressed in the pulp. BMP3 has been shown to promote bone formation (L. Fan et al., 2018)
Focusing on individual epithelial components, we identified several significantly differentially expressed genes between males and females (Table I) who have yet been shown to regulate the size or shape of skin appendages. We present those that are upregulated in the tissue discussed. The male upper follicle sheath expresses ATF3, the cAMP-dependent transcription factor may be involved in regulating aldosterone synthesis (Felizola et al., 2014). Aldosterone is associated with increased growth hormone levels in humans (Bielohuby et al., 2009). ATF over expression in mouse hair outer root sheath cells induced large, abnormal hair follicles (Wang et al., 2007); CSRNP1, Cysteine and serine rich nuclear protein 1 which may have tumor suppressor activity (Gingras, Pelletier, Boyd, & Ihle, 2007); MMP13, Matrix metalloproteinase 13 which is involved in embryonic development and tissue remodeling and has been implicated in scarless wound healing in nude mice (Gawronska-Kozak, 2011); SOCS3, the suppressor of cytokinase signaling which inhibits signaling through the JAK/STAT pathway blocks the formation of alopecia areata (Gao, Jin, & Wu, 2017); KRT20 immunostaining of sclerosing adnexal neoplasms was found to be a strong indicator of Desmoplastic trichoepithelioma (Evangelista & North, 2015) and SPRY3. In contrast, KRT6A and KRT8 were expressed exclusively in the female lower follicle sheath. The male lower follicle sheath expressed CILP2, cartilage intermediate layer protein 2, may be associated with collagen VI which regulates fibroblast motility in the skin (Bernardo et al., 2011; Theocharidis et al., 2016). The female papillar ectoderm expressed COL8A1, collagen type VIII alpha 1 is an extracellular matrix molecule; FLT4, Fms related tyrosine kinase 4 is involved in lymphangiogenesis and angiogenesis; HGF/SF, Hepatocyte growth factor/Scatter factor has been reported to promote hair growth (Lindner et al., 2000; Qi et al., 2016); MMRN1, Multimerin 1 is found in platelets and the endothelium of blood vessels; TFPI2, Tissue factor pathway inhibitor 2 which is associated with melanoma when methylated (Lo Nigro et al., 2013); and TIE1, Tyrosine kinase with immunoglobulin like and EGF like domains 1 is a marker of endothelial cells (Talavera-Adame et al., 2011); whereas the male PE epidermis expressed SERPINB2, Serpin family member 2 whose loss induced the impairment of skin barrier function and the formation of a defective stratum corneum (Schroder et al., 2016); and surprisingly, the androgen receptor (AR). We expected AR expression levels to be raised in male feather tissues. While in males CYR61, Cysteine rich angiogenic inducer 61 is involved in tissue remodeling and an inflammatory response (Schlage, Kockmann, Sabino, Kizhakkedathu, & Auf dem Keller, 2015; Pinru Wu, Ma, & Li, 2017). In the male pulp, ZNF536, the Zinc finger protein 536 is up-regulated. It has been shown to repress retinoic acid mediated transcription in the brain (Qin et al., 2009). These interesting molecules await future studies to establish a better understanding of their possible roles in sexual dimorphisms.
Discussion
Morpho-regulation
Birds have developed diverse feather forms, dramatically varying in size, shape, texture and color which has enabled them to live and thrive in diverse ecosystems throughout the world. Some of these disparate forms are based on genetic differences in different avian species. Even more interestingly is that the birds can generate different feather forms from the same follicles in different ages, and in response to sex hormones, seasons, etc. (Fig. 1), in the context of organ metamorphosis (C.-M. Chuong et al., 2013). Dr. Edelman has proposed a morpho-regulatory concept in which he suggested the activities of cell adhesions can be fine-tuned by morpho-regulatory molecules, so more complex cell interactions can be generated (Edelman, 1992). Here we develop this concept further to propose a quantitative morpho-regulatory process can help generate diverse feather forms for adaptation (Fig. 3B), although an evolutionary novel event will introduce qualitatively different feather properties. For example, the generation of a rachis converted radial to bilateral symmetry, or the generation of barbule hooklets led to the formation of the feather vane.
We show the basic feather morphology is produced by a core branching module. In phylogeny and ontogeny, more morpho-regulatory modules are imposed. The rachis forming module regulates the size and placement of the rachis within the feather. A barb generating module establishes invaginations of the epithelium within the feather follicle which later will form branches. The core branching module is also involved in branching morphogenesis. The position of the barb generative zone, relative to the rachis establishes feather asymmetry (Li et al., 2015, 2017). The level and range of their expression helps to produce temporal and regional specific feather forms. We further propose, upstream, there are enhancers regulating downstream molecules involved in these different morpho-regulatory modules. The activation of these modules is optional, i.e. they are not essential for the basic formation of the feather template but will change the forms of feathers. They modulate the expression of genes which embellish the basic core units and produce the observed morphological diversity. Upstream molecules regulating these modules are usually enhancers controlled by transcription factors, expressed in response to diverse environments. Here we analyzed the effects of hormones on feather diversity and then examined the differential expression of genes in both sexes to gain an understanding of molecular changes which could regulate various forms of feather morphogenesis. We show the proof of principle of this morpho-regulatory hypothesis. The next stage will be the elucidation of more specific molecular mechanisms.
Role of hormones
The role for hormones in avian gonad determination was first demonstrated by the injection of estradiol to fertilized chicken eggs that induced genetically male birds to form ovaries (Fry & Toone, 1981). Along similar trends, treating young chicks with aromatase inhibitors prior to gonad development caused sex reversal in hens and the levels of testosterone versus estradiol were indicative of male or female gonadal differentiation (Vaillant, Dorizzi, Pieau, & Richard-Mercier, 2001). While the sexually dimorphic, colorful and sometimes exotic feather morphologies draw a lot of interest, the involvement of hormones in their morphogenesis is still not clearly understood. To date, what is known is that the presence of aromatase which converts androgens to estrogens in the skin of some breeds (i.e., Golden Campine and Sebright Bantam) cause males to have a female feathering pattern (henny feathering) although they are genetically male (George, Matsumine, McPhaul, Somes, & Wilson, 1990; Matsumine, Herbst, Ou, Wilson, & McPhaul, 1991). Most interestingly, the region-specific control or aromatase, here working as a morpho-regulatory molecule, is mediated by a retroviral promoter (McPhaul, Matsumine, Herbst, & Wilson, 1991).
We tested the impact of different steroid hormone combinations on feather morphology. We treated adult roosters and hens with estradiol, testosterone or aromatase inhibitors. We expected that increasing testosterone in females, increasing estradiol in males or suppressing the conversion of androgens to estrogens in females might alter the sexual dimorphic phenotype. Our results show that while hormones can influence feather morphology, androgens do not seem to play a large role in this capacity. Rather, estradiol had a marked effect on feather morphology, converting male pattern feathers to resemble female feathers in terms of the overall shape. In males, exogenous estradiol induced a significant reduction in male saddle feather length. In females, exogenous testosterone induced a small increase in feather length. Femara induced a larger increase in feather length. However, this finding could be due to an increase in androgen or a decrease in estrogen levels.
We further explored how hormones might regulate the differential expression of genes in male and female chickens using transcriptome analyses. Our analysis provides a number of molecular clues that can be traced in the future to determine their possible role in regulating the morphology of sexually dimorphic feather forms. Not surprisingly, several of the molecules were involved in hormone metabolism and their receptors. NR4A2 and NR4A3 are up-regulated in all components of male feathers. Surprisingly, the AR was up-regulated in female compared to male dermal papilla and ESR1 was up-regulated in the male compared to the female pulp. These results are opposite to that expected for males and females and need to be further explored. Other molecules control tissue remodeling (MMP13), extracellular matrix (KRT8, KRT20, COL8A1), and branch formation (GDF10) and pigmentation (ASIP, SOX10, PMEL, MLANA, TYR, TYRP1). As for molecules regulating feather morphology, we find that the female feather epithelium expresses significantly elevated levels of BMP3, GREM1 and DKK2. The lower female follicle expresses elevated levels of WNT4, WNT16, KRT6A and KRT8. The female pulp expresses GDF10 and Pgo2. The female dermal papilla expresses elevated levels of Myosin. The upper follicle sheath did not express elevated levels of any of these molecules. The male collar epithelium expresses elevated levels of WNT2B and WNT7A, the upper follicle sheath expresses elevated levels of KRT20, the lower follicle sheath expresses elevated levels of SPRY3, and the dermal pulp expresses elevated levels of FOS and JUN. The papilla ectoderm did not express elevated levels of any of these molecules (Table 1).
Wnts, RSPO1, RSPO3 positively regulate while DKK2 and SFRP4 negatively regulate WNT signaling. BMPs stimulate while GREM1 suppresses BMP signaling. These and other pathways have a major influence on feather cycling. Other molecules influence the expression of GDF10 to influence rachis size. Taken together, these data suggest that stem cells give rise to cells in male or female birds that can then produce sexually dimorphic feather phenotypes. Exogenous hormone application to adult chickens suggest that hormones can influence feather morphology, feather length and feather pigmentation in a reversible fashion with each feather cycle (Fig. 5a–e). Here estradiol had the most dramatic effect on feather morphology inducing the female feather phenotype, even when estradiol was injected to male birds. Injecting testosterone to female birds or suppressing aromatase expression levels had no effect on feather phenotype. These results suggest that sexual dimorphic feather shapes, sizes and textures are regulated by the presence or absence of estradiol. Feather length was most dramatically influenced by estradiol although injection of testosterone did induce a small increase in female feather length. Testosterone also regulates aspects of pigmentation (Fig. 5d). PMEL, MLANA, TYR and TYRP1 were up-regulated in male feathers while ASIP was increased in female feathers.
Gynandromorphs
Although there is good evidence that sex hormones can regulate feather shapes at the level of the organ (a feather is a mini-organ), some investigators have identified a genetic component that bestows maleness or femaleness to individual cells. This cell autonomous sex identity was studied in three chickens that were morphologically male on one side and female on the other (gynandromorphs), generally but not absolutely, divided along the dorsal mid-line. It should be noted that although these chickens show regions of male and female body and feather morphology, they share a common circulatory system. These naturally occurring chickens were shown to be chimeric containing both male and female cells, although the majority of cells on the male side were male and the majority of cells on the female side were female (Fig. 4b).
After transplanting dermal cells from the male side to the female side or vice versa the donor cells maintained their identity and were secluded from the functional regions of the host gonad (Clinton, Zhao, Nandi, & McBride, 2012; Zhao et al., 2010). Gynandromorphs have been found in species other than chickens. In zebra finch (song birds) gynandromorphs’ differences were found in the sex associated neural song circuit residing in the left (male) versus right (female) side of the brain (Agate et al., 2003). The role of intrinsic genetic markers (Zhao et al., 2010) versus extrinsic hormones (C.-C. Chen, Plikus, Tang, Widelitz, & Chuong, 2015; Lindsay, Barron, Webster, & Schwabl, 2016) in regulating these sexual dimorphic feather shapes, sizes, textures and colors is still unclear. This is especially true since gonadectomy which eliminates the source of hormone secretion can alter feather characteristics (Lambeth & Smith, 2012), suggesting that hormones do play a role in sexual dimorphisms.
Lately, investigators have begun to discuss the role of epigenetic regulation in switching on a male or female developmental program (Deveson et al., 2017). Epigenetics involves the regulation of gene expression through the addition of adducts to DNA or to histone proteins around which the DNA is tightly wound. These modifications can control the accessibility of regions of the DNA to be transcribed into RNA. In general methylation of DNA silences the expression of the genes encoded within the methylated region. Hypomethylation leads to increased DNA expression. Another level of epigenetic control lies at the level of histone tail modifications. Some modifications form a closed DNA configuration that is inaccessible to protein interactions, blocking transcription while other modifications leave DNA in an open configuration enabling transcription to proceed (Jaenisch & Bird, 2003). These changes can occur at the level of the estrogen receptor or androgen receptor which could then commit cells toward a male or female state (Mann, Cortez, & Vadlamudi, 2011). In addition tissue specific sexually dimorphic DNA methylation patterns have been identified (McCormick et al., 2017). Other investigators have found that the Male-Hyper-Methylated (MHM) locus induces gene expression in females but silences genes in male chickens (Wright, Zimmer, Harrison, & Mank, 2015) and may function to regulate DMRT1 expression to regulate ovary or testis development (Roeszler, Itman, Sinclair, & Smith, 2012; Yang et al., 2016). One might speculate that the male and female sides of gynandromorphs may be regulated by epigenetic events, however, the role of epigenetic changes in avian cellular sex determination has not been completely investigated yet and should be one of the future directions.
In summary, skin appendages represent a complex morphogenesis system (Lai and Chuong, 2016; Chuong et al., 2013). Several molecular pathways have been found to be involved in shaping feather morphology (Li et al., 2017). Feather length is regulated by the duration of the growth phase during feather cycling (Mayer et al., 2004). Similarly, hair growth is known to be modulated by multi-levels of environment control (C.-C. Chen & Chuong, 2012; Plikus & Chuong, 2014). While there are several clues from our transcriptome data that suggest hormones can directly influence known feather morpho-regulatory pathways, specific interactions remain to be resolved. However, the complex and sexually dimorphic avian plumage patterns represent an experimentally tractable system for analyses. The work here provides a platform for future investigation on how sex hormones talk to core feather morphogenesis and core feather cycling control molecular circuits to regulate feather morphogenesis. The multi-scale morpho-regulatory pathways give rise to feathers with different shapes, sizes, textures and colors enabling them to take on different functions so birds can adapt and thrive in a variety of ecologies.
Supplementary Material
Figure Supplementary 1 Experimental design for hormone treatments and regenerated feather collections. After sexual maturity (forty-week-age), ten feathers were plucked from the saddle region of each hen and rooster of Black-feathered Taiwan Country chickens at Week 0. The sample collections and drug/hormone delivery time points are indicated in the above diagram. Feathers collected at Week 1 were for RNA-seq analysis and at Week 3 were for qPCR analysis. The fully regenerated feathers collected at Week 9 were shown in Fig. 5 (a)-(e). If multiple procedures were scheduled on the same date, the procedures were performed in the following order: (1) serum collection, (2) feather collection, (3) drug/hormone delivery.
Highlights.
Basic feather branching morphogenesis module can be modulated by sex hormone or other environmental factors to change the form, texture or colors.
Sex hormones can override genetic identity of cells or cell collectives and guide feather morphology
Sex hormones can affect molting and feather cycling
Hormones can modulate intrinsic factors shown to guide feather morphology
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers 42177, 47364, and 60306. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. It was also supported by the iEGG and Animal Biotechnology Center from the feature areas research center program within the framework of the high education sprout project by the Ministry of Education in Taiwan. We thank the USC Epigenome Center for sequencing; Yibu Chen and Meng Li of the USC Norris Medical Library Bioinformatics Service for assistance in RNA-Seq analysis.
Bibliography
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, … Arnold AP (2003). Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proceedings of the National Academy of Sciences of the United States of America, 100(8), 4873–4878. doi: 10.1073/pnas.0636925100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo BC, Belluoccio D, Rowley L, Little CB, Hansen U, & Bateman JF (2011). Cartilage intermediate layer protein 2 (CILP-2) is expressed in articular and meniscal cartilage and down-regulated in experimental osteoarthritis. The Journal of Biological Chemistry, 286(43), 37758–37767. doi: 10.1074/jbc.M111.248039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielohuby M, Roemmler J, Manolopoulou J, Johnsen I, Sawitzky M, Schopohl J, … Bidlingmaier M (2009). Chronic growth hormone excess is associated with increased aldosterone: a study in patients with acromegaly and in growth hormone transgenic mice. Experimental Biology and Medicine, 234(8), 1002–1009. doi: 10.3181/0901-RM-34 [DOI] [PubMed] [Google Scholar]
- Blank SK, Helm KD, McCartney CR, & Marshall JC (2008). Polycystic ovary syndrome in adolescence. Annals of the New York Academy of Sciences, 1135, 76–84. doi: 10.1196/annals.1429.005 [DOI] [PubMed] [Google Scholar]
- Bogart M (1987). Sex Determination: A Hypothesis Based on Steroid Ratios. Journal of Theoretical Biology, 128, 349–357. [DOI] [PubMed] [Google Scholar]
- Chen C-C, & Chuong CM (2012). Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. Journal of Dermatological Science, 66(1), 3–11. doi: 10.1016/j.jdermsci.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Plikus MV, Tang P-C, Widelitz RB, & Chuong CM (2015). The Modulatable Stem Cell Niche: Tissue Interactions during Hair and Feather Follicle Regeneration. Journal of Molecular Biology, 428(7), 1423–1440. doi: 10.1016/j.jmb.2015.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-F, Foley J, Tang P-C, Li A, Jiang TX, Wu P, … Chuong CM (2015). Development, regeneration, and evolution of feathers. Annual Review of Animal Biosciences, 3, 169–195. doi: 10.1146/annurev-animal-022513-114127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng D, Yan X, Qiu G, Zhang J, Wang H, Feng T, … Yue Z (2018). Contraction of basal filopodia controls periodic feather branching via Notch and FGF signaling. Nature Communications, 9(1), 1345. doi: 10.1038/s41467-018-03801-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, & Edelman GM (1985a). Expression of cell-adhesion molecules in embryonic induction. I. Morphogenesis of nestling feathers. The Journal of Cell Biology, 101(3), 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, & Edelman GM (1985b). Expression of cell-adhesion molecules in embryonic induction. II. Morphogenesis of adult feathers. The Journal of Cell Biology, 101(3), 1027–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Yeh CY, Jiang TX, & Widelitz RB (2012). Module-based complexity formation: periodic patterning in fethers and hairs. WIRES Developmental Biology, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Randall VA, Widelitz RB, Wu P, & Jiang T-X (2012). Physiological regeneration of skin appendages and implications for regenerative medicine. Physiology, 27(2), 61–72. doi: 10.1152/physiol.00028.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C-M, Yeh C-Y, Jiang T-X, & Widelitz R (2013). Module-based complexity formation: periodic patterning in feathers and hairs. Wiley Interdisciplinary Reviews. Developmental Biology, 2(1), 97–112. doi: 10.1002/wdev.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton M, Zhao D, Nandi S, & McBride D (2012). Evidence for avian cell autonomous sex identity (CASI) and implications for the sex-determination process? Chromosome Research, 20(1), 177–190. doi: 10.1007/s10577-011-9257-9 [DOI] [PubMed] [Google Scholar]
- Dawson DA, Dos Remedios N, & Horsburgh GJ (2016). A new marker based on the avian spindlin gene that is able to sex most birds, including species problematic to sex with CHD markers. Zoo Biology, 35(6), 533–545. doi: 10.1002/zoo.21326 [DOI] [PubMed] [Google Scholar]
- Deveson IW, Holleley CE, Blackburn J, Marshall Graves JA, Mattick JS, Waters PD, & Georges A (2017). Differential intron retention in Jumonji chromatin modifier genes is implicated in reptile temperature-dependent sex determination. Science Advances, 3(6), e1700731. doi: 10.1126/sciadv.1700731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM (1992). Morphoregulation. Developmental Dynamics, 193(1), 2–10. doi: 10.1002/aja.1001930103 [DOI] [PubMed] [Google Scholar]
- Evangelista MTP, & North JP (2015). Comparative analysis of cytokeratin 15, TDAG51, cytokeratin 20 and androgen receptor in sclerosing adnexal neoplasms and variants of basal cell carcinoma. Journal of Cutaneous Pathology, 42(11), 824–831. doi: 10.1111/cup.12546 [DOI] [PubMed] [Google Scholar]
- Fan L, Fan J, Liu Y, Li T, Xu H, Yang Y, … Zhao RC (2018). miR-450b Promotes Osteogenic Differentiation In Vitro and Enhances Bone Formation In Vivo by Targeting BMP3. Stem Cells and Development, 27(9), 600–611. doi: 10.1089/scd.2017.0276 [DOI] [PubMed] [Google Scholar]
- Fan SM-Y, Chang Y-T, Chen C-L, Wang W-H, Pan M-K, Chen W-P, … Lin S-J (2018). External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proceedings of the National Academy of Sciences of the United States of America, 115(29), E6880–E6889. doi: 10.1073/pnas.1719548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felizola SJA, Nakamura Y, Ozawa Y, Ono Y, Morimoto R, Midorikawa S, … Sasano H (2014). Activating transcription factor 3 (ATF3) in the human adrenal cortex: its possible involvement in aldosterone biosynthesis. The Tohoku Journal of Experimental Medicine, 234(4), 249–254. [DOI] [PubMed] [Google Scholar]
- Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, & Horsley V (2011). Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell, 146(5), 761–771. doi: 10.1016/j.cell.2011.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DM, & Toone CK (1981). DDT-induced feminization of gull embryos. Science, 213(4510), 922–924. [DOI] [PubMed] [Google Scholar]
- Gao Z, Jin Y-Q, & Wu W (2017). SOCS3 treatment prevents the development of alopecia areata by inhibiting CD8+ T cell-mediated autoimmune destruction. Oncotarget, 8(20), 33432–33443. doi: 10.18632/oncotarget.16504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawronska-Kozak B (2011). Scarless skin wound healing in FOXN1 deficient (nude) mice is associated with distinctive matrix metalloproteinase expression. Matrix Biology, 30(4), 290–300. doi: 10.1016/j.matbio.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George FW, Matsumine H, McPhaul MJ, Somes RG, & Wilson JD (1990). Inheritance of the henny feathering trait in the golden Campine chicken: evidence for allelism with the gene that causes henny feathering in the Sebright bantam. The Journal of Heredity, 81(2), 107–110. [DOI] [PubMed] [Google Scholar]
- Gingras S, Pelletier S, Boyd K, & Ihle JN (2007). Characterization of a family of novel cysteine- serine-rich nuclear proteins (CSRNP). Plos One, 2(8), e808. doi: 10.1371/journal.pone.0000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson U, Kerje S, Bed’hom B, Sahlqvist A-S, Ekwall O, Tixier-Boichard M, … Andersson L (2011). The Dark brown plumage color in chickens is caused by an 8.3-kb deletion upstream of SOX10. Pigment Cell & Melanoma Research, 24(2), 268–274. doi: 10.1111/j.1755-148X.2011.00825.x [DOI] [PubMed] [Google Scholar]
- Hamilton JB (1942). Male hormone stimulation as a prerequisite and an incitement in common baldness, 71, 451–453. [Google Scholar]
- Hardman JA, Haslam IS, Farjo N, Farjo B, & Paus R (2015). Thyroxine differentially modulates the peripheral clock: lessons from the human hair follicle. Plos One, 10(3), e0121878. doi: 10.1371/journal.pone.0121878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MP, Fallon JF, & Prum RO (2002). Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. The Journal of Experimental Zoology, 294(2), 160–176. doi: 10.1002/jez.10157 [DOI] [PubMed] [Google Scholar]
- Hartl M, & Bister K (1995). Specific activation in jun-transformed avian fibroblasts of a gene (bkj) related to the avian beta-keratin gene family. Proceedings of the National Academy of Sciences of the United States of America, 92(25), 11731–11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann-Heimbach S, Hochfeld LM, Paus R, & Nöthen MM (2016). Hunting the genes in male-pattern alopecia: how important are they, how close are we and what will they tell us? Experimental Dermatology, 25(4), 251–257. doi: 10.1111/exd.12965 [DOI] [PubMed] [Google Scholar]
- Hirst CE, Major AT, & Smith CA (2018). Sex determination and gonadal sex differentiation in the chicken model. The International Journal of Developmental Biology, 62(1–2–3), 153–166. doi: 10.1387/ijdb.170319cs [DOI] [PubMed] [Google Scholar]
- Jabbour SA (2013). Cutaneous Manifestations of Endocrine Disorders. American Journal of Clinical Dermatology, 4(5), 315–331. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, & Bird A (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics, 33 Suppl, 245–254. doi: 10.1038/ng1089 [DOI] [PubMed] [Google Scholar]
- Juhn M, & Gustavson RG (1930). The production of female genital subsidiary characters by injection of human placental hormones in fowls. J. Exptl. Zool, 56, 31–61. [Google Scholar]
- Kandyba E, Leung Y, Chen Y-B, Widelitz R, Chuong C-M, & Kobielak K (2013). Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proceedings of the National Academy of Sciences of the United States of America, 110(4), 1351–1356. doi: 10.1073/pnas.1121312110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M (2005). WNT2B: comparative integromics and clinical applications (Review). International Journal of Molecular Medicine, 16(6), 1103–1108. [PubMed] [Google Scholar]
- Kel AE, Gössling E, Reuter I, Cheremushkin E, Kel-Margoulis OV, & Wingender E (2003). MATCH: A tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Research, 31(13), 3576–3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopcic B, Maass T, Meyer E, Lehr HA, Metzger D, Chambon P, … Blessing M (2007). TGF-beta superfamily signaling is essential for tooth and hair morphogenesis and differentiation. European Journal of Cell Biology, 86(11–12), 781–799. doi: 10.1016/j.ejcb.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Lambeth LS, & Smith CA (2012). Disorders of sexual development in poultry. Sexual Development: Genetics, Molecular Biology, Evolution, Endocrinology, Embryology, and Pathology of Sex Determination and Differentiation, 6(1–3), 96–103. doi: 10.1159/000334059 [DOI] [PubMed] [Google Scholar]
- Lee YH, Nair S, Rousseau E, Allison DB, Page GP, Tataranni PA, … Permana PA (2005). Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia, 48(9), 1776–1783. doi: 10.1007/s00125-005-1867-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Chen M, Jiang T-X, Wu P, Nie Q, Widelitz R, & Chuong C-M (2013). Shaping organs by a wingless-int/Notch/nonmuscle myosin module which orients feather bud elongation. Proceedings of the National Academy of Sciences of the United States of America, 110(16), E1452–61. doi: 10.1073/pnas.1219813110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Figueroa S, Jiang T-X, Wu P, Widelitz R, Nie Q, & Chuong C-M (2017). Diverse feather shape evolution enabled by coupling anisotropic signalling modules with self-organizing branching programme. Nature Communications, 8, ncomms14139. doi: 10.1038/ncomms14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Lai Y-C, Figueroa S, Yang T, Widelitz RB, Kobielak K, … Chuong CM (2015). Deciphering principles of morphogenesis from temporal and spatial patterns on the integument. Developmental Dynamics, 244(8), 905–920. doi: 10.1002/dvdy.24281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, & Yue Z (2018). Coupling of apical-basal polarity and planar cell polarity to interpret the Wnt signaling gradient in feather development. Development, 145(17). doi: 10.1242/dev.162792 [DOI] [PubMed] [Google Scholar]
- Lin SJ, Foley J, Jiang TX, Yeh CY, Wu P, Foley A, … Chuong CM (2013). Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge. Science, 340(6139), 1442–1445. doi: 10.1126/science.1230374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner G, Menrad A, Gherardi E, Merlino G, Welker P, Handjiski B, … Paus R (2000). Involvement of hepatocyte growth factor/scatter factor and met receptor signaling in hair follicle morphogenesis and cycling. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 14(2), 319–332. [DOI] [PubMed] [Google Scholar]
- Lindsay WR, Barron DG, Webster MS, & Schwabl H (2016). Testosterone activates sexual dimorphism including male-typical carotenoid but not melanin plumage pigmentation in a female bird. The Journal of Experimental Biology, 219(Pt 19), 3091–3099. doi: 10.1242/jeb.135384 [DOI] [PubMed] [Google Scholar]
- Lo Nigro C, Wang H, McHugh A, Lattanzio L, Matin R, Harwood C, … Crook T (2013). Methylated tissue factor pathway inhibitor 2 (TFPI2) DNA in serum is a biomarker of metastatic melanoma. The Journal of Investigative Dermatology, 133(5), 1278–1285. doi: 10.1038/jid.2012.493 [DOI] [PubMed] [Google Scholar]
- Mann M, Cortez V, & Vadlamudi RK (2011). Epigenetics of estrogen receptor signaling: role in hormonal cancer progression and therapy. Cancers, 3(3), 1691–1707. doi: 10.3390/cancers3021691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumine H, Herbst MA, Ou SH, Wilson JD, & McPhaul MJ (1991). Aromatase mRNA in the extragonadal tissues of chickens with the henny-feathering trait is derived from a distinctive promoter structure that contains a segment of a retroviral long terminal repeat. Functional organization of the Sebright, Leghorn, and Campine aromatase genes. The Journal of Biological Chemistry, 266(30), 19900–19907. [PubMed] [Google Scholar]
- Mayer JA, Chuong C-M, & Widelitz R (2004). Rooster feathering, androgenic alopecia, and hormone-dependent tumor growth: what is in common? Differentiation; Research in Biological Diversity, 72(9–10), 474–488. doi: 10.1111/j.1432-0436.2004.07209003.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick H, Young PE, Hur SSJ, Booher K, Chung H, Cropley JE, … Suter CM (2017). Isogenic mice exhibit sexually-dimorphic DNA methylation patterns across multiple tissues. BMC Genomics, 18(1), 966. doi: 10.1186/s12864-017-4350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhaul MJ, Matsumine H, Herbst MA, & Wilson JD (1991). Aromatase expression in extragonadal tissues of the Sebright chicken is controlled by a retroviral promoter. Transactions of the Association of American Physicians, 104, 141–149. [PubMed] [Google Scholar]
- Michon F, Forest L, Collomb E, Demongeot J, & Dhouailly D (2008). BMP2 and BMP7 play antagonistic roles in feather induction. Development, 135(16), 2797–2805. doi: 10.1242/dev.018341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort RL, Jackson IJ, & Patton EE (2015). The melanocyte lineage in development and disease. Development, 142(7), 1387. doi: 10.1242/dev.123729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura H, Sato T, Sakuma R, Fukushima H, Matsuda T, & Ujita M (2015). Identification of distinctive interdomain interactions among ZP-N, ZP-C and other domains of zona pellucida glycoproteins underlying association of chicken egg-coat matrix. FEBS Open Bio, 5, 454–465. doi: 10.1016/j.fob.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osthaus B, Proops L, Long S, Bell N, Hayday K, & Burden F (2018). Hair coat properties of donkeys, mules and horses in a temperate climate. Equine Veterinary Journal, 50(3), 339–342. doi: 10.1111/evj.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens P, Han G, Li AG, & Wang X-J (2008). The role of Smads in skin development. The Journal of Investigative Dermatology, 128(4), 783–790. doi: 10.1038/sj.jid.5700969 [DOI] [PubMed] [Google Scholar]
- Paul MJ, George NT, Zucker I, & Butler MP (2007). Photoperiodic and hormonal influences on fur density and regrowth in two hamster species. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 293(6), R2363–9. doi: 10.1152/ajpregu.00520.2007 [DOI] [PubMed] [Google Scholar]
- Plikus MV, & Chuong C-M (2014). Macroenvironmental regulation of hair cycling and collective regenerative behavior. Cold Spring Harbor Perspectives in Medicine, 4(1), a015198. doi: 10.1101/cshperspect.a015198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, & Chuong C-M (2008). Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature, 451(7176), 340–344. doi: 10.1038/nature06457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Vollmers C, de la Cruz D, Chaix A, Ramos R, Panda S, & Chuong C-M (2013). Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proceedings of the National Academy of Sciences of the United States of America, 110(23), E2106–15. doi: 10.1073/pnas.1215935110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prum RO (2017). The evolution of beauty: How Darwin’s forgotten theory of mate choice shapes the animal world-- and us (First edition.). New York: Doubleday. [Google Scholar]
- Qi Y, Li M, Xu L, Chang Z, Shu X, & Zhou L (2016). Therapeutic role of human hepatocyte growth factor (HGF) in treating hair loss. PeerJ, 4, e2624. doi: 10.7717/peerj.2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Ren F, Xu X, Ren Y, Li H, Wang Y, … Chang Z (2009). ZNF536, a novel zinc finger protein specifically expressed in the brain, negatively regulates neuron differentiation by repressing retinoic acid-induced gene transcription. Molecular and Cellular Biology, 29(13), 3633–3643. doi: 10.1128/MCB.00362-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Dündar F, Diehl S, Grüning BA, & Manke T (2014). deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Research, 42(Web Server issue), W187–91. doi: 10.1093/nar/gku365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall VA, & Ebling FJ (1991). Seasonal changes in human hair growth. The British Journal of Dermatology, 124(2), 146–151. [DOI] [PubMed] [Google Scholar]
- Randall VA, Thornton MJ, Messenger AG, Hibberts NA, Loudon AS, & Brinklow BR (1993). Hormones and hair growth: variations in androgen receptor content of dermal papilla cells cultured from human and red deer (Cervus elaphus) hair follicles. The Journal of Investigative Dermatology, 101(1 Suppl), 114S–120S. [DOI] [PubMed] [Google Scholar]
- Randall Valerie Anne. (2007). Hormonal regulation of hair follicles exhibits a biological paradox. Seminars in Cell & Developmental Biology, 18(2), 274–285. doi: 10.1016/j.semcdb.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, & Smyth GK (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 26(1), 139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, & Oshlack A (2010). A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biology, 11(3), R25. doi: 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeszler KN, Itman C, Sinclair AH, & Smith CA (2012). The long non-coding RNA, MHM, plays a role in chicken embryonic development, including gonadogenesis. Developmental Biology, 366(2), 317–326. doi: 10.1016/j.ydbio.2012.03.025 [DOI] [PubMed] [Google Scholar]
- Scaglione A, Montemiglio LC, Parisi G, Asteriti IA, Bruni R, Cerutti G, … Vallone B (2017). Subcellular localization of the five members of the human steroid 5α-reductase family. Biochimie Open, 4, 99–106. doi: 10.1016/j.biopen.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlage P, Kockmann T, Sabino F, Kizhakkedathu JN, & Auf dem Keller U (2015). Matrix metalloproteinase 10 degradomics in keratinocytes and epidermal tissue identifies bioactive substrates with pleiotropic functions. Molecular & Cellular Proteomics, 14(12), 3234–3246. doi: 10.1074/mcp.M115.053520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Schmidt-Ullrich R, & Paus R (2009). The hair follicle as a dynamic miniorgan. Current Biology, 19(3), R132–42. doi: 10.1016/j.cub.2008.12.005 [DOI] [PubMed] [Google Scholar]
- Schroder WA, Anraku I, Le TT, Hirata TDC, Nakaya HI, Major L, … Suhrbier A (2016). Serpinb2 deficiency results in a stratum corneum defect and increased sensitivity to topically applied inflammatory agents. The American Journal of Pathology, 186(6), 1511–1523. doi: 10.1016/j.ajpath.2016.02.017 [DOI] [PubMed] [Google Scholar]
- Speir ML, Zweig AS, Rosenbloom KR, Raney BJ, Paten B, Nejad P, … Kent WJ (2016). The UCSC Genome Browser database: 2016 update. Nucleic Acids Research, 44(D1), D717–25. doi: 10.1093/nar/gkv1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera-Adame D, Ng TT, Gupta A, Kurtovic S, Wu GD, & Dafoe DC (2011). Characterization of microvascular endothelial cells isolated from the dermis of adult mouse tails. Microvascular Research, 82(2), 97–104. doi: 10.1016/j.mvr.2011.04.009 [DOI] [PubMed] [Google Scholar]
- Tangpricha V, & den Heijer M (2017). Oestrogen and anti-androgen therapy for transgender women. The Lancet. Diabetes & Endocrinology, 5(4), 291–300. doi: 10.1016/S2213-8587(16)30319-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharidis G, Drymoussi Z, Kao AP, Barber AH, Lee DA, Braun KM, & Connelly JT (2016). Type VI collagen regulates dermal matrix assembly and fibroblast motility. The Journal of Investigative Dermatology, 136(1), 74–83. doi: 10.1038/JID.2015.352 [DOI] [PubMed] [Google Scholar]
- Thornton MJ, Hibberts NA, Street T, Brinklow BR, Loudon AS, & Randall VA (2001). Androgen receptors are only present in mesenchyme-derived dermal papilla cells of red deer (Cervus elaphus) neck follicles when raised androgens induce a mane in the breeding season. The Journal of Endocrinology, 168(3), 401–408. [DOI] [PubMed] [Google Scholar]
- Ting-Berreth SA, & Chuong CM (1996). Local delivery of TGF beta2 can substitute for placode epithelium to induce mesenchymal condensation during skin appendage morphogenesis. Developmental Biology, 179(2), 347–359. doi: 10.1006/dbio.1996.0266 [DOI] [PubMed] [Google Scholar]
- Tsai S-Y, Clavel C, Kim S, Ang Y-S, Grisanti L, Lee D-F, … Rendl M (2010). Oct4 and klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells, 28(2), 221–228. doi: 10.1002/stem.281 [DOI] [PubMed] [Google Scholar]
- Vaillant S, Dorizzi M, Pieau C, & Richard-Mercier N (2001). Sex reversal and aromatase in chicken. The Journal of Experimental Zoology, 290(7), 727–740. [DOI] [PubMed] [Google Scholar]
- Wang A, Arantes S, Conti C, McArthur M, Aldaz CM, & MacLeod MC (2007). Epidermal hyperplasia and oral carcinoma in mice overexpressing the transcription factor ATF3 in basal epithelial cells. Molecular Carcinogenesis, 46(6), 476–487. doi: 10.1002/mc.20298 [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Jiang TX, Yu M, Shen T, Shen J-Y, Wu P, … Chuong C-M (2003). Molecular biology of feather morphogenesis: a testable model for evo-devo research. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 298(1), 109–122. doi: 10.1002/jez.b.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AE, Zimmer F, Harrison PW, & Mank JE (2015). Conservation of Regional Variation in Sex-Specific Sex Chromosome Regulation. Genetics, 201(2), 587–598. doi: 10.1534/genetics.115.179234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Einstein M, Geissler WM, Chan HK, Elliston KO, & Andersson S (1993). Expression cloning and characterization of human 17 beta-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20 alpha-hydroxysteroid dehydrogenase activity. The Journal of Biological Chemistry, 268(17), 12964–12969. [PubMed] [Google Scholar]
- Wu Ping, Ng CS, Yan J, Lai Y-C, Chen C-K, Lai Y-T, … Chuong C-M (2015). Topographical mapping of α- and β-keratins on developing chicken skin integuments: Functional interaction and evolutionary perspectives. Proceedings of the National Academy of Sciences of the United States of America, 112(49), E6770–9. doi: 10.1073/pnas.1520566112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Pinru, Ma G, & Li N (2017). The profile of Cyr61 expression data correlate to the skin inflammation in psoriasis. Data in Brief, 10, 487–491. doi: 10.1016/j.dib.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Deng J, Zheng J, Xia L, Yang Z, Qu L, … Yang N (2016). A Window of MHM Demethylation Correlates with Key Events in Gonadal Differentiation in the Chicken. Sexual Development: Genetics, Molecular Biology, Evolution, Endocrinology, Embryology, and Pathology of Sex Determination and Differentiation, 10(3), 152–158. doi: 10.1159/000447659 [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widelitz RB, & Chuong C-M (2002). The morphogenesis of feathers. Nature, 420(6913), 308–312. doi: 10.1038/nature01196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang TX, Wu P, Widelitz RB, & Chuong CM (2012). Sprouty/FGF signaling regulates the proximal-distal feather morphology and the size of dermal papillae. Developmental Biology, 372(1), 45–54. doi: 10.1016/j.ydbio.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang T-X, Widelitz RB, & Chuong C-M (2005). Mapping stem cell activities in the feather follicle. Nature, 438(7070), 1026–1029. doi: 10.1038/nature04222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jiang T-X, Widelitz RB, & Chuong C-M (2006). Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proceedings of the National Academy of Sciences of the United States of America, 103(4), 951–955. doi: 10.1073/pnas.0506894103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, McBride D, Nandi S, McQueen HA, McGrew MJ, Hocking PM, … Clinton M (2010). Somatic sex identity is cell autonomous in the chicken. Nature, 464(7286), 237–242. doi: 10.1038/nature08852 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure Supplementary 1 Experimental design for hormone treatments and regenerated feather collections. After sexual maturity (forty-week-age), ten feathers were plucked from the saddle region of each hen and rooster of Black-feathered Taiwan Country chickens at Week 0. The sample collections and drug/hormone delivery time points are indicated in the above diagram. Feathers collected at Week 1 were for RNA-seq analysis and at Week 3 were for qPCR analysis. The fully regenerated feathers collected at Week 9 were shown in Fig. 5 (a)-(e). If multiple procedures were scheduled on the same date, the procedures were performed in the following order: (1) serum collection, (2) feather collection, (3) drug/hormone delivery.