Abstract
Purpose of review.
Osteoarthritis (OA) is one of the most common and disabling forms of arthritis worldwide, with joint pain being a primary symptom. Given that clinical symptoms often show poor concordance with tissue damage in OA, processes other than joint remodeling likely play a role in the condition. Using the biopsychosocial model of pain as a guiding framework, the purpose of this review is to highlight the extra-articular mechanisms that contribute to pain and dysfunction in OA, with a specific focus on resilience.
Recent findings.
Whereas previous research has mostly focused on risk factors for worsening of OA pain, recently emerging evidence places greater emphasis on the identification of protective mechanisms that enhance pain adaptation and palliate the negative effects of joint pain.
Summary.
In view of this new and important research, more emphasis should be placed on endogenous pain modulation, and in particular, pain attenuation. The result of such work could serve as a basis for optimizing treatment in the OA population.
Keywords: Osteoarthritis, Mechanisms, Biopsychosocial, Risk, Vulnerability, Resilience
Introduction
Chronic pain is rapidly becoming one of the nation’s top public health concerns, affecting over 100 million U.S. adults and resulting in tremendous societal burden. Costs are estimated to supersede that of cancer, diabetes, and heart disease combined [1], and patients are often at risk for substantial physical disability and psychological comorbidities (e.g., anxiety, depression). By and large, osteoarthritis (OA) represents one of the most prevalent and disabling chronic pain conditions, with an estimated 26 million people in the U.S. being affected [2]. While any di-arthrodial joint of the body can be affected, OA most often occurs in the hands, knees, and hips, with incidence rates highest for the knee [3]. Women and adults over the age of 50 tend to be disproportionately affected by OA [4], and various demographic, lifestyle, physiological, and psychosocial factors have been implicated in its pathogenesis. Because OA is a chronic condition affecting the joint and surrounding tissues, radiological evidence of joint pathology is traditionally used for diagnosis. However, diagnosis is also strongly influenced by clinical symptomatology, with joint pain, aching, and stiffness being the most frequently reported symptoms [5].
When OA becomes symptomatic the effect is often staggering, leading to significant decrements in emotional and physical health, social functioning, and activities of daily living. OA pain is considered to be a leading cause of mobility impairment in older adults, with hip and knee OA ranked as the 11th highest contributors to global disability (from 291 conditions) [6]. In addition to these effects, OA pain results in substantial economic burden with annual direct and indirect costs totaling $5,700 per individual, as well as lost productivity due to reduced work capacity [7]. These rates are estimated to rise as a result of longer life expectancies, increased obesity prevalence, and the rapid growth of aging adults in the United States.
Biopsychosocial Model of Pain
Traditionally, a biomedical approach focusing on physiological contributions to symptoms has been the cornerstone to understanding OA and other pain conditions. The problem of the biomedical model is that despite often readily detectable pathology, there is a poor concordance between pain and pathology, especially in the context of OA [8]. In fact, individuals with a high degree of pain and disability from OA may show minimal signs of osteoarthritic changes on clinical exam or imaging. What is more, significant inter-individual differences exist in the experience of OA pain, even in the presence of similar joint abnormality, suggesting that factors other than pathophysiology contribute to pain-related outcomes.
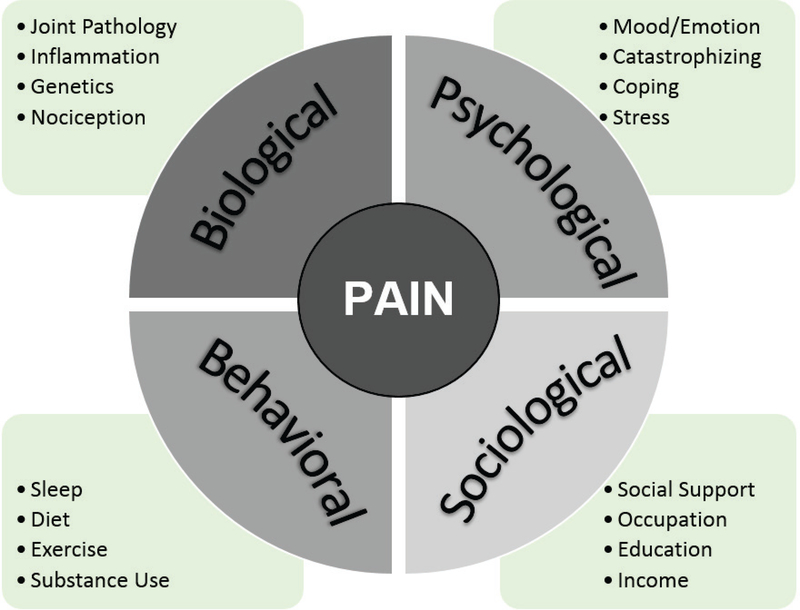
In contrast to the biomedical model, the prevailing biopsychosocial model recognizes the contribution of all relevant biological, psychological, sociological, and behavioral factors that dynamically interact with one another to generate the experience of pain (Figure 1). A fundamental tenet of this theory is that pain is affected by a combination of factors. Supporting this, considerable evidence suggests that pain and disability have profound influences on social and psychological functioning, and multiple psychosocial factors such as mood, personality traits, and coping styles can heighten the pain experience. While much of previous research has focused on vulnerability or risk factors that worsen pain-related outcomes, there is also increased recognition of protective mechanisms that mitigate the negative effects of chronic pain to enhance resilience.
Figure 1. Biopsychosocial Model of Pain.
Illustration of the biological, psychological, social, and behavioral factors that independently influence and dynamically interact with one another to impact the pain experience.
The Role of Resilience in Pain
Over the years, pain research has provided considerable evidence regarding vulnerability factors that worsen the risk for pain outcomes. Stemming from this research has been the proliferation of both pharmacological and psychological treatments of pain that focus on the reduction of negative symptoms, such as pain severity and negative mood. While many important answers have been gained from this research, less attention has been directed toward identifying factors that confer successful adaptation to pain. In light of this, recent years have seen a paradigm shift in search of positive factors that enhance resilience and promote optimal functioning. Much of the push in this direction has been derived from numerous studies demonstrating that not all individuals with persistent pain show the same pattern of disability and loss of functioning.
Broadly, resilience is a dynamic process resulting from the ability to adjust to challenges and sustain successful adaptability to adversity. The quantification of resilience can be challenging due to its many variations in definitions; however, recent models [9] conceptualize this construct as a process through which dispositional resources (e.g., trait positive affect, social ties) and mechanisms (e.g., state positive affect, positive social interactions) enhance adaptive pain coping, thereby resulting in recovery, sustainability, and growth – outcomes which are central to the characterization of a resilient system. It is important to note that resilience is not simply the opposite of vulnerability, nor does it mean the absence of pathology. In fact, people can have varying levels of both, and effects may be contingent upon the type or duration of stressor present.
In essence, individuals with greater levels of resilience have the ability to quickly rebound from physiological or emotional stress, persist in meaningful activities despite ongoing hardship, and experience personal growth as a result of the adversity [9]. While factors such as catastrophizing and depression may increase one’s risk for pain vulnerability, psychosocial facets including optimism and self-efficacy (among others) may promote greater pain-related resilience [10, 9]. However, this is not simply relegated to psychosocial variables, as numerous genetic, environmental, neurochemical, and behavioral factors have been found to confer resilience.
Mechanisms Underlying Osteoarthritis Pain
In the following sections, we briefly review the empirical literature by evaluating contributory mechanisms that underlie osteoarthritis pain and functioning. Using the framework of the biopsychosocial model, factors associated with vulnerability and increased risk for OA will be reviewed, as well as protective resources that attenuate risk and promote resilience in osteoarthritic conditions. While this review is not intended to be exhaustive, we will highlight the most salient factors with a specific focus directed toward recent discoveries implicated in the pathogenesis of and protection against negative pain outcomes in OA.
Biological Mechanisms
Biomechanical changes are a prime characteristic of OA development, including compromised hyaline articular cartilage in the joints, joint-line spacing, sub-chondral bone, development of osteophytes, cyst formation, corresponding damage to ligaments, and diminished joint/muscle strength [11]. Although it is generally conceived that the degree of joint and tissue damage should be proportional to the amount of pain and disability arising from OA, this is not always the case. In fact, other biological and neurobiological factors, beyond biomechanical and physiological changes, can confer risk of OA pain development [11]. For instance, peripheral mechanisms (e.g., sensitized nociceptors) can contribute to chronic pain development [12, 13]. Such peripheral sensitization can lead to hyperalgesia (heightened sensitivity/reactivity to painful stimuli and/or reduced pain threshold) and/or allodynia (pain in response to non-noxious stimuli) [14]. Further, abnormalities in central nervous system mechanisms such as central sensitization (hyperexcitability of central nervous system neurons) and decreased descending pain inhibition are thought to be associated with chronic pain risk, including OA pain development [13].
Like many other conditions, OA has a genetic basis, accounting for approximately 50% of hand and hip OA cases, but less so for knee OA [15]. Over the past several years, genome wide association studies (GWAS) have identified numerous genes and their associated single nucleotide polymorphisms (SNPs) as being linked to OA (as of 2013, one review identified 28 SNPs) [16, 17]. While a complete synopsis of these SNPs is beyond the scope of this review, it is suggested that the clinical course and progression of OA is influenced by genetic factors and their interactions with multiple environmental determinants.
Growing evidence also supports the relationship between leukocyte telomere length (LTL) and OA. Considered to be a marker of cellular aging, telomeres comprise regions of repetitive DNA that protect the ends of chromosomes from degradation and end-to-end fusion with neighboring chromosomes [18]. LTL is negatively associated with numerous health outcomes including mortality, chronic disease, and psychosocial stress [19]. In the context of OA, shorter telomere length was found in knee osteoarthritis patients with high levels of stress and pain [20], with a later study by the same authors largely confirming these results by observing shorter telomeres among individuals with the greatest burden of chronic knee pain [21]. Interestingly, a recent investigation demonstrated an association between angiogenic cytokines (i.e., hepatic growth factor, vascular endothelial growth factor, granulocyte-colony stimulating factor) and telomere length in knee OA, suggesting a potential role for these factors in cellular aging and OA pathogenesis [22].
The involvement of inflammation in OA is also well recognized, an effect partially attributable to systemic effects associated with aging and adiposity [23]. The strongest support for this relationship is derived from studies demonstrating a link between inflammation and OA pain/function [24], with measures of inflammation (e.g., interleukin-6, tumor necrosis factor alpha, C-reactive protein) independently predicting worsening knee pain [25] and emergence of radiographic knee OA over time [26]. Evidence also points to an association between joint inflammation and heightened experimental pain sensitivity in knee OA [4]. As mentioned, obesity appears to be a large contributing factor to OA prevalence and progression [26, 27]. While increased weight can place greater pressure on the joints, especially on the knee and hip, it has also been shown that metabolic factors, such as central adiposity, contribute to a low-grade inflammation that directly accelerates cartilage degradation [28]. For individuals undergoing weight loss, improvements in inflammatory cytokines, as well as pain and function have been observed [29].
A linkage between Omega-3 and Omega-6 polyunsaturated fatty acid (PUFA) levels and inflammation exists, since eicosanoids (which regulate inflammation) are derived from these fatty acids. Specifically, Omega-3 plays an anti-inflammatory role, while Omega-6 has pro-inflammatory properties [30]. Evidence suggests that in those individuals with or at risk for developing knee OA, Omega-3 fatty acid may serve a protective role, at least in terms of preventing patellofemoral cartilage damage, while Omega-6 fatty acids may have the opposite effect on synovial membranes [30].
Reduced Vitamin D is associated with enhanced sensitivity to pain, including greater pain severity and disability in knee OA [31]. Vitamin D levels can also interact with psychosocial and behavioral factors such as age, ethnicity, and weight. Recent evidence suggests that individuals with knee OA who had normal levels of Vitamin D reported less pain severity than those who were Vitamin D deficient. In terms of functional capacity, obese individuals with adequate Vitamin D were able to perform better on a task of lower extremity functioning, than obese individuals with inadequate levels of Vitamin D [32].
Additionally, sex differences in pain are present such that women tend to experience more pain and have higher rates of chronic pain, including OA-related pain [33, 34]. Moreover, experimental evidence suggests that sex differences in central sensitization may be a risk factor for knee OA, with women demonstrating more enhanced central sensitization compared to men [35]. Although psychosocial and cultural factors may play a role in these differences, sex is likely to contribute in some capacity.
Psychological Mechanisms
Negative Factors
Various psychological factors have been implicated in the development and maintenance of chronic pain conditions such as OA. Such factors likely contribute to the variability in pain severity found among OA patients, especially when divergence is noted between radiographic changes and pain report [36]. For example, negative affect (e.g., general distress or depression), anxiety, and maladaptive coping (e.g., catastrophizing) often result from the experience of persistent or chronic pain, although these psychological variables can also serve as risk factors leading to the development of chronic pain [37]. In addition to general psychological distress and pain facilitation, some symptoms of depression and anxiety (e.g., muscle tension) may even be directly related to physical findings in OA (e.g., joint stiffness/muscle tension) [36], as well as greater utilization of pain medication after total knee arthroplasty [38]. Factors such as helplessness, stress, and limited self-efficacy can influence OA development and progression [39]. Recent evidence suggests that roughly 20% of individuals with OA report symptoms of depression and/or anxiety [40], and higher levels of anxiety have been related to poorer physical health in those with knee OA [41]. Although it is currently unclear if rates of mood disturbance are higher than those of the general population, the relationship between negative affect (including depression) and stress/anxiety with pain make these psychological factors important to consider for OA risk [40].
Perhaps one of the most widely investigated pain-related psychological factors is catastrophizing – a cognitive–emotional process associated with rumination, magnification, and helplessness related to the pain experience. Individuals scoring high on catastrophizing have been found at risk for heightened pain severity and interference, disability, low functional performance, and negative affect [37]. A similar pattern also emerges for surgical outcomes, including arthroplasty, as pre-operative catastrophizing is a unique predictor of post-operative pain and physical functioning in OA [42–45]. Pain-related fear is also common among individuals with OA [46], and refers to excessive worry regarding pain-associated activity or (re)injury (i.e., kinesiophobia). Often this generates a maladaptive cycle whereby negative pain cognitions lead to activity avoidance, thus promoting physical deconditioning and maintenance of pain. Whereas this response may be adaptive after acute pain onset (i.e., facilitation of recovery), it can lead to increased OA pain severity, psychological disability, and functional impairment over time [41, 44, 47, 48], effects which seem to be independent of radiographic severity [47]. While catastrophizing and fear processes can be significant impediments to pain management, cognitive-behavioral and fear-exposure therapies can optimize treatment gains.
Though OA risk tends to increase with age, childhood factors may impact the onset of OA later in life. It is known that childhood traumas (e.g., abuse, neglect/maltreatment) often contribute to worse health and pain outcomes in adulthood. Research examining the role of psychosocial stressors on OA development suggests that individuals with a childhood history of two or more adverse psychosocial events are at greater risk of developing OA, which can occur in a dose-response manner [49].
Positive Factors
In contrast to maladaptive cognitive and affective processes, positive emotions have long been studied in terms of their health-promoting effects, with positive affect (PA) being an important mediator of pain adaptability (for reviews, see [50, 51]). PA is found to be protective against weekly increases in pain and negative affect (NA) during exacerbations of pain and stress [52], and appears to be a better predictor of daily OA pain than NA [53]. There is also evidence for conferred benefits to activity promotion, with higher daily step count observed among knee OA patients with higher PA, relative to individuals with low PA and depressive symptoms [54]. Similar to PA, optimism (generalized expectancy toward positive future outcomes) is also known to play a role in pain resilience, effects which are posited to occur from the interplay between positive appraisal and adaptive coping [55]. In OA patients, higher levels of optimism are associated with decreased clinical pain and higher life satisfaction [56], in addition to lower levels of pain catastrophizing and temporal summation (pain facilitatory measure) [57]. Moreover, in a sample of individuals with knee OA, decreased experimental pain sensitivity was observed among those who had high levels of optimism and low negative affect [58]. Comparable findings in surgical outcomes have also been noted; baseline optimism was a significant predictor of less acute pain and anxiety after total knee and hip arthroplasty [59], as well as lower pain severity (but not pain interference) 1-year following knee arthroscopy [60].
Additionally, there is considerable support for greater self-efficacy in the promotion of health and improved pain outcomes [61], although this may be reflected more so for disability than pain [62]. Self-efficacy is broadly defined as the perceived confidence in one’s abilities to produce desired outcomes [63], and is predictive of physical performance [64], emotional/physical health, self-reported physical function [44], and lower disability [64, 65]. Further, self-efficacy is known to be a potent mediator between resilience and physical functioning in knee OA [66], while a similar body of literature has linked self-efficacy to post-surgical functional ability [67] and pain-related recovery [68] after knee and hip replacement, respectively. However, results have also been inconclusive in this area as a recent review concluded that post-operative self-efficacy was a more robust predictor of functional outcomes, relative to pre-surgical values [69].
Pain acceptance and mindfulness have also emerged as significant contributors to pain resilience. While pain acceptance reflects a sustainability of life activities/goals and reduced efforts to control or avoid pain [70], mindfulness denotes increased nonjudgmental awareness and self-regulated attention toward one’s immediate experience [71]. Highlighting their influence, one study found that greater levels of pain acceptance in women with OA offset the relationship between negative affect and higher weekly pain severity [72]. Additionally, others have demonstrated that mindfulness was associated with adaptive psychosocial functioning and buffered the relationship between pain and perceived stress in patients with knee OA [73].
Behavioral Mechanisms
Physical activity plays an important role in disease prevention, along with symptom management of OA pain [74]. Whereas OA can result in muscle weakness or atrophy, exercise is able to increase muscle and joint health, in addition to reducing body weight [75]. Sedentary behavior is associated with poorer functional performance [76], while acute exercise has analgesic effects on experimental pain in knee OA patients [77] (although outcomes may be contingent upon differences in endogenous pain modulation) [78]. Intensity of exercise may also play a contributory role in OA progression as a recent study found that low and high levels of physical activity were associated with greater cartilage degeneration than engaging in moderate-intensity exercise [79]. Although the type of physical activity may differentially impact OA-related outcomes [80], regular exercise is generally considered an effective tool in reducing pain and disability in patients.
As may be expected, OA pain is related to sleep disturbance and there is evidence of central sensitization in patients with co-morbid insomnia [81]. Although this relationship is most significant in patients with affective disturbance and decreased social support, it is also seen in OA patients lacking psychosocial comorbidities [82–84]. Depression has been found to mediate the relationship between OA pain/disability and sleep disturbance in older adults [82], while high catastrophizing combined with low sleep efficiency seem to worsen central pain sensitivity [81]. Using behavioral approaches for sleep improvement (i.e., CBT) is promising for both acute and long-term pain reductions in OA patients [85–87].
Sociological Mechanisms
Research suggests that social and interpersonal processes such as low socioeconomic status (SES; including education, income, and occupation) and social support are independently associated with risk for OA, as well as pain outcomes [88]. For example, certain occupational demands can lead to increased potential for developing OA due to biomechanical force on the joints arising from strenuous or repetitive physical motions (e.g., lifting, kneeling, bending, etc.). Supporting this, some investigators found that physically demanding occupational tasks were associated with higher incidence of hip and knee OA; however, effects were more robust for symptomatic than radiographic changes [89]. A similar pattern of findings was observed in a recent large-scale study, whereby high occupational workload was a significant predictor of hip and knee OA, with risks increasing in relation to the number of years engaged in the occupation [90]. In addition, situational factors such as footwear, type of flooring (e.g., carpet vs. tile), and presence of stairs in an individual’s everyday work or home environment can impact the development of knee OA possibly due to mechanical loading on the knee [91].
Socioeconomic disadvantage is also a causative factor for unfavorable pain outcomes. In the National Health and Nutrition Examination Survey (NHANES), lower educational attainment was associated with symptomatic knee OA and self-reported arthritis (but not radiographic OA), even after adjustment for relevant risk factors [92]. Studies from the Johnston County Osteoarthritis Project have reported similar findings, both at the individual and community level. These investigators found that low educational achievement and community poverty (neighborhood household poverty rates >25%) were independent predictors of radiographic and symptomatic knee OA [93], while a later investigation by the same authors demonstrated that occupation and education were associated with self-reported knee pain and physical function, with higher rates of knee pain observed among those living in impoverished areas [94]. Comparable findings have also been noted in OA patients with hip pain and disability [95]. More recently, studies have observed greater incidence rates of hip and knee OA among individuals who were older (≥75 years), obese, had a previous hip/knee injury, and with an annual income less than $15,000 [96, 97]. While these studies support the role of socioeconomic measures in OA-related pain and disability, additional factors, such as obesity, may partially account for these findings [98].
Social support is one of the most widely explored determinants of health, with evidence supporting the beneficial role that social relationships have on positive emotions, effective pain coping efforts, and reduced disability. Illustrating this, higher levels of perceived social support have been found to moderate the relationship between pain and depression [56], and a positive affiliation with one’s partner is known to attenuate pain disability and strengthen pain coping responses during high-pain days in women with OA [99]. Further, higher emotional and instrumental (tangible assistance from others) support, as well as social companionship, were related to greater health-related quality of life among individuals with hip and knee OA [100]. Emerging evidence indicates that higher spousal empathy and perceived autonomy support for being active are associated with increased physical activity in patients with knee OA [101], whereas positive interpersonal functioning in OA buffers the relationship between pain intensity and negative affect [102]. Although extant research highlights the positive role that affiliative processes have on health outcomes, it is important to note that social relationships are not always supportive and may in fact, have detrimental effects on OA. For example, one study found that social strain was related to lower social support and higher levels of pain in individuals with knee OA [103], while another investigation observed an inverse relationship between negative social support and postsurgical improvements in pain and positive affect in patients with knee OA [104]. Taken together, these studies suggest that interpersonal relationships do not guarantee favorable effects on pain. Instead, the quality of the social milieu can yield widely differential effects on OA outcomes.
Conclusions
OA is a multifactorial condition consisting of numerous biopsychosocial factors that potentiate or ameliorate the risk for pain and disability. By and large, much of the extant literature has been problem-focused in nature, with an emphasis on vulnerability and risk factors associated with negative pain outcomes. However, this clearly presents a one-sided view of health as evidence also supports the important contributions of resilience and positive affect on pain. Namely, there is increased realization that individuals with chronic pain, including OA, have the capacity to adapt and manage their pain while simultaneously limiting their disability.
In light of this, we propose that research initiatives be directed toward the factors that mollify the adverse effects of pain. Moreover, recognition of the multiple, interactive processes that serve to facilitate or attenuate pain-related outcomes in OA will likely provide a more comprehensive picture of patient functioning that could ultimately inform clinical practice. From a treatment perspective, this will require moving beyond a traditional biomedical approach and instead target the numerous facets (both positive and negative) that comprise the pain experience (i.e., multidisciplinary treatment). Overall, it will be imperative for health care professionals to approach OA patient care with this in mind and encourage communication across all pain management specialties (e.g., physicians, psychologists, physiotherapists, social workers, etc).
Acknowledgments
Preparation of the article was supported by the National Institutes of Health/National Institute on Aging (K99AG052642) awarded to EJB. The authors have no other conflicts of interest to disclose.
Contributor Information
Emily J. Bartley, University of Florida, Department of Community Dentistry and Behavioral Science, Pain Research & Intervention Center of Excellence, 1395 Center Drive, Room D2-13, PO Box 100404, Gainesville, FL 32610, EBartley@dental.ufl.edu, 352-273-8934 (office).
Shreela Palit, The University of Tulsa, Department of Psychology, 800 South Tucker Drive, Tulsa, OK 74104, shreela-palit@utulsa.edu, 918-631-3565 (office).
Roland Staud, University of Florida, College of Medicine, Pain Research & Intervention Center of Excellence, PO Box 100221, Gainesville, FL, 32610, Roland.Staud@medicine.ufl.edu, 352-294-8213 (office).
References
- 1.RWt Gereau, KA Sluka, W Maixner, SR Savage, TJ Price, BB Murinson et al. A pain research agenda for the 21st century. The Journal of Pain. 2014;15(12):1203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and Rheumatism. 2008;58(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen KD, Golightly YM. Epidemiology of osteoarthritis: State of the evidence. Current Opinion in Rheumatology. 2015;27(3):276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neogi T The epidemiology and impact of pain in osteoarthritis. Osteoarthritis and Cartilage. 2013;21(9):1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. British Medical Bulletin. 2013;105:185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Annals of the Rheumatic Diseases. 2014;73(7):1323–30. [DOI] [PubMed] [Google Scholar]
- 7.Maetzel A, Li L, Pencharz J, Tomlinson G, Bombardier C. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: A comparative study. Annals of the Rheumatic Diseases. 2004;63(4):395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ et al. Discordance between pain and radiographic severity in knee osteoarthritis: Findings from quantitative sensory testing of central sensitization. Arthritis and Rheumatism. 2013;65(2):363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sturgeon JA, Zautra AJ. Resilience: A new paradigm for adaptation to chronic pain. Current Pain and Headache Reports. 2010;14(2):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sturgeon JA, Zautra AJ. Psychological resilience, pain catastrophizing, and positive emotions: Perspectives on comprehensive modeling of individual pain adaptation. Current Pain and Headache Reports. 2013;17(3):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salaffi F, Ciapetti A, Carotti M. The sources of pain in osteoarthritis: A pathophysiological review. Reumatismo. 2014;66(1):57–71. [DOI] [PubMed] [Google Scholar]
- 12.Arendt-Nielsen L, Graven-Nielsen T. Central sensitization in fibromyalgia and other musculoskeletal disorders. Current Pain and Headache Reports. 2003;7(5):355–61. [DOI] [PubMed] [Google Scholar]
- 13.Graven-Nielsen T, Arendt-Nielsen L. Peripheral and central sensitization in musculoskeletal pain disorders: An experimental approach. Current Rheumatology Reports. 2002;4(4):313–21. [DOI] [PubMed] [Google Scholar]
- 14.Staud R Peripheral pain mechanisms in chronic widespread pain. Bailliere’s Best Practice & Research in Clinical Rheumatology. 2011;25(2):155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Annals of Internal Medicine. 2000;133(8):635–46. [DOI] [PubMed] [Google Scholar]
- 16.Warner CS, Valdes MA. The genetics of osteoarthritis: A review. Journal of Functional Morphology and Kinesiology. 2016;1(1):140–53. [Google Scholar]
- 17.Hochberg MC, Yerges-Armstrong L, Yau M, Mitchell BD. Genetic epidemiology of osteoarthritis: Recent developments and future directions. Current Opinion in Rheumatology. 2013;25(2):192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–73. [DOI] [PubMed] [Google Scholar]
- 19.Shammas MA. Telomeres, lifestyle, cancer, and aging. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibille KT, Langaee T, Burkley B, Gong Y, Glover TL, King C et al. Chronic pain, perceived stress, and cellular aging: An exploratory study. Molecular Pain. 2012;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sibille KT, Chen H, Bartley EJ, Riley JL, Glover TL, King CD et al. Accelerated aging in adults with knee osteoarthritis pain: Consideration for frequency, intensity, time, and total pain sites. Pain Reports. 2017. doi:dx.doi.org/0.1097/PR9.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poonpet T, Saetan N, Tanavalee A, Wilairatana V, Yuktanandana P, Honsawek S. Association between leukocyte telomere length and angiogenic cytokines in knee osteoarthritis. International Journal of Rheumatic Diseases. 2017. doi: 10.1111/1756-185X.12988. [DOI] [PubMed] [Google Scholar]
- 23.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Therapeutic Advances in Musculoskeletal Disease. 2013;5(2):77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis and Cartilage. 2015;23(11):1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: A prospective cohort study. Annals of Rheumatic Diseases. 2013;72(4):535–40. [DOI] [PubMed] [Google Scholar]
- 26.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis and Rheumatism. 2009;60(7):2037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y et al. Body mass index and susceptibility to knee osteoarthritis: A systematic review and meta-analysis. Joint Bone Spine. 2012;79(3):291–7. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Hunter D, Xu J, Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis and Cartilage. 2015;23(1):22–30. Excellent overview of the association between inflammation and osteoarthritis, with a focus on metabolic mechanisms underlying meta-inflammation. [DOI] [PubMed] [Google Scholar]
- 29.Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. The Journal of the American Medical Association. 2013;310(12):1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker KR, Matthan NR, Lichtenstein A, Niu J, Guermazi A, Roemer F et al. Association of Plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: The MOST Study. Osteoarthritis and Cartilage. 2012;20(5):382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glover TL, Horgas AL, Fillingim RB, Goodin BR. Vitamin D status and pain sensitization in knee osteoarthritis: A critical review of the literature. Pain Management. 2015;5(6):447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glover TL, Goodin BR, King CD, Sibille KT, Herbert MS, Sotolongo AS et al. A cross-sectional examination of Vitamin D, obesity, and measures of pain and function in middle-aged and older adults with knee osteoarthritis. The Clinical Journal of Pain. 2015;31(12):1060–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartley EJ, Fillingim RB. Sex differences in pain: A brief review of clinical and experimental findings. British Journal of Anaesthesia. 2013;111(1):52–8. This review article provides an updated overview of the mechanisms underlying sex differences in pain, including a broad summary of the clinical and experimental findings in the literature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartley EJ, Palit S. Gender and pain. Current Anesthesiology Reports. 2016;6(4):344–53. [Google Scholar]
- 35.Bartley EJ, King CD, Sibille KT, Cruz-Almeida Y, Riley JL, Glover TL et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: Potential sex differences in central sensitization. Arthritis Care & Research. 2016;68(4):472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creamer P, Hochberg MC. The relationship between psychosocial variables and pain reporting in osteoarthritis of the knee. Arthritis & Rheumatism. 1998;11(1):60–5. [DOI] [PubMed] [Google Scholar]
- 37.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. The Journal of Pain. 2016;17(9 Suppl):T70–92. This paper is an excellent, comprehensive review of our current understanding of psychosocial factors in chronic pain development, and future directions for examining these factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh JA, Lewallen DG. Predictors of use of pain medications for persistent knee pain after primary total knee arthroplasty: A cohort study using an institutional joint registry. Arthritis Research & Therapy. 2012;14(6):R248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keefe FJ, Smith SJ, Buffington AL, Gibson J, Studts JL, Caldwell DS. Recent advances and future directions in the biopsychosocial assessment and treatment of arthritis. Journal of Consulting and Clinical Psychology. 2002;70(3):640–55. [DOI] [PubMed] [Google Scholar]
- 40.Stubbs B, Aluko Y, Phyo Kyaw M, Smith TO. Prevalence of depressive symptoms and anxiety in osteoarthritis: A systematic review and meta-analysis. Age & Ageing. 2016;45(2):228–35. This paper highlights updated research investigating the relationships between symptoms of depression and anxiety in osteoarthritis. [DOI] [PubMed] [Google Scholar]
- 41.Scopaz KA, Piva SR, Wisniewski S, Fitzgerald GK. Relationships of fear, anxiety, and depression with physical function in patients with knee osteoarthritis. Archives of Physical Medicine and Rehabilitation. 2009;90(11):1866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis GN, Rice DA, McNair PJ, Kluger M. Predictors of persistent pain after total knee arthroplasty: A systematic review and meta-analysis. British Journal of Anaesthesia. 2015;114(4):551–61. [DOI] [PubMed] [Google Scholar]
- 43.Burns LC, Ritvo SE, Ferguson MK, Clarke H, Seltzer Z, Katz J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: A systematic review. Journal of Pain Research. 2015;8:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helminen EE, Sinikallio SH, Valjakka AL, Vaisanen-Rouvali RH, Arokoski JP. Determinants of pain and functioning in knee osteoarthritis: A one-year prospective study. Clinical Rehabilitation. 2016;30(9):890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dave AJ, Selzer F, Losina E, Usiskin I, Collins JE, Lee YC et al. The association of pre-operative body pain diagram scores with pain outcomes following total knee arthroplasty. Osteoarthritis and Cartilage. 2017;25(5):667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunn AH, Schwartz TA, Arbeeva LS, Callahan LF, Golightly Y, Goode A et al. Fear of movement and associated factors among adults with symptomatic knee osteoarthritis. Arthritis Care & Research. 2017. doi: 10.1002/acr.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heuts PHTG, Vlaeyen JWS, Roelofs J, de Bie RA, Aretz K, van Weel C et al. Pain-related fear and daily functioning in patients with osteoarthritis. Pain. 2004;110(1–2):228–35. [DOI] [PubMed] [Google Scholar]
- 48.Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Waters SJ, Riordan PA et al. Pain catastrophizing and pain-related fear in osteoarthritis patients: Relationships to pain and disability. Journal of Pain and Symptom Management. 2009;37(5):863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Von Korff M, Alonso J, Ormel J, Angermeyer M, Bruffaerts R, Fleiz C et al. Childhood psychosocial stressors and adult onset arthritis: Broad spectrum risk factors and allostatic load. Pain. 2009;143(1–2):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finan PH, Garland EL. The role of positive affect in pain and its treatment. The Clinical Journal of Pain 2015;31(2):177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassett AL, Finan PH. The role of resilience in the clinical management of chronic pain. Current Pain and Headache Reports. 2016;20(6):39. doi: 10.1007/s11916-016-0567-7. This brief review summarizes the literature on positive activity interventions for chronic pain, including the putative mechanisms contributing to pain reduction across these therapies. [DOI] [PubMed] [Google Scholar]
- 52.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. Journal of Consulting and Clinical Psychology. 2005;73(2):212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finan PH, Quartana PJ, Smith MT. Positive and negative affect dimensions in chronic knee osteoarthritis: Effects on clinical and laboratory pain. Psychosomatic Medicine. 2013;75(5):463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White DK, Keysor JJ, Neogi T, Felson DT, LaValley M, Gross KD et al. When it hurts, a positive attitude may help: Association of positive affect with daily walking in knee osteoarthritis. Results from a multicenter longitudinal cohort study. Arthritis Care & Research. 2012;64(9):1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodin BR, Bulls HW. Optimism and the experience of pain: Benefits of seeing the glass as half full. Current Pain and Headache Reports. 2013;17(5):329. doi: 10.1007/s11916-013-0329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferreira VM, Sherman AM. The relationship of optimism, pain and social support to well-being in older adults with osteoarthritis. Aging & Mental Health. 2007;11(1):89–98. [DOI] [PubMed] [Google Scholar]
- 57.Goodin BR, Glover TL, Sotolongo A, King CD, Sibille KT, Herbert MS et al. The association of greater dispositional optimism with less endogenous pain facilitation is indirectly transmitted through lower levels of pain catastrophizing. The Journal of Pain. 2013;14(2):126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cruz-Almeida Y, King CD, Goodin BR, Sibille KT, Glover TL, Riley JL et al. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care & Research. 2013;65(11):1786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinto PR, McIntyre T, Ferrero R, Almeida A, Araujo-Soares V. Predictors of acute postsurgical pain and anxiety following primary total hip and knee arthroplasty. The Journal of Pain. 2013;14(5):502–15. [DOI] [PubMed] [Google Scholar]
- 60.Rosenberger PH, Kerns R, Jokl P, Ickovics JR. Mood and attitude predict pain outcomes following arthroscopic knee surgery. Annals of Behavioral Medicine. 2009;37(1):70–6. [DOI] [PubMed] [Google Scholar]
- 61.Jackson T, Wang Y, Wang Y, Fan H. Self-efficacy and chronic pain outcomes: A meta-analytic review. The Journal of Pain. 2014;15(8):800–14. [DOI] [PubMed] [Google Scholar]
- 62.Benyon K, Hill S, Zadurian N, Mallen C. Coping strategies and self-efficacy as predictors of outcome in osteoarthritis: A systematic review. Musculoskeletal Care. 2010;8(4):224–36. [DOI] [PubMed] [Google Scholar]
- 63.Bandura A Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84(2):191–215. [DOI] [PubMed] [Google Scholar]
- 64.Rejeski WJ, Miller ME, Foy C, Messier S, Rapp S. Self-efficacy and the progression of functional limitations and self-reported disability in older adults with knee pain. The Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2001;56(5):S261–5. [DOI] [PubMed] [Google Scholar]
- 65.Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: Role of psychosocial, local mechanical, and neuromuscular factors. Arthritis and Rheumatism. 2003;48(12):3359–70. [DOI] [PubMed] [Google Scholar]
- 66.Wright LJ, Zautra AJ, Going S. Adaptation to early knee osteoarthritis: The role of risk, resilience, and disease severity on pain and physical functioning. Annals of Behavioral Medicine. 2008;36(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wylde V, Dixon S, Blom AW. The role of preoperative self-efficacy in predicting outcome after total knee replacement. Musculoskeletal Care. 2012;10(2):110–8. [DOI] [PubMed] [Google Scholar]
- 68.Brembo EA, Kapstad H, Van Dulmen S, Eide H. Role of self-efficacy and social support in short-term recovery after total hip replacement: A prospective cohort study. Health and Quality of Life Outcomes. 2017;15(1):68. doi: 10.1186/s12955-017-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Magklara E, Burton CR, Morrison V. Does self-efficacy influence recovery and well-being in osteoarthritis patients undergoing joint replacement? A systematic review. Clinical Rehabilitation. 2014;28(9):835–46. [DOI] [PubMed] [Google Scholar]
- 70.McCracken LM. Learning to live with the pain: Acceptance of pain predicts adjustment in persons with chronic pain. Pain. 1998;74(1):21–7. [DOI] [PubMed] [Google Scholar]
- 71.Kabat-Zinn J An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4(1):33–47. [DOI] [PubMed] [Google Scholar]
- 72.Kratz AL, Davis MC, Zautra AJ. Pain acceptance moderates the relation between pain and negative affect in female osteoarthritis and fibromyalgia patients. Annals of Behavioral Medicine. 2007;33(3):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee AC, Harvey WF, Price LL, Morgan LPK, Morgan NL, Wang C. Mindfulness is associated with psychological health and moderates pain in knee osteoarthritis. Osteoarthritis and Cartilage. 2017;25(6):824–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennell KL, Hinman RS. A review of the clinical evidence for exercise in osteoarthritis of the hip and knee. Journal of Science and Medicine in Sport. 2011;14(1):4–9. [DOI] [PubMed] [Google Scholar]
- 75.Valderrabano V, Steiger C. Treatment and prevention of osteoarthritis through exercise and sports. Journal of Aging Research. 2010;2011:1–6. doi: 10.4061/2011/374653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J, Chang RW, Ehrlich-Jones L, Kwoh CK, Nevitt M, Semanik PA et al. Sedentary behavior and physical function: Objective evidence from the Osteoarthritis Initiative. Arthritis Care & Research. 2015;67(3):366–73. This cross-sectional study found that sedentary behavior was associated with worse physical functioning in adults with knee osteoarthritis, thus supporting the beneficial role of physical activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burrows NJ, Booth J, Sturnieks DL, Barry BK. Acute resistance exercise and pressure pain sensitivity in knee osteoarthritis: A randomised crossover trial. Osteoarthritis and Cartilage. 2014;22(3):407–14. [DOI] [PubMed] [Google Scholar]
- 78.Fingleton C, Smart KM, Doody CM. Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. The Clinical Journal of Pain. 2017;33(5):395–404. [DOI] [PubMed] [Google Scholar]
- 79.Lin W, Alizai H, Joseph GB, Srikhum W, Nevitt MC, Lynch JA et al. Physical activity in relation to knee cartilage T2 progression measured with 3 T MRI over a period of 4 years: Data from the Osteoarthritis Initiative. Osteoarthritis and Cartilage. 2013;21(10):1558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bartholdy C, Juhl C, Christensen R, Lund H, Zhang W, Henriksen M. The role of muscle strengthening in exercise therapy for knee osteoarthritis: A systematic review and meta-regression analysis of randomized trials. Seminars in Arthritis and Rheumatism. 2017. doi: 10.1016/j.semarthrit.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Campbell CM, Buenaver LF, Finan P, Bounds SC, Redding M, McCauley L et al. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care & Research. 2015;67(10):1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parmelee PA, Tighe CA, Dautovich ND. Sleep disturbance in osteoarthritis: Linkages with pain, disability, and depressive symptoms. Arthritis Care & Research. 2015;67(3):358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allen KD, Renner JB, Devellis B, Helmick CG, Jordan JM. Osteoarthritis and sleep: The Johnston County Osteoarthritis Project. The Journal of Rheumatology. 2008;35(6):1102–7. [PMC free article] [PubMed] [Google Scholar]
- 84.Louie GH, Tektonidou MG, Caban‐Martinez AJ, Ward MM. Sleep disturbances in adults with arthritis: Prevalence, mediators, and subgroups at greatest risk. Data from the 2007 National Health Interview Survey. Arthritis Care & Research. 2011;63(2):247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vitiello MV, McCurry SM, Shortreed SM, Baker LD, Rybarczyk BD, Keefe FJ et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. 2014;155(8):1547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith MT, Finan PH, Buenaver LF, Robinson M, Haque U, Quain A et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: A randomized, double-blind, active placebo-controlled clinical trial. Arthritis & Rheumatology. 2015;67(5):1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salwen JK, Smith MT, Finan PH. Mid-treatment sleep duration predicts clinically significant knee osteoarthritis pain reduction at 6 months: Effects from a behavioral sleep medicine clinical trial. Sleep. 2017;40(2). doi: 10.1093/sleep/zsw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luong ML, Cleveland RJ, Nyrop KA, Callahan LF. Social determinants and osteoarthritis outcomes. Aging Health. 2012;8(4):413–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allen KD, Chen JC, Callahan LF, Golightly YM, Helmick CG, Renner JB et al. Associations of occupational tasks with knee and hip osteoarthritis: The Johnston County Osteoarthritis Project. The Journal of Rheumatology. 2010;37(4):842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Andersen S, Thygesen LC, Davidsen M, Helweg-Larsen K. Cumulative years in occupation and the risk of hip or knee osteoarthritis in men and women: A register-based follow-up study. Occupational and Environmental Medicine. 2012;69(5):325–30. [DOI] [PubMed] [Google Scholar]
- 91.Hunt MA, Birmingham TB, Skarakis-Doyle E, Vandervoort AA. Towards a biopsychosocial framework of osteoarthritis of the knee. Disability and Rehabilitation. 2008;30(1):54–61. [DOI] [PubMed] [Google Scholar]
- 92.Hannan MT, Anderson JJ, Pincus T, Felson DT. Educational attainment and osteoarthritis: Differential associations with radiographic changes and symptom reporting. Journal of Clinical Epidemiology. 1992;45(2):139–47. [DOI] [PubMed] [Google Scholar]
- 93.Callahan LF, Cleveland RJ, Shreffler J, Schwartz TA, Schoster B, Randolph R et al. Associations of educational attainment, occupation and community poverty with knee osteoarthritis in the Johnston County (North Carolina) osteoarthritis project. Arthritis Research & Therapy. 2011;13(5):R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cleveland RJ, Luong ML, Knight JB, Schoster B, Renner JB, Jordan JM et al. Independent associations of socioeconomic factors with disability and pain in adults with knee osteoarthritis. BMC Musculoskeletal Disorders. 2013;14:297. doi: 10.1186/1471-2474-14-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knight JB, Callahan LF, Luong ML, Shreffler J, Schoster B, Renner JB et al. The association of disability and pain with individual and community socioeconomic status in people with hip osteoarthritis. The Open Rheumatology Journal. 2011;5:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moss AS, Murphy LB, Helmick CG, Schwartz TA, Barbour KE, Renner JB et al. Annual incidence rates of hip symptoms and three hip OA outcomes from a U.S. population-based cohort study: The Johnston County Osteoarthritis Project. Osteoarthritis and Cartilage. 2016;24(9):1518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Murphy LB, Moss S, Do BT, Helmick CG, Schwartz TA, Barbour KE et al. Annual incidence of knee symptoms and four knee osteoarthritis outcomes in the Johnston County Osteoarthritis Project. Arthritis Care & Research. 2016;68(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reyes C, Garcia-Gil M, Elorza JM, Mendez-Boo L, Hermosilla E, Javaid MK et al. Socio-economic status and the risk of developing hand, hip or knee osteoarthritis: A region-wide ecological study. Osteoarthritis and Cartilage. 2015;23(8):1323–9. Reports the highest prevalence rates of hand, hip, and knee osteoarthritis among socioeconomically disadvantaged areas, with obesity significantly contributing to increased risk. [DOI] [PubMed] [Google Scholar]
- 99.Taylor SS, Davis MC, Zautra AJ. Relationship status and quality moderate daily pain-related changes in physical disability, affect, and cognitions in women with chronic pain. Pain. 2013;154(1):147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ethgen O, Vanparijs P, Delhalle S, Rosant S, Bruyère O, Reginster J-Y. Social support and health-related quality of life in hip and knee osteoarthritis. Quality of Life Research. 2004;13(2):321–30. [DOI] [PubMed] [Google Scholar]
- 101.Martire LM, Stephens MA, Mogle J, Schulz R, Brach J, Keefe FJ. Daily spousal influence on physical activity in knee osteoarthritis. Annals of Behavioral Medicine. 2013;45(2):213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sturgeon JA, Zautra AJ, Arewasikporn A. A multilevel structural equation modeling analysis of vulnerabilities and resilience resources influencing affective adaptation to chronic pain. Pain. 2014;155(2):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sherman AM. Social relations and depressive symptoms in older adults with knee osteoarthritis. Social Science & Medicine. 2003;56(2):247–57. [DOI] [PubMed] [Google Scholar]
- 104.Stephens MA, Druley JA, Zautra AJ. Older adults’ recovery from surgery for osteoarthritis of the knee: Psychosocial resources and constraints as predictors of outcomes. Health Psychology. 2002;21(4):377–83. [DOI] [PubMed] [Google Scholar]