Abstract
Rationale
Although short sleep duration has been linked to unhealthy dietary patterns, little is known about the association of obstructive sleep apnea (OSA), a disorder characterized by sleep fragmentation and diet.
Study Objectives
Investigate associations between diet quality and OSA in the Multi-Ethnic Study of Atherosclerosis and assess whether reductions in slow-wave sleep (stage N3) and rapid eye movement (REM) sleep are potential mediators for these associations.
Methods
A diverse population (N = 1813) completed a food frequency questionnaire and underwent Type 2 in-home polysomnography, which included measurement of N3 and REM sleep and apnea–hypopnea index (AHI). Moderate-to-more severe OSA was defined as having an AHI > 15 events/hr.
Results
Participants were 53.9% female with a mean age of 68.3 (SD 9.1) years. Approximately 33.8% were categorized as having moderate-to-more severe OSA. In adjusted analyses, OSA was associated with lower intakes of whole grains, (β = −0.200, SE = 0.072, p < 0.01), higher intakes of red/processed meat, (β = −0.440, SE = 0.136, p < 0.01), and lower overall diet quality (β = −1.286, SE = 0.535, p = 0.02). Stage N3 sleep partially explained the associations between red/processed meat and overall diet quality score with OSA.
Conclusions
Moderate-to-more severe OSA is associated with a less healthy dietary profile that is partially explained by reduced N3 sleep. These findings suggest the opportunity to target sleep quality in interventions aimed at improving cardio-metabolic risk factors in patients with OSA.
Keywords: OSA, diet quality, slow-wave sleep, Alternative Health Eating Index
Statement of Significance
This paper investigates the associations between obstructive sleep apnea (OSA) and diet quality, characterized by the Alternative Healthy Eating Index-2010, and assesses sleep architecture, such as slow-wave sleep and rapid eye movement, as potential mediators of the association between OSA and diet quality. The study shows that OSA is associated with dietary intake of red/processed meat, whole grains, and overall diet quality and that reduced slow-wave sleep partially explains these associations. These findings raise the important possibility that by focusing on improving sleep quality, it may be possible to break the cycle of obesity–OSA–obesity and further suggests the need to address sleep quality as a way of improving OSA/CVD risk factors.
Introduction
There is a growing literature linking sleep quality and duration to nutrient intake [1]. Individuals with insomnia, poor sleep quality, or curtailed sleep report unhealthy diets. For example, compared with individuals who report good sleep quality, those who report poor sleep quality report lower intakes of vegetables and fish and higher intakes of confectionary, sugar-sweetened beverages, energy drinks, and carbohydrates as well as more frequently report skipping breakfast and eating irregularly [2, 3]. Curtailed sleep or short sleep duration are also associated with high intakes of fatty foods, carbohydrates, total calories, and snacks [4–8]. Mechanisms linking insufficient sleep with dietary intake include hedonic and hormonal factors [9]. A period of restricted sleep has been shown to enhance activation of hedonic stimulus processing in the brain, particularly in the insular cortex, orbitofrontal cortex, and dorsolateral prefrontal cortex [10–13]. Poor sleep quality and short sleep duration have also been found to affect hunger and satiety hormones, ghrelin and leptin, which are implicated in a causal association between insufficient sleep and increased propensity to consume food [14–17].
Sleep quality and duration may not only reflect the impact of behavioral factors influencing sleep, but also may reflect underlying sleep disorders, which have not been systematically studied in relation to diet. In particular, obstructive sleep apnea (OSA), a condition characterized by intermittent upper airway obstruction [18], causes sleep fragmentation as well as exposure to intermittent hypoxemia, factors that trigger sympathetic nervous system activation, insulin resistance, and other neurohumoral responses [19, 20]. The Multi-Ethnic Study of Atherosclerosis (MESA) provides an opportunity to characterize the association between OSA and dietary intake. This diverse cohort allows for the examination of objective sleep measures and dietary quality using the Alternative Healthy Eating Index – 2010 (AHEI-10), a validated tool used to assess conformity based on Dietary Guidelines [21]. We hypothesized that OSA, via effects on sleep architecture, would be associated with less healthy dietary patterns.
Methods
Study design
The Multi-Ethnic Study of Atherosclerosis is a longitudinal assessment of risk factors for the incidence and progression of cardiovascular disease in a multiethnic cohort of adults. The study was comprised of a population-based sample of 6814 men and women aged 45–84 years who were free of clinically recognized cardiovascular disease at baseline. Participants were studied every 2 years with in-clinic examinations, starting in July 2000, as described elsewhere [22]. The current analyses used data from the MESA Exam 5 and an associated MESA Sleep ancillary study.
Sleep measures
Between 2010 and 2013, participants who reported not regularly using oral devices, nocturnal oxygen, or nightly positive airway pressure devices were invited to participate in the MESA Sleep Ancillary Study. Of the 4077 potentially eligible individuals participating in Exam 5, 2261 MESA participants agreed to participate in the sleep study [23]. As described before, compared with the overall MESA cohort, participants in the sleep exam were somewhat more likely to be white, older, smokers, and to have hypertension but had a comparable prevalence of self-reported doctor-diagnosed sleep apnea [23]. Participants underwent in-home polysomnography (PSG) using a 15-channel monitor. The sleep data were centrally scored [23] and provided quantitative levels of overnight hypoxemia, arousal indices, apneas and hypopneas, and sleep architecture which included sleep stage distributions and total sleep time, as described elsewhere [24].The primary exposure variable was the apnea–hypopnea index (AHI), which was calculated as the average number of all apneas plus hypopneas associated with a 4% desaturation per hour of sleep [25]. Moderate-to-more severe OSA was defined as an AHI > 15 [24]. Short sleep duration (<6 hr/night) and long sleep duration (>9 hr/night) [26] were derived from the PSG assessment of total sleep time. Sleep staging was conducted following published guidelines by staff with high scorer reliability [27, 28]. Slow-wave sleep (stage N3) was defined as the percentage and absolute duration of total sleep time spent in stage N3 sleep. Rapid eye movement (REM) was defined as the percentage and total duration of total sleep time spent in stage REM sleep.
Dietary measures
Participants attending Exam 5 (2010–2012) completed a detailed multiple-choice food frequency questionnaire (FFQ) [21] that assessed the participant’s usual dietary intake over the past year. Dietary intake from the FFQ was then used to assess the dietary quality based on the AHEI-10 food group component and matrix score. The AHEI-10 uses a dietary scoring system that has been shown to predict risk of chronic diseases, such as cardiovascular disease [29–35]. The nutrient and food group components used to calculate the AHEI-10 were calculated at the MESA Coordinating Center blinded to other data. Briefly, the AHEI-10 represents the sum of 11 individual food components, each scored based on assessing the FFQ food group line item per day and then recalculated based on 1000 calories (kcals) of energy intake and the individual food components: vegetables, fruit, whole grains, sugar-sweetened beverages and fruit juices, nuts and legumes, red/processed meat, trans fat, long-chain fats, polyunsaturated fatty acids, sodium, and alcohol. Each food component is given a score that ranges from 0 to 10, with 10 considered the healthiest and summed for a total score [29, 30]. The following components: sugar-sweetened beverages and fruit juices, red/processed meat, trans fat, and sodium were reversed scored, meaning lower intake of these components received higher scores. The moderate category of alcohol intake received the maximum score. Those with too high or too low daily energy intake (>5000 or <500 kcal/day) were considered outliers and incomplete dietary questionnaires were excluded from the analysis [36].
Covariates
At the clinic examination, height, weight, waist circumference, and hip circumference were directly measured using standardized approaches, and sociodemographic and comorbidity data were obtained from questionnaires as described previously [22]. Age, sex, race/ethnicity, education level, income, diabetes, and physical activity were considered as potential confounders and were included in fully adjusted models. Age was assessed as a continuous variable. Race/ethnicity was categorized into four categories: non-Hispanic white, black, Hispanic, and Chinese. Education level was obtained using an 8-level scale and was further classified into the following four categories: less than high school, high school or graduate education diploma (GED), some college, and college degree or higher. Income was defined as the total gross family income. It was categorized into four categories: <$25000, $25–49999, $50–74999, and ≥$75000. Diabetes status was categorized into a dichotomous yes/no variable based on having a fasting glucose ≥126 mg/dL, taking insulin, or oral diabetes medication. Physical activity was derived from the Typical Week Physical Activity Survey (TWPAS), a validated questionnaire [37], that assesses daily activities associated with different physical intensity levels as well as the number of times this activity was performed and the average amount of time spent per day. Physical activity was categorized into three categories: low physical activity (<33% quartile), moderate physical activity (33%–67% quartile), and high physical activity (>67% quartile), using levels of moderate intensity physical activity, expressed as MET-min/day of energy expenditure [38].
Statistical analysis
Multivariate linear regression analysis was used to estimate the associations of OSA with the overall AHEI-10 score as well as its individual components (i.e. the diet scores were considered as dependent variables). Covariate adjustments included age, sex, race/ethnicity, education, and income. We also adjusted for diabetes given that it is associated with OSA and may influence dietary choices, and physical activity, as a proxy for general health habits associated with both diet and OSA [39]. Secondary analyses also adjusted for sleep duration and considered AHI as a continuous variable. Mediation analysis was used to assess stages N3 and REM sleep as potential mediators of the association between OSA (dichotomized) or AHI (continuously measured) and diet scores. Mediated effects were estimated using the method of Baron and Kenny, which estimates the indirect (mediated) effects as the difference between the total and direct effects, based on hypothesized causal associations [40] The point estimates and 95% confidence intervals for these estimates were calculated using resampling methods described by Mackinnon et al. [41]. All statistical analyses were conducted with SAS 9.4 statistical software.
Results with two-sided p < 0.05 were considered statistically significant.
Results
A total of 1813 individuals had both acceptable PSG studies and complete food intake data. The sample had a mean age of 68.3 years (standard deviation [SD] = 9.1 years); 37.8% were non-Hispanic white with the remaining black (27.5%), Hispanic (23.5%), and Chinese (11.2%); 53.9% were female. Approximately 33.8% of the study sample met the criterion for moderate-to-more severe OSA (AHI > 15). Individuals with OSA were more likely to be male, spend fewer minutes in N3 and REM sleep, and have shorter sleep duration, higher BMI, and greater waist circumference and waist-to-hip ratio relative to those without moderate-to-more severe OSA, all p < 0.01 (Table 1).
Table 1.
Study population characteristics by OSA (N = 1813), MESA Study 2010–2013
| Characteristic | With moderate-to-severe OSA (AHI > 15; n = 613) mean (SD) or number (%) | Without moderate-to-severe OSA (AHI ≤ 15) (n = 1200) mean (SD) or number (%) | P* |
|---|---|---|---|
| Age, year | 68.8 ± 8.9 | 68.1 ± 9.2 | 0.11 |
| Race | 0.02 | ||
| Non-Hispanic white | 210 (34.3%) | 476 (39.7%) | |
| Black | 161 (26.2%) | 338 (28.1%) | |
| Hispanic | 161 (26.3%) | 264 (22.0%) | |
| Chinese | 81 (13.2%) | 122 (10.2%) | |
| Sex | <0.01 | ||
| Male | 381 (62.1%) | 455 (37.9%) | |
| Female | 232 (37.9%) | 745 (62.1%) | |
| Education | 0.12 | ||
| Less than high school | 96 (15.7%) | 279 (23.8%) | |
| High school/GED | 93 (15.2%) | 338 (28.8%) | |
| Some college but no degree | 164 (26.7%) | 214 (18.3%) | |
| College degree or higher | 260 (42.4%) | 341 (29.1%) | |
| Income | 0.12 | ||
| <$25000 | 172 (28.6%) | 279 (23.8%) | |
| $25–49999 | 150 (24.9%) | 338 (28.8%) | |
| $50–74999 | 108 (17.9%) | 214 (18.3%) | |
| ≥$75000 | 172 (28.6%) | 341 (29.1%) | |
| Sleep duration | |||
| Short sleep (<6 hr per night) | 218 (35.6%) | 334 (27.8%) | <0.01 |
| Long sleep (>9 hr per night) | 12 (2.0%) | 18 (1.5%) | 0.47 |
| Smoking status | 0.12 | ||
| Current smoker | 33 (5.5%) | 88 (7.4%) | |
| Former/never | 573 (94.5%) | 1104 (92.6%) | |
| Physical activity, hours per day | <0.01 | ||
| Low | 227 (37.1%) | 335 (27.9%) | |
| Moderate | 189 (30.8%) | 417 (34.8%) | |
| High | 197 (32.1%) | 448 (37.3%) | |
| Body mass index, kg/m2 | 30.67 ± 5.94 | 27.78 ± 5.08 | <0.01 |
| Waist–hip ratio, cm | 0.962 ± 0.066 | 0.926 ± 0.090 | <0.01 |
| Alcohol use (currently drink alcohol) | 0.67 | ||
| Yes | 277 (45.3%) | 530 (44.2%) | |
| No | 335 (54.7%) | 669 (55.8%) | |
| Diabetes | <0.01 | ||
| Yes | 155 (25.5%) | 187 (15.7%) | |
| No | 452 (74.5%) | 1001 (84.3%) | |
| Time in N3 sleep, min | 29.46 ± 29.55 | 40.90 ± 34.76 | <0.01 |
| Time in REM sleep, min | 58.28 ± 29.29 | 71.00 ± 29.18 | <0.01 |
| Time in N3 sleep, % | 8.26 ± 7.97 | 10.97 ± 9.15 | <0.01 |
| Time in REM sleep, % | 16.29 ± 7.02 | 18.97 ± 6.29 | <0.01 |
*Chi-square or ANOVA tests for categorical or continuous variables (respectively).
In unadjusted analyses, moderate-to-more severe OSA was associated with a lower total AHEI-10 score (β = −2.278, SE = 0.521, p < 0.01), lower intake of fruits (β = −0.354, SE = 0.153, p = 0.021) and whole grains (β = −0.274, SE = 0.069, p < 0.01), and higher intake of red/processed meats (β = −0.543, SE = 0.133, p < 0.01), sugar-sweetened beverages and fruit juices (β = −0.433, SE = 0.196, p = 0.027), trans fat (β = −0.068, SE = 0.033, p = 0.038), and sodium (β = −0.521, SE = 0.159, p < 0.01), compared with those without moderate-to-more severe OSA.
In adjusted models, moderate-to-more severe OSA was associated with lower intakes of whole grains (β = −0.200, SE = 0.072, p < 0.01) and total AHEI-10 scores (β = −1.286, SE = 0.535, p = 0.02), and higher intakes of red/processed meat (β = −0.440, SE = 0.136, p < 0.01) (Table 2). These associations persisted when adjusting for both short and long sleep durations (long sleep duration not shown). Associations of OSA with fruits, sugar-sweetened beverages and fruit juices, trans fat, and sodium were no longer significant after adjusting for demographics, socioeconomic status, diabetes, and physical activity.
Table 2.
Differences in the overall AHEI-10 score and its individual dietary components according to OSA status (AHI > 15 vs. AHI ≤ 15) using multivariate linear regression, MESA Study 2010–2013
| Moderate-to-severe OSA (AHI > 15) vs. without moderate-to-severe OSA (AHI ≤ 15) | ||||||
|---|---|---|---|---|---|---|
| Model 1, n = 1813 | Model 2, n = 1752 | Model 3, n = 1752 | ||||
| β (SE) | P | β (SE) | P | β (SE) | P | |
| Vegetable score | −0.12 (0.14) | 0.38 | 0.047 (0.14) | 0.74 | 0.042 (0.14) | 0.77 |
| Fruit score | −0.35 (0.15) | 0.021 | −0.092 (0.16) | 0.56 | −0.087 (0.16) | 0.58 |
| Whole grain score | −0.27 (0.069) | <0.001 | −0.20 (0.072) | 0.0059 | −0.20 (0.073) | 0.0055 |
| Beverage score | −0.43 (0.20) | 0.027 | −0.39 (0.20) | 0.055 | −0.38 (0.20) | 0.064 |
| Nuts and legumes score | −0.20 (0.17) | 0.25 | −0.12 (0.18) | 0.52 | −0.17 (0.18) | 0.56 |
| Red/processed meat score | −0.54 (0.13) | <0.001 | −0.44 (0.14) | 0.0013 | −0.43 (0.14) | 0.0017 |
| Trans fat score | −0.068 (0.033) | 0.038 | −0.022 (0.034) | 0.52 | −0.022 (0.034) | 0.53 |
| Long-chain fats score | 0.20 (0.11) | 0.084 | 0.22 (0.12) | 0.061 | 0.21 (0.12) | 0.077 |
| Polyunsaturated fatty acids score | −0.10 (0.15) | 0.49 | −0.047 (0.15) | 0.76 | −0.052 (0.15) | 0.74 |
| Sodium score | −0.52 (0.16) | 0.0011 | −0.30 (0.16) | 0.063 | −0.29 (0.16) | 0.071 |
| Alcohol score | 0.14 (0.14) | 0.32 | 0.057 (0.14) | 0.69 | 0.062 (0.14) | 0.66 |
| Total AHEI-10 score | −2.28 (0.52) | <0.001 | −1.29 (0.54) | 0.016 | −1.26 (0.54) | 0.019 |
Model 1: Unadjusted; Model 2: Adjusted for age, sex, race, education, income, diabetes, and physical activity; Model 3: Model 2 + short sleep duration.
Bold p-values represent statistical significance at the 0.05 level.
Similar associations were seen when modeling AHI as a continuous variable (Supplementary Table 1). Of note, the association between higher intake of sugar-sweetened beverages and fruit juices and AHI level remained significant in adjusted models using AHI as a continuous variable, whereas it was no longer significant when AHI was used as a dichotomous variable.
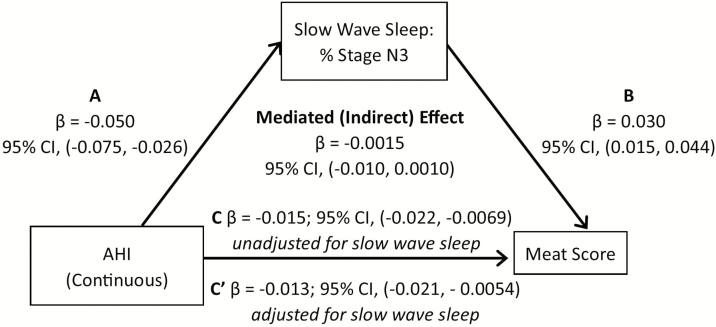
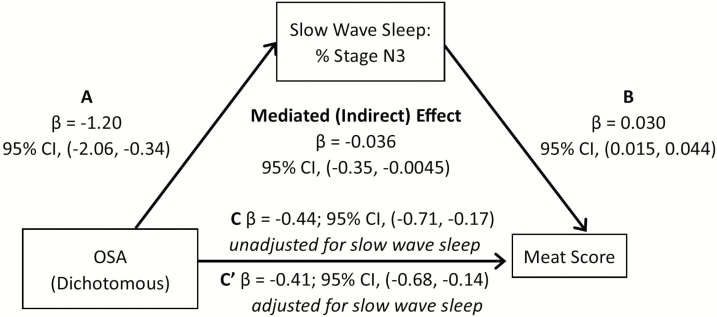
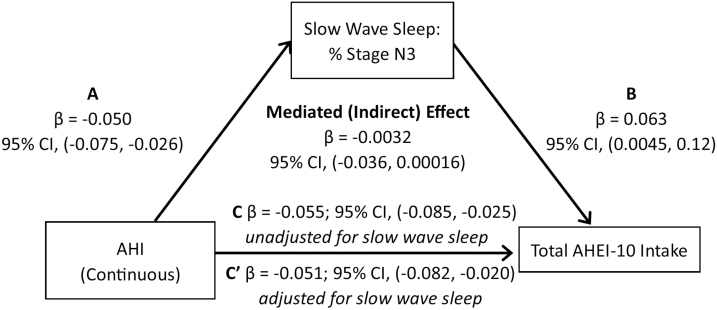
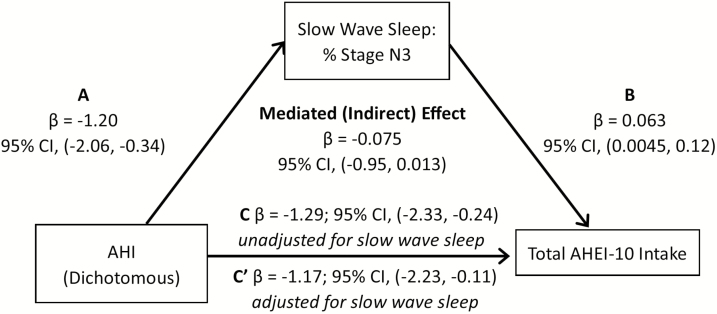
In the mediation analysis, we first examined whether duration of stage N3 and REM sleep mediated the association between AHI level and red/processed meat intake, when controlling for sex, race/ethnicity, education, income, age, physical activity, and diabetes. Percent sleep time spent in N3 sleep explained 9.25% of the association between AHI and red/processed meat score (95% CI: −0.021, −0.0054; Figure 1). A similar pattern was observed when OSA was based on a dichotomous definition (AHI > 15) (Figure 2). We also examined whether N3 and REM sleep duration explained the association between AHI and total AHEI-10 score. Our mediation analysis suggested that percent time in N3 sleep explained 7.4% of the association between AHI and overall diet score (Figure 3), with similar associations observed for OSA described as a binary variable (9.3% of the variation explained by N3; Figure 4). If we modeled N3 as absolute N3 sleep time in minutes rather than as a percentage of total sleep, N3 sleep explained 9.4% and 9.9% of the association between AHI and red/processed meat and total AHEI-10 score, respectively.
Figure 1.
Mediation Analysis: N3 as a mediator in the association of AHI or OSA with red/processed meat intake, MESA Study 2010–2013. (A–C) Mediation analysis demonstrating that the association of sleep apnea (modeled either as continuously or dichotomously expressed AHI) on meat score is partially mediated by slow-wave sleep. Sleep apnea is associated with both (A) N3 sleep and (C) meat score. (B) N3 sleep is also strongly associated with meat score. The mediated (indirect) effect is the total effect (pathway C) minus the direct effect (pathway C′) or the difference in the point estimate of sleep apnea on meat score without and with adjustment for N3 sleep.
Figure 2.
Mediation Analysis: N3 as a mediator in the association of AHI or OSA with red/processed meat intake, MESA Study 2010–2013. (A–C) Mediation analysis demonstrating that the association of sleep apnea (modeled either as continuously or dichotomously expressed AHI) on meat score is partially mediated by slow-wave sleep. Sleep apnea is associated with both (A) N3 sleep and (C) meat score. (B) N3 sleep is also strongly associated with meat score. The mediated (indirect) effect is the total effect (pathway C) minus the direct effect (pathway C′) or the difference in the point estimate of sleep apnea on meat score without and with adjustment for N3 sleep.
Figure 3.
Mediation analysis: N3 as a mediator in the association of AHI or OSA with total AHEI-10 intake, MESA Study 2010–2013. A-C, Mediation analysis demonstrating that the association of sleep apnea (modeled either as continuously or dichotomously expressed AHI) on total AHEI score is partially mediated by slow-wave sleep. Sleep apnea is associated with both (A) N3 sleep and (C) total AHEI score. (B) N3 sleep is also strongly associated with total AHEI score. The mediated effect is the total effect (pathway C) minus the direct effect (pathway C′) or the difference in the point estimate of sleep apnea on total AHEI score without and with adjustment for N3 sleep.
Figure 4.
Mediation analysis: N3 as a mediator in the association of AHI or OSA with total AHEI-10 intake, MESA Study 2010–2013. A-C, Mediation analysis demonstrating that the association of sleep apnea (modeled either as continuously or dichotomously expressed AHI) on total AHEI score is partially mediated by slow-wave sleep. Sleep apnea is associated with both (A) N3 sleep and (C) total AHEI score. (B) N3 sleep is also strongly associated with total AHEI score. The mediated effect is the total effect (pathway C) minus the direct effect (pathway C′) or the difference in the point estimate of sleep apnea on total AHEI score without and with adjustment for N3 sleep.
The mediation models did not identify significant explanatory associations for N3 sleep in the association between AHI and whole grain intake. REM sleep duration was not a significant explanatory variable between AHI and any measure of dietary intake.
Since the association between diet quality and risk of chronic diseases, such as cardiovascular disease, is mediated by adiposity [42], we also explored the consistency of our models after additionally adjusting for waist-to-hip ratio (Supplementary Table 2). When adjusting for waist-to-hip ratio, our results did not substantively change and OSA remained significantly associated with lower intake of whole grains (β = −0.815, SE = 0.075, p = 0.01) and total AHEI-10 score (β = −1.084, SE = 0.536, p = 0.04), and higher intake of red/processed meat (β = −0.431, SE = 0.137, p < 0.01). This suggests that adiposity did not entirely explain the association, despite its correlation with both sleep and dietary quality.
Discussion
This study provides novel data linking dietary quality with OSA. Compared with adults without moderate-to-more severe OSA, we found that individuals with moderate-to-more severe OSA had significantly poorer AHEI-10 total scores, due to lower whole grain intake and increased red/processed meat consumption. These associations persisted after adjusting for age, sex, race/ethnicity, education level, income, diabetes, and physical activity. Moreover, these findings were independent of sleep duration but were partially explained by reductions in stage N3 sleep, a sleep state associated with generally high parasympathetic tone, low sympathetic tone, and less fragmented sleep. These are the first data to the best of our knowledge that identify that a common sleep disorder (OSA) is associated with an unhealthy diet and suggest that reduced N3 sleep partially explains this association.
A growing literature indicates that adhering to the recommendation set forth in the Dietary Guidelines for Americans is associated with a lower risk of developing chronic diseases [21]. The recommendations show that adhering to a high consumption of high nutrient dense foods and low consumption of low density foods decreases the risk of developing cardiovascular disease and other major chronic diseases. The combination of low whole grain and high red/processed meat consumption is associated with numerous adverse health outcomes, such as cardiovascular disease, type-2 diabetes, and all-cause mortality [43–45].
Prior studies have shown associations between poor dietary habits and short sleep duration. A study of 410 female youths in Iran showed those who slept less than 6 hr per day were more likely to consume a lower amount of whole grains compared with those who slept more than 6 hr per night [46]. Individuals with symptoms of insomnia report lower intakes of vegetables and higher intakes of trans fat, sodium, and total energy consumption than individuals without symptoms of insomnia [2]. Several mechanisms have been invoked to explain this association between insufficient sleep and unhealthy eating, including dysregulation of appetite hormones and changes in central nervous system responses to food stimuli [9–13]. However, prior research has not examined the association between OSA and dietary intake.
Adjusted analyses showed that the associations with OSA were significant for total AHEI-10 score and scores for red/processed meats and whole grains. Although unadjusted analyses also showed associations with trans fat and sodium with OSA, those associations were attenuated after adjusting for age, sex, race/ethnicity, education level, income, diabetes, and physical activity. The clinical relevance of these associations is supported by the literature linking red/processed meat and whole grains with chronic diseases and mortality [43–45]. To the best of our knowledge, no study has previously reported an association between OSA and consumption of low whole grains and increased red/processed meat intake.
In adjusted analyses relating AHI and diet, we also observed associations with increasing AHI levels and higher consumption of sugar-sweetened beverages. Sugar-sweetened beverages have been identified as a significant contributor to rising rates of obesity [47]. Therefore, it is plausible that there are reinforcing mechanisms relating sleep apnea, high-caloric diets, and obesity.
Our study suggests that an important link between OSA and unhealthy diet may be through reduced N3 sleep. N3 sleep has long been recognized to play an important role in neurobehavioral functioning, including memory consolidation [48] and cognition [49]. In addition, N3 sleep significantly modulates a number of endocrine functions, including the hypothalamic–pituitary–adrenocortical system and growth hormone and prolactin release [50]. Experimental studies show that selective reductions in N3 sleep cause abnormalities in glucose tolerance and insulin secretion [51, 52] consistent with a causal role in the development of diabetes. Epidemiological studies have also shown that independent of sleep duration, decreased N3 sleep is associated with increased adiposity [53]. Reduced N3 sleep has been found to be associated with poor diet intake in a small experimental study of young adults. In that study, percentage of time spent in N3 sleep was inversely associated with fat and carbohydrate intake [54].
Reductions in N3 sleep occur commonly with aging and secondary to sleep fragmentation. Individuals with OSA experience repetitive respiratory disturbances and arousals, which increase time in Stage N1 sleep and reduce time in N3 and prolong the latency to the first epoch of N3 and REM sleep compared with those without sleep apnea [55]. In our study, N3 sleep was 25%–28% lower in individuals with OSA compared with others. A formal mediation analysis showed that N3 sleep duration explained approximately 6% to 9% of the association between AHI and red/processed meat score and total AHEI-10 score. These associations suggest the importance of poor sleep quality as a contributor to unhealthy eating patterns in individuals with OSA, and possibly secondarily, as a risk factor for obesity and cardiovascular disease in those patients.
In contrast to N3 sleep, we found no statistical evidence to support a potential mediatory role of reduced REM sleep linking OSA and dietary pattern. Although REM sleep is the sleep state associated with the highest sympathetic tone, which we postulated may associate with dietary patterns, there is little evidence that REM sleep patterns are strongly associated with endocrine function or appetite.
We carefully considered the challenge of covariate adjustment and adjusted for factors that a priori we considered to be common risk factors for diet and OSA. For example, sociodemographic factors are associated with both diet and OSA [23]. Prior analyses showed that increased physical activity was associated with OSA [56] and since physical activity may be a marker of other lifestyle factors, we also included this in our adjusted model. Diabetes was included as individuals with diabetes may alter their diet. Although we were reluctant to adjust for obesity (which may be an outcome of poor diet) due to concerns over introducing collider bias, in exploratory analysis inclusion of waist–hip ratio in exploratory analysis did not alter the results (Supplementary Table 2). Short sleep duration was adjusted for given its association with diet; however, its inclusion (models 3) did not appreciably influence the findings.
There are several clinical implications to our results that should be tested in future interventions. OSA is associated with obesity and weight loss may lead to significant improvements in the AHI [57]. However, behavioral interventions to improve energy balance have had limited success [58–60] and in fact, even with CPAP treatment, patients with OSA commonly gain weight [61]. OSA is associated with an increased prevalence of cardiovascular disease [62–64]. Therefore, it is important to identify modifiable factors that influence diet quality or otherwise influence cardiovascular risk factors in individuals with OSA. Diets characterized by increased intake of high-quality foods, such as whole grains, and decreased low-quality foods, such as red/processed meat, are ideal in reducing cardiovascular risk factors and other chronic diseases. The results of this analysis suggest that efforts to improve sleep quality, particularly N3 sleep duration, in OSA may be a potentially useful adjunctive strategy for addressing the chronic health problems associated with OSA.
Strengths of this study include the racial/ethnic diversity of the participants, relatively large sample size, and the use of rigorously collected objective sleep and standardized diet measures. The use of calculating dietary intake using the AHEI-10 guidelines corresponded to analyzing OSA, a chronic debilitating disease. Authors were blinded to the diet and sleep scoring.
The study also had several limitations. Although we used a well-validated dietary scoring tool, misclassification is possible and information on timing of meals was not available. As described earlier, we selected an a priori list of covariates in our adjusted model; nonetheless, we cannot exclude the possibility of unmeasured confounding, including those that may affect N3 sleep duration. We examined both total AHEI-10 score and its components, and it is possible that by examining multiple outcomes, we increase the likelihood of falsely rejecting the null hypothesis. Although less than 60% of the eligible participants participated in the sleep ancillary study, it is possible but unlikely that selection bias influenced the observed associations between sleep and diet [23]. The cross-sectional design limits causal inference and assessment of mediation [65]. A meta-analysis of data from the Nurses’ Health Study, the Nurses’ Health Study II, and the Health Professionals Follow-up Study showed that those who consume more unprocessed red meats and processed meats and consumed less whole grains experienced shorter sleep duration and increased weight gain [66], consistent with a causal association between insufficient sleep and obesogenic behaviors. It is plausible that diet could influence N3 sleep (rather than the reverse). Supporting this hypothesis, a prior study of 10 healthy young males, randomized to either a high-carbohydrate dinner or high-fat dinner, found those who ingested a high-carbohydrate dinner spent significantly less time in N3 sleep compared with those who are a high-fat dinner [67]. This causal direction raises the provocative idea that an unhealthy diet may predispose to OSA via effects on reducing N3, the sleep stage when breathing is typically most stable (and AHI is lowest).
Conclusion
This study provides novel evidence that sleep apnea is associated with dietary quality, with associations strongest for consumption of low whole grains, high red/processed meat, and overall low AHEI-10 score, and that the latter associations may be partially explained by reduction in N3 sleep. Although current interventions aim to reduce apnea and hypopnea frequency, our study suggests that additional efforts to improve sleep architecture may help improve the cardiovascular risk factor profile of patients with OSA. However, longitudinal studies and clinical trials are needed to clarify causal pathways and identify the effect of changes in N3 sleep on diet patterns in patients with OSA.
Supplementary Material
Supplementary material is available at SLEEP online.
Acknowledgments
We thank the other investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
MESA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001881, and DK06349. Funding support for the Sleep Polysomnography dataset was provided by grant HL56984. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Dr. Susan Redline is partly funded through the NHLBI (1R35 HL135818-01). Drs. Johnson-Morgan and Redline reports grants from NIH, during the conduct of this study. Dr. Shea reports grants from NHLBI, during the conduct of the study. Dr. St-Onge reports grants from NHLBI and the American Heart Association Go Red for Women Strategically Focused Research Network during the conduct of this study.
References
- 1. Grandner MA, et al. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite. 2013;64:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng FW, et al. Probable insomnia is associated with future total energy intake and diet quality in men. Am J Clin Nutr. 2016;104(2):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katagiri R, et al. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J Occup Health. 2014;56(5):359–368. [DOI] [PubMed] [Google Scholar]
- 4. Broussard JL, et al. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity (Silver Spring). 2016;24(1):132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nedeltcheva AV, et al. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spaeth AM, et al. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr. 2014;100(2):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. St-Onge MP, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94(2):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nishiura C, et al. Dietary patterns only partially explain the effect of short sleep duration on the incidence of obesity. Sleep. 2010;33(6):753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaput JP, et al. Increased food intake by insufficient sleep in humans: are we jumping the gun on the hormonal explanation?Front Endocrinol (Lausanne). 2014;5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. St-Onge MP, et al. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes (Lond). 2014;38(3):411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chaput JP. Sleep patterns, diet quality and energy balance. Physiol Behav. 2014;134:86–91. [DOI] [PubMed] [Google Scholar]
- 12. St-Onge MP. The role of sleep in the control of feeding behavior. In Watson, R, ed. Modulation of Sleep by Obesity, Diabetes, Age, and Diet. San Diego, CA: Elsevier; 2014. [Google Scholar]
- 13. St-Onge MP, et al. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95(4):818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. St-Onge MP, et al. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35(11):1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. St-Onge MP, et al. Sleep disturbances, body fat distribution, food intake and/or energy expenditure: pathophysiological aspects. Horm Mol Biol Clin Investig. 2014;17(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shechter A, et al. The role of sleep in the control of food intake. Am J Lifestyle Med. 2014;8(6):371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taheri S, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greenstone M, et al. Obstructive sleep apnoea. BMJ. 2014;348:g3745. [DOI] [PubMed] [Google Scholar]
- 19. Canessa N, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183(10):1419–1426. [DOI] [PubMed] [Google Scholar]
- 20. Engleman HM, et al. Sleep. 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chiuve SE, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bild DE, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 23. Chen X, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2015;38(6):877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flemons WW, Buysse D, Redline S, et al. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 25. Ruehland WR, et al. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32(2):150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watson NF, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Redline S, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3(2):169–200. [PubMed] [Google Scholar]
- 28. Silber MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121–131. [PubMed] [Google Scholar]
- 29. Feskanich D, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–796. [DOI] [PubMed] [Google Scholar]
- 30. Liu K, et al. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation. 2012;125(8):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belin RJ, et al. Diet quality and the risk of cardiovascular disease: the Women’s Health Initiative (WHI). Am J Clin Nutr. 2011;94(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fung TT, et al. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr. 2006;136(2):466–472. [DOI] [PubMed] [Google Scholar]
- 33. Fung TT, et al. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care. 2007;30(7):1753–1757. [DOI] [PubMed] [Google Scholar]
- 34. McCullough ML, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–1271. [DOI] [PubMed] [Google Scholar]
- 35. Reedy J, et al. Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2008;168(1):38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shah RV, et al. Diet and adipose tissue distributions: the Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2016;26(3):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ainsworth BE, et al. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–813. [DOI] [PubMed] [Google Scholar]
- 38. LaMonte MJ, et al. Physical activity, physical fitness, and Framingham 10-year risk score: the cross-cultural activity participation study. J Cardiopulm Rehabil. 2001;21(2):63–70. [DOI] [PubMed] [Google Scholar]
- 39. Reutrakul S, et al. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baron RM, et al. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 41. MacKinnon DP, et al. Distribution of the product confidence limits for the indirect effect: program PRODCLIN. Behav Res Methods. 2007;39(3):384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Frazier-Wood AC, et al. In cross-sectional observations, dietary quality is not associated with CVD risk in women; in men the positive association is accounted for by BMI. Br J Nutr. 2015;113(8):1244–1253. [DOI] [PubMed] [Google Scholar]
- 43. Aune D, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Larsson SC, et al. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol. 2014;179(3):282–289. [DOI] [PubMed] [Google Scholar]
- 45. Schwingshackl L, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haghighatdoost F, et al. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition. 2012;28(11-12):1146–1150. [DOI] [PubMed] [Google Scholar]
- 47. Malik VS, et al. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84(2):274–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Walker MP. The role of slow wave sleep in memory processing. J Clin Sleep Med. 2009;5(2 Suppl):S20–S26. [PMC free article] [PubMed] [Google Scholar]
- 49. Ferrara M, et al. Selective slow-wave sleep deprivation and time-of-night effects on cognitive performance upon awakening. Psychophysiology. 2000;37(4):440–446. [PubMed] [Google Scholar]
- 50. Van Cauter E, et al. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9 (Suppl 1):S23–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tasali E, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herzog N, et al. Selective slow wave sleep but not rapid eye movement sleep suppression impairs morning glucose tolerance in healthy men. Psychoneuroendocrinology. 2013;38(10):2075–2082. [DOI] [PubMed] [Google Scholar]
- 53. Rao MN, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group Association between sleep architecture and measures of body composition. Sleep. 2009;32(4):483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shechter A, et al. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303(9):R883–R889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ratnavadivel R, et al. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5(6):519–524. [PMC free article] [PubMed] [Google Scholar]
- 56. Billings ME, et al. Neighborhood walking environment and activity level are associated with OSA: the multi-ethnic study of atherosclerosis. Chest. 2016;150(5):1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peppard PE, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. [DOI] [PubMed] [Google Scholar]
- 58. Ammerman AS, et al. The efficacy of behavioral interventions to modify dietary fat and fruit and vegetable intake: a review of the evidence. Prev Med. 2002;35(1):25–41. [DOI] [PubMed] [Google Scholar]
- 59. Jeffery RW, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19(1S):5–16. [DOI] [PubMed] [Google Scholar]
- 60. Jeffery RW, et al. Strengthening behavioral interventions for weight loss: a randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61(6):1038–1045. [DOI] [PubMed] [Google Scholar]
- 61. Quan SF, et al. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. J Clin Sleep Med. 2013;9(10):989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharma N, et al. Obesity, cardiovascular disease and sleep disorders: insights into the rising epidemic. J Sleep Disord Ther. 2017;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ayas NT, et al. Cardiovascular consequences of obstructive sleep apnea. Curr Opin Cardiol. 2016;31(6):599–605. [DOI] [PubMed] [Google Scholar]
- 64. Wang X, et al. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;169(3):207–214. [DOI] [PubMed] [Google Scholar]
- 65. Maxwell SE, et al. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. 2007;12(1):23–44. [DOI] [PubMed] [Google Scholar]
- 66. Mozaffarian D, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yajima K, et al. Effects of nutrient composition of dinner on sleep architecture and energy metabolism during sleep. J Nutr Sci Vitaminol (Tokyo). 2014;60(2):114–121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.