Abstract.
Globally, pneumonia is the leading cause of death among children younger than 5 years old, with most deaths occurring in low-income countries. Rapid bedside tools to assist practitioners to accurately triage and risk-stratify these patients may improve clinical care and patient outcomes. We conducted a prospective cohort study of children with pneumonia admitted to two Ugandan hospitals to examine the predictive value of a single point-of-care lactate measurement using a commercially available handheld device, the Lactate Scout Analyzer. One hundred and fifty-five children were included, 90 (58%) male, with a median (interquartile range [IQR]) age of 11 (1.4–20) months. One hundred and twenty-five (81%) patients had chest indrawing, 133 (86%) were hypoxemic, and 75 (68%) had a chest x-ray abnormality. In-hospital mortality was 22/155 (14%). Median (IQR) admission lactate level was 2.4 (1.8–3.6) mmol/L among children who survived versus 7.2 (2.6–9.7) mmol/L among those who died (P < 0.001). Lactate was a better prognostic marker of mortality (area under receiver operator characteristic 0.76, 95% confidence interval: 0.69–0.87, P ≤ 0.001), than any single clinical sign or composite clinical risk score. Lactate level at admission of < 2.0, 2.0–4.0, and > 4.0 mmol/L accurately risk-stratified children, with 5-day mortality of 2%, 11% and 26%, respectively (P < 0.001). Slow lactate clearance also predicted subsequent mortality in children with repeated lactate measurements. Hand-held lactate measurement is a clinically informative and convenient tool in low-resource settings for triage and risk stratification of pediatric pneumonia.
Introduction
Pneumonia is the leading cause of death among children less than 5 years old.1 In 2015, an estimated 120 million cases of childhood pneumonia occurred globally, with an estimated 922,000 deaths.1 Most of these deaths occurred in resource-limited countries in sub-Saharan Africa and South Asia.2,3
Rapid and accurate triage of children with pneumonia is important for allocation of resources to patients at greatest risk of adverse outcomes.4,5 Currently, an integrated community case management (iCCM) program endorsed by the World Health Organization (WHO) employs community health workers (CHWs) to assess, triage, and treat pneumonia in rural and semi-urban settings based on clinical symptoms such as fast-breathing. This strategy increases access to antibiotic treatment in a timely fashion in these resource-limited settings. Community health workers also use signs such as altered consciousness and chest-wall indrawing to risk-stratify pneumonia severity, and make decisions about transferring patients for higher levels of care.6–8 Additional tools may assist CHWs in decision-making for children with pneumonia. Prognostic tools may be equally useful in the hospital setting, to assist trained health practitioners in assigning patients to emergency or intensive care units. In research studies, composite risk scores based on numerous clinical measurements are often used to assess disease severity and prognosis; however, these are complex, time-consuming, and require a well-trained and meticulous clinician.
Lactate, a product of anaerobic metabolism, may be used as an indicator of perfusion status and illness severity.9 Serum lactate is elevated in cases of tissue hypoperfusion and hypoxia due to different etiologies such as sepsis, shock, cardiac arrest, and trauma.9 Previous studies have demonstrated that elevated serum lactate is associated with an increased risk of patient mortality in these conditions.9–14 Recent studies have also shown lactate to be a valuable tool for predicting the risk of mortality in adult pneumonia patients.10,15–17 The use of point-of-care lactate measurement for rapid prognosis in pediatric pneumonia patients in low-resource setting, however, has not previously been reported.
We investigated the prognostic value of point-of-care lactate in a resource-limited hospital setting and compared its prognostic value to several composite clinical risk scores in children admitted to hospital with pneumonia.
Materials and Methods
We conducted a prospective cohort study of children younger than 5 years admitted to two hospitals in Uganda with clinical signs of pneumonia consistent with the WHO Integrated Management of Childhood Illness definition (cough or difficulty breathing plus tachypnea or chest indrawing).8 Children were excluded if there was a clinical suspicion of tuberculosis.
Study area and population.
This study was conducted at the Jinja Regional Referral Hospital and Kambuga District Hospital in Uganda. Uganda ranks 163rd of 188 countries in terms of the human development index18 and has a gross domestic product per capita of $660 USD.19 Uganda has an under-five mortality of 135/1,000 live births13 and pneumonia accounts for 15% of child deaths in this area, making it the leading cause of pediatric death.20
Study procedures.
For each child admitted to the hospital, information was collected regarding the child’s demographics, presenting signs and symptoms, vital signs, physical examination findings, results of laboratory tests, treatments during admission, and outcome (survival or death). Lactate was measured at admission and daily during hospitalization using a point-of-care device, the Lactate Scout Analyzer (Sports Resource Group, Inc., Minneapolis, MN). The Lactate Scout Analyzer takes < 10 seconds to measure serum lactate.21 The following clinical risk scores were also calculated for each patient: Fluid as Expansive Supportive Therapy Pediatric Emergency Triage (FEAST PET), Bedside Pediatric Emergency Warning System (PEWS), Pediatric Early Death Index for Africa (PEDIA), Lambarene Organ Dysfunction Score (LODS), Signs of Inflammation in Children that can Kill (SICK), and the Respiratory Index of Severity in Children (RISC).22–27 Fluid as Expansive Supportive Therapy Pediatric Emergency Triage (range 0–10) was calculated by the weighted sum of the following clinical variables: temperature ≤ 37°C (+1); heart rate < 80 minutes−1 (+2) or ≥ 80 to < 105 minutes−1 (+1) or ≥ 220 minutes−1 (+2); capillary refill time ≥ 2 seconds (+1); conscious level prostrate (+1) or coma (+2); severe pallor (+1); respiratory distress (+1); lung crepitations (+1); and weak pulse (+1).22 Pediatric Early Warning System (PEWS) (score range 0–26) was calculated by the weighted sum of the following clinical variables: heart rate, systolic blood pressure, capillary refill, pulses, bolus fluids, and respiratory rate. Weightings for PEWS variables were age-dependent and ranged from 0 to 4.23 Pediatric Early Death Index for Africa (score range 0–7) was calculated by the weighted sum of the following clinical variables: jaundice (+1); subcostal indrawing (+1); prostration with seizures (+1); prostration without seizures (+3); altered consciousness with seizures (+2); altered consciousness without seizures (+3); wasting (+1); and kwashiorkor (+1).24 Lambarene Organ Dysfunction Score (score range 0–3) was calculated by the sum of the following clinical variables: coma (+1), prostration (+1), and deep breathing (+1).25 Signs of Inflammation in Children that can Kill (score range 0–9.8) was calculated by the weighted sum of the following clinical variables: age < 1 month (+2.2) or < 12 months (+1.0) or < 5 years (+0.3); temperature > 38°C or < 36°C (+1.2); heart rate > 160 minutes−1 for infants or > 150 minutes−1 for children (+0.2); respiratory rate > 60 minutes−1 for infants or > 50 minutes−1 for children (+0.4); systolic blood pressure < 65 mmHg for infants or < 75 mmHg for children (+1.2); oxygen saturation < 90% (+1.4); capillary refill time > 3 seconds (+1.2); and level of consciousness less than “alert” (+2.0).26 Respiratory Index of Severity in Children (score range 0–6) was calculated by the weighted sum of the following clinical variables: oxygen saturation ≤ 90% (+3) or chest indrawing (+2); wheezing (−2) points; refusal to feed (+1); and WHO weight for age z-score ≤ −3 (+2) or −3 < z ≤ −2 (+1).27 Children were treated according to WHO guidelines.19 The definition of lactate clearance was adapted from a recent study by Aramburo et al.28 Slow lactate clearance among patients with elevated lactate at baseline (≥ 2.5 mmol/L) was defined as failure to normalize lactate and clearance half-life of ≥ 10.8 hours (corresponding to clearance of least 40% of baseline lactate by 8 hours, assuming first-order clearance kinetics).28
Ethical approval and consent to participate.
Ethics approval for this study was obtained from the University Health Network, University of Toronto (Toronto, Canada), and the Uganda National Council for Science and Technology, Makerere University Research Ethics Committee (Kampala, Uganda). All participating children had a parent or caregiver that provided written informed consent.
Statistical analysis.
Data analyses were performed using GraphPad Prism version 6 (GraphPad Software, Inc., La Jolla, CA, 2012), and R (R Core Team, Vienna, Austria).29 To examine associations between variables; non-parametric methods (Mann–Whitney U test) were used for continuous data, and the two-tailed Pearson Chi-Square or Fisher’s exact test were used for categorical data, as appropriate. Lactate and clinical risk scores as prognostic markers of mortality were analyzed using receiver–operator characteristic (ROC) curves. Survival, stratified by admission lactate level, was studied using Kaplan-Meier analysis. We analyzed longitudinal lactate levels in patients with linear mixed effects (LMEs) models to account for repeated lactate measurements over time using R29 and lme4.30 As fixed effects, we entered time since admission and survival group (death or survival) without interaction term into the model. We modeled intercepts and slopes for each subject as random effects. Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. P-values were obtained by likelihood ratio tests of the full model against the model without survival group and against the model without time as fixed effect, respectively.
Results
We enrolled 155 children admitted with WHO-defined clinical pneumonia from September 2013 to July 2015. Clinical characteristics of the cohort are shown in Table 1. Seven (4%) of children had concurrent diagnoses or comorbidities, including two with trisomy 21, two with diarrhea and dehydration, one with congenital heart disease, one with birth asphyxia, and one with neonatal sepsis. In-hospital mortality was 22/155 (14%).
Table 1.
Clinical characteristics of children hospitalized with pneumonia
| Entire cohort (n = 155) | Non-survivors (n = 22) | Survivors (n = 133) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Female | 90 (58) | 10 (45) | 80 (60) | 0.20 |
| Age (years), median (IQR) | 11 (1.4–20) | 3 (0.02–17) | 12 (3–22) | 0.058 |
| History | ||||
| Cough | 136 (88) | 14 (63) | 122 (92) | < 0.001 |
| Difficulty breathing | 140 (90) | 20 (91) | 120 (80) | > 0.99 |
| Lethargy | 25 (16) | 9 (41) | 16 (12) | < 0.001 |
| Convulsions | 31 (20) | 3 (14) | 28 (21) | 0.57 |
| Vomiting | 46 (30) | 6 (27) | 40 (30) | 0.77 |
| Unable to feed/drink | 57 (37) | 11 (50) | 46 (35) | 0.17 |
| Clinical examination | ||||
| Weight (kg), median (IQR) | 8 (5–10) | 6 (3.8–10) | 8 (6–10) | 0.10 |
| Underweight* | 22 (14) | 5 (23) | 17 (13) | 0.22 |
| Temperature (°C), median (IQR) | 38 (37–38) | 37 (36–37) | 38 (37–38) | 0.063 |
| Fever† | 76 (49) | 7 (32) | 69 (52) | 0.081 |
| Blood pressure (mmHg)‡ | ||||
| Systolic, median (IQR) | 90 (90–100) | 90 (85–100) | 90 (90–100) | 0.54 |
| Diastolic, median (IQR) | 50 (50–50) | 50 (44–60) | 50 (50–50) | 0.67 |
| Heart rate (bpm), median (IQR) | 163 (151–176) | 156 (145–174) | 163 (154–177) | 0.31 |
| Tachycardia§ | 92 (59) | 8 (36) | 84 (63) | 0.033 |
| Respiratory rate (bpm), median (IQR) | 65 (52–78) | 62 (51–85) | 66 (52–77) | 0.72 |
| Tachypnea§ | 136 (88) | 20 (91) | 118 (89) | > 0.99 |
| Hypoxemia (SaO2 < 90%) | 133 (86) | 21 (95) | 112 (84) | 0.21 |
| Altered level of consciousness‖ | 32 (21) | 10 (45) | 22 (17) | 0.002 |
| Deep breathing | 131 (85) | 18 (82) | 113 (85) | 0.75 |
| Nasal flaring | 117 (75) | 17 (77) | 100 (75) | > 0.99 |
| Intercostal retractions | 119 (76) | 18 (82) | 101 (76) | 0.79 |
| Subcostal retractions | 125 (81) | 20 (91) | 105 (79) | 0.25 |
| Wheeze | 27 (17) | 2 (9.1) | 25 (19) | 0.37 |
| Stridor | 17 (11) | 2 (9.1) | 15 (11) | > 0.99 |
| Crackles on auscultation | 78 (50) | 7 (32) | 71 (53) | 0.052 |
| Laboratory assessments | ||||
| CXR¶ | 0.57 | |||
| Normal | 36 (32) | 3 (43) | 33 (32) | |
| Lobar consolidation | 22 (20) | 2 (29) | 20 (19) | |
| Other | 53 (48) | 2 (29) | 51 (49) | |
| Hypoglycemia# | 12 (8) | 6 (27) | 6 (4.5) | 0.002 |
| Lactate (mmol/L), median (IQR) | 2.6 (1.9–4.6) | 7.2 (2.6–9.7) | 2.4 (1.8–3.6) | < 0.001 |
| < 2.0 | 13 (8.4) | 0 (0) | 13 (9.8) | |
| 2.0–4.0 | 103 (66) | 10 (45) | 93 (70) | |
| > 4.0 | 39 (25) | 12 (55) | 27 (20) | |
| Diagnoses | ||||
| Severe pneumonia or very severe disease** | 141 (91) | 22 (100) | 119 (89) | 0.22 |
| Malaria with respiratory distress†† | 9 (5.8) | 2 (9.1) | 7 (5.3) | 0.62 |
| HIV seropositive with pneumonia‡‡ | 5 (3.9) | 2 (9.1) | 3 (2.2) | 0.10 |
| Treatment | ||||
| Antibiotics§§ | ||||
| Azithromycin | 9 (5.8) | 0 (0) | 9 (6.8) | 0.36 |
| Ceftriaxone | 143 (92) | 18 (82) | 125 (94) | 0.070 |
| Supplemental oxygen | 140 (90) | 22 (100) | 118 (89) | 0.13 |
| IV fluid administration | 43 (28) | 12 (55) | 31 (23) | 0.002 |
IQR = interquartile range. Data represent n (%) unless otherwise specified.
* Weight-for-age below -3 standard deviation.31
† Axillary temperature > 37.5°C.
‡ Blood pressure was recorded for 73/155 (47%) of patients.
§ Vital sign > 99 percentile for age.32
‖ Any one of the following: fails to watch or follow with eyes, fails to localize to painful stimulus, or fails to cry or verbalize appropriately with pain.
¶ CXR was performed on 111/155 (72%) of patients.
# Random blood glucose < 3 mmol/L.
** Presence of any general danger sign (vomiting, convulsions, unable to feed/drink, altered consciousness, stridor, or hypoxemia).19
†† Malaria diagnosed by positive histidine-rich protein-2 and parasite lactate dehydrogenase on rapid diagnostic test, and positive blood smear.
‡‡ human immunodeficiency virus testing was performed on one of these patients were under 18 months. Eighteen percent missing data.
§§ All patients were treated with antibiotics. In addition to ceftriaxone and azithromycin, antibiotic treatment included penicillin, ampicillin, cloxacillin, and/or gentamicin.
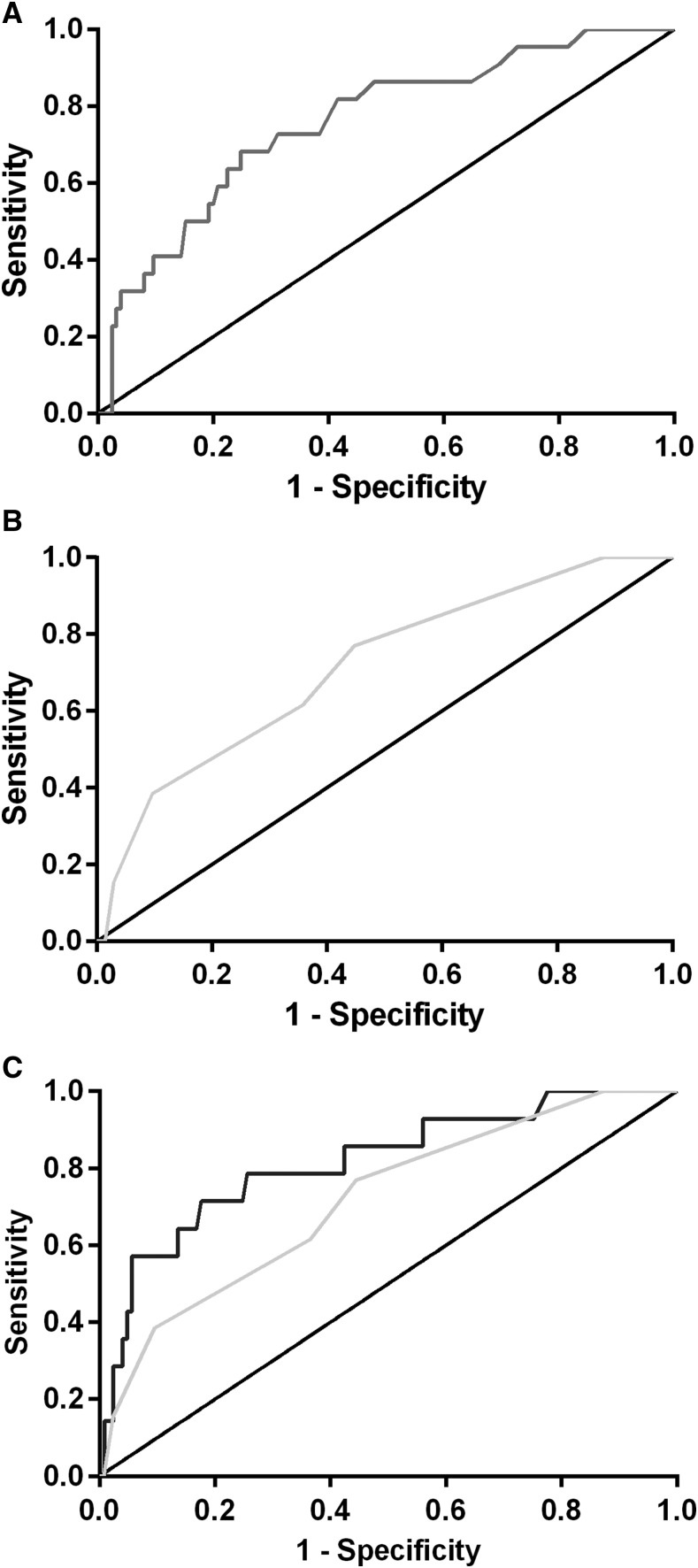
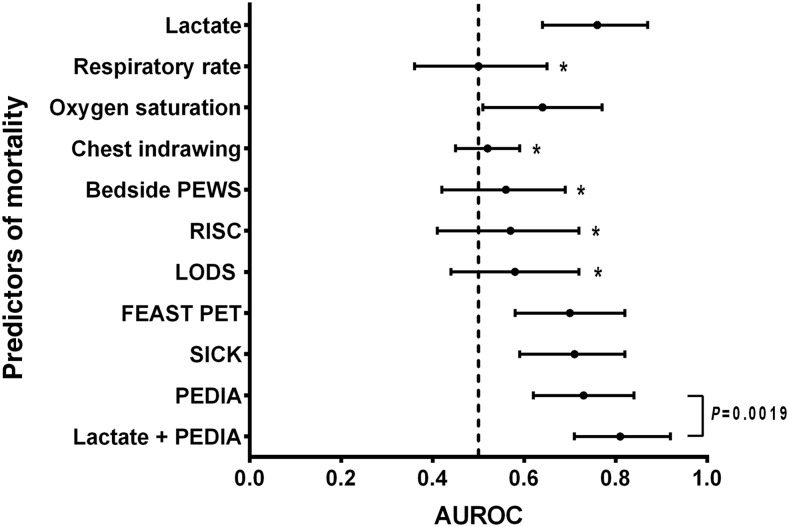
Lactate levels measured using a handheld device from a fingerprick blood test at admission were statistically different in children who died, compared with those who survived (median [interquartile range] 7.2 [2.6–9.7] mmol/L versus 2.4 [1.8–3.6] mmol/L, P = 0.0001, Table 1). Lactate at admission discriminated well between fatal and nonfatal outcomes, with area under the ROC curve (AUROC) statistically significantly different from chance alone (AUROC = 0.76, 95% CI: 0.65–0.87, P = 0.0001, Figure 1A). We compared lactate with other predictors of mortality using ROC analysis, including individual clinical parameters and previously validated composite clinical risk scores (Table 2, Figure 2).22,23,25,26,31 Lactate had a statistically significantly greater AUROC than respiratory rate, chest indrawing, RISC, PEWS, and LODS (P < 0.05 for all comparisons). Although the AUROC for lactate was numerically superior to oxygen saturation, FEAST PET, SICK, and PEDIA, these differences did not reach statistical significance (P > 0.05 for all comparisons). Using logistic regression analysis to construct a predictive model of mortality with both lactate and PEDIA, we found that lactate improved predictive ability beyond PEDIA alone (P = 0.0019, Figure 1C).
Figure 1.
Handheld lactate measurement taken at hospital admission accurately discriminates fatal from nonfatal pediatric pneumonia. Shown are receiver–operator characteristic (ROC) curves for (A) lactate (area under ROC [AUROC] 0.76), (B) Composite clinical score Pediatric Early Death Index for Africa (PEDIA) (AUROC 0.73), and (C) PEDIA augmented with lactate (AUROC 0.81). Of note, the predictive value of lactate + PEDIA was statistically greater than PEDIA alone (P = 0.0019).
Table 2.
Discriminative power of lactate, selected clinical parameters, and composite clinical scores as predictors of mortality
| Predictors | AUROC (95% CI) | P-value | Optimum threshold | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|---|
| Lactate | 0.76 (0.64–0.87) | 0.00013 | 3.4 mmol/L | 0.68 (0.45–0.86) | 0.74 (0.66–0.82) | 0.31 (0.23–0.4) | 0.93 (0.88–0.96) |
| Respiratory rate | 0.50 (0.36–0.65) | 0.99 | 56 minutes−1 | 0.59 (0.36–0.79) | 0.28 (0.2–0.36) | 0.12 (0.086–0.16) | 0.80 (0.70–0.88) |
| Oxygen saturation | 0.64 (0.51–0.77) | 0.036 | 72% | 0.59 (0.36–0.79) | 0.14 (0.082–0.21) | 0.1 (0.074–0.14) | 0.67 (0.51–0.79) |
| Chest indrawing | 0.52 (0.45–0.59) | 0.63 | – | 0.91 (0.71–0.99) | 0.13 (0.076–0.2) | 0.15 (0.13–0.17) | 0.89 (0.68–0.97) |
| Bedside PEWS* | 0.56 (0.42–0.69) | 0.41 | 12 | 0.64 (0.41–0.83) | 0.47 (0.38–0.55) | 0.16 (0.12–0.22) | 0.89 (0.81–0.93) |
| RISC† | 0.57 (0.41–0.72) | 0.32 | 3.5 | 0.5 (0.28–0.72) | 0.71 (0.62–0.78) | 0.22 (0.15–0.32) | 0.90 (0.85–0.93) |
| LODS‡ | 0.58 (0.44–0.72) | 0.16 | 1.5 | 0.50 (0.28–0.72) | 0.73 (0.65–0.80) | 0.23 (0.16–0.34) | 0.90 (0.85–0.93) |
| FEAST PET§ | 0.70 (0.58–0.82) | 0.0022 | 4.5 | 0.41 (0.21–0.64) | 0.89 (0.82–0.94) | 0.37 (0.23–0.55) | 0.90 (0.86–0.93) |
| SICK‖ | 0.71 (0.59–0.82) | 0.002 | 3.7 | 0.68 (0.45–0.86) | 0.72 (0.64–0.80) | 0.29 (0.21–0.38) | 0.93 (0.88–0.96) |
| PEDIA¶ | 0.73 (0.62–0.84) | < 0.0001 | 2.5 | 0.59 (0.36–0.79) | 0.78 (0.70–0.85) | 0.31 (0.22–0.42) | 0.92 (0.87––0.95) |
| Lactate + PEDIA# | 0.81 (0.71–0.92) | < 0.0001 | −1.4 | 0.68 (0.45–0.86) | 0.87 (0.80–0.92) | 0.47 (0.34–0.60) | 0.94 (0.90–0.97) |
NPV = negative predictive value; PPV = positive predictive value.
* Bedside Pediatric Early Warning System (PEWS).23
† Respiratory Index of Severity in Children (RISC).27
‡ Lambarene Organ Dysfunction Score (LODS).25
§ Fluid as Expansive Supportive Therapy Pediatric Emergency Triage (FEAST PET).22
‖ Signs of Inflammation in Children that can Kill (SICK).26
¶ Pediatric Early Death Index for Africa (PEDIA).31
# Logistic regression model using both PEDIA and lactate as mortality predictors.
Figure 2.
Admission lactate measurement is superior or equivalent to clinical signs and composite clinical risk scores in discriminating fatal from nonfatal pediatric pneumonia. Points indicate the estimate of the area under receiver operator characteristic (AUROC) for each predictor, and error bars represent the 95% CI. The asterisk (*) denotes predictors for which the AUROC for lactate was statistically significantly greater (P < 0.05). FEAST PET = fluid as expansive supportive therapy pediatric emergency triage; PEDIA = pediatric early death index for Africa; RISC = respiratory index of severity in children; SICK = signs of inflammation in children that can kill.
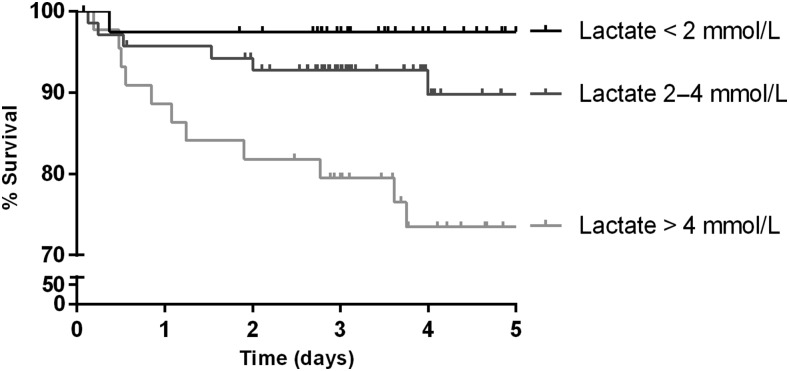
Lactate levels at admission accurately risk-stratified children (Figure 3). Mortality 5-days after admission was 2%, 11%, and 26% among children with lactate of < 2.0, 2.0–4.0, and > 4.0 mmol/L, respectively (P = 0.001). Using LMEs models to analyze the longitudinal course, lactate levels decreased over time (rate 1.1 [95% CI: 0.62–1.5] mmol/L per day, P < 0.0001) and were higher overall in children who died (estimated difference 2.2 [95% CI: 0.98–3.4] mmol/L, P = 0.0005).
Figure 3.
Admission handheld lactate measurement predicts in-hospital mortality. Kaplan–Meier curves show the survival of patients stratified by admission lactate level. Patients were right-censored at the time of hospital discharge or transfer to another facility. Differences between survival curves were statistically significant (P = 0.001).
Following the method of Aramburo et al.,28 slow lactate clearance predicted subsequent mortality. Among 75 children with elevated initial lactate (≥ 2.5 mmol/L) and at least two lactate measurements, 7/22 (32%) with slow lactate clearance had a fatal outcome compared with 6/53 (11%) patients with efficient lactate clearance (P = 0.046).
Discussion
This study demonstrated that a rapid and convenient point-of-care lactate measurement using a handheld, commercially available device is an accurate predictor of mortality among children admitted to hospital with pneumonia in a low-income country. The prognostic value of the admission lactate measurement was equivalent or superior to any clinical sign or composite severity score. Slow lactate clearance predicted subsequent mortality. Simple bedside tools such as lactate could improve the triage and ultimately the management and outcomes of children suffering from pneumonia, especially in a community setting and/or African hospitals with limited access to resources.
Our findings are consistent with several studies demonstrating that high lactate is a marker of poor prognosis in adult pneumonia.9,10,15–17 Mortality in our study (2% and 26% in patients with admission lactate of < 2.0 and > 4.0 mmol/L, respectively) was comparable with a previous report in adults (1.5% and 22.4% for lactate levels of < 2.0 and > 4.0 mmol/L, respectively).10 Serial lactate levels collected during hospitalization were significantly higher in those who died, consistent with previous studies evaluating lactate clearance to document treatment response.9
Prognosis for children with pneumonia may have clinical utility in resource-limited settings in at least two contexts: 1) where minimally trained CHWs use iCCM to triage children for referral or administer oral antibiotics on an outpatient basis; and 2) where trained clinicians in hospitals with a heavy case load and limited access to a central laboratory need to triage cases at highest risk of adverse outcome to high-dependency units.
Our study included only hospitalized children; therefore, these data cannot be easily extrapolated to the community setting. Nonetheless, it is tempting to speculate how a prognostic tool such as handheld lactate could be integrated within iCCM algorithms to augment CHW performance. CHWs play an important role in low-income countries to assess and treat children with pneumonia.8 They use clinical criteria such as cough, cyanosis, chest indrawing, and decreased oral intake to assessseverity.8 Children with non-severe pneumonia are treated in their communities with oral antibiotics, whereas children with severe or very severe pneumonia are transferred to the nearest hospital for further management.4 Prioritizing death reduction, these guidelines predict disease severity with high sensitivity at the expense of specificity.4 However, clinical signs, even among experts, are intrinsically imprecise, and lay CHWs may not accurately count the respiratory rate or appropriately categorize children with pneumonia.29 We showed that a simple handheld lactate measurement was equivalent or superior to any individual clinical sign or composite clinical score in predicting survival outcome (Figure 2). In particular, lactate was superior to tachypnea and chest indrawing, two commonly used signs in the iCCM algorithm.6 The sensitivity of lactate in our study was 0.68 (0.45–0.86), which is comparable with the iCCM algorithm, and specificity was 0.75 (0.67–0.82), which is superior to that of current practices.4 Higher specificity has the potential to significantly improve efficiencies, reduce antibiotic overuse, and mitigate the economic burden of pneumonia.4 Although the use of handheld lactate has potential utility at the community level, our study enrolled hospitalized patients and should be extrapolated with caution to the outpatient setting.
With respect to the hospital setting, handheld lactate measurement could augment a trained health worker’s ability to triage children with pneumonia. Lactate used in combination with the best composite clinical score (PEDIA), increased the accuracy of mortality prediction (Figure 1). Among clinical scores for the prediction of mortality, PEDIA discriminated best between survivors and nonsurvivors in our study. Pediatric early death index for Africa was developed and validated in a cohort of more than 8,000 children admitted to a district hospital in Kenya from 1998 to 2001. In this study, the PEDIA score, derived from a small number of simple clinical signs, accurately predicted outcome, with AUROC of 0.82–0.93 for immediate, early, and late death.31 Similar to our findings, a previous study comparing the prognostic ability of lactate and the confusion, urea, respiratory rate, blood pressure, >65 years of age score in adult pneumonia patients similarly showed lactate’s ability to improve clinical prediction.10 Although a combined score may be difficult to practically implement because of the complexity of PEDIA, this finding provides proof-of-concept that lactate has incremental predictive value, when used together with available clinical data for the prediction of mortality. Lactate clearance had prognostic value in our study, as in a recently published secondary analysis of the FEAST study,28 suggesting that serial lactate measurements could be used to monitor response to therapy in pediatric pneumonia.
Limitations of our study include the observational study design, which demonstrated an association between admission lactate level and subsequent mortality but could not show whether actionable findings could alter management and patient outcomes. Future experimental studies (e.g., randomized controlled trials) should examine whether handheld lactate can guide clinical practice and modify patient outcomes. The sample size, follow-up duration, and number of hospitals included may limit the generalizability of this study. Findings should be confirmed in larger series in low-income community-based settings and high-income countries. The use of a handheld point-of-care device, the Lactate Scout analyzer, instead of conventional laboratory methods to measure circulating lactate levels, may not be seen as a weakness because we have previously shown the accuracy of this device in the field.18 Furthermore, the lack of access to a central laboratory in many low-resource settings implies that our findings could be applied widely in similar contexts. The cost of the Lactate Scout analyzer (approximately USD $350), and the consumable strips (approximately USD $2.42 each) may be an impediment to the use of this test in resource-limited hospitals. However, if applied selectively to critically ill children to guide triage and resource allocation, the prognostic information gained from the test may be worth the additional cost. Cost-effectiveness and/or “willingness-to-pay” studies are warranted to investigate this hypothesis.
Here, we show that handheld point-of-care lactate measurement provides equivalent or superior prediction of mortality than any single clinical sign or composite clinical risk score in children hospitalized with pneumonia. Point-of-care lactate is efficient, objective, and can be used to provide accurate risk stratification to guide allocation of scarce health-care resources in resource-limited settings. Given its accuracy in predicting mortality, lactate can be used to replace or augment clinical parameters which are currently being used. Integrating point-of-care lactate measurement within pneumonia management algorithms may be a promising strategy to reduce global childhood mortality, particularly in low-income countries in Africa and Asia, where 99% of global deaths related to pediatric pneumonia occur.3,4
Acknowledgments:
We would like to thank the patients and parents for their participation. We would also like thank the hospital staff for their support in caring for the children.
REFERENCES
- 1.Wang H, et al. 2014. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 957–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy AL, Lafferty EI, Lovegrove FE, Krudsood S, Tangpukdee N, Liles WC, Kain KC, 2009. Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar J 8: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung DT, Chisti MJ, Pavia AT, 2016. Prevention and control of childhood pneumonia and diarrhea. Pediatr Clin North Am 63: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naydenova E, Tsanas A, Howie S, Casals-Pascual C, De Vos M, 2016. The power of data mining in diagnosis of childhood pneumonia. J R Soc Interface 13: 20160266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang H, et al. 2014. Discovery and validation of biomarkers to guide clinical management of pneumonia in African children. Clin Infect Dis 58: 1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott JA, et al. Pneumonia Methods Working Group , 2012. The definition of pneumonia, the assessment of severity, and clinical standardization in the pneumonia etiology research for child health study. Clin Infect Dis 54(Suppl 2): S109–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO/UNICEF Joint Statement, Integrated Community Case Management (iCCM) , 2012. Integrated Community Case Management. An Equity-Focused Strategy to Improve Access to Essential Treatment Services for Children. Available at: http://www.who.int/maternal_child_adolescent/documents/statement_child_services_access_whounicef.pdf. Accessed April 21, 2016.
- 8.World Health Organization (WHO) , 2014. Revised WHO Classification and Treatment of Childhood Pneumonia at Health Facilities. Available at: www.who.int. Accessed April 21, 2016.
- 9.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW, 2013. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc 88: 1127–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YX, Li CS, 2015. Lactate on emergency department arrival as a predictor of mortality and site-of-care in pneumonia patients: a cohort study. Thorax 70: 404–410. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW, 2005. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med 45: 524–528. [DOI] [PubMed] [Google Scholar]
- 12.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD, 2009. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 37: 1670–1677. [DOI] [PubMed] [Google Scholar]
- 13.Juneja D, Singh O, Dang R, 2011. Admission hyperlactatemia: causes, incidence, and impact on outcome of patients admitted in a general medical intensive care unit. J Crit Care 26: 316–320. [DOI] [PubMed] [Google Scholar]
- 14.Khosravani H, Shahpori R, Stelfox HT, Kirkpatrick AW, Laupland KB, 2009. Occurrence and adverse effect on outcome of hyperlactatemia in the critically ill. Crit Care 13: R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenzen FS, Kutschan U, Meiswinkel N, Schulte-Hubbert B, Ewig S, Kolditz M, 2017. Admission lactate predicts poor prognosis independently of the CRB/CURB-65 scores in community-acquired pneumonia. Clin Microbiol Infect 24: 306.e1–306.e6. [DOI] [PubMed] [Google Scholar]
- 16.Demirel B, 2017. Lactate levels and pneumonia severity index are good predictors of in-hospital mortality in pneumonia. Clin Respir J 12: 991–995. [DOI] [PubMed] [Google Scholar]
- 17.Gwak MH, Jo S, Jeong T, Lee JB, Jin YH, Yoon J, Park B, 2015. Initial serum lactate level is associated with inpatient mortality in patients with community-acquired pneumonia. Am J Emerg Med 33: 685–690. [DOI] [PubMed] [Google Scholar]
- 18.Hawkes M, Conroy AL, Opoka RO, Namasopo S, Liles WC, John CC, Kain KC, 2014. Performance of point-of-care diagnostics for glucose, lactate, and hemoglobin in the management of severe malaria in a resource-constrained hospital in Uganda. Am J Trop Med Hyg 90: 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakrishna B, Graham SM, Phiri A, Mankhambo L, Duke T, 2012. Lactate as a predictor of mortality in Malawian children with WHO-defined pneumonia. Arch Dis Child 97: 336–342. [DOI] [PubMed] [Google Scholar]
- 20.Mulholland K, 2007. Childhood pneumonia mortality—a permanent global emergency. Lancet 370: 285–289. [DOI] [PubMed] [Google Scholar]
- 21.Bonafide CP, Brady PW, Keren R, Conway PH, Marsolo K, Daymont C, 2013. Development of heart and respiratory rate percentile curves for hospitalized children. Pediatrics 131: e1150–e1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George EC, et al. 2015. Predicting mortality in sick African children: the FEAST Paediatric Emergency Triage (PET) score. BMC Med 13: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parshuram, 2009. Development and initial validation of the bedside paediatric early warning system score. Crit Care 13: R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkley JA, Ross A, Mwangi I, Osier FH, Mohammed M, Shebbe M, Lowe BS, Marsh K, Newton CR, 2003. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ 326: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helbok R, et al. 2009. The Lambarene Organ Dysfunction Score (LODS) is a simple clinical predictor of fatal malaria in African children. J Infect Dis 200: 1834–1841. [DOI] [PubMed] [Google Scholar]
- 26.Bhal S, Tygai V, Kumar N, Sreenivas V, Puliyel JM, 2006. Signs of inflammation in children that can kill (SICK score): preliminary prospective validation of a new non-invasive measure of severity-of-illness. J Postgrad Med 52: 102–105. [PubMed] [Google Scholar]
- 27.Hooli S, Colbourn T, Lufesi N, Costello A, Nambiar B, Thammasitboon S, Makwenda C, Mwansambo C, McCollum ED, King C, 2016. Predicting hospitalised paediatric pneumonia mortality risk: an external validation of RISC and mRISC, and local tool development (RISC-Malawi) from Malawi. PLoS One 11: e0168126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aramburo A, et al. 2018. Lactate clearance as a prognostic marker of mortality in severely ill febrile children in east Africa. BMC Med 16: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukanga D, Babirye R, Peterson S, Pariyo GW, Ojiambo G, Tibenderana JK, Nsubuga P, Kallander K, 2011. Can lay community health workers be trained to use diagnostics to distinguish and treat malaria and pneumonia in children? Lessons from rural Uganda. Trop Med Int Health 16: 1234–1242. [DOI] [PubMed] [Google Scholar]
- 30.Bates D, Maechler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. J Stat Software 67: 1–48. [Google Scholar]
- 31.Berkley JA, Ross A, Mwangi I, Osier FH, Mohammed M, Shebbe M, Lowe BS, Marsh K, Newton CR, 2003. Prognostic indicators of early and late death in children admitted to district hospital in Kenya: cohort study. BMJ 326: 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming S, Thompson M, Stevens R, Heneghan C, Plüddemann A, Maconochie I, Tarassenko L, Mant D, 2011. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 377: 1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]