Abstract
Exposure to anthrax leaves susceptible hosts at prolonged risk of infection since spores can persist in vivo for months before germinating to cause life-threatening disease. Anthrax vaccine adsorbed (AVA, the licensed US vaccine) induces immunity too slowly to protect susceptible individuals post-exposure. Antibiotics prevent the proliferation of vegetative bacilli but do not block latent spores from germinating. Thus, anthrax-exposed individuals must remain on antibiotic therapy for months to eliminate the threat posed by delayed spore germination. Unfortunately, long-term antibiotic treatment is poorly tolerated and frequently discontinued. This work explores whether administering a single dose of a long-acting antibiotic (Dalbavancin) combined with a rapidly immunogenic vaccine/adjuvant combination can provide seamless protection from anthrax with minimal patient compliance. Results show that significant protection is achieved by delivering a single dose of this therapeutic combination any time before through 3 days after anthrax exposure.
Keywords: Anthrax, CpG ODN, Vaccine, Dalbavancin, Protection
1. Introduction
Bacillus anthracis is an aerobic gram-positive bacterium found naturally in wild and domesticated animals [1]. Anthrax spores designed for aerosol delivery were released by bioterrorists in 2001, resulting in morbidity, mortality, and widespread panic [2]. Once inhaled by a susceptible host, B. anthracis spores can germinate immediately or remain dormant for months before activating to cause disease [3–5]. Thus, individuals exposed to anthrax require treatment to prevent infection following both immediate and delayed spore germination.
The bioterror attack of 2001 exposed ≈10,000 individuals to anthrax. To prevent disease in this largely unvaccinated population, 2 months of twice daily Ciprofloxacin (a broad spectrum antibiotic) was recommended [6,7]. A majority of the target population failed to complete this course of therapy, largely due to the side effects associated with prolonged oral antibiotic usage [8–10]. This is consistent with a multitude of studies documenting the problems associated with self-medication [11,12], and highlighted the need for a treatment strategy that provided effective protection against anthrax infection with minimal patient compliance [2].
Vaccination provides a useful and cost effective method of reducing susceptibility to anthrax [13]. Anthrax vaccine adsorbed (AVA) is the sole vaccine licensed to prevent human anthrax in the US. It is prepared by adsorbing the culture filtrate of an attenuated toxinogenic non-encapsulated strain of B. anthracis (V770-NP1-R) onto aluminum hydroxide [14]. The protective immune response induced by AVA is primarily mediated by antibodies (Ab) against protective Ag (PA), a critical component of the anthrax toxin. Anti-PA Abs inhibit spore germination, improve the phagocytosis/killing of spores by macrophages, and neutralize the toxin [15–18]. Unfortunately, AVA requires a series of six immunizations over 18 months to elicit and maintain protective anti-PA titers [19]. This schedule induces immunity too slowly to protect individuals recently exposed to anthrax and has been associated with a variety of undesirable side effects [20–23].
Studies in mice, rhesus macaques and humans show that the protection induced by AVA can be accelerated and magnified by the addition of CpG oligonucleotides (ODN) as adjuvants [24–26]. CpG ODN interact with Toll-like receptor 9 expressed by B cells and plasmacytoid dendritic cells [27–30], improving antigen presentation and triggering the production of Th1 and pro-inflammatory chemokines and cytokines (including IFNγ, IL-6, IL-12, IL-18 and TNF[H9251]) [27,28,31,32]. In mice, a single dose of CpG-adjuvanted AVA induces immunity against both aerosol and systemic challenge within 10 days of administration that persists for more than 1 year [25]. However, both AVA and CpG-adjuvanted AVA are ineffective when delivered after anthrax spore challenge, as proliferating bacteria reach toxic levels before protective immunity can develop [25].
Recognizing this problem, Vietri et al. demonstrated that macaques challenged with anthrax were protected if vaccinated with AVA within 2 h of infection and then treated for 2 weeks with oral Ciprofloxacin [33]. Building upon that work, we examined the utility of administering a single dose of the long-acting lipoglycopeptide antibiotic Dalbavancin in conjunction with CpG-adjuvanted AVA. Dalbavancin effectively targets gram-positive bacteria (including anthrax) and has a much longer half life than Ciprofloxacin [34–36]. Thus, a single dose of Dalbavancin can protect an exposed animal for weeks rather than days, providing sufficient time for the co-administered CpG-adjuvanted AVA to induce a durable protective immune response.
2. Materials and methods
2.1. Reagents
Phosphorothioate CpG ODN 1555 (GCTAGACGTTAGCGT) and 1466 (TCAACGTTGA) were synthesized at the Center for Biologics core facility (Bethesda, MD). All ODN were free of endotoxin and protein contamination. Dalbavancin was a kind gift from Pfizer, Inc. AVA was obtained from BioPort Corporation (East Lansing, MI). Recombinant PA (rPA) was provided by USAMRIID (Fort Detrick, MD) and prepared as described [37]. The toxinogenic (pXO1+), non-encapsulated (pXO2−) Sterne vaccine strain spores of B. anthracis were obtained from the culture collection of USARMIID. Spores were prepared and stored as previously described [38].
2.2. Animals
Specific pathogen free female A/J mice were obtained from the NCI (Frederick, MD). They were housed in sterile micro-isolator cages in a barrier environment and studied at 6–12 weeks of age. All animal experiments were conducted using ACUC approved protocols, and challenge studies were performed in a BL-2 facility.
2.3. Immunization and challenge studies
Mice were immunized intraperitoneally (i.p.) with 10 μl of AVA + 20 μg of CpG ODN in a final volume of 100 μl. Mice were treated i.p. with 150 μg of Dalbavancin in 100 μl. The dose of Dalbavancin was selected based on published safety data [34–36] and preliminary survival studies (data not shown). In some experiments, the Dalbavancin was mixed with serum pooled from hyperimmunized A/J mice. This serum had an IgG anti-PA titer >1:400,000. 100 μl of this serum delivered within 24 h of challenge protected 100% of naive mice from anthrax infection. When co-administered, 10 μl of AVA + 20 μg of CpG ODN was combined with 150 μg of Dalbavancin in a final volume of 100 μl of saline or serum.
Mice were challenged i.p. with 30 LD50 of Sterne strain anthrax spores suspended in 0.2 ml of sterile phosphate-buffered saline (PBS) (1 LD50 = 1.1 × 103 spores). Individual animals were vaccinated and/or treated once from 30 days prior to infection through 3 days after infection. Details of each experiment are provided in the figure legends. Survival was monitored for 2–3 weeks. In some cases, mice were re-challenged after 6 weeks with 30 LD50 of Sterne strain anthrax spores.
2.4. Statistics
Differences in survival were evaluated using chi-square analysis of Kaplan–Meier curves.
3. Results
3.1. Dalbavancin protects mice from anthrax infection
Initial studies evaluated whether a single dose of Dalbavancin could protect A/J mice from challenge with Sterne strain anthrax spores. A/J mice were selected for study because these animals (i) are highly susceptible to anthrax challenge due to a defect in their complement cascade and (ii) generate an anti-PA response to AVA ± CpG ODN similar to that of other species, including humans [24,26,39–41]. As previously shown, this animal model provides a rigorous and reproducible measure of vaccine-induced protection following systemic or aerosol challenge [24,25].
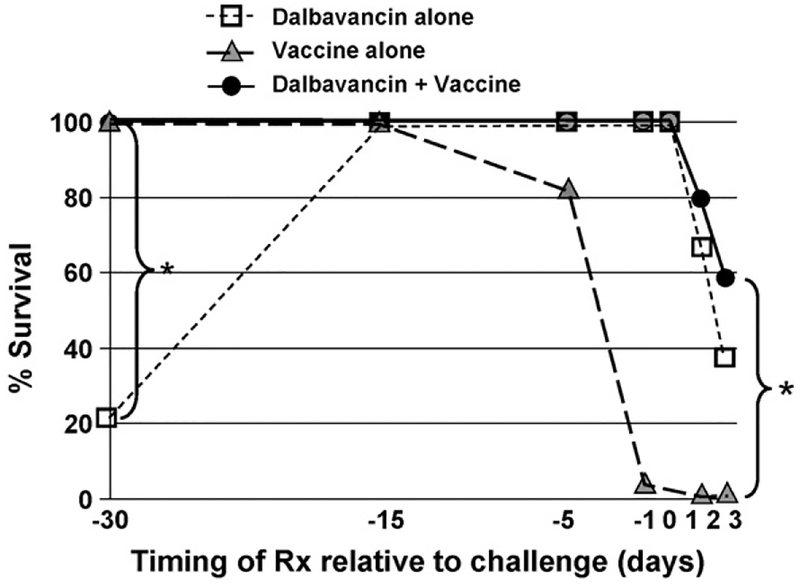
As seen in Fig. 1, 150 μg of Dalbavancin administered from 0 to 15 days prior to challenge protected all mice from 30 LD50 of anthrax. Based on a reported serum half life of ≈60 h in mice [42], this finding suggests that Dalbavancin is protective at a serum concentration consistent with the in vitro MIC90 of 0.25 μg/ml [42]. A single dose of Dalbavancin failed to protect if administered 30 or more days prior to challenge (Fig. 1).
Fig. 1.
Protection against anthrax infection is optimized by adding Dalbavancin to CpG-adjuvanted AVA. A/J mice were challenged with 30 LD50 of Sterne strain anthrax spores on day 0. Animals were treated once i.p. with 150 μg of Dalbavancin (□), 10 μl of AVA + 20 μg of CpG ODN (▲), or both (●) in the period from 30 days before to 3 days after challenge. Survival was monitored for 3 weeks. Data represent the combined results from three independent experiments involving a total of 10–18 mice/group. *p < .02 comparing combination therapy with either single therapy.
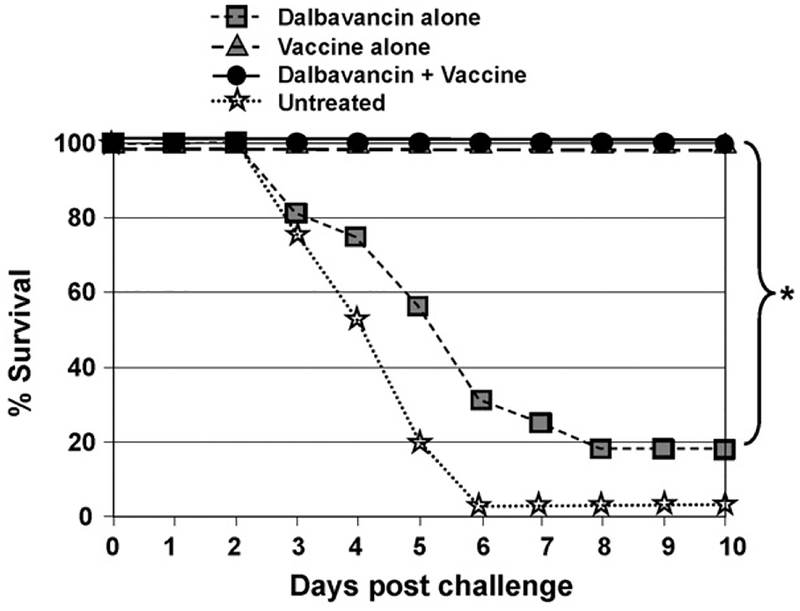
When Dalbavancin was injected from 0 to 24 h after infection, all mice survived (Fig. 1). When delivered 2–3 days after infection, survival significantly exceeded that of challenged control mice (p < .02), although nearly half of the mice still succumbed to infection (Fig. 1). Dalbavancin was ineffective if administered 4 or more days post-challenge, by which time all animals were toxic (a majority of control animals were dead by day 5, see Fig. 2). Animals that survived infection after treatment with Dalbavancin alone were re-challenged 6 weeks later. >80% of these mice succumbed to the second infection, establishing that Dalbavancin did not induce long-lasting immunity (Fig. 2).
Fig. 2.
Vaccination, but not Dalbavancin treatment, induces long-term protection against infection. A/J mice were treated and challenged as described in Fig. 1. Surviving mice were re-challenged 6 weeks later with 30 LD50 of Sterne strain anthrax spores. Data represent the combined results from three independent experiments involving a total of 16–18 mice/group.*p < .01.
3.2. Seamless protection is provided when Dalbavancin is co-administered with CpG-adjuvanted AVA
Previous reports established that mice vaccinated with CpG-adjuvanted AVA developed protective immunity much faster than animals vaccinated with AVA alone [24,26]. Consistent with those results, the adjuvanted vaccine induced partial protection within 5 days of administration and complete protection by day 10 (Fig. 1). Vaccinated animals remained resistant to infection when re-challenged many weeks later, consistent with recent evidence that a single dose of CpG-adjuvanted AVA provides protection for >1 year (Fig. 2) [43]. As expected, mice vaccinated less than 5 days prior to challenge (or at any time post-challenge) died from infection, as the proliferating bacilli reached toxic levels before protective immunity could develop (Fig. 1) [24,26].
To examine whether antibiotic therapy could prevent disease during the period before vaccine-induced immunity was achieved, mice were immunized with CpG-adjuvanted AVA and treated 1 h later with Dalbavancin. As seen in Fig. 1, this therapeutic strategy resulted in seamless protection against anthrax when the combination was administered any time before through 1 day after challenge (p < .001). If treatment was delayed until 2–3 days after challenge, survival was significantly improved when compared to controls, with less than a third of animals dying from a 30 LD30 spore challenge (p < .02, Fig. 1). The protection provided by this combination therapy persisted when animals were re-challenged 6 weeks later (p < .01, Fig. 2). Thus, the combination of CpG-adjuvanted AVA plus Dalbavancin provided the long-term benefits of vaccine-induced immunity coupled with the short term protection mediated by antibiotic therapy.
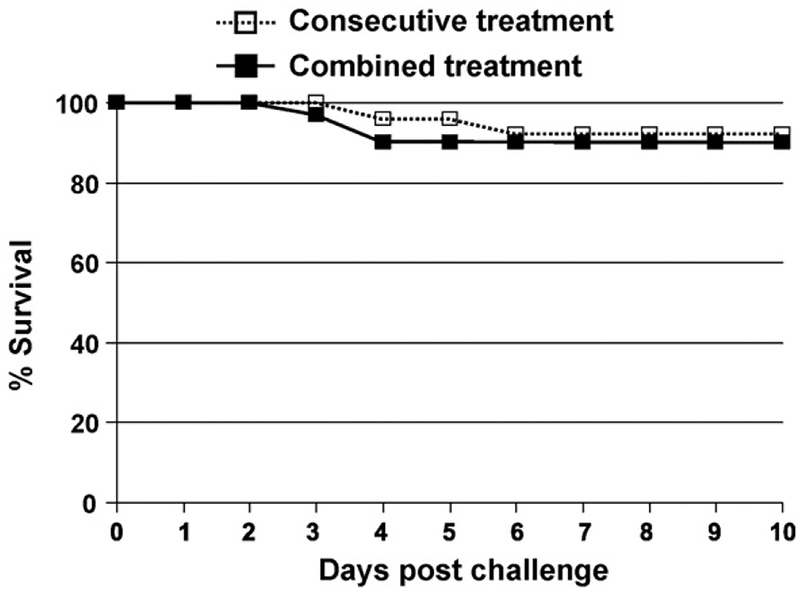
Subsequent studies established that these two agents were effective when combined and delivered simultaneously in a single syringe. As seen in Fig. 3, a one-time injection of vaccine plus Dalbavancin provided the same level of protection as serial injections of these two agents.
Fig. 3.
Dalbavancin and CpG-adjuvanted AVA can be co-administered in a single syringe. A/J mice were challenged with 30 LD50 of Sterne strain anthrax spores on day 0. Animals were treated i.p. with 10 μl of AVA + 20 μg of CpG ODN anytime from 15 days before through 2 days after challenge. 150 μg of Dalbavancin was co-administered with vaccine in the same syringe (■) or delivered 30 min later in a separate syringe (□). Survival was monitored for 3 weeks post-challenge. Results show the combined survival of all mice in each treatment group (N = 25/treatment from two independent experiments).
3.3. Anti-PA Abs do not improve the protection provided by Dalbavancin plus CpG-adjuvanted AVA
The combination of CpG-adjuvanted AVA plus Dalbavancin did not protect mice with advanced disease (i.e., those treated >3 days after challenge with 30 LD50 of anthrax). This was not surprising, since antibiotic therapy is rarely successful in animals with toxic septicemia [44].
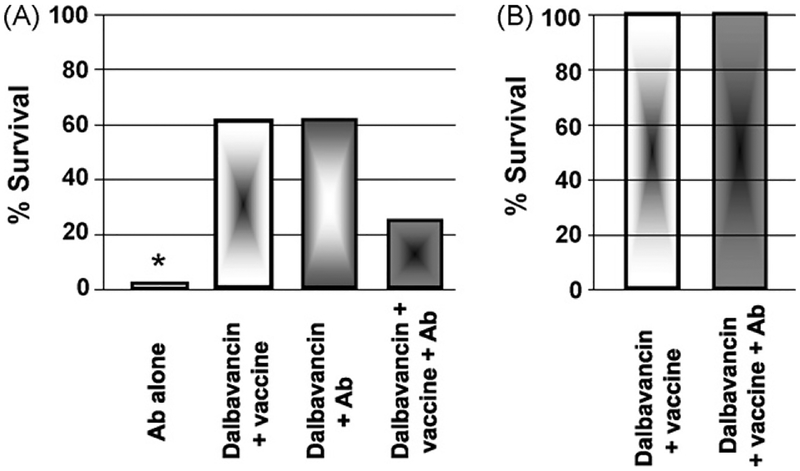
While high-titered anti-PA Abs are protective when administered within 1 day of challenge [44,45], some have speculated that administering toxin neutralizing Abs might improve survival late in the infectious process [40,46–48]. To test this hypothesis, A/J mice were challenged with 30 LD50 of Sterne strain anthrax and treatment initiated 3 days later with high titered IgG anti-PA antiserum (alone or mixed with Dalbavancin CpG-adjuvanted vaccine). As seen in Fig. 4A, antibody treatment ± alone had no effect on survival when initiated on day 3 post challenge. Abs also had no significant effect on the protection provided by the combination of Dalbavancin + vaccine when administered either before or after infection (Fig. 4A and B).
Fig. 4.
Anti-PA Abs do not improve the protection conferred by Dalbavancin plus CpG-adjuvanted AVA. (A) A/J mice were challenged with 30 LD50 of Sterne strain anthrax spores. 3 days later, they were treated with 150 μg of Dalbavancin plus CpG-adjuvanted AVA and/or 100 μl of high-titered anti-PA antiserum. This quantity of antiserum was protective if administered within 24 h of challenge. (B) A/J mice were immunized with CpG-adjuvanted AVA plus Dalbavancin alone or combined with 100 μl of high-titered anti-PA antiserum. These mice were challenged 1 month later with 30 LD50 of Sterne strain anthrax spores. The percent improvement in survival vs. controls is shown (N = 8–10 mice/group). *p < .01 vs. animals treated with Dalbavancin.
4. Discussion
The mortality, morbidity and panic caused by the release of anthrax spores in 2001 established the utility of this pathogen as a weapon of bioterror. It also established the need for a simple and rapid treatment that could reduce the immediate and long term threat posed by anthrax exposure with minimal patient compliance. Current results suggest that administering a single dose of CpG-adjuvanted AVA combined with Dalbavancin achieves these goals.
AVA requires six immunizations delivered over 18 months to induce and maintain protective Ab titers in humans. This immunization regimen is associated with deleterious side effects including joint pain, gastrointestinal disorders and pneumonia [20–22]. Previous studies in mice, macaques and humans demonstrate that protective Ab titers are induced more rapidly and maintained for longer periods when immunostimulatory CpG ODN are added to AVA [24–26,41,43]. CpG ODN trigger cells that express TLR9, inducing the functional maturation of professional APCs and the generation of immune responses characterized by increased Ab secretion and the production of pro-inflammatory and Th1 cytokines/chemokines [28,31,32,49–51]. Of particular importance, CpG-adjuvanted AVA induces protective immunity within 10 days of administration (significantly faster than AVA alone [24,25]). Current findings show that a single dose of the long-acting antibiotic Dalbavancin protects against anthrax for up to 15 days (Fig. 1). Thus, combining Dalbavancin with CpG-adjuvanted AVA provided seamless protection against both the immediate and long-term threat posed by anthrax infection (Fig. 1).
Immunizing individuals exposed to anthrax and then treating them with antibiotics is not a novel idea. Indeed, a recent report by Vietri et al. described the benefits of this strategy in macaques challenged with 1000 LD50 of anthrax [33]. They vaccinated half of the animals with AVA, and treated all of the animals twice daily with oral Ciprofloxacin starting 2 h after exposure. While 100% of the macaques that were vaccinated and treated with antibiotics survived, 5/9 of the unvaccinated macaques succumbed to infection once antibiotic therapy was discontinued. These findings provided an important proof of principle, but the approach described by Vietri et al. failed to (1) utilize the best available antibiotic/vaccine regimen, (2) evaluate the effect of vaccine alone on infection, (3) examine the persistence of protective immunity, or (4) monitor the effect of therapy initiated days rather than hours after anthrax challenge (it required several days to diagnose and treat most individuals involved in the 2001 bioterror attacks [9]).
Building upon the strategy of Vietri et al., we substituted CpG-adjuvanted AVA for AVA, since animal and human studies show that the adjuvanted vaccine induces protective immunity significantly faster than AVA alone [24,25]. In addition, we substituted a single dose of the long acting injectable lipoglycopeptide antibiotic Dalbavancin for twice daily oral Ciprofloxacin, thereby eliminating the need for continued patient compliance and eliminating the side effects associated with prolonged oral antibiotic usage. All studies were conducted in a well established murine model that enabled different treatment regimens to be evaluated at multiple time points relative to challenge [52]. This A/J mouse model provides immunogenicity results predictive of the response of other species (including humans) to vaccination and yields protection data relevant to both systemic and aerosol anthrax spore challenge [24–26,40,52].
The combination of CpG-adjuvanted AVA plus Dalbavancin significantly improved survival when delivered up to 3 days post-challenge (Fig. 1). However efficacy was lost if treatment was delayed until the mice became toxic (on day 4). Some speculate that administering toxin neutralizing Abs might be of benefit late in the disease process [44,45]. While anti-PA Abs are protective when administered within 24 h of challenge, we found no report of efficacy at later time points [40,46–48]. As seen in Fig. 4, anti-PA Abs did not enhance the survival of septic animals, nor did they magnify or extend the protection provided by CpG-adjuvanted AVA plus Dalbavancin. It thus appears that anti-PA Abs, which are costly to produce and require i.v. administration, represent a suboptimal approach to countering the threat posed by anthrax. In contrast, Dalbavancin combined with CpG-adjuvanted AVA significantly reduced the risk from both immediate and delayed germination of anthrax spores. Further evaluation of the safety and utility of this single-dose combination therapy for individuals exposed to (or at high risk of exposure) to anthrax appears warranted.
Acknowledgments
The assertions herein are the private ones of the authors and are not to be construed as official or as reflecting the views of DTRA or the NCI at large. Support for this work was provided in part by the Joint Science and Technology Office for Chemical and Biological Defense of the Defense Threat Reduction Agency. The authors thank Pfizer Global Medical Business Operations, Groton, CT, for kindly providing the Dalbavancin pure substance used in these studies, and Drs. Constance Rothermel and Howard Young for their helpful suggestions.
Abbreviations:
- AVA
anthrax vaccine adsorbed
- Ab
antibody
- ODN
oligodeoxynucleotide
- Ag
antigen
- PA
protective antigen
- APC
antigen presenting cell
- LD50
50% lethal dose
References
- [1].Hanna P Anthrax pathogenesis and host response. Curr Top Microbiol Immunol 1998;225:13–35. [DOI] [PubMed] [Google Scholar]
- [2].Lane HC, Montagne JL, Fauci AS. Bioterrorism: a clear and present danger. Nat Med 2001;7(December (12)):1271–3. [DOI] [PubMed] [Google Scholar]
- [3].Ross JM. The pathogenesis of anthrax following the administration of spores by the respiratory route. J Pathol Bacteriol 1957;73:485–94. [Google Scholar]
- [4].Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J Hyg 1956;54:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Glassman HN. Industrial inhalation anthrax: discussion. Bateriol Rev 1966;30:657–9. [Google Scholar]
- [6].Occupational health guidelines for remediation workers at Bacillus anthracis-contaminated sites—United States, 2001–2002. MMWR Morb Mortal Wkly Rep 2002;51(September (35)):786–9. [PubMed] [Google Scholar]
- [7].Stern EJ, Uhde KB, Shadomy SV, Messonnier N. Conference report on public health and clinical guidelines for anthrax. Emerg Infect Dis 2008;14(April (4)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Meropol SB, Chan KA, Chen Z, Finkelstein JA, Hennessy S, Lautenbach E, et al. Adverse events associated with prolonged antibiotic use. Pharmacoepidemiol Drug Saf 2008;17(May (5)):523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jefferds MD, Laserson K, Fry AM, Roy S, Hayslett J, Grummer-Strawn L, et al. Adherence to antimicrobial inhalational anthrax prophylaxis among postal workers, Washington, DC, 2001. Emerg Infect Dis 2002;8(October (10)):1138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shepard CW, Soriano-Gabarro M, Zell ER, Hayslett J, Lukacs S, Goldstein S, et al. Antimicrobial postexposure prophylaxis for anthrax: adverse events and adherence. Emerg Infect Dis 2002;8(October (10)):1124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique. J Am Med Assoc 1989;261(June (22)):3273–7. [PubMed] [Google Scholar]
- [12].Harter JG, Peck CC, Chronobiology. Suggestions for integrating it into drug development. Ann NY Acad Sci 1991;618:563–71. [DOI] [PubMed] [Google Scholar]
- [13].Friedlander AM, Brachman PS. Anthrax In: Plotkin SA, Mortimer EA, editors. Vaccines. Philadelphia: PA, W.B. Saunders; 1998. pp. 729–39. [Google Scholar]
- [14].Ivins BE, Welkos SL. Recent advances in the development of an improved, human anthrax vaccine. Eur J Epidemiol 1988;4(March (1)):12–9. [DOI] [PubMed] [Google Scholar]
- [15].Chowdhury K, Light SE, Garon CF, Ito Y, Israel MA. A cloned polyoma DNA fragment representing the 5′ half of the early gene region is oncogenic. J Virol 1980;36(November (2)):566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Little SF, Ivins BE. Molecular pathogenesis of Bacillus anthracis infection. Microb Infect 1999;1(February (2)):131–9. [DOI] [PubMed] [Google Scholar]
- [17].Welkos S, Little S, Friedlander A, Fritz D, Fellows P. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 2001;147(June (Pt 6)): 1677–85. [DOI] [PubMed] [Google Scholar]
- [18].Welkos SL, Friedlander AM. Comparative safety and efficacy against Bacillus anthracis of protective antigen and live vaccines in mice. Microb Pathog 1988;5(August (2)):127–39. [DOI] [PubMed] [Google Scholar]
- [19].Pittman PR, Gibbs PH, Cannon TL, Friedlander AM. Anthrax vaccine: short-term safety experience in humans. Vaccine 2001;20(December (5–6)):972–8. [DOI] [PubMed] [Google Scholar]
- [20].Brachman PS, Gold H, Plotkin SA, Fedety FR, Werrin M, Ingraham NR. Field evaluation of a human anthrax vaccine. Am J Public Health 1962;52:632–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ready T US soldiers refuse to fall in line with anthrax vaccination scheme. Nat Med 2004;10(February (2)):112. [DOI] [PubMed] [Google Scholar]
- [22].Geier DA, Geier MR. Anthrax vaccination and joint related adverse reactions in light of biological warfare scenarios. Clin Exp Rheumatol 2002;20(March (2)):217–20. [PubMed] [Google Scholar]
- [23].Lange JL, Lesikar SE, Rubertone MV, Brundage JF. Comprehensive systematic surveillance for adverse effects of anthrax vaccine adsorbed, US Armed Forces, 1998–2000. Vaccine 2003;21(April (15)):1620–8. [DOI] [PubMed] [Google Scholar]
- [24].Xie H, Gursel I, Ivins BE, Singh M, O’Hagan DT, Ulmer JB, et al. CpG oligodeoxynucleotides adsorbed onto polylactide-co-glycolide microparticles improve the immunogenicity and protective activity of the licensed anthrax vaccine. Infect Immun 2005;73(February (2)):828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Klinman DM, Currie D, Lee G, Grippe V, Merkel T. Systemic but not mucosal immunity induced by AVA prevents inhalational anthrax. Microb Infect 2007;9(October (12–13)):1478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Klinman DM, Xie H, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the licensed anthrax vaccine. Ann NY Acad Sci 2006;1082(October):137–50. [DOI] [PubMed] [Google Scholar]
- [27].Krieg AM, Yi A, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995;374: 546–8. [DOI] [PubMed] [Google Scholar]
- [28].Klinman DM, Yi A, Beaucage SL, Conover J, Krieg AM. CpG motifs expressed by bacterial DNA rapidly induce lymphocytes to secrete IL-6, IL-12 and IFNg. Proc Natl Acad Sci USA 1996;93:2879–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Takeshita F, Leifer CA, Gursel I, Ishii K, Takeshita S, Gursel M, et al. Cutting edge: role of toll-like receptor 9 in CpG DNA-induced activation of human cells. J Immunol 2001;167(7):3555–8. [DOI] [PubMed] [Google Scholar]
- [30].Hemmi H, Takeuchi O, Kawai T, Sato S, Sanjo H, Matsumoto M, et al. A toll-like receptor recognizes bacterial DNA. Nature 2000;408:740–5. [DOI] [PubMed] [Google Scholar]
- [31].Ballas ZD, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol 1996;157:1840–7. [PubMed] [Google Scholar]
- [32].Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-gamma production by stimulation of IL-12 and tumor necrosis factor-alpha. Cell Immunol 1996;167:72–8. [DOI] [PubMed] [Google Scholar]
- [33].Vietri NJ, Purcell BK, Lawler JV, Leffel EK, Rico P, Gamble CS, et al. Short-course postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalational anthrax. Proc Natl Acad Sci USA 2006;103(May (20)):7813–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Goldstein EJ, Citron DM, Merriam CV, Warren Y, Tyrrell K, Fernandez HT. In vitro activities of dalbavancin and nine comparator agents against anaerobic gram-positive species and corynebacteria. Antimicrob Agents Chemother 2003;47(June (6)):1968–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Streit JM, Fritsche TR, Sader HS, Jones RN. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn Microbiol Infect Dis 2004;48(February (2)):137–43. [DOI] [PubMed] [Google Scholar]
- [36].Leighton A, Gottlieb AB, Dorr MB, Jabes D, Mosconi G, VanSaders C, et al. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother 2004;48(March (3)):940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ivins BE, Pitt ML, Fellows PF, Farchaus JW, Benner GE, Waag DM, et al. Comparative efficacy of experimental anthrax vaccine candidates against inhalation anthrax in rhesus macaques. Vaccine 1998;16(July (11–12)):1141–8. [DOI] [PubMed] [Google Scholar]
- [38].Ivins BE, Welkos SL, Knudson GB, Little SF. Immunization against anthrax with aromatic compound-dependent (Aro-) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect Immun 1990;58(February> (2)):303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Harvill ET, Lee G, Grippe VK, Merkel TJ. Complement depletion renders C57BL/6 mice sensitive to the Bacillus anthracis Sterne strain. Infect Immun 2005;73(July (7)):4420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Klinman DM, Xie H, Little SF, Currie D, Ivins BE. CpG oligonucleotides improve the protective immune response induced by the anthrax vaccination of rhesus macaques. Vaccine 2004;22(July (21–22)):2881–6. [DOI] [PubMed] [Google Scholar]
- [41].Rynkiewicz D, Rathkopf M, Ransom J, et al. Marked enhancement of antibody response to anthrax vaccine adsorbed with CpG 7909 in healthy volunteers. ICAAC; abstract LB-25, 2005. [DOI] [PubMed] [Google Scholar]
- [42].Heine HS, Bassett J, Miller L. In vitro and in vivo activity of dalbavancin against Bacillus anthracis. In: 45th Interscience Conference on Antimicrobial agents 2005. (abstract A-1435). [Google Scholar]
- [43].Tross D, Klinman DM. Effect of CpG oligonucleotides on vaccine-induced B cell memory. J Immunol 2008;181(August (8)):5785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Baillie LW. Past, imminent and future human medical countermeasures for anthrax. J Appl Microbiol 2006;101(September (3)):594–606. [DOI] [PubMed] [Google Scholar]
- [45].Casadevall A Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg Infect Dis 2002;8(August (8)):833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kelly CD, O’Loughlin C, Gelder FB, Peterson JW, Sower LE, Cirino NM. Rapid generation of an anthrax immunotherapeutic from goats using a novel nontoxic muramyl dipeptide adjuvant. J Immune Based Ther Vaccines 2007;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vitale L, Blanset D, Lowy I, O’Neill T, Goldstein J, Little SF, et al. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect Immun 2006;74(October 10):5840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Herrmann JE, Wang S, Zhang C, Panchal RG, Bavari S, Lyons CR, et al. Passive immunotherapy of Bacillus anthracis pulmonary infection in mice with antisera produced by DNA immunization. Vaccine 2006;24(July (31–32)):5872–80. [DOI] [PubMed] [Google Scholar]
- [49].Klinman DM. Adjuvant activity of CpG oligodeoxynucleotides. Int Rev Immunol 2006;25(May (3–4)):135–54. [DOI] [PubMed] [Google Scholar]
- [50].Sparwasser T, Koch E, Vabulas RM, Heeg K, Lipford GB, Ellwart JW, et al. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol 1998;28(6):2045–54. [DOI] [PubMed] [Google Scholar]
- [51].Gursel M, Verthelyi D, Klinman DM. CpG oligodeoxynucleotides induce human monocytes to mature into functional dendritic cells. Eur J Immunol 2002;32(September (9)):2617–22. [DOI] [PubMed] [Google Scholar]
- [52].Loving CL, Kennett M, Lee GM, Grippe VK, Merkel TJ. Murine aerosol challenge model of anthrax. Infect Immun 2007;75(June (6)):2689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]