Abstract
Tragopogon graminifolius DC. is a perennial plant from the family Asteraceae which grows in West parts of Iran. Several biological activities like antimicrobial, antioxidant and anti-inflammatory effects are reported for the plant. The aim of this study was to assess the wound healing activity of standardized extract from T. graminifolius (TG) aerial parts. Topical standardized TG extract with 5% and 10% concentrations in eucerine base was assessed for its healing properties on second degree burn in rats during a 14-day period. Biomarkers of oxidative damage including total antioxidant power, lipid peroxidation and total thiol molecules of the skin tissue samples were also evaluated. Results showed that 10%TG had the best efficacy with 80 ± 3% wound closure and tissue repair in comparison to negative control (p < 0.05). Significant reduction of tissue oxidative stress biomarkers was also observed. Histological analyses confirmed wound healing activity of TG extract, as well. Considering the antioxidative stress and anti-inflammatory activities of TG, explained by the high content of phenolic compounds of the plant, standardized TG extract could be considered as a natural remedy for the treatment of burn wounds. Further clinical studies are suggested to confirm the effectiveness of TG as a wound healing agent.
Keywords: Tragopogon graminifolius, Second-degree burn, Oxidative stress, Medicinal plant
Graphical abstract
1. Introduction
Skin is a vital organ which performs several functions such as thermoregulation, homeostasis, metabolic, neurosensory and immunologic functions. It is also a physical barrier against infections; thus, when it is injured, pathogens can have a direct access to the deep tissues.1
Burns, defined as tissue damage by heat or corrosive chemicals, are one of the most common causes of skin injuries. Extensive tissue damaged during burns impairs angiogenesis, collagen reorganization, granulation tissue formation and induces free radical-mediated damage which results in delayed tissue repair. Recovery from burn wound needs long hospital stay, expensive medications and long rehabilitation period.2 As one of the first lines of treatment, topical antibacterial agents are used to prevent infection; though, they can cause allergic reactions which postpone the healing process.3,4 Another treatment approach is to use recombinant growth factors and tissue engineered wound dressings which are too expensive for a relatively high number of the patients; thus, investigations for the discovery of new wound healing agents are still required.
One of the available options in burn wounds management are medicinal plants which provide a wide area of research for scientists due to the vast diversity of phytochemicals with antioxidant, anti-inflammatory and immunomodulatory effects.5, 6, 7
Tragopogon graminifolius DC. is a perennial plant from the family Asteraceae which grows in the West parts of Iran and is locally known as “Sheng”. In Persian medicine, T. graminifolius (TG) has been known as a hemostatic, wound healing, detoxifying and hepatoprotective agent, and is used for several gastrointestinal complications. Studies showed that different species of the genus Tragopogon contain phytochemicals such as flavonoids including luteolin, vitexin, isovitexin, vicenin −1 and 2, and orientin.8, 9, 10 Farzaei et al. reported the presence of five phenolic compounds including p-coumaric acid, ferulic acid, catechin, gallic acid and caffeic acid in the hydroalcoholic extract of T. graminifolius aerial parts.11 TG essential oil contains n-hexadecanoic acid, β-caryophyllene, heneicosane and nonanal as major components. The plant also showed significant antioxidant properties.11 As oxidative stress has a crucial role in the pathogenesis of burns and wounds, potent antioxidant activity is of a great interest for the treatment of skin tissue injuries. TG have demonstrated different pharmacological activities in recent studies including amelioration of TNBS-induced inflammatory bowel disease as well as ethanol-induced peptic ulcer disease in rats.12,13
Due to previously reported anti-inflammatory and antioxidant properties of TG, we hypothesized that the plant can be used as a wound healing agent in skin damage. The goal of the present study is to investigate the wound healing properties of standardized extract from TG aerial parts in animal model of second-degree burn wound along with the assessment of oxidative damage biomarkers in skin tissue.
2. Method and materials
2.1. Chemicals
Ethanol, distilled water, formalin 10%, ketamine, xylazine, normal saline (0.9%), eucerine (400%), hematoxylin, eosin, silver sulfadiazine 1% cream USP, and sodium bicarbonate were supplied from Merck company (Germany).
2.2. Plant material and preparation of standardized extract
TG was collected in July 2012 from Kermanshah province, West of Iran and was authenticated by Dr. F. Attar (Department of Biology, Faculty of Sciences, University of Tehran). A voucher specimen (No.43603) was deposited in the Central Herbarium of University of Tehran.
Aerial parts of TG were air-dried in shadow and powdered by mechanical grinder. Powdered aerial parts (100 g) were extracted 3 times with 80% ethanol. The obtained extracts were mixed and the solvent was evaporated under reduced pressure using rotary evaporator.14 As described in our previous report,11 the extract was standardized based on five phenolic compounds (gallic acid, p-coumaric acid, Ferulic acid, catechin and caffeic acid) using high performance liquid chromatography (HPLC) method and the standardized extract was used for further experiments.
2.3. Ointment preparation
Eucerine is a well-known emulsion base which can absorb a high portion of aqueous or oily solutions and thus, can be used for the preparation of several types of creams and ointments.15 Ointments were prepared with concentration of 5% and 10% of the extract in eucerine. Silver sulfadiazine 1% and eucerine were used as positive and negative controls, respectively.
2.4. Burn wound induction
Twenty four male Wistar rats, weighing 200–250 g, were divided into 4 groups of 6 animals in each group. The rats were individually housed under standard animal lab condition (25 ± 1 °C, humidity 60%, 12 h light/12 h dark cycles) and fed with standard laboratory food and water ad libitum. Treatments include TG 10% extract (TG10), TG 5% extract (TG5), silver sulfadiazine (C+) and eucerine (C-). All animal experiments were according to laboratory animal ethics guidelines.
Anesthesia was induced by intraperitoneal injection of pentobarbital (50 mg/kg). Dorsal parts of animals were shaved and burn wounds were created on the shaved area of rats using a burn set with an aluminum rod (1.5 cm) with 110 °C heat and exposed for 10 s under 1 atm pressure. Animals were kept separately (one in a cage). Treatment started 24 h after burn wound induction. Ointments were daily applied to cover all over the wound and the wound dressed by a regular dressing. Dressing was changed every 24 h for 14 days.
Using a digital camera, wound areas were photographed every 24 h and a ruler was used as scale. At the end of the study, pictures of each day were analyzed by Adobe Photoshop CS3 software to measure wound contraction. Wound contraction rate was calculated by the following formula:
| Wound contraction (%) = [(wound size of the induction day – wound size of the specific day)/wound size of the induction day] × 100 |
At the day of 14, all rats were sacrificed and skin tissues of the burnt area were collected. Each sample were cut into pieces; one part was kept in formalin 10% for evaluation of histological changes. The other half was preserved in −80 C° for assessing total thiol molecules (TTM), lipid peroxidation (LPO) and total antioxidant power (TAP).
2.5. Histological analysis
Hematoxylin and eosin were used to stain tissue sections and microscopic images of tissue were taken under × 400 magnification.14
2.6. Tissue oxidative stress biomarkers assessment
Samples were floated in phosphate-buffered saline and then homogenized with Heidolph Silent Crusher M (Germany). Then, the samples were centrifuged at 3000 g at 4 °C for 15 min and super natal fluid was kept for further analyses.16
2.7. TTM assessment
For TTM assessment, the collected super natal fluid was mixed with Tris-EDTA buffer (EDTA 20 mM, Tris base 0.25 M pH: 8.2) and the DTNB reaction with total sulfhydryl groups to achieve a chromogen material and the absorbance was measured at 412 nm.16,17
2.8. LPO assessment
For the determination of LPO, reaction of 2-thiobarbituric acid reducing substance (TBARS) method with lipid peroxides was used. Tissue extract was mixed with trichloroacetic acid (20%) and the precipitant was dissolved with H2SO4 (0.05 M). After that, 2-Thiobarbituric acid (TBA, 0.2% in 2 M sodium sulfate) was added and heated for 30 min in boiling water bath. The final mixture was reacted with n-butanol and the absorbance was measured at 532 nm (ELISA reader, Biotek, Germany).16
2.9. TAP assessment
Total antioxidant power was measured by assessing the ability of tissue extract to reduce Fe3+ to Fe2+. FRAP reagent (25 ml of sodium acetate (300 mM), 2.5 ml 2,4,6-tripyridyl-s-triazine (TPTZ) (10 mM) and 2.5 ml FeCl3.6H2O (20 mM) (pH 3.6)) was mixed with super natal fluid and the absorbance was determined at 593 nm.16,18
2.10. Statistical analysis
Results were expressed as the mean ± standard deviation. All collected data was analyzed with one-way ANOVA test (SPSS 15 software) and P < 0.05 showed statistical significance.
3. Results
3.1. Wound closure and healing process
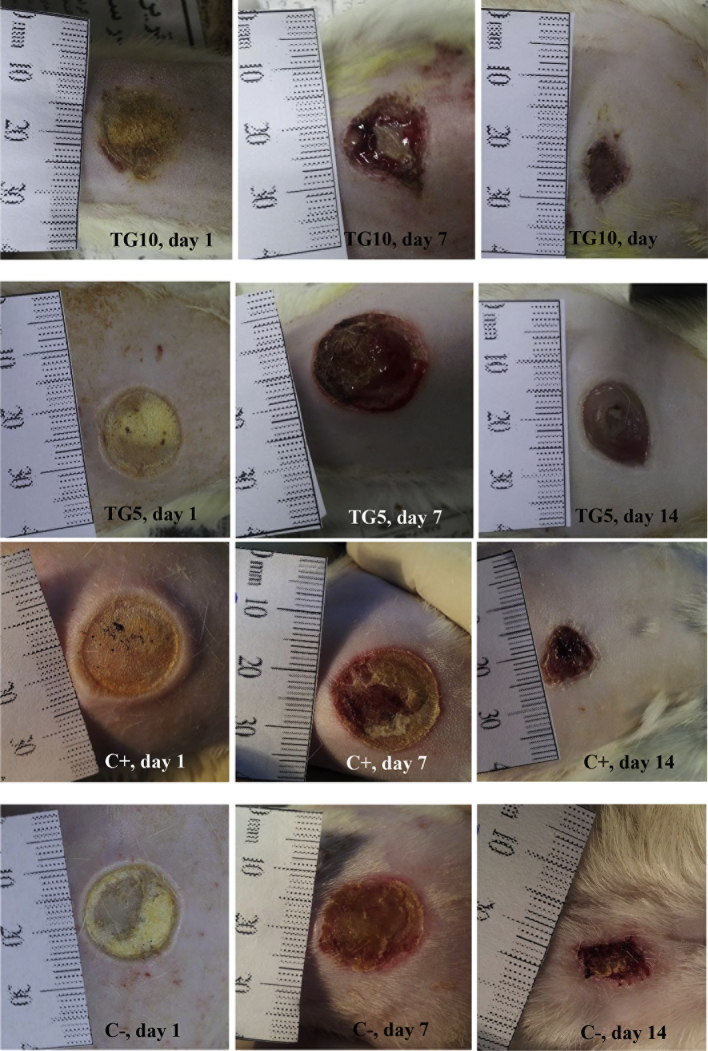
Table 1 shows the wound closure percentage in the last day of experiment. In negative control group, the wound area reached to nearly half of the zero day wound size. Ointment of 5% TG showed a significantly better wound healing effect in comparison with negative control (p < 0.05). TG 10% ointment showed the best wound healing effect with 80 ± 0.3 reduction in wound size compared with the day of wound induction which was significantly higher than negative control (p < 0.01). This effect was numerically (but not significantly) better than silver sulfadiazine (Table 1). Fig. 1 shows the macro-area of the wounds in different groups.
Table 1.
Wound contraction rate (%) in the 14th day of animal experiment.
| 14th day | Treatment |
|---|---|
| 57.80 ± 5.71 | C- |
| 78.80 ± 3.96** | C+ |
| 73 ± 0.1* | 5% TG |
| 80 ± 0.3** | 10% TG |
Values are expressed as mean ± standard deviation for each group. Significant differences in comparison to C-: *: P < 0.05, **: P < 0.01, TG5: Tragopogon graminifolius extract (5%), TG10: T. graminifolius extract(10%).
Fig. 1.
Macro-area of wounds in 1st, 7th, and 14th day of experiment. TG: Tragopogon graminifolius.
3.2. Histological analysis
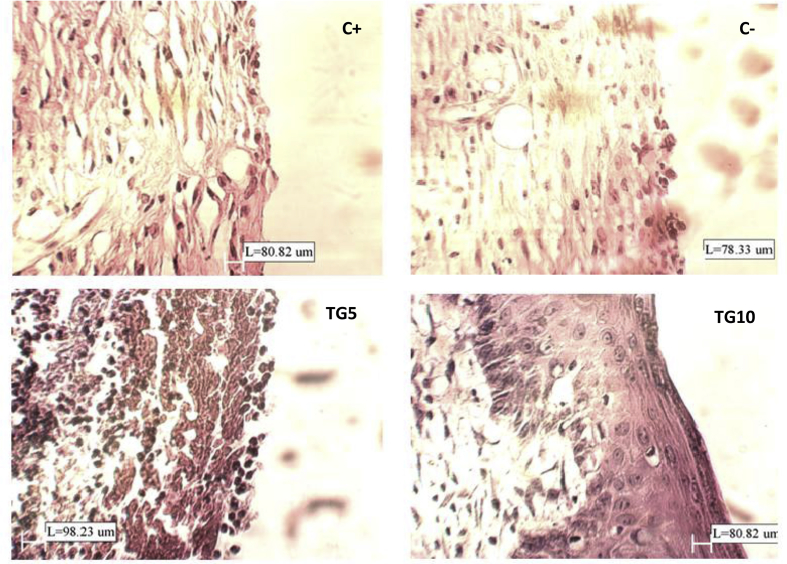
The collected tissue samples of each group on 14th day were assessed under × 400 magnification. One photograph of each group is shown in Fig. 2.
Fig. 2.
Microscopic images of skin tissue samples of 14th day with magnification of × 400.
C+: PMNs population decrease and epithelium regeneration is observable in wound edge but not in wound center (neovascularization: +).
C-: high density of blood vessels and no re-epithelialization, only reduction in PMNs and macrophages (neovascularization: +).
TG5: high density of blood vessels and connective cells, no healing and extreme bleeding in dermis layer (neovascularization: ++++).
TG10: re-generation is almost complete, only stratum keratinosum need to be complete.
In C+ group, the number of polymorphonuclears (PMNs) was decreased in the wound edge. Basal epithelium regeneration was observed in wound margins, but not in wound center. Fibroblasts density was relatively high and collagen bundles had an irregular appearance.
In C-, re-epithelialization was not occurred, but the number of macrophages and PMNs were started to decrease and blood vessels had a high density.
In TG5 group, despite the 14 days of treatment, there was an extreme bleeding and no healing in dermis layer was occurred. Blood vessels and connective cells density was high.
In TG10 group, epidermis was regenerated and different layers of dermis was repaired, but stratum keratinosum was not yet completely formed.
3.3. Tissue oxidative stress biomarkers
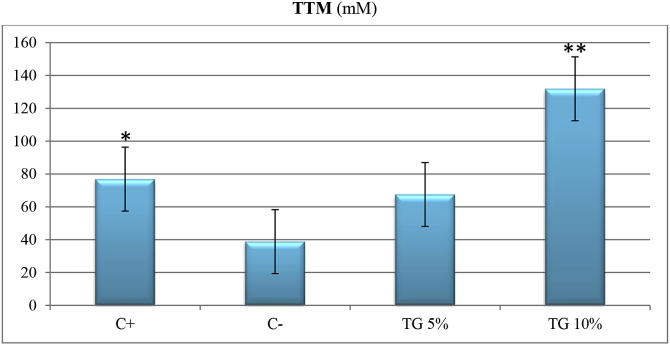
Silver sulfadiazine 1% and TG extract with concentration of 10% increased TTM compared with negative control with P < 0.05; whereas TG5 could not significantly increase TTM in comparison with C- (P > 0.05). As shown in Fig. 3, TG10 increased TTM even more than positive control (P < 0.05) (Fig. 3).
Fig. 3.
Comparing effect of different treatments on total thiol content of skin tissue on day 14.
TTM: total thiol molecules. C-: negative control, C+: positive control, TG5: 5% extract ointment, TG10: 10% extract ointment. Significant difference in comparison to negative control: *: P < 0.05, **: P < 0.01, ***: P < 0.001.
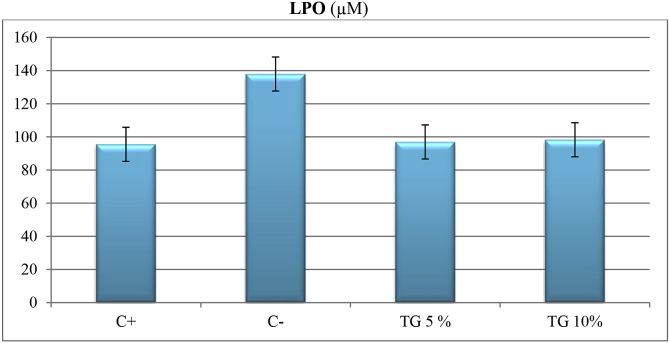
In LPO test, TG5, TG10 and C+ significantly decreased lipid peroxidation in skin tissue compared with negative control (P < 0.01) (Fig. 4).
Fig. 4.
Comparing effect of different treatment on lipid peroxidation in skin tissue on day 14.
LPO: lipid peroxidation, C-: negative control, C+: positive control, TG5: 5% extract ointment, TG10: 10% extract ointment. Significant difference in comparison to negative control: *: P < 0.05, **: P < 0.01, ***: P < 0.001.
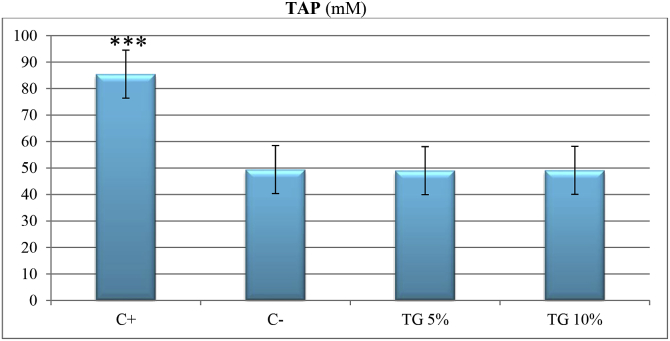
In TAP test, neither TG5 nor TG10 were able to increase total antioxidant capacity of skin tissue (P > 0.05). Positive control significantly increased total antioxidant capacity of skin tissue in comparison to all other groups (P < 0.001) (Fig. 5).
Fig. 5.
Comparison of different treatments on total antioxidant power of skin tissue samples on day 14.
TAP: total antioxidant power, C-: negative control, C+: positive control, TG5: 5% extract ointment, TG10: 10% extract ointment. Significant difference in comparison to negative control: *: P < 0.05, **: P < 0.01, ***:P < 0.001.
4. Discussion
Burn wound healing is a complicated process with several stages which finally results in dermis and epidermis tissue repair. Inflammatory response, occurring in a short time after injury, mainly starts with infiltration of granulocytes or PMN leukocytes and creates free radicals in inflammation site which could damage cells and postpone the healing process.19,20
Topical antibiotics such as silver sulfadiazine are among the drugs used for the management of burn wound; however, finding new drugs with higher efficacy and lower adverse effects is still considered as a priority.21
About 1–3% of modern medicines are specified to wound repair; whereas nearly 30% of preparations in traditional medicine are proposed for skin disorders which indicates high potential of traditional medicine to suggest new drugs for wound care.22,23
Different species of Tragopogon have been used for their astringent, antitussive and antiulcer properties, and demonstrated beneficial effects in wound healing.24,25 T. graminifolius aerial parts and roots have been used in Persian medicine for their anti-inflammatory, antiseptic, anti-hemorrhagic and for skin damages, especially wounds.13,26,27
To the best of our knowledge, there is no previous study on the wound healing properties of T. graminifolius or other species of the genus Tragopogon. In the present study, the wound healing activity of TG extract was assessed on second degree burn for the first time. Considering wound closure percentage, both concentrations of TG topical ointment showed significant healing effect compared with negative control group. In addition, TG 10% showed the best wound closure effect among all assessed treatments. Histological analysis also confirmed wound healing activity of the extract as TG 10% represented the best repair and regeneration in different layers of skin in comparison to the other treatments.
Oxidative stress has an important role in the pathogenesis of several types of tissue damage, including burn wounds and ideal wound healing agents are supposed to show potent antioxidant activity in order to overcome the burst of free radicals produced as the result of tissue damage.28 Despite of no significant change in TAP test, marked alteration in other biomarkers of oxidative stress measured in the skin tissue of wound area, including LPO and TTM, represented in vivo antioxidant activity of TG extract. It is demonstrated that a normal wound healing process depends on a balance between free radicals and antioxidants. Overproduction of free radicals cause delayed and impaired wound healing; thus, antioxidants play a crucial role in healing process of skin injuries.29 Sakarcan et al. (2005) also investigated the role of oxidative stress in animal model of burn wound using systemic Ginkgo biloba L. and demonstrated lower level of lipid peroxidation and improved endogenous antioxidant defense mechanisms; thus, our study is in line with previous reports describing the importance of antioxidant activity for wound healing agents.30
As previously mentioned, TG extract is rich in polyphenolic compounds like gallic acid, p-coumaric acid, ferulic acid, caffeic acid and catechin which have demonstrated antioxidant and anti-inflammatory effects. There are several reports on wound healing effects of polyphenol-rich herbal extracts or their isolated polyphenolic compounds.31 Vafi et al. reported wound healing activity of Hypericum scabrum L. and Lythrum salicaria L. which contained a high amount of flavonoids.32 Another study on Malva sylvestris L. also reported wound healing properties of the plant to be partially attributed to its polyphenolic compounds33 A polyherbal preparation containing M. sylvestris, Rosa damascena Mill., and Solanum nigrum L. which is rich in polyphenolic compounds such as tannins were also effective for the treatment of bur wounds.34Therefore, the healing activity of TG extracts, at least in part, can be attributed to its high polyphenolic content. Future studies on the polyphenol-rich fractions or isolated compounds are needed to confirm this hypothesis.
5. Conclusion
TG aerial parts extract ointment with concentration of 10% had significant beneficial effects on animal second-degree burn wound model in regard to wound closure, histological evaluations and biomarkers of oxidative stress. Thus, the extract can be considered as a future treatment for healing of burn wounds. Future animal studies, as well as clinical trials are essential to demonstrate the safety and efficacy of TG extract as a topical wound healing agent in human.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Singer A.J., Clark R.A.F. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Arturson G. Pathophysiology of the burn wound and pharmacological treatment. The Rudi Hermans lecture. Burns. 1996;22:255–274. doi: 10.1016/0305-4179(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 3.Thomas G.W., Rael L.T., Bar-Or R. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma. 2009;66:82–90. doi: 10.1097/TA.0b013e31818b146d. [DOI] [PubMed] [Google Scholar]
- 4.Lee A.R.C., Leem H., Lee J., Park K.C. Reversal of silver sulfadiazine-impaired wound healing by epidermal growth factor. Biomaterials. 2005;26:4670–4676. doi: 10.1016/j.biomaterials.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 5.Shetty S., Udupa S., Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in rats. Evid Based Complement Altern Med. 2008;5:95–101. doi: 10.1093/ecam/nem004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahramsoltani R., Farzaei M.H., Abdolghaffari A. Evaluation of phytochemicals, antioxidant and burn wound healing activities of Cucurbita moschata Duchesne fruit peel. Iran J Basic Med Sci. 2017;20(7):798–805. doi: 10.22038/IJBMS.2017.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A., Upadhyay N.K., Sawhney R.C., Kumar R. A poly herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair Regen. 2008;16:784–790. doi: 10.1111/j.1524-475X.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- 8.Kroschewsky J.R., Mabry T.J., Markham K.R., Alston R.E. Flavonoids from the genus Tragopogon (compositae) Phytochemistry. 1969;8:1495–1498. [Google Scholar]
- 9.Sareedenchai V., Ganzera M., Ellmerer E., Lohwasser U., Zidorn C. Phenolic compounds from Tragopogon porrifolius L. Biochem Systemat Ecol. 2009;37:234–236. [Google Scholar]
- 10.Kucekova Z., Mlcek J., Humpolicek P., Rop O., Valasek P., Saha P. Phenolic compounds from Allium schoenoprasum, Tragopogon pratensis and Rumex acetosa and their antiproliferative effects. Molecules. 2011;16:9207–9217. doi: 10.3390/molecules16119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farzaei M.H., Rahimi R., Attar F. Chemical composition, antioxidant and antimicrobial activity of essential oil and extracts of Tragopogon graminifolius, a medicinal herb from Iran. Nat Prod Commun. 2014;9(1):121–124. [PubMed] [Google Scholar]
- 12.Farzaei M.H., Ghasemi-Niri S.F., Abdolghafari A.H. Biochemical and histopathological evidence on the beneficial effects of Tragopogon graminifolius in TNBS-induced colitis. Pharm Biol. 2015;53(3):429–436. doi: 10.3109/13880209.2014.923004. [DOI] [PubMed] [Google Scholar]
- 13.Farzaei M.H., Khazaei M., Abbasabadi Z., Feyzmahdavi M., Mohseni G.R. Protective effect of Trogopogon graminifolius DC. against ethanol induced gastric ulcer in rat. Iran Red Crescent Med J. 2013;15(9):813–816. doi: 10.5812/ircmj.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamedi S., Arian A.A., Farzaei M.H. Evaluation of gastroprotective effects of aqueous stem bark extract of Ziziphus jujuba L. against HCl/ethanol-induced gastric mucosal injury in rats. J Tradit Chin Med. 2015;35:666–670. doi: 10.1016/s0254-6272(15)30157-6. [DOI] [PubMed] [Google Scholar]
- 15.Anwar Y., Lowenstein E.J. Eucerin: a revolutionary formulation still going strong for over a century. Skinmed. 2016;14(6):437–439. [PubMed] [Google Scholar]
- 16.Navaei-Nigjeh M., Rahimifard M., Pourkhalili N. Multi-organ protective effects of cerium oxide nanoparticle/selenium in diabetic rats: evidence for more efficiency of nanocerium in comparison to metal form of cerium. Asian J Anim Vet Adv. 2012;7(7):605–612. [Google Scholar]
- 17.Sethi J., Sood S., Seth S., Talwar A. Evaluation of hypoglycemic and antioxidant effect of Ocimum sanctum. Indian J Clin Biochem. 2004;19(2):152–155. doi: 10.1007/BF02894276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdolghaffari A.H., Baghaei A., Moayer F. On the benefit of Teucrium in murine colitis through improvement of toxic inflammatory mediators. Hum Exp Toxicol. 2010;29(4):287–295. doi: 10.1177/0960327110361754. [DOI] [PubMed] [Google Scholar]
- 19.Martin P., Leibovich S.J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 2005;15(11):599–607. doi: 10.1016/j.tcb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Kumar V., Abbas A.K., Fautso N., Mitchell R.N. Elsevier Health sciences; Philadelphia: 2015. Robbin Basic Pathology. [Google Scholar]
- 21.Atiyeh B.S., Costagliola M., Hayek S.N., Dibo S.A. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 2007;33:139–148. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Bahramsoltani R., Farzaei M.H., Rahimi R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: an integrative review. Arch Dermatol Res. 2014;306(7):601–617. doi: 10.1007/s00403-014-1474-6. [DOI] [PubMed] [Google Scholar]
- 23.Kumar B., Vijayakumar M., Govindarajan R. Ethnopharmacological approaches to wound healing-exploring medicinal plants of India. J. Ethnopharmacol. 2007;114(2):103–113. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Guarrera P.M. Food medicine and minor nourishment in the folk traditions of Central Italy. Fitoterapia. 2003;74:515–544. doi: 10.1016/s0367-326x(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 25.Singh K.N., Lal B. Ethnomedicines used against four common ailments by the tribal communities of Lahaul-Spiti in western Himalaya. Journal of Ethnopharmacology. J Ethnopharmacol. 2008;115(1):147–159. doi: 10.1016/j.jep.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Aghili MH, Makhzan-al-Advia. Rahimi R, Ardekani MRS, Farjadmand F. (Eds). Tehran University of Medical Sciences, Tehran, 2009; 700.
- 27.Farzaei M.H., Rahimi R., Abbasabadi Z., Abdollahi M. An evidence-based review on medicinal plants used for the treatment of peptic ulcer in traditional Iranian medicine. Int J Pharmacol. 2013;9:108–124. [Google Scholar]
- 28.Schäfer M., Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008;58(2):165–171. doi: 10.1016/j.phrs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Fitzmaurice S.D., Sivamani R.K., Isseroff R.R. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharmacol Physiol. 2011;24(3):113–126. doi: 10.1159/000322643. [DOI] [PubMed] [Google Scholar]
- 30.Sakarcan A., Sehirli O., Velioglu-Ovünç A. Ginkgo biloba extract improves oxidative organ damage in a rat model of thermal trauma. J Burn Care Rehabil. 2005;26(6):515–524. doi: 10.1097/01.bcr.0000185115.17261.50. [DOI] [PubMed] [Google Scholar]
- 31.Ratz-Łyko A., Arct J., Majewski S., Pytkowska K. Influence of polyphenols on the physiological processes in the skin. Phytother Res. 2015;24(9):509–517. doi: 10.1002/ptr.5289. [DOI] [PubMed] [Google Scholar]
- 32.Vafi F., Bahramsoltani R., Abdollahi M. Burn wound healing activity of Lythrum salicaria L. and Hypericum scabrum L. Wounds. 2016 pii: WNDS20160929–2. [PubMed] [Google Scholar]
- 33.Nasiri E., Hosseinimehr S.J., Azadbakht M., Akbari J., Enayati-Fard R., Azizi S. Effect of Malva sylvestris cream on burn injury and wounds in rats. Avicenna J Phytomed. 2015;5(4):341–354. [PMC free article] [PubMed] [Google Scholar]
- 34.Fahimi S., Abdollahi M., Mortazavi S.A., Hajimehdipoor H., Abdolghaffari A.H., Rezvanfar M.A. Wound healing activity of a traditionally used poly herbal product in a burn wound model in rats. Iran Red Crescent Med J. 2015;17(9) doi: 10.5812/ircmj.19960. e19960. [DOI] [PMC free article] [PubMed] [Google Scholar]