Abstract
Research findings implicate cerebral glutamate in the pathophysiology of schizophrenia, including genetic studies reporting associations with glutamatergic neurotransmission. The extent to which aberrant glutamate levels can be explained by genetic factors is unknown, and if glutamate can serve as a marker of genetic susceptibility for schizophrenia remains to be established. We investigated the heritability of cerebral glutamate levels and whether a potential association with schizophrenia spectrum disorders could be explained by genetic factors. Twenty-three monozygotic (MZ) and 20 dizygotic (DZ) proband pairs con- or discordant for schizophrenia spectrum disorders, along with healthy control pairs (MZ = 28, DZ = 18) were recruited via the National Danish Twin Register and the Psychiatric Central Register (17 additional twins were scanned without their siblings). Glutamate levels in the left thalamus and the anterior cingulate cortex (ACC) were measured using 1[H]-magnetic resonance spectroscopy at 3 Tesla and analyzed by structural equation modeling. Glutamate levels in the left thalamus were heritable and positively correlated with liability for schizophrenia spectrum disorders (phenotypic correlation, 0.16, [0.02–0.29]; p = 0.010). The correlation was explained by common genes influencing both the levels of glutamate and liability for schizophrenia spectrum disorders. In the ACC, glutamate and glx levels were heritable, but not correlated to disease liability. Increases in thalamic glutamate levels found in schizophrenia spectrum disorders are explained by genetic influences related to the disease, and as such the measure could be a potential marker of genetic susceptibility, useful in early detection and stratification of patients with psychosis.
Subject terms: Schizophrenia, Predictive markers, Diagnostic markers, Genetics research
Introduction
Schizophrenia constitutes a heterogeneous phenotype with a complex etiology, but clinical, animal, and genetic studies have supported the involvement of glutamate metabolism in its pathophysiology [1–5]. However, the extent to which aberrant glutamate concentration and disease liability are founded in common genes remains to be established.
Glutamatergic disturbances have been suggested to precede the dopaminergic changes observed in schizophrenia [6], or to represent a separate pathophysiological entity [7]. In vivo, differences in concentrations of glutamate, glutamine, or glx (glutamate + glutamine) can be investigated with proton magnetic resonance spectroscopy (MRS). Findings depend on the region(s) studied [8] and are putatively influenced by age [1], and antipsychotic medication status [8, 9]. Compared with healthy controls (HCs) higher levels of glutamate and glutamine in the thalamus have been found in both the antipsychotic naive [10–12] and in the medicated state [13], whereas unaltered glutamate, glutamine, and glx levels are described in chronic, as well as in minimally treated and medication-free patients [14–17]. Moreover a recent meta-analysis found glutamine to be increased and glutamate unaltered in the thalamus [2]. Similarly, the findings in the anterior cingulate cortex (ACC) are diverse. Here glutamate is found unaltered in antipsychotic naive [11, 18] and minimally treated [17, 19, 20] patients with schizophrenia, but reports of increased glutamine levels in antipsychotic naive patients [11, 18] are supported by findings of increased glx levels in the overlapping medial prefrontal cortical region of unmedicated patients ([21, 22]). Unaltered glutamine levels in first episode [23] and lower glx levels in antipsychotic naive patients [24] are also reported in prefrontal cortex. Studies in chronic patients have generally found decreased [13, 25, 26] or unaltered [27, 28] levels of glutamate and glutamine in the ACC. For a comprehensive meta-analysis, see [2].
Only one previous MRS study has reported on cerebral glutamate levels in twins discordant for schizophrenia, but did not estimate heritability [29]. Lutkenhoff and colleagues have found indications of decreased glutamate levels in the medial prefrontal cortex of probands and unaffected co-twins, suggesting that glutamate may be a state-independent marker for schizophrenia liability and be altered in unaffected family members. MRS studies of clinical high-risk subjects have reported lower thalamic (but not ACC) glutamate levels relative to controls [30–32]. In contrast, in familial high-risk subjects higher glx was reported in the thalamus [33]. The latter result is in accordance with findings in patients and suggests a specific familial and likely genetic, influence on glutamate in this region. Genetic influences on cerebral glutamate were also detected in an MRS twin study in children with autism spectrum disorder where glx was found to be heritable in the thalamus [34]. Since schizophrenia itself is highly heritable [35], and abnormal glutamate levels appear to be influenced by genetic factors, it is possible that the same genes influence both traits. To our knowledge, this has not been tested in a discordant twin study design [36, 37].
Other metabolites ordinarily estimated with 1[H] (proton)-MRS at 3 Tesla are N-acetyl aspartate (NAA), choline (Cho), creatinine (Cr), and myo-inositol (MI). The heritability of these metabolites has been studied in a group of older healthy twins (mean age 72 years) [38] where Batouli et al. found all metabolites (glutamate was not measured) to be significantly heritable in the posterior cingulate cortex. Heritability was most pronounced for NAA, a marker of neuronal integrity. MRS studies in patients with chronic schizophrenia have reported lower levels of NAA in the frontal lobe, the thalamus, the temporal lobe, and the hippocampus. Cr, Cho, and MI levels have generally been found to be unaltered in schizophrenia (reviewed in [39–41]).
In the present study, we investigated whether glutamate levels in the left thalamus and the ACC are heritable and altered in schizophrenia spectrum disorder (ICD-10 F2x.x), and whether glutamate levels and liability for disease are influenced by common genetic effects. In secondary analyses, we performed the same calculations in a subsample including only proband pairs with an ICD-10 F20.x schizophrenia diagnosis, to investigate if the results are consistent within the schizophrenia spectrum. In addition to glutamate, we investigated the heritability and correlation with disease for NAA, Cho, Cr, and MI.
Materials and methods
Study approval was obtained from the National Committee on Health Research ethics (H-2-2010-1289) and the Data Protection Agency (2010-41-5468).
Recruitment
The current study population is part of a larger study (Vulnerability Indicators of Psychosis). Results from other modalities in this overall study will be presented elsewhere. By linking the Danish Civil Registration System [42] with The Danish Twin Register [43] and The Danish Psychiatric Central Research Register [44], we identified all twin pairs in Denmark con- or discordant for a schizophrenia spectrum diagnosis (Main or secondary lifetime diagnosis in ICD-8: 295, 297, 298.29, 298.39, 298.89, 298.99, 299.05, 299.09, 301.09, 301.29, or in ICD-10: DF2x.x). Restrictions of the overall study population (n = 902 twin pairs) according to the following criteria: (i) 18–60 years old, (ii) both twins alive, (iii) both twins resided in Denmark, (iv) no participation in another research project within the last 2 years (due to restrictions by the Danish Twin Register) identified a final study population of 61 monozygotic (MZ) and 143 dizygotic (DZ) proband twin pairs eligible for study inclusion. All MZ proband pairs were contacted; those who participated were matched on sex and age to DZ proband pairs, as well as MZ and DZ HC pairs. DNA testing confirmed zygosity in 94% of the participants (micro-array by Infinium PsychArray v1.1, Illumina, San Diego, USA). Register-based information on zygosity was used if live DNA was not available.
Participants
A total of 219 subjects agreed to participate, from whom 11 were excluded according to the criteria listed below and 13 did not conclude MRS, leaving a total of 195 participants for the current study. The study population consisted of 23 complete MZ and 20 complete DZ twin pairs con- or discordant for a schizophrenia spectrum diagnosis (see Table S1 for proband subdiagnoses and co-twin diagnoses), and 28 complete MZ and 18 complete DZ HC pairs. Seventeen additional twins were scanned without their siblings (Table 1). All DZ pairs were same sex pairs. Of the unaffected co-twins, five (10%) had a psychiatric diagnosis. In the MZ group, one had depression, one had phobia, and one had Asperger syndrome, and in the DZ group one had depression and one had a personality disorder (Table S1). Inclusion criteria for proband pairs are described under “Recruitment”. HC pairs furthermore had to be mentally healthy and match proband pairs with regard to age, sex, and zygosity. General exclusion criteria: serious head trauma (loss of consciousness >5 min), addiction to drugs/alcohol, serious physical illness, and pregnancy. Additional exclusion criteria for HC pairs: no major psychosis or affective diagnosis in first-degree relatives (F2x.x, F30, F31, and F32.3). Semi-structured diagnostic interviews were conducted by Schedules for Clinical Assessment in Neuropsychiatry (SCAN) [45] and Comprehensive Assessment of At-Risk Mental State (CAARMS) [46]. SCAN interviews were only performed for HC pairs if symptoms were detected on CAARMS.
Table 1.
Demographic presentation of participants by group
| PR MZ | PR DZ | Co MZ | Co DZ | HC MZ | HC DZ | p-Value* | |
|---|---|---|---|---|---|---|---|
| Frequencya, N = 195 | 30 (4) | 23 (3) | 24 (4) | 25 (5) | 56 | 37 (1) | n.s. |
| Age, mean (SD) | 38.6 (9.8) | 43.2 (9.5) | 39.9 (10.9) | 42.2 (10.2) | 40.7 (11.2) | 41.2 (9.7) | n.s. |
| Sex, male/female (% male) | 19/11 (63) | 11/12 (48) | 14/10 (58) | 11/14 (44) | 26/30 (46) | 22/15 (60) | n.s. |
| Con-/discordant (N) | 7/24 | 1/23 | 0/25 | 0/25 | – | – | n.s. |
| Handedness (right/non-right) | 28/3 | 19/5 | 22/3 | 21/4 | 49/9 | 37/3 | n.s. |
| Years of education, mean (SD) | 13.4 (2.9)b | 13.4 (2.5)b | 13.9 (3.3)c | 14.6 (3.9) | 15.4 (3.0) | 16.5 (2.6) | p < 0.05 |
| Age at first diagnosis, mean (SD) | 26.7 (8.6)d | 24.0 (5.6) | – | – | – | – | p < 0.05 |
| Years since diagnosis, mean (SD) | 11.4 (6.3)e | 18.4 (10.0) | – | – | – | – | p < 0.05 |
| Antipsychotic medication (% of patients, their current mean daily dose) | |||||||
| Amisulpride | 6%, 225 mg | 4%, 300 mg | – | – | – | – | – |
| Aripiprazole | 13%, 11 mg | 8%, 15 mg | – | – | – | – | – |
| Chlorprothixene | 3%, 50 mg | 17%, 143 mg | – | – | – | – | – |
| Clozapine | 3%, 350 mg | 17%, 225 mg | – | – | – | – | – |
| Haloperidol | 0% | 8%, 11 mg | – | – | – | – | – |
| Olanzapine | 16%, 15 mg | 8%, 16 mg | – | – | – | – | – |
| Paliperidone | 3%, 9 mg | 4%, 3 mg | – | – | – | – | – |
| Perphenazine | 3%, 24 mg | 4%, 8 mg | – | – | – | – | – |
| Quetiapine | 16%, 75 mg | 25%, 167 mg | – | – | – | – | – |
| Ziprasidone | 3%, 120 mg | 4%, 160 mg | – | – | – | – | – |
| Zuclopenthixol | 6%, 9 mg | 4%, 36 mg | – | – | – | – | – |
| % Of patients currently using any antipsychotic medication | 52% | 71% | 0% | 0% | 0% | 0% | – |
| Psychopathology assessed by the Positive and Negative Syndrome Scale (PANSS) | |||||||
| Positive, mean (SD) | 15.0 (5.8)f | 14.1 (6.4)f | 8.8 (2.5) | 9.1 (3.6) | 7.3 (0.9) | 7.1 (0.4) | p < 0.05 |
| Negative, mean (SD) | 16.7 (7.4)f | 15.5 (7.4)f | 9.5 (2.9) | 9.3 (3.6) | 8.2 (3.3) | 7.8 (1.1) | p < 0.05 |
| General, mean (SD) | 31.2 (9.4)f | 31.1 (9.7)f | 20.4 (4.2) | 19.9 (6.2) | 17.3 (2.6) | 16.8 (1.5) | p < 0.05 |
| Total, mean (SD) | 62.9 (19.9)f | 60.6 (20.7)f | 38.6 (7.8) | 38.3 (11.5) | 32.7 (6.2) | 31.7 (1.7) | p < 0.05 |
| Global assessment of function (GAF-F) | |||||||
| Mean (SD) | 47.9 (20.7)g | 50.1 (21.5)g | 78.2 (14.9)h | 82.3 (16.0) | 92.4 (6.9) | 91.8 (8.4) | p < 0.05 |
PR proband F2x.x International Classification of Disease version 10 (ICD-10) definition of schizophrenia spectrum disorders (schizophrenia, schizotypal and delusional disorders), MZ monozygotic, DZ dizygotic, Co unaffected co-twin of proband, HC healthy control, SD standard deviation; age at first diagnosis and years since diagnosis are calculated from first psychiatric diagnosis
aNumber of subjects in the group scanned without co-twin
*One-way ANOVA followed by Hochberg post-hoc test:
b PR < HC
cCo MZ < HC DZ
dPR MZ > PR DZ
ePR MZ < PR DZ
fPR > Co and HC
gPR < Co and HC
hCo Mz < DZ and HC
1H-MRS data acquisition
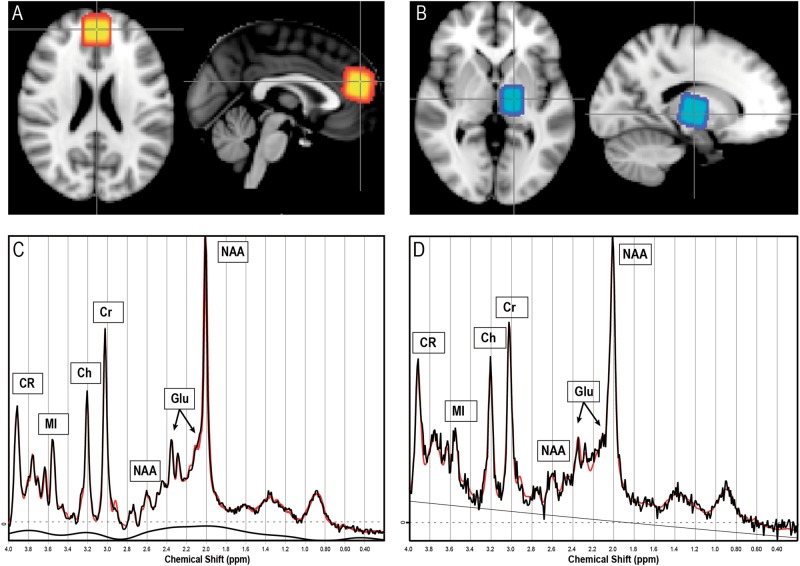
1H-MRS data were acquired on a 3-Tesla Philips Achieva system (Philips, Best, The Netherlands) equipped with a 32-channel head coil (Invivo, Orlando, Florida). The system was upgraded approximately halfway through inclusion, but care was taken to balance groups before and after the upgrade, and all twin pairs were scanned on the same or nearly the same date avoiding any twin pair being scanned across the upgrade. Eight volunteers were scanned before and after the upgrade for which, no significant differences were found in metabolite levels (by paired t-test). One ACC voxel (20 × 20 × 20 mm3) was positioned bilaterally covering Brodman areas 24 and 32. Another voxel was positioned in the left thalamus (15 × 20 × 20 mm3), see Figs. 1a, b for voxel placement.
Fig. 1.
Voxel positioning and metabolite spectra. Voxel positioning: average voxel placement for all subjects shown in model space, with lighter areas indicating higher overlap. Voxel location in the anterior cingulate cortex (a) and in the left thalamus (b). Representative spectrum with raw (in black) and fitted data (in red) from the anterior cingulate cortex (c) and the left thalamus (d)
Spectra were acquired using a Point-Resolved Spectroscopy Sequence (PRESS) (TR = 3000 ms, TE = 30 ms, 96 averages) and analyzed using LCModel version 6.3-1 J (Provencher 1993). Spectral quality allowed for reliable assessment of glutamate (predefined primary measure), glx (glutamate + glutamine, for comparison with studies reporting on glx), NAA, total Cho (GPC + PCh), creatine (Cr + PCr), and MI, see Figs. 1c, d for representative spectra. Internal non-water suppressed acquisition was used as a reference and all metabolite values were corrected for cerebrospinal fluid contamination as described in Supplementary Material.
Quality control of MRS measures
A total of 15 spectra in the ACC and 13 in the thalamus were discarded due to acquisition error or by visual inspection, leaving a total of 180 spectra in the ACC and 182 in the thalamus (Table S2 and S3). The full-width at half maximum (FWHM) of the unsuppressed water resonance was below 12 Hz for all spectra and the Cramer-Rao Lower bound (CRLB) for all metabolites included in the analysis were below 12%. Quality control procedures including FWHM, CRLB, and signal to noise ratio (SNR) analyses are described in detail in the Supplementary Material.
Statistical analyses
Demographic variables, clinical characteristics, and metabolite levels are reported as frequencies, mean values (standard deviations), or percentages in Table 1 and Table S3. Outlier detection excluded metabolite levels differing >3 standard deviations from the mean of the total sample, see Table S2 and S3.
To disentangle the genetic and environmental influences on a trait, the twin model uses the fact that MZ twins share (almost) 100% of their genes, whereas DZ twins share on average 50% of their segregating genes. If MZ twins are more alike than DZ twins, this indicates that a genetic factor explains variation between individuals. If MZ and DZ twins are alike to an equal extent, as indicated by significant, but not different correlations between members of a twin pair within the MZ and DZ groups, then common environmental factors are assumed to play a role. The model including both these factors is usually referred to as an ACE model, including A (additive genetic), C (common environmental) and E (unique environmental, including measurement error) factors [37]. Heritability h2 can then be defined as the proportion of variance explained by additive genetic factors (A/(A + C + E)). Correspondingly, effects of common and unique environmental factors can be determined by c2 = C/(A + C + E) and e2 = E/(A + C + E), respectively. Cross-trait cross-twin correlations (in this case between disease liability and metabolites) within the MZ and DZ groups provide information on the genetic overlap between two traits. A phenotypic correlation rph can therefore be separated into a genetic rph-a, a common environmental rph-c, and a unique environmental component rph-e. The genetic component rph-a represents the correlation that would have been observed if only genetic factors were taken into account. It is a function of the genetic correlation, indicating whether the same genetic factors are explaining variation between individuals for both traits, and the heritability of both individual traits [47]. The significance of variance components was determined by comparing the difference between −2∗log-likelihood of the full model and −2∗log-likelihood of a model in which the h2, c2 or both for metabolite concentration were constrained at zero, which follows a mixture of chi-square distributions [48]. If the familial component (h2 + c2) was significant but not h2 or c2, we then determined the best fitting model (either AE or CE for metabolite concentration) based on the Akaike Information Criterion (AIC). Significance of correlations was based on the 95% confidence intervals.
The OpenMx software package (version 2.3.1; [49]) installed on the R platform (version 3.1.2) was used for twin model setup and analyses via structural equation modeling (SEM). The liability threshold for schizophrenia spectrum disorder was fixed in correspondence with an overall population prevalence of 1.85% [35]. Heritability (h2) of schizophrenia spectrum disorder was fixed at 73% and unique environmental influences on liability for schizophrenia spectrum disorder were assumed to be 27% (based on estimates in the Danish population [35]). All metabolite variables (six metabolites in two structures) were corrected for age, sex, scanner upgrade (using a binary variable for correction), and gray matter fraction using a linear model (effects of these variables on metabolites are listed in Supplementary Table S4). Significance of correlations was based on 95% confidence intervals. Further description of genetic modeling can be found in the Supplementary Material. Glutamate levels corrected for effects of gender, age, and scanner upgrade (residuals) were submitted to general linear model analyses to determine the influences of duration of illness, PANSS total scores, handedness (Edinburgh Handedness Inventory), and use of current antipsychotic medication (binary variable) separately.
Results
Heritability of metabolites
Heritability estimates and contributions of common and unique environmental influences from the full ACE model for metabolites can be found in Table 2. Significant genetic influences were found in the full ACE model for both glutamate (16%) and glx (31%) in the thalamus, Cho in both the ACC (38%), and the thalamus (60%), and Cr (37%) and MI (33%) in the ACC. Based on a significant familial variance component and the AIC, the AE model was the best fitting model for glutamate, glx, Cho in the thalamus and the ACC, and NAA, MI and Cr in the ACC, showing heritability estimates of 16 to 60%. Notably, in the AE model a significant contribution of genetic factors was found for glutamate and glx not only in the thalamus, but also in the ACC; see Table 2.
Table 2.
Estimates of heritability, environmental influences, and correlation of metabolite levels to liability for schizophrenia spectrum disorder (F2x.x)
| Metabolite | Model output full ACE model | Model output best fitting model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| h2 [CI] | c2 [CI] | e2 [CI] | Correlation SZ spectrum liability— metabolite concentration | p Familial | Best | h2 [CI] | c2 [CI] | e2 [CI] | ||
| Left thalamus | Glutamate (N = 181) | 0.16** [0.01 to 0.36] | 0.00 [0 to 0.24] | 0.84 [0.64 to 0.98] | 0.16* [0.02 to 0.29] | 0.010 | AE | 0.16** [0.02 to 0.36] | — | 0.84 [0.64 to 0.98] |
| Glx (N = 178) | 0.31** [0.01 to 0.49] | 0.00 [0 to 0.30] | 0.69 [0.51 to 0.90] | 0.11 [−0.04 to 0.25] | 0.003 | AE | 0.31*** [0.10 to 0.49] | — | 0.69 [0.51 to 0.89] | |
| NAA (N = 182) | 0.00 [0 to 0.27] | 0.23 [0 to 0.39] | 0.77 [0.61 to 0.96] | −0.06 [−0.20 to 0.08] | 0.063 | E | — | — | 1.00 | |
| Choline (N = 181) | 0.60** [0.19 to 0.73] | 0.00 [0 to 0.36] | 0.40 [0.27 to 0.58] | −0.08 [−0.23 to 0.06] | < 0.001 | AE | 0.60*** [0.42 to 0.73] | — | 0.40 [0.27 to 0.58] | |
| Creatine (N = 182) | 0.00 [0 to 0.45] | 0.29 [0 to 0.46] | 0.70 [0.54 to 0.88] | −0.03 [−0.17 to 0.11] | 0.016 | CE | — | 0.29** [0.11 to 0.45] | 0.71 [0.55 to 0.89] | |
| MI (N = 182) | 0.00 [0 to 0.34] | 0.20 [0 to 0.38] | 0.80 [0.62 to 1] | 0.12 [−0.02 to 0.26] | 0.133 | E | — | — | 1.00 | |
| Anterior cingulate cortex | Glutamate (N = 175) | 0.29 [0.00 to 0.49] | 0.00 [0 to 0.37] | 0.70 [0.51 to 0.94] | 0.01 [−0.13 to 0.14] | 0.018 | AE | 0.29** [0.06 to 0.49] | — | 0.71 [0.51 to 0.94] |
| Glx (N = 173) | 0.31 [0 to 0.50] | 0.00 [0 to 0.39] | 0.69 [0.50 to 0.91] | −0.07 [−0.21 to 0.07] | 0.024 | AE | 0.31** [0.09 to 0.50] | — | 0.69 [0.50 to 0.91] | |
| NAA (N = 180) | 0.27 [0 to 0.55] | 0.12 [0 to 0.47] | 0.62 [0.45 to 0.82] | −0.16* [−0.29 to −0.02] | 0.027 | AE | 0.39*** [0.20 to 0.55] | — | 0.61 [0.45 to 0.80] | |
| Choline (N = 179) | 0.38* [0.01 to 0.58] | 0.00 [0 to 0.16] | 0.62 [0.42 to 0.86] | −0.10 [−0.24 to 0.05] | 0.010 | AE | 0.38** [0.14 to 0.58] | — | 0.62 [0.42 to 0.86] | |
| Creatine (N = 180) | 0.37* [0.01 to 0.56] | 0.00 [0 to 0.34] | 0.63 [0.44 to 0.87] | −0.25*** [−0.38 to −0.11] | 0.005 | AE | 0.37** [0.12 to 0.56] | — | 0.63 [0.44 to 0.87] | |
| MI (N = 180) | 0.33* [0.07 to 0.52] | 0.00 [0 to 0.17] | 0.67 [0.48 to 0.88] | −0.07 [−0.21 to 0.08] | 0.020 | AE | 0.33** [0.12 to 0.52] | — | 0.67 [0.48 to 0.88] | |
Model output full ACE model (left part): The model output for the bivariate model in which the full ACE model was assumed for metabolite concentration. Heritability estimates (h2), common environmental influences (c2) and unique environmental influences (e2) are reported with confidence intervals (CI), together with the estimated association between the liability for schizophrenia (SZ) spectrum disorders and metabolite concentration. Significant variance components or correlations are displayed in bold. When a familial component (A + C, p familial) acting on the metabolite concentration was significant, the best fitting model (AE, CE, or E) was determined based on the AIC criterion. Model output best fitting model (right part): The model output for the best fitting model for the metabolite. In all cases, the heritability of liability for schizophrenia spectrum disorders was fixed at 73%, and unique environmental influences were 27%. Prevalence of schizophrenia spectrum disorders was fixed at 1.85%. Spectra with poor quality (e.g., CRLB > 20%) and outliers of > 3 standard deviations were excluded, the number of subjects included in each analysis is denoted by N. Significant results are displayed in bold.
ACC anterior cingulate cortex, Glx glutamate and glutamine combined, NAA N-acetyl aspartate, MI myo-inositol
*p-Value < 0.05
**p-Value < 0.01
***p-Value < 0.001
Associations of metabolites with schizophrenia spectrum disorder (F2x.x)
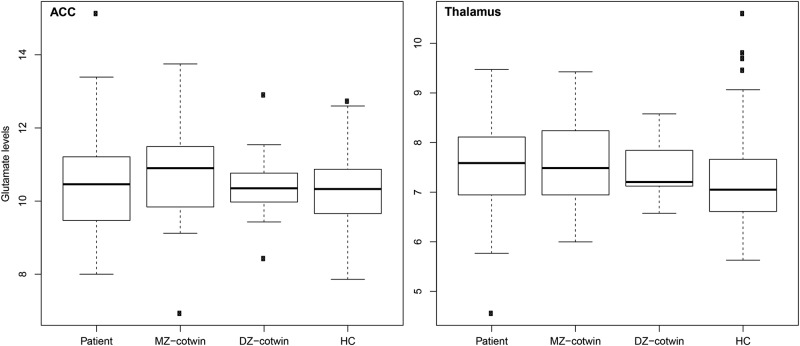
Glutamate concentrations in the thalamus were positively correlated with liability for schizophrenia spectrum disorder (r = 0.16; p = 0.03) due to a significant positive genetic part of the correlation (rph-a = 0.21 [0.04 to 0.36]). This reflects a significant pattern of elevated glutamate levels in patients and MZ co-twins compared with DZ co-twins and even more so compared with HCs in the thalamus (see box plots in Fig. 2 and Figure S2 (of residuals used for analyses) in Supplementary Material. Data for glx are presented in Figure S3). The effect is detected by using structural equation modeling even though there was no significant effect of group when using analysis of variance (ANOVA). Metabolite concentrations in the ACC were negatively correlated with schizophrenia spectrum liability for NAA (r = −0.16; p = 0.02) and for Cr (r = −0.25; p = 0.006). For NAA, the correlation was attributable to unique environmental effects (rph-e = −0.15 [−0.25 to −0.03]). For Cr, the correlation was due to a significant negative genetic part of the correlation (rph-a = −0.17 [−0.33 to −0.01]). See Table 2, for overview.
Fig. 2.
Glutamate levels across twin groups. Box plots showing the glutamate levels in institutional units/H2O for patients; monozygotic (MZ) co-twins; dizygotic (DZ) co-twins, and control twins (HC), in the anterior cingulate cortex (ACC) and the left thalamus. The whiskers indicate 1.5 times the interquartile range. By using structural equation modeling, a significant pattern of increased glutamate levels in MZ co-twins compared with DZ co-twins and even more so compared with healthy controls was found in the left thalamus. The glutamate levels in the patients are comparable to those in MZ co-twins in the left thalamus but numerically lower in the ACC
Correlations to clinical measures and current use of antipsychotic medication
We did not find duration of illness, PANSS total scores, handedness, or current medication to influence levels of glutamate neither in the ACC nor in the left thalamus of patients (all p > 0.05).
Additional analyses of probands with narrow schizophrenia diagnosis (F20.x)
We repeated our analyses excluding the proband pairs in which the patients did not meet the narrow F20.x criterion for schizophrenia. The main results from these analyses include very similar findings of heritability for glutamate (19%) and glx (36%) in the left thalamus. However, significant correlation to disease liability could only be established for Cr in the ACC in this smaller sample (see Supplementary Material for a description and Table S5 for results).
Quality control of MRS measures
In the ACC, a significant effect of group was detected for SNR, with post-hoc test showing a lower SNR for MZ probands and MZ Co-twins compared with DZ Co-twins and HC, see Table S2 in Supplementary Material.
Discussion
We found glutamate levels in the ACC and the left thalamus to be heritable (h2 = 29 and 16%, respectively) and positively correlated to schizophrenia spectrum liability in the left thalamus. In subsequent analyses, the correlation to disease liability proved to be carried by common genes influencing both glutamate levels in the left thalamus and liability for schizophrenia spectrum disorder. From this, it can be inferred that the aberrant increased glutamate levels found in the left thalamus of patients can be explained by genetic factors. A finding of common genetic influences on disease liability and increased glutamate levels in the left thalamus means that glutamate levels in unaffected MZ co-twins are increased compared with unaffected DZ co-twins and even more so compared with HCs [50] (see Fig. 2). The glutamate and glx levels in the MZ co-twins were comparable to those in patients.
The finding of common genetic influences on glutamate levels in the thalamus and disease in our study is supported by a report of higher glx levels in the thalamus of subjects at increased familial risk for schizophrenia [33], and a recent study linking higher glx levels to glutamate-related genes associated with risk for schizophrenia [5]. The current heritability estimates are in line with the heritability estimate of glx in the thalamus reported in a twin study in children with autism and controls [34], suggesting a generalizability of the heritability estimate across populations. The present study could not confirm exploratory findings from a MRS study of 2 MZ and 12 DZ twin pairs discordant for schizophrenia describing decreased glutamate levels in the ACC of patients and unaffected co-twins [29]. Our current findings do not support glutamate in ACC as a trait marker for schizophrenia risk, since no significant differences between groups were found in the ACC, and glutamate levels in MZ co-twins were numerically higher than in HC while patients had lower levels (possibly due to effects of antipsychotic medication).
Glutamatergic alterations in the thalamus have long been hypothesized to play a central part in the pathophysiology of schizophrenia [51, 52]. This is supported by studies of antipsychotic naive first episode [10, 11], as well as chronic [13] schizophrenia patients, reporting higher glutamate and glutamine levels in the thalamus, though the metabolites are found unaltered in a study of minimally treated patients at 4 Tesla [17] and a meta-analysis only found alterations of glutamine in patients with schizophrenia [2]. Together with the current data, the evidence points to a possible dysfunction of glutamate metabolism in the thalamus in patients with schizophrenia irrespective of the phase of the illness. An effect that, according to current findings of shared genetic influences on glutamate levels in thalamus and disease liability, may be carried by genetic factors. The absence of an association between the ACC glutamate levels and disease in our study is in line with a recent meta-analysis [2]. Voxel position could influence results in ACC since findings of increased glutamate or glx levels are reported in studies of a more ventral (pregenual) voxel [21, 53], whereas unaltered metabolite levels are reported in a more dorsal voxel placement [19, 20], similar to the one in the current study. In the ACC, we found no significant difference in glutamate or glx levels between groups but numerically the unaffected MZ co-twins had the highest concentration and patients had the lowest (see Figure S2 and S3 in Supplementary Material). This could point to unique environment, such as lifestyle (e.g., tobacco use [54, 55]) or other disease- or treatment-related processes decreasing glutamate levels in patients. Reductions of glutamate and glx have been reported after antipsychotic treatment [22, 56, 57], but in the current study we did not find a significant influence of current antipsychotic medication on glutamate levels, possibly due to limited statistical power. No significant effects of disease on glx levels were detected in the current study, possibly due to glutamine being less reliably quantified with a PRESS sequence [58].
Significant estimates of heritability were also found for NAA (h2 = 39%), Cr (h2 = 37%), and MI (h2 = 33%) in the ACC, and Cho in both the thalamus (h2 = 60%) and the ACC (h2 = 38%). Common environmental influence was significant for Cr (c2 = 29%) in the thalamus. Although the scanned brain regions are different, our findings are in accordance with a study of elderly healthy twins estimating heritability of these metabolites (mean age 72). Using a 1.5 Tesla MRI scanner, Batouli et al. [38] found NAA (h2 = 72%), Cho (h2 = 33%), Cr (h2 = 51%), and MI (h2 = 55%) to be heritable in the posterior cingulate cortex. In the MRS study of children with autism, contradictory to the current findings in left thalamus, Cho was not found to be heritable but NAA and MI were found heritable in the thalamus bilaterally [34]. These discrepancies with our findings could possibly be due to differences in age, although this is not currently supported. Among these metabolites, we found a negative correlation with schizophrenia spectrum liability for NAA and Cr in the ACC. The present findings are in part in agreement with earlier studies in chronic patients. Two meta-analyses [39, 40] have found decreased levels of NAA in the frontal cortex and the thalamus of patients with schizophrenia, but no significant differences of Cr. The correlation between schizophrenia spectrum liability and NAA, generally considered to be a marker for neuronal health [59], and Cr, involved in neuronal metabolism [60], could point to an altered neuronal viability and energy expenditure in schizophrenia. The negative correlation between schizophrenia spectrum liability and Cr is explained by genetic influences and emphasizes this metabolite as a candidate genetic marker.
By using register-based inclusion, this study largely avoids inclusion bias and enables effective match on age and sex. The study is limited by including the broader schizophrenia spectrum, but in the analyses of the subgroup of patients with a narrow schizophrenia diagnosis (ICD-10 F20.x as opposed to F2x.x) the observed heritability results were consistent with results based on the total sample. This corresponds to earlier work from our group finding comparable heritability estimates of schizophrenia spectrum disorder 73% and schizophrenia 79% in the largest twin sample to date [35]. The variability in duration of illness was partly addressed by correcting the data for age of the included subjects. Medication of the probands with antipsychotic compounds is a limitation, but work in progress from our own group, as well as the work from others [2, 10, 11, 13] suggest that abnormalities of glutamate metabolism are present in both antipsychotic naive and chronic patients. Moreover, in the present study we did not find an effect of current use of antipsychotic medication on glutamate levels. Quality control showed a smaller SNR for MZ proband pairs compared with DZ co-twins and HC (Table S2) potentially leading to an underestimation of genetic effects.
It should be noted that the co-twins, though unaffected by schizophrenia spectrum disorders, are to some extent influenced by other psychiatric disease processes (Table S1). Furthermore, reservations due to the inability to distinguish glutamate in the synapse from vesicular or metabolic glutamate are relevant, since the current MRS techniques do not provide this possibility. However, a recent study found increases in glutamate levels in the ACC upon activation by a cognitive task [61], suggesting that the measured glutamate may be an indicator of glutamatergic signaling. Finally, on account of noise being included in unique environmental influences, we cannot disentangle true unique environment from noise.
In conclusion, the present study provides estimates of heritability for cerebral glutamate levels in the left thalamus and the ACC and finds a genetic association between the glutamate levels in the left thalamus and liability for schizophrenia spectrum disorder in the same cohort. Together, this establishes alterations in thalamic glutamate levels as a possible genetic marker for schizophrenia.
Funding and disclosure
The study was funded by The Lundbeck Foundation (grant no. 25-A2701 and R155-2013-16337) and C.S.L. was supported by a grant from The Mental Health Services – Capital Region of Denmark. Dr. Glenthøj is the leader of a Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), which is partially financed by an independent grant from the Lundbeck Foundation, based on international review and partially financed by the Mental Health Services in the Capital Region of Denmark, the University of Copenhagen, and other foundations. Her group has also received a research grant from Lundbeck A/S for another independent investigator initiated study. All grants are the property of the Mental Health Services in the Capital Region of Denmark and administrated by them. She has no other conflicts to disclose. The authors declare no competing interests.
Electronic supplementary material
Acknowledgements
The authors would like to acknowledge the valuable contribution of Mikkel Erlang Sørensen and Helle Schæbel in the recruitment and data collection process, and Lisbeth Jensen for keeping everything running smoothly.
Competing interests
The authors declare no competing interestst.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Christian Stefan Legind, Brian Villumsen Broberg.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0236-0).
References
- 1.Marsman A, van den Heuvel MP, Klomp DWJ, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull. 2013;39:120–9. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merritt K, Egerton A, Kempton MJ, Taylor MJ, Mcguire PK. Nature of glutamate alterations in schizophrenia a meta-analysis of proton magnetic resonance spectroscopy studies. JAMA Psychiatry. 2016;73:1–10. doi: 10.1001/jamapsychiatry.2016.0442. [DOI] [PubMed] [Google Scholar]
- 3.Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014. 10.1038/nature13595. [DOI] [PMC free article] [PubMed]
- 4.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bustillo JR, Patel V, Jones T, Jung R, Payaknait N, Qualls C, et al. Risk-conferring glutamatergic genes and brain glutamate plus glutamine in schizophrenia. Front Psychiatry. 2017;8:1–9. doi: 10.3389/fpsyt.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycine-site agonists. Neuropsychopharmacology. 2004;29:300–7. doi: 10.1038/sj.npp.1300313. [DOI] [PubMed] [Google Scholar]
- 7.Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37:2515–21. doi: 10.1038/npp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poels EMP, Kegeles LS, Kantrowitz JT, Javitt DC, Lieberman JA, Abi-Dargham A, et al. Glutamatergic abnormalities in schizophrenia: a review of proton MRS findings. Schizophr Res. 2014;152:325–32. doi: 10.1016/j.schres.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egerton A, Bhachu A, Merritt K, McQueen G, Szulc A, McGuire P. Effects of antipsychotic administration on brain glutamate in schizophrenia: a systematic review of longitudinal 1H-MRS studies. Front Psychiatry. 2017;8:1–7. doi: 10.3389/fpsyt.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bojesen K, Jessen K, Sigvard A, Jensen MB, Rostrup E, Broberg BV, et al. 87. Glutamate and GABA in antipsychotic-naive schizophrenia and association with treatment outcome. Schizophr Bull. 2017;43:S48–S48. doi: 10.1093/schbul/sbx021.126. [DOI] [Google Scholar]
- 11.Théberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–6. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 12.Théberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, et al. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. Br J Psychiatry. 2007;191:325–34. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- 13.Théberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RWJ, Rajakumar N, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160:2231–3. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- 14.Galińska B, Szulc A, Tarasów E, Kubas B, Dzienis W, Czernikiewicz A, et al. Duration of untreated psychosis and proton magnetic resonance spectroscopy (1H-MRS) findings in first-episode schizophrenia. Med Sci Monit. 2009;15:CR82–88. [PubMed] [Google Scholar]
- 15.Szulc A, Galinska B, Tarasow E, Dzienis W, Kubas B, Konarzewska B, et al. The effect of risperidone on metabolite measures in the frontal lobe, temporal lobe, and thalamus in schizophrenic patients. A proton magnetic resonance spectroscopy (1 H MRS) study. Pharmacopsychiatry. 2005;38:214–9. doi: 10.1055/s-2005-873156. [DOI] [PubMed] [Google Scholar]
- 16.Szulc A, Galinska B, Tarasow E, Waszkiewicz N, Konarzewska B, Poplawska R, et al. Proton magnetic resonance spectroscopy study of brain metabolite changes after antipsychotic treatment. Pharmacopsychiatry. 2011;44:148–57. doi: 10.1055/s-0031-1279739. [DOI] [PubMed] [Google Scholar]
- 17.Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, et al. 1H-MRS at 4 Tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15:629–36. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartha R, Williamson PC, Drost DJ, Malla A, Carr TJ, Cortese L, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959–65. doi: 10.1001/archpsyc.1997.01830220085012. [DOI] [PubMed] [Google Scholar]
- 19.Egerton A, Broberg BV., Haren N van, Merritt K, Barker GJ, Lythgoe DJ, et al. Response to initial antipsychotic treatment in first episode psychosis is related to anterior cingulate glutamate levels: a multicentre1H-MRS study (OPTiMiSE). Mol Psychiatry 2018;1–11. 10.1038/s41380-018-0082-9 [DOI] [PubMed]
- 20.Tibbo PG, Bernier D, Hanstock CC, Seres P, Lakusta B, Purdon SE. 3-T proton magnetic spectroscopy in unmedicated first episode psychosis: a focus on creatine. Magn Reson Med. 2013;69:613–20. doi: 10.1002/mrm.24291. [DOI] [PubMed] [Google Scholar]
- 21.Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69:449–59. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 22.la Fuente-Sandoval C de, Reyes-Madrigal F, Mao X, León-Ortiz P, Rodríguez-Mayoral O, Jung-Cook H, et al. Prefrontal and sStriatal gamma-aminobutyric acid levels and the effect of antipsychotic treatment in first-episode psychosis patients. Biol Psychiatry. 2018;83:475–483. [DOI] [PMC free article] [PubMed]
- 23.Yang Z, Zhu Y, Song Z, Mei L, Zhang J, Chen T, et al. Comparison of the density of gamma-aminobutyric acid in the ventromedial prefrontal cortex of patients with first-episode psychosis and healthy controls. Shanghai Arch Psychiatry. 2015;27:341–8. doi: 10.11919/j.issn.1002-0829.215130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Wang J, Tang Y, Zhang T, Cui H, Xu L, et al. Reduced γ-aminobutyric acid and glutamate+glutamine levels in drug-naïve patients with first-episode schizophrenia but not in those at ultrahigh risk. Neural Plast. 2016;2016. 10.1155/2016/3915703. [DOI] [PMC free article] [PubMed]
- 25.Natsubori T, Inoue H, Abe O, Takano Y, Iwashiro N, Aoki Y, et al. Reduced frontal glutamate+glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia. Schizophr Bull. 2014;40:1128–39. doi: 10.1093/schbul/sbt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tayoshi S, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, ichi Iga J, et al. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS) Schizophr Res. 2009;108:69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Marsman A, Mandl RCW, Klomp DWJ, Bohlken MM, Boer VO, Andreychenko A, et al. GABA and glutamate in schizophrenia: a 7 T 1H-MRS study. NeuroImage Clin. 2014;6:398–407. doi: 10.1016/j.nicl.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Öngür D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biol Psychiatry. 2010;68:667–70. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutkenhoff ES, van Erp TG, Thomas MA, Therman S, Manninen M, Huttunen MO, et al. Proton MRS in twin pairs discordant for schizophrenia. Mol Psychiatry. 2010;15:308–18. doi: 10.1038/mp.2008.87. [DOI] [PubMed] [Google Scholar]
- 30.Byun MS, Choi JS, Yoo SY, Kang DH, Choi CH, Jang DP, et al. Depressive symptoms and brain metabolite alterations in subjects at ultra-high risk for psychosis: a preliminary study. Psychiatry Investig. 2009;6:264–71. doi: 10.4306/pi.2009.6.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egerton A, Stone JM, Chaddock CA, Barker GJ, Bonoldi I, Howard RM, et al. Relationship between brain glutamate levels and clinical outcome in individuals at ultra high risk of psychosis. Neuropsychopharmacology. 2014;39:2891–9. doi: 10.1038/npp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, et al. Glutamate dysfunction in people with prodromal symptoms of ppsychosis: relationship to gray matter volume. Biol Psychiatry. 2009;66:533–9. doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 33.Tandon N, Bolo NR, Sanghavi K, Mathew IT, Francis AN, Stanley JA, et al. Brain metabolite alterations in young adults at familial high risk for schizophrenia using proton magnetic resonance spectroscopy. Schizophr Res. 2013;148:59–66. doi: 10.1016/j.schres.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Hegarty JP, Gu M, Spielman DM, Cleveland SC, Hallmayer JF, Lazzeroni LC, et al. A proton MR spectroscopy study of the thalamus in twins with autism spectrum disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;81:153–60. doi: 10.1016/j.pnpbp.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilker R, Helenius D, Fagerlund B, Skytthe A, Christensen K, Werge TM, et al. Heritability of schizophrenia and schizophrenia spectrum based on the nationwide Danish Twin Register. Biol Psychiatry. 2018;83:492–8. [DOI] [PubMed]
- 36.Falconer D, Mackay T. Introduction to quantitative genetics. Essex, UK: Longman Group Ltd.; 1996. [Google Scholar]
- 37.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat Rev Genet. 2002;3:872–82. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 38.Batouli SAH, Sachdev PS, Wen W, Wright MJ, Suo C, Ames D, et al. The heritability of brain metabolites on proton magnetic resonance spectroscopy in older individuals. Neuroimage. 2012;62:281–9. doi: 10.1016/j.neuroimage.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 39.Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, et al. Neurometabolites in schizophrenia and bipolar disorder - a systematic review and meta-analysis. Psychiatry Res. 2012;203:111–25. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwerk A, Alves FDS, Pouwels PJW, Van Amelsvoort T. Metabolic alterations associated with schizophrenia: a critical evaluation of proton magnetic resonance spectroscopy studies. J Neurochem. 2014;128:1–87. doi: 10.1111/jnc.12398. [DOI] [PubMed] [Google Scholar]
- 41.Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–62. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen CB, Gøtzsche H, Møller JOslash, Mortensen PB. The Danish civil registration system A cohort of eight million persons. Dan Med Bull. 2006;53:441–9. [PubMed] [Google Scholar]
- 43.Skytthe A, Ohm Kyvik K, Vilstrup Holm N, Christensen K. The Danish twin registry. Scand J Public Health. 2011;39:75–78. doi: 10.1177/1403494810387966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39:54–57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- 45.Wing J, Babor T, T B, Burke J, Cooper J, Giel R, et al. SCAN schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–93. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 46.Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39:964–71. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 47.Toulopoulou T, Picchioni M, Rijsdijk F, Hua-Hall M, Ettinger U, Sham P, et al. Substantial genetic overlap between neurocognition and schizophrenia. Arch Gen Psychiatry. 2007;64:1348. doi: 10.1001/archpsyc.64.12.1348. [DOI] [PubMed] [Google Scholar]
- 48.Dominicus A, Skrondal A, Gjessing HK, Pedersen NL, Palmgren J. Likelihood ratio tests in behavioral genetics: problems and solutions. Behav Genet. 2006;36:331–40. doi: 10.1007/s10519-005-9034-7. [DOI] [PubMed] [Google Scholar]
- 49.Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76:306–17. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Haren NEM, Rijsdijk F, Schnack HG, Picchioni MM, Toulopoulou T, Weisbrod M, et al. The genetic and environmental determinants of the association between brain abnormalities and schizophrenia: the schizophrenia twins and relatives consortium. Biol Psychiatry. 2012;71:915–21. doi: 10.1016/j.biopsych.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlsson A. The neurochemical circuitry of schizophrenia. Pharmacopsychiatry. 2006;39:10–14. doi: 10.1055/s-2006-931483. [DOI] [PubMed] [Google Scholar]
- 52.Carlsson M, Carlsson A. Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr Bull. 1990;16:425–32. doi: 10.1093/schbul/16.3.425. [DOI] [PubMed] [Google Scholar]
- 53.Bartha R. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54:959. doi: 10.1001/archpsyc.1997.01830220085012. [DOI] [PubMed] [Google Scholar]
- 54.Durazzo TC, Meyerhoff DJ, Mon A, Abé C, Gazdzinski S, Murray DE. Chronic cigarette smoking in healthy middle-aged individuals is associated with decreased regional brain n-acetylaspartate and glutamate levels. Biol Psychiatry. 2016;79:481–8. doi: 10.1016/j.biopsych.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallinat J, Lang UE, Jacobsen LK, Bajbouj M, Kalus P, Von Haebler D, et al. Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3 T. J Clin Psychopharmacol. 2007;27:80–84. doi: 10.1097/JCP.0b013e31802dffde. [DOI] [PubMed] [Google Scholar]
- 56.Goto N, Yoshimura, ReijiKakeda S, Nishimura J, Moriya J, Hayashi K, et al. Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–22. [DOI] [PMC free article] [PubMed]
- 57.De La Fuente-Sandoval C, León-Ortiz P, Azcárraga M, Stephano S, Favila R, Díaz-Galvis L, et al. Glutamate levels in the associative striatum before and after 4 weeks of antipsychotic treatment in first-episode psychosis: a longitudinal proton magnetic resonance spectroscopy study. JAMA Psychiatry. 2013;70:1057–66. doi: 10.1001/jamapsychiatry.2013.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Veenendaal TM, Backes WH, Van Bussel FCG, Edden RAE. Glutamate quantification by PRESS or MEGA-PRESS: validation, repeatability, and concordance. Magn Reson Imaging. 2018;48:107–14. [DOI] [PubMed]
- 59.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AMA. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Béard E, Braissant O. Synthesis and transport of creatine in the CNS: importance for cerebral functions. J Neurochem. 2010;115:297–313. doi: 10.1111/j.1471-4159.2010.06935.x. [DOI] [PubMed] [Google Scholar]
- 61.Taylor R, Schaefer B, Densmore M, Neufeld RWJ, Rajakumar N, Williamson PC, et al. Increased glutamate levels observed upon functional activation in the anterior cingulate cortex using the Stroop Task and functional spectroscopy. Neuroreport. 2015;26:107–12. doi: 10.1097/WNR.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.