Abstract
Spinal cord injury is a devastating condition that is followed by long and often unsuccessful recovery after trauma. The state of the art approach to manage paralysis and concomitant impairments is rehabilitation, which is the only strategy that has proven to be effective and beneficial for the patients over the last decades. How rehabilitation influences the remodeling of spinal axonal connections in patients is important to understand, in order to better target these changes and define the optimal timing and onset of training. While clinically the answers to these questions remain difficult to obtain, rodent models of rehabilitation like bicycling, treadmill training, swimming, enriched environments or wheel running that mimic clinical rehabilitation can be helpful to reveal the axonal changes underlying motor recovery. This review will focus on the different animal models of spinal cord injury rehabilitation and the underlying changes in neuronal networks that are improved by exercise and rehabilitation.
Keywords: remodeling, exercise, wheel running, treadmill, detour circuit, propriospinal neuron, corticospinal tract, raphespinal tract, reticulospinal tract, activity, recovery, central nervous system
Introduction
A trauma to the spinal cord is a life changing event, which leaves most patients impaired or paralyzed throughout lifetime due to the limited capacity of the central nervous system to heal and the restricted number of therapeutic options until today. Spinal cord injuries (SCI) are most often caused by accidents during labor, recreation or involving motor vehicles (Armour et al., 2016). SCI is characterized by partial or total loss of motor and sensory function below the level of the injury resulting in individual disabilities that depend on the level and severity of the lesion. A complete severance of the spinal cord leads to a permanent and irreversible loss of all neuronal connections caudal to the level of the injury and functional recovery is highly improbable. Incomplete injuries, e.g., with spinal cord tissue sparing, have a potential for some level of recovery, the extent of which depends on the location and magnitude of neuronal sparing. About 42% of all spinal cord injuries are considered functionally complete with the patient presenting without any motor and sensory function below the affected area. Interestingly it is thought that only 14.3% of all SCIs are anatomically complete, which means that around 25–30% of all injuries deemed functionally complete also have some extend of spared connections that could potentially be harvested with the right intervention (Kakulas, 2004). Locomotor rehabilitation is the only known intervention which is beneficial for affected individuals, but the optimal timing and extent of locomotor exercise as well as the underlying neuronal mechanisms resulting in motor improvements remain poorly understood (Cote et al., 2017).
To write this review on rehabilitative strategies in humans and rodents we conducted a PubMed search for animal models of rehabilitation, detour circuit formation and human rehabilitative strategies and their outcomes. The database search for human and rodent rehabilitation was conducted using always the term spinal cord injury (MeSH Terms) and rehabilitation and setting the species filter to either humans or other animals and then screening the title or abstract for rodent models. General information on SCI was searched by that term in combination with the desired term (e.g. cause). To search more in depth for a rehabilitative paradigm the term (e.g. swimming) was combined with spinal cord injury. Publications on detour circuit formation and plasticity were searched by combining these two terms with spinal cord injury. All results were then screened for suitability by title and abstract to fit the topic and the model. We focused in general rather on recent findings if possible.
Rehabilitative Strategies for Patient Locomotor Recovery Following SCI
Rehabilitation in patients with motor complete SCI primarily aims at improving quality of life and secondary consequences of SCI such as circulation, wheelchair transfer and joint mobility as motor recovery is unlikely. In motor incomplete patients the main aim of rehabilitation is functional recovery, in particular regaining stepping ability or hand function, a way toward daily independence. A functional baseline is determined around 3 weeks after the injury and recovery is most likely to occur within the first year post trauma (Kakulas, 2004). Motor incomplete individuals show sustained functional recovery with treadmill training, the most standard rehabilitation strategy. With older interventions such as training on parallel bars about 50% of patients that were initially wheelchair bound managed to walk independently again. In contrast, body weight supported or unsupported treadmill rehabilitation now allows up to 92% of incompletely-injured patients with residual motor function to achieve this. Chronic patients (1 year or more post injury) can also benefit from treadmill training with 78% of participants becoming progressively wheelchair independent and sustained functional improvements after the end of rehabilitation (Wernig et al., 1995, 1998). In general an early onset of rehabilitation after hospital discharge and a higher training intensity seem to be favorable to foster maximal functional recovery, but there is no specific guidelines regarding optimal intensity and onset of rehabilitation (Wernig et al., 1995; Harkema et al., 2012; Brazg et al., 2017; Burns et al., 2017). While more training sessions over a prolonged time seem to result in a heightened functional recovery, most abilities seem to rather be regained early and then level with time (Morrison et al., 2018). Robotic body weight assisted treadmill training or exoskeleton assisted step training have emerged recently as new approaches for human rehabilitation, but have so far failed to demonstrate better results than rehabilitation with treadmill and assisted step training, without a moving treadmill (Fisahn et al., 2016; Burns et al., 2017; Mehrholz et al., 2017). While stem cell transplantation into the injury site seems to be safe, it has yet to show beneficial effects for patients (Oh et al., 2016; Anderson et al., 2017; Curt et al., 2017) unless combined with rehabilitation (Zhu et al., 2016). Combining epidural electrical stimulation - a stimulus delivered by an electrical array implanted epidurally over the spinal segments L1–S1 - with treadmill rehabilitation seems to be the most promising approach to date. Recent studies have generated very promising results in particular to significantly improve spasticity after SCI (Gomes-Osman et al., 2016; Minassian et al., 2016; Nagel et al., 2017). Recently it was reported that patients with chronic motor complete injuries that had not seen improvements with locomotor training alone, were able to achieve over-ground walking or, at least, independent standing, following intense (between 80 and 280 sessions) locomotor treadmill training with body-weight support and spinal cord epidural electrical stimulation (Angeli et al., 2018). Likewise, dynamic and intense task-specific training in the presence of epidural electrical stimulation, performed on a patient with a clinically complete injury resulted in bilateral stepping on a treadmill and enabled independent stepping with a walker (Gill et al., 2018). Many crucial questions relative to the timing, intensity and underlying mechanisms of the functional recovery still remain currently unanswered, stirring a need for further research on exercise, rehabilitation and training after spinal cord injury.
Animal Models of Rehabilitation in SCI
As rehabilitation is the only known intervention that can improve functional recovery following clinical SCI, scientists have aimed at mimicking training-induced recovery to uncover the underlying mechanisms leading to these sustained improvements. First experiments used de-cerebrated cats (i.e., spinal cats) which recover stepping ability without central input with treadmill training due to intraspinal circuits for stepping named central pattern generator (Lovely et al., 1986, 1990; Rossignol and Bouyer, 2004; Martinez et al., 2013). However, as stepping ability does not spontaneously recover in completely transected rodents, monkeys and humans, cats may not be the best model organism to study functional recovery following SCI. Non-human primates are most alike to the human situation, but their use in research is controversial and limited (Abbott, 2014; Friedli et al., 2015). Most animal research is therefore conducted in rodents, either rats or mice, due to their broad availability and the possibility of genetic and surgical interventions. The necessity of movement and exercise for functional recovery following injury in rodents was demonstrated using a reverse approach. Rats received a mild contusion to the spinal cord or a partial transection and showed functional recovery over time when returned to their home cages and allowed to move around freely. A second group of rats was fixed in custom built ‘wheelchairs’. These interventions immobilized the impaired hindlimbs of the animals in a ‘dragging-like’ or sitting position for a considerable amount of time. These rats did not show functional recovery, but rather their hindlimb motor abilities worsened over time most likely due to neglect of muscles and neuronal connections. After 8 weeks, wheelchairs were removed and the animals were allowed to move freely. These animals then started to show some behavioral recovery, but failed to reach levels comparable to previously unrestrained animals (Caudle et al., 2011). Similarly, animals that were previously restrained in tubes following injury, showed behavioral recovery at the time tubes were removed and animals were allowed to move around freely (Little et al., 1991). Identical results were demonstrated with unilateral corticospinal tract (CST) lesions that impair fine forelimb function. If the impaired forelimb was fixed in a cast it showed no improvement in fine motor function, if the intact forelimb was fixed in a cast and the animal was forced to rely completely on the injured forelimb, full motor recovery to baseline level was achieved (Maier et al., 2008). These studies underline the necessity and importance of a timely onset of movement and exercise after injury to trigger functional locomotor recovery.
Rodent models of hindlimb animal rehabilitation after SCI can be classified into voluntary and forced paradigms. Voluntary training encompasses enriched environments and wheel running while forced training options involve for example treadmill walking, bicycling or swimming (Figure 1A). Treadmill training with or without body weight assistance is the most commonly used forced training paradigm experimentally as it mimics fairly well the training administered in humans after SCI and can, with body weight assistance, also be administered to severely lesioned animals. Rodents show increased functional recovery with treadmill training after incomplete SCI (Shah et al., 2013; Sun et al., 2013; Jung et al., 2016) while there is only very limited recovery in the open field locomotion Basso, Beattie, Bresnahan score (Basso et al., 1995) in case of complete transections (Ilha et al., 2011). Interestingly, quadrupedal treadmill step training was demonstrated to be superior to stepping with hindlimbs only (Shah et al., 2013) and premature onset of training at 1 or 2 days after injury was shown to limit recovery, if compared to onset at 3 days post SCI (Yin et al., 2013). Low training intensity seems to be a major pitfall of experimental treadmill training: for example, a typical protocol consists of 20 minutes on the treadmill for 5–7 days a week (Sun et al., 2013; Yin et al., 2013; Hayashibe et al., 2016) with alternative protocols ranging from 5 to 30 minutes training a day (Gomez-Pinilla et al., 2001; Robert et al., 2010). It was recently shown that combining treadmill rehabilitation with pharmacological and electrical modulations in the lumbar spinal cord can lead to the re-establishment of walking ability in completely transected rats, a major step towards future therapy for completely paralyzed patients (van den Brand et al., 2012). Other forced rehabilitative strategies are less commonly used. Bicycling is a fairly rare forced but passive method used in rodents. In this rehabilitation model the animal’s hindlimbs are typically moved by a custom made device. This training approach is rather non-physiological and unusual for rodents and does not directly train stepping ability which translates in slight gait improvements after injury (3 points on the Basso, Beattie, Bresnahan score, less effective than treadmill training; Bose et al., 2012). Another rather scarcely used approach is swimming, which, in contrast to bicycling, is restricted to incomplete spinal cord injuries, and also provides body weight support to the animal analogous to treadmill training. Swimming, especially with proprioceptive sensory feedback, improves motor recovery after incomplete SCI, albeit less effectively than treadmill training (Magnuson et al., 2009; Smith et al., 2009; Robert et al., 2010). Those studies as well as another recent work also pinpoint the fact that sensory as well as muscle spindle feedback is crucial for motor recovery, which is a common attribute of all rehabilitation models (Smith et al., 2009; Takeoka et al., 2014).
Figure 1.
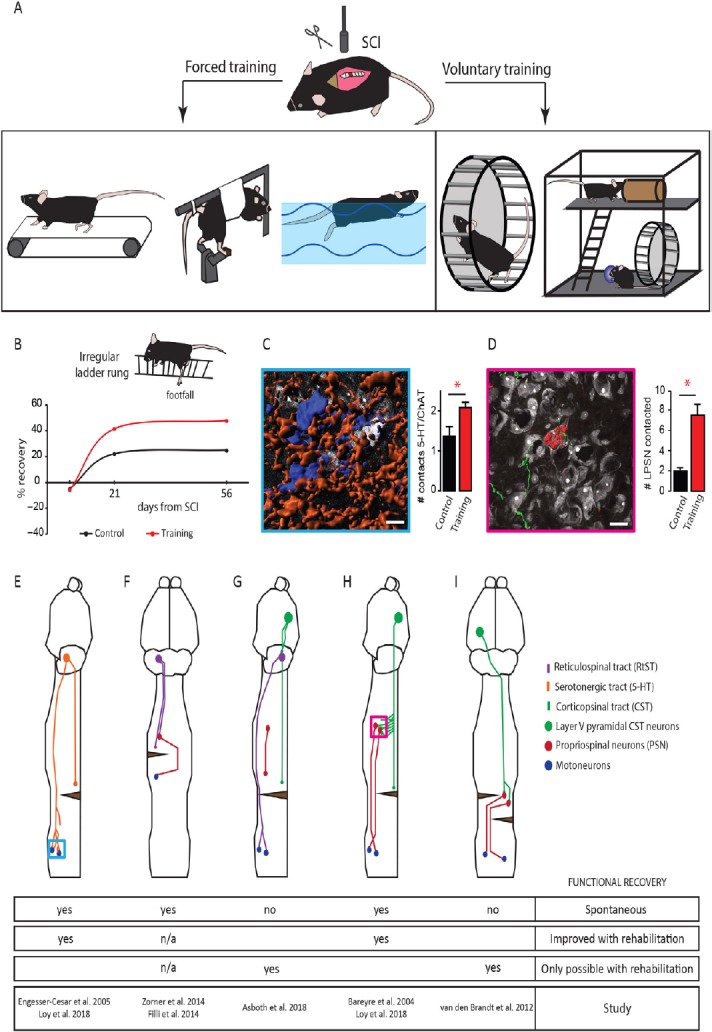
Overview of animal models of spinal cord injury (SCI) rehabilitation and schematic representations of several detour circuits paradigms in SCI.
(A) Overview of several experimental approaches used to rehabilitate hindlimb function following SCI in rodents. Rehabilitation can be divided into forced approaches (from left to right): treadmill walking, passive bicycling and swimming and voluntary strategies (from left to right): wheel running and enriched environments. (B) Schematic representation of the percentage of recovery obtained in the irregular ladder rung test with voluntary wheel running. (C) Representative rendering (left) of lumbar motoneurons (blue) contacted by serotonergic fibers (orange) and quantification of the number of contacts between serotonergic (5-HT) fibers and motoneurons 2 weeks after SCI without and with training on an irregular wheel (right). Displayed in white are non-positive neurons. (D) Representative rendering (left) of a corticospinal tract collateral (green) contacting a long propriospinal neuron (red) in the cervical spinal cord 2 weeks after dorsal midthoracic hemisection and quantification of the number of long propriospinal neurons contacted 2 weeks after lesion without and with training on an irregular running wheel (right). Displayed in white are non-positive neurons labeled. Scale bars: 20 µm. *P < 0.05. # is an abbreviation for number. (E) Schematic representation of the remodeling of serotonergic fibers in the spinal cord following dorsal midthoracic hemisection. Blue inset refers to image in panel C. (F) Schematic representation of the sprouting and detour circuit formation of the reticulospinal tract following a cervical hemilesion. (G) Schematic representation of a detour circuit of the corticospinal tract via the reticulospinal tract following a near complete thoracic lesion. (H) Schematic representation of the corticospinal tract-long propriospinal neuron detour circuit following midthoracic dorsal hemisection. Pink inset refers to image in panel D. (I) Schematic representation of a corticospinal-propriospinal detour circuit following temporally and spatially separated complete lesion of the spinal cord (staggered hemisection). ChAT: Choline acetyltransferase; LPSN: left propriospinal neuron.
In sharp contrast to those forced rehabilitation models, wheel running is a voluntary elective behavior in mice which can be seen in the wild if a wheel is provided (Meijer and Robbers, 2014). There are several advantages of voluntary wheel training when compared to treadmill training: wheel running is a self-motivated and thereby non stressful training paradigm. Motivation increases functional recovery via the nucleus accumbens neuronal pathway (Sawada et al., 2015) and in contrast to forced exercise, wheel running does not increase corticosteroid levels, as does forced treadmill running in unlesioned animals, indicating high stress levels during training (Erschbamer et al., 2006; Brown et al., 2007; Ke et al., 2011). High corticosteroid levels while having shown beneficial effects following spinal cord injury can also trigger undesired side effects which might also hamper recovery (Bydon et al., 2014; Han et al., 2014). The main limitation of voluntary rehabilitation is that it can only be used in cases of incomplete lesions, as fully paralyzed animals are unable to self-train. However, wheel running has been shown to affect post-injury functional recovery differently depending on (i) the wheel itself, (ii) the running intensity and (iii) the starting time from injury (Van Meeteren et al., 2003; Engesser-Cesar et al., 2005; Erschbamer et al., 2006; Loy et al., 2018). Wheel-based rehabilitation with regularly-spaced rungs, late onset running post-injury or low training intensity initially failed to show increased functional recovery after SCI (Engesser-Cesar et al., 2005; Erschbamer et al., 2006). However, voluntary wheel running improved functional recovery when a flat surface wheel - in which the rung surface is covered with a plastic to generate an even running surface - was used (Engesser-Cesar et al., 2007). Recently, we used wheels with irregularly-spaced rungs to also train the corticospinal tract, a supraspinal pathway known to control skilled placed movements. In this study we showed that we could detect a significant improvement of functional recovery including coordination, measured by the RotaRod, and fine paw placement, measured by the irregular LadderRung (Loy et al., 2018; Figure 1B). In general, studies with a high running intensity (about 5 km a day) showed robust behavioral improvements after SCI (Van Meeteren et al., 2003; Engesser-Cesar et al., 2005; Loy et al., 2018). Enriched environments also represent another model of voluntary rehabilitation that was shown to increase functional recovery after SCI. Interestingly most enriched environment paradigms include a running wheel and show comparable results to rehabilitation using wheel running (Lankhorst et al., 2001; Erschbamer et al., 2006).
Activity-Induced Neuroplasticity Following SCI
One general hallmark of all rehabilitative strategies in animals is the upregulation of brain-derived neurotrophic factor (BDNF) (Neeper et al., 1996; Ke et al., 2011; Wang et al., 2015; Jung et al., 2016)) and blockage of BDNF signaling on its receptor TrkB (tropomyosin receptor kinase B) abolishes the positive effect of rehabilitation on functional recovery (Li et al., 2018). In the brain it was demonstrated that BDNF mediates activity-dependent neuronal plasticity and learning in health and disease (Griesbach et al., 2004; Cheng et al., 2014; Leal et al., 2014). In contrast to the peripheral nervous system which can show extensive axonal regeneration, the injured spinal cord can only exhibit limited levels of plasticity, which is characterized by the remodeling of spared connections and severed axons. Axonal regeneration and re-growth across the lesion site, which was long considered the gold standard to cure paralysis, is, in fact, hardly seen spontaneously in the injured central nervous system. However it can be achieved to a limited extent with genetic modifications, pharmaceutical interventions or extracellular matrix degeneration, but even though functional benefits remain small (Bradbury et al., 2002; Liu et al., 2010; Ruschel et al., 2015). In contrast, increased sprouting and plasticity of axonal connections can trigger remodeling of brain-spinal cord connectivity. How rehabilitation - which, as mentioned above, triggers the expression of molecules involved in plasticity - can modulate and improve brain-spinal cord connectivity is a crucial question. In the rest of the review we will focus on the changes in supraspinal-spinal connectivity triggered by experimental rehabilitative procedures with a focus on the serotonergic, the reticulospinal and the corticospinal tracts.
Serotonergic neurons reside mostly in the raphe nucleus and other brainstem areas but can be found in limited number directly in the spinal cord (Ballion et al., 2002). The complete loss of central serotonergic input to spinal motor neurons results in paralysis after injury and post-injury application of serotonin or restoration of endogenous serotonin levels lead to acute modulation of spinal networks, thereby facilitating movement (Hashimoto and Fukuda, 1991; Fong et al., 2005). Using voluntary wheel training on a flat surface wheel, the serotonergic tract was shown to increase its fiber outgrowth caudal of an incomplete spinal cord lesion (Engesser-Cesar et al., 2007). Likewise an increase of contact formation between serotonergic fibers and cholinergic motoneurons in the lumbar spinal cord was recently reported using an irregular running wheel training paradigm following incomplete spinal cord injury (Loy et al., 2018; Figure 1C and E). Strikingly, the combination of rehabilitation with serotonin agonists results in acute improvement in motor capabilities, but the serotonergic effect is lost sub-acutely whereas the rehabilitation effect persists (Fong et al., 2005). It is to note that the high complexity of the serotonergic systems that encompasses not only intraspinal and supraspinal neurons, but also many different receptors and a high plasticity make the serotonergic system more complex to study than other motor systems. Non-pharmacological contributions of serotonergic neurons to locomotor recovery with or without rehabilitation remain poorly understood and further studies are needed to clarify the role of serotonergic remodeling in SCI recovery.
The reticulospinal originates in the brainstem and terminates mostly in the ventral horn in the spinal cord in rodents (Liang et al., 2016). In contrast to the CST which mediates fine locomotor control (Lawrence and Kuypers, 1968b), the reticulospinal tract is mainly involved in gross movements and postural control during locomotion (Lawrence and Kuypers, 1968a; Baker, 2011). After a unilateral spinal cord lesion reticulospinal tract projections of the contralateral brainstem show compensatory plasticity by crossing the midline towards the denervated site or contacting double-midline crossing propriospinal interneurons to re-relay the information from the brainstem motor center to the denervated spinal cord caudal of the lesion, a phenomenon that is accompanied by functional motor recovery (Figure 1F; Filli et al., 2014; Zorner et al., 2014). This relay pathway via propriospinal interneurons forms a detour circuit to circumvent the lesioned area of the spinal cord and reconnects the central neuron to it’s appropriate target and thereby restores function (Bareyre et al., 2004; Filli et al., 2014). The reticulospinal tract can also serve as relay circuit after spinal cord injury by forming a cortico-reticulospinal detour circuit. In the case of a severe contusion injury few white matter tract fibers of the reticulospinal tract are spared and these fibers can serve as a long distance relay for the CST, which forms new connections on the neurons of the reticulospinal tract, enabling functional recovery of motor function. This circuit re-organization only occurs with rehabilitation and neuromodulation and without treadmill training animals could not regain stepping ability. Trained animals which recovered voluntary stepping ability retained their improvements even after the termination of training and without neuromodulation, arguing for a persistent exercise-induced re-wiring of locomotor circuits (Figure 1G; Asboth et al., 2018).
The CST is one important motor tract in rodents and primates and mostly mediates skilled placement and fine paw or hand movements. Following injury, studies have shown spontaneous sprouting of fibers (Weidner et al., 2001; Bareyre et al., 2004). Spontaneous compensatory sprouting of the CST could either be mediated by uninjured remaining fibers that change their target innervation to compensate for lost connections - which can be increased by forced use of the de-innervated limb (Weidner et al., 2001; Maier et al., 2008) - or injured axons of the CST can form a new circuit by establishing connections with new partners (Bareyre et al., 2004; Lang et al., 2013). In this later case, after a midthoracic spinal cord injury that transects the main dorsal and dorsolateral CST, animals loose stepping ability that spontaneously recovers with time if the ventral half of the spinal cord remains intact. This is due to the formation of specific detour circuits in which the CST contacts long descending propriospinal neurons rostral of the lesion that then in turn contact and relay the information via their spared ventral axons to the lumbar motor neurons enabling the animal to regain walking ability (Figure 1D and H; Bareyre et al., 2004; Jacobi et al., 2015). The formation of the CST-long descending propriospinal neuron detour circuit after lesion is initiated 10 days post injury when the CST fibers exit the tract and enter the intermediate and ventral gray matter in the cervical spinal cord rostral of the lesion. These fibers grow and form contacts on long descending propriospinal neurons within the first 3 to 4 weeks after injury which are then refined over the following period of time (Lang et al., 2012). We could recently show that rehabilitation provided via irregular running wheels accelerates and increases the CST-long descending propriospinal neuron detour circuit formation, as robust outgrowth of corticospinal collaterals in the grey matter and contact formation on long descending propriospinal neurons is already established 14 days after injury (Loy et al., 2018). The amount of contacts and the number of recruited long descending propriospinal neurons further increases over time if the animals are constantly self-training in irregular spaced running wheels (Figure 1D and H). These irregularly-spaced running wheels might serve as a trigger for the remodeling of the CST, as precise digit control is CST mediated and is needed for the animal to train on the wheel (Lawrence and Kuypers, 1968b; Loy et al., 2018) which would then argue that task-specific training is efficient to improve a given locomotor performance. We can only speculate about the molecular factors triggered by irregularly-spaced running wheels but it seems reasonable to believe that molecules such as BDNF or insulin-like growth factor 1 already described following regular voluntary wheel running could serve as trigger for the remodeling (Cetinkaya et al., 2013; Venezia et al., 2016). Treadmill training and wheel running also result in increased fiber density and length around the lesion arguing, not only for long distance remodeling, but also for local sprouting effects (Hayashibe et al., 2016; Loy et al., 2018). In contrast to partial spinal cord lesions, staggered hemi-lesions timely and segmentally spaced do not show spontaneous functional recovery. However, with a combinatory approach of treadmill rehabilitation with an electrochemical prosthesis in the lumbar spinal cord, stepping ability can be restored even if all direct descending connections from the brain are severed. Anatomically this is achieved by an intraspinal detour circuit of the CST via propriospinal interneurons (Figure 1I; van den Brand et al., 2012). CST detour circuit formation is enhanced and improved with exercise and neuronal re-wiring seems to be the underlying cause for this sustained improved recovery after spinal cord injury. Moreover a CST- detour circuit seems conserved across species and was recently shown to mediate functional recovery in monkeys (Friedli et al., 2015), arguing that maximizing the formation of this circuit could contribute greatly to human recovery.
Conclusion
Significant progress towards the understanding of the most suitable training paradigms to trigger the maximal functional recovery has been made in the recent years. Different models of animal rehabilitation have generated results reminiscent of clinical rehabilitation, in particular in the case of incomplete lesions. These models have provided considerable insight into which neuronal tracts are the target of rehabilitative strategies and have given us first insights on how neuronal plasticity can be promoted using physical training in patients. Therapies of complete or nearly complete spinal cord injuries, combining rehabilitation with epidural stimulation, pharmacological agents or neuromodulation, have shown great potential experimentally and have even started to be translated into clinical cases. Efficient translation from bench to bedside is however still ongoing and the subject of intense efforts (Garcia-Alias et al., 2009; Zorner and Schwab, 2010; Gerasimenko et al., 2015; Chen et al., 2017; Rejc et al., 2017).
Additional file: Open peer review report 1 (117.3KB, pdf) .
Footnotes
Conflicts of interest: The authors claim no existing conflicts of interest, financial or otherwise.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Mitsuhiro Enomoto, Tokyo Medical and Dental University, Japan.
Funding: Work in FMB laboratory is supported by grants from the Deutsche Forschungsgemeinschaft (DFG, SFB870), by the Munich Center for Neurosciences (MCN) and the Wings for Life foundation. FMB is also supported by the Munich Center for Systems Neurology (DFG, SyNergy; EXC 1010).
P-Reviewer: Enomoto M; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Abbott A. Biomedicine: the changing face of primate research. Nature. 2014;506:24–26. doi: 10.1038/506024a. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD, Guest JD, Dietrich WD, Bartlett Bunge M, Curiel R, Dididze M, Green BA, Khan A, Pearse DD, Saraf-Lavi E, Widerstrom-Noga E, Wood P, Levi AD. Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J Neurotrauma. 2017;34:2950–2963. doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 3.Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, Ferreira CK, Harkema SJ. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379:1244–1250. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- 4.Armour BS, Courtney-Long EA, Fox MH, Fredine H, Cahill A. Prevalence and causes of paralysis-United States, 2013. Am J Public Health. 2016;106:1855–1857. doi: 10.2105/AJPH.2016.303270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, Baud L, Pidpruzhnykova G, Anderson MA, Shkorbatova P, Batti L, Pages S, Kreider J, Schneider BL, Barraud Q, Courtine G. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018;21:576–588. doi: 10.1038/s41593-018-0093-5. [DOI] [PubMed] [Google Scholar]
- 6.Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballion B, Branchereau P, Chapron J, Viala D. Ontogeny of descending serotonergic innervation and evidence for intraspinal 5-HT neurons in the mouse spinal cord. Brain Res Dev Brain Res. 2002;137:81–88. doi: 10.1016/s0165-3806(02)00414-5. [DOI] [PubMed] [Google Scholar]
- 8.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 9.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 10.Bose PK, Hou J, Parmer R, Reier PJ, Thompson FJ. Altered patterns of reflex excitability, balance, and locomotion following spinal cord injury and locomotor training. Front Physiol. 2012;3:258. doi: 10.3389/fphys.2012.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 12.Brazg G, Fahey M, Holleran CL, Connolly M, Woodward J, Hennessy PW, Schmit BD, Hornby TG. Effects of training intensity on locomotor performance in individuals with chronic spinal cord injury: a randomized crossover study. Neurorehabil Neural Repair. 2017;31:944–954. doi: 10.1177/1545968317731538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DA, Johnson MS, Armstrong CJ, Lynch JM, Caruso NM, Ehlers LB, Fleshner M, Spencer RL, Moore RL. Short-term treadmill running in the rat: what kind of stressor is it? J Appl Physiol (1985) 2007;103:1979–1985. doi: 10.1152/japplphysiol.00706.2007. [DOI] [PubMed] [Google Scholar]
- 14.Burns AS, Marino RJ, Kalsi-Ryan S, Middleton JW, Tetreault LA, Dettori JR, Mihalovich KE, Fehlings MG. Type and timing of rehabilitation following acute and subacute spinal cord injury: a systematic review. Global Spine J. 2017;7:175s–194s. doi: 10.1177/2192568217703084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bydon M, Lin J, Macki M, Gokaslan ZL, Bydon A. The current role of steroids in acute spinal cord injury. World Neurosurg. 2014;82:848–854. doi: 10.1016/j.wneu.2013.02.062. [DOI] [PubMed] [Google Scholar]
- 16.Caudle KL, Brown EH, Shum-Siu A, Burke DA, Magnuson TS, Voor MJ, Magnuson DS. Hindlimb immobilization in a wheelchair alters functional recovery following contusive spinal cord injury in the adult rat. Neurorehabil Neural Repair. 2011;25:729–739. doi: 10.1177/1545968311407519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cetinkaya C, Sisman AR, Kiray M, Camsari UM, Gencoglu C, Baykara B, Aksu I, Uysal N. Positive effects of aerobic exercise on learning and memory functioning, which correlate with hippocampal IGF-1 increase in adolescent rats. Neurosci Lett. 2013:177–181. doi: 10.1016/j.neulet.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Chen K, Marsh BC, Cowan M, Al’Joboori YD, Gigout S, Smith CC, Messenger N, Gamper N, Schwab ME, Ichiyama RM. Sequential therapy of anti-Nogo-A antibody treatment and treadmill training leads to cumulative improvements after spinal cord injury in rats. Exp Neurol. 2017;292:135–144. doi: 10.1016/j.expneurol.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Cheng MY, Wang EH, Woodson WJ, Wang S, Sun G, Lee AG, Arac A, Fenno LE, Deisseroth K, Steinberg GK. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci U S A. 2014;111:12913–12918. doi: 10.1073/pnas.1404109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cote MP, Murray M, Lemay MA. Rehabilitation strategies after spinal cord injury: inquiry into the mechanisms of success and failure. J Neurotrauma. 2017;34:1841–1857. doi: 10.1089/neu.2016.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curt A, Levi AD, Schwab JM. Challenges to translation and the hippocratic oath by premature termination of spinal cord stem cell–based trials. JAMA Neurol. 2017;74:635–636. doi: 10.1001/jamaneurol.2017.0318. [DOI] [PubMed] [Google Scholar]
- 22.Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- 23.Engesser-Cesar C, Ichiyama RM, Nefas AL, Hill MA, Edgerton VR, Cotman CW, Anderson AJ. Wheel running following spinal cord injury improves locomotor recovery and stimulates serotonergic fiber growth. Eur J Neurosci. 2007;25:1931–1939. doi: 10.1111/j.1460-9568.2007.05469.x. [DOI] [PubMed] [Google Scholar]
- 24.Erschbamer MK, Pham TM, Zwart MC, Baumans V, Olson L. Neither environmental enrichment nor voluntary wheel running enhances recovery from incomplete spinal cord injury in rats. Exp Neurol. 2006;201:154–164. doi: 10.1016/j.expneurol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Filli L, Engmann AK, Zorner B, Weinmann O, Moraitis T, Gullo M, Kasper H, Schneider R, Schwab ME. Bridging the gap: a reticulo-propriospinal detour bypassing an incomplete spinal cord injury. J Neurosci. 2014;34:13399–13410. doi: 10.1523/JNEUROSCI.0701-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisahn C, Aach M, Jansen O, Moisi M, Mayadev A, Pagarigan KT, Dettori JR, Schildhauer TA. The effectiveness and safety of exoskeletons as assistive and rehabilitation devices in the treatment of neurologic gait disorders in patients with spinal cord injury: a systematic review. Global Spine J. 2016;6:822–841. doi: 10.1055/s-0036-1593805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedli L, Rosenzweig ES, Barraud Q, Schubert M, Dominici N, Awai L, Nielson JL, Musienko P, Nout-Lomas Y, Zhong H, Zdunowski S, Roy RR, Strand SC, van den Brand R, Havton LA, Beattie MS, Bresnahan JC, Bezard E, Bloch J, Edgerton VR, et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci Transl Med. 2015;7:302ra134. doi: 10.1126/scitranslmed.aac5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- 30.Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, Reggie Edgerton V. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med. 2015;58:225–231. doi: 10.1016/j.rehab.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, Beck LA, Sayenko DG, Van Straaten MG, Drubach DI, Veith DD, Thoreson AR, Lopez C, Gerasimenko YP, Edgerton VR, Lee KH, Zhao KD. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018 doi: 10.1038/s41591-018-0175-7. doi: 10.1038/s41591-018-0175-7. [DOI] [PubMed] [Google Scholar]
- 32.Gomes-Osman J, Cortes M, Guest J, Pascual-Leone A. A systematic review of experimental strategies aimed at improving motor function after acute and chronic spinal cord injury. J Neurotrauma. 2016;33:425–438. doi: 10.1089/neu.2014.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- 34.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 35.Han Z, Lat I, Pollard SR. Safety and efficacy of corticosteroid use in neurologic trauma. J Pharm Pract. 2014;27:487–495. doi: 10.1177/0897190013516500. [DOI] [PubMed] [Google Scholar]
- 36.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil. 2012;93:1508–1517. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto T, Fukuda N. Contribution of serotonin neurons to the functional recovery after spinal cord injury in rats. Brain Res. 1991;539:263–270. doi: 10.1016/0006-8993(91)91630-j. [DOI] [PubMed] [Google Scholar]
- 38.Hayashibe M, Homma T, Fujimoto K, Oi T, Yagi N, Kashihara M, Nishikawa N, Ishizumi Y, Abe S, Hashimoto H, Kanekiyo K, Imagita H, Ide C, Morioka S. Locomotor improvement of spinal cord-injured rats through treadmill training by forced plantar placement of hind paws. Spinal Cord. 2016;54:521–529. doi: 10.1038/sc.2015.186. [DOI] [PubMed] [Google Scholar]
- 39.Ilha J, Centenaro LA, Broetto Cunha N, de Souza DF, Jaeger M, do Nascimento PS, Kolling J, Ben J, Marcuzzo S, Wyse AT, Gottfried C, Achaval M. The beneficial effects of treadmill step training on activity-dependent synaptic and cellular plasticity markers after complete spinal cord injury. Neurochem Res. 2011;36:1046–1055. doi: 10.1007/s11064-011-0446-x. [DOI] [PubMed] [Google Scholar]
- 40.Jacobi A, Loy K, Schmalz AM, Hellsten M, Umemori H, Kerschensteiner M, Bareyre FM. FGF22 signaling regulates synapse formation during post-injury remodeling of the spinal cord. The EMBO J. 2015;34:1231–1243. doi: 10.15252/embj.201490578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung SY, Seo TB, Kim DY. Treadmill exercise facilitates recovery of locomotor function through axonal regeneration following spinal cord injury in rats. J Exerc Rehabil. 2016;12:284–292. doi: 10.12965/jer.1632698.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kakulas BA. Neuropathology: the foundation for new treatments in spinal cord injury. Spinal Cord. 2004;42:549–563. doi: 10.1038/sj.sc.3101670. [DOI] [PubMed] [Google Scholar]
- 43.Ke Z, Yip SP, Li L, Zheng XX, Tong KY. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS One. 2011;6:e16643. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang C, Guo X, Kerschensteiner M, Bareyre FM. Single collateral reconstructions reveal distinct phases of corticospinal remodeling after spinal cord injury. PLoS One. 2012;7:e30461. doi: 10.1371/journal.pone.0030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang C, Bradley PM, Jacobi A, Kerschensteiner M, Bareyre FM. STAT3 promotes corticospinal remodelling and functional recovery after spinal cord injury. EMBO Rep. 2013;14:931–937. doi: 10.1038/embor.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lankhorst AJ, ter Laak MP, van Laar TJ, van Meeteren NL, de Groot JC, Schrama LH, Hamers FP, Gispen WH. Effects of enriched housing on functional recovery after spinal cord contusive injury in the adult rat. J Neurotrauma. 2001;18:203–215. doi: 10.1089/08977150150502622. [DOI] [PubMed] [Google Scholar]
- 47.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968a;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968b:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Leal G, Comprido D, Duarte CB. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology. 2014;76(Pt C):639–656. doi: 10.1016/j.neuropharm.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Wu Q, Xie C, Wang C, Wang Q, Dong C, Fang L, Ding J, Wang T. Blocking of BDNF-TrkB signaling inhibits the promotion effect of neurological function recovery after treadmill training in rats with spinal cord injury. Spinal Cord. 2018 doi: 10.1038/s41393-018-0173-0. doi: 10.1038/s41393-018-0173-0. [DOI] [PubMed] [Google Scholar]
- 51.Liang H, Watson C, Paxinos G. Terminations of reticulospinal fibers originating from the gigantocellular reticular formation in the mouse spinal cord. Brain Struct Funct. 2016;221:1623–1633. doi: 10.1007/s00429-015-0993-z. [DOI] [PubMed] [Google Scholar]
- 52.Little JW, Harris RM, Lerner SJ. Immobilization impairs recovery after spinal cord injury. Arch Phys Med Rehabil. 1991;72:408–412. [PubMed] [Google Scholar]
- 53.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- 55.Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res. 1990;514:206–218. doi: 10.1016/0006-8993(90)91417-f. [DOI] [PubMed] [Google Scholar]
- 56.Loy K, Schmalz A, Hoche T, Jacobi A, Kreutzfeldt M, Merkler D, Bareyre FM. Enhanced voluntary exercise improves functional recovery following spinal cord injury by impacting the local neuroglial injury response and supporting the rewiring of supraspinal circuits. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5544. doi: 10.1089/neu.2017.5544. [DOI] [PubMed] [Google Scholar]
- 57.Magnuson DS, Smith RR, Brown EH, Enzmann G, Angeli C, Quesada PM, Burke D. Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabil Neural Repair. 2009;23:535–545. doi: 10.1177/1545968308331147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maier IC, Baumann K, Thallmair M, Weinmann O, Scholl J, Schwab ME. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez M, Delivet-Mongrain H, Rossignol S. Treadmill training promotes spinal changes leading to locomotor recovery after partial spinal cord injury in cats. J Neurophysiol. 2013;109:2909–2922. doi: 10.1152/jn.01044.2012. [DOI] [PubMed] [Google Scholar]
- 60.Mehrholz J, Harvey LA, Thomas S, Elsner B. Is body-weight- supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord. 2017;55:722–729. doi: 10.1038/sc.2017.31. [DOI] [PubMed] [Google Scholar]
- 61.Meijer JH, Robbers Y. Wheel running in the wild. Proc Biol Sci. 2014;281:20140210. doi: 10.1098/rspb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minassian K, McKay WB, Binder H, Hofstoetter US. Targeting lumbar spinal neural circuitry by epidural stimulation to restore motor function after spinal cord injury. Neurotherapeutics. 2016;13:284–294. doi: 10.1007/s13311-016-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morrison SA, Lorenz D, Eskay CP, Forrest GF, Basso DM. Longitudinal recovery and reduced costs after 120 sessions of locomotor training for motor incomplete spinal cord injury. Arch Phys Med Rehabil. 2018;99:555–562. doi: 10.1016/j.apmr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Nagel SJ, Wilson S, Johnson MD, Machado A, Frizon L, Chardon MK, Reddy CG, Gillies GT, Howard MA. Spinal cord stimulation for spasticity: historical approaches, current status, and future directions. Neuromodulation. 2017;20:307–321. doi: 10.1111/ner.12591. [DOI] [PubMed] [Google Scholar]
- 65.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 66.Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. 2016;78:436–447. doi: 10.1227/NEU.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 67.Rejc E, Angeli CA, Atkinson D, Harkema SJ. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci Rep. 2017;7:13476. doi: 10.1038/s41598-017-14003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robert AA, Al Jadid MS, Bin Afif S, Al Sowyed AA, Al-Mubarak S. The effects of different rehabilitation strategies on the functional recovery of spinal cord injured rats: an experimental study. Spine. 2010;35:E1273–1277. doi: 10.1097/BRS.0b013e3181e3fc5f. [DOI] [PubMed] [Google Scholar]
- 69.Rossignol S, Bouyer L. Adaptive mechanisms of spinal locomotion in cats. Integr Comp Biol. 2004;44:71–79. doi: 10.1093/icb/44.1.71. [DOI] [PubMed] [Google Scholar]
- 70.Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, Peitz M, Brustle O, Norenberg MD, Blesch A, Weidner N, Bunge MB, Bixby JL, Bradke F. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 2015;348:347–352. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sawada M, Kato K, Kunieda T, Mikuni N, Miyamoto S, Onoe H, Isa T, Nishimura Y. Function of the nucleus accumbens in motor control during recovery after spinal cord injury. Science. 2015;350:98–101. doi: 10.1126/science.aab3825. [DOI] [PubMed] [Google Scholar]
- 72.Shah PK, Garcia-Alias G, Choe J, Gad P, Gerasimenko Y, Tillakaratne N, Zhong H, Roy RR, Edgerton VR. Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain. 2013;136:3362–3377. doi: 10.1093/brain/awt265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith RR, Brown EH, Shum-Siu A, Whelan A, Burke DA, Benton RL, Magnuson DS. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma. 2009;26:1017–1027. doi: 10.1089/neu.2008-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun T, Ye C, Wu J, Zhang Z, Cai Y, Yue F. Treadmill step training promotes spinal cord neural plasticity after incomplete spinal cord injury. Neural Regen Res. 2013;8:2540–2547. doi: 10.3969/j.issn.1673-5374.2013.27.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell. 2014;159:1626–1639. doi: 10.1016/j.cell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 76.van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 77.Van Meeteren NL, Eggers R, Lankhorst AJ, Gispen WH, Hamers FP. Locomotor recovery after spinal cord contusion injury in rats is improved by spontaneous exercise. J Neurotrauma. 2003;20:1029–1037. doi: 10.1089/089771503770195876. [DOI] [PubMed] [Google Scholar]
- 78.Venezia AC, Guth LM, Sapp RM, Spangenburg EE, Roth SM. Sex-dependent and independent effects of long-term voluntary wheel running on Bdnf mRNA and protein expression. Physiol Behav. 2016;156:8–15. doi: 10.1016/j.physbeh.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H, Liu NK, Zhang YP, Deng L, Lu QB, Shields CB, Walker MJ, Li J, Xu XM. Treadmill training induced lumbar motoneuron dendritic plasticity and behavior recovery in adult rats after a thoracic contusive spinal cord injury. Exp Neurol. 2015;271:368–378. doi: 10.1016/j.expneurol.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wernig A, Nanassy A, Muller S. Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord. 1998;36:744–749. doi: 10.1038/sj.sc.3100670. [DOI] [PubMed] [Google Scholar]
- 82.Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur J Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 83.Yin Y, Sun W, Li Z, Zhang B, Cui H, Deng L, Xie P, Xiang J, Zou J. Effects of combining methylprednisolone with rolipram on functional recovery in adult rats following spinal cord injury. Neurochem Int. 2013;62:903–912. doi: 10.1016/j.neuint.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 84.Zhu H, Poon W, Liu Y, Leung GK-K, Wong Y, Feng Y, Ng SCP, Tsang KS, Sun DTF, Yeung DK, Shen C, Niu F, Xu Z, Tan P, Tang S, Gao H, Cha Y, So KF, Fleischaker R, Sun D, et al. Phase I–II clinical trial assessing safety and efficacy of umbilical cord blood mononuclear cell transplant therapy of chronic complete spinal cord injury. Cell Transplant. 2016;25:1925–1943. doi: 10.3727/096368916X691411. [DOI] [PubMed] [Google Scholar]
- 85.Zorner B, Schwab ME. Anti-Nogo on the go: from animal models to a clinical trial. Ann N Y Acad Sci 1198. 2010;Suppl 1:E22–34. doi: 10.1111/j.1749-6632.2010.05566.x. [DOI] [PubMed] [Google Scholar]
- 86.Zorner B, Bachmann LC, Filli L, Kapitza S, Gullo M, Bolliger M, Starkey ML, Rothlisberger M, Gonzenbach RR, Schwab ME. Chasing central nervous system plasticity: the brainstem’s contribution to locomotor recovery in rats with spinal cord injury. Brain. 2014;137:1716–1732. doi: 10.1093/brain/awu078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


