Abstract
Objectives:
Structural and functional MRI studies have consistently documented cortical and subcortical abnormalities in patients with Juvenile Myoclonic Epilepsy (JME). However, little is known about how these structural abnormalities emerge from the time of epilepsy onset and how network interactions between and within cortical and subcortical regions may diverge in youth with JME compared to typically developing children.
Methods:
We examined prospective co-variations of volumetric differences derived from high resolution structural MRI during the first two years of epilepsy diagnosis in a group of youth with JME (n=21) compared to healthy controls (n=22). We indexed developmental brain changes using graph theory by computing network metrics based on the correlation of the cortical and subcortical structural covariance near the time of epilepsy and two years later.
Results:
Over two years, normally developing children showed modular cortical development and network integration between cortical and subcortical regions. In contrast, children with JME developed a highly correlated and less modular cortical network, which was atypically dissociated from subcortical structures. Furthermore, the JME group also presented higher clustering and lower modularity indices than controls, indicating weaker modules or communities. The local efficiency in JME was higher than controls across the majority of cortical nodes. Regarding network hubs, controls presented a higher number than youth with JME that were spread across the brain with ample representation from the different modules. In contrast, children with JME showed a lower number of hubs that were mainly from one module and were comprised mostly of subcortical structures.
Significance:
Youth with JME prospectively developed a network of highly correlated cortical regions dissociated from subcortical structures during the first 2 years of epilepsy onset. The cortical-subcortical network dissociation provides converging insights on the disparate literature of cortical and subcortical abnormalities found in previous studies.
Keywords: Juvenile Myoclonic Epilepsy (JME), graph theory, development, brain volumes, MRI
INTRODUCTION
Juvenile Myoclonic Epilepsy (JME) is the most common generalized epilepsy (GE) syndrome in children with a typical onset during late-childhood to adolescent years1–3. JME is known to be associated with cognitive complications including aspects of executive function4–6, but also more broadly adversely affected neuropsychological status7, increased rate of psychiatric complications8, and problematic long term social function and employment status9,10.
One of the hallmarks of this idiopathic syndrome is that brain structural abnormalities are not found in routine magnetic resonance imaging (MRI) studies11. However, when investigating brain integrity with quantitative techniques a diversity of abnormalities has been reported in, for example, brain structure, metabolism, connectivity, and function11–13, 4, with several of these abnormalities linked to disrupted cognition13–15,4,6 attesting to their clinical significance. There are very few prospective investigations of cognitive and brain developmental course in youth with new or recent-onset JME. In one recent investigation, cortical developmental abnormalities were reported including greater cortical volumes compared to healthy controls in the superior frontal gyrus, sensorimotor region, temporal, parieto-occipital, fusiform, and retrosplenial regions, as well as the posterior cingulate cortex16. Aside from this study and in addition to our own work in epilepsy17, prospective investigations of this type have been carried out in other disorders including patients with 22q11.2DS18 and prospective RS-fMRI changes in older adults undergoing meditation training19.
These abnormalities provide only a small part of the story regarding brain development in this epilepsy population. For example, conventional methodologies can inform discrepancies in certain brain regions regarding thickness, area, and volume, however, they do not expand on the relative importance of specific regions within the whole brain network nor about their potential influence on the development of other related brain regions. In this investigation graph theory methodologies were used to investigate longitudinal brain development in children with JME. Graph theory analyses have provided insights regarding the covariance of cognitive testing in children with epilepsy20, as well as cortical volumes cross-sectionally in healthy children21 and in pediatric and adult patients with epilepsy22–23. This method allows a global assessment by providing information regarding how associated brain regions co-vary with each other and with the relatively important brain regions in the network. Such areas, called network hubs, are regions that provide valuable information about the configuration of a network. Here we investigated covariance networks in the prospective development of cortical and subcortical structures in children with JME compared to healthy controls based on volumetric differences as an index of brain development. Specifically, we investigated the covariance of the difference between diverse brain regions during the first two years following epilepsy diagnosis. How different brain regions developmentally co-vary provides information regarding the degree to which development is heterogeneous or homogeneous within cortical and subcortical regions as well as between them—a novel way of investigating brain development. We hypothesized that this method would provide a comprehensive network-based understanding of anticipated differences in brain development and organization in youth with JME compared to normally developing children.
METHODS
Participants
Twenty-one pediatric participants with recent-onset JME were recruited for this study, along with 22 age- and sex-matched healthy controls. All participants underwent T1 volumetric MRI scans at baseline and two years later as part of this prospective controlled cohort investigation with rolling recruitment. Children were recruited from pediatric neurology clinics at three medical centers (University of Wisconsin, Madison, WI; Marshfield Clinic, Marshfield, WI; and Dean Clinic, Madison, WI). Inclusion criteria included diagnosis of epilepsy within the past 12 months, no other developmental disabilities (e.g., intellectual impairment, autism), no other neurological disorder, and normal clinical MRI. We did not exclude children on the basis of psychiatric comorbidities (including attention-deficit/hyperactivity disorder [ADHD]) or learning disabilities. A pediatric neurologist certified by the American Board of Psychiatry and Neurology diagnosed individuals with JME according to the International League Against Epilepsy (ILAE) international classification of epilepsy24. Specifically, all children with JME presented: (1) EEG showing 4–6 Hz polyspike and slow-wave generalized discharges; and (2) history of myoclonic jerks, with or without generalized tonic–clonic seizures. Among the psychiatric comorbidities experienced by the group of patients, seven had Axis I diagnosis (some with more than one diagnosis), including three diagnoses of anxiety, three ADHD, four depression, one psychosis, and one adjustment disorder. Regarding the medications taken by the children, 13 were on valproate, 4 on lamotrigine, and 4 on levetiracetam. One of the patients on levetiracetam was also on valproate. All children with epilepsy were attending regular schools at the time of baseline evaluations and IQ was within normal levels.
Criteria for inclusion of control participants include no neurological disorders, no history of seizures, no history of any classic precipitating injury (e.g., febrile seizures), no previous loss of consciousness for >5 min, and no other family history of a first-degree relative with epilepsy or febrile convulsions. Further details and rationale for selection criteria for participants can be found in previous publications25. Details regarding demographic and clinical characteristics of the study participants are provided in Table 1. All patients and controls were seen at baseline and 2 years later and all procedures were conducted by research staff.
Table 1:
Demographic and Clinical Characteristics
| Control (n=22) | JME (n=21) | |
|---|---|---|
| Age* (mean ± SD) | 11.3 ± 2.1 | 15.6 ± 2.7 |
| Sex (F/M) | 12/10 | 12/9 |
| SES (mean ± SD) | 4.4 ± 1.6 | 4.3 ± 2.0 |
| Grade* (mean ± SD) | 5.2 ± 2.1 | 9.5 ± 2.9 |
| IQ (mean ± SD) | 109.2 ± 11.2 | 101.5 ± 12.8 |
|
Epilepsy duration (months,
mean ± SD) |
- | 8.5 ± 3.6 |
Significantly different between groups. SES=socioeconomic status based on mother’s education.
The project protocol was reviewed and approved by the institutional review board of the University of Wisconsin School of Medicine and Public Health. Families and children gave written informed consent or assent, respectively, on the day of the study.
MRI acquisition and processing
MR images were obtained on a 1.5-T GE Signa MRI scanner (GE Healthcare, Waukesha, WI, USA). T1-weighted images were acquired using a three-dimensional (3D) spoiled gradient recall (SPGR). The imaging parameters were: repetition time (TR) = 24 ms, echo time (TE) = 5 ms, flip angle = 40°, thickness = 1.5 mm, slices = 124, slice plane = coronal, field of view (FOV) = 20 cm, matrix = 256 × 256.
High-resolution structural MRI was acquired to investigate cortical volumes in children with epilepsy. Processing of T1-weighted images were performed with the software Freesurfer (http://freesurfer.net) (version 5.3) using the recon-all pipeline, which includes motion correction, non-uniform intensity normalization, Talairach transform computation, intensity normalization, skull stripping, among others26–28. We performed automatic cortical surface parcellation and subcortical structures segmentation in a subject-specific manner, using Freesurfer. The cortical parcellations were based on the Desikan-Killiany probabilistic atlas. Cortical volume changes were calculated for each subject using the Freesurfer’s processing stream for longitudinal images. This stream calculates measures such as the rate of change and the percentage of change between longitudinal acquisitions without being biased to any time point by registering them together and creating a subject-specific template (base image). This reduces random variations and increases the robustness of results29. Once both baseline and follow-up scans were longitudinally processed, the change between time points normalized to the baseline evaluation ((TP2-TP1)/TP1) was calculated in order to investigate the network of volume changes between different cortical and subcortical regions.
Matrix creation and graph theory measures
After calculating the difference between time points normalized to the baseline evaluation for each participant, a weighted undirected matrix of 85 nodes (68 cortical, and 17 subcortical structures) was calculated, based on the correlation coefficients of the covariance between nodal volume changes for each group. To discern statistically significant group differences, each group matrix was resampled by replacement (i.e. bootstrapped) a total of 500 times. Given that results from graph theory measures can occur by chance alone, the null distribution was calculated based on 500 random matrices with the same number of nodes and degree distribution as the pertinent graphs. P-values were corrected for multiple comparisons (Bonferroni correction) for each of the global and local measures. Matrices were corrected for intracranial volumes (ICV). In order to be able to discern group differences, matrices were proportionally thresholded, representing a way to ensure that only the strongest (highest weighted) percentage of links forms the graph. However, given that graphs may at times fail to fully connect at sparse thresholds (relatively low number of links) we combined the use of proportional thresholding with the Minimum Spanning Tree (MST) as its backbone (see reference 20 for details). In short, the MST is a subgraph of the network that connects all nodes in the graph while using the strongest weights between them without forming cycles or loops. Adding the proportional threshold would then build up on the rest of the connections between nodes. For the remainder of this manuscript, each graph threshold represents a combination of MST and proportional thresholding, indexed by the density level. For example, a density level of 15% would be the MST plus a proportional threshold of 15%. It might also be referenced as a hybrid threshold. Graph theory measures were obtained from each resampled matrix at the same threshold and averages were used for analyses.
In this study, global measures such as transitivity, global efficiency, and modularity index were calculated in order to investigate the properties of network segregation, integration, and configuration, respectively. Transitivity characterizes the level of segregation of the network; therefore, investigating how clustered the nodes are, in graph form. Global and local efficiency were calculated to examine network integration. Global efficiency is defined as the average of the inverse of the shortest paths in the network30. Therefore, high global efficiency represents high integration of communication in the network. In contrast, local efficiency is a metric of interactions between the neighbors of a node30. Finally, modularity or community structure indexes the configuration of a network into segregated communities. Given that the modularity algorithm provides a statistical estimate for each output31, we calculated modularity 1000 times for each group and the highest proportion was chosen as the number of modules in that group. Next, we used the Force Atlas algorithm, an open source software Gephi (https://gephi.org), for the 2D visualization of modularity or community structure on each group (attraction strength=5, repulsion strength=2000, gravity=30). These global metrics were calculated over a range of topological thresholds to ensure that the results were not driven by graph density.
Betweenness centrality (BC) was investigated here in order to identify the hubs of the networks, and it was calculated for each group at a hybrid threshold of 35%, which was the density level at which both groups presented full graph connectedness. The hubs are those nodes that are critical for the configuration of the network32. In the case of BC, it measures the relevance of a node for the communication between other nodes in the network33. Nodes with high BC facilitate global integrative processes by serving as “highways” to ease “traffic” flow in the network32. Hubs for each group were identified as those nodes with BC higher than one standard deviation above the mean.
RESULTS
The JME and control groups were similar in age, (F(1,57)=0.017, p=0.895), sex (χ2=1.184; p=0.277), socioeconomic status (SES) (F(1,57)=1.002, p=0.321), and IQ (F(1,57)=0.086, p=0.77). Student’s t-test were carried out for the continuous variables and Chi-square test for categorical one (sex). Further, ICV was included as a covariate on the matrices calculations.
Adjacency matrices and modularity
Unthresholded adjacency matrices were calculated for the average matrices of each group (Figure 1). As can be seen in Figure 1, the group with JME is presenting a highly correlated matrix of cortical regions (nodes 1–68) and sparse associations with subcortical structures (nodes 69–85). In contrast, controls show integration between cortical and subcortical regions.
Figure 1:
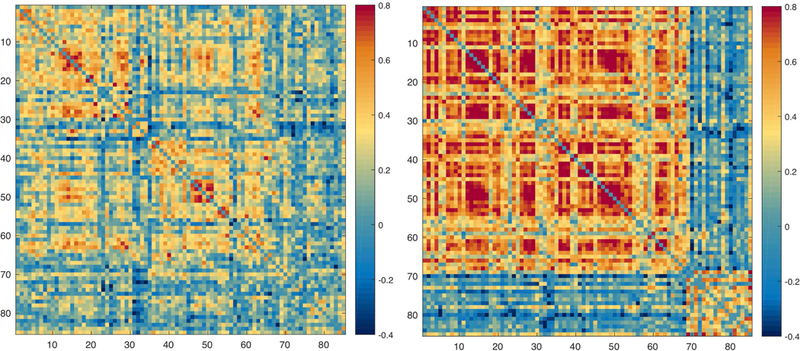
Adjacency matrices of percentage change correlations in controls (left) and JME (right). The ordering of nodes is the same as in Table 1S.
Figure 2 depicts the modularity for each group calculated at a threshold or graph density of 35%. It can be seen that the JME group showed three closely associated modules (red, orange, and yellow) with low association to subcortical structures (blue nodes in Figure 2, right).
Figure 2:
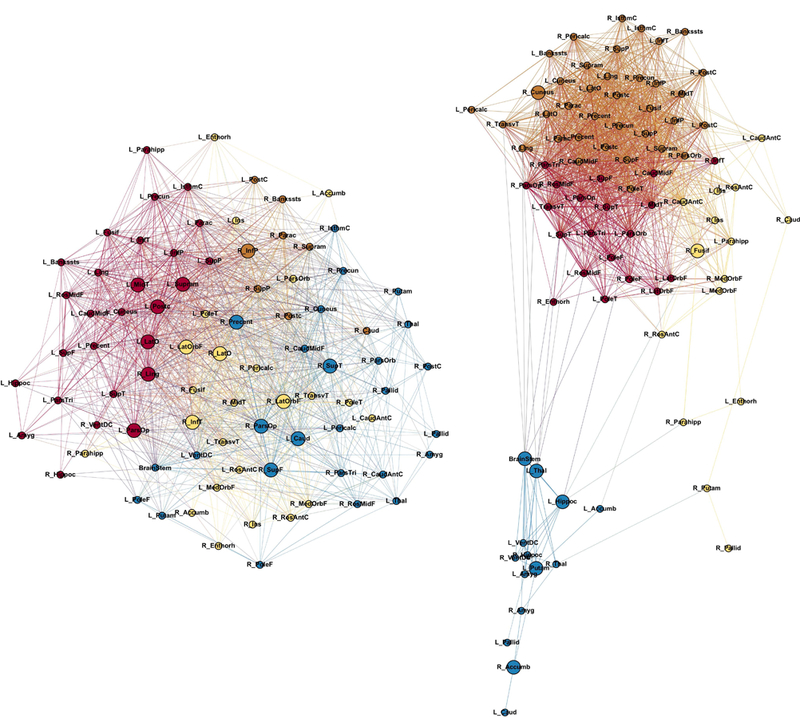
Modularity in controls (left) and JME (right). Node abbreviations are the same as in Table 1S. Same color nodes belong to the same module. Bigger nodes represent the hubs of the network as calculated using BC. The spatial distribution of nodes was calculated using the force-atlas graph algorithm, where nodes that demonstrated stronger connections are located closer in space, while nodes with fewer connections tend to be farther in space. Calculated at a hybrid threshold of 35%.
Global Measures
Figure 3 shows the global measures calculated over a range of density thresholds. As can be seen, participants with JME are presenting higher transitivity compared to controls; therefore, the JME group exhibits greater segregation. Regarding global efficiency, JME also presents with higher efficiency than controls across thresholds. Finally, the JME group shows a significantly lower modularity index than controls indicating weaker modules or communities. Altogether, these findings indicate that the JME group showed greater divergence of the global network configuration compared to healthy controls.
Figure 3:
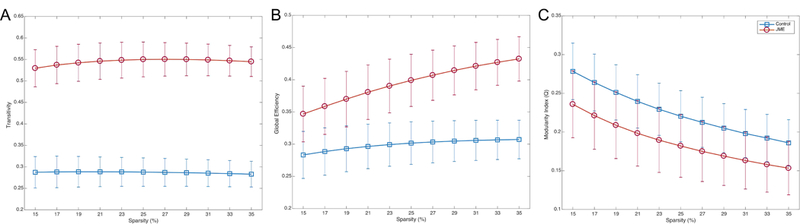
Transitivity (left), global efficiency (middle), and modularity index (right) in controls (blue), and JME (red). Error bars represent the standard deviation. Groups were statistically significant after Student’s t-test between themselves and against zero; corrected for multiple comparisons (Bonferroni correction).
In order for readers to know how different are the results developmentally (this study) and at each time point (baseline and two-year follow-up), additional analyses were carried out for the global measures at each time point and for both groups and can be found in the supporting documentation 2.
Regional Measures
As shown in Figure 4, JME presented the highest local efficiency across the majority of cortical nodes (Figure 4). However, although controls had lower values in general, they presented relatively constant local efficiency across nodes while JME although higher on average were irregular, especially in subcortical structures.
Figure 4:
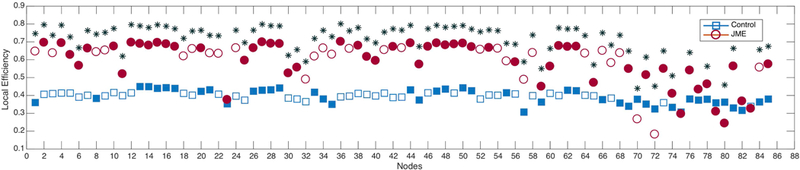
Local efficiency in controls (blue) and JME (red). Filled markers represent statistical significance against random. *Statistically significant between groups after Student’s t-test; corrected for multiple comparisons (Bonferroni correction). Calculated at a hybrid threshold of 35%.
Regarding the hubs of the network, controls presented a high number across the brain (16 hubs) while JME showed only 7 hubs. Furthermore, controls had ample representation of hubs from the different modules and lobes of the brain. In the JME group, hubs were mainly from one module, comprised mostly of subcortical structures (Figure 5), and excluded the parietal lobe and prefrontal regions entirely.
Figure 5:
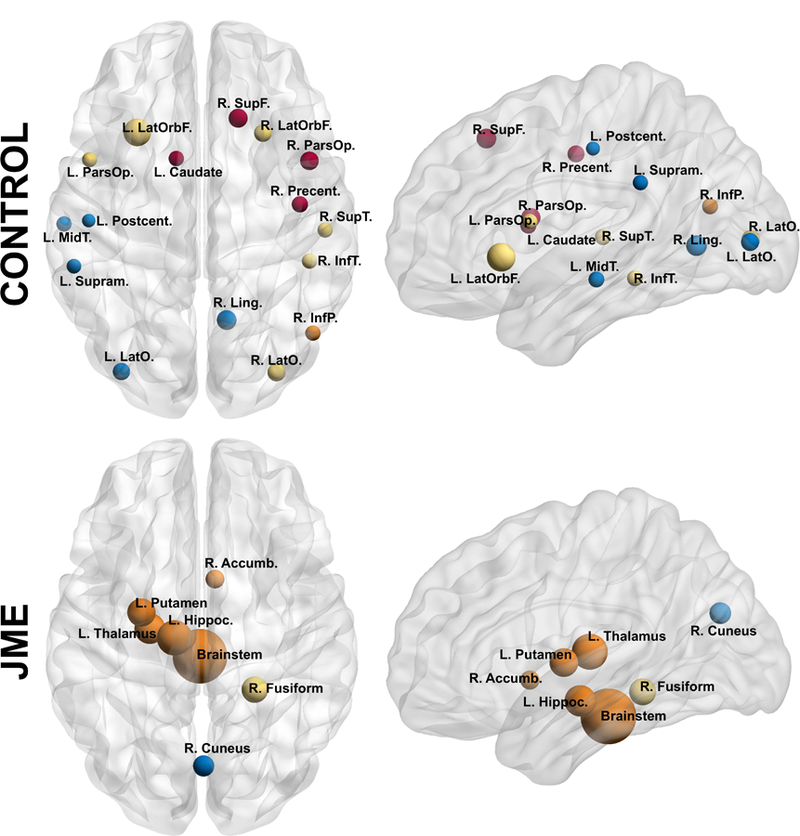
Nodes with high betweenness centrality (BC) in controls (top) and JME (bottom) at their approximate anatomical location. Nodes with the same color represent the same module. Labels are the node abbreviations from Table 1S. Calculated at a hybrid threshold of 35%.
DISCUSION
This study investigated the covariance of prospective volumetric development across cortical and subcortical structures in youth with new/recent onset JME, as a network based on graph theory analyses. Our data showed that JME patients exhibited marked differences compared to controls in terms of global and regional network science metrics as well as ultimate network configuration. We used a novel approach to investigate prospective development, something previously performed to examine cognitive network development in children with epilepsy20.
Network configuration
Regarding the overall network configuration based on the prospective adjacency matrices and community structure, children with JME presented a network of highly correlated cortical regions that were unassociated with subcortical structures. In addition to striking cortical and subcortical disengagement, cortical regions appeared to be highly correlated among themselves in one large module. These findings indicate a brain that is developing in a non-modular fashion, suggesting that development is not organized but following an abnormal non-modular course. Longitudinal studies of cortical development in normally developing children using conventional methods have shown that cortical development does not occur homogeneously across the brain, rather, different regions mature at different rates34–35, indicating modular development. Modular development indicates that different groups of functional regions in the brain develop in synchrony and do not necessarily develop similarly to other groups of functional regions. This kind of development indicates specialization36. In direct contrast, the JME group presented with a highly non-modular development of their cortical/subcortical covariance networks, possibly indicating suboptimal maturation.
Cortical and subcortical dissociation has recently been reported in cross-sectional research of patients with temporal lobe epilepsy (TLE), suggesting that this may be a generalizable finding and principle in epilepsy37. Cortical and subcortical coordination is critical for decision making and inhibition and conflict resolution – problems common in JME38. Working memory also involves dynamic dialog between cortical and subcortical regions, specifically frontal and mesial temporal networks39. Working memory is poor in JME and this poor cortical-subcortical association may be a potential contributor.
Global and Regional Measures
Regarding global network development, JME children presented the highest segregation and integration compared to controls. Such lower segregation in controls has been reported previously in cross sectional investigations22. However, the modularity index in controls was significantly higher than JME, which presented very low values across density levels. Such low modularity index confirms that JME brains are not developing efficiently.
In terms of local efficiency, children with JME showed the highest level of structural covariance among cortical regions, indicating higher than expected developmental associations in the cerebral cortex. However, the local efficiency across nodes was not constant as in controls, possibly indicating sparse regions that are not engaging with the rest of the network. Specifically, the regions showing close-to-zero values of local efficiency were the left insula, temporal regions (bilateral entorhinal and pericalcarine regions), as well as subcortical areas (left caudate, left pallidum, left accumbens, right amygdala, right accumbens, and right ventral DC). Therefore, even though the JME developmental network expresses high local associations, not all regions are as correlated to the rest of the network, while in controls all regions show similar association to each other—another indication of disrupted developmental processes in JME.
Network hubs
Regarding the most important regions for the configuration of the network, controls presented a high number of hubs across the brain. Moreover, representation from different modules was evident while JME participants showed the majority of hubs from only one module. Furthermore, JME patients did not share any hubs with the controls. Furthermore, controls presented a similar proportion of hubs across lobes, while JME mostly presented subcortical regions as hubs, with controls showing only the left pallidum as a subcortical hub.
Interestingly, the majority of nodes serving as hubs in the JME network did not belong to the conglomerate of cortical regions, but derived mainly from subcortical areas. Specifically, only three hubs seemed to be holding together the extensive cortical regions (left superior temporal, right caudal middle frontal, and right caudal anterior cingulate) while the rest (majority) were bridging cortical and subcortical areas. These results could indicate that the development of subcortical regions is more closely associated within themselves, possibly causing them to act like a sub-network that is not developmentally influenced by the remaining brain regions. This apparent cortical-subcortical dissociation is in line with the well-known thalamic abnormality in JME40. However, our findings indicate that JME is not a simply a disorder of frontothalamic derangement. The subcortical dissociation involves diverse structures including the putamen, the amygdala, and nucleus accumbens. These findings may indicate impaired cortical-subcortical information flow, possibly underlying impaired cognition15.
Notable is that children with JME were missing hubs from the frontal and parietal regions (Figure 5). Frontal and parietal networks are emerging as important circuits for working memory and attention and their absence is noteworthy41. The frontal abnormality is well appreciated in JME but disconnection to the parietal region has not been previously noted and may represent another under-appreciated network deficit in JME that contributes to the well known dysexecutive function4–6.
Furthermore, the identified widespread network disruptions may be contributors to the increasingly recognized generalized cognitive dysfunction7, increased rate of psychiatric complications8, and problematic long term social function and employment status9,10. Linking early identified abnormalities in brain and brain development to these important comorbidities will be important tasks for the future.
Study Limitations
This study has some limitations. One limitation is the use of only one epilepsy group, which does not allow the investigation of the specificity of findings. We have also performed the same analysis on children with Benign Epilepsy with Centrotemporal Spikes (BECTS); however, we have not included the BECTS patients, analyses and findings in the present manuscript as it would change the nature and intent of the paper as well as make it considerably more complicated and dense. A manuscript describing network function in children with BECTS is under preparation. But some of the differences between JME and BECTS is lower modularity and efficiency in the former. Another limitation is that patients were treated with AEDs, which might influence the results. Because this is a prospective observational study it becomes essentially impossible to isolate AED effects. While it is possible to examine drug-naïve new-onset JME patients, a prospective study of untreated children with JME is untenable. The important point is that this cohort of children with new onset/recently diagnosed JME were treated at baseline as they were 8 months post-diagnosis (on average), so there is some confounding of time with treatment.
CONCLUSIONS
Youth with new and recent onset JME demonstrated atypical prospective development in a span of two years as investigated using graph theory—a novel application to this literature. This conclusion is supported by their low modular development, evident developmental disengagement of cortical from subcortical regions, and abnormal global measures when compared to healthy controls. This apparent cortical-subcortical morphological dissociation might be related to the well-known thalamic abnormality in JME; however, since more subcortical regions are involved, this might indicate lack of synchronization between cortical and subcortical areas more generally. Overall, youth with JME develop differently from controls from a neuroanatomical perspective and use of network science provides interesting insights into these differences.
Supplementary Material
Highlights.
Over two years, normally developing children showed modular cortical development and network integration between cortical and subcortical regions.
Children with JME developed a highly correlated and less modular cortical network, which was atypically dissociated from subcortical structures.
The local efficiency in JME was higher than controls across the majority of cortical nodes.
Regarding network hubs, children with JME showed a lower number of hubs that were mainly from one module and were comprised mostly of subcortical structures.
ACKNOWLEDGMENTS
All phases of this study were supported by the National Institute of Neurological Disorders and Stroke (NINDS) 3RO1–44351 and by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. We thank Raj Sheth MD, Monica Koehn MD, and Jason Dozier MD for study participation and recruitment of participants. Also greatly appreciated are Dace Almane, Melissa Hanson, Kate Young, and Bjorn Hanson for overall study coordination, participant recruitment, cognitive assessment, and data management.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Financial disclosures
The authors report no conflicts of interest.
REFERENCES
- 1.Panayiotopoulos CP. A Clinical Guide to Epileptic Syndromes and their Treatment: Based on the New ILAE Diagnostic Scheme. Bladon Medical Pub 2002, Oxfordshire, UK. [Google Scholar]
- 2.Larsson K, Eeg-Olofsson O. A population based study of epilepsy in children from a Swedish county. Eur J Paediatr Neurol 2006;10:107–13. [DOI] [PubMed] [Google Scholar]
- 3.Sidenvall R, Forsgren L, Heijbel J. Prevalence and characteristics of epilepsy in children in northern Sweden. Seizure 1996;5:139–46. [DOI] [PubMed] [Google Scholar]
- 4.Wanndschneider B, Thompson PJ, Vollmar C, et al. Frontal lobe function and structure in juvenile myoclonic epilepsy: a comprehensive review of neuropsychological and imaging data. Epilepsia 2012;53:2091–98. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury FA, Elwes RD, Koutroumanidis M, et al. Impaired cognitive function in idiopathic generalized epilepsy and unaffected family members: an epilepsy endophenotype. Epilepsia 2014;55:835–40. [DOI] [PubMed] [Google Scholar]
- 6.Pulsipher DT, Seidenberg M, Guidotti L, et al. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia 2009;50:1210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loughman A, Bowden SC, D’Souza W. Cognitive functioning in idiopathic generalised epilepsies: a systematic review and meta-analysis. Neurosci Biobehav Rev 2014;43:20–34. Epub 2014 Mar 14. Review. [DOI] [PubMed] [Google Scholar]
- 8.De Araujo Filho GM, Yacubian EM. Juvenile myoclonic epilepsy: psychiatric comorbidity and impact on outcome. Epilepsy Behav 2013;28 Suppl 1:S74–80. [DOI] [PubMed] [Google Scholar]
- 9.Camfield CS, Camfield PR. Juvenile myoclonic epilepsy 25 years after seizure onset: a population-based study. Neurology 2009;73:1041–45. [DOI] [PubMed] [Google Scholar]
- 10.Schneider-von Podewils F, Gasse C, Geithner J, et al. Clinical predictors of the long-term social outcome and quality of life in juvenile myoclonic epilepsy: 20–65 years of follow-up. Epilepsia 2014;55:322–30. [DOI] [PubMed] [Google Scholar]
- 11.Koepp MJ, Thomas RH, Wandschneider B, et al. Concepts and controversies of juvenile myoclonic epilepsy: still an enigmatic epilepsy. Expert Rev Neurother 2014;14:819–31. [DOI] [PubMed] [Google Scholar]
- 12.Cao B, Tang Y, Li J, et al. A meta-analysis of voxel-based morphometry studies on gray matter volume alteration in juvenile myoclonic epilepsy. Epilepsy Res 2013;106:370–7. [DOI] [PubMed] [Google Scholar]
- 13.Caeyenberghs K, Powell HW, Thomas RH, et al. Hyperconnectivity in juvenile myoclonic epilepsy: a network analysis. Neuroimage Clin 2014;7:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cevik N, Koksal A, Dogan VB, et al. Evaluation of cognitive functions of juvenile myoclonic epileptic patients by magnetic resonance spectroscopy and neuropsychiatric cognitive tests concurrently. Neurol Sci 2016;37:623–7. [DOI] [PubMed] [Google Scholar]
- 15.O’Muircheartaigh J, Vollmar C, Barker GJ, et al. Focal structural changes and cognitive dysfunction in juvenile myoclonic epilepsy. Neurology 2011;76:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JJ, Dabbs K, Riley JD, et al. Neurodevelopment in new-onset juvenile myoclonic epilepsy over the first 2 years. Ann Neurol 2014;76:660–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Ramos C, Jackson D, Dabbs K, Jones J, Hsu D, Stafstrom C, Zawadzki L, Koehn M, Seidenberg M, Prabhakaran V, Hermann B. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia 2015b. October;56(10):1615–22. doi: 10.1111/epi.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandini C, Zöller D, Scariati E, Padula MC, Schneider M, Schaer M, Van De Ville D, Eliez S. Development of Structural Covariance From Childhood to Adolescence: A Longitudinal Study in 22q11.2DS. Front Neurosci 2018. May 18;12:327. doi: 10.3389/fnins.2018.00327. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotier FA, Zhang R, Lee TMC. A longitudinal study of the effect of short-term meditation training on functional network organization of the aging brain. Sci Rep 2017. April 4;7(1):598. doi: 10.1038/s41598-017-00678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Ramos C, Lin JJ, Prabhakaran V, Hermann BP. Developmental reorganization of the cognitive network in pediatric epilepsy. PLoS ONE 2015a; 10(10): e0141186. doi: 10.1371/journal.pone.0141186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lerch JP, Worsley K, Shaw WP, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage 2006;31:993–1003. [DOI] [PubMed] [Google Scholar]
- 22.Bonilha L, Tabesh A, Dabbs K, et al. Neurodevelopmental alterations of large-scale structural networks in children with new-onset epilepsy. Hum Brain Mapp 2014;35:3661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernhardt BC, Chen Z, He Y, et al. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex 2011;21(9):2147–57. [DOI] [PubMed] [Google Scholar]
- 24.ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy 1989. Epilepsia 1989;30:389–399. [DOI] [PubMed] [Google Scholar]
- 25.Hermann B, Jones J, Sheth R, et al. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain 2006;129:2609–2619. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences USA 2000;97:11044–11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions in Medical Imaging; 2001;20:70–80. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex, 2004;14:11–22. [DOI] [PubMed] [Google Scholar]
- 29.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage 2011;57:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Zuo X, He Y. Graph-based network analysis of resting-state functional MRI. Front Syst Neurosci 2010;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blondel VD, Guillaume J-L, Lambiotte R, et al. Fast unfolding of communities in large networks. J. Stat. Mech 2008, P10008. [Google Scholar]
- 32.Sporns O, Honey CJ, Kötter R. Identification and classification of hubs in brain networks. PLoS One 2007; 2:e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boccaletti S, Latora V, Moreno Y, et al. Complex networks: Structure and dynamics. Phys Rep 2006. February;424:175–308. [Google Scholar]
- 35.Raznahan A, Lerch JP, Lee N, et al. Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron 2011;72:873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 2008;28:3586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitano H Biological robustness. Nat. Rev. Genet 2004;5, 826–837. [DOI] [PubMed] [Google Scholar]
- 38.Englot DJ, D’Haese PF, Konrad PE, et al. Functional connectivity disturbances of the ascending reticular activating system in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 2017;88:925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hell F, Taylor PCJ, Mehrkens JH, et al. Subthalamic stimulation, oscillatory activity and connectivity reveal functional role of STN and network mechanisms during decision making under conflict. Neuroimage 2018;171:222–233. [DOI] [PubMed] [Google Scholar]
- 40.Johnson EL, Adams JN, Solbakk AK, et al. Dynamic frontotemporal systems process space and time in working memory. PLoS Biol 2018;16:e2004274 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craiu D What is special about the adolescent (JME) brain? Epilepsy Behav 2013. July;28 Suppl 1:S45–51. [DOI] [PubMed] [Google Scholar]
- 42.Johnson EL, Dewar CD, Solbakk AK, et al. Bidirectional Frontoparietal Oscillatory Systems Support Working Memory. Curr Biol 2017;27:1829–1835.e4. Epub 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


