Abstract
Parkinson’s disease (PD) is characterized by the presence of inflammation-mediated dopaminergic neurodegeneration in the substantia nigra. Inflammatory mediators from activated microglia, astrocytes, neurons, T-cells and mast cells mediate neuroinflammation and neurodegeneration. Administration of neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induces PD like motor deficits in rodents. 1-methyl-4-phenylpyridinium (MPP+), a toxic metabolite of MPTP activates glial cells, neurons and mast cells to release neuroinflammatory mediators. Glia maturation factor (GMF), mast cells and proteinase activated receptor-2 (PAR-2) are implicated in neuroinflammation. Alpha-synuclein which induces neurodegeneration increases PAR-2 expression in the brain. However, the exact mechanisms are not yet understood. In this study, we quantified inflammatory mediators in the brains of MPTP-administered wild type (Wt), GMF-knockout (GMF-KO) and mast cell knockout (MC-KO) mice. Additionally, we analyzed the effect of MPP+, GMF and mast cell proteases on PAR-2 expression in astrocytes and neurons in vitro. Results show that the levels of interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α) and the chemokine (C-C motif) ligand 2 (CCL2) were lesser in the brains of GMF-KO mice and MC-KO mice when compared to Wt. mice brain after MPTP administration. Incubation of astrocytes and neurons with MPP+, GMF and mouse mast cell protease-6 (MMCP-6) and MMCP-7 increased the expression of PAR-2. Our studies show that the absence of mast cells and GMF reduce the expression of neuroinflammatory mediators in the brain. We conclude that GMF along with mast cell interactions with glial cells and neurons during neuroinflammation can be explored as a new therapeutic target for PD and other neuroinflammatory disorders.
Keywords: Astrocytes, cytokines, glia maturation factor, mast cells, mouse mast cell proteases, Parkinson’s disease, PAR-2
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disease due to the degeneration of dopaminergic neurons in the substantia nigra and decreased dopamine level in the striatum [1]. PD is the second most common neurodegenerative disorder affecting nearly one million people in the United States of America and 10 million worldwide (Parkinson’s Foundation, New York, NY). About 60,000 peoples are diagnosed with PD each year in the USA. The incidence of PD increases with age and men are about 1.5 times more prone to PD than women. About 4% of people with PD are diagnosed before the age of 50 years. It costs about $25 billion per year to combat PD in the USA. PD affects movement and there are no disease-specific treatment options available as the exact cause and the disease mechanisms are still not yet clearly understood [2–4]. PD is a movement disorder and the current dopaminergic medication causes significant side effects to the patients [2]. Although the exact cause of PD remains unknown, exposure to environmental chemicals such as pesticides and heavy metals accumulation in the brains are reported to cause PD in humans and animals [5]. Administration of neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxydopamine (6-OHDA), paraquate and rotenone mimics several PD symptoms in primates and rodents [6, 7]. MPTP-administration increases alpha-synuclein (α-synuclein) expression in the brains of rodents [8]. Lewy bodies (LB) are implicated in the development, survival and functioning of dopaminergic neurons and α-synuclein is the major component of LB and a potential therapeutic target in PD [9]. Aggregation of α-synuclein in neurons forms LB that spreads through the brain and induces neurodegeneration in the substantia nigra in PD [10, 11]. Recent reports indicate that innate and adaptive immune responses are involved in the pathogenesis of PD [12–14]. Inflammatory cytokines, chemokines and other neuroinflammatory mediators including interleukin-1beta (IL-1β), tumor necrosis factor-alpha (TNF-α), chemokine (C-C motif) ligand 2 (CCL2) are shown to be involved in the pathogenesis of PD [15–18].
We have shown that glia maturation factor (GMF), a brain specific protein is involved in the neuroinflammation and neurodegeneration [19–22]. Further, we have shown that GMF activates glial cells to release many neuroinflammatory mediators which induce neurodegeneration and that the absence of GMF reduced these effects [23–28]. Mast cells are innate immune cells which interact with glia and neurons and exacerbate neuroinflammation [29]. We and others have previously shown that mast cells are critical in neuroinflammatory conditions by releasing pre-stored and newly generated inflammatory mediators including proteases [30–33]. Proteinase activated receptors (PARs) are G-protein coupled receptors that are expressed in both peripheral system and central nervous system (CNS) that induce neuroinflammation [8]. There are four types of PARs such as PAR-1, PAR-2, PAR-3 and PAR-4. PAR-2 expression is increased in the brains of Multiple sclerosis (MS), PD and Alzheimer’s disease (AD). PARs are activated by proteases including the tryptase released from the activated mast cells. PAR-2 activation leads to the activation of mitogen-activated protein kinases (MAPKs) and nuclear factor kappa-B (NF-kB) pathways that are involved in the generation of inflammatory cytokines and chemokines. MPTP administration increased the expression of PAR-2 in the substantia nigra and the blockade of PAR-2 reduced the synthesis of α-synuclein and the activation of NF-kB in rats [8]. However, interaction between MPP+, GMF and mast cell proteases on the regulation of PAR-2 expression in glia and neurons are not yet understood. Therefore, in the present study, we investigated whether the absence of GMF or mast cells would reduce neuroinflammation in MPTP administered mice. Additionally, we examined whether 1-methyl-4-phenylpyridinium (MPP+), a metabolite of MPTP along with GMF and mast cell proteases can induce the expression of PAR-2. We demonstrate that the absence of GMF or mast cells reduced the expression of inflammatory mediators in the brain and PAR-2 in the glial cells.
MATERIALS AND METHODS
Reagents and antibodies
DuoSet enzyme-linked immunosorbent assay (ELISA) kits for mouse chemokine CCL2/JE/MCP-1, IL-1β, and TNF-α as well as recombinant mouse mast cell protease-6 (MMCP-6) and MMCP-7 were purchased from R&D Systems (Minneapolis, MN). Dulbecco’s phosphate buffered saline (DPBS), Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 (Ham) (DMEM F12), penicillin/streptomycin and fetal bovine serum (FBS) were obtained from Life Technologies (Grand Island, NY). Cell culture flasks and tissue culture plates were purchased from Costar (Corning Incorporated, and Corning, NY). MPTP, MPP+, anti-microtubule associated protein-2 (MAP2) rabbit polyclonal antibody and poly-D-lysine were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit polyclonal glial fibrillary acidic protein (GFAP) antibody (Proteintech, Rosemont, IL), anti-PAR-2 mouse monoclonal antibody (SAM11) (Abcam, Cambridge, MA), goat anti-mouse 568 (red), goat anti-rabbit 488 (green), goat anti-mouse IgG Texas red antibodies were purchased from Vector Laboratories (Burlingame, CA) and Calbiochem (Burlington, MA). Recombinant GMF was obtained from the Vector core facility (University of Iowa, Iowa City, IA). GMF-knockout (GMF-KO) mice were previously developed in our laboratory and a colony of these mice were maintained for our studies [24, 34, 35]. Mast cell knockout (MC-KO) mice (WBB6/F1-KitW/KitWv) and Wild type (Wt) C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME).
MPTP-administered mouse model of PD
MPTP was administered to the mice as per the standard published safety procedures [36]. These mice were maintained and the experiments were conducted at the Harry S. Truman Memorial Veterans Hospital (Columbia, MO) in accordance with the NIH guidelines approved by the Animal Care and Use Committees at the VA Hospital (Columbia, MO). Briefly, adult Wt. mice, GMF-KO mice, and MC-KO mice (4 to 5/group) were given four intraperitoneal (i.p) injections of MPTP (15 mg/kg body weight) at 2 hrs intervals for acute effects as per the standard procedures [36, 37]. Control mice were given an equal volume of sterile phosphate buffered saline (PBS) only instead of MPTP. Rota rod and grip strength tests for locomotor behavioral tests were performed to assess the presence of PD symptoms after MPTP administration as we have reported previously [37]. Then, the mice were euthanized by cervical dislocation on day 7 after MPTP administration for the procurement of brain for inflammatory cytokines and chemokine TNF-α, IL-1β and CCL2 quantification by ELISA.
Inflammatory cytokines and chemokine quantification in the brain lysates
Brain lysates were prepared from the brains of Wt. mice, GMF-KO mice and MC-KO mice by tissue homogenization and sonication methods with protease and phosphatase inhibitors in HEPES lysis buffer. Protein concentration in the lysates was quantified using BCA assay method (Pierce, Rockford, IL). TNF-α, IL-1β and CCL2 levels were measured in the brain lysates consisting of 50 to 100 μg protein per sample using DuoSet ELISA kits as recommended by the manufacturer. Finally, the ELISA microplates were read on a microplate reader (Molecular Devices, Sunnyvale, CA) as we have previously reported [38, 39].
Mouse primary astrocytes and glia-neurons mixed culture
Wt. pregnant mice (C57BL/6) were euthanized on 16th to 18th day of gestation and the fetal brains were subsequently obtained. Primary astrocytes were isolated from the fetal brains and cultured in vitro, as we have reported previously [39]. Pure astrocytes were obtained by removing the microglia after shaking the flasks. Astrocytes were grown in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in a 5% CO2 and 95% air atmosphere in tissue culture flasks and on poly-D-lysine coated coverslips in 24 well culture plates [23, 40]. Glia-neurons (mixed culture) were grown in DMEM F-12 containing 5% FBS, 5% horse serum and 1% penicillin/streptomycin at 37 °C in a 5% CO2 and 95% air atmosphere in 25 cm2 or 75 cm2 tissue culture flasks and in poly-D-lysine coated coverslips in 24 well plates as reported previously [41]. Cells grown on poly-D-lysine coated cover glass were used for immunofluorescence staining of PAR-2, GFAP and MAP2 expression.
Cell stimulation with MPP+, GMF and MMCPs for PAR-2 expression
Primary astrocytes and glia-neurons were grown to on poly-D-lysine coated cover glasses that were placed in 24 well tissue culture plates. These cells were incubated with MPP+ (10 µM), recombinant GMF (100 ng/ml), MMCP-6 (100 ng/ml) or MMCP-7 (100 ng/ml) for 48 hrs in 0.1% serum supplemented medium. The culture medium was then removed and the cells were washed with PBS and fixed with 4% paraformaldehyde for immunofluorescence staining.
Double immunofluorescence detection of GFAP and MAP2 with PAR-2 in astrocytes and Neurons
Primary astrocytes and glia-neurons grown on cover glasses were incubated with MPP+,GMF, MMCP-6 and MMCP-7 and then fixed with 4% paraformaldehyde. Double immunofluorescence labeling was performed using the polyclonal antibody to GFAP (1:250) or polyclonal antibody for MAP2 (1:1000) along with monoclonal antibody to PAR-2 (1:100) as we have reported previously [38, 42–44]. Astrocytes were stained for the astrocytic marker GFAP and neurons were stained for the neuronal marker MAP2. Briefly, the cells were incubated overnight with primary antibodies at 4ᵒC. Following this, they were incubated with a mixture of Alexa Flour 488 goat anti-rabbit IgG and Alexa Flour 568 goat anti-mouse IgG/goat anti-mouse Texas red secondary antibodies (1:500) for one hr at room temperature for double immunofluorescence labeling. After washing with DPBS, the cover slips with cells were lifted from the wells and mounted onto the microscope slides, dried and viewed with a confocal fluorescent microscope (Leica Microsystems GmbH, Germany; Harry S. Truman Memorial Veterans Hospital, Columbia, MO). Photomicrographs were acquired using an oil immersion objective (63x) as we have previously reported [38].
Statistical analysis
All the ELISA results were analyzed by GraphPad InStat 3 software. Results were provided as mean ± SEM. Data were analyzed using One-way Analysis of Variance (ANOVA) and the post hoc test Tukey-Kramer multiple comparison analysis to determine statistically significant differences between the groups. A p-value of <0.05 was considered as statistically significant.
RESULTS
Decreased levels of cytokines TNF-α and IL-1β, and chemokine CCL2 in the brain lysates of MC-KO mice administered with MPTP
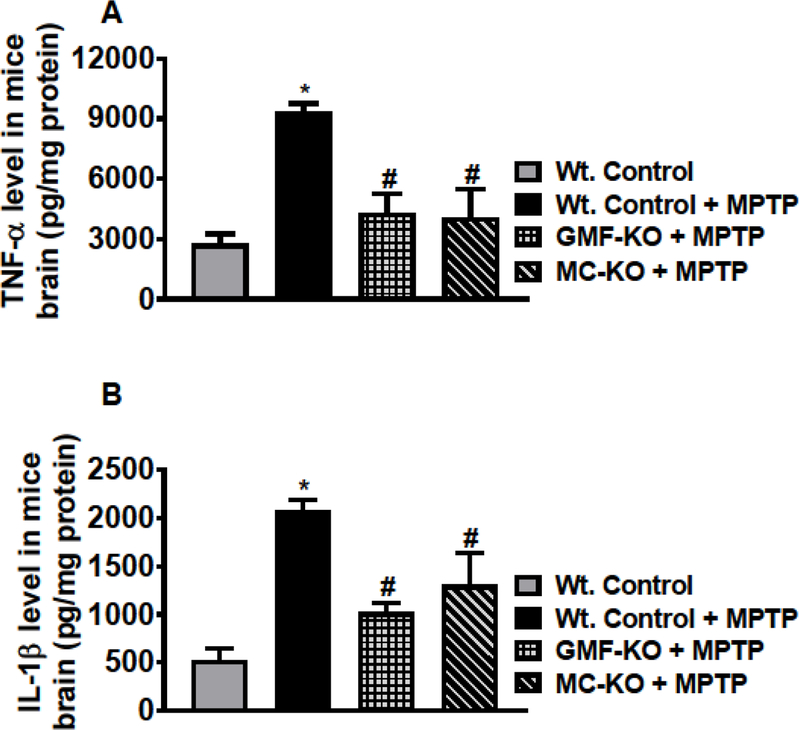
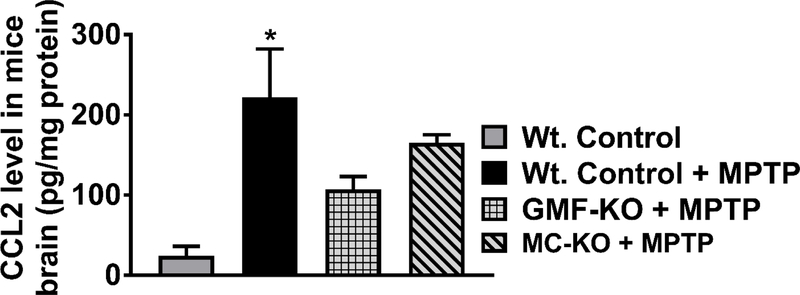
The levels of TNF-α, IL-1β and CCL2 were quantified by ELISA in the brain lysates prepared from MPTP administered Wt. mice, GMF-KO mice, MC-KO mice as well as from untreated control mice. MPTP-administration significantly increased TNF-α level in the Wt. mice as compared to the control untreated mice (Fig. 1A, n=4–5/group). However, there is no significant increase of TNF-α in both GMF-KO mice and MC-KO mice. In these groups of mice, TNF-α levels remained significantly reduced (p<0.05) as compared to Wt. mice treated with MPTP (Fig. 1A). Similarly, MPTP administration significantly increased (p<0.05) IL-1β level in Wt. mice as compared to control mice (Fig. 1B). Similar to TNF-α, both GMF-KO mice and MC-KO show significantly reduced levels of IL-1β as compared to Wt. mice after MPTP administration (Fig. 1B, n=3–4/group). Further, we also measured chemokine CCL2 in the brain lysates in the groups as we mentioned above in the text. Our results show that CCL2 level increased in the brains of Wt. mice administered with MPTP (Fig. 2, n=3/group). Both GMF-KO and MC-KO brains show reduced levels of CCL2 as compared with Wt. mice with MPTP administration. Our results indicate that absence of GMF and mast cells reduce the release of TNF-α, IL-β and CCL2 in MPTP administered mice.
Fig. 1.
Mast cell deficiency decreases the levels of cytokines TNF-α and IL-1β in the brain lysates of MC-KO mice after MPTP administration. TNF-α and IL-1β were measured by ELISA in the brain lysates of MPTP administered Wt. mice, GMF-KO mice, MC-KO mice and untreated control mice. MPTP-administration significantly increased TNF-α level in the Wt. mice as compared to the control mice (A, n=4–5/group). However, there is no significant increase of TNF-α in both GMF-KO mice and MC-KO mice as compared with control mice. In these groups of mice, TNF-α levels remain significantly reduced (p<0.05) as compared to Wt. mice treated with MPTP (A). Further, MPTP administration significantly increased (p<0.05) IL-1β level in Wt. mice as compared to untreated control mice (B, n=3–4/group). Both GMF-KO mice and MC-KO mice show significantly reduced (p<0.05) IL-1β levels as compared to the Wt. mice after MPTP administration. *,# p<0.05, *Vs Wt. Control, #Vs Wt. + MPTP, ANOVA and Tukey-Kramer post hoc –––––test.
Fig. 2.
Mast cell deficiency decreases chemokine CCL2 level in the brain lysates of MC-KO mice after MPTP administration. CCL2 level was measured by ELISA in the brain lysates of MPTP administered Wt. mice, GMF-KO mice, MC-KO mice and untreated control mice (n=3/group). Our results show that CCL2 level increased in Wt. mice brain administered with MPTP as compared with control mice. Both GMF-KO mice and MC-KO mice show reduced levels CCL2 as compared with Wt. mice with MPTP administration. *p<0.05, Vs. Wt. Control, ANOVA and Tukey-Kramer post hoc test.
Decreased levels of TNF-α and IL-1β in the serum of MC-KO mice administered with MPTP
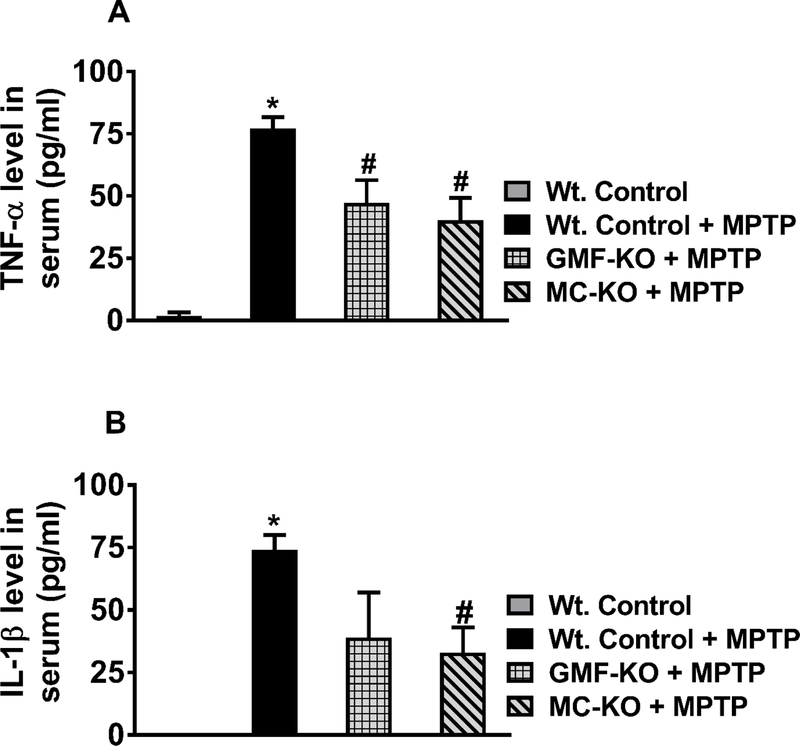
In addition to quantification in brain lysates, we also quantified TNF-α and IL-1β levels in the serum of MPTP-administered Wt. mice, GMF-KO mice, MC-KO mice and compared with serum from untreated control mice (n=4/group). Wt. mice administered with MPTP show significantly increased (p<0.05) TNF-α (Fig. 3A) as well as IL-1β (Fig. 3B) levels in the serum as compared with control mice serum. Additionally, both GMF-KO mice and MC-KO mice show significantly decreased (p<0.05) TNF-α level in the serum as compared with the level in MPTP-administered Wt. mice serum (Fig. 3A). However, serum IL-1β level is significantly reduced (p<0.05) only in MC-KO mice as compared with Wt. mice administered with MPTP (Fig. 3B). These results further show that the absence of GMF and mast cells can also reduce the serum levels of TNF-α and IL-1β compared to MPTP administered mice.
Fig. 3.
Mast cell deficiency decreases serum levels of TNF-α and IL-1β in MC-KO mice. We measured serum levels of TNF-α and IL-1β in MPTP administered Wt. mice, GMF-KO mice, MC-KO mice and untreated control mice (n=3–4/group). Wt. mice administered with MPTP show significantly increased (p<0.05) serum levels of TNF-α (A) and IL-1β (B) as compared with serum levels of untreated control mice. Both GMF-KO mice and MC-KO mice show significantly decreased (p<0.05) serum TNF-α level as compared with the serum level in MPTP-administered Wt. mice (A). However, serum IL-1β has been significantly reduced (p<0.05) only in MC-KO mice as compared with Wt. mice administered with MPTP (B). *,# p<0.05, *Vs Wt. Control, #Vs Wt. + MPTP, ANOVA and Tukey-Kramer post hoc test.
MPP+, GMF, MMCP-6 and MMCP-7 upregulate the expression of PAR-2 in mouse primary astrocytes as detected by double immunofluorescence
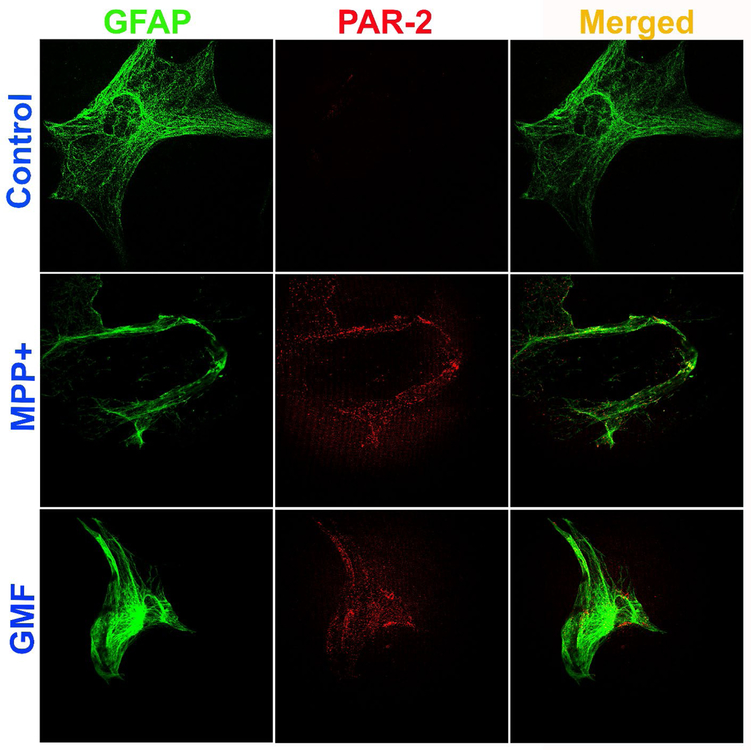
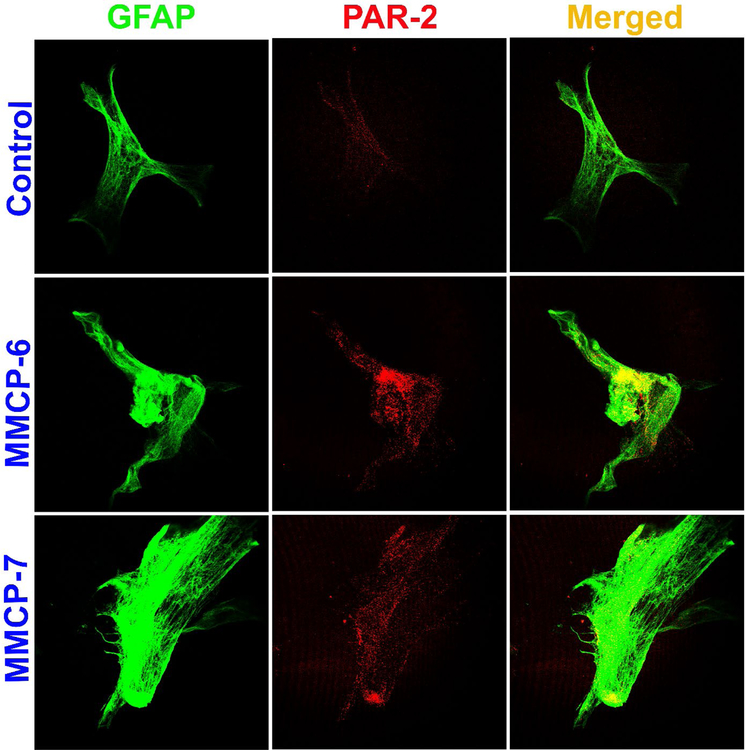
Mouse primary astrocytes were grown and incubated with MPP+ (10 μM), GMF (100 ng/ml), MMCP-6 (100 ng/ml) or MMCP-7 (100 ng/ml) for 48 hrs and the expression of PAR-2 and GFAP were analyzed by double immunofluorescence staining. Representative images (n=3) show that astrocytes incubated with MPP+ and GMF for 48 hrs increased the expression of PAR-2 (red fluorescence) as compared with untreated control cells (Fig. 4). GFAP expression is shown by the green fluorescence (Fig. 4). Merged images show co-localization of PAR-2 with GFAP. Similarly, incubation of astrocytes with mast cell proteases MMCP-6 and MMCP-7 increased the expression of PAR-2 (red fluorescence) as compared with untreated control cells (Fig. 5). Astrocyte marker GFAP is shown by the green fluorescence (Fig. 5). Untreated control astrocytes show reduced expression of PAR-2 as compared to treated astrocytes (Fig. 4 and Fig. 5). Merged images show co-localization of GFAP and PAR-2. These results indicate that MPP+, GMF, MMCP-6 and MMCP-7 induce PAR-2 expression in astrocytes that plays an important role in the neuroinflammation of the brain.
Fig. 4.
MPP+ and GMF upregulate the expression of PAR-2 in mouse primary astrocytes as determined by double immunofluorescence staining. Astrocytes were incubated with MPP+ (10 μM) or GMF (100 ng/ml) for 48 hrs and the expression of PAR-2 and GFAP were analyzed by immunofluorescence staining (n=3). The cells were incubated with monoclonal antibody for PAR-2 and polyclonal primary antibodies for GFAP followed by incubation with Alexa Flour 488 goat anti-rabbit IgG and Alexa Flour 568 goat anti-mouse IgG secondary antibodies. Then the cover glass with cells were lifted from the wells and mounted on to the microscope glass slides, dried and viewed using a confocal microscope. Representative images show that astrocytes incubated with MPP+ and GMF increased the expression of PAR-2 (red fluorescence) as compared with untreated control cells. Astrocyte marker GFAP is shown by the green fluorescence. Merged images show co-localization of PAR-2 and GFAP in the astrocytes. Photomicrographs original magnification = 630x.
Fig. 5.
Mast cell proteases MMCP-6 and MMCP-7 upregulate the expression of PAR-2 in mouse primary astrocytes as determined by double immunofluorescence staining. Astrocytes were incubated with MMCP-6 and MMCP-7 for 48 hrs and the expression of PAR-2 and GFAP were analyzed by immunofluorescence staining as mentioned previously (n=3). Representative images show that astrocytes incubated with MMCP-6 and MMCP-7 increased the expression of PAR-2 (red fluorescence) as compared with untreated control cells. Astrocyte marker GFAP is shown with green fluorescence. Merged images show co-localization of PAR-2 and GFAP in the astrocytes. Photomicrographs original magnification = 630x.
MPP+, GMF, MMCP-6 and MMCP-7 upregulate the expression of PAR-2 in mouse primary neurons as detected by double immunofluorescence
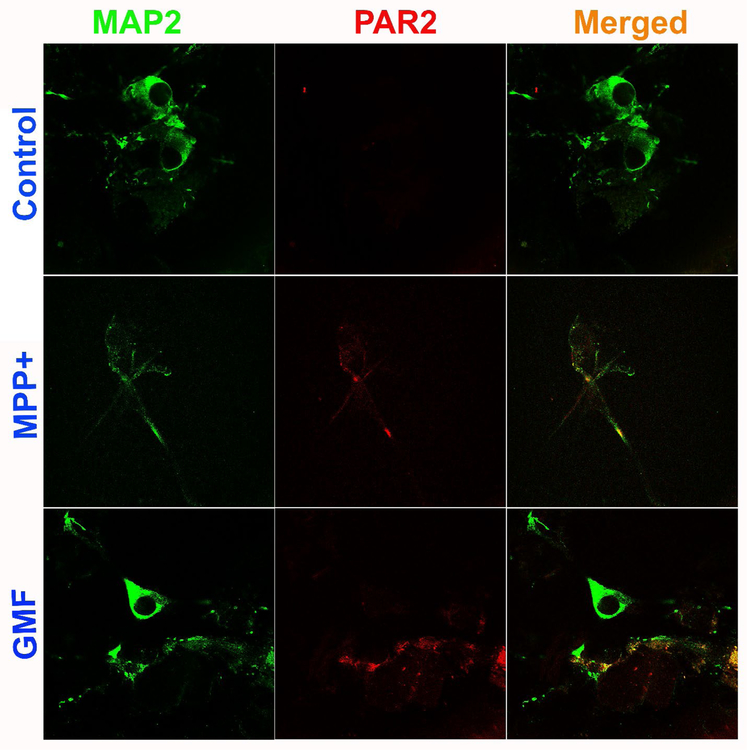
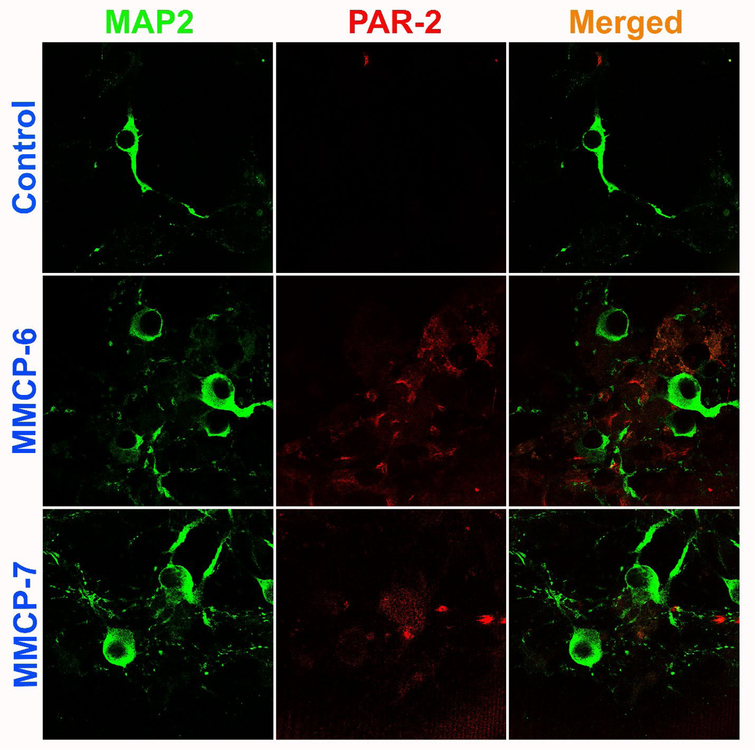
Mouse primary neurons were grown and incubated with MPP+ (10 μM), GMF (100 ng/ml), MMCP-6 (100 ng/ml) or MMCP-7 (100 ng/ml) for 48 hrs and the expression of PAR-2 and MAP2 were analyzed by double immunofluorescence staining. Representative images (n=3) show that neurons incubated with MPP+ and GMF for 48 hrs increased the expression of PAR-2 (red fluorescence) as compared with control untreated cells (Fig. 6). MAP2 expression is shown by the green fluorescence (Fig. 6). Similarly, incubation of neurons with mast cell proteases MMCP-6 and MMCP-7 increased the expression of PAR-2 (red fluorescence) as compared with un-treated control cells (Fig. 7). Neuronal marker MAP2 is shown by the green fluorescence (Fig. 7). Untreated control neurons show less expression of PAR-2 as compared to treated cells (Fig. 6 and Fig. 7). Merged images show co-localization of MAP2 and PAR-2. Our results show that MPP+, GMF, MMCP-6 and MMCP-7 induce neuronal PAR-2 expression that can lead to neuroinflammation and neurodegeneration in the brain.
Fig. 6.
MPP+ and GMF upregulate the expression of PAR-2 in mouse primary neurons as detected by double immunofluorescence. Mouse primary neurons were grown and incubated with MPP+ (10 μM) or GMF (100 ng/ml) for 48 hrs and the expression of PAR-2 and MAP2 were analyzed by double immunofluorescence staining as mentioned previously. Representative images (n=3) show that neurons incubated with MPP+ or GMF for 48 hrs increased the expression of PAR-2 (red fluorescence) as compared with control untreated cells. MAP2 expression is shown by green fluorescence. Merged images show co-localization of MAP2 and PAR-2. Photomicrographs original magnification = 630x.
Fig. 7.
Mast cell proteases MMCP-6 and MMCP-7 upregulate the expression of PAR-2 in mouse primary neurons as detected by double immunofluorescence. Mouse primary neurons were grown and incubated with MMCP-6 (100 ng/ml) or MMCP-7 (100 ng/ml) for 48 hrs and the expression of PAR-2 and MAP2 were analyzed by double immunofluorescence staining as mentioned previously. Representative images (n=3) show that both MMCP-6 and MMCP-7 increased the neuronal expression of PAR-2 (red fluorescence) as compared with untreated control cells. Neuronal marker MAP2 is shown by green fluorescence. Merged images show co-localization of MAP2 and PAR-2. Photomicrographs original magnification = 630x.
DISCUSSION
In the present study, we investigated whether MPTP administration would show decreased levels of inflammatory mediators in the brain and serum of MC-KO mice and GMF-MO mice as compared with Wt. mice. We found significant reduction in the levels of TNF-α, IL-1β and CCL2 levels in both MC-KO mice as well as in GMF-KO mice when compared to the Wt. mice after MPTP administration. Wt. mice showed significantly increased levels of these inflammatory mediators in the brain and serum after MPTP administration when compared to untreated control mice. Increased level of α-synuclein is associated with increased expression of PAR-2 in the brains of PD patients. Since MPP+, GMF and mouse mast cell proteases are implicated in neuroinflammation, we further investigated whether MPP+, GMF and mouse mast cell proteases increase the expression of PAR-2 in astrocytes and neurons in vitro. We found that incubation of mouse primary astrocytes and neurons with MPP+, GMF, MMCP-6 and MMCP-7 for 48 hrs increased the expression of PAR-2 as detected by double immunofluorescence staining. Administration of MPTP is known to produce PD like motor disorders in the animal models of PD. In the present study, MPTP treated animals showed motor abnormalities as determined by Rota Rod and Grip tests (results not shown) as we have previously reported [37, 45]. We have previously reported reduced neuroinflammation in GMF-KO mice [25]. GMF is a multifunctional, brain-predominant protein first discovered purified, sequenced, and cloned in our laboratory [23, 27, 46–48]. GMF is primarily expressed in the CNS where it activates glia and neurons to release multiple neuroinflammatory mediators that induce and upregulate neuroinflammation and neurodegeneration in neurodegenerative diseases [23, 24, 35, 40, 49]. We have also shown that GMF expression is increased in the brains of AD [22, 42, 43, 50, 51]. In the present study, we used GMF-KO mice as a control to compare the effects in MC-KO mice after MPTP administration. Mast cells are involved in the pathogenesis of neurodegenerative diseases such as experimental autoimmune encephalomyelitis (EAE), PD and AD [30, 31, 52–54]. Mast cells are early responders after brain injury by releasing prestored inflammatory mediators including TNF-α and proteases MMCP-6 and MMCP-7 [39, 55–57]. Though mast cells are fewer in numbers in the CNS, they can release significant amounts of inflammatory mediators. Moreover, mast cells can accumulate at sites of inflammation, and mast cell progenitors migrate from distant sites, organs, and increasing mast cell numbers depending upon the inflammatory status.
We measured cytokines TNF-α and IL-1β as well as chemokine CCL2 in the brain lysates from Wt. mice, GMF-KO mice, MC-KO mice, and untreated control mice. These cytokines and chemokine mediate neuroinflammation and implicated in the pathogenesis of PD. Moreover, both IL-1β and TNF-α in higher concentrations can directly induce neuronal damage, and through activating microglia and astrocytes to release neuroinflammatory and neurotoxic mediators in the brain [30, 58, 59]. CCL2 induces the accumulation of immune cells at the site of inflammation and increases the blood-brain barrier permeability in neurodegenerative diseases [60, 61]. Our results show that TNF-α, IL-1β and CCL2 are increased in Wt. mice administered with MPTP as compared to control mice. Furthermore, we show that the levels of these cytokines and chemokine are lower in both GMF-KO mice as well as MC-KO mice as compared with Wt. MPTP-administered mice. This may because the absence of GMF reduced the activation of glial cells to release inflammatory mediators in the brain after MPTP administration. The reduced levels of TNF-α, IL-1β and CCL2 in MC-KO mice after the MPTP administration may be because absence of mast cells reduced the release of TNF-α, IL-1β and CCL2 upon activation. Inflammatory mediators released from mast cells can activate glial cells to release additional inflammatory mediators that induce neuroinflammation and neurodegeneration [30, 62]. Thus, absence of mast cells can reduce the levels of TNF-α, IL-1β and CCL2 in MPTP administered mice as seen in this study. Mast cells are an important source of TNF-α, IL-1β, CCL2 and other mediators [30, 63, 64]. We have previously shown that MPP+, the metabolite of the neurotoxin MPTP and the neuroinflammatory protein GMF activate mast cells in vitro to release several neuroinflammatory cytokines and chemokines [65, 66].
Mast cell protease can activate brain cells through PAR-2 pathways as astrocytes, microglia and neurons express only low levels of PAR-2 for neuroprotective physiological functions [67]. It has been shown that mast cell protease tryptase activates microglia to release TNF-α and IL-6 [68].We have previously demonstrated that the mast cell proteases MMCP-6 and MMCP-7 activate glia and neurons and release inflammatory mediators including CCL2, matrix metalloproteinase-3 (MMP-3), IL-33 that are implicated in neuroinflammation and neurodegeneration [38, 39]. PAR-2 is abundantly expressed in cortical layers, hippocampus, thalamus, hypothalamus, and striatum [67]. However, the expression of PAR-2 increases in neuroinflammatory conditions including MPTP induced PD in rats [8]. Activation of PAR-2 induces neuroinflammation mediated neuronal death [69]. Reactive astrocytes show increased expression of PAR-1 and PAR-2 [70]. PARs activate ERK1/2, JNK, and p38 MAPKs to induce cellular responses in the brain cells that also induces the secretion of inflammatory cytokines and chemokines [67]. Previous study has shown that mast cell proteases upregulate neuroinflammation via activating glial cells and neurons to release TNF-α and IL-6 through PAR-2 expression [71]. PAR-2 expressed on glia, neurons and mast cells upregulate neuroinflammation in the CNS [72–75]. Additionally, tryptase induces the recruitment and accumulation of mast cells at the site of inflammation through the activation of PAR-2 [76]. In the present study, we have analyzed the expression of PAR-2 in astrocytes and neurons after incubation with MPP+, GMF or mast cell proteases such as MMCP-6 and MMCP-7. Here, we have demonstrated that both MPP+ and GMF, as well as mast cell proteases, increase the expression of PAR-2 in primary astrocytes and neurons.
Conclusions
MPTP administration increases the expression of TNF-α, IL-1β and CCL2 levels in the brains of Wt. mice. However, these levels were reduced in the brains of MC-KO mice as well as in GMF-KO mice. Additionally, both TNF-α as well as IL-1β levels were reduced in the serum of MPTP administered mice when compared to levels in the serum of wt. mice. Furthermore, MPP+, GMF and mast cell proteases increases the expression of PAR-2 in Wt. mouse primary astrocytes and neurons. We propose that GMF and mast cell interactions with glial cells and neurons during neuroinflammation be explored as a new therapeutic target in PD and other neuroinflammatory conditions.
ACKNOWLEDGMENTS
This work was supported by Veteran Affairs Merit Award I01BX002477 and National Institutes of Health Grants AG048205 & NS073670 to AZ.
REFERENCES
- [1].Zeng XS, Geng WS, Jia JJ, Chen L, Zhang PP (2018) Cellular and Molecular Basis of Neurodegeneration in Parkinson Disease. Front Aging Neurosci 10, 109 10.3389/fnagi.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stoker TB, Barker RA (2018) Regenerative Therapies for Parkinson’s Disease: An Update. BioDrugs 32, 357–366. [DOI] [PubMed] [Google Scholar]
- [3].Chen X, Gumina G, Virga KG (2018) Recent Advances in Drug Repurposing for Parkinson’s Disease. Curr Med Chem 10.2174/0929867325666180719144850. [DOI] [PubMed]
- [4].Ramirez-Zamora A (2018) Parkinson Disease: Current and Emerging Treatment Strategies. J Clin Psychiatry 79, 10.4088/JCP.PP17030TX1C. [DOI] [PubMed] [Google Scholar]
- [5].Fleming SM (2017) Mechanisms of Gene-Environment Interactions in Parkinson’s Disease. Curr Environ Health Rep 4, 192–199. [DOI] [PubMed] [Google Scholar]
- [6].Vingill S, Connor-Robson N, Wade-Martins R (2017) Are rodent models of Parkinson’s disease behaving as they should? Behav Brain Res 352, 133–141. [DOI] [PubMed] [Google Scholar]
- [7].Morissette M, Di Paolo T (2018) Non-human primate models of PD to test novel therapies. J Neural Transm (Vienna) 125, 291–324. [DOI] [PubMed] [Google Scholar]
- [8].Liu P, Sun L, Zhao XL, Zhang P, Zhao XM, Zhang J (2014) PAR2-mediated epigenetic upregulation of alpha-synuclein contributes to the pathogenesis of Parkinsons disease. Brain Res 1565, 82–89. [DOI] [PubMed] [Google Scholar]
- [9].Yan F, Chen Y, Li M, Wang Y, Zhang W, Chen X, Ye Q (2018) Gastrointestinal nervous system alpha-synuclein as a potential biomarker of Parkinson disease. Medicine (Baltimore) 97, e11337 10.1097/MD.0000000000011337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fecchio C, Palazzi L, de Laureto PP (2018) alpha-Synuclein and Polyunsaturated Fatty Acids: Molecular Basis of the Interaction and Implication in Neurodegeneration. Molecules 23, 10.3390/molecules23071531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rodriguez L, Marano MM, Tandon A (2018) Import and Export of Misfolded alpha-Synuclein. Front Neurosci 12, 344 10.3389/fnins.2018.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gelders G, Baekelandt V, Van der Perren A (2018) Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J Immunol Res 2018, 4784268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Altmann DM (2018) Neuroimmunology and neuroinflammation in autoimmune, neurodegenerative and psychiatric disease. Immunology 154, 167–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Skaper SD, Facci L, Zusso M, Giusti P (2018) An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front Cell Neurosci 12, 72 10.3389/fncel.2018.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bassani TB, Vital MA, Rauh LK (2015) Neuroinflammation in the pathophysiology of Parkinson’s disease and therapeutic evidence of anti-inflammatory drugs. Arq Neuropsiquiatr 73, 616–623. [DOI] [PubMed] [Google Scholar]
- [16].Stojkovska I, Wagner BM, Morrison BE (2015) Parkinson’s disease and enhanced inflammatory response. Exp Biol Med (Maywood) 240, 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chao Y, Wong SC, Tan EK (2014) Evidence of inflammatory system involvement in Parkinson’s disease. Biomed Res Int 2014, 308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].More SV, Kumar H, Kim IS, Song SY, Choi DK (2013) Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators Inflamm 2013, 952375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lim R, Miller JF, Zaheer A (1989) Purification and characterization of glia maturation factor beta: a growth regulator for neurons and glia. Proc Natl Acad Sci U S A 86, 3901–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kaplan R, Zaheer A, Jaye M, Lim R (1991) Molecular cloning and expression of biologically active human glia maturation factor-beta. J Neurochem 57, 483–490. [DOI] [PubMed] [Google Scholar]
- [21].Zaheer A, Fink BD, Lim R (1993) Expression of glia maturation factor beta mRNA and protein in rat organs and cells. J Neurochem 60, 914–920. [DOI] [PubMed] [Google Scholar]
- [22].Zaheer S, Thangavel R, Sahu SK, Zaheer A (2011) Augmented expression of glia maturation factor in Alzheimer’s disease. Neuroscience 194, 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zaheer A, Mathur SN, Lim R (2002) Overexpression of glia maturation factor in astrocytes leads to immune activation of microglia through secretion of granulocyte-macrophage-colony stimulating factor. Biochem Biophys Res Commun 294, 238–244. [DOI] [PubMed] [Google Scholar]
- [24].Zaheer A, Sahu SK, Wu Y, Zaheer A, Haas J, Lee K, Yang B (2007) Diminished cytokine and chemokine expression in the central nervous system of GMF-deficient mice with experimental autoimmune encephalomyelitis. Brain Res 1144, 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zaheer A, Zaheer S, Sahu SK, Yang B, Lim R (2007) Reduced severity of experimental autoimmune encephalomyelitis in GMF-deficient mice. Neurochem Res 32, 39–47. [DOI] [PubMed] [Google Scholar]
- [26].Zaheer S, Wu Y, Yang X, Thangavel R, Sahu SK, Zaheer A (2012) Efficient down-regulation of glia maturation factor expression in mouse brain and spinal cord. Neurochem Res 37, 1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zaheer A, Zaheer S, Sahu SK, Knight S, Khosravi H, Mathur SN, Lim R (2007) A novel role of glia maturation factor: induction of granulocyte-macrophage colony-stimulating factor and pro-inflammatory cytokines. J Neurochem 101, 364–376. [DOI] [PubMed] [Google Scholar]
- [28].Kempuraj D, Khan MM, Thangavel R, Xiong Z, Yang E, Zaheer A (2013) Glia maturation factor induces interleukin-33 release from astrocytes: implications for neurodegenerative diseases. J Neuroimmune Pharmacol 8, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hendriksen E, van Bergeijk D, Oosting RS, Redegeld FA (2017) Mast cells in neuroinflammation and brain disorders. Neurosci Biobehav Rev 79:119–133. 10.1016/j.neubiorev.2017.05.001. [DOI] [PubMed] [Google Scholar]
- [30].Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, Zahoor H, Saeed D, Natteru PA, Iyer S, Zaheer A (2017) Brain and Peripheral Atypical Inflammatory Mediators Potentiate Neuroinflammation and Neurodegeneration. Front Cell Neurosci 11, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Skaper SD, Facci L, Zusso M, Giusti P (2017) Neuroinflammation, Mast Cells, and Glia: Dangerous Liaisons. Neuroscientist 23, 478–498. [DOI] [PubMed] [Google Scholar]
- [32].Skaper SD, Facci L, Giusti P (2013) Mast cells, glia and neuroinflammation: partners in crime? Immunology 141, 314–327. 10.1111/imm.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D (2012) Mast cells and inflammation. Biochim Biophys Acta 1822, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lim R, Zaheer A, Khosravi H, Freeman JH Jr., Halverson HE, Wemmie JA, Yang B (2004) Impaired motor performance and learning in glia maturation factor-knockout mice. Brain Res 1024, 225–232. [DOI] [PubMed] [Google Scholar]
- [35].Zaheer S, Wu Y, Yang X, Ahrens M, Sahu SK, Zaheer A (2012) Clinical course of myelin oligodendrocyte glycoprotein 35–55 induced experimental autoimmune encephalomyelitis is aggravated by glia maturation factor. Neurochem Int 60, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M (2001) The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem 76, 1265–1274. [DOI] [PubMed] [Google Scholar]
- [37].Khan MM, Zaheer S, Thangavel R, Patel M, Kempuraj D, Zaheer A (2015) Absence of Glia Maturation Factor Protects Dopaminergic Neurons and Improves Motor Behavior in Mouse Model of Parkinsonism. Neurochem Res 40, 980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kempuraj D, Thangavel R, Selvakumar GP, Ahmed ME, Zaheer S, Raikwar SP, Zahoor H, Saeed D, Dubova I, Giler G, Herr S, Iyer SS, Zaheer A (2018) Mast Cell Proteases Activate Astrocytes and Glia-Neurons and Release Interleukin-33 by Activating p38 and ERK1/2 MAPKs and NF-kappaB. Mol Neurobiol 10.1007/s12035-018-1177-7. [DOI] [PMC free article] [PubMed]
- [39].Kempuraj D, Selvakumar GP, Zaheer S, Thangavel R, Ahmed ME, Raikwar S, Govindarajan R, Iyer S, Zaheer A (2018) Cross-Talk between Glia, Neurons and Mast Cells in Neuroinflammation Associated with Parkinson’s Disease. J Neuroimmune Pharmacol 13, 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zaheer A, Yorek MA, Lim R (2001) Effects of glia maturation factor overexpression in primary astrocytes on MAP kinase activation, transcription factor activation, and neurotrophin secretion. Neurochem Res 26, 1293–1299. [DOI] [PubMed] [Google Scholar]
- [41].Lee Y, Aono M, Laskowitz D, Warner DS, Pearlstein RD (2004) Apolipoprotein E protects against oxidative stress in mixed neuronal-glial cell cultures by reducing glutamate toxicity. Neurochem Int 44, 107–118. [DOI] [PubMed] [Google Scholar]
- [42].Thangavel R, Bhagavan SM, Ramaswamy SB, Surpur S, Govindarajan R, Kempuraj D, Zaheer S, Raikwar S, Ahmed ME, Selvakumar GP, Iyer SS, Zaheer A (2018) Co-Expression of Glia Maturation Factor and Apolipoprotein E4 in Alzheimer’s Disease Brain. J Alzheimers Dis 61, 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ahmed ME, Iyer S, Thangavel R, Kempuraj D, Selvakumar GP, Raikwar SP, Zaheer S, Zaheer A (2017) Co-Localization of Glia Maturation Factor with NLRP3 Inflammasome and Autophagosome Markers in Human Alzheimer’s Disease Brain. J Alzheimers Dis 60, 1143–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Selvakumar GP, Iyer SS, Kempuraj D, Raju M, Thangavel R, Saeed D, Ahmed ME, Zahoor H, Raikwar SP, Zaheer S, Zaheer A (2018) Glia Maturation Factor Dependent Inhibition of Mitochondrial PGC-1alpha Triggers Oxidative Stress-Mediated Apoptosis in N27 Rat Dopaminergic Neuronal Cells. Mol Neurobiol 55, 7132–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Khan MM, Kempuraj D, Thangavel R, Zaheer A (2013) Protection of MPTP-induced neuroinflammation and neurodegeneration by Pycnogenol. Neurochem Int 62, 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lim R, Zaheer A, Lane WS (1990) Complete amino acid sequence of bovine glia maturation factor beta. Proc Natl Acad Sci U S A 87, 5233–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lim R, Zaheer A (1991) Structure and function of glia maturation factor beta. Adv Exp Med Biol 296, 161–164. [DOI] [PubMed] [Google Scholar]
- [48].Zaheer S, Wu Y, Sahu SK, Zaheer A (2011) Suppression of neuro inflammation in experimental autoimmune encephalomyelitis by glia maturation factor antibody. Brain Res 1373, 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Raikwar SP, Thangavel R, Dubova I, Selvakumar GP, Ahmed ME, Kempuraj D, Zaheer SA, Iyer SS, Zaheer A (2018) Targeted Gene Editing of Glia Maturation Factor in Microglia: a Novel Alzheimer’s Disease Therapeutic Target. Mol Neurobiol 10.1007/s12035-018-1068-y. [DOI] [PMC free article] [PubMed]
- [50].Thangavel R, Kempuraj D, Stolmeier D, Anantharam P, Khan M, Zaheer A (2013) Glia maturation factor expression in entorhinal cortex of Alzheimer’s disease brain. Neurochem Res 38, 1777–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A (2012) Expression of glia maturation factor in neuropathological lesions of Alzheimer’s disease. Neuropathol Appl Neurobiol 38, 572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shaik-Dasthagirisaheb YB, Conti P (2016) The Role of Mast Cells in Alzheimer’s Disease. Adv Clin Exp Med 25, 781–787. [DOI] [PubMed] [Google Scholar]
- [53].Traina G (2017) Mast cells in the brain - Old cells, new target. J Integr Neurosci [DOI] [PubMed]
- [54].Dong H, Zhang X, Qian Y (2014) Mast cells and neuroinflammation. Med Sci Monit Basic Res 20, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Walker ME, Hatfield JK, Brown MA (2012) New insights into the role of mast cells in autoimmunity: evidence for a common mechanism of action? Biochim Biophys Acta 1822, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yehya M, Torbey MT (2017) The Role of Mast Cells in Intracerebral Hemorrhage. Neurocrit Care 28, 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gu Y, Yang DK, Spinas E, Kritas SK, Saggini A, Caraffa A, Antinolfi P, Saggini R, Conti P (2015) Role of TNF in mast cell neuroinflammation and pain. J Biol Regul Homeost Agents 29, 787–791. [PubMed] [Google Scholar]
- [58].Allan SM, Rothwell NJ (2003) Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci 358, 1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Brough D, Rothwell NJ, Allan SM (2015) Interleukin-1 as a pharmacological target in acute brain injury. Exp Physiol 100, 1488–1494. [DOI] [PubMed] [Google Scholar]
- [60].Madrigal JL, Caso JR (2014) The chemokine (C-C motif) ligand 2 in neuroinflammation and neurodegeneration. Adv Exp Med Biol 824, 209–219. [DOI] [PubMed] [Google Scholar]
- [61].Bose S, Cho J (2013) Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch Pharm Res 36, 1039–1050. [DOI] [PubMed] [Google Scholar]
- [62].Skaper SD, Giusti P, Facci L (2012) Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J 26, 3103–3117. [DOI] [PubMed] [Google Scholar]
- [63].Mekori YA, Metcalfe DD (2000) Mast cells in innate immunity. Immunol Rev 173, 131–140. [DOI] [PubMed] [Google Scholar]
- [64].Mukai K, Tsai M, Saito H, Galli SJ (2018) Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 282, 121–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kempuraj D, Thangavel R, Yang E, Pattani S, Zaheer S, Santillan DA, Santillan MK, Zaheer A (2015) Dopaminergic Toxin 1-Methyl-4-Phenylpyridinium, Proteins alpha-Synuclein and Glia Maturation Factor Activate Mast Cells and Release Inflammatory Mediators. PLoS One 10, e0135776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kempuraj D, Thangavel R, Fattal R, Pattani S, Yang E, Zaheer S, Santillan DA, Santillan MK, Zaheer A (2016) Mast Cells Release Chemokine CCL2 in Response to Parkinsonian Toxin 1-Methyl-4-Phenyl-Pyridinium (MPP(+)). Neurochem Res 41, 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Luo W, Wang Y, Reiser G (2007) Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res Rev 56, 331–345. [DOI] [PubMed] [Google Scholar]
- [68].Zhang X, Wang Y, Dong H, Xu Y, Zhang S (2016) Induction of Microglial Activation by Mediators Released from Mast Cells. Cell Physiol Biochem 38, 1520–1531. [DOI] [PubMed] [Google Scholar]
- [69].Smith-Swintosky VL, Cheo-Isaacs CT, D’Andrea MR, Santulli RJ, Darrow AL, Andrade-Gordon P (1997) Protease-activated receptor-2 (PAR-2) is present in the rat hippocampus and is associated with neurodegeneration. J Neurochem 69, 1890–1896. [DOI] [PubMed] [Google Scholar]
- [70].Pompili E, Nori SL, Geloso MC, Guadagni E, Corvino V, Michetti F, Fumagalli L (2004) Trimethyltin-induced differential expression of PAR subtypes in reactive astrocytes of the rat hippocampus. Brain Res Mol Brain Res 122, 93–98. [DOI] [PubMed] [Google Scholar]
- [71].Khedr M, Abdelmotelb AM, Pender SLF, Zhou X, Walls AF (2018) Neutrophilia, gelatinase release and microvascular leakage induced by human mast cell tryptase in a mouse model: Lack of a role of protease-activated receptor 2 (PAR2). Clin Exp Allergy 48, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cottrell GS, Amadesi S, Schmidlin F, Bunnett N (2003) Protease-activated receptor 2: activation, signalling and function. Biochem Soc Trans 31, 1191–1197. [DOI] [PubMed] [Google Scholar]
- [73].Saito T, Bunnett NW (2005) Protease-activated receptors: regulation of neuronal function. Neuromolecular Med 7, 79–99. [DOI] [PubMed] [Google Scholar]
- [74].Rothmeier AS, Ruf W (2012) Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol 34, 133–149. [DOI] [PubMed] [Google Scholar]
- [75].Zhang S, Zeng X, Yang H, Hu G, He S (2012) Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell Physiol Biochem 29, 931–940. [DOI] [PubMed] [Google Scholar]
- [76].Liu X, Wang J, Zhang H, Zhan M, Chen H, Fang Z, Xu C, Chen H, He S (2016) Induction of Mast Cell Accumulation by Tryptase via a Protease Activated Receptor-2 and ICAM-1 Dependent Mechanism. Mediators Inflamm 2016, 6431574. [DOI] [PMC free article] [PubMed] [Google Scholar]