Abstract
Introduction: Renal ischemia-reperfusion injury (IRI) causes renal functional alterations which may lead to permanent renal impairment. β-caryophyllene (BCP), a natural bicyclic sesquiterpene, is an important constituent of many edible plants including spices. It is an FDA-approved food additive which possesses potent anti-inflammatory and antioxidant activities and has been shown to protect against chemotherapeutics-induced organ toxicities and against ischemic injuries to heart, liver and brain. Oxidative stress and inflammation is a main accompaniment of renal dysfunction, however, the effect of BCP on IRI-induced renal dysfunction has not been investigated yet and therefore the aim of this study was to investigate the effect of BCP on IRI-induced renal dysfunction. Methods: Wistar rats underwent left renal warm ischemia for 40 minutes. G-BCP (n=13) received oral BCP (50 mg/kg/day) dissolved in a vehicle starting 7 days prior to IRI and continued 7 days thereafter when the renal functions of both kidneys and the markers of oxidative stress and pro-inflammatory cytokines were measured. G-Vx (n=13) underwent similar protocol but received vehicle only. Results: IRI affected hemodynamic (renal blood flow and glomerular filtration rate) and tubular (urine volume, total and fractional urinary sodium excretion) parameters in the left ischemic kidney in G-Vx. Though, BCP did not affect any of these alterations in the ischemic kidney (P>0.05 for all). However, it attenuated the alterations in malondialdehyde (MDA) and glutathione (GSH) in the left ischemic kidney in G-BCP compared to G-Vx (9.3±2.1 vs. 4.8±1.0, P=0.047 and 18.1±2.5 vs. 13.6±1.7, P=0.09, respectively). Conclusion: Our study results demonstrate that BCP attenuated the alterations in some of the oxidative stress markers. However, these were not translated in to the protective effects on the haemodynamic and tubular glomerular functions when measured seven days post-IRI. This suggests that BCP has a weak reno-protective effect under ischemic conditions.
Keywords: β-caryophyllene, ischemia-reperfusion injury, renal functions
Introduction
Renal ischemia-reperfusion injury (IRI) is an invariable consequence of several conditions such as resuscitation following systemic hypotension and procedures including renal transplantation, aortic cross clamping and partial nephrectomy [1]. IRI causes renal functional alterations that may eventually result in renal impairment [2-4]. The efforts to decrease ischemia-reperfusion injury have been the subject of many previously performed studies and it’s apparent that several agents and modified technique attenuate the ischemia-reperfusion induced renal injury [3,5-9].
β-caryophyllene (BCP), a natural bicyclic sesquiterpene, is an important constituent of many edible plants including spices such as oregano, cinnamon, cloves, ginger, black pepper and rosemary. It is one of the chemical compounds that contribute to the spiciness of black pepper. BCP is an FDA-approved food additive and is one of the major component of cannabis sativa and Sativex, an approved drug in European countries and Canada [10]. BCP is notable for having a cyclobutane ring, a rarity in nature which contributes to the anti-inflammatory activity of β-caryophyllene.
BCP has been shown to exhibit anti-inflammatory and antioxidant actions and confer organo-protective effects against chemicals and drugs as well as ischemic injuries to brain, liver and heart in experimental studies [11]. BCP has been shown to ameliorate ulcerative colitis, hepatitis, endometriosis, peptic ulcer, neuropathic pain, cerebral ischemia-reperfusion injury and other neurological conditions [11-15]. Recently, BCP has been shown to attenuate nephrotoxicity induced by a chemotherapeutic drug cisplatin [16]. The recent renoprotective effect of BCP against cisplatin, and its effect against ischemic-reperfusion injury of other organs prompted us to investigate the effect of BCP on the kidney dysfunction following ischemia-reperfusion injury in a rat model of warm ischemia-reperfusion injury. We investigated the effect of BCP on renal hemodynamic and tubular functions as well as on oxidative stress markers and biomarkers of inflammation.
Materials and methods
Studies were performed in male Wistar rats weighing 234-263 g at the time of IRI. Rats were housed in standard cages and kept in a 12-hour light-dark cycle at 20°C. They were fed a standard rat chow and had free access to water. Animals were fasted for 12 hours before the experimental procedures but had water ad libitum. The experimental protocol was approved by the Institutional animal research ethics committee.
Ischemia-reperfusion injury
Under aseptic conditions, the animals were anesthetized with ketamine hydrochloride (70 mg/kg, intraperitoneally, Pantex Holland B.V., Holland) and Pentobarbital Sodium (20 mg/kg, intraperitoneally, Sigma Life Science, St Louis, USA). The left renal artery was then exposed via a flank incision and occluded using microvascular non-traumatic bulldog clamp. Following a warm ischemia of 40 min, the microvascular clamp was removed to allow reperfusion. At the end, the wound was closed in layers.
Caryophyllene/vehicle administration
β-caryophyllene was dissolved in olive oil and administered orally by gavage immediately after preparation as single daily dose of 50 mg/kg/day. The reatment was started 7 days before institution of ischemia and continued for 7 days thereafter until the terminal experiment.
Experimental groups
Animals were divided into two groups: 1. G-BCP (n=13): Rats received β-caryophyllene and underwent renal ischemia for 40 min. 2. G-Vx (n=13): Rats received only the vehicle and underwent renal ischemia for 40 min and received only the vehicle (olive oil).
Surgical procedure in the terminal experiment
All rats underwent terminal experiment seven days following IRI. Animals were anaesthetised with pentobarbital sodium (45 mg/kg, intraperitoneally; Sigma Life Science, St Louis, USA) and the trachea was cannulated. The right femoral vein was then cannulated with polyethylene tubing (PE-50) and anaesthesia was maintained by a continuous infusion of pentobarbital sodium (15 mg/kg/hr) and a sustaining infusion of 0.9% saline was established at a rate of 50 µl/min using an infusion pump. The left femoral artery was cannulated with similar tubing used in the femoral vein and the tip of the cannula was positioned just below the level of the left renal artery. The cannula was connected to a pressure transducer (Memscap, Skoppum, Norway). The blood pressure signal was amplified using a bridge Amp (AD Instruments, Castle Hill, Australia), digitised using Power Lab 4/30 and Lab Chart version 6 software (AD Instruments, Australia) and displayed on a computer screen. The arterial cannula was also used to obtain blood samples throughout the procedure as required. Both kidneys were exposed through a midline abdominal incision and the upper ureters were cannulated with polyethylene tubing (PE-10) for the collection of urine into pre-weighed micro-capped tubes. The urine volume was determined gravimetrically.
On completion of surgery, the sustaining infusion of 0.9% saline was replaced by one composed of Fluorescein isothiocyanate-inulin (FITC-inulin, Sigma-Aldrich, St Louis, USA) (2.5 mg/ml) and para-aminohippuric acid (PAH, Sigma-Aldrich, MO, USA) (0.4% w/v) in 0.9% saline. A priming dose of 2 ml of the same solution was infused over 2 minutes. Animals were allowed 45 minutes to equilibrate before being subjected to the experimental protocol.
Experimental protocol and assays
The experimental protocol consisted of two 20-minute clearance periods. Arterial blood samples (0.4 ml) taken at the beginning and end of the clearance periods were immediately centrifuged. Plasma samples (125 µl) were frozen to be assayed later. The plasma was replaced by an equal volume of saline and the erythrocytes were re-suspended by gentle vortexing and returned to the animal. The hematocrit was determined. Finally, after euthanizing the animals, the kidneys were removed, weighed and prepared for biochemical studies (vide infra).
Urine and plasma samples were assayed for sodium level using a flame photometer (Corning, Halstead, Essex, England). Glomerular filtration rate (GFR) was estimated from the clearance of inulin. Renal blood flow (RBF) was calculated using the formula [RBF=ERPF/(1-hematocrit)], where the PAH clearance was used to estimate ERPF (effective renal plasma flow). The values of GFR, RBF, urine volume (UV), urinary sodium (UNaV) and fractional excretion of sodium (FENa) were calculated as the average of the two clearance periods and were corrected for kidney weight.
Biochemical studies
Following the terminal experiment, the kidneys were quickly removed and placed on ice and immediately snap frozen in liquid nitrogen for future analysis. Upon finishing all the terminal experiments, the right and left kidney from all rats were collected from each group and homogenized in potassium chloride buffer containing Tris-HCl 10 mM, NaCl 140 mM, KCl 300 mM, EDTA 1 mM, Triton X-100 0.5% at pH 8.0 supplemented with protease and phosphatase inhibitor. The tissue homogenates of the samples were centrifuged at 14000×g for 20 min at 4°C to obtain the post-mitochondrial supernatant (PMS) for estimation of some of the oxidative stress markers and pro-inflammatory cytokines using spectrophotometric measurements and enzyme-linked immunosorbent assay (ELISA).
Protein estimation
The protein contents in the samples were estimated using the Pierce BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA) following the manufacturer’s instructions.
Measurement of malondialdehyde (MDA)
The tissue level of MDA was measured using commercially available kit from North West Life science (Vancouver, WA, USA). Briefly, the samples or calibrators (250 µl) were incubated in the presence of acid reagent and thiobarbituric acid (250 µl) and mixed vigorously using vortex mixer. The samples were incubated for 60 min at 60°C and then centrifuged at 10,000×g for 2-3 min. The resultant reaction mixture was transferred to cuvette and the spectra were recorded at 532 nm. The results were expressed as µM MDA/mg protein.
Measurement of glutathione
The amount of glutathione (GSH) was estimated using the manufacturer’s instructions of the commercially available kit. The samples were first de-proteinized with 5-sulfosalicylic acid solution (5%) and centrifuged to remove the precipitated protein. The supernatant was used to estimate the amount of GSH. The samples or standards (10 µl) were incubated with 150 µl of working mixture (assay buffer+5,5’-Dithiobis (2-nitrobenzoic acid) + glutathione reductase) in a 96 well plate for 5 min. Diluted NADPH solution (50 µl) was added to the each well and mixed properly. The absorbance of the samples was recorded at 412 nm using the microplate reader with the kinetics for 5 min. The results were expressed as µM GSH/mg protein.
Estimation of nitrite levels
The nitrite levels were estimated by commercially available kit (R&D system, Minneapolis, MN, USA). Briefly, the sample or nitrite standard (50 µl) were added with reaction diluent (50 µl) in 96 well plate supplied by manufacturer. Subsequently, Griess reagent (50 µl) was added to the each well and mixed by gentle tapping on the side of the plate. The plate was incubated for 10 min at room temperature and the absorbance was measured at 540 nm using the microplate reader. The nitrite levels were expressed as µmol/mg protein.
Estimation of pro-inflammatory cytokines
The levels of pro-inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α) were estimated using the commercially available ELISA kits purchased from R&D system, Minneapolis, MN, USA. Briefly, 96-well plate was coated with the diluted capture antibody (100 µl) for overnight at room temperature. Each well was aspirated and washed with the wash buffer (0.05% tween 20 in PBS 0.01 M pH 7.4). The plate was blocked by adding reagent diluent (containing 1% bovine serum albumin in PBS (300 µl) for 1 hr and washed with wash buffer. The samples or standards (100 µl) were added to the well and incubated for 2 hrs. Each well was exchanged with the detection antibody (100 µl) and then incubated for 2 hrs at room temperature. The wells were then exchanged with working solution (1:200) of streptavidin horseradish peroxidase (100 µl) and further incubated for 20 min. The wells were exchanged with substrate solution (100 µl) and incubated for 20 min. Stop solution containing 2N H2SO4 (50 µl) was added and the plate was gently tapped to ensure proper mixing. The absorbance of each well was measured immediately at 450 nm using microplate reader. The results were expressed as pg/mg protein.
Statistical analysis
Statistical analysis was performed using SPSS V16.0. Results were expressed as means ± SEM. One-way factorial ANOVA was used for comparison of variables between the two groups and between the control and ischemic kidneys within each group. P value less than 0.05 was considered statistically significant.
Results
The mean arterial blood pressure and heart rate in G-Vx and G-BCP were similar (109±3 vs. 109±2, P=0.94 and 435±7 vs. 428±7, P=0.47).
Glomerular and tubular functions
In G-Vx, left RBF, seven days following IRI, was 45% of the right RBF (3.11±0.58 vs. 6.88±0.75, P=0.001). Similarly, left GFR was 38% that of the right GFR (0.29±0.06 vs. 0.77±0.06, P=0.0001) (Figure 1). With the decrease in both RBF and GFR, the FENa in the left kidney was significantly higher than the right kidney (2.56±0.96 vs. 0.54±0.12, P=0.03). However, both UV and UNaV were similar in the two kidneys (8.45±1.80 vs. 10.45±2.17, and 1.29±0.33 vs. 2.13±0.74, respectively, P>0.05 for both) (Figure 2).
Figure 1.
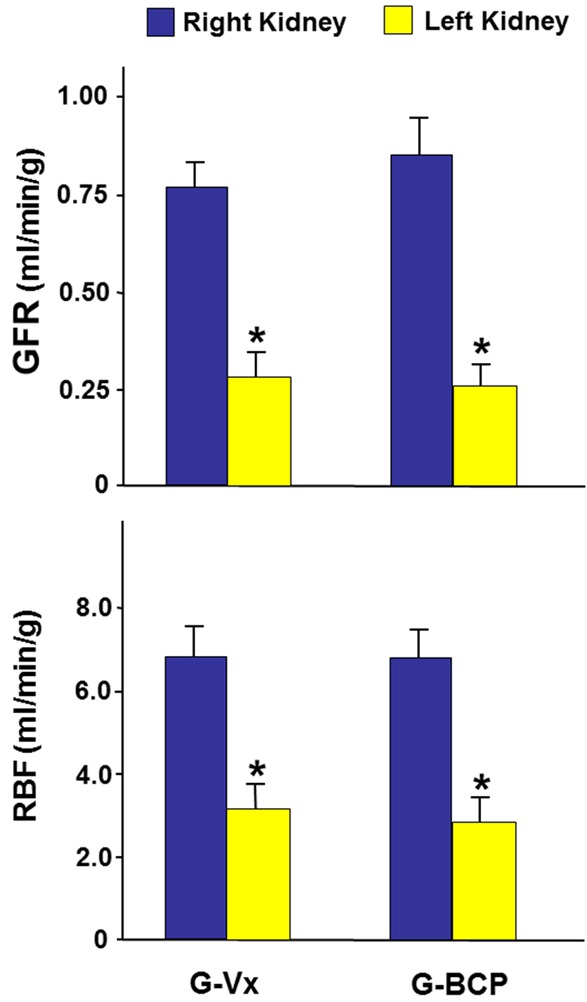
The glomerular filtration rate (GFR) and renal blood flow (RBF) in the right and left kidneys in G-Vx and G-BCP following left renal ischemia. Values represent mean ± SEM. *Indicates statistical significance between the right and left kidney within the same group.
Figure 2.
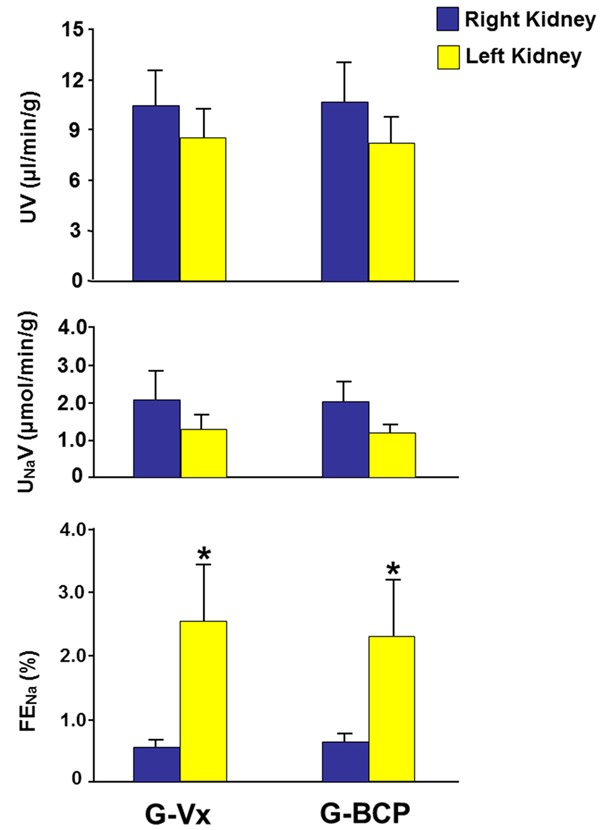
The tubular functional parameters including urine volume (UV), urinary sodium (UNaV) and fractional excretion of sodium (FENa) in both kidneys in G-Vx and G-BCP following left renal ischemia. Values represent mean ± SEM. *Indicates statistical significance between the right and left kidney within the same group.
In G-BCP which received the BCP, the RBF and GFR in the left kidney were also significantly lower that of the right kidney (2.85±0.55 vs. 6.84±0.69 and 0.26±0.06 vs. 0.85±0.09, respectively, P=0.001 for both) (Figure 1). As shown in Figure 2, the FENa of the left kidney were higher than that of the right kidney (2.32±0.92 vs. 0.63±0.13 P=0.04). However, the UV and UNaV of the left kidney were similar to those of the right kidney (8.18±1.58 vs. 10.74±2.31, and 1.21±0.21 vs. 2.03±0.51, respectively, P>0.05 for both).
When G-BCP was compared to G-Vx, all variables in the right non-ischemic kidneys in both groups were similar (P>0.05 for all variables). Similarly, all variables in the left ischemic kidney were also similar in both groups (P>0.05 for all).
Oxidative stress markers
As depicted in Figure 3, in G-Vx, the tissue level of MDA in the left kidney was higher than that of the right kidney (9.3±2.1 vs. 3.9±0.9, P=0.04). However, the level of both GSH and nitric oxide were similar in both kidneys (13.6±1.7 vs. 16.3±1.5 and 13.5±2.6 vs. 11.1±2.7, P>0.05 for both). In G-BCP, all MDA, GSH and nitrite were similar when the left and right kidneys were compared (4.8±1.0 vs. 3.9±1.2, 18.1±2.5 vs. 16.1±1.2, and 11.7±1.3 vs. 11.1±1.6, respectively, P>0.05 for all).
Figure 3.
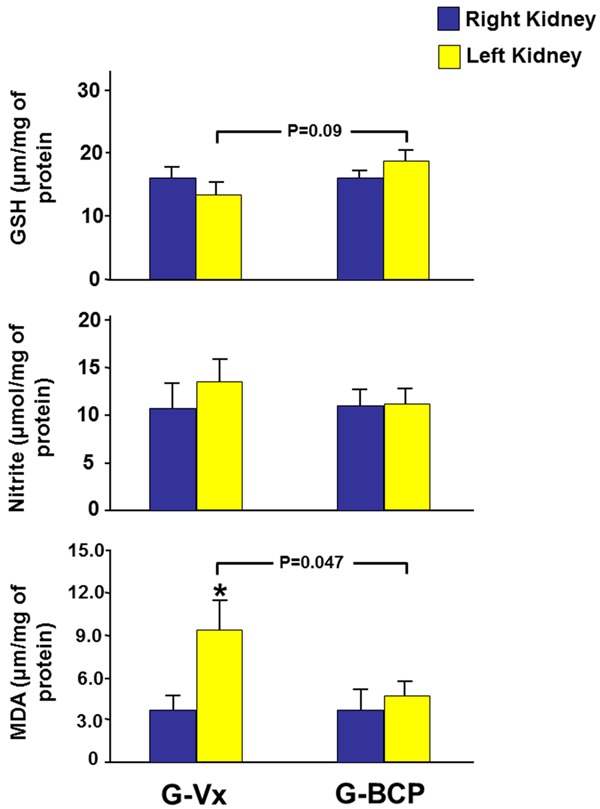
The tissue level of the oxidative stress markers malondialdehyde (MDA), glutathione (GSH) and nitrite in both kidneys in G-Vx and G-BCP. Values represent mean ± SEM. *Indicates statistical significance between the right and left kidney within the same group whereas the bar indicate the level of significance between the left kidneys in both groups.
When the right kidneys in G-BCP and G-Vx were compared, all the MDA, GSH and nitrite levels were similar in the two groups. Comparing the left kidneys in both groups, MDA was lower in the G-BCP (9.3±2.1 vs. 4.8±1.0, P=0.047). GSH in the left kidney was higher in the G-BCP but this did not reach statistical significance (18.1±2.5 vs. 13.6±1.7, P=0.09). The left nitrite level was similar in both groups (Figure 3).
Pro-inflammatory cytokines
In G-Vx, the tissue level of both TNF-α and IL-1β in the left ischemic kidney were significantly lower than the right kidney (391±87 vs. 789±46, P=0.001 and 1382±115 vs. 1829±88, P=0.008, respectively) (Figure 4).
Figure 4.
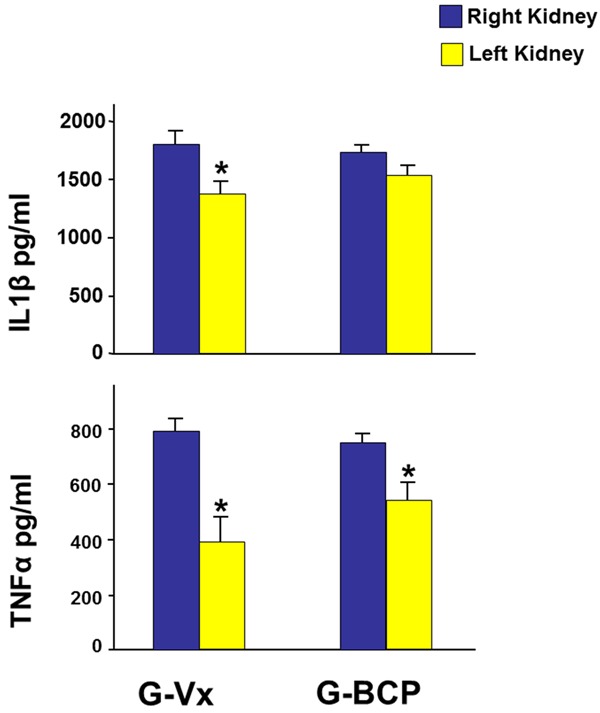
The tissue level of the pro-inflammatory cytokines TNF-α and IL-1β in both kidneys in G-Vx and G-BCP seven days following reperfusion and using oral β-caryophyllene. Values represent mean ± SEM. *Indicates statistical significance between the right and left kidney within the same group.
In G-BCP, a similar trend was observed; as TNF-α was also significantly lower in the left kidney (558±61 vs. 758±26, P=0.008) and the IL-1β in left kidney was lower than the right one but this did not reach statistical significance (1537±97 vs. 1753±52, P=0.06).
When the right kidneys in the two groups were compared, both TNF-α and IL-1β were similar. There were also no differences between the left kidneys in both groups.
To rule out the possibility of issues related to the dosing and the route of drug delivery, we also performed another set of experiments wherein age-matched rats underwent similar protocol i.e. left renal ischemia for 40 minutes. The treated animals, G-BCP-IP (n=7) received β-caryophyllene 25 mg/kg dissolved in 0.5 ml of olive oil twice daily intra-peritoneally starting 24 hours prior to the ischemia and continued thereafter until the measurement of tissue level of the inflammatory biomarkers 24 hours following the reperfusion in contrary to the seventh day post-IRI as in the main study protocol. The control group, G-VX-IP (n=7) underwent similar protocol but received only intraperitoneal injection of the vehicle. As demonstrated in Figure 5, in G-Vx-IP, the tissue level of TNF-α in the left ischemic kidney was significantly lower than the right kidney (557±55 vs. 800±42, P=0.005). IL-1β was also lower in the left kidney but did not reach statistical significance (2865±66 vs. 3145±134, P=0.08). Similarly, in G-BCP-IP, TNF-α was significantly lower in the left kidney (487±46 vs. 733±39, P=0.002). IL-1β was also lower in the left kidney but did not reach statistical significance (2702±104 vs. 3012±153, P=0.09). When the right kidneys in the two groups were compared, both TNF-α and IL-1β were similar. There were also no differences when the left kidneys in both groups were compared.
Figure 5.
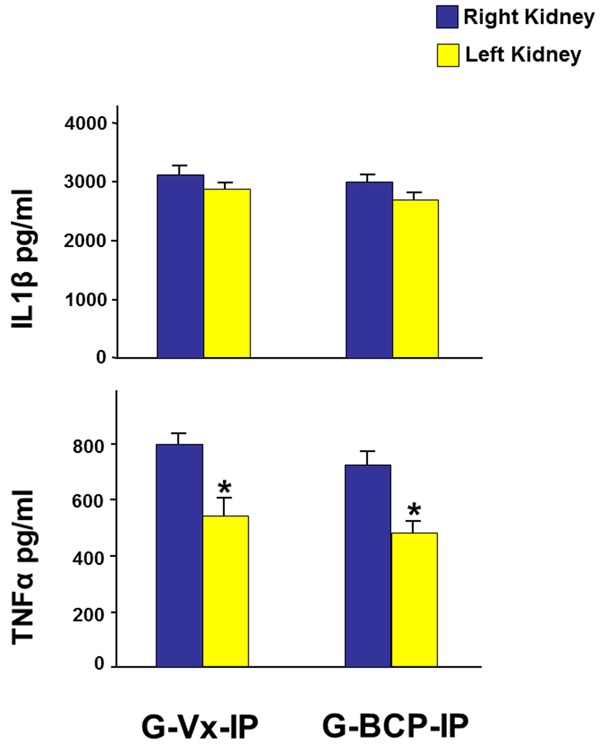
The tissue level of the pro-inflammatory cytokines TNF-α and IL-1β in both kidneys in G-Vx-IP and G-BCP-IP 24 hours following reperfusion and using intra-peritoneal β-caryophyllene. Values represent mean ± SEM. *Indicates statistical significance between the right and left kidney within the same group.
Discussion
In the current study, we have investigated for the first time, the effect of β-caryophyllene on the renal dysfunction following ischemia-reperfusion injury. The administration of β-caryophyllene prior to and following IRI has some protective effects on some of the oxidative stress markers. However, it seems that the effects were not strong enough to be reflected on the ischemia-induced alterations in renal functional hemodynamic and tubular parameters.
The renal dysfunction observed in IRI is caused by both ischemia and reperfusion injury. The pathogenesis of the injury appears to be multifactorial and is associated with hypoxia and consequent excessive production of reactive oxygen species and other oxidative stress markers. Subsequent to oxidative stress, there is also an induction of inflammatory response that is characterized by increased production of several pro-inflammatory cytokines such as TNF-α, Il-1β and IL-6 [17-21].
In the current study, despite the attenuation of some of the oxidative stress markers, β-caryophyllene did not significantly affect the renal hemodynamic or tubular parameters which were similar in the two groups. From the present data, it is difficult to determine the exact reason for the lack of improvement in the renal functions; however, this lack of effect is unlikely to be due to bioavailability issues because similar protective doses were showed to be protective by other investigators [12,22]. Moreover, the attenuation of the rise in the tissue levels of MDA observed in G-BCP group indicates that the drug was bioavailable.
In this study, it can also be argued that, the renal functions and other biomarkers were measured relatively too long after the induction of acute injury i.e. seven days after the ischemic insult that is the time when most of the acute injury markers may have returned to normal level. To rule out this possibility and the possibility of issues related to the dosing and the route of drug delivery, we also performed another set of experiments wherein age-matched rats underwent similar protocol but received an intra-peritoneal β-caryophyllene instead of oral route. The results showed similar findings to those obtained in the main experiment when the β-caryophyllene was administered orally and the biomarkers were measured seven days post-IRI. Collectively, the data obtained with intraperitoneal or oral doses of β-caryophyllene demonstrate the weak protective effect of β-caryophyllene in this model. The probable reason for this could be due to either the severity of the 40-minutes warm ischemia-induced injury or the weak effect of β-caryophyllene on the the inflammatory mediators involved in the early alterations observed in ischemia-reperfusion injury or both.
This lack of improvement in the clinically more relevant renal functional parameters despite the attenuation in the alterations of some of the biomarkers of renal injury has been reported previously with other therapeutic agents in ischemia-reperfusion injury [18] and other renal conditions [23,24]. It is imperative to believe that this might apply to other therapeutic agents in other organs. Indeed, there is large number of studies in the literature which demonstrated the beneficial protective effects of many therapeutic agents on injury-induced markers in various organs. The vast majority of these studies, however, did not further investigate the effect of these agents on the more relevant clinical parameters. Additionally, it is not known with certainty if these early changes in the acute injury markers would be reflected on clinical indices long-term.
β-caryophyllene has significantly attenuated the rise in the MDA level in the left ischemic kidney in the G-BCP compared to G-Vx when measured seven days following the IRI but it had only weakly affected the level of GSH in the ischemic kidney when the two groups were compared. This is probably due to the fact that the tissue level of GSH was similar in both the ischemic and control kidneys in both groups. GSH is an endogenous free radical scavenger and the lack of increase in the tissue level of GSH following IRI could possibly be due to the over-consumption of GSH during the breakdown of free radicals. Similar findings were reported in other tissues [25]. Collectively, the findings of this study confirm the antioxidant protective effect of β-caryophyllene, although weak, which has been reported previously in other renal conditions such as cisplatin-induced nephrotoxicity [16].
In conclusion, the administration of β-caryophyllene before and during as well after ischemia-reperfusion injury appears to have a marginal protective effect on the IRI-induced alterations in some of the biomarkers of acute renal injury but did not significantly affect the hemodynamic or tubular renal functional parameters.
Acknowledgements
This study was funded by the College of Medicine & Health Sciences, United Arab Emirates University.
Disclosure of conflict of interest
None.
Abbreviations
- IRI
ischemia-reperfusion injury
- GFR
glomerular filtration rate
- RBF
renal blood flow
- UV
urine volume
- UNaV
urinary sodium
- FENa
fractional excretion of sodium
- PCR
polymerase chain reaction
- MDA
malondialdehyde
- GSH
glutathione
- TNF-α
tumour necrosis factor-alpha
- IL-1β
interleukin-1β
- SEM
standard error of the mean
References
- 1.Weight SC, Bell PR, Nicholson ML. Renal ischaemia--reperfusion injury. Br J Surg. 1996;83:162–70. [PubMed] [Google Scholar]
- 2.Chatauret N, Badet L, Barrou B, Hauet T. Ischemia-reperfusion: from cell biology to acute kidney injury. Prog Urol. 2014;24(Suppl 1):S4–12. doi: 10.1016/S1166-7087(14)70057-0. [DOI] [PubMed] [Google Scholar]
- 3.Hammad FT, Davis G, Zhang XY, Wheatley AM. Endotelin ETA and ETB receptor antagonism during cold preservation in renal transplantation. Transplantation. 2001;71:619–27. doi: 10.1097/00007890-200103150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Lerman L, Textor SC. Pathophysiology of ischemic nephropathy. Urol Clin North Am. 2001;28:793–803. ix. doi: 10.1016/s0094-0143(01)80034-3. [DOI] [PubMed] [Google Scholar]
- 5.Goes N, Urmson J, Ramassar V, Halloran PF. Ischemic acute tubular necrosis induces an extensive local cytokine response. Evidence for induction of interferon-gamma, transforming growth factor-beta 1, granulocyte-macrophage colony-stimulating factor, interleukin-2, and interleukin-10. Transplantation. 1995;59:565–72. [PubMed] [Google Scholar]
- 6.Hammad FT, Wheatley AM, Davis G. Role of endothelin ET(A) receptor antagonism in the post-transplant renal response to angiotensin II in the rat. Exp Physiol. 2001;86:365–72. doi: 10.1113/eph8602137. [DOI] [PubMed] [Google Scholar]
- 7.Troppmann C, Gruessner AC, Papalois BE, Sutherland DE, Matas AJ, Benedetti E, Gruessner RW. Delayed endocrine pancreas graft function after simultaneous pancreas-kidney transplantation. Incidence, risk factors, and impact on long-term outcome. Transplantation. 1996;61:1323–30. doi: 10.1097/00007890-199605150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Unal S, Ozmen S, DemIr Y, Yavuzer R, LatIfoğlu O, Atabay K, Oguz M. The effect of gradually increased blood flow on ischemia-reperfusion injury. Ann Plast Surg. 2001;47:412–6. doi: 10.1097/00000637-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Yang CW, Kim BS, Kim J, Ahn HJ, Park JH, Jin DC, Kim YS, Bang BK. Preconditioning with sodium arsenite inhibits apoptotic cell death in rat kidney with ischemia/reperfusion or cyclosporine-induced Injuries. The possible role of heat-shock protein 70 as a mediator of ischemic tolerance. Exp Nephrol. 2001;9:284–94. doi: 10.1159/000052623. [DOI] [PubMed] [Google Scholar]
- 10.Sibbald B. Conditional okay for cannabis prescription drug. CMAJ. 2005;172:1672. doi: 10.1503/cmaj.050628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calleja MA, Vieites JM, Montero-Meléndez T, Torres MI, Faus MJ, Gil A, Suárez A. The antioxidant effect of beta-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br J Nutr. 2013;109:394–401. doi: 10.1017/S0007114512001298. [DOI] [PubMed] [Google Scholar]
- 12.Bento AF, Marcon R, Dutra RC, Claudino RF, Cola M, Leite DF, Calixto JB. beta-Caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARgamma pathway. Am J Pathol. 2011;178:1153–66. doi: 10.1016/j.ajpath.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Javed H, Azimullah S, Haque ME, Ojha SK. Cannabinoid type 2 (CB2) receptors activation protects against oxidative stress and neuroinflammation associated dopaminergic neurodege-neration in rotenone model of parkinson’s disease. Front Neurosci. 2016;10:321. doi: 10.3389/fnins.2016.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ojha S, Javed H, Azimullah S, Haque ME. beta-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol Cell Biochem. 2016;418:59–70. doi: 10.1007/s11010-016-2733-y. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Sun T, Wang X. Activation of type 2 cannabinoid receptors (CB2R) promotes fatty acid oxidation through the SIRT1/PGC-1alpha pathway. Biochem Biophys Res Commun. 2013;436:377–81. doi: 10.1016/j.bbrc.2013.05.108. [DOI] [PubMed] [Google Scholar]
- 16.Horváth B, Mukhopadhyay P, Kechrid M, Patel V, Tanchian G, Wink DA, Gertsch J, Pacher P. β-Caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic Biol Med. 2012;52:1325–33. doi: 10.1016/j.freeradbiomed.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR. Early kidney TNF-alpha expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol Regul Integr Comp Physiol. 1999;277:R922–R929. doi: 10.1152/ajpregu.1999.277.3.R922. [DOI] [PubMed] [Google Scholar]
- 18.Hammad FT, Al-Salam S, Lubbad L. Lubbad, curcumin provides incomplete protection of the kidney in ischemia reperfusion injury. Physiol Res. 2012;61:503–11. doi: 10.33549/physiolres.932376. [DOI] [PubMed] [Google Scholar]
- 19.Hammad FT, Lubbad L. The effect of thymoquinone on the renal functions following ischemia-reperfusion injury in the rat. Int J Physiol Pathophysiol Pharmacol. 2016;8:152–159. [PMC free article] [PubMed] [Google Scholar]
- 20.Furuichi K, Wada T, Iwata Y, Kokubo S, Hara A, Yamahana J, Sugaya T, Iwakura Y, Matsushima K, Asano M, Yokoyama H, Kaneko S. Interleukin-1-dependent sequential chemokine expression and inflammatory cell infiltration in ischemia-reperfusion injury. Crit Care Med. 2006;34:2447–55. doi: 10.1097/01.CCM.0000233878.36340.10. [DOI] [PubMed] [Google Scholar]
- 21.Haq M, Norman J, Saba SR, Ramirez G, Rabb H. Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol. 1998;9:614–9. doi: 10.1681/ASN.V94614. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes ES, Passos GF, Medeiros R, da Cunha FM, Ferreira J, Campos MM, Pianowski LF, Calixto JB. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur J Pharmacol. 2007;569:228–36. doi: 10.1016/j.ejphar.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 23.Hammad FT, Lubbad L. Does curcumin protect against renal dysfunction following reversible unilateral ureteric obstruction in the rat? Eur Surg Res. 2011;46:188–93. doi: 10.1159/000324414. [DOI] [PubMed] [Google Scholar]
- 24.Hammad FT, Lubbad L. The effect of epigallocatechin-3-gallate on the renal dysfunction in the obstructed kidney in the rat. Int J Physiol Pathophysiol Pharmacol. 2017;9:119–126. [PMC free article] [PubMed] [Google Scholar]
- 25.Nemmar A, Al Hemeiri A, Al Hammadi N, Yuvaraju P, Beegam S, Yasin J, Elwasila M, Ali BH, Adeghate E. Early pulmonary events of nose-only water pipe (shisha) smoking exposure in mice. Physiol Rep. 2015;3 doi: 10.14814/phy2.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]


